Abstract
Background:
Clinicians make a medical diagnosis by recognizing diagnostic possibilities, often using memories of prior examples. These memories, called exemplars, reflect specific symptom combinations in individual patients, yet most clinical studies report how symptoms aggregate in populations. We studied how symptoms of acute myocardial infarction combine in individuals as symptom phenotypes and how symptom phenotypes are distributed in women and men.
Methods:
In this analysis of the SILVER-AMI Study, we studied 3041 patients (1346 women and 1645 men) ≥75 years old with acute myocardial infarction. Each patient had a standardized in-person interview during the acute myocardial infarction admission to document the presenting symptoms, which enabled a thorough examination of symptom combinations in individuals. Specific symptom combinations defined symptom phenotypes and distributions of symptom phenotypes were compared in women and men using Monte Carlo permutation testing and repeated subsampling.
Results:
There were 1469 unique symptom phenotypes in the entire SILVER-AMI cohort of acute myocardial infarction patients. There were 831 unique symptom phenotypes in women, as compared with 819 in men, which was highly significant, given the larger number of men than women in the study (p<0.0001). Women had significantly more symptom phenotypes than men in almost all acute myocardial infarction subgroups.
Conclusions:
Older patients with acute myocardial infarction have enormous variation in symptom phenotypes. Women reported more symptoms and had significantly more symptom phenotypes than men. Appreciation of the diversity of symptom phenotypes may help clinicians recognize the less common phenotypes that occur more often in women.
Keywords: Gender disparities, diagnostic reasoning, acute myocardial infarction
Introduction
Making a diagnosis for an individual patient typically starts with a clinician using pattern recognition to generate a list of diagnostic possibilities.1–4 Novice students, lacking clinical experience, use causal knowledge of diseases to recognize diagnostic possibilities, whereas expert diagnosticians rely more on clinical experience including memories of specific clinical instances. 5,6 When an instance such as a diagnostic encounter is stored in long-term memory, it is remembered using a knowledge structure called an exemplar.7,8
Because exemplars are memories of patients with specific feature combinations, it may be important to study how symptoms combine in patients as symptom phenotypes. Most clinical reports of a diagnosis describe how symptoms aggregate at the population level and not how symptoms combine at the individual patient level as symptom phenotypes.
The Comprehensive Evaluation of Risk Factors in Older Patients with Acute Myocardial Infarction (SILVER-AMI) study was a cohort study that enrolled older patients (≥75 years old) hospitalized with acute myocardial infarction.9 This study provided an opportunity to examine symptom phenotypes in patients with acute myocardial infarction because all study participants underwent structured interviews that systematically recorded patient’s symptoms. Accordingly, our objective was to examine symptom phenotypes in older acute myocardial infarction patients in SILVER-AMI, to study the variation in symptom phenotypes and to compare the variation and distribution of symptom phenotypes in women and men.
Methods
Study Population.
The SILVER-AMI study prospectively collected information on 3041 participants from 94 hospitals in the United States.10–12 Institutional Review Boards at each institution approved the study and all participants provided informed consent. Eligible participants were patients ≥75 years old who met the criteria for the Third Universal Definition of acute myocardial infarction.13 Patients were enrolled in the study at the time of hospitalization and underwent a comprehensive, face-to-face, structured interview at baseline by a local research coordinator. Further abstraction of medical records was performed by the Yale Coordinating Center for in-depth chart review of medications, cardiac procedures, and adverse events. A primary objective of the SILVER-AMI study was to assess functional impairments, including those in mobility, activities of daily living, and cognition. Mobility impairment was assessed using the Timed UP and Go (TUG) test, and activities of daily living were assessed by asking about pre-hospital function.14 Cognitive status was assessed as part of the face-to-face baseline interview using the telephone interview for cognitive status (TICS). Cognitive impairment was defined by a TICS score of <27.15
Symptoms.
Each patient had a standardized in-person interview by a trained, local research coordinator during the acute myocardial infarction admission. Patients were specifically asked if they experienced chest pain, neck or throat pain, jaw, teeth, or mouth pain, shoulder or arm pain, back pain between the shoulder blades, sweating or diaphoresis, lightheadedness, confusion, numbness or tingling, blurry vision, shortness of breath, cough, indigestion or heartburn, stomach or epigastric pain or pressure, nausea, vomiting, belching, anxiety, stress or agitation, ankle swelling, weakness or fatigue, a fall, or other symptoms not listed. Thus, there were 22 symptoms that could combine in individual patients as unique symptom phenotypes.
Statistical Analysis.
