Abstract
Background:
Interest in therapeutic applications of exogenous ketones has grown significantly, spanning patients with heart failure to endurance athletes. Exogenous ketones engender significant effects on cardiac function in heart failure and provide an ergogenic benefit in athletes. The aim of this study was to assess the effects of exogenous ketones on cardiac function in healthy participants.
Methods:
In a single-arm intervention study, 20 fasting, healthy participants underwent comprehensive echocardiography (two-dimensional, Doppler, and strain) before and 30 min after weight-based oral ketone ester administration. The relationship between changes in log-transformed biomarker levels and change in absolute global longitudinal strain (GLS) was assessed using linear regression.
Results:
The mean age was 30 ± 7 years, 50% were women, 45% were nonwhite, and the average body mass index was 24.3 ± 3.1 kg/m2. Ketone ingestion acutely elevated β-hydroxybutyrate levels from a median of 0.13 mmol/L (interquartile range, 0.10–0.37 mmol/L) to 3.23 mmol/L (interquartile range, 2.40–4.97 mmol/L) (P < .001). After ketone ester consumption, systolic blood pressure, heart rate, biventricular function, left ventricular GLS, and left atrial (LA) strain all augmented, while systemic vascular resistance decreased. Displayed as mean change, increases in ejection fraction (3.1%; 95% CI, 2.0%−4.2%; P < .001), GLS (2.0%; 95% CI, 1.4%−2.7%; P < .001), right ventricular S′ (1.1 cm/sec; 95% CI, 0.4–1.8 cm/sec; P = .004), LA reservoir strain (7%; 95% CI, 3%−12%; P = .005), and LA contractile strain (4%; 2%−6%; P = .001) were observed. During robustly achieved ketosis, change in GLS was inversely associated with change in nonesterified fatty acids (P = .019).
Conclusions:
In a single-arm study, systolic blood pressure, heart rate, biventricular function, and LV and LA strain acutely augmented after ketone ester ingestion in healthy, fasting participants, similar to several effects observed in the failing heart. These data may provide supporting data for the ergogenic benefits observed in athletes and may become increasingly relevant with exogenous ketone consumption across a variety of cardiovascular and noncardiovascular applications.
Keywords: Ketones, Ketone ester, Insulin, Echocardiography, Metabolism
Interest in the cardiovascular therapeutic applications of exogenous ketosis has grown significantly in recent years, spanning endurance athletes to patients with heart failure (HF).1–4 In a crossover trial of highly trained endurance athletes, ketone ester (KE) ingestion enhanced exercise performance while also reducing glycolytic flux.4 Moreover, in a crossover trial of patients with HF with reduced ejection fraction, exogenous ketone delivery engendered several resting echocardiographic and hemodynamic effects, including amelioration of left ventricular (LV) systolic function with a robust augmentation in LV ejection fraction (LVEF), an increase in heart rate, and a decrease in systemic vascular resistance (SVR).3 Whether these same effects occur in healthy volunteers, however, has not been well elucidated. Given that exogenous ketone consumption also shows therapeutic promise across a number of noncardiovascular diseases (neurologic, endocrine, and metabolic),5–10 understanding off-target effects on the myocardium is critical. Therefore, we sought to assess the echocardiographic effects of nutritional ketosis, specifically hypothesizing that acute KE administration would augment LV systolic function, qualitatively similar to effects observed in HF.3
METHODS
Study Design
We enrolled participants aged 18 to 60 years at the University of Pennsylvania between January 2020 and January 2021 (NCT04275453).11 We excluded individuals with any reported history, or risk factors, for cardiovascular disease (including hypertension, hyperlipidemia, and diabetes mellitus), those currently on a ketogenic diet, and pregnant or breastfeeding women. A total of 20 individuals underwent comprehensive echocardiography before and after KE administration. The study was approved by an institutional review committee, and informed consent was obtained.
Metabolite and Hormone Level Analysis
Venous blood was collected via peripheral phlebotomy before and 30 min after KE ingestion. Serum β-hydroxybutyrate (BHB) levels were then determined on freshly processed samples using a Beckman-Coulter analyzer (AU5800 or AU680; Beckman-Coulter, Brea, CA). The reportable range for BHB is 0.02 to 13.5 mmol/L, and the coefficient of variation through multilevel, internal quality control ranges from 0.8% to 2.6%.
Insulin, glucagon, and nonesterified fatty acid (NEFA) were analyzed in bulk at the Penn Radioimmunoassay and Biomarkers Core. Blood samples were collected into tubes on ice containing ethylenediamine tetra-acetic acid and protease inhibitor cocktail (Sigma-Aldrich, St. Louis, MO). Samples were centrifuged at 4°C, separated, and frozen at −80°C for subsequent analysis. NEFA levels (range, 0.125–2.9 mmol/L) were measured in duplicate using enzymatic colorimetry (Wako Chemicals, Richmond, VA). Plasma insulin (range, 2–100 mIU/mL) and glucagon (range, 20–400 pg/mL) were measured in duplicate using double-antibody radioimmunoassay (Millipore, Billerica, MA).
Patient Preparation
Participants were permitted to eat ad lib during the day before the study visit and fasted starting at midnight before echocardiography. During the visit, participants received weight-based dosing (714 mg/kg) of (R)-3-hydroxybutyl (R)-3-hydroxybutyrate (H.V.M.N., Miami, FL), which has been extensively studied for the purposes of achieving ketosis.5–7
Briefly, this KE undergoes rapid enzymatic hydrolysis ultimately to form BHB (and other ketone bodies), achieving high BHB levels (maximum concentration 3.3 mmol/L) within a short time frame (time to maximum concentration 2.5 hours), and is eliminated quickly (half-life 0.8 hours), as demonstrated previously.5 As substantial concentrations are obtained even within 30 min, and to minimize external influences, we performed repeat echocardiography at this time point after KE consumption.
