Introduction
Adoptive transfer of T cells (ACT) that are genetically engineered to express a chimeric antigen receptor (CAR) specific for a cell surface molecule, or a native T cell receptor (TCR) that recognizes peptide/MHC complexes can be effective in refractory malignancies and hold promise in many cancers[1–6]. ACT for solid tumors can be challenging because many target antigens are expressed on normal tissues, leading to a risk of on target off tumor toxicity[7–10]. Furthermore, efficacy can be compromised because some tumor cells may lack antigen expression and escape recognition[11–14], or T cells may become dysfunctional due to chronic antigen stimulation in the tumor microenvironment[15–18]. Natural signaling pathways in T cells integrate numerous inputs that dictate the functional response suggesting that synthetic controls that mirror how T cells integrate signals might better regulate effector functions for safety and efficacy[19,20]. An early cell engineering approach attempted to improve safety by limiting on target, off tumor recognition using split chimeric receptors linked to binding domains specific for different tumor antigens. Co-expressing a suboptimal CAR specific for one tumor antigen with a chimeric costimulatory receptor specific for a second tumor antigen limited full T cell activation to encounters with target cells that expressed both antigens[21]. An alternative approach co-expressed an inhibitory CAR specific for a molecule on normal cells, thereby abrogating signaling if the normal cell also expressed the target of the activating CAR[22]. These studies served as proof of principle that T cell recognition could be tuned by integrating multiple inputs but were not evaluated in rigorous models or developed clinically. Recent advances have enabled the application of more complex “AND”, “OR” and “NOT” logic gates in T cells that can act in a cell intrinsic or extrinsic fashion to precisely regulate effector functions and overcome barriers in ACT (Table 1). The focus of this review will be to discuss these developments and their promise for controlling therapeutic T cells.
Table 1.
Applications of synthetic biology to improve adoptive T cell therapy.
| Challenge | Solutions |
|---|---|
| Antigen loss or heterogeneity | Multi-specific, universal and ‘OR’ gated receptors that specify constellations of antigens that can be recognized |
| Normal tissue expression | ‘AND’ and ‘NOT’ gated cell extrinsic or intrinsic controls |
| Toxicity due to T cell activation | Tune recognition with user controlled small molecule controls |
| T cell dysfunction | Imposing intermittent rest to receptor signaling through small molecule control or regulated receptor expression |
Multi-specific and universal chimeric receptors to overcome tumor antigen loss
Engineering T cells to recognize a single molecule on B cell malignancies can lead to the outgrowth of antigen-negative tumor cells that escape recognition[11,12,14]. Surface antigen expression on solid tumors can be heterogeneous, and outgrowth of antigen-negative variants has been observed with CAR and TCR engineered T cells[23–26]. Targeting multiple antigens with a TCR engineered product is challenging due to the potential for mispairing of TCR chains and competition for signaling molecules if more than one TCR is expressed[27,28]. Unlike TCRs, multi-specific CAR T cells that target two or more antigens can be readily developed. Multiple binding moieties separated by flexible linkers can be incorporated in a single tandem receptor, different CAR transgenes can be encoded in a multi-cistronic vector, or CAR T cells recognizing a single Ag can be engineered to secrete bi-specific T cell engagers that bind to alternative Ag(s) and activate T cells by binding to CD3[29–34]. It is necessary to optimize the stoichiometry of a tandem receptor to efficiently recognize more than one target molecule on the tumor and vector size can reduce gene delivery efficiency. Despite these limitations, such “OR” gated CAR T cells are being evaluated for B cell malignancies to determine whether depth and durability of tumor elimination can be improved[35].
Targeting multiple antigens with a single engineered T cell has also been accomplished using universal receptors. Targeting ligands such as scFvs or antibodies that bind to tumor cells form a bridge to T cells expressing a receptor capable of binding the ligand[36]. This was first described using a biotin-binding immune receptor consisting of dimeric avidin linked via a transmembrane domain to 4–1BB and CD3ζ signaling domains in T cells, enabling recognition of tumor cell antigens bound with biotinylated antibodies[37,38]. Numerous universal receptors were designed to overcome limitations of the avidin-biotin system, including expression of a high-affinity CD16 4–1BB/CD3ζ chimeric receptor that binds Fc fragments and redirects T cell specificity to target cells bound by monoclonal antibodies[39]; grafting a neoepitope (PNE, FITC or E5B9) onto tumor targeting monoclonal antibodies that can be recognized by a CAR designed with a PNE, FITC or E5B9 specific scFv respectively[40–43]; and the Spycatcher/Spytag system to redirect T cells to tumor cells[44]. An elegant universal strategy for gating of T cell recognition uses a two-component receptor system designed by linking a leucine zipper extracellular domain to an intracellular signaling domain expressed in T cells (zipCAR) and one or more tumor-targeting scFv adaptors (zipFv) constructed by fusing a cognate leucine zipper and an scFv[45]. This approach can respond to multiple antigens and could tune T cell activation strength by titrating administration of scFv adaptors to mitigate toxicity. Universal receptors provide modular platforms capable of ‘OR’ and possibly ‘AND’ gated recognition of multiple antigens with a single CAR T cell product and are well suited for clinical applications in hematologic malignancies to combat antigen loss[46]. These approaches have the potential to reduce toxicity but the immunogenicity of some universal CAR approaches may limit T cell persistence. Multi-specific targeting and universal CAR approaches have already advanced to the clinic and safety and efficacy data is eagerly awaited.
