In severe aortic stenosis (AS), the timing of surgical aortic valve replacement or transcatheter aortic valve replacement (TAVR) is currently determined by the hemodynamic status of the valve and symptoms, but it is the myocardial damage caused by myocardial fibrosis (MF) that determines outcome after successful intervention (1). Furthermore, dual AS and cardiac amyloidosis (CA) have been detected in 13% to 16% of elderly patients with AS referred for TAVR, adding to the case for myocardial assessment in AS (2). The detection of MF and CA was previously limited to invasive biopsy or cardiovascular magnetic resonance (CMR); neither is suitable for elderly patients referred for TAVR, the dominant mode of treatment in many countries. Contemporary work-up for TAVR includes cardiac computed tomography (CT), where quantification of extracellular volume by CT (ECVCT) can be performed without additional contrast. We sought to noninvasively evaluate the association of MF with outcome in patients with severe AS having excluded concomitant CA.
Patients with severe AS ≥75 years of age referred for TAVR underwent ECVCT as part of the ATTRact-AS (The Role of Occult Cardiac Amyloid in the Elderly With Aortic Stenosis) study (NCT03029026) protocol (3). This study complied with the Declaration of Helsinki and local ethics and site approvals; all patients provided written informed consent. Patients underwent 99mTc-3,3-diphosphono-1,2-propanodicarboxylic acid (DPD) scintigraphy to exclude CA, a TAVR computed tomographic protocol (SOMATOM Force, Siemens Healthineers) with ECVCT (3), echocardiography, and N-terminal pro–brain natriuretic peptide and high-sensitivity troponin T (hsTnT) measurement. The additional ECVCT acquisitions were precontrast baseline and 3 minutes postcontrast pseudoequilibrium axial shuttle-mode scans, triggered 250 ms after the R wave, analyzed using a dedicated ECVCT prototype for automatic 3-dimensional analysis (Siemens Healthineers). Managing clinicians were blinded to results of ECVCT. All-cause mortality was captured through national mortality registry interrogation. Extended Cox regression with temporal stratification was performed. Survival analysis was performed in R version 4.0.3 (R Foundation for Statistical Computing).
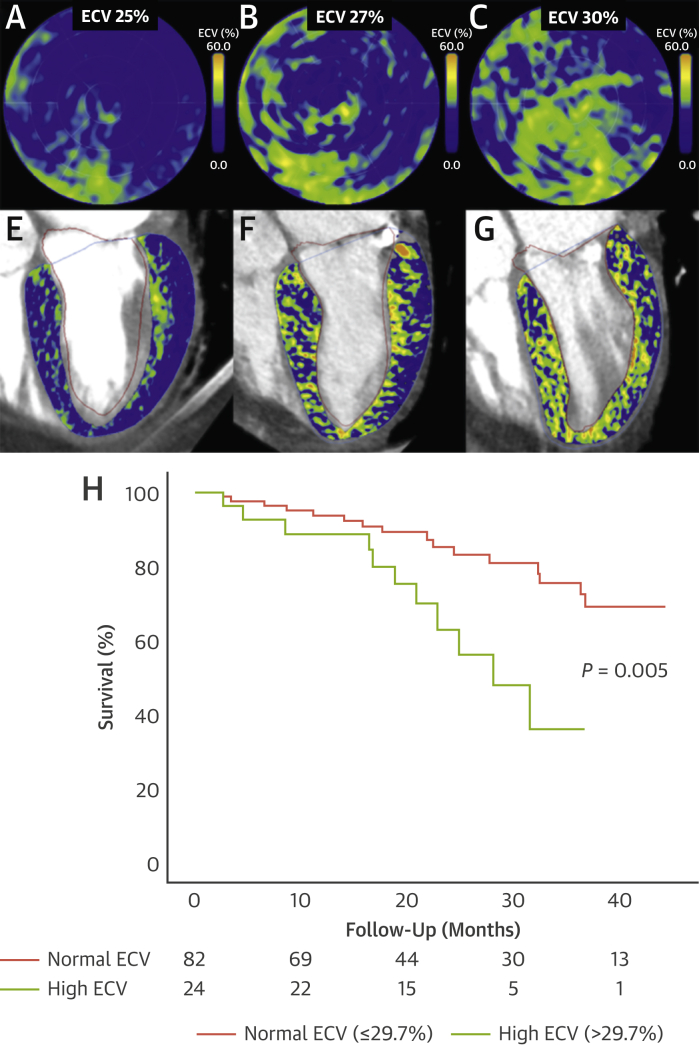
One hundred fifty patients underwent ECVCT and DPD scintigraphy, of whom 18 (12%) were excluded with dual AS-CA; 20 (15%) did not undergo TAVR. One hundred twelve patients (mean age 85 ± 5 years, 49% men) with severe AS (peak aortic valve jet velocity 4.1 ± 0.6 m/s, aortic valve area 0.7 ± 0.2 cm2) and a median ECVCT of 28.0% (IQR: 26.3%-29.7%) were included (Figure 1).
Figure 1.