Echocardiography
Two-dimensional, Doppler, and M-mode echocardiograms were obtained before KE administration and 30 min after KE administration by credentialed sonographers using guideline-based protocols.12 Each set of measurements was made in a blinded fashion by a single cardiologist, board certified in echocardiography with experience in strain analysis, using Syngo Dynamics (Siemens Medical Solutions, Malvern, PA) according to guidelines.13 LVEF was assessed using the biplane method of disks. Stroke volume was calculated by multiplying the LV outflow tract velocity-time integral and cross-sectional area of the LV outflow tract. Left atrial (LA) minimum and maximum volumes were measured as previously described.14 Echocardiographic estimation of SVR was calculated as the difference between mean arterial pressure and right atrial pressure estimate (from the inferior vena cava), divided by cardiac output (product of stroke volume and heart rate at the time of LV outflow tract velocity-time integral measurement).15 Pulmonary artery systolic pressure was calculated as 4 × (peak velocity of the tricuspid regurgitant jet)2 + estimated right atrial pressure from the inferior vena cava.13,15 Pulmonary vascular resistance was calculated using the method of Abbas et al.16
Speckle-tracking strain analysis using semiautomated techniques with subsequent manual adjustment was performed using TomTec 2D Cardiac Performance Analysis (TomTec, Unterschleissheim, Germany). LV global longitudinal strain (GLS) was measured from the apical four-, three-, and two-chamber views and is reported as the positive absolute percentage for ease of display. LA strain speckle-tracking measurements were made with R-R wave electrocardiographic gating to define the cardiac cycle in the apical four-chamber view.17 Because the ventricular cycle was the reference point, all LA strain values are also reported as positive absolute percentages. LA reservoir strain was defined as peak average LA strain and corresponded to ventricular systole, while peak average LA contractile strain was measured during atrial systole. LA conduit strain was calculated as the difference of reservoir and contractile strain. To assess intraobserver reliability, we repeated strain measurements on 20 echocardiograms for LA strain and LV strain, separated by at least 3 weeks from the initial measurement. Estimates of the intraclass correlation coefficient were 0.93 for LA reservoir strain, 0.91 for LA contractile strain, and 0.81 for LV GLS. Coefficients of variation were 5.4% (LA reservoir strain), 8.5% (LA contractile strain), and 4.4% (LV GLS).
Statistical Analysis
Baseline characteristics are described using mean ± SD, medians with interquartile ranges (IQRs), or percentages as appropriate for the levels of measurement and distributions of the variables. Biomarker levels before and after KE were compared using the Wilcoxon signed rank test because levels were right skewed. Echocardiographic parameters before and after KE administration were compared using paired t testing. We performed mixed-effects modeling to assess whether changes in strain were independent of the change in heart rate, modeling participant as a random effect. We assessed whether the change in LV systolic function (the primary hypothesis) was associated with the changes in log-transformed BHB, insulin, and NEFA levels using linear regression, adjusting for baseline log-transformed biomarker levels and baseline echocardiographic values. Analyses were performed using Stata version 14 (StataCorp, College Station, TX). A two-sided P value < .05 was considered to indicate statistical significance.
RESULTS
Baseline Characteristics
The baseline characteristics of the 20 study participants who completed 40 echocardiographic examinations are shown in Table 1.11 The average age was 30 ± 7 years, 50% were women, and 45% were nonwhite. The average body mass index (24.3 ± 3.1 kg/m2) and blood pressure (119 ±12/75 ± 9 mm Hg) fell within normal ranges (Tables 1 and 2). Laboratory values were consistent with a healthy cohort. BHB levels acutely increased 30 min after KE administration (median, 0.13 mmol/L [IQR, 0.10–0.37 mmol/L] to 3.23 [IQR, 2.40–4.97 mmol/L]; P < .001). While insulin levels also acutely augmented (median, 10.9 mIU/L [IQR, 8.4–14.0 mIU/L] to 18.8 [IQR, 15.7–27.0 mIU/L]; P < .001), NEFA levels decreased (median, 0.41 mmol/L [IQR, 0.31–0.57 mmol/L] to 0.30 mmol/L [IQR, 0.17–0.43 mmol/L]; P = .019) with KE. There was no change in glucagon (P = .28).
Table 1.
Baseline clinical characteristics of the study participants (n = 20)
| Value | |
|---|---|
|
| |
| Age, y | 30 ± 7 |
| Women | 10 (50) |
| Race | |
| White | 11 (55) |
| Black | 4 (20) |
| Asian | 4 (20) |
| Other | 1 (5) |
| Average number of alcohol drinks (per week) | 6.7 ± 3.3 |
| Current smoking | 1 (5) |
| Body mass index, kg/m2 | 24.3 ± 3.1 |
| Laboratory testing | |
| Creatinine, mg/dL | 0.8 ± 0.1 |
| Glucose, mg/dL | 81 ± 10 |
| Hemoglobin, g/dL | 13.2 ± 1.6 |
| Low-density lipoprotein cholesterol, mg/dL | 102 ± 22 |
| High-density lipoprotein cholesterol, mg/dL | 55 ± 12 |
| Triglycerides, mg/dL | 78 ± 24 |
| BHB before KE, mmol/L | 0.13 (0.10–0.37) |
| BHB after KE, mmol/L | 3.23 (2.40–4.97) |
| Insulin before KE, mIU/L | 10.9 (8.4–14.0) |
| Insulin after KE, mIU/L | 18.8 (15.7–27.0) |
| Glucagon before KE, pg/mL | 65.5 (49.5–77.5) |
| Glucagon after KE, pg/mL | 62.5 (48.5–75.0) |
| NEFA before KE, mmol/L | 0.41 (0.31–0.57) |
| NEFA after KE, mmol/L | 0.30 (0.17–0.43) |
Data are expressed as mean ± SD, number (percentage), or median (IQR) (for right-skewed distributions).