Symptoms obtained by the standardized interviews were analyzed by combining the symptoms in individual patients into combinations that defined symptom phenotypes and by creating subsets of patients with unique symptom phenotypes. The distribution of symptom phenotypes was compared in women and men and stratified by subgroups that might affect presenting symptoms: acute myocardial infarction type (ST-segment-elevation myocardial infarction [STEMI] or non-ST-segment-elevation myocardial infarction [NSTEMI]), receipt of emergent or urgent percutaneous coronary intervention (PCI) or no PCI, presentation time of ≥6 hours from onset of symptoms or <6 hours, presence or absence of diabetes, presence or absence of chest pain as a presenting symptom, and presence or absence of cognitive, mobility, or activities of daily living impairment.
The variation and distributions of symptom phenotypes were analyzed using SAS/STAT Version 14.3 (SAS, Cary, NC). The SILVER-AMI population had unequal numbers of female (44.2%) and male (55.8%) patients. This imbalance could bias the comparison of the number of symptom phenotypes in women and men because different sample sizes could randomly affect the ability to detect rare symptom phenotype in a smaller group of patients. Therefore, two analytical approaches were used to compare women and men: Monte Carlo permutation tests16 and repeated subsampling,17 as described previously.18 Monte Carlo permutation testing was the primary method for analyzing the differences in the numbers of symptom phenotypes between women and men because it compared the observed differences to that which would have been expected if the differences in the number of phenotypes were simply due to the different sample sizes. Repeated subsampling allowed further analysis of the overlap in symptom phenotypes between women and men.
For the Monte Carlo permutation analysis, patients’ sexes were randomly permuted to generate 99,999 data sets reflecting the null hypothesis that any difference in the number of phenotypes was due to the unequal sample sizes for female and male patients rather than due to the effect of sex. These datasets plus the dataset that was actually observed in SILVER-AMI resulted in an ensemble of 100,000 datasets, a number that was deemed adequate for analysis. This analysis created an empirical distribution of the differences in phenotypes for significance testing. The median of the empirical distribution was interpreted as an estimate of the difference in the number of phenotypes between women and men that would have been expected due to the differences in sample sizes alone and the p-value was the proportion of the empirical distribution showing a difference as large or larger than the difference between sexes observed in the original SILVER-AMI data.
For the repeated subsampling analysis, 100,000 subsamples of 500 women and 500 men were randomly generated. The numbers of distinct phenotypes in each subsample of women and men were compared with Student t-test (with Satterthwaite correction for unequal variances). Logistic regression was performed on the repeated sub-samples to determine if the number of symptoms in the phenotype predicted if a phenotype was unique to either women or men.
Distributions of patients in subgroups were compared in women and men using chi-square analysis. Symptom counts in women and men were compared using the Wilcoxon-Mann-Whitney rank-sum test.
Results
There were 3041 patients in the SILVER-AMI study; 1346 (44.2%) were women. The average age was 82.1 years in women and 81.1 years in men (p<0.0001). Of the women, 1134 (84.2%) were White, 162 (12.0%) were Black, and 50 (3.7%) were other race, as compared with men where 1534 (90.5%) were White, 88 (5.2%) were Black, and 73 (4.3%) were other race (p<0.0001). Of the women, 46 (3.4%) were Hispanic, as compared with 41 (2.4%) of the men.
The average number of symptoms per patient was 4.2±2.8 in women and 3.6±2.5 in men (p<0.0001). The frequencies for individual symptoms at the population level are listed in Table 1. Chest pain was more commonly reported in men, whereas pain in other areas including the neck, jaw, arm, and back were more commonly reported in women. Indigestion, nausea and vomiting were also more commonly reported in women. Frequencies of other individual symptoms were not different between women and men.
Table 1.
Frequencies of Symptoms at the Population Level in Women and Men.
