Abstract
The aging brain is shaped by many structural and functional alterations. Recent cross-disciplinary efforts have uncovered powerful and integrated adaptive mechanisms that promote brain health and prevent functional decline during aging. Here, we review some of the most robust adaptive mechanisms and how they can be engaged to protect, and restore the aging brain.
Introduction
Aging of the brain is associated with the accumulation of molecular damage and cellular defects that gradually decrease mental capacity and predispose to disease. Many studies have shown that these age-related changes are neither linear nor homogenous and vary greatly from person to person, with some individuals being exceptionally resilient during aging [1,2]. Furthermore, a subset of cognitive abilities are typically preserved, whereas others, such as inductive reasoning, verbal fluency, and executive attention, may improve with age [3,4].
Converging evidence from neuroimaging, cognitive psychology, and neuropathology suggests that the aging brain is adaptable and resilient. The brain can build a neural reserve throughout life, facilitated by environmental factors such as education or physical activity, that may prevent or delay functional decline due to aging and pathology. When confronted with pathological insults, the aging brain can activate stress resistance pathways to actively suppress pathology or limit its deleterious consequences. The aging brain can also compensate for decreased performance by relying on alternative neural strategies and reorganizing networks to maintain functional homeostasis [5,6].
These observations in humans have led to the development of animal models from invertebrates to mammals that have explored the mechanisms of brain aging. Collectively, studies in humans and animal models have identified adaptive mechanisms that can protect against functional decline. Furthermore, these mechanisms can be engaged by a growing list of physiological and pharmacological interventions (Figure 1). Taken together, these cross-disciplinary efforts show that the aging brain is not only characterized by molecular damage and functional decline, but also by adaptive changes that maintain neural homeostasis and prevent disease. Here, we review some of the most robust adaptive mechanisms and examine how they can be recruited therapeutically to delay brain aging and the onset of functional decline.
Figure 1. Adaptive mechanisms of brain aging.
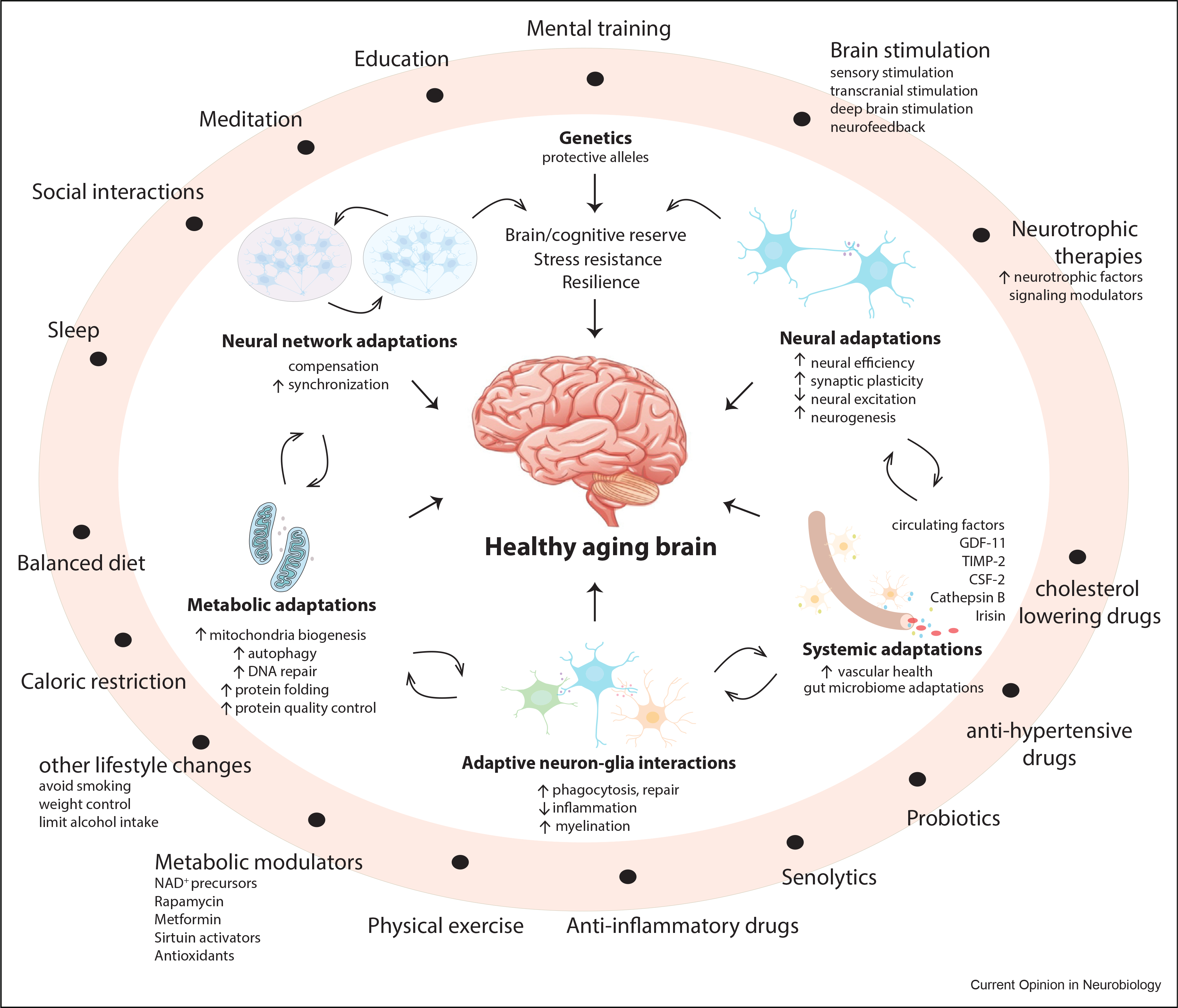
Physiologic adaptive mechanisms (inside white circle) and those that can be recruited by lifestyle and therapeutic interventions (outer light red ring) promote stress resistance and functional integrity of the aging brain. Physiologic adaptive mechanisms include neural network changes, increased synaptic plasticity, and reduced neural excitation, as well as broader metabolic and systemic adaptations that involve vascular function and circulating factors. Genetic factors, such as the protective APOE2 allele, may also engage adaptive mechanisms. Adaptive mechanisms can also be recruited by physiologic and pharmacologic interventions (outer ring). Examples of induced physiologic adaptations are those engaged by lifestyle choices and changes, such as diet and physical activity, caloric restriction, education, mental training, meditation, social interactions, and sleep. Pharmacologic and therapeutic interventions target metabolic, neuroprotective, and inflammatory pathways. Other approaches target senescent cells, blood pressure, and vascular health or mentally stimulate the aging brain.
Metabolic adaptations in the aging brain
The evolution of mental representations underlying abstract thought has been made possible by structural changes in the human cerebral cortex, as well as augmented cerebral energy metabolism [7]. The over-abundance of food in some parts of the world, coupled with a significant reduction in physical exercise, is likely having a significant impact on the metabolism of the aging brain [8]. Neural pathways that for most of human history have evolved to adapt to food scarcity may have become disengaged by increased caloric intake and decreased physical activity, accelerating structural and functional decline during brain aging and the onset of pathology [8].