Table 2.
Hemodynamic and echocardiographic parameters before and after KE administration
| Before KE (n = 20) | After KE (n = 20) | Difference (95% CI) | P | |
|---|---|---|---|---|
|
| ||||
| Vital signs | ||||
| Systolic blood pressure, mm Hg | 119 ± 12 | 124 ± 11 | 5 (1 to 9) | .023 |
| Diastolic blood pressure, mm Hg | 75 ± 9 | 76 ± 9 | 1 (−2.4 to 4) | .58 |
| Mean arterial pressure, mm Hg | 90 ± 9 | 92 ± 8 | 2 (−1 to 5) | .12 |
| Pulse pressure, mm Hg | 44 ± 8 | 48 ± 10 | 4 (−1 to 9) | .12 |
| Heart rate, beats/min | 62 ± 12 | 71 ± 13 | 9 (6 to 12) | <.001 |
| Left ventricle | ||||
| End-diastolic dimension, cm | 4.78 ± 0.37 | 4.71 ± 0.44 | −0.07 (−0.19 to 0.06) | .26 |
| End-systolic dimension, cm | 3.34 ± 0.31 | 3.10 ± 0.31 | −0.24 (−0.33 to −0.15) | <.001 |
| End-diastolic volume, mL | 112 ± 19 | 115 ± 24 | 3 (−2 to 7) | .27 |
| End-systolic volume, mL | 46 ± 11 | 44 ± 11 | −3 (−5 to 0) | .02 |
| Biplane LVEF, % | 59.0 ± 4.0 | 62.2 ± 3.6 | 3.1 (2.0 to 4.2) | <.001 |
| LVOT VTI, cm | 22.0 ± 2.8 | 23.7 ± 3.1 | 1.7 (0.9 to 2.4) | <.001 |
| Stroke volume, mL | 74 ± 11 | 80 ± 12 | 6 (3 to 8) | <.001 |
| SVR, dynes · sec/cm5 | 1,562 ± 263 | 1,310 ± 252 | −252 (−364 to −140) | <.001 |
| Absolute value of LV GLS, % | 21.1 ± 1.8 | 23.1 ± 2.0 | 2.0 (1.4 to 2.7) | <.001 |
| Mitral inflow E velocity, cm/sec | 75 ± 17 | 80 ± 16 | 5 (−1 to 11) | .09 |
| Mitral inflow A velocity, cm/sec | 40 ± 12 | 53 ± 10 | 13 (10 to 17) | <.001 |
| E/A ratio | 2.00 ± 0.58 | 1.55 ± 0.33 | −0.45 (−0.61 to −0.30) | <.001 |
| Mitral E deceleration time, msec | 191 ± 30 | 182 ± 32 | −8 (−25 to 8) | .30 |
| Septal E’ velocity, cm/sec | 11.9 ± 2.3 | 12.7 ± 2.3 | 0.8 (0.2 to 1.3) | .013 |
| Lateral E’ velocity, cm/sec | 15.7 ± 2.7 | 16.6 ± 3.7 | 0.8 (−0.0 to 1.7) | .06 |
| Septal E/e’ ratio | 6.4 ± 1.5 | 6.4 ± 1.4 | 0.0 (−0.4 to 0.5) | .88 |
| Lateral E/e’ ratio | 4.8 ± 0.9 | 5.0 ± 1.2 | 0.2 (−0.3 to 0.7) | .40 |
| Left atrium | ||||
| Maximal volume index, mL/m2 | 27.8 ± 6.1 | 26.6 ± 6.5 | −1.2 (−3.6 to 1.2) | .31 |
| Minimal volume index, mL/m2 | 10.3 ± 3.0 | 9.0 ± 3.5 | −1.3 (−2.2 to −0.4) | .006 |
| Reservoir strain, % | 41 ± 7 | 49 ± 12 | 7 (3 to 12) | .005 |
| Conduit strain, % | 30 ± 6 | 34 ± 10 | 4 (−1 to 8) | .11 |
| Contractile strain, % | 11 ± 4 | 15 ± 5 | 4 (2 to 6) | .001 |
| Right ventricle | ||||
| TAPSE, cm | 2.48 ± 0.31 | 2.72 ± 0.42 | 0.25 (0.13 to 0.37) | <.001 |
| S’ velocity, cm/sec | 12.4 ± 1.8 | 13.5 ± 1.9 | 1.1 (0.4 to 1.8) | .004 |
| Fractional area change, % | 42 ± 7 | 46 ± 7 | 4 (0.9 to 7) | .013 |
| RVOT VTI, cm | 14.8 ± 2.3 | 16.8 ± 2.8 | 2.0 (1.1 to 2.8) | <.001 |
| E’ velocity, cm/sec | 15.0 ± 2.2 | 14.6 ± 2.6 | −0.4 (−1.5 to 0.7) | .46 |
| Pulmonary artery systolic pressure, mm Hg | 25 ± 6 | 24 ± 7 | −1 (−5 to 2) | .43 |
| RVOT VTI acceleration time, msec | 154 ± 22 | 161 ± 23 | 7 (−6 to 20) | .27 |
| Pulmonary vascular resistance, Wood units | 1.73 ± 0.32 | 1.53 ± 0.35 | −0.21 (−0.34 to −0.07) | .004 |
LVOT, LV outflow tract; RVOT, right ventricular outflow tract; TAPSE, tricuspid annular planar systolic excursion; VTI, velocity-time integral.