Complex cell extrinsic logic gating of T cell recognition using protein switches
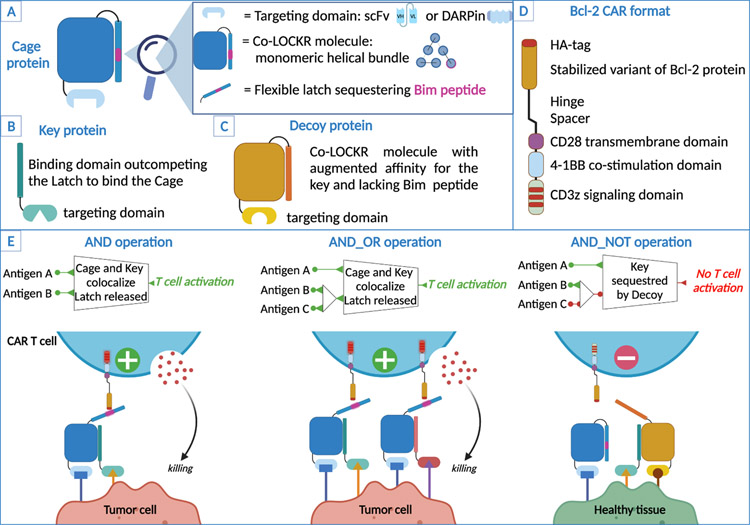
A novel extension of the universal CAR approach uses fully synthetic protein switches compatible with CAR recognition and capable of complex ‘OR’, ‘AND’, and ‘NOT’ logic operations. This system uses colocalization-dependent orthogonal proteins (Co-LOCKR) composed of a structural “Cage” protein with a “Latch” domain that holds a functional peptide in an inactive conformation until binding of a separate “Key” protein. Key binding induces a conformational change exposing the peptide for recognition by a universal CAR [47]. For CAR T cells, Co-LOCKR switches were designed with a caged Bim peptide, which when exposed, can be recognized by T cells expressing a CAR designed with a Bcl-2 ectodomain linked to CD28/CD3ζ signaling domains. Cage and Key proteins target cell surface antigens using scFv or DARPin recognition domains and T cells are only activated when Cage and Key are colocalized at the cell surface by binding domains attached to each Co-LOCKR component (Fig. 1). Using binders specific for Her2, EpCAM and EGFR, Co-LOCKR targeting could direct ‘AND’ and ‘OR’ gated T cell recognition of tumor cell lines expressing various constellations of these antigens, and ‘NOT’ logic was achieved with a Decoy Key linked to EGFR that had a higher affinity for the Cage[47]. Thus, Co-LOCKR could integrate multiple inputs to instruct T cell specificity in a fashion not yet achieved with other approaches. This technology has similar limitations to other universal CARs including immunogenicity of component molecules, the need to optimize synapse formation between T cell and target cell, and determine appropriate pharmacokinetics for in vivo applications. Translation to the clinic of this cell extrinsic strategy requires further development but is certainly an intriguing approach to provide adaptability and safety in tumor targeting.
Figure 1. Components and logic-gating operations of the Co-LOCKR system.
A) Schematic of the Cage protein. The Cage protein is composed of a targeting domain (either an scFv or a DARPin) linked to a LOCKR molecule designed using Rosetta[80,81]. Co-LOCKR is monomeric and less prone to aggregation because of shorter helices and improved hydrophobic packing. The Cage contains a Latch that sequesters a Bim peptide. B) Schematic of the Key protein. The Key protein is composed of a targeting domain (scfv or DARPin) and a binding domain specific to the Co-LOCKR protein. The key binds to the same region than the Latch on the Co-LOCKR molecule but with higher affinity and therefore outcompetes the Latch while both the Cage and the Key are co-localized at the surface of a target cell. C) Schematic of the Decoy protein. The Decoy protein is an affinity tuned Cage that doesn’t sequester Bim and has a stronger affinity for the Key than the Cage molecule that sequesters Bim, thereby preventing the Key from exposing Bim. D) Schematic of the Bcl-2 CAR construct that recognizes Bim. E: Co-LOCKR reagents and Bcl-2 CAR T cells perform AND, AND_OR and AND_NOT logic-gating operations and tumor cells or healthy tissue. AND operation: The Cage and Key proteins bind to their target antigens at the surface of the tumor cell, the Key then outcompetes the Latch exposing Bim for recognition by CAR T cells. AND_OR operations: Two Keys specific for 2 antigens can independently induce activation of the CAR T cells. AND_NOT operation: The Decoy protein binds to its target antigen and sequesters the Key thus preventing Bim exposure and Bcl-2 CAR T cells activation.
Cell intrinsic regulation with SynNotch receptors
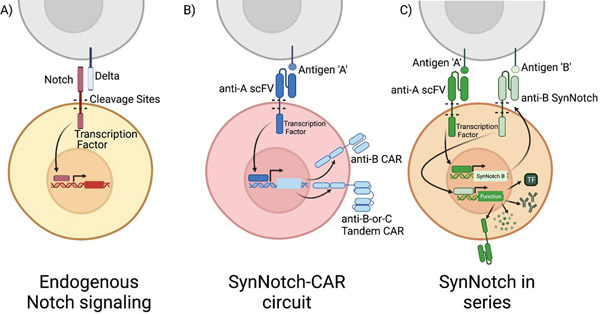
An alternative to protein switches is to regulate T cell specificity with chimeric synthetic Notch (SynNotch) receptors. All cells respond to environmental cues through cell surface receptors that determine transcriptional and functional responses. Engagement of the extracellular domain of Notch by cellular ligands results in cleavage of the receptor in its transmembrane domain and release of the intracellular domain that directs transcription of Notch regulated genes, making it ideal for generating synthetic signaling circuits in T cells[48]. The Notch pathway could be altered by either replacing the extracellular recognition domain or the cleaved intracellular domain[49]. Based on this, the Lim lab generated diverse chimeric SynNotch receptors that retain the cleavage domain, but replaces the extracellular domain with a recognition domain specific for a cell surface antigen, and the intracellular domain with a transcription factor that is released upon ligand binding and enters the nucleus to initiate transcription of an introduced transgene[50]. In principle, a SynNotch receptor can release any user-defined transcription factor, linking antigen recognition with a distinct transcriptional program that controls the cell’s response (Fig. 2). Applications of SynNotch receptors include regulating the expression of a CAR specific for a second antigen using a Gal4VP64 transcription factor to drive CAR expression. In this way, T cell effector function depends on recognizing two antigens, thereby limiting off tumor toxicity. This was demonstrated by engineering T cells to express a SynNotch receptor specific for a cell surface GFP antigen and a CD19 4–1BB/ζ CAR transgene under control of Gal4VP64. In NSG mice implanted in separate flanks with K562 cells expressing both surface GFP and CD19, and with K562 cells expressing only CD19, these T cells controlled the growth of the tumor expressing both GFP and CD19, but not the tumor expressing only CD19[51].
Figure 2. Logic gating using SynNotch receptors.