Studies in animal models have identified conserved metabolic pathways that play major adaptive roles during aging, including those mediated by insulin/insulin-like growth factor signaling and the mammalian target of rapamycin (mTOR), AMPK, and sirtuin enzymes [9]. These adaptive pathways can be engaged by lifestyle changes, such as increased physical exercise, altered diet, or fasting regimens, and can also be targeted pharmacologically by drugs, such as nicotinamide derivatives, rapamycin, and metformin (Figure 2).
Figure 2. Metabolic adaptations in the aging brain.
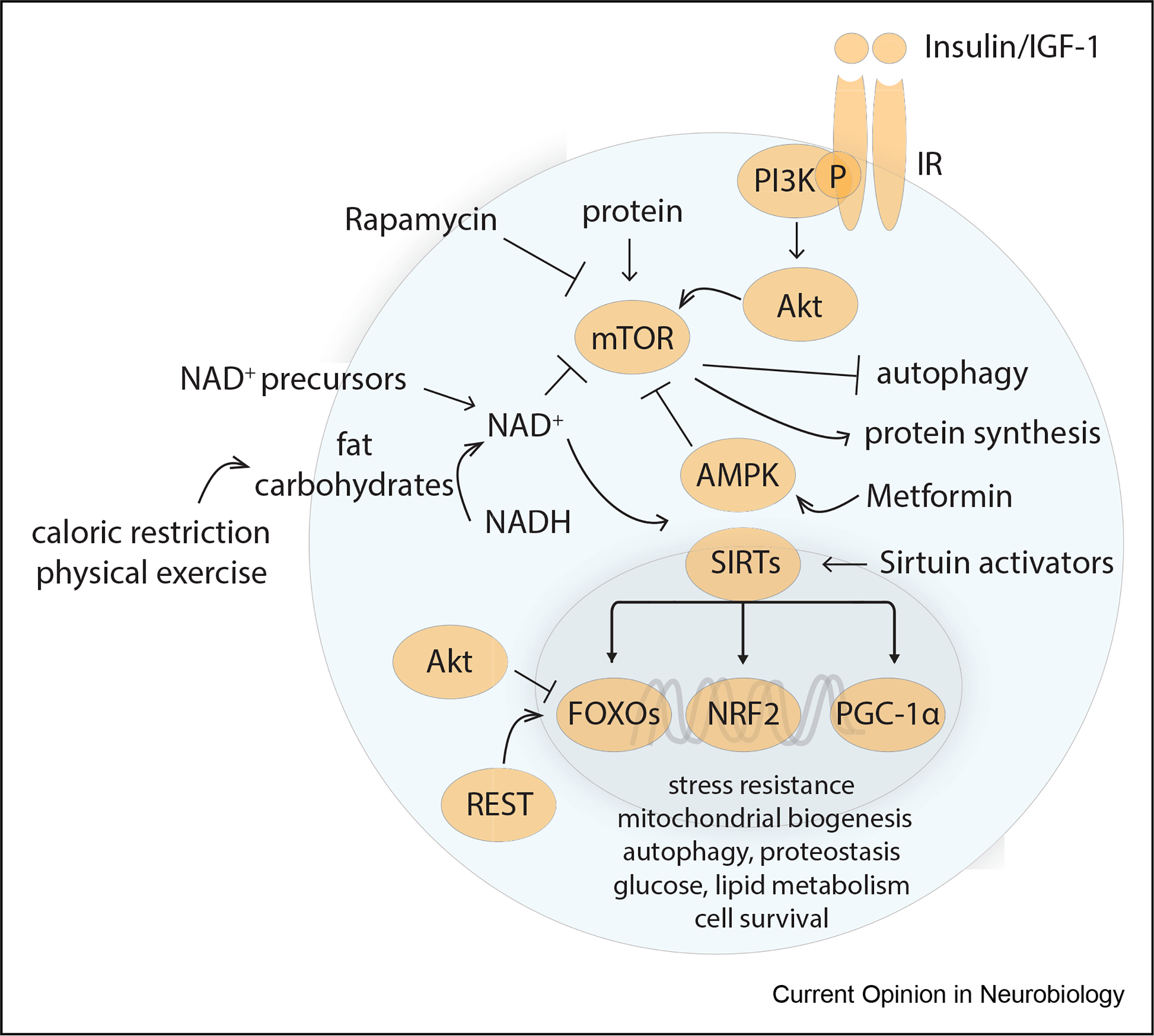
Several conserved metabolic regulators can be engaged physiologically or therapeutically. Fasting, exercise, downregulation of insulin/insulin-like growth factor 1 (IGF1–1) signaling, and decreased protein and amino acid levels inhibit the activity of mammalian target of rapamycin (mTOR). This results in inhibition of protein synthesis and stimulation of autophagy. Physical exercise and fasting also increase nicotinamide adenine dinucleotide (NAD+) production, which serves as a critical redox cofactor for metabolism and ATP generation. NAD+ also serves as cofactor for sirtuins. Nuclear localization of sirtuins (SIRTs) leads to deacetylation of target genes, such as Fork-head box O (FOXO) proteins, peroxisome proliferator-activated receptor γ coactivator 1α (PGC-1α), and nuclear factor erythroid 2-related factor 2 (NRF2). FOXO1-dependent transcription can also be activated by REST, a transcriptional repressor that is activated in the aging brain and correlates with longevity. FOXOs, PGC-1α, and NRF2 activate the transcription of genes that promote stress resistance, autophagy, proteostasis, DNA repair, mitochondrial biogenesis, and cell survival. Increased AMP and decreased ATP during fasting activate AMP kinase (AMPK). AMPK can also be activated pharmacologically by metformin. NAD precursors such as nicotinamide mononucleotide can pharmacologically increase cellular NAD+ levels.
Metabolic changes induced by physical exercise and fasting, such as increased fat metabolism and ketone generation, can have a major impact on neural networks and the onset of disease [8]. For example, reliance on glucose as a fuel can destabilize neural networks, while a metabolic switch to increased utilization of ketone bodies stabilizes them [10]. A similar metabolic switch takes place during sleep [11], highlighting its beneficial effects for the aging brain.
Adaptive metabolic changes have profound effects on brain aging. They can be neuroprotective and neuro-restorative by stimulating the release of neurotrophic factors, increasing mitochondrial biogenesis, promoting autophagy and DNA repair, and activating stress resistance pathways [8]. Thus, physiologic and pharmacologic engagement of adaptive metabolic mechanisms may delay brain aging and prevent the onset of age-related neurodegenerative disorders.
Neural network adaptation in the aging brain
The brain has the ability to build a ‘reserve’ of neural resources over time that can prevent or delay the onset of dysfunction and pathology during aging. A number of contributors to cognitive reserve have been identified, including genetic and environmental factors, such as lifelong education, physical activity, and engagement in mentally stimulating activity [5]. The neurobiological substrates of cognitive reserve remain elusive. The available evidence suggests that education may promote cognitive reserve by increasing synaptic density in the hippocampus [12] and by promoting global functional connectivity between regions of the neocortex [13]. The cellular interactions and molecular mechanisms underlying cognitive reserve remain to be determined.
Neural network changes may compensate for loss of function due to aging or pathology. Several compensatory mechanisms involving neural networks that may play adaptive roles in the aging brain have been identified [5]. For example, aged individuals may compensate for cortical atrophy by activating neural networks associated with focused attention to maintain reading ability [14] or by further activating cortical areas to augment working memory [15]. The aging brain can also recruit new networks to functionally compensate for deficits in others. For example, recruitment of the rhinal cortex can compensate for decreased hippocampal activity during the memory retrieval [16]. Furthermore, neural networks can be reorganized to maintain performance. For example, episodic memory is associated with unilateral mostly right-sided activation of the preforntal cortex in young adults, but bilateral activation in aged individuals who continue to perform well [17] (Figure 3a). Thus, adaptation of neural networks may preserve a high level of cognitive function during aging.