| Symptom | Women | Men | p value |
|---|---|---|---|
| Chest Pain | 72.8 | 78.2 | 0.0006 |
| Neck/Throat Pain | 17.4 | 9.6 | <0.0001 |
| Jaw Pain | 13.5 | 7.9 | <0.0001 |
| Shoulder/Arm Pain | 34.8 | 28 | <0.0001 |
| Interscapular Pain | 22 | 11.9 | <0.0001 |
| Diaphoresis | 22.3 | 21.7 | 0.6914 |
| Lightheaded | 19.5 | 16.6 | 0.0455 |
| Confusion | 6.3 | 5.8 | 0.5928 |
| Numbness/Tingling | 7.5 | 6.7 | 0.3919 |
| Blurred Vision | 3.5 | 4.2 | 0.3015 |
| Dyspnea | 45.5 | 44.8 | 0.7413 |
| Cough | 11.1 | 10.0 | 0.3716 |
| Indigestion | 19.6 | 16.4 | 0.0221 |
| Epigastric Pain | 11.1 | 10.1 | 0.4050 |
| Nausea | 25.0 | 15.1 | <0.0001 |
| Vomiting | 11.5 | 6.2 | <0.0001 |
| Belching | 7.1 | 7.1 | 1.0000 |
| Anxiety, stress, agitation | 19.7 | 18.2 | 0.3273 |
| Ankle Edema | 6.5 | 5.4 | 0.2158 |
| Weakness/Fatigue | 28.9 | 26.7 | 0.1781 |
| Fall | 2.5 | 3.1 | 0.3810 |
| Other symptom not listed | 10 | 8.1 | 0.0739 |
Examination of how symptoms combine at the individual level revealed that there were 1469 unique symptom phenotypes in the entire SILVER-AMI cohort of patients. There were 831 unique symptom phenotypes in women, as compared with 819 in men. The observed difference of 12 greater phenotypes in women was highly statistically significant because men would have been expected to have 158 more phenotypes than women under the null that the difference was due to different sample sizes rather than sex (permutation p<0.0001).
The differences between women and men in the number of symptom phenotypes in subgroups are listed in Table 2. Women had significantly more symptom phenotypes than men in all subgroups except Hispanic patients, and patients with cognitive impairment and activities of daily living impairment. Chest pain was the most frequently reported symptom in both women and men but was not a reported symptom significantly more often women than men (Table 1). Women had significantly more symptom phenotypes than men regardless of whether chest pain was one of the reported symptoms (Table 2).
Table 2.
Distribution of Women and Men in Subgroups and Results of the Monte Carlo Permutation Analysis.
| Subgroup | Distribution of Patients | Monte Carlo Permutation Tests | |||
|---|---|---|---|---|---|
| Women | Men | Observed Difference | Difference under the null | p value | |
| Overall (n=3041) | 1346 (44%) | 1695 (56%) | 12 | 158 | .00001 |
| White (n=2668, 89%) | 1134 (43%) | 1534 (57%) | −46 | −183 | .00001 |
| Non-white (n=325, 11%) | 194 (60%) | 131 (40%) | 64 | 45 | .00483 |
| Hispanic (n=87, 3%) | 46 (53%) | 41 (47%) | 0 | 4 | .91014 |
| NSTEMI (n=2244, 74%) | 968 (43%) | 1276 (57%) | −25 | −143 | .00001 |
| STEMI (n=797, 26%) | 378 (47%) | 419 (53%) | 19 | −25 | .00018 |
| Delay (n=1289, 43%) | 580 (45%) | 709 (55%) | 9 | −68 | .00002 |
| No Delay (n=1736, 57%) | 761 (44%) | 975 (56%) | −19 | −107 | .00001 |
| PCI (n=1738, 57%) | 758 (44%) | 980 (56%) | −7 | −118 | .00001 |
| No PCI (n=1303, 43%) | 588 (45) | 715 (55%) | 0 | −62 | .00009 |
| DM (n=1128, 37%) | 495 (44%) | 632 (56%) | −16 | −73 | .00013 |
| No DM (n=1913, 63%) | 850 (44%) | 1063 (56%) | 7 | −105 | .00001 |
| Chest Pain (n=2306, 76%) | 980 (42%) | 1326 (58%) | −17 | −152 | .00001 |
| No Chest Pain (n=735, 24%) | 366 (50%) | 369 (50%) | 29 | −2 | .00842 |
| Cognitive Impairment (n=512, 17%) | 277 (54%) | 235 (46%) | 36 | 26 | .17871 |
| No Cognitive Impairment (n=2479, 82%) | 1037 (42%) | 1442 (58%) | −40 | −189 | .00001 |
| Mobility Impairment (n=1064, 35%) | 580 (55%) | 484 (45%) | 101 | 54 | .00092 |
| No Mobility Impairment (n=1493, 49%) | 550 (37%) | 943 (63%) | −128 | −200 | .00002 |
| ADL Impairment (n=420, 14%) | 247 (59%) | 173 (41%) | 67 | 53 | .05246 |
| No ADL Impairment (n=2620, 86%) | 1099 (42%) | 1521 (58%) | −57 | −191 | .00001 |
NSTEMI=non-ST elevation myocardial infarction, STEMI=ST-elevation myocardial infarction, PCI=emergent or urgent percutaneous coronary intervention, DM=diabetes mellitus, ADL=activities of daily living.