Data are expressed as mean ± SD.
Hemodynamic and Echocardiographic Effects of Exogenous Ketones
Table 2 displays the hemodynamic and echocardiographic effects of KE before and after administration. LV size was within normal limits for all participants. One participant was found to have a mildly reduced LVEF (47%) before KE administration, which was previously unknown.
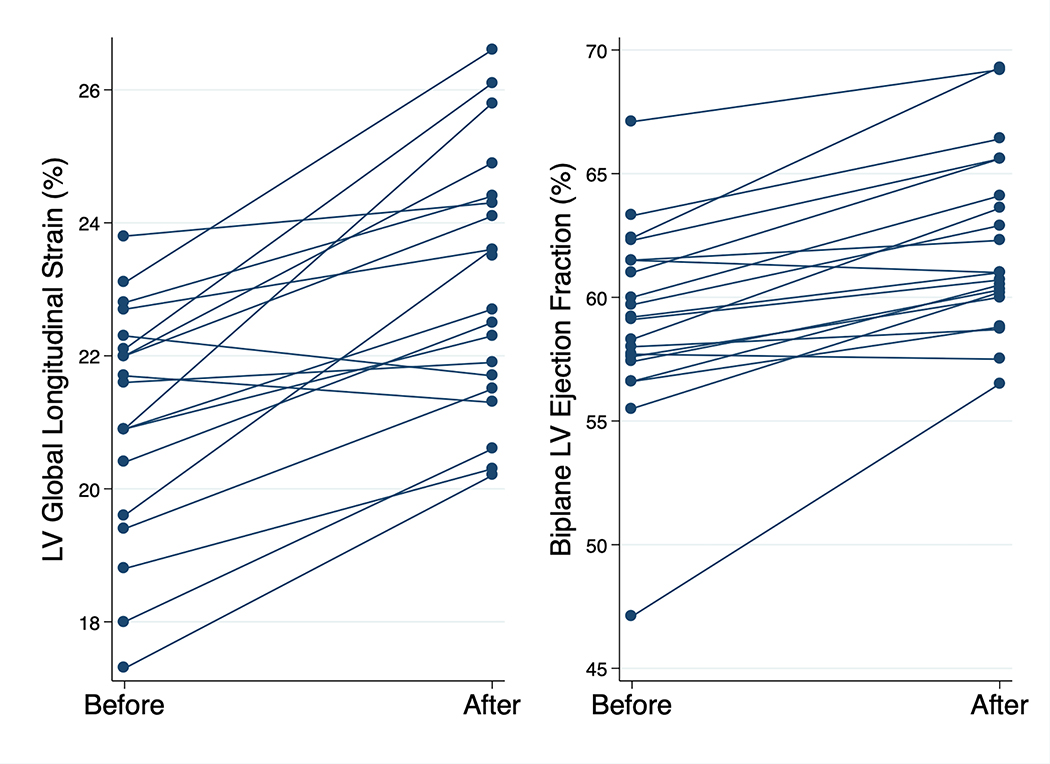
Increases in systolic blood pressure (mean, 5 mm Hg; 95% CI, 1 to 9 mm Hg; P = .023) and heart rate (mean, 9 beats/min; 95% CI, 6 to 12 beats/min; P < .001) occurred after KE administration, with a trend toward an increase in pulse pressure (P = .12). LV systolic function acutely augmented, including increases in stroke volume (mean, 6 mL; 95% CI, 3 to 8 mL; P < .001), LVEF (mean, 3.1%; 95% CI, 2.0% to 4.2%; P < .001), and GLS (mean, 2.0%; 95% CI, 1.4% to 2.7%; P < .001). The augmentation of GLS was independent of the change in heart rate (adjusted β coefficient = 1.8%, P < .001). In this context, SVR decreased (mean, −252 dynes · sec/cm5; 95% CI, −364 to −140 dynes · sec/cm5; P < .001). Individual line plots for changes in LVEF and GLS are shown in Figure 1. There was normalization of LVEF (57%) in the one participant with an initially mildly reduced LVEF (47%); excluding this outlier still revealed similar LVEF increases in the other 19 participants (2.8%). Diastolic filling assessment revealed an increase in mitral inflow A velocity, with a subsequently lower E/A ratio (P < .001 for both comparisons). Septal e′ velocity was higher (mean, 0.8 cm/sec; 95% CI, 0.2 to 1.3 cm/sec; P = .013), while lateral e′ velocity was nonsignificantly higher (mean, 0.8 cm/sec; 95% CI, −0.0 to 1.7 cm/sec; P = .06), after KE. There was no change in estimated filling pressures as reflected by the E/e′ ratio. Although there was no statistically significant difference in LA maximum volume index (P = .31), LA minimum volume index was lower after KE (mean, −1.3 mL/m2; 95% CI, −2.2 to −0.4 mL/m2; P = .006). LA strain analysis showed increases in reservoir strain (mean, 7%; 95% CI, 3% to 12%; P = .005) and contractile strain (mean, 4%; 95% CI, 2% to 6%; P = .001), and these changes were also independent of the change in heart rate (adjusted P < .05 for both comparisons).