Examples of ECVCT
(A-C) Extracellular volume quantification by computed tomography (ECVCT) polar maps. (E-G) Four-chamber contrast computed tomography with fused ECVCT color map. (H) Kaplan-Meier survival curves in lone aortic stenosis over a median follow-up period of 21 months by global ECVCT.
Over a follow-up of 21 months (IQR: 15-35 months), 27 deaths (24%) occurred. Only ECVCT (HR: 1.246; P = 0.004) and right ventricular function (tricuspid annular plane systolic excursion; HR: 0.372; P = 0.017) were univariate Cox regression predictors. Excluding patients with focal ECV elevation likely corresponding to myocardial infarction (n = 13 [12%]), ECVCT remained associated with outcome (P = 0.02), with a similar HR (1.22 vs 1.25). On multivariate extended Cox regression, short-term mortality hazard doubled as hsTnT doubled, and medium-term mortality (>1.5 years) doubled for every 2% increase in ECVCT and every 4-mm reduction in tricuspid annular plane systolic excursion (P < 0.0001).
ECVCT as part of the preprocedural cardiac CT predicts outcome after TAVR even after exclusion of dual AS-CA. ECVCT therefore serves as a robust screening tool not only for the detection of AS-CA (3) but also for risk stratification in lone AS. ECVCT as a marker of MF predicts medium-term outcome together with right heart function, whereas short-term outcome is predicted by myocardial injury (hsTnT). Compared with CMR, ECVCT is a faster alternative, offering 3-dimensional isotropic, whole-heart ECV quantification, and it can be integrated into routine preprocedural CT for TAVR without additional iodine contrast, with minimal additional radiation and scan time. Although limited by a small number of events restricting adjustment for confounders, the key strength is the exclusion of 1 in 7 patients with AS-CA, who would have fallen in the high ECVCT category (3). Previous studies showing an association of ECVCT with adverse clinical outcomes post-TAVR could not differentiate fibrosis from amyloidosis as cause of ECV elevation (4), a key missing diagnostic step to target appropriate management (ie, amyloidosis-targeted vs fibrosis-targeted therapeutics) (5). So what is the role of ECVCT in patients under evaluation for TAVR? First, it can be used to identify patients with concomitant AS-CA in order to offer amyloidosis-specific therapies after TAVR. Second, ECVCT can aid in risk stratification according to fibrosis burden, potentially guiding clinicians as to the optimal timing of TAVR, with elevated ECVCT prompting consideration of intervention prior to symptom onset, similar to how such findings might be handled after the detection of elevated ECV on CMR (EVoLVeD [Early Valve Replacement Guided by Biomarkers of LV Decompensation in Asymptomatic Patients With Severe AS]; NCT03094143).
Footnotes
†Drs Pugliese and Treibel are joint last authors. Dr Scully was supported by a British Heart Foundation Clinical Research Training Fellowship (FS/16/31/32185). Dr Patel is supported by an unrestricted educational grant from Edwards Lifesciences and a British Heart Foundation Clinical Research Training Fellowship (FS/19/48/34523). Dr Klotz is a consultant for Siemens Healthineers. Dr Thornton is supported by a British Heart Foundation Clinical Research Training Fellowship (FS/CRTF/21/24128). Dr Saberwal is supported by an educational grant from Siemens Healthineers. Dr Haberland is an employee of Siemens Healthineers. Drs Moon and Treibel are directly and indirectly supported by the University College London Hospitals National Institute for Health Research (NIHR) Biomedical Research Centre and Biomedical Research Unit at University College London Hospitals and Barts, respectively. Dr Pugliese has received research support from Siemens Healthineers; and this work forms part of the translational research portfolio of the NIHR Cardiovascular Biomedical Research Centre at Barts Heart Centre, which is supported and funded by the NIHR. Dr Treibel is funded by a British Heart Foundation Intermediate Research Fellowship (FS/19/35/34374) and previously received consultancy fees from Toshiba Medical Systems Europe. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
References
- 1.Everett R.J., Treibel T.A., Fukui M., et al. Extracellular myocardial volume in patients with aortic stenosis. J Am Coll Cardiol. 2020;75:304–316. doi: 10.1016/j.jacc.2019.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nitsche C., Scully P.R., Patel K.P., et al. Prevalence and outcomes of concomitant aortic stenosis and cardiac amyloidosis. J Am Coll Cardiol. 2021;77:128–139. doi: 10.1016/j.jacc.2020.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Scully P.R., Patel K.P., Saberwal B., et al. Identifying cardiac amyloid in aortic stenosis: ECV quantification by CT in TAVR patients. J Am Coll Cardiol Img. 2020;13(10):2177–2189. doi: 10.1016/j.jcmg.2020.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tamarappoo B., Han D., Tyler J., et al. Prognostic value of computed tomography-derived extracellular volume in TAVR patients with low-flow low-gradient aortic stenosis. J Am Coll Cardiol Img. 2020;13:2591–2601. doi: 10.1016/j.jcmg.2020.07.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maurer M.S., Schwartz J.H., Gundapaneni B., et al. Tafamidis treatment for patients with transthyretin amyloid cardiomyopathy. N Engl J Med. 2018;379:1007–1016. doi: 10.1056/NEJMoa1805689. [DOI] [PubMed] [Google Scholar]