Figure 3. Neural adaptations in the aging brain.
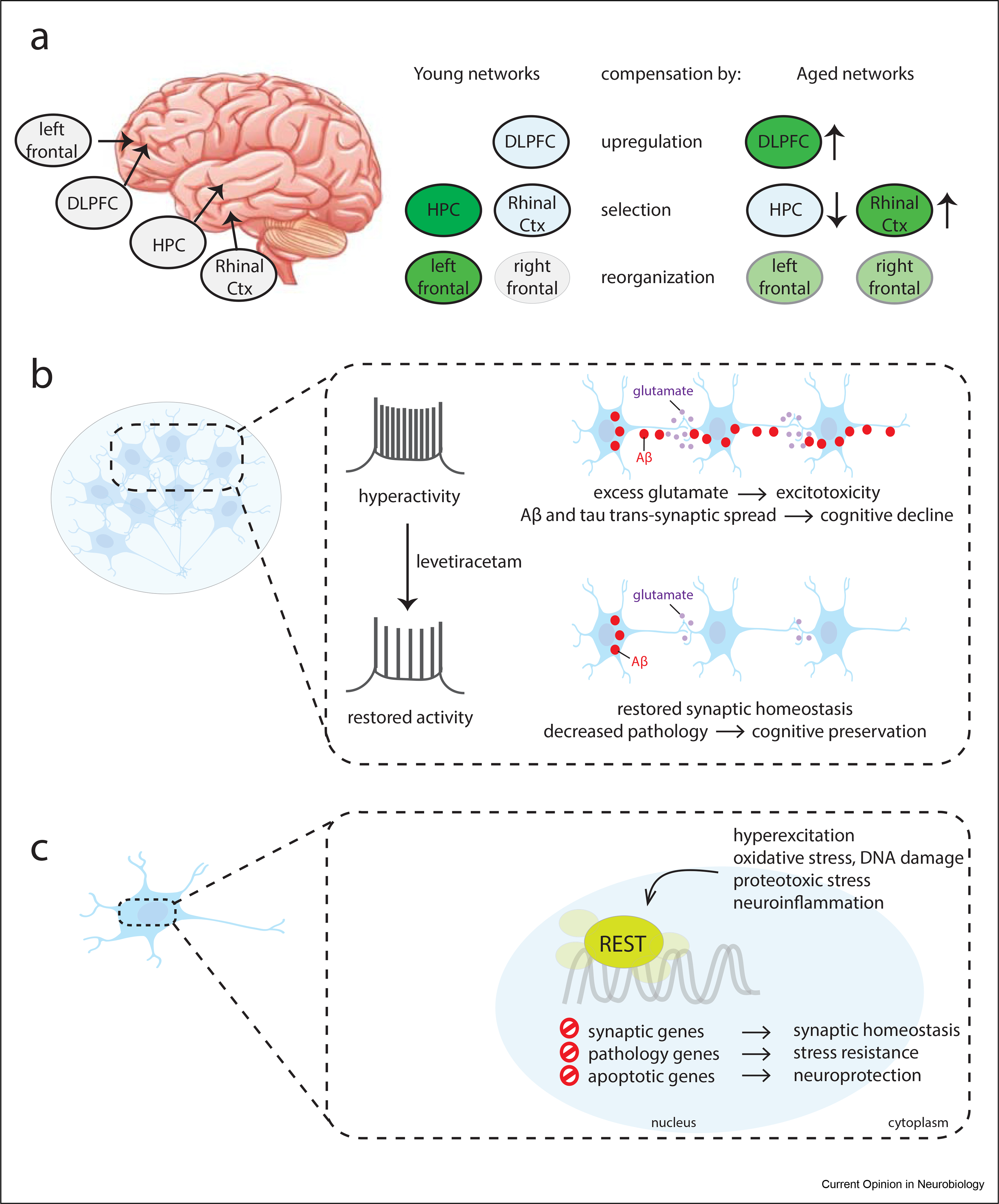
(a) Neural network compensatory adaptations preserve neural network homeostasis during aging. Three broad age-related neural mechanisms of compensation in the aging brain have been described: (1) Some neural networks can increase activity during aging, such as the dorsolateral prefrontal cortex (DLPFC) that is involved in working memory. (2) Neural networks that are not typically involved in a task can be recruited, such as the recruitment of the rhinal cortex to augment the function of the hippocampus (HPC) in recognition memory. (3) Neural networks can be reorganized in the aging brain to improve performance. For example, the frontal cortex can become activated bilaterally in aged individuals to perform executive function tasks, in contrast to the unilateral frontal activation pattern that occurs in young individuals. (b) Reducing neural excitation may protect against hyperexcitation and also reduce the transsynaptic spread of misfolded proteins, such as Aβ and tau, which contribute to the pathogenesis of AD. (c) The molecular mechanism of reduced neural excitation in a well-adapted aging brain may involve the transcriptional repressor REST, which is activated in aging neurons. AD, Alzheimer disease.
In addition to physiological adaptations, interventions that target neural networks may lead to adaptive changes during aging. Cognitive training and social enhancement interventions improve cognition, mental and physical health, independent living, and overall well-being in older adults and are associated with significant changes in brain function, as assessed by neuroimaging [18].
Targeting neural networks might also impact neurodegenerative disorders. A recently developed noninvasive protocol called gamma entrainment using sensory stimuli (GENUS) uses visual, auditory or multisensory stimulation to induce gamma neural oscillations across brain regions. GENUS activates microglial, astrocytic, and vascular pathways and recruits neural circuits, resulting in improved cognitive function and decreased Aβ and tau pathology in Alzheimer disease (AD) mouse models [19–22]. The detailed molecular and cellular mechanisms engaged by GENUS and their consequences for cognitive function in normal and cognitively impaired individuals are subjects of ongoing study.
Combinatorial approaches involving diet, physical exercise, noninvasive brain stimulation, and pharmacological approaches may engage multiple adaptive mechanisms at once to protect and preserve function in the aging brain [23,24]. Further research is necessary to dissect the cellular and molecular mechanisms of these adaptations and determine how they can be used therapeutically.
Neural networks modulate stress resistance and lifespan
Recent studies suggest that changes in the excitation state of neural networks play important adaptive roles during aging through modulation of stress resistance and resilience pathways (Figure 3). Evidence that reduced neural activity is an adaptive mechanism during aging comes from analysis of the aging brain transcriptome. The first transcriptome signature of the aging human brain showed significant downregulation of synaptic genes in the cortex of healthy older individuals [25]. A subsequent study found that excitatory synaptic gene expression decreases further in cognitively intact individuals with longer lifespans. Another study showed that increased gene expression in excitatory hippocampal neurons predicts cortical thinning in the aging population, including individuals with mild cognitive impairment (MCI) and AD [26]. These observations suggest somewhat counterintuitively that reduced synaptic gene expression predicts a longer lifespan among healthy aging individuals [27]. In the nematode C. elegans, pharmacologic or genetic inhibition of neural excitation was shown to extend lifespan, whereas increased neural excitation shortened lifespan [27], providing experimental evidence that reduced neural excitation may be a conserved adaptive mechanism that delays aging.
