Abstract
Heavy drinking and HIV infection are independently associated with damage to the brain’s white matter. The purpose of the current study was to investigate whether current alcohol consumption, HIV infection, and associated characteristics were associated with indices of white matter microstructural integrity in people living with HIV (PLWH) and seronegative individuals. PLWH and controls were categorized as non-drinkers, moderate drinkers, or heavy drinkers. White matter fractional anisotropy (FA), radial diffusivity (RD), and axial diffusivity (AD) were assessed using diffusion tensor imaging (DTI). Voxelwise analyses using tract-based spatial statistics were followed by confirmatory region-of-interest (ROI) analyses. Data from 108 participants (62 PLWH, 46 controls) were suitable for analysis. Average age (±standard deviation) was 45.2±11.1 years, and the sample was 42% female. The majority of PLWH were on antiretroviral therapy (94%) and were virally suppressed (69%). PLWH and controls did not differ on substance use. Heavier alcohol intake was significantly associated with lower FA and higher RD in widespread areas. Heavy drinking was significantly associated with higher AD in a small region. The main effect of HIV was not significant, but a significant HIV-age interaction was observed. Follow-up ROI analyses confirmed the main effect of drinking group and HIV-age interaction. In conclusion, results are consistent with a dose-dependent association of alcohol use with lower white matter microstructural coherence. Concordance between FA and RD findings suggests dysmyelination as a mechanism. Findings underscore the need to address unhealthy alcohol use in HIV-positive and seronegative individuals, the consequences of which may be exacerbated by aging.
Keywords: magnetic resonance imaging, diffusion tensor imaging, alcohol, heavy drinking, HIV infection, white matter
Introduction
Alcohol use is common in people living with HIV infection (PLWH): 63% report alcohol use in the past year, and 16% endorse heavy drinking in the past month (Centers for Disease Control and Prevention, 2019). Although alcohol use disorder (AUD) and HIV infection have distinct neuropathological mechanisms, abnormality in the brain’s white matter is a hallmark injury of both conditions (Monnig et al, 2013b; O’Connor et al, 2017). Diffusion tensor imaging (DTI) is an application of magnetic resonance imaging (MRI) that quantifies directionality of water diffusion to allow inferences about microstructural tissue properties (Basser and Pierpaoli, 1996). DTI has helped to illuminate the functional significance of brain white matter, showing that intact white matter microstructure is vitally important to cognitive function. Population-based studies have identified white matter as a key contributor to general intelligence in community samples, specifically through its support of information processing speed (Penke et al, 2012). In studies of healthy aging, DTI metrics strongly mediate numerous higher-order cognitive functions, including fluid intelligence, executive function, memory, and flexibility (Borghesani et al, 2013; Coelho et al, 2021; Kievit et al, 2014; Madden et al, 2009; Penke et al, 2010; Vernooij et al, 2009).
DTI provides valuable information about white matter abnormalities in individuals with AUD and HIV, prior to the appearance of gross lesions or atrophy. AUD is associated with white matter damage in corpus callosum, corona radiata, thalamic pathways, superior longitudinal fasciculus, and internal capsule (Alhassoon et al, 2012; Pfefferbaum et al, 2006; Pfefferbaum et al, 2009; Yeh et al, 2009). Similarly, HIV infection is associated with white matter abnormality in corticocortical and corticolimbic fibers (Cole et al, 2018; Gongvatana et al, 2011; Gongvatana et al, 2009; Nir et al, 2014; Seider et al, 2016). White matter damage in HIV infection manifests during the acute stage (Ragin et al, 2015) and is not fully mitigated by combined antiretroviral therapy (ART) (O’Connor and Zeffiro, 2018). Research also shows that DTI metrics predict cognition in the context of HIV infection and AUD. Higher MD in the corpus callosum, corona radiata, optic radiation, and internal capsule has been associated with neurocognitive impairment in PLWH (Gongvatana et al, 2009). In another study of PLWH, lower FA of the corpus callosum and uncinate fasciculus was associated with slower processing speed and lower accuracy in an attention-emotion task (Schulte et al, 2012). In AUD, DTI metrics correlate with psychomotor speed, balance, memory, and executive function (Crespi et al, 2020; Pfefferbaum et al, 2009; Trivedi et al, 2013).
Prior reports are mixed as to whether HIV clinical characteristics, such as nadir CD4 or duration of infection, predict white matter compromise. In addition, findings vary as to whether aging and HIV infection interact to promote accelerated aging of brain white matter. Some studies have reported an interaction of aging and HIV infection (i.e., accelerated aging) on brain white matter microstructure (Kuhn et al, 2019; Seider et al, 2016), whereas others have found augmented white matter aging in PLWH in the absence of an interaction per se (Cole et al, 2018; Kuhn et al, 2018; Su et al, 2016).
Associations of heavy alcohol use with white matter metrics in the context of HIV infection are another important area of investigation, yet most HIV-focused studies have excluded individuals with current or past AUD. One study found that PLWH with co-occurring AUD had greater white matter deficits in corpus callosum compared to controls or to individuals with HIV or AUD alone (Pfefferbaum et al, 2007). Our group previously found that lifetime diagnosis of AUD accounted for abnormal diffusivity in frontal white matter (Gullett et al, 2018). Given the high prevalence of alcohol use in PLWH (Williams et al, 2016), there is a pressing need to further investigate whether current alcohol use is associated with white matter abnormality in this population.
This study aimed to quantify the independent and concomitant associations of heavy drinking and HIV infection with white matter microstructure. Participants were 108 individuals enrolled in a study on brain dysfunction associated with HIV and alcohol use through the Alcohol Research Center on HIV (1P01AA019072). The cohort included PLWH and a seronegative control group, with a full range of current drinking behavior (i.e., non-drinkers, moderate drinkers, and heavy drinkers) represented in both groups. We predicted that HIV infection and current heavy drinking each would be associated with white matter microstructural abnormalities. At the same time, current drinking status can be a weak representation of lifetime alcohol use, particularly when individuals stop drinking due to health problems or negative consequences. Because there are significant gaps in understanding of the effects of current versus past drinking on brain health, we also examined lifetime history of AUD. Finally, based on prior research by our group and others (Cohen et al, 2010; Gongvatana et al, 2011; Seider et al, 2016; Sexton et al, 2014), we also expected that older age in all participants and select clinical characteristics in PLWH [e.g., CD4 nadir, detectable viral load, Hepatitis C virus (HCV) coinfection] would be associated with white matter microstructural compromise.
Method
Participants
Data were collected in 2010–2015 at the baseline visit of a longitudinal project sponsored by Brown University’s Alcohol Research Center on HIV (ARCH). Individuals were recruited from an HIV clinic in Providence, RI, based on review of medical records and clinical interviews. Seronegative individuals treated at or referred by patients of the clinic were recruited as a control group with similar sociodemographic characteristics. Inclusion/exclusion criteria for the seropositive and seronegative groups were the same, except for criteria pertaining to HIV disease status. After screening and enrollment, participants completed a detailed behavioral assessment and MRI scan. HCV status was determined by antibody test followed by confirmatory HCV RNA testing. All participants provided informed consent for research activities, which were approved by the Institutional Review Boards of Brown University and The Miriam Hospital/Lifespan. This report includes all participants from this cohort with usable baseline DTI data; different analyses to address distinct research questions have been reported previously in a related sample (Gullett et al, 2018).
Inclusion criteria:
1) age 21–70 years; 2) HIV serostatus documented by ELISA, confirmed by Western blot and plasma HIV RNA or a second antibody test.
Exclusion criteria:
1) history of significant pre-existing neurological brain disease, including Alzheimer’s disease, stroke, seizure disorder, and traumatic brain injury with loss of consciousness >10 minutes; 2) severe psychiatric illness (i.e., of severity that may compromise ability to provide informed consent); 3) end-stage disease (life expectancy <12 months); 4) pregnancy; 5) contraindication for MRI scanning (e.g., non-removable ferromagnetic material in the body); 6) opportunistic CNS infection (e.g., toxoplasmosis) or neoplasm; 7) history of ascites, encephalopathy, esophageal variceal bleeding, hepatorenal syndrome or evidence of severe liver disease; 8) current cocaine, stimulant, or opioid use disorder, or any intravenous drug use.
Measures
Participants completed in-house questionnaires on medical history and smoking status. Current and past AUD were assessed using the Mini-International Neuropsychiatric Interview (MINI) (Sheehan et al, 1998). Other psychiatric diagnoses were assessed by self-report. The Timeline Followback (TLFB) interview was used to collect data on alcohol and other drug use over the previous 90 days (Sobell and Sobell, 1992). TLFB data were used to classify participants into drinking groups as follows: non-drinkers reported no alcohol consumption; moderate drinkers reported average weekly alcohol intake at low-risk levels (≤7 drinks/week for women and ≤14 drinks/week for men); and heavy drinkers reported weekly intake above low-risk levels (National Institute on Alcohol Abuse and Alcoholism, 2010).