Figure 1.
Changes in LV systolic function before and after KE. Line plots showing before and after values for absolute values of LV GLS and LVEF.
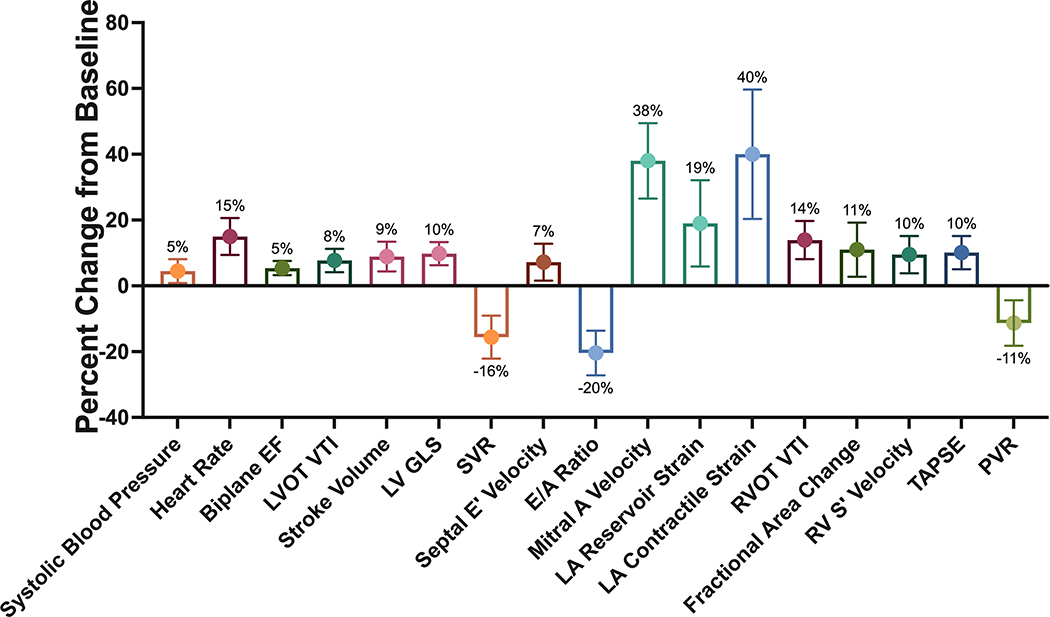
Right ventricular systolic function likewise augmented, including an increase in tricuspid annular planar systolic excursion (mean, 0.25 cm; 95% CI, 0.13 to 0.37 cm; P < .001), S′ velocity (mean, 1.1 cm/sec; 95% CI, 0.4 to 1.8 cm/sec; P = .004), fractional area change (mean, 4%; 0.9% to 7%; P = .013), and right ventricular outflow tract velocity-time integral (mean, 2.0 cm; 95% CI, 1.1 to 2.8 cm; P < .001). There was no difference in the estimated pulmonary artery systolic pressure (P = .43) or right ventricular outflow tract acceleration time (P = .27). Estimated pulmonary vascular resistance decreased after KE (mean, −0.21 Wood units; 95% CI, −0.34 to −0.07 Wood units; P = .004). The percentage changes in hemodynamic and echocardiographic parameters after KE administration are shown in Figure 2.
Figure 2.
Summary of ketogenic effects on cardiac function. The percentage change and 95% CI are shown for the effect of exogenous ketones on several resting cardiac parameters of interest that were significantly associated with KE administration. GLS was transformed using absolute values. EF, Ejection fraction; LVOT, LV outflow tract; PVR, pulmonary vascular resistance; RV, right ventricular; RVOT, right ventricular outflow tract; TAPSE, tricuspid annular planar systolic excursion; VTI, velocity-time integral.
Using linear regression, decrease in log-transformed NEFA was associated with increase in GLS (β coefficient = −1.70, P = .019) but not significantly associated with change in LVEF (β coefficient = −1.90, P = .052). There were no significant relationships between changes in ketone (BHB) and insulin levels and change in LVEF or GLS (P > .05 for all comparisons).
Adverse Events
Mild (grade 1) adverse events occurred in nine participants, which were mostly gastrointestinal (nausea or heartburn, n = 7), including one participant who experienced emesis. Two participants reported headache.
DISCUSSION
In a study of participants free of known cardiovascular disease, we found that administration of KE was associated with several hemodynamic and echocardiographic effects. After exogenously inducing systemic ketosis, biventricular function, LA strain, and heart rate all increased, while estimated SVR decreased. LVEF normalized following KE in one participant with incidentally detected mild systolic dysfunction. Our findings suggest that exogenous ketone consumption in healthy participants may have qualitatively similar (though quantitatively smaller) effects on cardiac function compared with patients with HF.3 Combined with reductions in SVR, these hemodynamic effects may also contribute to the ergogenic effects of KE observed in athletes.4
Exogenous ketones have several cardiovascular effects across the spectrum of cardiovascular health in preclinical and clinical studies, similar to what we observed here.1,3,18–20 The increase in heart rate has been observed in other studies,3,19 and it remains unclear whether this is compensatory to a vasodilatory effect of ketones or perhaps related to changes in autonomic tone. We likewise observed both an increase in heart rate and a decrease in SVR. We also noted a decrease in estimated pulmonary vascular resistance without a change in the acceleration time (a surrogate of mean pulmonary artery pressure), suggesting that the increase in flow was matched by a decrease in resistance. Increases in biventricular function (including an 8% absolute increase in LVEF) have been reported in the failing heart,3 while the increase in LVEF was smaller in our study (3.1%). The augmentation of systolic function has also been observed after ketone infusion compared with placebo in volunteers free of heart disease.3 Interestingly, one participant with an incidentally discovered reduced LVEF (47%) experienced normalized function following KE, similar to the effect size observed in HF.3 It is important to note that exogenous ketosis may render differential effects on echocardiographic function than endogenous ketosis (i.e., achieved by a ketogenic diet). The ketogenic diet generally results in less substantial increases in ketosis and also increases free fatty acids,11 which for example may impair diastolic function.21 Additionally, ketosis is achieved acutely with exogenous ketones, whereas a time dependence is required for endogenous ketosis. However, dedicated study of the echocardiographic effects of endogenous ketosis is warranted.