Neural excitation and network efficiency in healthy older adults
Many studies have documented that reduced neural activation associated with cognitive tasks correlates with better performance in defined neuropsychometric tests (reviewed in the study by Neubauer and Fink [28]). This has led to the proposal that decreased neural and synaptic activity indicate increased neural efficiency [28]. This idea is supported by the observation that decreased neural activation is associated with training-induced acquisition of expertise in a particular cognitive domain [5]. Importantly, increased neural efficiency may represent a form of acquired cognitive reserve [29]. Two studies showed that greater cognitive reserve — linked to education and occupational attainment — was associated with lower cortical neural activity during speech comprehension [30] and visual encoding [31] tasks in aged individuals. These observations suggest that reduced task-associated neural activation may be a manifestation of more efficient neural network function. Moreover, more efficient neural network function may be an important component of the cognitive reserve that maintains and protects the aging brain.
Can reduced neural excitation and increased neural efficiency delay the onset of cognitive aging in healthy individuals? Combined cognitive and physical training or exergaming — playing a computer game controlled by body movements that provide augmented feedback — was associated with reduced cortical activation in older individuals and improved performance in multiple cognitive domains [32,33]. Additional studies are required to obtain greater insight into the adaptive mechanisms that are engaged by these interventions and how they improve cognitive function.
Reduced neural excitation as an adaptive mechanism in cognitively impaired individuals
Decreased neural excitation may improve cognitive function in individuals with MCI by preventing the transsynaptic spread of misfolded proteins, such as Aβ and tau (Figure 3b) [34–38]. Treatment with the anticonvulsant levetiracetam reduced hyperexcitation and improved memory in AD mouse models [39,40]. Furthermore, levetiracetam treatment prevented hippocampal hyperexcitation in patients with MCI and improved cognitive function [41]. In a recent single-blinded randomized controlled trial, a combination of cognitive and physical training significantly reduced cortical activation in individuals with MCI and improved cognitive function [42]. Thus, interventions that decrease neural excitation may be neuroprotective at early stages of cognitive decline.
How does reduced neural excitation protect the aging brain?
Genetic experiments in C.elegans showed that the effects of neural excitation on the regulation of aging and lifespan were mediated by orthologs of the mammalian transcriptional repressor REST. REST recruits other corepressors that modify histones and remodel chromatin to suppress the transcription of target genes, many of which are involved in synaptic function and signaling [43]. REST is activated in the aging human brain and is associated with extended longevity [27,44]. Activation of the REST ortholog spr-4 reduces neural excitation and increases longevity in C. elegans [27]. Furthermore, REST can be activated by a variety of stressors, including oxidative stress, DNA damage, and Aβ [44], as well as neuro-inflammatory stimuli [45], to mediate stress resistance and restore neural network homeostasis (Figure 3c) [46]. These findings suggest that downregulation of neural excitation, mediated by REST, is a conserved stress response which is engaged adaptively in the aging brain.
Activation of REST in the aging brain may protect against degenerative changes associated with aging and prevent the onset of AD. REST represses many genes involved in AD pathology and neurodegeneration [44]. Moreover, REST is depleted in the AD brain [44], as well as in brain-derived exosomes [47,48] and plasma [49] from patients with AD relative to cognitively intact controls. Interestingly, in older adults at risk for developing AD, mindfulness meditation training significantly elevated plasma REST levels [49]. Furthermore, in aged mice, physical activity increased REST expression in the hippocampus [50]. Thus, REST may be activated by noninvasive interventions. Taken together, these observations suggest that REST activation in the aging brain is an adaptive mechanism that may protect against age-related pathology and neurodegeneration.
Adaptive neuron–glia interactions
Glial cells play important adaptive roles in the aging brain. Astrocytes promote synaptic and neuronal homeostasis [51], sculpt synapses [52], and release neurotrophic factors to protect synapses, including during the earliest stages of Aβ accumulation [53]. Microglia protect neurons by acting as phagocytes (Figure 4a), sculpting processes and synapses, and releasing neurotrophic factors and regulatory cytokines [54–56]. Glial cells engage in extensive cross talk with neurons and other glia, as well as endothelial and peripheral immune cells that likely impact the aging brain [57].
Figure 4. Adaptation of the neuron–glia interface in the aging brain.
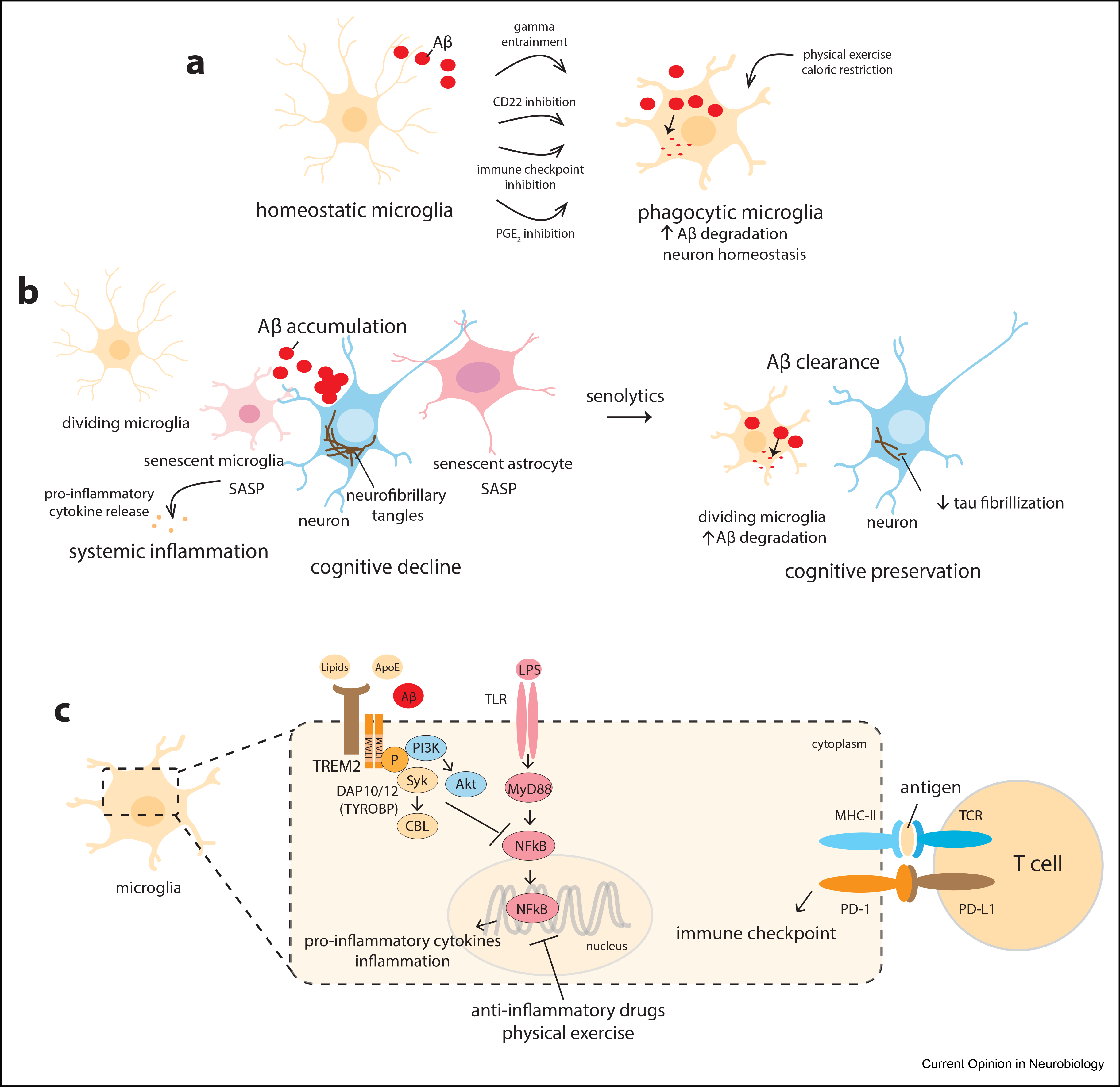
(a) Homeostatic microglia become phagocytic in the presence of protein aggregates and degenerating cells. Gamma entrainment can recruit and augment microglial phagocytic activity. Inhibition of the CD22 receptor and the other indicated interventions can restore beneficial microglial activity and reduce proinflammatory responses. (b) Senescent microglia and astrocytes exhibit the senescence-associated secretory phenotype (SASP) with the release of proinflammatory mediators. Senescent cells do not efficiently clear misfolded proteins, such as Aβ and tau, leading to their accumulation and spread. Strategies that eliminate senescent glial cells, such as the use of senolytics, can allow the bystander glial cells to more efficiently clear misfolded proteins, leading to decreased pathology and potentially improving cognitive function. (c) TREM2 is a microglial receptor that interacts with lipids, lipoproteins, and Aβ and activates pathways that restrict proinflammatory NF-κB signaling. NF-κB is activated by a variety of proinflammatory stimuli, including cytokines, infection, stress, and circadian disturbance. Inhibition of NF-κB signaling by anti-inflammatory drugs, blockade of immune checkpoints, or physical exercise leads to reduced inflammation and increased microglial phagocytic activity.