MRI Acquisition
Imaging data were collected on a Siemens MAGNETOM Trio 3T scanner as described in a previous publication (Gullett et al, 2018). Briefly, whole-brain T1-weighted images were collected in 255 interleaved axial 1-mm slices with TR=1.90 s, TE=2.98 s, and field of view=256×256 mm. Structural T1 images were processed using the Freesurfer automated pipeline (Fischl et al, 2002). Whole-brain DTI were collected in 69 interleaved axial 2-mm slices with TR=1.10 s, TE=103 ms, and field of view=128×128 mm. Diffusion data were collated into a dataset with 64 b=1000m/s2 volumes, 10 b=5 mm/s2 volumes, and 1 b=0 volume, for a total of 75 volumes per scan.
DTI Processing and Analysis
Processing:
Diffusion weighted images were denoised using the LPCA filter (Manjón et al, 2013) and corrected for motion by linearly aligning all diffusion weighted image volumes to the b0 image. T1-weighted images were denoised using the nonlocal means filter (Coupe et al, 2008) and underwent N3 intensity inhomogeneity normalization (Sled et al, 1998) and brain extraction (Fischl, 2012). T1 images were linearly aligned to diffusion images, and diffusion images were then non-linearly warped to their respective T1-weighted scans to correct for echo-planar imaging (EPI) induced susceptibility artifacts (Avants et al, 2011). Diffusion gradient directions were rotated to accommodate linear registrations.
Quality check:
DTI scans were collected from a total of 132 participants. From the 132 datasets, 20 had motion/scanner artifact, and 4 had extreme outlier values >3 SDs from the group mean. After excluding those 24 datasets, 108 DTI datasets were available for analysis. Excluded datasets did not differ by HIV status [Pearson’s χ2(1)=.002, p=.962].
Voxelwise analyses:
Tract-based spatial statistics (TBSS) from the FMRIB Software Library [FSL; (Jenkinson et al, 2012)] were used to implement voxelwise statistics on the mean FA skeleton (Smith et al, 2006). The mean FA skeleton was derived from participants’ data after non-linear registration and transformation. The FA skeleton image was thresholded at 0.20. Axial diffusivity (AD) and radial diffusivity (RD) were analyzed in a parallel manner using FSL’s script for non-FA files. All voxelwise analyses utilized the randomise program with threshold-free cluster enhancement, which corrects for multiple comparisons across spatial images (Winkler et al, 2014).
Region of interest (ROI) analyses:
ROIs were calculated from the white matter skeleton using the JHU-ICBM-DTI-81 atlas (Mori et al, 2008). Informed by previous research on HIV and alcohol effects reviewed above, we analyzed 21 ROIs (4 midline, 17 bilateral), as follows: genu, body, and splenium of corpus callosum; fornix; corticospinal tract; medial lemniscus; cerebral peduncles; anterior, posterior, and retrolenticular internal capsule; anterior, superior, and posterior corona radiata; posterior thalamic radiation; sagittal striatum; external capsule; cingulate gyrus; cingulum hippocampal part; fornix/stria terminalis; superior longitudinal fasciculus; uncinate fasciculus.
Statistical Analysis
Demographic and clinical characteristics:
Group differences in demographic and clinical attributes were assessed with t-tests or one-way analysis of variance (ANOVA) for continuous variables and Pearson’s χ2 test for categorical variables.
TBSS voxelwise analyses:
Our approach was to test predictor variables on a whole-brain voxelwise basis using TBSS (Smith et al, 2006). TBSS allows for testing a contrast of interest (e.g., HIV-seropositive vs. HIV-seronegative) while controlling for other variables entered into the model.
Primary hypothesis test.
We tested main effects of HIV status, age, drinking group, sex, smoking status, and marijuana use (i.e., frequency on the TLFB) on FA of the white matter skeleton. Sex, smoking status, and marijuana status were included as control variables in this analysis due to white matter effects reported previously (Filbey et al, 2014; Inano et al, 2011; Liang et al, 2018; Orr et al, 2016). Drinking group was coded as 0=non-drinker, 1=moderate drinker, and 2=heavy drinker.
Interaction analyses.
In two separate TBSS analyses, we tested the interaction of HIV status with A) age and B) drinking group on white matter FA. This analysis was conducted without additional variables included in the model and was followed by a confirmatory ROI analysis with relevant control variables (see below).
AD and RD analyses.
Finally, we conducted whole-brain TBSS analyses on AD and RD using parallel methods to the primary hypothesis test. These analyses tested main effects of HIV status, age, drinking group, sex, smoking status, and marijuana use on AD or RD of the white matter skeleton.
Additional FA, AD, and RD analyses.
In order to evaluate possible confounders, we conducted three additional analyses that included all predictor variables above (i.e., HIV status, age, drinking group, sex, smoking status, marijuana use) plus the following variables in separate analyses: A) mental health conditions; B) illicit drug use; or C) lifetime AUD. Each of these three potential confounders was tested as a predictor of FA, AD, and RD values in separate TBSS analyses. The mental health conditions variable was a count variable reflecting how many of the following diagnoses were self-reported by the participant: depression, anxiety, schizophrenia, bipolar disorder, ADHD. These conditions were selected due to the high prevalence of depression in PLWH (Rubin and Maki, 2019) and to recent evidence linking anxiety, schizophrenia, bipolar disorder, and ADHD with accelerated brain aging (Amen et al, 2018). The illicit drug use variable was a dichotomous variable (0=no, 1=yes) representing whether the participant reported any use of cocaine, hallucinogens, sedatives, opioids, or stimulants on the 90-day TLFB. Lifetime AUD was a dichotomous variable (0=no, 1=yes) determined from the MINI and captured both current and remote AUD.
ROI analyses:
Significant voxelwise findings were followed with confirmatory ROI analyses. Univariate ANOVAs were used to probe the main effect of drinking group on FA of ROIs identified in the voxelwise analysis. Averaged left and right hemisphere ROIs were analyzed unless voxelwise analyses indicated a unilateral effect. These tests used HIV, drinking group, age, and marijuana use as predictors of individual ROI FA values. Smoking status and sex were not included in ANOVA models because these variables were not significant in voxelwise analyses. For significant models, follow-up contrasts were performed to assess pairwise differences between drinking groups. Separately, multivariate ANOVA was used to examine the HIV-age interaction in all ROIs simultaneously, given the extent of the interaction in voxelwise analysis. The multivariate analysis used HIV, age, drinking group, marijuana use, and the HIV-age interaction as predictors and the 21 ROIs as dependent variables.
Clinical characteristics:
Within-group analyses in PLWH tested clinical characteristics as predictors of FA, AD, and RD values in the 21 ROIs using multiple regression in participants who had complete data on the predictor variables (n=56). Clinical characteristics of interest were 1) CD4 nadir; 2) current CD4; 3) HCV status; 4) detectable HIV RNA, using a threshold of 75 copies/ml; 5) length of infection, which was implemented as unstandardized residuals from a regression with age as the independent variable and length of infection as the dependent variable. The residualized variable was necessary to differentiate length of infection from age because these observed variables were highly correlated (r=.603, p<.0001). The resulting residualized variable was not correlated with age (r=.000, p=1.000). Bonferroni adjustment for multiple comparisons was applied to regression models for a critical p-value=.00238 (i.e., .05/21 ROIs).
Post hoc tests:
We considered that differential lifetime alcohol exposure (across non-drinkers and moderate drinkers) or HIV disease history (across all groups) might have confounded differences according to drinking group. As post hoc analyses, we tested A) whether non-drinkers and moderate drinkers differed on lifetime AUD; and B) whether, within PLWH, the three drinking groups differed on infection length, CD4 nadir, current CD4, detectable viral load, or HCV co-infection.
Results
Participant Characteristics
As shown in Table 1, PLWH and controls did not differ on education, race/ethnicity, smoking status, current drinking status, current or past AUD diagnosis, average drinks per week, or percent days using marijuana. Compared to controls, PLWH had a higher mean age and a lower proportion of women. Participants reported use of non-prescribed drugs other than cannabis (i.e., cocaine, opioids, sedatives, stimulants, hallucinogens) on <2% of days.