Among diastolic parameters, the increase in mitral inflow A velocity was most marked. As mitral inflow A velocity can reflect LA systolic function but is also related to a number of variables that were also affected by KE in our study (transvalvular flow and heart rate), we performed LA strain analysis. We observed that LA contractile strain was augmented by approximately 40% following KE, which may be consistent with an intrinsic increase in LA function with exogenous ketones. The increase in LA reservoir strain is likely related to the increase in LA contractile strain, as supported by the difference observed in LA minimum volumes without a statistically significant difference in LA maximum volumes. The relevance of LA dysfunction to patients with atrial fibrillation17 and HF, particularly with preserved LVEF,22 has been well described. Whether therapeutic ketosis may be a novel strategy for conditions characterized by atrial myopathy merits further study.
Notably, we did not observe a dose-response relationship between achieved level of ketosis (or insulin) and change in echocardiographic parameters. Because we used a relatively high weight-based dose of KE to achieve ketosis in this study, it is possible that a ceiling effect was reached in participants with already normal myocardial function. The use of lower doses of KE may elucidate either threshold or dose-response relationships to myocardial function, as observed in the failing heart.3 In an exploratory analysis that should be considered hypothesis-generating, we did find that change in GLS was inversely associated with change in NEFA; whether this suggests that reducing NEFA may be a mechanism of benefit of KE warrants further study.
We observed increases in LV systolic function (LVEF and LV GLS) in our study of healthy volunteers, though these were modest. Several mechanisms may underpin these findings. First, ketone-induced vasodilation may augment myocardial performance and/or enhance ventricular-arterial coupling. Although this may contribute to the changes observed, systolic blood pressure increased and diastolic blood pressure did not decrease, suggesting that the increase in flow was not matched by the decrease in resistance. Second, the increase in heart rate may cause frequency-dependent inotropy, also known as the staircase phenomenon of Bowditch. However, given the modest increase in heart rate, we suspect that this contribution to contractility is not substantial. Third, because ketone body oxidation is largely unregulated and proportionate to substrate availability,23,24 metabolic modulation with exogenous KE and provision of a more energetically efficient fuel source may improve ventricular performance.25 Fourth, the increase in insulin, which may be related to counterregulatory feedback in the face of systemic ketosis,26 could engender increases in myocardial contractility, though we did not observe a relationship between change in LV systolic function and change in insulin levels. Finally, it is possible that the gastrointestinal side effects engendered by exogenous ketones (as observed in our study) contributed to the hemodynamic effects we observed, though these side effects were typically transient, and the post-KE echocardiogram was obtained 30 min after ingestion.
Our findings, if confirmed in placebo-controlled (and nonfasting) states, could have several implications. First, they provide data on the cardiovascular effects of exogenous ketones in individuals taking ketones for ergogenic benefits, though more research specifically during exercise will be warranted. As research expands on their use in diabetes and neurological conditions, these data will provide relevant cardiovascular safety data as well. Second, the increase in heart rate (which may be vasodilatory or less likely direct sympathetic stimulation) may not be desirable in all patients, and understanding this in relation to patients with arrhythmias will be important. Conversely, the increase in LA mechanics may actually be helpful in patients with atrial arrhythmias in whom atrial dysfunction is common. Finally, our results maybe complementary to recent interest in metabolic modulation as a mechanism of benefit of the ketogenic sodium-glucose cotransporter-2 inhibitors. Although sodium-glucose cotransporter-2 inhibitors engender a number of salutary cardiovascular, renal, and metabolic effects, it is reasonable to speculate that the resulting ketosis (albeit modest) may contribute to their benefit, particularly in HF.1
There were several limitations, as our study did not incorporate a control arm (passive or active), which would provide more definitive evidence of the effects of ketones on myocardial function, particularly as meal consumption can affect echocardiographic measurements.27,28 Additionally, although preload augmentation with the KE drink could theoretically explain some of our findings, participants received a modest volume orally through KE consumption (137 mL on average). Furthermore, these echocardiographic effects were assessed at rest, and dedicated study during exercise would be useful given known ergogenic effects of KE. Finally, only an acute assessment of these echocardiographic effects was performed, and therefore the duration of the effect is unknown. Given the short half-life of the compound, however, we suspect that the echocardiographic effects are likewise short in duration. Strengths of our study include comprehensive echocardiographic phenotyping, including LA and LV strain analysis, and integrated metabolite and hormonal data.
CONCLUSION
Acute KE administration was associated with significant echocardiographic effects among participants free of known cardiovascular disease, including augmentation of biventricular function, LA strain, and heart rate, and decrease in SVR. Our findings, if confirmed in controlled studies, suggest that the clinical effects of exogenous ketones may not be limited to the failing heart and, further, may provide mechanistic links for the ergogenic benefits observed in athletes.
HIGHLIGHTS.