A) Endogenous Notch signaling: The Notch receptor is activated by the binding of its extracellular domain to delta family ligands on the surface of interacting cells. Physical binding results in cleavage and release of the Notch receptor’s intracellular domain, which is then free to translocate to the nucleus where it can activate transcription of target genes. B) SynNotch-CAR circuit: The endogenous Notch receptor can be altered to recognize antigen with a single chain variable fragment (scFV) allowing for user-defined targeting of binding. The intracellular domain of Notch is replaced with a Gal4 DNA binding domain. A chimeric antigen receptor under the control of a Gal4 is then expressed only when the SynNotch receptor binds its antigen (‘A’). This serial expression imposes a two-antigen restriction on T cell activation. First the SynNotch receptor must bind ‘A’ in order to express an anti-’B’ CAR, then the CAR must bind antigen ‘B’ in order to activate the T cell. The cell must interact in both A and B to activate. Further inputs can be added by altering the antigen targeting of the downstream CAR. A tandem CAR activated by SynNotch can provide ‘OR’ logic gated T cell recognition of either antigen C or D. C) SynNotch in Series: The SynNotch system can be used to direct T cell function and do so under multiple-antigen restriction. Expression of two SynNotch receptors in series imposes a two-antigen requirement on the expression of a particular transcriptional program. Recognition of the primary SynNotch receptor’s cognate antigen, ‘A,’ leads to transcription factor cleavage and expression of a secondary SynNotch receptor targeting ‘B.’ Binding of B results in cleavage of a second transcription factor which can control expression of a diverse array of genes that impact T cell function, including expression of transcription factors, therapeutic antibodies, cytokines or CARs.
While expression of a CAR in series with a Notch receptor can impose a two-antigen requirement, this is not a strict ‘AND’ condition. Both antigens need not be present on the same cell since the engagement of the SynNotch receptor can drive expression of the CAR through interaction with one cell, and then target the same or a different cell that expresses the CAR target. Rather than ‘AND’ gating, SynNotch applies ‘If-then’ logic and a consequence is less stringent control of CAR-mediated killing of normal tissue, particularly since once expressed, the CAR has a half-life of 8 hours[51]. This is a limitation when tumor cells are near normal cells that express the CAR target. Indeed, this design of SynNotch did not prevent toxicity to normal cells when the tumor cells were adjacent to normal cells expressing the same CAR antigen[10].
Despite this limitation, combinatorial logic achieved with SynNotch circuits can increase the types of tumors targeted using CAR T cell therapies. Single antigens cannot distinguish many solid tumors from healthy tissues and including a second or third antigen requirement significantly improves the discriminatory power of T cells[52]. Furthermore, targeting a single antigen can fail to clear tumors with heterogenous antigen expression. An example of both challenges is seen in T cell therapy of glioblastoma. Mutant EGFRvIII on glioblastoma is tumor-specific but heterogeneously expressed, while other targets, including ephrin type-A receptor 2 (EphA2) and interleukin 13 receptor a2 (IL13Ra2), are more homogenous but also expressed in normal cells. Choe and colleagues demonstrated that a SynNotch receptor that recognizes the tumor-specific antigen EGFRvIII and induces expression of a tandem CAR specific for EphA2 or IL13Ra2 can improve targeting. SynNotch T cells controlled growth of implanted tumors with heterogenous EGFRvIII expression better than T cells constitutively expressing either an EGFRvIII CAR or the tandem EphA2 IL12Ra2 CAR[53]. SynNotch can also function in parallel for more complex circuits, where the activation of the first receptor leads to the expression of a second SynNotch receptor that controls the desired cell function[54].
In addition to precise tumor targeting, intermittent expression of a CAR driven by a SynNotch circuit may improve T cell function by preventing the development of T cell exhaustion due to tonic activation of a constitutively expressed CAR[55]. Thus, SynNotch can add stringency to T cell recognition, potentially expanding the types of tumors treatable with CAR T cells and improving T cell function by restricting CAR expression. Still, the logic imposed through SynNotch has limitations, including on-target off-tumor targeting of nearby healthy tissue and the use of foreign transcription factors. Continued engineering of SynNotch circuits is likely to resolve these limitations, making this a promising logic gated approach for next generation T cell therapies. It is anticipated that clinical trials to test SynNotch logic gating will soon be initiated.
Logic gating using small molecules
The administration of small molecule drugs to provide temporal, spatial and/or qualitative control of T cell activity may mitigate severe toxicities of T cell therapy such as graft versus host disease (GVHD), cytokine release syndrome (CRS) and immune effector cell-associated neurotoxicity syndrome (ICANS), and improve T cell function. An early iteration of this was developed to control GVHD after adoptive cell transfer of donor lymphocytes to treat patients relapsing with EBV-induced lymphoma following allogenic bone marrow transplantation. Donor lymphocytes were genetically modified to express herpes simplex virus thymidine kinase and were effectively eliminated by administration of ganciclovir in patients developing severe GVHD[56]. Small molecule regulation can be applied to TCR and CAR based cell therapies such that activation requires both an autonomous input (i.e. antigen recognition) and a user-controlled input (i.e. small molecule ), providing broad control over T cell function (Fig. 3). This approach was initially conceived to reduce CRS and ICANS directly related to CAR T cell activation and to limit on-target off-tumor recognition. An inducible active domain of caspase 9 fused to a mutated drug-binding domain of FBKP protein was shown to rapidly and efficiently trigger cell-death following administration of the dimerizing agent AP1903[57–59]. Truncated EGFR (tEGFR), CD20 sequences or a myc-tag were included in TCR and CAR designs to permit antibody-mediated cellular depletion following administration of respective antibodies[60–65]. These strategies can be engineered to be somewhat tunable, but eliminate CAR T cells irreversibly and abrogate any anti-tumor effects of the therapy[66].
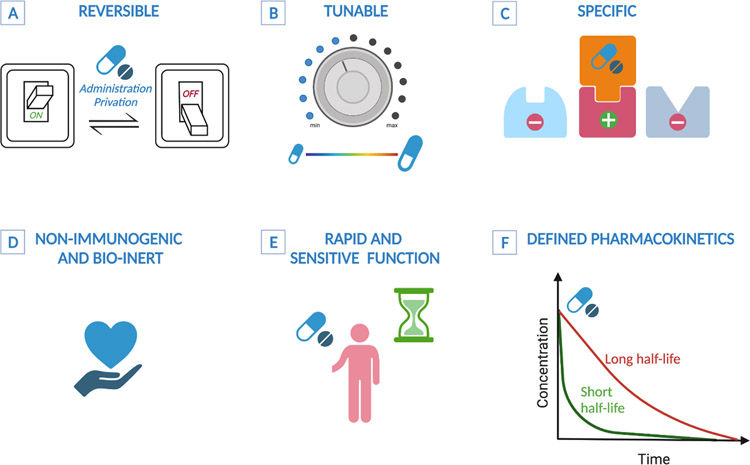
Figure 3. Desired features of small molecule regulation of T cell functions.