Table 1:
Demographic and clinical characteristics (N=108)
| Entire sample (N=108) | PLWH (n=62) | HIV-seronegative (n=46) | p-value for test of group difference | |
|---|---|---|---|---|
| Age | 45.2 ± 11.1 | 48.9 ± 8.6 | 40.3 ± 12.2 | p < .001* |
| Education | 13.7 ± 2.8 | 13.3 ± 2.9 | 14.3 ± 2.5 | p = .076 |
| Sex (% female) | 42% | 29% | 59% | p = .002* |
| Race/ethnicity | 68% White, 24% African-American, 7% Hispanic/Latino, 1% Other | 66% White, 23% African-American, 10% Hispanic/Latino, 1% Other | 70% White, 26% African-American, 4% Hispanic/Latino | p = .586 |
| Current smoker | 42% | 43% | p = .873 | |
| Years since HIV diagnosis | ---- | 18.8 ± 18.2 | ---- | ---- |
| On ART | ---- | 93.5% | ---- | ---- |
| Virally suppressed (HIV RNA ≤75 copies/ml)a | ---- | 69.4% | ---- | ---- |
| Current CD4 counta | ---- | 581 ± 275 | ---- | ---- |
| CD4 nadira | ---- | 214 ± 171 | ---- | ---- |
| HCV co-infectiona | ---- | 19% | ---- | ---- |
| Current drinking status | 28% non-drinker, 55% moderate drinker, 17% heavy drinker | 32% non-drinker, 53% moderate drinker, 15% heavy drinker | 22% non-drinker, 58% moderate drinker, 20% heavy drinker | p = .450 |
| Diagnosis of alcohol use disorder (AUD) | 27% never AUD, 57% remote AUD, 16% current AUD | 35% never AUD, 50% remote AUD, 15% current AUD | 21% never AUD, 63% remote AUD, 16% current AUD | p = .265 |
| Average drinks per weekb | 6.9 ± 13.8 | 7.0 ± 14.7 | 6.9 ± 12.7 | p = .967 |
| Percent days using marijuanab | 12.7 ± 27.5 | 17.1 ± 30.4 | 6.9 ± 22.1 | p = .055 |
| ADHDa,c | 1% | 2% | 0% | p = .388 |
| Anxietya,c | 54% | 61% | 43% | p = .068 |
| Bipolar Disordera,c | 7% | 8% | 7% | p = .768 |
| Depressiona,c | 55% | 68% | 37% | p = .001 |
| Schizophreniaa,c | 1% | 2% | 0% | p = .388 |
Notes on missing data: 4 participants were missing HIV viral load, 3 were missing CD4 nadir, 4 were missing current CD4, 6 were missing HCV co-infection, and 2 were missing mental health conditions.
In the past 90 days, per the Timeline Followback Interview.
Mental health conditions were self-reported.
significant group difference at p < .05.
In the sample of PLWH, 94% were on ART, and 69% were virally suppressed. CD4 count (581±275) and CD4 nadir (214±171) were relatively high, indicating a lack of severe immunosuppression in general. HCV coinfection was present in 19% of PLWH. PLWH self-reported depression, but not other mental health conditions, at a higher rate than seronegative controls, consistent with depression as a neuropsychiatric complication of HIV (Rubin and Maki, 2019).
TBSS Voxelwise Results
Primary hypothesis test:
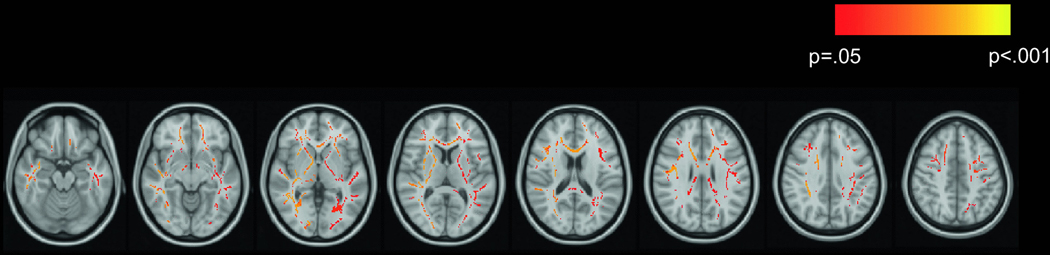
The TBSS main effects analysis tested HIV status, age, drinking group, sex, smoking status, and marijuana use as predictors of white matter skeleton FA. Age, drinking group, and marijuana use showed significant main effects. Main effects of HIV status, sex, and smoking status were not significant. Older age was associated with lower FA over the majority (approximately 71%) of the white matter skeleton (Figure 1A). Drinking group was associated with FA over a large portion (approximately 31%) of the white matter skeleton (Figure 1B). As expected, heavier drinking was associated with lower FA. Tracts affected by the significant main effect of drinking group included the genu, body, and splenium of corpus callosum; left retrolenticular part of the internal capsule; anterior, superior, and posterior corona radiata; posterior thalamic radiation; left external capsule; cingulate gyrus; cingulum hippocampal part; and superior longitudinal fasciculus. Greater frequency of marijuana use was associated with higher FA in several tracts, including anterior, superior, and posterior corona radiata; superior longitudinal fasciculus; right cingulate gyrus; genu and splenium of corpus callosum; anterior limb of internal capsule; and external capsule (Figure 1C).
Figure 1.
Main effects of age, alcohol, and marijuana on FA
Effects of age, drinking group, and marijuana use on FA are displayed in red-yellow on the MNI152 T1 template. In all analyses, drinking group was coded such that a negative association indicates that heavier drinking was associated with lower FA. A) Negative association of age with FA; B) negative association of drinking group with FA; C) positive association of marijuana use frequency with FA.
Interaction analyses:
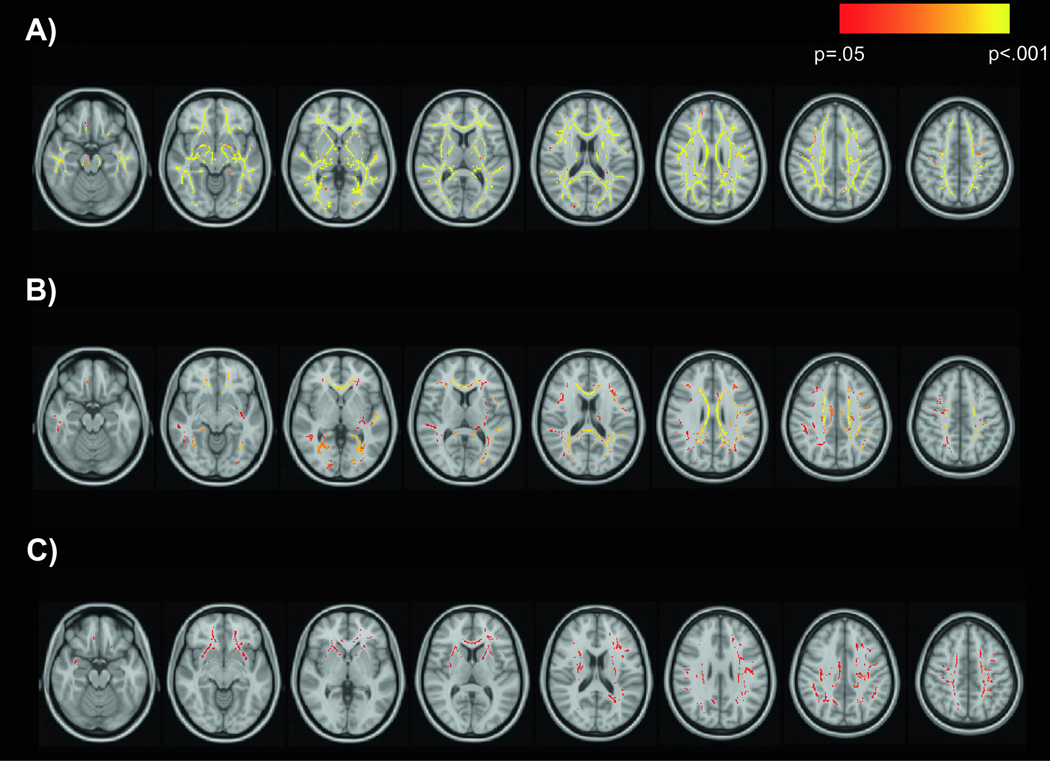
Next, TBSS was used to test interactions of HIV with age and drinking group on white matter skeleton FA. There was a significant HIV-age interaction in which the effect of age on FA was significantly more negative in the HIV group than in the control group. The HIV-age interaction affected FA over a large portion of the white matter skeleton (Figure 2). Important to note, this finding qualifies the significant main effect of age and null main effect of HIV reported above. Tracts affected by the HIV-age interaction included anterior and superior corona radiata; left posterior corona radiata; body, genu, and splenium of corpus callosum; anterior limb, posterior limb, and retrolenticular part of internal capsule; external capsule; superior longitudinal fasciculus; posterior thalamic radiation; sagittal striatum; fornix/stria terminalis (bilateral). The interaction of HIV with drinking group was not significant.
Figure 2.
Interactive effects of HIV and age on FA
The interaction of HIV status and age on FA is displayed in red-yellow on the MNI152 T1 template. Significant voxels are those in which the effect of age on white matter FA was significantly more negative in the HIV group than in the control group.
AD and RD analyses:
We conducted parallel voxelwise analyses on AD and RD to better understand FA differences. Results for AD were limited (Figure 3). Older age was associated with lower AD in the left retrolenticular part of the internal capsule (Figure 3A), and heavier alcohol use was associated with higher AD in left anterior and superior corona radiata and left anterior and posterior limbs of the internal capsule (Figure 3B). Findings for RD were widespread and showed a high degree of spatial overlap with FA results above. Older age (Figure 3C) and heavier drinking (Figure 3D) were associated with higher RD over the majority of the white matter skeleton. In addition, marijuana use was associated with lower RD in right cingulate gyrus, superior longitudinal fasciculus, splenium of corpus callosum, left anterior corona radiata, anterior limb of internal capsule, and external capsule (Figure 3E).