Converging evidence suggests that several approaches may be necessary to fully engage glial adaptive mechanisms in the aging brain. First, glial cells must be recruited and activated. Upregulation of the CD22 receptor by aging microglia impairs their phagocytic activity, and inhibition of CD22 restores microglial phagocytosis and cognitive function in aged 5xFAD mice [58]. Activation of phagocytosis is a two-step process in which initial downregulation of microglial checkpoints is followed by a signaling cascade triggered by TREM2 [59], a critical microglial receptor that is genetically linked to AD [60]. Strategies that inhibit immune checkpoints may unleash the full adaptive potential of microglia in the aging brain (Figure 4c) [51]. Interestingly, blockade of the PD-1/PD-L1 immune checkpoint in T lymphocytes can induce a T-cell effector response, leading to increased macrophage recruitment to the brain, suppression of Aβ and tau pathology, and memory recovery in mouse models of neurodegeneration [61,62].
Senescent glial cells exhibit a proinflammatory senescence-associated secretory phenotype that may exacerbate pathology in the aging brain (Figure 4b) [63]. Removal of senescent astrocytes by targeted expression of caspase 8 mitigated tau pathology and restored cognitive function in a mouse model of tauopathy [64]. Senescent cells may also shorten lifespan. Removal of naturally occurring senescent cells in aging mice extended lifespan and reduced tumorigenesis [65]. Senolytic agents remove senescent cells by selective induction of apoptosis [66]. However, further development of this strategy is needed to optimally target these drugs without impairing physiological processes, such as wound healing [67].
Aging macrophages and microglia show impaired glucose metabolism, which contributes to systemic inflammation and cognitive decline. Targeted inhibition of prostaglandin E2 signaling in microglia restores microglial energetics and improves synaptic plasticity and memory in mouse models [68]. These findings suggest that anti-inflammatory interventions can improve function in the aging brain. One such lifestyle intervention is sustained physical exercise; a systematic review of 13 clinical trials found that physical exercise reduced inflammatory markers in people of all ages and was particularly effective in reducing inflammation in older individuals [69].
An intriguing aspect of the neuron–glia interface is its effects on systemic aging and lifespan. The aging hypothalamus exhibits increased microglial proliferation and release of TNFα, which in turn suppresses neuronal release of gonadotropin-releasing hormone. Microglial deletion of the upstream NF-κβ regulator IKK-β prevented the accumulation of TNFα, increased gonadotropin-releasing hormone production, and reduced brain and systemic aging [70]. These findings suggest that microglia–neuron cross talk in the hypothalamus contributes to neuroendocrine control of the aging process [71].
Peripheral factors that rejuvenate the aging brain
An exciting area of research is the identification of circulating factors that affect the aging brain. Several factors that promote homeostasis and improve aspects of brain function in aged animal models have been identified (Figure 1). These factors include exercise-induced metabolites and myokines, microbial metabolites, and molecules secreted by immune cells. Circulating factors in young animals can be transferred to aged animals by an experimental approach known as parabiosis, in which the peripheral circulations of the two animals are surgically linked [72]. Circulating factors such as GDF-11, CSF-2, and cathepsin B have been shown to affect diverse neural stem cells [73], neurons, and glial cells [74]. Interestingly, umbilical cord plasma also provides a reservoir of proteins that can have beneficial effects in the aging brain. One example is umbilical cord-derived TIMP-2, a protease inhibitor that was found to improve hippocampal synaptic plasticity and memory in aged mice [75]. Irisin is an exercise-induced hormone that might preserve cognitive functions in aged mice. Furthermore, peripheral delivery of irisin reduced neuroinflammation and improved memory in AD mouse models [76]. Hence, circulating factors may contribute to adaptive mechanisms in the aging brain, although their physiological significance and therapeutic potential remain to be determined.
Conclusion
The discovery of adaptive mechanisms in the aging brain and their detailed characterization in animal models suggest that aspects of brain aging and pathology may be modifiable. This has potentially important implications for future strategies to prevent and treat age-related neurodegenerative disorders. An intriguing finding is that neural and glial adaptive mechanisms may also delay systemic aging and increase longevity, raising the possibility that the brain is a central regulator of the aging process. Therapeutic interventions that engage these adaptive mechanisms may therefore have global effects on aging, and protect against not only neurodegenerative disorders, but also systemic age-related diseases, such as cancer, diabetes, cardiovascular disease and metabolic syndrome.
Several challenges remain to be addressed in order to fully harness the power of brain adaptive mechanisms. First, the cellular and molecular intricacies of brain adaptation during aging must be understood in greater depth. Despite their central roles in the aging brain, the molecular underpinnings of cognitive reserve and compensatory mechanisms are poorly understood. Second, the effects of naturally occurring human genetic diversity on brain adaptation must be thoroughly investigated, as they may provide important clues for personalized therapeutic approaches. Third, the potential benefits and risks of many lifestyle, dietary, and therapeutic approaches must be rigorously examined. Finally, the relevance of adaptive mechanisms to the onset and progression of neurodegenerative disorders must be better understood to harness these pathways for a healthy aging brain.
Acknowledgements
This study was supported by NIH grants RO1AG046174 and RO1MH113279, and grants from the Glenn Foundation for Medical Research and The Ludwig Family Foundation to B.A.Y.
Footnotes
Conflict of interest statement
Nothing declared.