A) Reversible: regulation dependent on small molecule administration should be reversible and alternate between ON and OFF states based on administration/privation cycles. B) Tunable: amplitude of T cell functions should correlate to the concentration of the small compound. C) Specificity: the small molecule should be specifc for its target on T cells. D) Bio-inert and non-immunogenic: ensures tolerability and repeated administration. E) Rapid and sensitive: the small molecule should rapidly diffuse after infusion and be highly sensitive for its target. F) Defined pharmacokinetics: to guide a desired effect, for example a long half-life is preferred for a prolonged period of active regulation and a short half-life when rapid and dynamic regulation is preferred.
More recently, this strategy was employed to control the strength and duration of activation and preserve T cell anti-tumor functions. Wu et al redesigned the CAR construct as two membrane anchored subunits that spatially separated the antigen recognition domain from the CD3ζ signaling domain and required a small molecule to dimerize these components into a functional receptor (“ON-switch” CARs).[67] The CAR disassembled in the absence of the dimerizing agent but rapidly reassembled after administration of the rapamycin analog AP21967. This system recapitulated an ‘AND’ gate requiring both the presence of the targeted antigen and the small molecule to confer T cells with anti-tumor activity. Interestingly, T cell functions were tunable and could be titrated depending on the small-molecule concentration. Additional versatility of the ON-switch CAR design was demonstrated by redirecting recognition of a second tumor antigen using a soluble scFv that also contained an FKBP12 domain and was pre-loaded with rapamycin (termed “plug-in”)[68]. The plug-in molecule dimerized the membrane-anchored CAR moiety containing the signaling domains with the soluble scFv, thereby inducing T cell activation. Therefore, in addition to improving safety, small molecule administration could prevent tumor escape/relapse mediated by antigen loss. Small molecule dimerization of receptors has also been used as a rheostat to regulate the number of receptors that are activated, potentially limiting on target off tumor toxicity[69]. In this approach, two low-affinity CARs specific for two distinct antigens require heterodimerization for T cell activation, thereby restricting recognition of two tumor antigens by administration of a small molecule. This approach increases specificity for the tumor cells over healthy tissues and improves safety of CAR T cell therapy.
A recent study described an “ON-switch” strategy applicable to both CAR and TCR transgenic T cells. In order to create a universal strategy, the signaling cascade common to both TCRs and CARs relying on phosphorylation of ITAM domains and recruitement of Zap70 via its SH2 domains following antigen triggering was targeted. This approach regulates T cell activation by expression of a chimeric protein consisting of a fusion of the SH2 domains of Zap70 for binding to CD3ζ and the inhibitory tail of the PD-1 receptor to inhibit TCR functions, linked to an HCV protease and a degron sequence (SMASh-tag). In the absence of a protease inhibitor, the HCV protease cleaves a linker between the SMASh tag and the inhibitory domains preventing protein degradation and therefore the Zap70-PD-1 protein is recruited to the CAR or TCR and inhibits T cell functions. Administration of an HCV protease inhibitor leads to degradation of the fusion protein and restores T cell function in a reversible and titrable mannercite.
There are limitations to ON-switch CARs and TCRs. The small molecule needs to be provided at a sufficient level and duration in vivo to maintain CAR expression and anti-tumor functions. Dosing will depend on the half-life and the biodistribution of the various dimerizing compounds. The development of synthetic bio-inert candidates with optimized pharmacokinetics as alternatives to rapalog and rapamycin are also needed to avoid the immunosuppressive effects of these agents.
CAR expression can alternatively be negatively regulated using small molecules to induce receptor degradation rather than assembly. One approach capitalizes on the description of a ligand induced degradation domain (LID) where a cryptic degron is incorporated into a mutated variant of FKBP12[70,71]. This mutant possesses a secondary structure that allows for high affinity and specific binding of an engineered ligand named Shield-1. LID was fused to the C-terminal end of the CAR construct and addition of Shield-1 induced exposure of the degradation domain and subsequent proteasomal degradation of the CAR. CAR expression and T cell functions were linearly proportional to Shield-1 concentration. Some toxicities following CAR T cell infusion could be controlled in mouse models with this approach, however maximum reduction of CAR expression following Shield-1 administration and recovery of CAR expression after drug washout were both achieved after 24h suggesting this system might be insufficient to control severe acute toxicities.
Transient inhibition of TCR or CAR signaling or CAR expression might counter T cell exhaustion in solid tumor models. The concept of providing periods of rest from T cell signaling to preserve function or prevent toxicities was suggested by studies using the tyrosine kinase inhibitor dasatinib, which rapidly and reversibly inhibits Lck and Zap70[72–74].
Activation of CAR T cells could be selectively regulated by fusing a degradation domain (DD) to the N terminus of the CAR leading to constant degradation of the receptor, unless Shield-1 was provided to inhibit degradation and restore CAR expression[75]. This approach could prevent signaling during generation of T cells engineered with a tonically signaling CAR that drives T cell dysfunction in the absence of ligand binding, preserving T cell function and preventing the epigenetic changes that occur with T cell exhaustion. These studies provide compelling proof of principle that small molecule control of T cell function and CAR expression can improve the therapeutic window in a variety of clinical applications. The efficacy and safety of these approaches require well designed clinical trials that assess safety, biologic and clinical endpoints.
Conclusions
The success of genetically modified T cells in cancer therapy has provided unprecedented opportunities for novel approaches to regulate cell specificity and function using synthetic biology. To succeed, these efforts to improve on biology should be grounded in a fundamental understanding of positive and negative signaling pathways in T cells, synapse formation, and T cell differentiation states[76–79]. This is a challenging endeavor and the lack of a complete understanding of cell signaling networks and animal models that fully recapitulate the barriers in human tumor therapy to test logic gated recognition approaches remain significant barriers. However, with future advances, the field of synthetic biology holds promise for improving the safety and specificity of immunotherapy.