Figure 3.
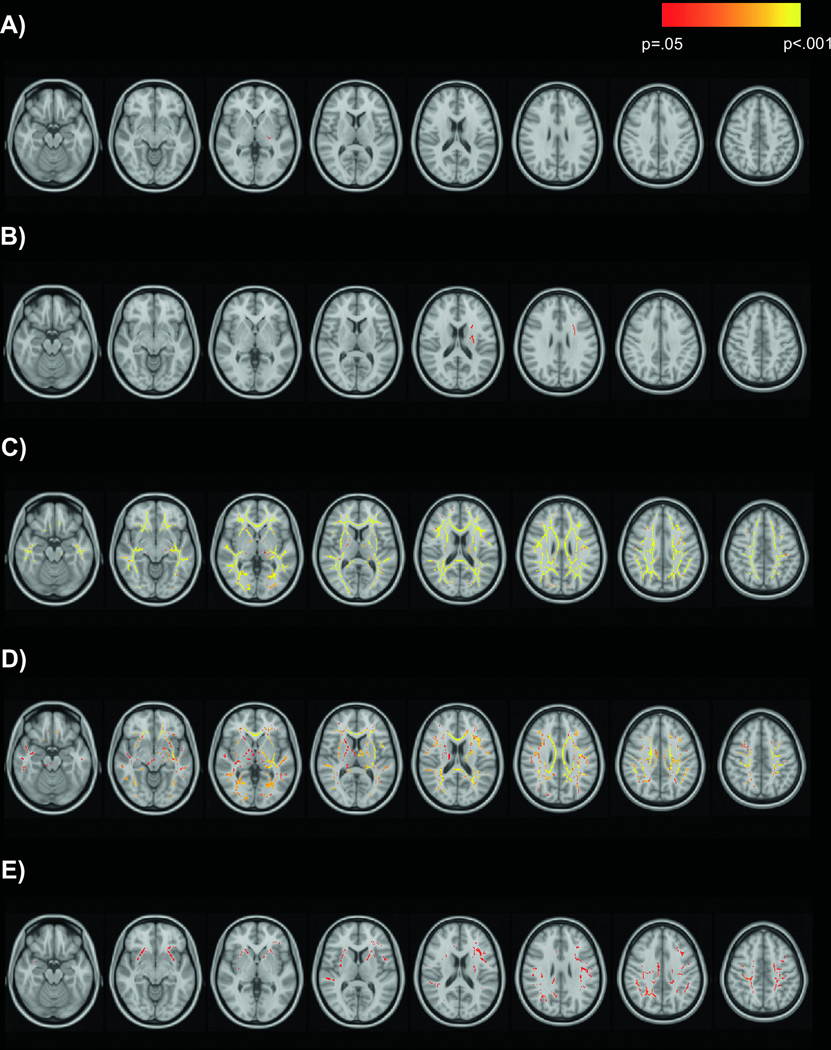
Main effects of age, alcohol, and marijuana on AD and RD
Effects of age and drinking group on AD and effects of age, drinking group, and marijuana use on RD are displayed in red-yellow on the MNI152 T1 template. A) Negative association of age with AD; B) positive association of drinking group with AD; C) positive association of age with RD; D) positive association of drinking group with RD; E) negative association of marijuana use with RD.
Additional FA, AD, and RD analyses: These analyses to evaluate possible effects of mental health conditions, illicit drug use, or lifetime AUD diagnosis on FA, AD, and RD values yielded no significant results (all p’s > .08). Addition of these variables to TBSS models did not change the significance of any effects reported above.
ROI Results
Comparison of drinking groups:
Univariate ANOVAs probed the main effect of drinking group on FA of the 12 ROIs identified in the voxelwise analysis above, with HIV, age, and marijuana use included as predictors. Of the 12 ROIs tested in the ANOVA framework, there was a significant effect of drinking group on 6 ROIs (Table 2 and Figure 4). These were the genu, body, and splenium of corpus callosum; posterior thalamic radiation; left external capsule; and cingulate gyrus. In all of these ROIs, non-drinkers had significantly higher FA than heavy drinkers, with medium to large effect sizes for group differences. In addition, non-drinkers had significantly higher FA than moderate drinkers in the genu and body of corpus callosum, with effect sizes of small magnitude. Moderate drinkers had significantly higher FA than heavy drinkers in the body of corpus callosum, posterior thalamic radiation, and left external capsule, with medium to large effect sizes observed for these differences.
Table 2.
Univariate analyses of FA in ROIs
| HIV (p-value) | Drinking group (p-value) | Age (p-value) | Marijuana use (p-value) | Significant pairwise contrasts | |
|---|---|---|---|---|---|
| Genu of corpus callosum | .700 | .008* | <.001* | .189 | Non-drinker > moderate drinker, p = .022, Cohen’s d = .331; Non-drinker > heavy drinker, p = .003, Cohen’s d = .789 |
| Body of corpus callosum | .092 | <.001* | <.001* | .618 | Non-drinker > moderate drinker, p = .037, Cohen’s d = .278; Non-drinker > heavy drinker, p <.001, Cohen’s d = .990; Moderate drinker > heavy drinker, p = .006, Cohen’s d = .740 |
| Splenium of corpus callosum | .852 | .044* | .001* | .329 | Non-drinker > heavy drinker, p = .013, Cohen’s d = .627 |
| Retrolenticular part of internal capsule (left) | .230 | .300 | <.001* | .418 | ---- |
| Anterior corona radiata | .701 | .143 | <.001* | .105 | ---- |
| Superior corona radiata | .416 | .157 | <.001* | .023* | ---- |
| Posterior corona radiata | .587 | .136 | .001* | .111 | ---- |
| Posterior thalamic radiation | .305 | .045* | <.001* | .945 | Non-drinker > heavy drinker, p =.033, Cohen’s d = .609; Moderate drinker > heavy drinker, p = .016, Cohen’s d = .644 |
| External capsule (left) | .597 | .012* | .001* | .057 | Non-drinker > heavy drinker, p = .003, Cohen’s d = .798; Moderate drinker > heavy drinker, p =.043, Cohen’s d = .575 |
| Cingulate gyrus | .345 | .032* | .117 | .028* | Non-drinker > heavy drinker, p =.011, Cohen’s d = .602 |
| Cingulum hippocampal part | .914 | .064 | .132 | .293 | ---- |
| Superior longitudinal fasciculus | .610 | .060 | <.001* | .040* | ---- |
p < .05
Figure 4.
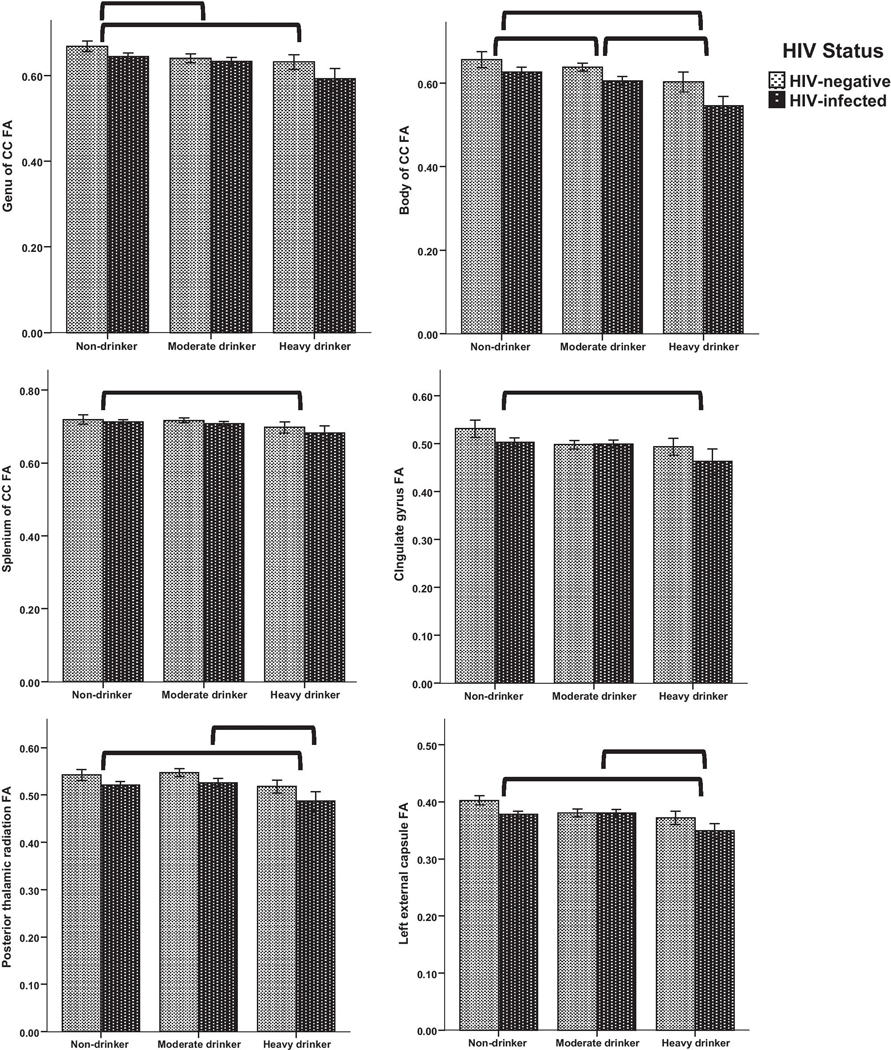
Drinking group differences in FA of 6 ROIs
FA differed significantly by drinking group in six ROIs. Brackets indicate significant pairwise differences (p<.05) by drinking group. Non-drinkers had significantly higher FA values than heavy drinkers in each ROI. Although FA values for HIV-positive participants were qualitatively lower in most ROIs, there was no significant effect of HIV status or interaction of HIV status with drinking group. Bars represent mean ± 1 standard error.