References
Papers of particular interest, published within the period of review, have been highlighted as:
•• of outstanding interest
- 1.James BD, Bennett DA: Causes and patterns of dementia: an update in the era of redefining Alzheimer’s disease. Annu Rev Publ Health 2019, 40:65–84. [DOI] [PubMed] [Google Scholar]
- 2.Sun FW, Stepanovic MR, Andreano J, Barrett LF, Touroutoglou A, Dickerson BC: Youthful brains in older adults: preserved neuroanatomy in the default mode and salience networks contributes to youthful memory in superaging. J Neurosci 2016, 36:9659–9668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Salthouse T: Consequences of age-related cognitive declines. Annu Rev Psychol 2012, 63:201–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Verissimo J, Verhaeghen P, Goldman N, Weinstein M, Ullman MT: Evidence that ageing yields improvements as well as declines across attention and executive functions<!——>. Nat Hum Behav 2021, 10.1038/s41562-021-01169-7. Online ahead of print. [DOI] [PubMed] [Google Scholar]
- 5.Cabeza R, Albert M, Belleville S, Craik FIM, Duarte A, Grady CL, Lindenberger U, Nyberg L, Park DC, Reuter-Lorenz PA, et al. : Maintenance, reserve and compensation: the cognitive neuroscience of healthy ageing. Nat Rev Neurosci 2018, 19: 701–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stern Y, Barnes CA, Grady C, Jones RN, Raz N: Brain reserve, cognitive reserve, compensation, and maintenance: operationalization, validity, and mechanisms of cognitive resilience. Neurobiol Aging 2019, 83:124–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Arnsten AFT, Datta D, Preuss TM: Studies of aging nonhuman primates illuminate the etiology of early-stage Alzheimer’s-like neuropathology: an evolutionary perspective. Am J Primatol 2021: e23254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mattson MP, Moehl K, Ghena N, Schmaedick M, Cheng A: Intermittent metabolic switching, neuroplasticity and brain health. Nat Rev Neurosci 2018, 19:63–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Satoh A, Imai SI, Guarente L: The brain, sirtuins, and ageing. Nat Rev Neurosci 2017, 18:362–374. [DOI] [PubMed] [Google Scholar]
- 10.Mujica-Parodi LR, Amgalan A, Sultan SF, Antal B, Sun X, Skiena S, Lithen A, Adra N, Ratai EM, Weistuch C, et al. : Diet modulates brain network stability, a biomarker for brain aging, in young adults. Proc Natl Acad Sci U S A 2020, 117: 6170–6177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aalling NN, Nedergaard M, DiNuzzo M: Cerebral metabolic changes during sleep. Curr Neurol Neurosci Rep 2018, 18:57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Piras F, Cherubini A, Caltagirone C, Spalletta G: Education mediates microstructural changes in bilateral hippocampus. Hum Brain Mapp 2011, 32:282–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Franzmeier N, Duering M, Weiner M, Dichgans M, Ewers M: Alzheimer’s Disease Neuroimaging I: left frontal cortex connectivity underlies cognitive reserve in prodromal Alzheimer disease. Neurology 2017, 88:1054–1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fitzhugh MC, Braden BB, Sabbagh MN, Rogalsky C, Baxter LC: Age-related atrophy and compensatory neural networks in reading comprehension. J Int Neuropsychol Soc 2019, 25: 569–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cappell KA, Gmeindl L, Reuter-Lorenz PA: Age differences in prefontal recruitment during verbal working memory maintenance depend on memory load. Cortex 2010, 46:462–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Daselaar SM, Fleck MS, Dobbins IG, Madden DJ, Cabeza R: Effects of healthy aging on hippocampal and rhinal memory functions: an event-related fMRI study. Cerebr Cortex 2006, 16:1771–1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cabeza R, Anderson ND, Locantore JK, McIntosh AR: Aging gracefully: compensatory brain activity in high-performing older adults. Neuroimage 2002, 17:1394–1402. [DOI] [PubMed] [Google Scholar]
- 18.Ballesteros S, Kraft E, Santana S, Tziraki C: Maintaining older brain functionality: a targeted review. Neurosci Biobehav Rev 2015, 55:453–477. [DOI] [PubMed] [Google Scholar]
- 19.Iaccarino HF, Singer AC, Martorell AJ, Rudenko A, Gao F, Gillingham TZ, Mathys H, Seo J, Kritskiy O, Abdurrob F, et al. : Gamma frequency entrainment attenuates amyloid load and modifies microglia. Nature 2016, 540:230–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Martorell AJ, Paulson AL, Suk HJ, Abdurrob F, Drummond GT, Guan W, Young JZ, Kim DN, Kritskiy O, Barker SJ, et al.: Multisensory gamma stimulation Ameliorates Alzheimer’s-associated pathology and improves cognition. Cell 201. 9, 177: 256–271 e222. •• A combination of visual and auditory stimulation induces gamma oscillations throughout the neocortex. This type of gamma entrainment leads to microglial, astrocytic and vascular responses that reduce Aβ deposition and improve cognition in the 5xFAD mouse model of Alzheimer’s disease.
- 21.Adaikkan C, Middleton SJ, Marco A, Pao PC, Mathys H, Kim DN, Gao F, Young JZ, Suk HJ, Boyden ES, et al. : Gamma entrainment binds higher-order brain regions and offers neuroprotection. Neuron 2019, 102:929–943 e928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Adaikkan C, Tsai LH: Gamma entrainment: impact on neurocircuits, glia, and therapeutic opportunities. Trends Neurosci 2020, 43:24–41. [DOI] [PubMed] [Google Scholar]
- 23.Brem AK, Sensi SL: Towards combinatorial approaches for preserving cognitive fitness in aging. Trends Neurosci 2018, 41:885–897. [DOI] [PubMed] [Google Scholar]
- 24.Austad SN, Wood MA, Villeda SA, Voss JL, Sahay A, Albert M: Innovative approaches in cognitive aging. Neurobiol Aging 2019, 83:150–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lu T, Pan Y, Kao SY, Li C, Kohane I, Chan J, Yankner BA: Gene regulation and DNA damage in the ageing human brain. Nature 2004, 429:883–891. [DOI] [PubMed] [Google Scholar]
- 26.Vidal-Pineiro D, Parker N, Shin J, French L, Grydeland H, Jackowski AP, Mowinckel AM, Patel Y, Pausova Z, Salum G, et al. : Cellular correlates of cortical thinning throughout the lifespan. Sci Rep 2020, 10:21803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zullo JM, Drake D, Aron L, O’Hern P, Dhamne SC, Davidsohn N, Mao CA, Klein WH, Rotenberg A, Bennett DA, et al.: Regulation of lifespan by neural excitation and REST. Nature 201. 9, 574: 359–364. •• This sudy identifies reduced neural excitation as a correlate of increased longevity in humans and the transcription factor REST as a regulator of neural activity during aging. REST suppresses the expression of genes involved in synaptic function and neurotransmission, and reduces neural excitation in C. elegans and mice. Moreover, suppression of neural excitation by an ortholog of REST in C. elegans results in a longer lifespan. These observations connect neural circuit activity to orgnanismal aging, and suggest that these processes are transcriptionally regulated.