Women were more broadly distributed in the symptom phenotype subgroups than men. Among women, 279 (21%) were in one of the top 10 symptom phenotype subgroups, as compared with 517 (31%) of men (p<0.0001). Only 5% of women had the most common symptom phenotype of chest pain alone, as compared with 11% of men. The top 10 symptom phenotypes are listed in Table 3. The top two phenotypes (chest pain and chest pain plus dyspnea) were the same in both women and men. Two of the top ten phenotypes occurred in only women or men and the remaining 6 of the top ten phenotypes occurred in both women and men but in different order of frequency.
Table 3.
Rates of the Top 10 Symptom Phenotypes in Women and Men.
| Women | Men | ||
|---|---|---|---|
| Symptom | Rate | Symptom | Rate |
| Chest pain | 5% | Chest pain | 11% |
| Chest pain, dyspnea | 3% | Chest pain, dyspnea | 6% |
| None | 3% | Chest pain, arm pain | 3% |
| Dyspnea | 3% | None | 3% |
| Chest pain, arm pain | 2% | Dyspnea | 2% |
| Chest pain, interscapular pain | 1% | Chest pain, diaphoresis | 2% |
| Chest pain, arm pain, interscapular pain | 1% | Chest pian, dyspnea, weakness | 1% |
| Arm pain | 1% | Chest pain, indigestion | 1% |
| Chest pain, dyspnea, weakness | 1% | Chest pain, arm pain, dyspnea | 1% |
| Chest pain, arm pain, dyspnea | 1% | Chest pain, interscapular pain | 1% |
| Top 10 phenotypes* | 21% | Top 10 phenotypes | 31% |
p<0.0001
The top 4 symptoms reported by both women and men were chest pain, shoulder or arm pain, dyspnea, and weakness. In women, 91.2% of the patients had one or more of those 4 symptoms and 92.9% of the phenotypes included one or more of those 4 symptoms, as compared with 92.1% of the patients and 93.8% of the phenotypes in men (NS).
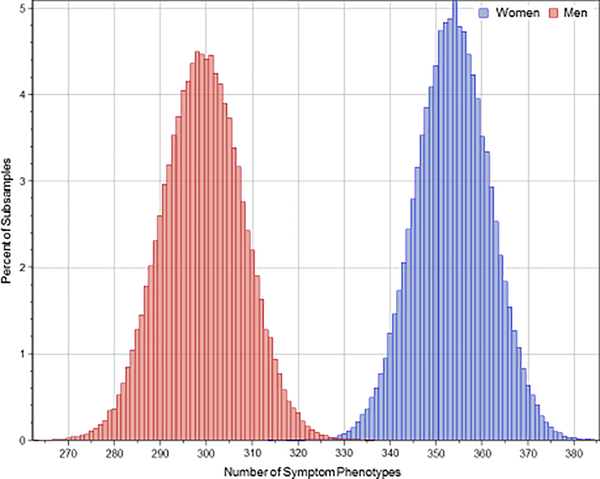
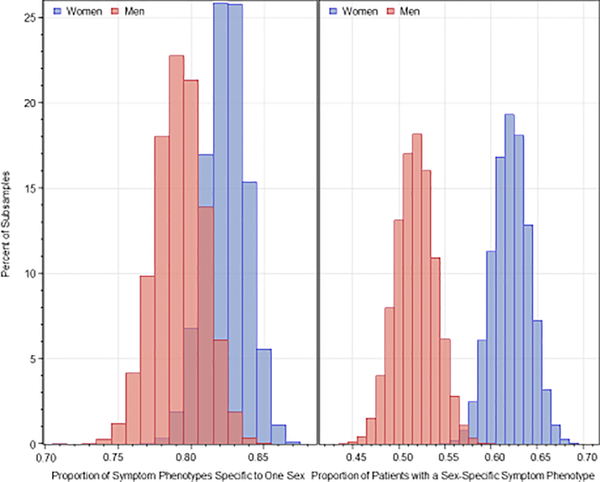
Repeated subsample analysis also showed significantly more symptom phenotypes in women than men, as shown in Figure 1. The average number of phenotypes in repeated subsampling was 353.6±8.1 in women and 299.3±8.8 in men (p<0.0001). Repeated subsample analysis showed that 82.4±1.4% of the symptom phenotypes constituting 62.1±2.0% of women were present only in women and not in men, and 79.2±1.7% of symptom phenotypes constituting 51.8±2.2% of men were present only in men and not in women (Figure 2). Logistic regression showed that the probability of overlap in symptom phenotypes in women and men decreased with the number of symptoms in the phenotype. The odds ratios accompanying a one-symptom increase were 0.398 (95% CI=0.398–0.399) for women (p<0.001) and 0.401 (95% CI=0.401–0.402) for men (p<0.001).
Figure 1.