HIGHLIGHTS.
Synthetic antigen receptors that mimic T cell signaling can redirect T cells to target tumors.
Achieving selective and complete recognition of tumor cells with T cells can be challenging.
Synthetic receptors with cell extrinsic or instrinsic controls provide logic to cell recognition.
Customized receptors can precisely instruct T cell function, improve safety and overcome antigen escape.
Acknowledgements
We acknowledge funding support from the NIHR01 CA114536 (SRR), the Department of Defence BC190327P1 (SRR). SS was supported by a Special Fellow award from The Leukemia & Lymphoma Society.
Footnotes
Competing interests: S.R.R. and A.I.S. are inventors on patents related to cellular immunotherapy and hold equity in Lyell Immunopharma.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Neelapu SS, Locke FL, Bartlett NL, Lekakis LJ, Miklos DB, Jacobson CA, Braunschweig I, Oluwole OO, Siddiqi T, Lin Y, et al. : Axicabtagene Ciloleucel CAR T-Cell Therapy in Refractory Large B-Cell Lymphoma. N Engl J Med 2017, 377:2531–2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schuster SJ, Svoboda J, Chong EA, Nasta SD, Mato AR, Anak O, Brogdon JL, Pruteanu-Malinici I, Bhoj V, Landsburg D, et al. : Chimeric Antigen Receptor T Cells in Refractory B-Cell Lymphomas. N Engl J Med 2017, 377:2545–2554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abramson JS, Palomba ML, Gordon LI, Lunning MA, Wang M, Arnason J, Mehta A, Purev E, Maloney DG, Andreadis C, et al. : Lisocabtagene maraleucel for patients with relapsed or refractory large B-cell lymphomas (TRANSCEND NHL 001): a multicentre seamless design study. Lancet 2020, 396:839–852. [DOI] [PubMed] [Google Scholar]
- 4.Raje N, Berdeja J, Lin Y, Siegel D, Jagannath S, Madduri D, Liedtke M, Rosenblatt J, Maus MV, Turka A, et al. : Anti-BCMA CAR T-Cell Therapy bb2121 in Relapsed or Refractory Multiple Myeloma. N Engl J Med 2019, 380:1726–1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nagarsheth NB, Norberg SM, Sinkoe AL, Adhikary S, Meyer TJ, Lack JB, Warner AC, Schweitzer C, Doran SL, Korrapati S, et al. : TCR-engineered T cells targeting E7 for patients with metastatic HPV-associated epithelial cancers. Nat Med 2021, 27:419–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.D’Angelo SP, Melchiori L, Merchant MS, Bernstein D, Glod J, Kaplan R, Grupp S, Tap WD, Chagin K, Binder GK, et al. : Antitumor Activity Associated with Prolonged Persistence of Adoptively Transferred NY-ESO-1 (c259)T Cells in Synovial Sarcoma. Cancer Discov 2018, 8:944–957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lamers CH, Sleijfer S, Vulto AG, Kruit WH, Kliffen M, Debets R, Gratama JW, Stoter G, Oosterwijk E: Treatment of metastatic renal cell carcinoma with autologous T-lymphocytes genetically retargeted against carbonic anhydrase IX: first clinical experience. J Clin Oncol 2006, 24:e20–22. [DOI] [PubMed] [Google Scholar]
- 8.Morgan RA, Yang JC, Kitano M, Dudley ME, Laurencot CM, Rosenberg SA: Case report of a serious adverse event following the administration of T cells transduced with a chimeric antigen receptor recognizing ERBB2. Mol Ther 2010, 18:843–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Srivastava S, Riddell SR: Chimeric Antigen Receptor T Cell Therapy: Challenges to Bench-to-Bedside Efficacy. J Immunol 2018, 200:459–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Srivastava S, Salter AI, Liggitt D, Yechan-Gunja S, Sarvothama M, Cooper K, Smythe KS, Dudakov JA, Pierce RH, Rader C, et al. : Logic-Gated ROR1 Chimeric Antigen Receptor Expression Rescues T Cell-Mediated Toxicity to Normal Tissues and Enables Selective Tumor Targeting. Cancer Cell 2019, 35:489–503 e488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sotillo E, Barrett DM, Black KL, Bagashev A, Oldridge D, Wu G, Sussman R, Lanauze C, Ruella M, Gazzara MR, et al. : Convergence of Acquired Mutations and Alternative Splicing of CD19 Enables Resistance to CART-19 Immunotherapy. Cancer Discov 2015, 5:1282–1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gardner R, Wu D, Cherian S, Fang M, Hanafi LA, Finney O, Smithers H, Jensen MC, Riddell SR, Maloney DG, et al. : Acquisition of a CD19-negative myeloid phenotype allows immune escape of MLL-rearranged B-ALL from CD19 CAR-T-cell therapy. Blood 2016, 127:2406–2410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shah NN, Highfill SL, Shalabi H, Yates B, Jin J, Wolters PL, Ombrello A, Steinberg SM, Martin S, Delbrook C, et al. : CD4/CD8 T-Cell Selection Affects Chimeric Antigen Receptor (CAR) T-Cell Potency and Toxicity: Updated Results From a Phase I Anti-CD22 CAR T-Cell Trial. J Clin Oncol 2020, 38:1938–1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Samur MK, Fulciniti M, Aktas Samur A, Bazarbachi AH, Tai YT, Prabhala R, Alonso A, Sperling AS, Campbell T, Petrocca F, et al. : Biallelic loss of BCMA as a resistance mechanism to CAR T cell therapy in a patient with multiple myeloma. Nat Commun 2021, 12:868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Blank CU, Haining WN, Held W, Hogan PG, Kallies A, Lugli E, Lynn RC, Philip M, Rao A, Restifo NP, et al. : Defining ‘T cell exhaustion’. Nat Rev Immunol 2019, 19:665–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen J, Lopez-Moyado IF, Seo H, Lio CJ, Hempleman LJ, Sekiya T, Yoshimura A, Scott-Browne JP, Rao A: NR4A transcription factors limit CAR T cell function in solid tumours. Nature 2019, 567:530–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sen DR, Kaminski J, Barnitz RA, Kurachi M, Gerdemann U, Yates KB, Tsao HW, Godec J, LaFleur MW, Brown FD, et al. : The epigenetic landscape of T cell exhaustion. Science 2016, 