- 28.Neubauer AC, Fink A: Intelligence and neural efficiency. Neurosci Biobehav Rev 2009, 33:1004–1023. [DOI] [PubMed] [Google Scholar]
- 29.Barulli D, Stern Y: Efficiency, capacity, compensation, maintenance, plasticity: emerging concepts in cognitive reserve. Trends Cognit Sci 2013, 17:502–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bosch B, Bartres-Faz D, Rami L, Arenaza-Urquijo EM, Fernandez-Espejo D, Junque C, Sole-Padulles C, Sanchez-Valle R, Bargallo N, Falcon C, et al. : Cognitive reserve modulates taskinduced activations and deactivations in healthy elders, amnestic mild cognitive impairment and mild Alzheimer’s disease. Cortex 2010, 46:451–461. [DOI] [PubMed] [Google Scholar]
- 31.Sole-Padulles C, Bartres-Faz D, Junque C, Vendrell P, Rami L, Clemente IC, Bosch B, Villar A, Bargallo N, Jurado MA, et al. : Brain structure and function related to cognitive reserve variables in normal aging, mild cognitive impairment and Alzheimer’s disease. Neurobiol Aging 2009, 30:1114–1124. [DOI] [PubMed] [Google Scholar]
- 32.Nishiguchi S, Yamada M, Tanigawa T, Sekiyama K, Kawagoe T, Suzuki M, Yoshikawa S, Abe N, Otsuka Y, Nakai R, et al. : A 12-week physical and cognitive exercise program can improve cognitive function and neural efficiency in community-dwelling older adults: a randomized controlled trial. J Am Geriatr Soc 2015, 63:1355–1363. [DOI] [PubMed] [Google Scholar]
- 33.Liao YY, Chen IH, Hsu WC, Tseng HY, Wang RY: Effect of exergaming versus combined exercise on cognitive function and brain activation in frail older adults: a randomised controlled trial. Ann Phys Rehabil Med 2021, 64:101492. [DOI] [PubMed] [Google Scholar]
- 34.Zott B, Simon MM, Hong W, Unger F, Chen-Engerer HJ, Frosch MP, Sakmann B, Walsh DM, Konnerth A: A vicious cycle of beta amyloid-dependent neuronal hyperactivation. Science 2019, 365:559–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Haberman RP, Branch A, Gallagher M: Targeting neural hyperactivity as a treatment to stem progression of late-onset Alzheimer’s disease. Neurotherapeutics 2017, 14:662–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Leal SL, Landau SM, Bell RK, Jagust WJ: Hippocampal activation is associated with longitudinal amyloid accumulation and cognitive decline. Elife 2017, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Huijbers W, Schultz AP, Papp KV, LaPoint MR, Hanseeuw B, Chhatwal JP, Hedden T, Johnson KA, Sperling RA: Tau accumulation in clinically normal older adults is associated with hippocampal hyperactivity. J Neurosci 201. 9, 39:548–556. •• This neuroimaging study shows that the accumulation of tau in aging individuals with normal cognitive function is associated with hyperactivity of hippocampal neural networks during memory encoding. These findings provide further evidence that neural network hyperactivation, driven by the accumulation of misfolded proteins, is an early pathogenic event that may lead to cognitve decline and Alzheimer’s disease.
- 38.Busche MA, Hyman BT: Synergy between amyloid-beta and tau in Alzheimer’s disease. Nat Neurosci 2020, 23:1183–1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sanchez PE, Zhu L, Verret L, Vossel KA, Orr AG, Cirrito JR, Devidze N, Ho K, Yu GQ, Palop JJ, et al. : Levetiracetam suppresses neuronal network dysfunction and reverses synaptic and cognitive deficits in an Alzheimer’s disease model. Proc Natl Acad Sci U S A 2012, 109:E2895–E2903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shi JQ, Wang BR, Tian YY, Xu J, Gao L, Zhao SL, Jiang T, Xie HG, Zhang YD: Antiepileptics topiramate and levetiracetam alleviate behavioral deficits and reduce neuropathology in APPswe/PS1dE9 transgenic mice. CNS Neurosci Ther 2013, 19:871–881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bakker A, Krauss GL, Albert MS, Speck CL, Jones LR, Stark CE, Yassa MA, Bassett SS, Shelton AL, Gallagher M: Reduction of hippocampal hyperactivity improves cognition in amnestic mild cognitive impairment. Neuron 2012, 74:467–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liao YY, Tseng HY, Lin YJ, Wang CJ, Hsu WC: Using virtual reality-based training to improve cognitive function, instrumental activities of daily living and neural efficiency in older adults with mild cognitive impairment. Eur J Phys Rehabil Med 2020, 56:47–57. [DOI] [PubMed] [Google Scholar]
- 43.Hwang JY, Zukin RS: REST, a master transcriptional regulator in neurodegenerative disease. Curr Opin Neurobiol 2018, 48: 193–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lu T, Aron L, Zullo J, Pan Y, Kim H, Chen Y, Yang TH, Kim HM, Drake D, Liu XS, et al. : REST and stress resistance in ageing and Alzheimer’s disease. Nature 2014, 507:448–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Buffolo F, Petrosino V, Albini M, Moschetta M, Carlini F, Floss T, Kerlero de Rosbo N, Cesca F, Rocchi A, Uccelli A, et al. : Neuroinflammation induces synaptic scaling through IL-1beta-mediated activation of the transcriptional repressor REST/NRSF. Cell Death Dis 2021, 12:180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pecoraro-Bisogni F, Lignani G, Contestabile A, Castroflorio E, Pozzi D, Rocchi A, Prestigio C, Orlando M, Valente P, Massacesi M, et al. : REST-dependent presynaptic homeostasis induced by chronic neuronal hyperactivity. Mol Neurobiol 2018, 55:4959–4972. [DOI] [PubMed] [Google Scholar]
- 47.Goetzl EJ, Boxer A, Schwartz JB, Abner EL, Petersen RC, Miller BL, Carlson OD, Mustapic M, Kapogiannis D: Low neural exosomal levels of cellular survival factors in Alzheimer’s disease. Ann Clin Transl Neurol 2015, 2:769–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Abner EL, Jicha GA, Shaw LM, Trojanowski JQ, Goetzl EJ: Plasma neuronal exosomal levels of Alzheimer’s disease biomarkers in normal aging. Ann Clin Transl Neurol 2016, 3:399–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ashton NJ, Hye A, Leckey CA, Jones AR, Gardner A, Elliott C, Wetherell JL, Lenze EJ, Killick R, Marchant NL: Plasma REST: a novel candidate biomarker of Alzheimer’s disease is modified by psychological intervention in an at-risk population. Transl Psychiatry 2017, 7:e1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dallagnol KMC, Remor AP, da Silva RA, Prediger RD, Latini A, Aguiar AS Jr: Running for REST: physical activity attenuates neuroinflammation in the hippocampus of aged mice. Brain Behav Immun 2017, 61:31–35. [DOI] [PubMed] [Google Scholar]
- 51.Castellani G, Schwartz M: Immunological features of non-neuronal brain cells: implications for Alzheimer’s disease immunotherapy. Trends Immunol 2020, 41:794–804. [DOI] [PubMed] [Google Scholar]
- 52.Lee JH, Kim JY, Noh S, Lee H, Lee SY, Mun JY, Park H, Chung WS: Astrocytes phagocytose adult hippocampal synapses for circuit homeostasis. Nature 2021, 590:612–617. [DOI] [PubMed] [Google Scholar]
- 53.Du Z, Song Y, Chen X, Zhang W, Zhang G, Li H, Chang L, Wu Y: Knockdown of astrocytic Grin2a aggravates beta-amyloid-induced memory and cognitive deficits through regulating nerve growth factor. Aging Cell 2021:e13437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hanisch UK, Kettenmann H: Microglia: active sensor and versatile effector cells in the normal and pathologic brain. Nat Neurosci 2007, 10:1387–1394. [DOI] [PubMed] [Google Scholar]
- 55.Colonna M, Butovsky O: Microglia function in the central nervous system during health and neurodegeneration. Annu Rev Immunol 2017, 35:441–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bartels T, De Schepper S, Hong S: Microglia modulate neurodegeneration in Alzheimer’s and Parkinson’s diseases. Science 2020, 370:66–69. [DOI] [PubMed] [Google Scholar]
- 57.Linnerbauer M, Wheeler MA, Quintana FJ: Astrocyte crosstalk in CNS inflammation. Neuron 2020, 108:608–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Pluvinage JV, Haney MS, Smith BAH, Sun J, Iram T, Bonanno L, Li L, Lee DP, Morgens DW, Yang AC, et al.: CD22 blockade restores homeostatic microglial phagocytosis in ageing brains. Nature 201. 9, 568:187–192. •• A CRISPR-Cas9 knockout screen identified the CD22 receptor as a negative regulator of microglial phagocytosis. CD22 is upregulated in aged microglia and inhibits microglial phagocytic activity. Inhibition of CD22 restores phagocytic activity and promotes the clearance of myelin debris, oligomeric Aβ, and α-synuclein fibrils. Moreover, continuous brain deilvery of CD22 blocking antibody improved cognitive function in aging normal mice. These observations suggest that relieving the CD22 blockade can restore the normal function of microglia in the aging brain.