Analysis of HIV-age interaction:
The HIV-age interaction was significant in the overall multivariate model, F(21,81)=2.218, p=.006. In follow-up univariate tests, the HIV-age interaction was significant for 7 of the 21 ROIs: anterior limb of internal capsule, posterior limb of internal capsule, posterior thalamic radiation, external capsule, cingulate gyrus, superior longitudinal fasciculus, and uncinate fasciculus (Table 3).
Table 3.
Multivariate analysis of HIV-age interaction
| F(1,101) | p-value | |
|---|---|---|
| Genu of corpus callosum | 2.948 | .089 |
| Body of corpus callosum | 1.450 | .231 |
| Splenium of corpus callosum | 1.169 | .282 |
| Fornix | .643 | .425 |
| Corticospinal tract | .083 | .774 |
| Medial lemniscus | 2.007 | .160 |
| Cerebral peduncles | .060 | .807 |
| Anterior limb of internal capsule | 6.530 | .012* |
| Posterior limb of internal capsule | 4.114 | .045* |
| Retrolenticular part of internal capsule | 2.457 | .120 |
| Anterior corona radiata | 3.732 | .056 |
| Superior corona radiata | .484 | .488 |
| Posterior corona radiata | .780 | .379 |
| Posterior thalamic radiation | 4.203 | .043* |
| Sagittal striatum | 3.661 | .059 |
| External capsule | 5.349 | .023* |
| Cingulate gyrus | 5.334 | .023* |
| Cingulum hippocampal part | 1.431 | .234 |
| Fornix/stria terminalis | .643 | .425 |
| Superior longitudinal fasciculus | 8.407 | .005* |
| Uncinate fasciculus | 6.188 | .014* |
p < .05
HIV Clinical Characteristics and DTI Metrics
HIV clinical characteristics were not significantly associated with FA in any ROI. After correction for multiple comparisons, regression models were significant for AD in the corticospinal tract (p=.000827) and cerebral peduncles (p=.002373) and for RD in the corticospinal tract (p=.000390). Results are shown in Table 4. Detectable HIV RNA, current CD4 count, and/or HCV co-infection were significant variables. Detectable HIV RNA was significantly associated with lower AD of corticospinal tract (p=.037) and lower AD of cerebral peduncles (p=.029). Higher current CD4 was significantly associated with lower AD of corticospinal tract (p=.020), lower AD of cerebral peduncles (p=.013), and lower RD of corticospinal tract (p=.028). HCV co-infection was significantly associated with higher AD (p=.003) and higher RD (p=.001) of corticospinal tract.
Table 4.
Significant regression models predicting DTI metrics using HIV clinical characteristics in PLWH (n=56)
| Overall modela | Length of infectionb | CD4 nadir | Detectable HIV RNAc | Current CD4 | HCV co-infectionc | |
|---|---|---|---|---|---|---|
| AD of corticospinal tract | F(5,50)=5.028, p=.000827 | β=.067, p=.577 | β=−.020, p=.885 | β=−.278, p=.037* | β=−.356, p=.020* | β=.389, p=.003* |
| AD of cerebral peduncles | F(5,50)=4.330, p=.002373 | β=.003, p=.981 | β=−.230, p=.118 | β=−.299, p=.029* | β=−.390, p=.013* | β=.066, p=.607 |
| RD of corticospinal tract | F(5,50)=5.539, p=.000390 | β=−.007, p=.954 | β=−.048, p=.731 | β=−.178, p=.168 | β=−.329, p=.028* | β=.445, p=.001* |
Notes:
Significant predictor at p<.05.
Overall models were considered significant at p<.00238 after Bonferroni correction for multiple tests.
Length of infection was implemented as unstandardized residuals from a regression with age as the independent variable and length of infection as the dependent variable because these variables were highly correlated.
Detectable HIV RNA and HCV co-infection were coded 0=absent, 1=present.
Post Hoc Tests
These analyses evaluated whether lifetime alcohol exposure (across non-drinkers and moderate drinkers) or HIV disease history (across all groups) differed according to drinking group. As a post hoc analysis, we tested A) whether non-drinkers and moderate drinkers differed on lifetime AUD; and B) whether, within PLWH, the three drinking groups differed on infection length, CD4 nadir, current CD4, detectable viral load, or HCV co-infection. Lifetime AUD rates were high in both non-drinkers (60%) and moderate drinkers (72%); rates did not differ between these two groups [χ2(1)=1.246, p=.264]. Across all drinking groups, PLWH did not differ on length of infection [F(2,59)=1.579, p=.215], nadir CD4 [F(2,56)=.242, p=.786], current CD4 [F(2,55)=.894, p=.415], detectable viral load [χ2(2)=.386, p=.824], or HCV co-infection [χ2(2)=1.009, p=.604]. Thus, drinking group differences in white matter microstructure were not confounded by differential lifetime AUD rates or HIV clinical characteristics. In summary, ROI analyses were consistent with a dose-dependent association of greater alcohol consumption with lower FA in corpus callosum, posterior thalamic radiation, and left external capsule.
Discussion
In this sample of PLWH and seronegative individuals, current alcohol use, age, and the interaction of HIV with age predicted diminished quality of white matter microstructure. TBSS whole-brain analyses showed widespread effects of drinking status and age on white matter, along with a fairly extensive HIV-age interaction. Follow-up ROI analyses confirmed significant effects of drinking group in corpus callosum (all segments), cingulate gyrus, posterior thalamic radiation, and left external capsule. In addition, ROI analyses confirmed significant HIV-age interaction effects in anterior and posterior limbs of internal capsule, posterior thalamic radiation, external capsule, cingulate gyrus, superior longitudinal fasciculus, and uncinate fasciculus. We did not observe a main effect of HIV status, and HIV clinical characteristics, including HCV coinfection, were not associated with FA of any white matter tract. However, we did observe limited associations of current CD4 count, detectable viral load, and HCV co-infection with AD and/or RD of corticospinal tract and cerebral peduncles. This study adds to previous research by demonstrating that current drinking status had strong associations with white matter microstructure in both PLWH and healthy controls. Further, associations of current drinking behavior with white matter characteristics were not better accounted for by AUD history, HIV clinical characteristics, mental health conditions, or illicit drug use.
To our knowledge, our study is the first to examine whole-brain white matter microstructure in relation to current drinking behavior in PLWH. Results are consistent with a previous study reporting corpus callosum abnormalities in PLWH with comorbid AUD, compared to individuals with HIV only or lifetime AUD only (Pfefferbaum et al, 2007). Our results are differentiated from previous findings by linking white matter deficits to current drinking behavior, as opposed to lifetime AUD diagnosis. Categorizing participants based on current weekly alcohol intake, we found that non-drinkers had higher FA than heavy drinkers in genu, body, and splenium of corpus callosum; posterior thalamic radiation; left external capsule; and cingulate gyrus. Moderate drinkers exhibited FA values that were intermediate to non-drinkers and heavy drinkers. Specifically, moderate drinkers had lower FA than non-drinkers in genu and body of corpus callosum and higher FA than heavy drinkers in posterior thalamic radiation and left external capsule. These results are consistent with a dose-dependent relationship of current alcohol intake with markers of poorer white matter microstructural integrity (see Table 2 and Figure 4). Results were not altered by including lifetime AUD diagnosis in the analysis. Moreover, we did not observe an interaction of HIV status with drinking group, indicating that associations of alcohol use with white matter FA did not differ for PLWH versus seronegative controls. As seen in Figure 4, FA values were qualitatively higher for HIV-negative individuals in the ROIs that showed drinking group differences, despite the absence of a significant interaction of HIV with drinking group. The absence of an interaction may be related to high variability within groups, especially heavy drinkers, as reflected in the relatively wide standard error bars. We did not probe the interaction of HIV with drinking group in the follow-up ANOVAs to the TBSS analysis, consistent with our strategy of using ROIs only for confirmatory testing to reduce the likelihood of Type I error.