Distribution of the number of symptom phenotypes in women and men with 100,000 subsamples with a bootstrap size of 500.
Figure 2.
Distributions of the proportion of phenotypes specific to one sex in the left panel and proportion of patients with a sex-specific phenotype in the right panel.
Discussion
The SILVER-AMI study provided an opportunity to analyze how symptoms combine as symptom phenotypes in individual patients with acute myocardial infarction. Our analysis revealed marked variation with a total of 1469 unique symptom phenotypes among older acute myocardial infarction patients and significantly more symptom phenotypes in women than men. A larger number of symptom phenotypes was seen in women in all the subgroups of acute myocardial infarction that were analyzed, except Hispanic patients, and patients with cognitive impairment and activities of daily living impairment where the smaller subgroups may have lacked sufficient numbers to capture the enough phenotypes to show a significant difference between women and men. These results echo the findings in younger patients in the VIRGO Study analysis18 and extends the findings to a cohort of older patients with acute myocardial infarction.
At the population level, the top reported symptoms by both women and men were chest pain, shoulder or arm pain, dyspnea, and weakness. Over 90% of both women and men and over 90% of the phenotypes in both women and men had one or more of these symptoms. The difference in women and men, appeared to be primarily explained by the other reported symptoms. Women were more likely than men to report 8 other symptoms, as compared with men who only reported chest pain more commonly than women (Table 1). As a result of reporting additional symptoms, women showed greater diversity of symptom combinations.
Clinicians make medical diagnoses using a process of hypothesis generation and hypothesis testing and the process evolves and improves as clinicians gain experience.1–4 This process is usually informed by population-level statistics about the most common symptoms, but how symptoms combine at the individual level may be more informative and may provide insights that could help clinicians better navigate the diagnostic process and improve diagnostic accuracy. To our knowledge, this study and the prior VIRGO analysis18 are the first studies to analyze how symptoms combine in individuals as symptom phenotypes.
Analysis of symptom phenotypes may have implications for understanding diagnostic error. The higher number of symptom phenotypes observed in women in SILVER-AMI, as well as in VIRGO, may be a source of error for diagnosticians. Rare phenotypes may be more difficult to recognize, and because they occur more commonly in women, this could lead to more missed diagnoses in women. Although missed diagnosis rates are hard to calculate because of difficulty establishing a denominator representing all patients under consideration for a specific diagnosis, one prior study suggested that women have a higher rate of missed diagnosis,19 which could lead to worse outcomes that have been observed in women with acute myocardial infarction.20 The increased variability in symptom phenotypes in women might provide an explanation for the higher rates of missed diagnoses in women with acute myocardial infarction.
Analysis of symptom phenotypes may also have implications for understanding the diagnostic process. With experience, clinicians store memories of individual diagnostic instances in episodic memory as exemplars.7,21–24 Episodic memory encodes direct memories of experiences (in contrast to semantic memory which encodes abstracted knowledge about concepts and meaning).25 Accumulated memories of exemplars give clinicians an implicit sense of the range of variation within a diagnostic category and the relative prevalence of a diagnosis.26 Diagnostic exemplars are retrieved from memory quickly and intuitively.27–29 The ease of retrieval depends on the strength of the association, the number of common features, and the recency or vividness of the memory.30,31
Other frameworks for knowledge representation in addition to exemplars have also been proposed including semantic networks and prototypes.7,21,22 Prototypical or classic descriptions of diagnoses are often discussed and presented as textbook examples. For acute myocardial infarction, the prototype might be a patient with chest pain, radiating to the arm, with shortness of breath and diaphoresis. Interestingly, that specific combination of symptoms occurred in less than 1% of the SILVER-AMI and VIRGO patients.18 The SILVER-AMI data indicate that the prototype for older acute myocardial infarction patients would include weakness rather than diaphoresis as one of the four most common symptoms. This combination of symptoms also was very uncommon, occurring in less than 1% of the SILVER-AMI patients. Although our analysis shows that the classic prototype for acute myocardial infarction is not a common phenotype, one or more of the prototypical symptoms occurred in over 90% of patients and phenotypes in both women and men, suggesting that looking for phenotypes that contain one or more of the prototypical symptoms would be a better diagnostic strategy than looking for the specific phenotype that matches the prototype.