- 59.Keren-Shaul H, Spinrad A, Weiner A, Matcovitch-Natan O, Dvir-Szternfeld R, Ulland TK, David E, Baruch K, Lara-Astaiso D, Toth B, et al. : A unique microglia type Associated with restricting development of Alzheimer’s disease. Cell 2017, 169:1276–1290 e1217. [DOI] [PubMed] [Google Scholar]
- 60.Colonna M, Wang Y: TREM2 variants: new keys to decipher Alzheimer disease pathogenesis. Nat Rev Neurosci 2016, 17: 201–207. [DOI] [PubMed] [Google Scholar]
- 61. Rosenzweig N, Dvir-Szternfeld R, Tsitsou-Kampeli A, Keren-Shaul H, Ben-Yehuda H, Weill-Raynal P, Cahalon L, Kertser A, Baruch K, Amit I, et al.: PD-1/PD-L1 checkpoint blockade harnesses monocyte-derived macrophages to combat cognitive impairment in a tauopathy mouse model. Nat Commun 2019, 10:465. •• Blockade of the PD-1/PD-L1 immune checkpoint, via systemic administration of either anti-PD-1 or anti-PD-L1 antibodies increased the recruitment of monocyte-derived macrophages to the brain parenchyma and prevented pathology, reduced inflammation, and improved cognitive function in 5xFAD amyloid and K257T/P301S-hTau tauopathy mouse models. Thus, blockade of the PD-1/PD-L1 immune checkpoint may be a novel approach to augmenting beneficial functions of microglia in the aging brain.
- 62.Baruch K, Deczkowska A, Rosenzweig N, Tsitsou-Kampeli A, Sharif AM, Matcovitch-Natan O, Kertser A, David E, Amit I, Schwartz M: PD-1 immune checkpoint blockade reduces pathology and improves memory in mouse models of Alzheimer’s disease. Nat Med 2016, 22:135–137. [DOI] [PubMed] [Google Scholar]
- 63.Sikora E, Bielak-Zmijewska A, Dudkowska M, Krzystyniak A, Mosieniak G, Wesierska M, Wlodarczyk J: Cellular senescence in brain aging. Front Aging Neurosci 2021, 13:646924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bussian TJ, Aziz A, Meyer CF, Swenson BL, van Deursen JM, Baker DJ: Clearance of senescent glial cells prevents tau-dependent pathology and cognitive decline. Nature 2018, 562: 578–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Baker DJ, Childs BG, Durik M, Wijers ME, Sieben CJ, Zhong J, Saltness RA, Jeganathan KB, Verzosa GC, Pezeshki A, et al. : Naturally occurring p16(Ink4a)-positive cells shorten healthy lifespan. Nature 2016, 530:184–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Di Micco R, Krizhanovsky V, Baker D, d’Adda di Fagagna F: Cellular senescence in ageing: from mechanisms to therapeutic opportunities. Nat Rev Mol Cell Biol 2021, 22:75–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hara Y, McKeehan N, Fillit HM: Translating the biology of aging into novel therapeutics for Alzheimer disease. Neurology 2019, 92:84–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Minhas PS, Latif-Hernandez A, McReynolds MR, Durairaj AS, Wang Q, Rubin A, Joshi AU, He JQ, Gauba E, Liu L, et al.: Restoring metabolism of myeloid cells reverses cognitive decline in ageing. Nature 202. 1, 590:122–128. •• Activation of the macrophage and microglial EP2 receptor by prostaglandin E2 in aged mice leads to reduced glucose utilization and mitochondrial respiration in myeloid cells and increases inflammation. Genetic and pharmacologic inhibition of EP2 signaling improves myeloid bioenergetics, reduces inflammation, enhances synaptic plasticity, and restores cognitive function in aged mice. Thus, restoration of macrophage and microglial metabolism can have major effects on brain function during aging.
- 69.Cronin O, Keohane DM, Molloy MG, Shanahan F: The effect of exercise interventions on inflammatory biomarkers in healthy, physically inactive subjects: a systematic review. QJM 2017, 110:629–637. [DOI] [PubMed] [Google Scholar]
- 70.Zhang G, Li J, Purkayastha S, Tang Y, Zhang H, Yin Y, Li B, Liu G, Cai D: Hypothalamic programming of systemic ageing involving IKK-beta, NF-kappaB and GnRH. Nature 2013, 497: 211–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gabuzda D, Yankner BA: Physiology: inflammation links ageing to the brain. Nature 2013, 497:197–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wyss-Coray T: Ageing, neurodegeneration and brain rejuvenation. Nature 2016, 539:180–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Navarro Negredo P, Yeo RW, Brunet A: Aging and rejuvenation of neural stem cells and their niches. Cell Stem Cell 2020, 27: 202–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Pluvinage JV, Wyss-Coray T: Systemic factors as mediators of brain homeostasis, ageing and neurodegeneration. Nat Rev Neurosci 2020, 21:93–102. [DOI] [PubMed] [Google Scholar]
- 75.Castellano JM, Mosher KI, Abbey RJ, McBride AA, James ML, Berdnik D, Shen JC, Zou B, Xie XS, Tingle M, et al. : Human umbilical cord plasma proteins revitalize hippocampal function in aged mice. Nature 2017, 544:488–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Islam MR, Valaris S, Young MF, Haley EB, Luo R, Bond SF, Mazuera S, Kitchen RR, Caldarone BJ, Bettio LEB, et al. : Exercise hormone irisin is a critical regulator of cognitive function. Nat Metab 2021, 3:1058–1070. [DOI] [PMC free article] [PubMed] [Google Scholar]