We followed these findings with post hoc comparisons to investigate whether background characteristics may have confounded FA differences between drinking groups. The very high rates of lifetime AUD in both non-drinkers (60%) and moderate drinkers (72%) are noteworthy, but groups did not differ from each other on lifetime AUD. Moreover, HIV clinical characteristics and HCV coinfection rates did not differ for nondrinking, moderate drinking, and heavy drinking PLWH. Therefore, we conclude that current drinking behavior had a dose-dependent association with white matter deficits that was not confounded by past alcohol exposure or clinical characteristics. Longitudinal research shows that DTI metrics of white matter microstructure actually improve with cessation of drinking in individuals with AUD (Pfefferbaum et al, 2014). Although the current study did not measure change over time, our results are consistent with the possibility that white matter damage abates with abstinence from alcohol and remains stable with effective HIV treatment.
As expected, age was another major predictor of white matter microstructural integrity in this sample of individuals aged 21–70 years. HIV infection by itself was not a significant predictor of FA, but the HIV-age interaction was widespread in voxelwise analyses (see Figure 2). In follow-up ROI analyses, we localized the HIV-age interaction to the anterior limb of internal capsule, posterior limb of internal capsule, posterior thalamic radiation, external capsule, cingulate gyrus, superior longitudinal fasciculus, and uncinate fasciculus (see Table 3). These results are consistent with our previous work. In this cohort, current alcohol use and age were stronger predictors of neurocognitive functioning than HIV infection (Cohen et al, 2019). In another cohort of PLWH, age was a stronger predictor of DTI outcomes than HIV clinical characteristics (Gongvatana et al, 2011). Moreover, our group reported similar main effects of age and HIV-age interactions on white matter microstructure in an independent cohort of PLWH and controls (Seider et al, 2016). Important to note, our finding of an HIV-age interaction does not speak to whether the rate of white matter microstructural decline over time differs by HIV status. Although several studies show greater-than-expected white matter microstructural decline for chronological age in PLWH, e.g., (Kuhn et al, 2018; Seider et al, 2016), it is not yet clear whether PLWH experience accelerated decline over time. One longitudinal study found no evidence of accelerated white matter decline in virally suppressed PLWH relative to matched controls over a two-year period (Cole et al, 2018).
Alcohol-related FA and RD effects were diffuse, affecting all cortical lobes and many subcortical structures. These results underscore the utility of whole-brain approaches to neurobiological correlates of alcohol use. Associations of alcohol use with lower AD were limited to left anterior and superior corona radiata and left anterior and posterior limbs of the internal capsule. Overall, locations of HIV-age and alcohol effects are consistent with previous research. In whole-brain longitudinal studies, aging effects on white matter microstructure are most marked in frontal and parietal lobes (Sexton et al, 2014; Vinke et al, 2018). Similarly, alcohol appears to target superior, frontal, and limbic white matter regions (Crews, 2008; Monnig et al, 2013a; Pfefferbaum et al, 2009). Some mechanisms of neurodegeneration, such as oxidative stress and neuroinflammation, are common to HIV, aging, and heavy drinking (Crews, 2008; Monnig, 2016). However, it is not known at present why certain white matter regions may be more vulnerable than others. The combination of diffuse and focal effects suggests multiple mechanisms contributed to observed effects.
AD and RD findings suggest that effects of age, drinking group, and marijuana use on FA largely were related to RD, rather than AD. AD and RD reflect the magnitude of water diffusion parallel and perpendicular to the primary orientation, respectively. The association of heavier drinking with higher AD along the left anterior and superior corona radiata and left anterior and posterior limbs of the internal capsule suggests that axonal integrity is negatively affected by current alcohol use. This finding is consistent with our previous analysis in a related cohort of PLWH that linked AUD to elevated AD (Gullett et al, 2018), yet contrary to what has been reported in other DTI studies on AUD (Monnig et al, 2013a). Associations of both heavy drinking and age with higher RD were widespread and largely overlapped with the corresponding FA voxelwise maps. Experimental studies have shown that demyelination or even milder myelin damage can cause elevated RD (Song et al, 2002; Song et al, 2005). Thus, our results suggest that poorer myelination status and/or myelin quality may contribute to lower FA associated with alcohol use and older age.
Although marijuana was not a primary focus of this study, we found that greater frequency of marijuana use was associated with higher FA and lower RD in several white matter regions. Higher FA and lower RD often have favorable interpretations in clinical scenarios, and it is possible that the anti-inflammatory effects of cannabis could confer some benefit for white matter health. However, there are several reasons why our results cannot be interpreted as showing a beneficial effect of marijuana use on brain white matter. First, average frequency of marijuana use was relatively low (approximately 13% of the past 90 days), such that this study did not capture effects of daily use. Second, our data do not speak to quantity or chronicity of marijuana use. Third, marijuana frequency and alcohol quantity were not at all correlated (r=.043, p=.658), meaning that results should not be construed as a possible “protective” effect of marijuana use in the context of heavy drinking. Individuals who use marijuana recreationally have shown subtle differences in white matter microstructure and subcortical morphometry (Orr et al, 2016). Further research on white matter effects of marijuana in PLWH is warranted.
HIV clinical characteristics showed limited associations with AD of corticospinal tract and cerebral peduncles and RD of corticospinal tract. The absence of associations between HIV clinical characteristics and FA was somewhat surprising but is not out of line with previous research. Whereas some studies have linked current/nadir CD4 or HCV co-infection to white matter microstructural integrity (Cysique et al, 2017; Gongvatana et al, 2011), others have failed to find such associations, e.g., (Heaps-Woodruff et al, 2016; Nir et al, 2014), or even have linked CD4 recovery to white matter abnormality (Fennema-Notestine et al, 2013). Thus, the clinical significance of these associations is unknown. Because our sample was recruited from an HIV clinic, all participants were engaged in care. The majority of participants were on ART and were virally suppressed, and their current and nadir CD4 counts were relatively high. Clinical characteristics of this sample were highly consistent with the US population of PLWH (Centers for Disease Control and Prevention, 2019). Under severe immunosuppression and/or untreated infection, we speculate that clinical characteristics would be more strongly related to white matter microstructure. The absence of white matter findings according to HIV status is a departure from some previous studies in similar populations (Cole et al, 2018; Gongvatana et al, 2009). However, other studies with comparable samples reported no main effect of HIV status (Liang et al, 2018; Pfefferbaum et al, 2007; Seider et al, 2016). It may be that mixed results are attributable to methodological differences, particularly how age and alcohol use are accounted for.
Decrements in cognition are observed in HIV/aging and heavy drinking (Heaton et al, 2010; Sullivan et al, 2010). Although we did not examine the clinical or functional significance of individual differences in white matter microstructure, a large body of literature links DTI metrics of white matter to cognitive performance, particularly fluid functions such as executive function and processing speed (e.g., (Coelho et al, 2021; Penke et al, 2012; Vernooij et al, 2009). As the average age of PLWH increases, avoiding behaviors associated with white matter deterioration will be key to maintaining cognitive abilities and functional independence. For these reasons, alcohol use warrants further attention as a likely contributor to white matter deterioration in PLWH.
A notable limitation of this study is the absence of HCV infection data in controls. Without HCV labs we were unable to investigate the possible effect of HCV status on white matter microstructure in the sample as a whole. On the other hand, we did not find a significant effect of HCV co-infection for any white matter ROI within the HIV-infected group. Mental health conditions were assessed by self-report rather than a structured clinical interview due to time constraints. The absence of a relationship between mental health conditions and white matter microstructure should be taken as tentative in light of this limitation. In addition, we used a more conservative criterion for HIV control (<75 copies/ml) than currently is applied in clinical practice (<200 copies/ml). However, applying the less conservative threshold would result in reclassification of <5% of participants.
Conclusions
Alcohol use was the only malleable factor associated with white matter microstructural deficits identified in the current study. Our study underscores the importance of matching groups on and examining effects of alcohol and drug use, rather than simply excluding individuals with current substance use disorders. Given the prevalence of heavy drinking in both the general population and in PLWH, findings also speak to the importance of ensuring access to effective treatments for AUD. Additional research to investigate the association of change in drinking with white matter health would be informative for preventing and treating alcohol-related pathology.
Acknowledgments
Role of the funding source: Study sponsors had no role in study design; in the collection, analysis and interpretation of data; in the writing of the report; or in the decision to submit the article for publication.