354:1165–1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Martinez M, Moon EK: CAR T Cells for Solid Tumors: New Strategies for Finding, Infiltrating, and Surviving in the Tumor Microenvironment. Front Immunol 2019, 10:128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Roybal KT, Lim WA: Synthetic Immunology: Hacking Immune Cells to Expand Their Therapeutic Capabilities. Annu Rev Immunol 2017, 35:229–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lim WA, June CH: The Principles of Engineering Immune Cells to Treat Cancer. Cell 2017, 168:724–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kloss CC, Condomines M, Cartellieri M, Bachmann M, Sadelain M: Combinatorial antigen recognition with balanced signaling promotes selective tumor eradication by engineered T cells. Nat Biotechnol 2013, 31:71–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fedorov VD, Themeli M, Sadelain M: PD-1- and CTLA-4-based inhibitory chimeric antigen receptors (iCARs) divert off-target immunotherapy responses. Sci Transl Med 2013, 5:215ra172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.O’Rourke DM, Nasrallah MP, Desai A, Melenhorst JJ, Mansfield K, Morrissette JJD, Martinez-Lage M, Brem S, Maloney E, Shen A, et al. : A single dose of peripherally infused EGFRvIII-directed CAR T cells mediates antigen loss and induces adaptive resistance in patients with recurrent glioblastoma. Sci Transl Med 2017, 9(399):eaaa0984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tchou J, Zhao Y, Levine BL, Zhang PJ, Davis MM, Melenhorst JJ, Kulikovskaya I, Brennan AL, Liu X, Lacey SF, et al. : Safety and Efficacy of Intratumoral Injections of Chimeric Antigen Receptor (CAR) T Cells in Metastatic Breast Cancer. Cancer Immunol Res 2017, 5:1152–1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tran E, Robbins PF, Lu YC, Prickett TD, Gartner JJ, Jia L, Pasetto A, Zheng Z, Ray S, Groh EM, et al. : T-Cell Transfer Therapy Targeting Mutant KRAS in Cancer. N Engl J Med 2016, 375:2255–2262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Paulson KG, Voillet V, McAfee MS, Hunter DS, Wagener FD, Perdicchio M, Valente WJ, Koelle SJ, Church CD, Vandeven N, et al. : Acquired cancer resistance to combination immunotherapy from transcriptional loss of class I HLA. Nat Commun 2018, 9:3868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ahmadi M, King JW, Xue SA, Voisine C, Holler A, Wright GP, Waxman J, Morris E, Stauss HJ: CD3 limits the efficacy of TCR gene therapy in vivo. Blood 2011, 118:3528–3537. [DOI] [PubMed] [Google Scholar]
- 28.van Loenen MM, de Boer R, Amir AL, Hagedoorn RS, Volbeda GL, Willemze R, van Rood JJ, Falkenburg JH, Heemskerk MH: Mixed T cell receptor dimers harbor potentially harmful neoreactivity. Proc Natl Acad Sci U S A 2010, 107:10972–10977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zah E, Lin MY, Silva-Benedict A, Jensen MC, Chen YY: T Cells Expressing CD19/CD20 Bispecific Chimeric Antigen Receptors Prevent Antigen Escape by Malignant B Cells. Cancer Immunol Res 2016, 4:498–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hegde M, Mukherjee M, Grada Z, Pignata A, Landi D, Navai SA, Wakefield A, Fousek K, Bielamowicz K, Chow KK, et al. : Tandem CAR T cells targeting HER2 and IL13Ralpha2 mitigate tumor antigen escape. J Clin Invest 2016, 126:3036–3052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ruella M, Barrett DM, Kenderian SS, Shestova O, Hofmann TJ, Perazzelli J, Klichinsky M, Aikawa V, Nazimuddin F, Kozlowski M, et al. : Dual CD19 and CD123 targeting prevents antigen-loss relapses after CD19-directed immunotherapies. J Clin Invest 2016, 126:3814–3826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.de Larrea CF, Staehr M, Lopez AV, Ng KY, Chen Y, Godfrey WD, Purdon TJ, Ponomarev V, Wendel HG, Brentjens RJ, et al. : Defining an Optimal Dual-Targeted CAR T-cell Therapy Approach Simultaneously Targeting BCMA and GPRC5D to Prevent BCMA Escape-Driven Relapse in Multiple Myeloma. Blood Cancer Discov 2020, 1:146–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Balakrishnan A, Rajan A, Salter AI, Kosasih PL, Wu Q, Voutsinas J, Jensen MC, Pluckthun A, Riddell SR: Multispecific Targeting with Synthetic Ankyrin Repeat Motif Chimeric Antigen Receptors. Clin Cancer Res 2019, 25:7506–7516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Choi BD, Yu X, Castano AP, Bouffard AA, Schmidts A, Larson RC, Bailey SR, Boroughs AC, Frigault MJ, Leick MB, et al. : CAR-T cells secreting BiTEs circumvent antigen escape without detectable toxicity. Nat Biotechnol 2019, 37:1049–1058. [DOI] [PubMed] [Google Scholar]
- 35.Shah NN, Johnson BD, Schneider D, Zhu F, Szabo A, Keever-Taylor CA, Krueger W, Worden AA, Kadan MJ, Yim S, et al. : Bispecific anti-CD20, anti-CD19 CAR T cells for relapsed B cell malignancies: a phase 1 dose escalation and expansion trial. Nat Med 2020, 26:1569–1575. [DOI] [PubMed] [Google Scholar]
- 36.Minutolo NG, Hollander EE, Powell DJ Jr.: The Emergence of Universal Immune Receptor T Cell Therapy for Cancer. Front Oncol 2019, 9:176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Urbanska K, Lanitis E, Poussin M, Lynn RC, Gavin BP, Kelderman S, Yu J, Scholler N, Powell DJ Jr.: A universal strategy for adoptive immunotherapy of cancer through use of a novel T-cell antigen receptor. Cancer Res 2012, 72:1844–1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lohmueller JJ, Ham JD, Kvorjak M, Finn OJ: mSA2 affinity-enhanced biotin-binding CAR T cells for universal tumor targeting. Oncoimmunology 2017, 7:e1368604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kudo K, Imai C, Lorenzini P, Kamiya T, Kono K, Davidoff AM, Chng WJ, Campana D: T lymphocytes expressing a CD16 signaling receptor exert antibody-dependent cancer cell killing. Cancer Res 2014, 74:93–103. [DOI] [PubMed] [Google Scholar]