These findings may have implications for how we teach and for how we might improve the diagnostic process.33–38 Teachers and clinicians should acknowledge that there is enormous variation in the patterns of presenting symptoms for acute myocardial infarction and the variation is greater in women. Knowledge of exemplar theory may help learners make the most of their experience by encouraging them to intentionally focus on categorizing each diagnostic encounter to make each encounter more memorable. Cognitive psychology studies have emphasized the importance of numerous examples for learning.39,40 The large number of symptom phenotypes suggest that extensive experience may be necessary to assure expertise in the diagnosis of acute myocardial infarction.41,42 Finally, there may be opportunities to apply computerized alerts or decision-support tools to help clinicians recognize rare and complex phenotypes.43–45
This study has several limitations. The available data only provide the ability to analyze patients with the established diagnosis of acute myocardial infarction. It would be intriguing to also analyze patients with suspected acute myocardial infarction where the diagnosis was missed to determine whether there are specific symptom combinations that might obscure the diagnosis of acute myocardial infarction. As noted above, defining a representative sample of all possible diagnostic encounters is difficult, so designing a study that compared the symptom phenotypes in patients with and without a documented diagnosis would be very difficult. Although this study may lack a “control group” of patients with suspected acute myocardial infarction who had an alternate diagnosis, the study does provide a robust opportunity to evaluate variation of symptom combinations in patients with established acute myocardial infarction. Finally, our study does not provide a mechanism to explain the observed differences in the number of symptom phenotypes between women and men. The differences could be due to biologic sex differences in acute myocardial infarction pathophysiology or pain perception or social differences in how symptoms are reported, and this is an interesting area for further study.
In summary, by analyzing how symptoms combine at the individual level, we have demonstrated that there is enormous variation in symptom phenotypes in acute myocardial infarction and women have significantly more symptom phenotypes than men. Examining how symptoms combine as symptom phenotypes provides important insights into how clinicians recognize diagnoses in individual patients and how individual diagnostic encounters add to a clinician’s experiential knowledge. These findings may have important implications for clinicians, learners, and teachers, which may ultimately improve diagnostic accuracy and help address gender disparities in the diagnosis of acute myocardial infarction.
Clinical Significance.
Clinical studies usually report how symptoms aggregate in populations, not how symptoms combine in individuals, yet clinicians observe symptom combinations in individuals as symptom phenotypes.
In the Silver-AMI registry of 3041 AMI patients, there were 1469 symptom phenotypes of AMI, demonstrating enormous variability.
There were significantly more symptom phenotypes in women.
These findings have implications that could improve diagnostic accuracy and gender disparities in the diagnosis of AMI.
Sources of Funding
This research was supported by grant R01 HL115295 from the National Heart, Lung, and Blood Institute. This SILVER-AMI study was conducted at the Yale Claude D. Pepper Older Americans Independence Center (grant P30 AG021342).
Declaration of Competing Interest
In the past three years, Dr. Krumholz received expenses and/or personal fees from UnitedHealth, IBM Watson Health, Element Science, Aetna, Facebook, the Siegfried and Jensen Law Firm, Arnold and Porter Law Firm, Martin/Baughman Law Firm, F-Prime, and the National Center for Cardiovascular Diseases in Beijing. He is a co-founder of Refactor Health and HugoHealth, and had grants and/or contracts from the Centers for Medicare & Medicaid Services, Medtronic, U.S. Food and Drug Administration, Johnson & Johnson, Foundation for a Smoke-Free World, State of Connecticut Department of Public Health, and the Shenzhen Center for Health Information. Dr. Brush receives royalties from Dementi Milestone Publishing for the book “The Science of the Art of Medicine: A Guide to Medical Reasoning.” Dr. Chaudhry serves as a reviewer for the CVS Caremark State of Connecticut Clinical Program. The other authors report no disclosures.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Elstein AS, Shulman LS, Sprafka SA: Medical Problem Solving: An Analysis of Clinical Reasoning. Cambridge, MA: Harvard University Press; 1978. [Google Scholar]