Funding acknowledgments:
This research was supported by National Institute on Alcohol Abuse and Alcoholism grants K23AA024704 (PI: Monnig), P01AA019072 (PI: Monti), K05AA019681 (PI: Monti), U01-AA020797–09 (Co-I: Gullett), and K01AA025306 (PI: Porges); by National Institute of Biomedical Imaging and Bioengineering grants U54EB020403 (PI: Thompson) and P41EB015922 (PI: Thompson); by National Institute on Aging grants R01AG059874 (PI: Jahanshad) and T32AG058507 (PI: Toga); by National Institute of General Medical Sciences grant P20GM130414 (PI: Monti); and by the National Institute of Allergy and Infectious Diseases grant to the Providence/Boston Center for AIDS Research (P30AI042853). Coauthors NJ, PT, and TN also received partial grant support from Biogen, Inc., for research unrelated to this work.
Bibliography
- Alhassoon OM, Sorg SF, Taylor MJ, Stephan RA, Schweinsburg BC, Stricker NH, Gongvatana A, Grant I (2012). Callosal white matter microstructural recovery in abstinent alcoholics: a longitudinal diffusion tensor imaging study. Alcohol Clin Exp Res 36: 1922–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amen DG, Egan S, Meysami S, Raji CA, George N (2018). Patterns of Regional Cerebral Blood Flow as a Function of Age Throughout the Lifespan. J Alzheimers Dis 65: 1087–1092. [DOI] [PubMed] [Google Scholar]
- Avants BB, Tustison NJ, Song G, Cook PA, Klein A, Gee JC (2011). A reproducible evaluation of ANTs similarity metric performance in brain image registration. Neuroimage 54: 2033–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basser PJ, Pierpaoli C (1996). Microstructural and physiological features of tissues elucidated by quantitative-diffusion-tensor MRI. J Magn Reson B 111: 209–19. [DOI] [PubMed] [Google Scholar]
- Borghesani PR, Madhyastha TM, Aylward EH, Reiter MA, Swarny BR, Schaie KW, Willis SL (2013). The association between higher order abilities, processing speed, and age are variably mediated by white matter integrity during typical aging. Neuropsychologia 51: 1435–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention (2019). Behavioral and clinical characteristics of persons with diagnosed HIV infection. Medical Monitoring Project, United States, 2017 Cycle (June 2017-May 2018). [Google Scholar]
- Coelho A, Fernandes HM, Magalhães R, Moreira PS, Marques P, Soares JM, Amorim L, Portugal-Nunes C, Castanho T, Santos NC, Sousa N (2021). Signatures of white-matter microstructure degradation during aging and its association with cognitive status. Sci Rep 11: 4517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen RA, Gullett JM, Porges EC, Woods AJ, Lamb DG, Bryant VE, McAdams M, Tashima K, Cook R, Bryant K, Monnig M, Kahler CW, Monti PM (2019). Heavy Alcohol Use and Age Effects on HIV-Associated Neurocognitive Function. Alcohol Clin Exp Res 43: 147–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen RA, Harezlak J, Schifitto G, Hana G, Clark U, Gongvatana A, Paul R, Taylor M, Thompson P, Alger J, Brown M, Zhong J, Campbell T, Singer E, Daar E, McMahon D, Tso Y, Yiannoutsos CT, Navia B (2010). Effects of nadir CD4 count and duration of human immunodeficiency virus infection on brain volumes in the highly active antiretroviral therapy era. J Neurovirol 16: 25–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole JH, Caan MWA, Underwood J, De Francesco D, van Zoest RA, Wit FWNM, Mutsaerts HJMM, Leech R, Geurtsen GJ, Portegies P, Majoie CBLM, Schim van der Loeff MF, Sabin CA, Reiss P, Winston A, Sharp DJ, Collaboration CiRtAC(2018). No Evidence for Accelerated Aging-Related Brain Pathology in Treated Human Immunodeficiency Virus: Longitudinal Neuroimaging Results From the Comorbidity in Relation to AIDS (COBRA) Project. Clin Infect Dis 66: 1899–1909. [DOI] [PubMed] [Google Scholar]
- Coupe P, Yger P, Prima S, Hellier P, Kervrann C, Barillot C (2008). An optimized blockwise nonlocal means denoising filter for 3-D magnetic resonance images. IEEE Trans Med Imaging 27: 425–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crespi C, Galandra C, Canessa N, Manera M, Poggi P, Basso G (2020). Microstructural damage of white-matter tracts connecting large-scale networks is related to impaired executive profile in alcohol use disorder. Neuroimage Clin 25: 102141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crews FT (2008). Alcohol-related neurodegeneration and recovery: mechanisms from animal models. Alcohol Res Health 31: 377–88. [PMC free article] [PubMed] [Google Scholar]
- Cysique LA, Soares JR, Geng G, Scarpetta M, Moffat K, Green M, Brew BJ, Henry RG, Rae C (2017). White matter measures are near normal in controlled HIV infection except in those with cognitive impairment and longer HIV duration. J Neurovirol 23: 539–547. [DOI] [PubMed] [Google Scholar]
- Fennema-Notestine C, Ellis RJ, Archibald SL, Jernigan TL, Letendre SL, Notestine RJ, Taylor MJ, Theilmann RJ, Julaton MD, Croteau DJ, Wolfson T, Heaton RK, Gamst AC, Franklin DR Jr, Clifford DB, Collier AC, Gelman BB, Marra C, McArthur JC, McCutchan JA, Morgello S, Simpson DM, Grant I (2013). Increases in brain white matter abnormalities and subcortical gray matter are linked to CD4 recovery in HIV infection. J Neurovirol 19: 393–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filbey FM, Aslan S, Calhoun VD, Spence JS, Damaraju E, Caprihan A, Segall J (2014). Long-term effects of marijuana use on the brain. Proc Natl Acad Sci U S A 111: 16913–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B (2012). FreeSurfer. Neuroimage 62: 774–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, van der Kouwe A, Killiany R, Kennedy D, Klaveness S, Montillo A, Makris N, Rosen B, Dale AM (2002). Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron 33: 341–55. [DOI] [PubMed] [Google Scholar]
- Gongvatana A, Cohen RA, Correia S, Devlin KN, Miles J, Kang H, Ombao H, Navia B, Laidlaw DH, Tashima KT (2011). Clinical contributors to cerebral white matter integrity in HIV-infected individuals. J Neurovirol 17: 477–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gongvatana A, Schweinsburg BC, Taylor MJ, Theilmann RJ, Letendre SL, Alhassoon OM, Jacobus J, Woods SP, Jernigan TL, Ellis RJ, Frank LR, Grant I (2009). White matter tract injury and cognitive impairment in human immunodeficiency virus-infected individuals. J Neurovirol 15: 187–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gullett JM, Lamb DG, Porges E, Woods AJ, Rieke J, Thompson P, Jahanshad N, Nir TM, Tashima K, Cohen RA (2018). The Impact of Alcohol Use on Frontal White Matter in HIV. Alcohol Clin Exp Res 42: 1640–1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaps-Woodruff JM, Wright PW, Ances BM, Clifford D, Paul RH (2016). The impact of human immune deficiency virus and hepatitis C coinfection on white matter microstructural integrity. J Neurovirol 22: 389–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton RK, Clifford DB, Franklin DR, Woods SP, Ake C, Vaida F, Ellis RJ, Letendre SL, Marcotte TD, Atkinson JH, Rivera-Mindt M, Vigil OR, Taylor MJ, Collier AC, Marra CM, Gelman BB, McArthur JC, Morgello S, Simpson DM, McCutchan JA, Abramson I, Gamst A, Fennema-Notestine C, Jernigan TL, Wong J, Grant I, CG (2010). HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy: CHARTER Study. Neurology 75: 2087–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inano S, Takao H, Hayashi N, Abe O, Ohtomo K (2011). Effects of age and gender on white matter integrity. AJNR Am J Neuroradiol 32: 2103–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkinson M, Beckmann CF, Behrens TE, Woolrich MW, Smith SM (2012). FSL. Neuroimage 62: 782–90. [DOI] [PubMed] [Google Scholar]