- 40.Rodgers DT, Mazagova M, Hampton EN, Cao Y, Ramadoss NS, Hardy IR, Schulman A, Du J, Wang F, Singer O, et al. : Switch-mediated activation and retargeting of CAR-T cells for B-cell malignancies. Proc Natl Acad Sci U S A 2016, 113:E459–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ma JS, Kim JY, Kazane SA, Choi SH, Yun HY, Kim MS, Rodgers DT, Pugh HM, Singer O, Sun SB, et al. : Versatile strategy for controlling the specificity and activity of engineered T cells. Proc Natl Acad Sci U S A 2016, 113:E450–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tamada K, Geng D, Sakoda Y, Bansal N, Srivastava R, Li Z, Davila E: Redirecting gene-modified T cells toward various cancer types using tagged antibodies. Clin Cancer Res 2012, 18:6436–6445. [DOI] [PubMed] [Google Scholar]
- 43.Dieckmann-Schuppert A, Bause E, Schwarz RT: Studies on O-glycans of Plasmodium-falciparum-infected human erythrocytes. Evidence for O-GlcNAc and O-GlcNAc-transferase in malaria parasites. Eur J Biochem 1993, 216:779–788. [DOI] [PubMed] [Google Scholar]
- 44.Minutolo NG, Sharma P, Poussin M, Shaw LC, Brown DP, Hollander EE, Smole A, Rodriguez-Garcia A, Hui JZ, Zappala F, et al. : Quantitative Control of Gene-Engineered T-Cell Activity through the Covalent Attachment of Targeting Ligands to a Universal Immune Receptor. J Am Chem Soc 2020, 142:6554–6568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cho JH, Collins JJ, Wong WW: Universal Chimeric Antigen Receptors for Multiplexed and Logical Control of T Cell Responses. Cell 2018, 173:1426–1438 e1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wermke M, Kraus S, Ehninger A, Bargou RC, Goebeler ME, Middeke JM, Kreissig C, von Bonin M, Koedam J, Pehl M, et al. : Proof of concept for a rapidly switchable universal CAR-T platform with UniCAR-T-CD123 in relapsed/refractory AML. Blood 2021, 137:3145–3148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lajoie MJ, Boyken SE, Salter AI, Bruffey J, Rajan A, Langan RA, Olshefsky A, Muhunthan V, Bick MJ, Gewe M, et al. : Designed protein logic to target cells with precise combinations of surface antigens. Science 2020, 369:1637–1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kopan R, Ilagan MX: The canonical Notch signaling pathway: unfolding the activation mechanism. Cell 2009, 137:216–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Braune EB, Lendahl U: Notch -- a goldilocks signaling pathway in disease and cancer therapy. Discov Med 2016, 21:189–196. [PubMed] [Google Scholar]
- 50.Morsut L, Roybal KT, Xiong X, Gordley RM, Coyle SM, Thomson M, Lim WA: Engineering Customized Cell Sensing and Response Behaviors Using Synthetic Notch Receptors. Cell 2016, 164:780–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Roybal KT, Rupp LJ, Morsut L, Walker WJ, McNally KA, Park JS, Lim WA: Precision Tumor Recognition by T Cells With Combinatorial Antigen-Sensing Circuits. Cell 2016, 164:770–779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dannenfelser R, Allen GM, VanderSluis B, Koegel AK, Levinson S, Stark SR, Yao V, Tadych A, Troyanskaya OG, Lim WA: Discriminatory Power of Combinatorial Antigen Recognition in Cancer T Cell Therapies. Cell Syst 2020, 11:215–228 e215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Choe JH, Watchmaker PB, Simic MS, Gilbert RD, Li AW, Krasnow NA, Downey KM, Yu W, Carrera DA, Celli A, et al. : SynNotch-CAR T cells overcome challenges of specificity, heterogeneity, and persistence in treating glioblastoma. Sci Transl Med 2021, 13(591):eabe7378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Williams JZ, Allen GM, Shah D, Sterin IS, Kim KH, Garcia VP, Shavey GE, Yu W, Puig-Saus C, Tsoi J, et al. : Precise T cell recognition programs designed by transcriptionally linking multiple receptors. Science 2020, 370:1099–1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hyrenius-Wittsten A, Su Y, Park M, Garcia JM, Alavi J, Perry N, Montgomery G, Liu B, Roybal KT: SynNotch CAR circuits enhance solid tumor recognition and promote persistent antitumor activity in mouse models. Sci Transl Med 2021, 13(591):eabd8836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bonini C, Ferrari G, Verzeletti S, Servida P, Zappone E, Ruggieri L, Ponzoni M, Rossini S, Mavilio F, Traversari C, et al. : HSV-TK gene transfer into donor lymphocytes for control of allogeneic graft-versus-leukemia. Science 1997, 276:1719–1724. [DOI] [PubMed] [Google Scholar]
- 57.Hoyos V, Savoldo B, Quintarelli C, Mahendravada A, Zhang M, Vera J, Heslop HE, Rooney CM, Brenner MK, Dotti G: Engineering CD19-specific T lymphocytes with interleukin-15 and a suicide gene to enhance their anti-lymphoma/leukemia effects and safety. Leukemia 2010, 24:1160–1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Di Stasi A, Tey SK, Dotti G, Fujita Y, Kennedy-Nasser A, Martinez C, Straathof K, Liu E, Durett AG, Grilley B, et al. : Inducible apoptosis as a safety switch for adoptive cell therapy. N Engl J Med 2011, 365:1673–1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.de Witte MA, Jorritsma A, Swart E, Straathof KC, de Punder K, Haanen JB, Rooney CM, Schumacher TN: An inducible caspase 9 safety switch can halt cell therapy-induced autoimmune disease. J Immunol 2008, 180:6365–6373. [DOI] [PubMed] [Google Scholar]