Ketosis may affect biventricular function, LV and LA strain, and heart rate.
The increase in GLS was inversely related to the change in NEFAs.
Whether these findings afford ergogenic or outcome benefit in HF merits attention.
ACKNOWLEDGMENTS
We thank Dr. Heather Collins and Huong-Lan Nguyen of the University of Pennsylvania Diabetes Research Center Radioimmunoassay and Biomarkers Core for performance of the assays.
This work was funded by an American Society of Nuclear Cardiology Institute for the Advancement of Nuclear Cardiology award (to Dr. Selvaraj). Funding for statistical support was provided by the Penn Cardiovascular Disease Fellowship Innovation Fund. Laboratory analyses were also supported in part by Public Health Services Research Grant P30 DK19525 (University of Pennsylvania Diabetes Research Center Radioimmunoassay and Biomarkers Core). Research reported in this publication was supported by the Institute for Translational Medicine and Therapeutics of the Perelman School of Medicine at the University of Pennsylvania. D.P.K. was funded by NIH R01HL151345.
Dr. Selvaraj receives research support from the Doris Duke Charitable Foundation (Physician Scientist Fellowship Award 2020061), the Measey Foundation, the Institute for Translational Medicine and Therapeutics (Junior Investigator Preliminary/Feasibility Grant Program award and Translational Bio-Imaging Center award), and the American Society of Nuclear Cardiology (Institute for the Advancement of Nuclear Cardiology award). Dr. Margulies receives research support from Amgen and serves as an adviser for Bristol Myers Squibb and Pfizer.
Abbreviations
- BHB
β-hydroxybutyrate
- GLS
Global longitudinal strain
- HF
Heart failure
- IQR
Interquartile range
- KE
Ketone ester
- LA
Left atrial
- LV
Left ventricular
- LVEF
Left ventricular ejection fraction
- NEFA
Nonesterified fatty acid
- SVR
Systemic vascular resistance
Contributor Information
Senthil Selvaraj, Division of Cardiology, Department of Medicine, Perelman School of Medicine, University of Pennsylvania, Philadelphia, Pennsylvania; Cardiovascular Institute, Perelman School of Medicine, University of Pennsylvania, Philadelphia, Pennsylvania.
Ray Hu, Division of Cardiology, Department of Medicine, Perelman School of Medicine, University of Pennsylvania, Philadelphia, Pennsylvania.
Mahesh K. Vidula, Division of Cardiology, Department of Medicine, Perelman School of Medicine, University of Pennsylvania, Philadelphia, Pennsylvania.
Supritha Dugyala, Division of Radiology, Perelman School of Medicine, University of Pennsylvania, Philadelphia, Pennsylvania.
Ann Tierney, Department of Biostatistics, Perelman School of Medicine, University of Pennsylvania, Philadelphia, Pennsylvania.
Bonnie Ky, Division of Cardiology, Department of Medicine, Perelman School of Medicine, University of Pennsylvania, Philadelphia, Pennsylvania; Division of Biostatistics, Epidemiology and Informatics, Perelman School of Medicine, University of Pennsylvania, Philadelphia, Pennsylvania.
Kenneth B. Margulies, Division of Cardiology, Department of Medicine, Perelman School of Medicine, University of Pennsylvania, Philadelphia, Pennsylvania; Cardiovascular Institute, Perelman School of Medicine, University of Pennsylvania, Philadelphia, Pennsylvania.
Svati H. Shah, Division of Cardiology, Department of Medicine, Duke University School of Medicine, Durham, North Carolina; Duke Molecular Physiology Institute, Duke University School of Medicine, Durham, North Carolina.
Daniel P. Kelly, Cardiovascular Institute, Perelman School of Medicine, University of Pennsylvania, Philadelphia, Pennsylvania.
Paco E. Bravo, Division of Cardiology, Department of Medicine, Perelman School of Medicine, University of Pennsylvania, Philadelphia, Pennsylvania; Division of Radiology, Perelman School of Medicine, University of Pennsylvania, Philadelphia, Pennsylvania.