- Kievit RA, Davis SW, Mitchell DJ, Taylor JR, Duncan J, Henson RN (2014). Distinct aspects of frontal lobe structure mediate age-related differences in fluid intelligence and multitasking. Nat Commun 5: 5658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn T, Jin Y, Huang C, Kim Y, Nir TM, Gullett JM, Jones JD, Sayegh P, Chung C, Dang BH, Singer EJ, Shattuck DW, Jahanshad N, Bookheimer SY, Hinkin CH, Zhu H, Thompson PM, Thames AD (2019). The joint effect of aging and HIV infection on microstructure of white matter bundles. Hum Brain Mapp 40: 4370–4380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn T, Kaufmann T, Doan NT, Westlye LT, Jones J, Nunez RA, Bookheimer SY, Singer EJ, Hinkin CH, Thames AD (2018). An augmented aging process in brain white matter in HIV. Hum Brain Mapp 39: 2532–2540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang H, Chang L, Chen R, Oishi K, Ernst T (2018). Independent and Combined Effects of Chronic HIV-Infection and Tobacco Smoking on Brain Microstructure. J Neuroimmune Pharmacol 13: 509–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madden DJ, Spaniol J, Costello MC, Bucur B, White LE, Cabeza R, Davis SW, Dennis NA, Provenzale JM, Huettel SA (2009). Cerebral white matter integrity mediates adult age differences in cognitive performance. J Cogn Neurosci 21: 289–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manjón JV, Coupé P, Concha L, Buades A, Collins DL, Robles M (2013). Diffusion weighted image denoising using overcomplete local PCA. PLoS One 8: e73021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monnig MA (2016). Immune activation and neuroinflammation in alcohol use and HIV infection: evidence for shared mechanisms. Am J Drug Alcohol Abuse: 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monnig MA, Caprihan A, Yeo RA, Gasparovic C, Ruhl DA, Lysne P, Bogenschutz MP, Hutchison KE, Thoma RJ (2013a). Diffusion tensor imaging of white matter networks in individuals with current and remitted alcohol use disorders and comorbid conditions. Psychol Addict Behav 27: 455–65. PMCID: PMC3374918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monnig MA, Tonigan JS, Yeo RA, Thoma RJ, McCrady BS (2013b). White matter volume in alcohol use disorders: a meta-analysis. Addict Biol 18: 581–92; PMCID: PMC3390447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori S, Oishi K, Jiang H, Jiang L, Li X, Akhter K, Hua K, Faria AV, Mahmood A, Woods R, Toga AW, Pike GB, Neto PR, Evans A, Zhang J, Huang H, Miller MI, van Zijl P, Mazziotta J (2008). Stereotaxic white matter atlas based on diffusion tensor imaging in an ICBM template. Neuroimage 40: 570–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Institute on Alcohol Abuse and Alcoholism (2010). Rethinking Drinking: Alcohol and Your Health. Services USDoHaH, (ed): Bethesda, MD. [Google Scholar]
- Nir TM, Jahanshad N, Busovaca E, Wendelken L, Nicolas K, Thompson PM, Valcour VG (2014). Mapping white matter integrity in elderly people with HIV. Hum Brain Mapp 35: 975–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connor EE, Jaillard A, Renard F, Zeffiro TA (2017). Reliability of White Matter Microstructural Changes in HIV Infection: Meta-Analysis and Confirmation. AJNR Am J Neuroradiol 38: 1510–1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connor EE, Zeffiro TA (2018). Brain Structural Changes following HIV Infection: Meta-Analysis. AJNR Am J Neuroradiol 39: 54–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orr JM, Paschall CJ, Banich MT (2016). Recreational marijuana use impacts white matter integrity and subcortical (but not cortical) morphometry. Neuroimage Clin 12: 47–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penke L, Maniega SM, Bastin ME, Valdés Hernández MC, Murray C, Royle NA, Starr JM, Wardlaw JM, Deary IJ (2012). Brain white matter tract integrity as a neural foundation for general intelligence. Mol Psychiatry 17: 1026–30. [DOI] [PubMed] [Google Scholar]
- Penke L, Muñoz Maniega S, Murray C, Gow AJ, Hernández MC, Clayden JD, Starr JM, Wardlaw JM, Bastin ME, Deary IJ (2010). A general factor of brain white matter integrity predicts information processing speed in healthy older people. J Neurosci 30: 7569–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfefferbaum A, Adalsteinsson E, Sullivan EV (2006). Supratentorial profile of white matter microstructural integrity in recovering alcoholic men and women. Biol Psychiatry 59: 364–72. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Rosenbloom M, Rohlfing T, Sullivan EV (2009). Degradation of association and projection white matter systems in alcoholism detected with quantitative fiber tracking. Biol Psychiatry 65: 680–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfefferbaum A, Rosenbloom MJ, Adalsteinsson E, Sullivan EV (2007). Diffusion tensor imaging with quantitative fibre tracking in HIV infection and alcoholism comorbidity: synergistic white matter damage. Brain 130: 48–64. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Rosenbloom MJ, Chu W, Sassoon SA, Rohlfing T, Pohl KM, Zahr NM, Sullivan EV (2014). White matter microstructural recovery with abstinence and decline with relapse in alcohol dependence interacts with normal ageing: a controlled longitudinal DTI study. Lancet Psychiatry 1: 202–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragin AB, Wu Y, Gao Y, Keating S, Du H, Sammet C, Kettering CS, Epstein LG (2015). Brain alterations within the first 100 days of HIV infection. Ann Clin Transl Neurol 2: 12–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin LH, Maki PM (2019). HIV, Depression, and Cognitive Impairment in the Era of Effective Antiretroviral Therapy. Curr HIV/AIDS Rep 16: 82–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulte T, Müller-Oehring EM, Sullivan EV, Pfefferbaum A (2012). White matter fiber compromise contributes differentially to attention and emotion processing impairment in alcoholism, HIV-infection, and their comorbidity. Neuropsychologia 50: 2812–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seider TR, Gongvatana A, Woods AJ, Chen H, Porges EC, Cummings T, Correia S, Tashima K, Cohen RA (2016). Age exacerbates HIV-associated white matter abnormalities. J Neurovirol 22: 201–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sexton CE, Walhovd KB, Storsve AB, Tamnes CK, Westlye LT, Johansen-Berg H, Fjell AM (2014). Accelerated changes in white matter microstructure during aging: a longitudinal diffusion tensor imaging study. J Neurosci 34: 15425–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, Hergueta T, Baker R, Dunbar GC (1998). The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry 59 Suppl 20: 22–33;quiz 34–57. [PubMed] [Google Scholar]
- Sled JG, Zijdenbos AP, Evans AC (1998). A nonparametric method for automatic correction of intensity nonuniformity in MRI data. IEEE Trans Med Imaging 17: 87–97. [DOI] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Johansen-Berg H, Rueckert D, Nichols TE, Mackay CE, Watkins KE, Ciccarelli O, Cader MZ, Matthews PM, Behrens TE (2006). Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. Neuroimage 31: 1487–505. [DOI] [PubMed] [Google Scholar]
- Sobell LC, Sobell MB (1992). In: Measuring Alcohol Consumption: Psychosocial and Biological Methods. Allen J, Litten RZ, (eds). Humana Press: Totowa, NJ, pp 41–72. [Google Scholar]
- Song SK, Sun SW, Ramsbottom MJ, Chang C, Russell J, Cross AH (2002). Dysmyelination revealed through MRI as increased radial (but unchanged axial) diffusion of water. Neuroimage 17: 1429–36. [DOI] [PubMed] [Google Scholar]
- Song SK, Yoshino J, Le TQ, Lin SJ, Sun SW, Cross AH, Armstrong RC (2005). Demyelination increases radial diffusivity in corpus callosum of mouse brain. Neuroimage 26: 132–40. [DOI] [PubMed] [Google Scholar]
- Su T, Caan MW, Wit FW, Schouten J, Geurtsen GJ, Cole JH, Sharp DJ, Vos FM, Prins M, Portegies P, Reiss P, Majoie CB (2016). White matter structure alterations in HIV-1-infected men with sustained suppression of viraemia on treatment. Aids 30: 311–22. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Harris RA, Pfefferbaum A (2010). Alcohol’s effects on brain and behavior. Alcohol Research & Health 33: 127–143. [PMC free article] [PubMed] [Google Scholar]
- Trivedi R, Bagga D, Bhattacharya D, Kaur P, Kumar P, Khushu S, Tripathi RP, Singh N (2013). White matter damage is associated with memory decline in chronic alcoholics: a quantitative diffusion tensor tractography study. Behav Brain Res 250: 192–8. [DOI] [PubMed] [Google Scholar]
- Vernooij MW, Ikram MA, Vrooman HA, Wielopolski PA, Krestin GP, Hofman A, Niessen WJ, Van der Lugt A, Breteler MM (2009). White matter microstructural integrity and cognitive function in a general elderly population. Arch Gen Psychiatry 66: 545–53. [DOI] [PubMed] [Google Scholar]
- Vinke EJ, de Groot M, Venkatraghavan V, Klein S, Niessen WJ, Ikram MA, Vernooij MW (2018). Trajectories of imaging markers in brain aging: the Rotterdam Study. Neurobiol Aging 71: 32–40. [DOI] [PubMed] [Google Scholar]
- Williams EC, Hahn JA, Saitz R, Bryant K, Lira MC, Samet JH (2016). Alcohol Use and Human Immunodeficiency Virus (HIV) Infection: Current Knowledge, Implications, and Future Directions. Alcohol Clin Exp Res 40: 2056–2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler AM, Ridgway GR, Webster MA, Smith SM, Nichols TE (2014). Permutation inference for the general linear model. Neuroimage 92: 381–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh PH, Simpson K, Durazzo TC, Gazdzinski S, Meyerhoff DJ (2009). Tract-Based Spatial Statistics (TBSS) of diffusion tensor imaging data in alcohol dependence: abnormalities of the motivational neurocircuitry. Psychiatry Res 173: 22–30. [DOI] [PMC free article] [PubMed] [Google Scholar]