- 60.Wang X, Chang WC, Wong CW, Colcher D, Sherman M, Ostberg JR, Forman SJ, Riddell SR, Jensen MC: A transgene-encoded cell surface polypeptide for selection, in vivo tracking, and ablation of engineered cells. Blood 2011, 118:1255–1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Paszkiewicz PJ, Frassle SP, Srivastava S, Sommermeyer D, Hudecek M, Drexler I, Sadelain M, Liu L, Jensen MC, Riddell SR, et al. : Targeted antibody-mediated depletion of murine CD19 CAR T cells permanently reverses B cell aplasia. J Clin Invest 2016, 126:4262–4272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Serafini M, Manganini M, Borleri G, Bonamino M, Imberti L, Biondi A, Golay J, Rambaldi A, Introna M: Characterization of CD20-transduced T lymphocytes as an alternative suicide gene therapy approach for the treatment of graft-versus-host disease. Hum Gene Ther 2004, 15:63–76. [DOI] [PubMed] [Google Scholar]
- 63.Philip B, Kokalaki E, Mekkaoui L, Thomas S, Straathof K, Flutter B, Marin V, Marafioti T, Chakraverty R, Linch D, et al. : A highly compact epitope-based marker/suicide gene for easier and safer T-cell therapy. Blood 2014, 124:1277–1287. [DOI] [PubMed] [Google Scholar]
- 64.Kieback E, Charo J, Sommermeyer D, Blankenstein T, Uckert W: A safeguard eliminates T cell receptor gene-modified autoreactive T cells after adoptive transfer. Proc Natl Acad Sci U S A 2008, 105:623–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Griffioen M, van Egmond HM, Barnby-Porritt H, van der Hoorn MA, Hagedoorn RS, Kester MG, Schwabe N, Willemze R, Falkenburg JH, Heemskerk MH: Genetic engineering of virus-specific T cells with T-cell receptors recognizing minor histocompatibility antigens for clinical application. Haematologica 2008, 93:1535–1543. [DOI] [PubMed] [Google Scholar]
- 66.Duong MT, Collinson-Pautz MR, Morschl E, Lu A, Szymanski SP, Zhang M, Brandt ME, Chang WC, Sharp KL, Toler SM, et al. : Two-Dimensional Regulation of CAR-T Cell Therapy with Orthogonal Switches. Mol Ther Oncolytics 2019, 12:124–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wu CY, Roybal KT, Puchner EM, Onuffer J, Lim WA: Remote control of therapeutic T cells through a small molecule-gated chimeric receptor. Science 2015, 350:aab4077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Leung WH, Gay J, Martin U, Garrett TE, Horton HM, Certo MT, Blazar BR, Morgan RA, Gregory PD, Jarjour J, et al. : Sensitive and adaptable pharmacological control of CAR T cells through extracellular receptor dimerization. JCI Insight 2019, 5(11):e124430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Salzer B, Schueller CM, Zajc CU, Peters T, Schoeber MA, Kovacic B, Buri MC, Lobner E, Dushek O, Huppa JB, et al. : Engineering AvidCARs for combinatorial antigen recognition and reversible control of CAR function. Nat Commun 2020, 11:4166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Richman SA, Wang LC, Moon EK, Khire UR, Albelda SM, Milone MC: Ligand-Induced Degradation of a CAR Permits Reversible Remote Control of CAR T Cell Activity In Vitro and In Vivo. Mol Ther 2020, 28:1600–1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bonger KM, Chen LC, Liu CW, Wandless TJ: Small-molecule displacement of a cryptic degron causes conditional protein degradation. Nat Chem Biol 2011, 7:531–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Schade AE, Schieven GL, Townsend R, Jankowska AM, Susulic V, Zhang R, Szpurka H, Maciejewski JP: Dasatinib, a small-molecule protein tyrosine kinase inhibitor, inhibits T-cell activation and proliferation. Blood 2008, 111:1366–1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mestermann K, Giavridis T, Weber J, Rydzek J, Frenz S, Nerreter T, Mades A, Sadelain M, Einsele H, Hudecek M: The tyrosine kinase inhibitor dasatinib acts as a pharmacologic on/off switch for CAR T cells. Sci Transl Med 2019, 11(499):eaau5907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Weber EW, Lynn RC, Sotillo E, Lattin J, Xu P, Mackall CL: Pharmacologic control of CAR-T cell function using dasatinib. Blood Adv 2019, 3:711–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Weber EW, Parker KR, Sotillo E, Lynn RC, Anbunathan H, Lattin J, Good Z, Belk JA, Daniel B, Klysz D, et al. : Transient rest restores functionality in exhausted CAR-T cells through epigenetic remodeling. Science 2021, 372(6537):eaba1786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Voisinne G, Kersse K, Chaoui K, Lu L, Chaix J, Zhang L, Goncalves Menoita M, Girard L, Ounoughene Y, Wang H, et al. : Quantitative interactomics in primary T cells unveils TCR signal diversification extent and dynamics. Nat Immunol 2019, 20:1530–1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Li X, Wang R, Fan P, Yao X, Qin L, Peng Y, Ma M, Asley N, Chang X, Feng Y, et al. : A Comprehensive Analysis of Key Immune Checkpoint Receptors on Tumor-Infiltrating T Cells From Multiple Types of Cancer. Front Oncol 2019, 9:1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Demetriou P, Abu-Shah E, Valvo S, McCuaig S, Mayya V, Kvalvaag A, Starkey T, Korobchevskaya K, Lee LYW, Friedrich M, et al. : A dynamic CD2-rich compartment at the outer edge of the immunological synapse boosts and integrates signals. Nat Immunol 2020, 21:1232–1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Philip M, Schietinger A: Heterogeneity and fate choice: T cell exhaustion in cancer and chronic infections. Curr Opin Immunol 2019, 58:98–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Langan RA, Boyken SE, Ng AH, Samson JA, Dods G, Westbrook AM, Nguyen TH, Lajoie MJ, Chen Z, Berger S, et al. : De novo design of bioactive protein switches. Nature 2019, 572:205–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Leaver-Fay A, Tyka M, Lewis SM, Lange OF, Thompson J, Jacak R, Kaufman K, Renfrew PD, Smith CA, Sheffler W, et al. : ROSETTA3: an object-oriented software suite for the simulation and design of macromolecules. Methods Enzymol 2011, 487:545–574. [DOI] [PMC free article] [PubMed] [Google Scholar]