- 2.Neufeld VR, Norman GR, Feightner JW and Barrows HS. Clinical problem-solving by medical students: a cross-sectional and longitudinal analysis. Med Educ. 1981;15:315–22. [DOI] [PubMed] [Google Scholar]
- 3.Barrows HS, Norman GR, Neufeld VR and Feightner JW. The clinical reasoning of randomly selected physicians in general medical practice. Clin Invest Med. 1982;5:49–55. [PubMed] [Google Scholar]
- 4.Kassirer JP and Gorry GA. Clinical problem solving: a behavioral analysis. Ann Intern Med. 1978;89:245–55. [DOI] [PubMed] [Google Scholar]
- 5.Schmidt HG, Norman GR and Boshuizen HP. A cognitive perspective on medical expertise: theory and implication. Acad Med. 1990;65:611–21. [DOI] [PubMed] [Google Scholar]
- 6.Schmidt HG and Rikers RM. How expertise develops in medicine: knowledge encapsulation and illness script formation. Med Educ. 2007;41:1133–9. [DOI] [PubMed] [Google Scholar]
- 7.Bordage G and Zacks R. The structure of medical knowledge in the memories of medical students and general practitioners: categories and prototypes. Med Educ. 1984;18:406–16. [DOI] [PubMed] [Google Scholar]
- 8.Medin DL. Concepts and conceptual structure. Am Psychol. 1989;44:1469–81. [DOI] [PubMed] [Google Scholar]
- 9.Dodson JA, Geda M, Krumholz HM, Lorenze N, Murphy TE, Allore HG, Charpentier P, Tsang SW, Acampora D, Tinetti ME, Gill TM and Chaudhry SI. Design and rationale of the comprehensive evaluation of risk factors in older patients with AMI (SILVER-AMI) study. BMC Health Serv Res. 2014;14:506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nanna MG, Hajduk AM, Krumholz HM, Murphy TE, Dreyer RP, Alexander KP, Geda M, Tsang S, Welty FK, Safdar B, Lakshminarayan DK, Chaudhry SI and Dodson JA. Sex-Based Differences in Presentation, Treatment, and Complications Among Older Adults Hospitalized for Acute Myocardial Infarction: The SILVER-AMI Study. Circ Cardiovasc Qual Outcomes. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hajduk AM, Saczynski JS, Tsang S, Geda ME, Dodson JA, Ouellet GM, Goldberg RJ and Chaudhry SI. Presentation, Treatment, and Outcomes of Older Adults Hospitalized for Acute Myocardial Infarction According to Cognitive Status: The SILVER-AMI Study. Am J Med. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gupta A, Tsang S, Hajduk A, Krumholz HM, Nanna MG, Green P, Dodson JA and Chaudhry SI. Presentation, Treatment, and Outcomes of the Oldest-Old Patients with Acute Myocardial Infarction: The SILVER-AMI Study. Am J Med. 2021;134:95–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thygesen K, Alpert JS, Jaffe AS, et al. Third universal definition of myocardial infarction. J Am Coll Cardiol. 2012;60:1581–98. [DOI] [PubMed] [Google Scholar]
- 14.Savva GM, Donoghue OA, Horgan F, O’Regan C, Cronin H and Kenny RA. Using timed up-and-go to identify frail members of the older population. J Gerontol A Biol Sci Med Sci. 2013;68:441–6. [DOI] [PubMed] [Google Scholar]
- 15.Moylan T, Das K, Gibb A, Hill A, Kane A, Lee C, Toye D, Wolstencroft K, Fail M and Stott DJ. Assessment of cognitive function in older hospital inpatients: is the Telephone Interview for Cognitive Status (TICS-M) a useful alternative to the Mini Mental State Examination? Int J Geriatr Psychiatry. 2004;19:1008–9. [DOI] [PubMed] [Google Scholar]
- 16.Upton G and Cook I A Dictionary of Statistics (3 ed.) Oxford, UK: Oxford University Press; 2014, page 323. [Google Scholar]
- 17.Politis DM, Romano JP, and Wolf M Subsampling. New York: Springer; 1999. [Google Scholar]
- 18.Brush JE Jr., Krumholz HM, Greene EJ and Dreyer RP. Sex Differences in Symptom Phenotypes Among Patients With Acute Myocardial Infarction. Circ Cardiovasc Qual Outcomes. 2020;13:e005948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pope JH, Aufderheide TP, Ruthazer R, Woolard RH, Feldman JA, Beshansky JR, Griffith JL and Selker HP. Missed diagnoses of acute cardiac ischemia in the emergency department. The New England journal of medicine. 2000;342:1163–70. [DOI] [PubMed] [Google Scholar]
- 20.Vaccarino V, Rathore SS, Wenger NK, Frederick PD, Abramson JL, Barron HV, Manhapra A, Mallik S, Krumholz HM and National Registry of Myocardial Infarction I. Sex and racial differences in the management of acute myocardial infarction, 1994 through 2002. The New England journal of medicine. 2005;353:671–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Custers EJ, Regehr G and Norman GR. Mental representations of medical diagnostic knowledge: a review. Acad Med. 1996;71:S55–61. [DOI] [PubMed] [Google Scholar]