REFERENCES
- 1.Selvaraj S, Kelly DP, Margulies KB. Implications of altered ketone metabolism and therapeutic ketosis in heart failure. Circulation 2020;141: 1800–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Evans M, Cogan KE, Egan B. Metabolism of ketone bodies during exercise and training: physiological basis for exogenous supplementation. J Physiol 2017;595:2857–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nielsen R, Moller N, Gormsen LC, Tolbod LP, Hansson NH, Sorensen J, et al. Cardiovascular effects of treatment with the ketone body 3-hydroxybutyrate in chronic heart failure patients. Circulation 2019;139:2129–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cox PJ, Kirk T, Ashmore T, Willerton K, Evans R, Smith A, et al. Nutritional ketosis alters fuel preference and thereby endurance performance in athletes. Cell Metab 2016;24:256–68. [DOI] [PubMed] [Google Scholar]
- 5.Clarke K, Tchabanenko K, Pawlosky R, Carter E, Todd King M, Musa-Veloso K, et al. Kinetics, safety and tolerability of (R)-3-hydroxybutyl (R)-3-hydroxybutyrate in healthy adult subjects. Regul Toxicol Pharmacol 2012;63:401–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shivva V, Cox PJ, Clarke K, Veech RL, Tucker IG, Duffull SB. The population pharmacokinetics of D-beta-hydroxybutyrate following administration of (R)-3-hydroxybutyl (R)-3-hydroxybutyrate. AAPS J 2016;18:678–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stubbs BJ, Cox PJ, Evans RD, Santer P, Miller JJ, Faull OK, et al. On the metabolism of exogenous ketones in humans. Front Physiol 2017;8:848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Myette-Cote E, Caldwell HG, Ainslie PN, Clarke K, Little JP. A ketone monoester drink reduces the glycemic response to an oral glucose challenge in individuals with obesity: a randomized trial. Am J Clin Nutr 2019;110:1491–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stubbs BJ, Cox PJ, Evans RD, Cyranka M, Clarke K, de Wet H. A ketone ester drink lowers human ghrelin and appetite. Obesity (Silver Spring) 2018;26:269–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Norwitz NG, Jaramillo JG, Clarke K, Soto A. Ketotherapeutics for neurodegenerative diseases. Int Rev Neurobiol 2020;155:141–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Selvaraj S, Margulies KB, Dugyala S, Schubert E, Tierney A, Arany Z, et al. Comparison of Exogenous Ketone Administration versus Dietary Carbohydrate Restriction on Myocardial Glucose Suppression: A Crossover Clinical Trial. J Nucl Med 2021. 10.2967/jnumed.121.262734. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mitchell C, Rahko PS, Blauwet LA, Canaday B, Finstuen JA, Foster MC, et al. Guidelines for performing a comprehensive transthoracic echocardiographic examination in adults: recommendations from the American Society of Echocardiography. J Am Soc Echocardiogr 2019;32:1–64. [DOI] [PubMed] [Google Scholar]
- 13.Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr 2015;28:1–39.e14. [DOI] [PubMed] [Google Scholar]
- 14.Badano LP, Miglioranza MH, Mihaila S, Peluso D, Xhaxho J, Marra MP, et al. Left atrial volumes and function by three-dimensional echocardiography: reference values, accuracy, reproducibility, and comparison with two-dimensional echocardiographic measurements. Circ Cardiovasc Imaging 2016;9:e004229. [DOI] [PubMed] [Google Scholar]
- 15.Rudski LG, Lai WW, Afilalo J, Hua L, Handschumacher MD, Chandrasekaran K, et al. Guidelines for the echocardiographic assessment of the right heart in adults: a report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J Am Soc Echocardiogr 2010;23:685–713. [DOI] [PubMed] [Google Scholar]
- 16.Abbas AE, Fortuin FD, Schiller NB, Appleton CP, Moreno CA, Lester SJ. A simple method for noninvasive estimation of pulmonary vascular resistance. J Am Coll Cardiol 2003;41:1021–7. [DOI] [PubMed] [Google Scholar]
- 17.Patel RB, Delaney JA, Hu M, Patel H, Cheng J, Gottdiener J, et al. Characterization of cardiac mechanics and incident atrial fibrillation in participants of the Cardiovascular Health Study. JCI Insight 2020;5:e141656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Horton JL, Davidson MT, Kurishima C, Vega RB, Powers JC, Matsuura TR, et al. The failing heart utilizes 3-hydroxybutyrate as a metabolic stress defense. JCI Insight 2019;4:e124079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gormsen LC, Svart M, Thomsen HH, Sondergaard E, Vendelbo MH, Christensen N, et al. Ketone body infusion with 3-hydroxybutyrate reduces myocardial glucose uptake and increases blood flow in humans: a positron emission tomography study. J Am Heart Assoc 2017;6: e005066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yurista SR, Chong C-R, Badimon JJ, Kelly DP, de Boer RA, Westenbrink BD. Therapeutic potential of ketone bodies for patients with cardiovascular disease. J Am Coll Cardiol 2021;77:1660–9. [DOI] [PubMed] [Google Scholar]
- 21.Leichman JG, Aguilar D, King TM, Vlada A, Reyes M, Taegtmeyer H. Association of plasma free fatty acids and left ventricular diastolic function in patients with clinically severe obesity. Am J Clin Nutr 2006;84: 336–41. [DOI] [PubMed] [Google Scholar]
- 22.Santos AB, Roca GQ, Claggett B, Sweitzer NK, Shah SJ, Anand IS, et al. Prognostic relevance of left atrial dysfunction in heart failure with preserved ejection fraction. Circ Heart Fail 2016;9:e002763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Murashige D, Jang C, Neinast M, Edwards JJ, Cowan A, Hyman MC, et al. Comprehensive quantification of fuel use by the failing and nonfailing human heart. Science 2020;370:364–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Monzo L, Sedlacek K, Hromanikova K, Tomanova L, Borlaug BA, Jabor A, et al. Myocardial ketone body utilization in patients with heart failure: the impact of oral ketone ester. Metabolism 2021;115:154452. [DOI] [PubMed] [Google Scholar]
- 25.Kashiwaya Y, King MT, Veech RL. Substrate signaling by insulin: a ketone bodies ratio mimics insulin action in heart. Am J Cardiol 1997;80: 50A–64A. [DOI] [PubMed] [Google Scholar]
- 26.Madison LL, Mebane D, Unger RH, Lochner A. The hypoglycemic action of ketones. II. Evidence for a stimulatory feedback of ketones on the pancreatic beta cells. J Clin Invest 1964;43:408–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dieden A, Gardinger Y, Hlebowicz J, Bjorgell O, Dencker M. Effect of food intake on left and right ventricular systolic tissue Doppler measurements. Clin Physiol Funct Imaging 2016;36:396–400. [DOI] [PubMed] [Google Scholar]
- 28.Gardinger Y, Dieden A, Hlebowicz J, Bjorgell O, Dencker M. Effect of food intake on myocardial performance index. Cardiovasc Ultrasound 2017; 15:10. [DOI] [PMC free article] [PubMed] [Google Scholar]