- 22.Patel VL, Groen GJ. Knowledge based solution strategies in medical reasoning. Cognitive Science. 1986;10(1):91–116. [Google Scholar]
- 23.Brooks LR, Norman GR and Allen SW. Role of specific similarity in a medical diagnostic task. J Exp Psychol Gen. 1991;120:278–87 [DOI] [PubMed] [Google Scholar]
- 24.Norman G, Young M and Brooks L. Non-analytical models of clinical reasoning: the role of experience. Med Educ. 2007;41:1140–5 [DOI] [PubMed] [Google Scholar]
- 25.Tulving E, Episodic and semantic memory in Tulving E, Donaldson W, Bower GH. Organization of memory New York,: Academic Press; 1972. [Google Scholar]
- 26.Weber EU, Bockenholt U, Hilton DJ and Wallace B. Determinants of diagnostic hypothesis generation: effects of information, base rates, and experience. J Exp Psychol Learn Mem Cogn. 1993;19:1151–64. [DOI] [PubMed] [Google Scholar]
- 27.Brush JE Jr., Sherbino J and Norman GR. How Expert Clinicians Intuitively Recognize a Medical Diagnosis. Am J Med. 2017;130:629–634. [DOI] [PubMed] [Google Scholar]
- 28.Evans JS. Dual-processing accounts of reasoning, judgment, and social cognition. Annu Rev Psychol. 2008;59:255–78. [DOI] [PubMed] [Google Scholar]
- 29.Kahneman D: Thinking, fast and slow. New York: Farrar, Straus and Giroux; 2011. [Google Scholar]
- 30.Logan GD Toward an instance theory of automatization. Psychological Review. 1988; 95(4), 492–527. [Google Scholar]
- 31.Shiffrin RM and Schneider W. Automatic and controlled processing revisited. Psychol Rev. 1984;91:269–76. [PubMed] [Google Scholar]
- 32.Ericsson KA. The Cambridge handbook of expertise and expert performance. Second edition. ed. Cambridge, United Kingdom; New York, NY, USA: Cambridge University Press; 2018. [Google Scholar]
- 33.National Academies of Science, Engineering, and Medicine. 2015. Improving Diagnosis in Health Care. Washington, DC: The National Academies Press. 10.17226/2194 [DOI] [Google Scholar]
- 34.Regehr G and Norman GR. Issues in cognitive psychology: implications for professional education. Acad Med. 1996;71:988–1001. [DOI] [PubMed] [Google Scholar]
- 35.Norman G Research in medical education: three decades of progress. BMJ. 2002;324:1560–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Eva KW. What every teacher needs to know about clinical reasoning. Med Educ 2004; 39:98–106. [DOI] [PubMed] [Google Scholar]
- 37.Bowen JL. Educational strategies to promote clinical diagnostic reasoning. N Engl J Med. 2006;355:2217–25. [DOI] [PubMed] [Google Scholar]
- 38.Schmidt HG and Mamede S. How to improve the teaching of clinical reasoning: a narrative review and a proposal. Med Educ. 2015;49:961–73. [DOI] [PubMed] [Google Scholar]
- 39.Ross BH (1987). This is like that: The use of earlier problems and the separation of similarity effects. Journal of Experimental Psychology: Learning, Memory, and Cognition, 13(4), 629–639. [Google Scholar]
- 40.Eva KW, Neville AJ and Norman GR. Exploring the etiology of content specificity: factors influencing analogic transfer and problem solving. Acad Med. 1998;73:S1–5. [DOI] [PubMed] [Google Scholar]
- 41.Fuster V, Halperin JL, Williams ES, Cho NR, Iobst WF, Mukherjee D and Vaishnava P. COCATS 4 Task Force 1: Training in Ambulatory, Consultative, and Longitudinal Cardiovascular Care. J Am Coll Cardiol. 2015;65:1734–53. [DOI] [PubMed] [Google Scholar]
- 42.Brush JE Jr. and Oetgen WJ. Maintenance of Competence in Cardiovascular Training and Practices: Worth the Effort? Methodist Debakey Cardiovasc J. 2020;16:199–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Berner ES, Webster GD, Shugerman AA, Jackson JR, Algina J, Baker AL, Ball EV, Cobbs CG, Dennis VW, Frenkel EP and et al. Performance of four computer-based diagnostic systems. N Engl J Med. 1994;330:1792–6. [DOI] [PubMed] [Google Scholar]
- 44.Friedman CP, Elstein AS, Wolf FM, Murphy GC, Franz TM, Heckerling PS, Fine PL, Miller TM and Abraham V. Enhancement of clinicians’ diagnostic reasoning by computer-based consultation: a multisite study of 2 systems. JAMA. 1999;282: 1851–6. [DOI] [PubMed] [Google Scholar]
- 45.Graber ML, Kissam S, Payne VL, Meyer AN, Sorensen A, Lenfestey N, Tant E, Henriksen K, Labresh K and Singh H. Cognitive interventions to reduce diagnostic error: a narrative review. BMJ Qual Saf. 2012;21:535–57. [DOI] [PubMed] [Google Scholar]