Abstract
Background
Population‐based data about cerebral venous sinus thrombosis (CVST) are limited.
Objectives
To investigate the epidemiology of CVST in the United States.
Patients/Methods
Three administrative data systems were analyzed: the 2018 Healthcare Cost and Utilization Project National Inpatient Sample (NIS) the 2019 IBM MarketScan Commercial and Medicare Supplemental Claims Database, and the 2019 IBM MarketScan Multi‐state Medicaid Database. CVST, thrombocytopenia, and numerous comorbidities were identified using the International Classification of Diseases, Tenth Revision, Clinical Modification codes. Incidence rates of CVST and CVST with thrombocytopenia were estimated (per 100,000 total US population [NIS] and per 100,000 population aged 0 to 64 years covered by relevant contributing health plans [MarketScan samples]). Comorbidity prevalence was estimated among CVST cases versus total inpatients in the NIS sample. Recent pregnancy prevalence was estimated for the Commercial sample.
Results
Incidence rates of CVST in NIS, Commercial, and Medicaid samples were 2.85, 2.45, and 3.16, respectively. Incidence rates of CVST with thrombocytopenia were 0.21, 0.22, and 0.16, respectively. In all samples, CVST incidence increased with age; however, peak incidence was reached at younger ages in females than males. Compared with the general inpatient population, persons with CVST had higher prevalences of hemorrhagic stroke, ischemic stroke, other venous thromboembolism (VTE), central nervous system infection, head or neck infection, prior VTE, thrombophilia, malignancy, head injury, hemorrhagic disorder, and connective tissue disorders. Women aged 18 to 49 years with CVST had a higher pregnancy prevalence than the same‐aged general population.
Conclusions
Our findings provide recent and comprehensive data on the epidemiology of CVST and CVST with thrombocytopenia.
Keywords: comorbidity, hemorrhagic stroke, incidence, intracranial sinus thrombosis, ischemic stroke thrombocytopenia, prevalence, United States
Essentials.
Comprehensive analyses of the epidemiology of cerebral venous sinus thrombosis (CVST) are needed.
Three large administrative data systems were used to investigate epidemiology of CVST in the United States.
Incidence of CVST was ≈3 in 100,000. Incidence of CVST with thrombocytopenia was lower, at ≈0.2 in 100,000.
Our findings have applicability in understanding the epidemiology of CVST and CVST with thrombocytopenia.
1. INTRODUCTION
Beginning in February 2021, cases of cerebral venous sinus thrombosis (CVST), a rare type of thrombosis that occurs in the venous sinuses of the brain, were reported in several European countries in persons who had recently received the Oxford‐AstraZeneca coronavirus disease 2019 (COVID‐19) vaccine (ChAdOx1 nCoV‐19). 1 , 2 , 3 Those affected also had thrombocytopenia, and many had high levels of antibodies against antigenic complexes of platelet factor 4, a finding seen in heparin‐induced thrombocytopenia (HIT); however, the patients in the vaccine‐associated case clusters had not received heparin. Investigators described the condition as vaccine‐induced immune thrombotic thrombocytopenia (VITT). 1 , 2 From March 2 through April 25, 2021, 17 cases of thrombosis with thrombocytopenia syndrome (TTS) after receipt of the Johnson & Johnson/Janssen COVID‐19 vaccine (Ad26.COV2.S) were reported to the Vaccine Adverse Event Reporting System, a national program to monitor vaccine safety in the United States. 4 , 5 Fourteen of 17 cases had CVST with thrombocytopenia. CVST has also been reported in association with COVID‐19 infection up to 21 days after diagnosis 6 , 7 , 8 In one study, the incidence of CVST among persons confirmed to have COVID‐19 was more than twice as high as the CVST incidence in persons with influenza and more than six times as high as the incidence in persons who had received a messenger RNA COVID‐19 vaccine. 9
Limited data are available on the epidemiology of CVST in the general population. Incidence estimates from studies of various European and Australian populations range from 1.30 to 1.57 per 100,000 persons per year. 10 , 11 , 12 In the United States, there is a reported increasing trend of the incidence of CVST from 1.37 per 100,000 in 2005 to 2.62 per 100,000 in 2016 among hospitalized adults aged ≥18 year. 13 More in‐depth analyses of data available for two states (New York and Florida) indicated that these trends remained after adjustment for age and sex. 13 In stratified analyses, incidence rates were highest among women aged 18 to 44 years. 13
CVST accounts for an estimated 0.5% to 0.7% of all strokes, and, in contrast to arterial strokes, CVST has been observed more commonly in younger adults and children. 14 Among adults, it is also much more commonly reported among women than men, with an estimated female‐to‐male ratio of 3:1. 10 , 13 Among children, the sex ratio appears balanced, likely due to the absence of sex‐specific risk factors among children. 15 , 16 Risk factors reported in association with CVST include thrombophilia (both genetic and acquired); infection; trauma; malignancy; medications such as steroids; inflammatory, endocrine, and hematologic disorders; and sex‐specific risk factors such as pregnancy and oral contraceptives. 17 , 18 However, information on CVST risk factors is primarily from studies assessing CVST clinical cases reported to registries without comparison to the prevalence of medical risk factors in the general hospitalized population.
More recent and comprehensive analyses of the epidemiology of CVST in the general US population are needed to better understand the incidence, demographic profile, and comorbidities associated with the very rare but serious subset of CVST cases—those with co‐occurring thrombocytopenia. Therefore, we conducted analyses of persons hospitalized with CVST using data from three large administrative data systems. The use of multiple data sources allowed for side‐by‐side comparisons of data obtained from independent samples. The large sample sizes of all three data sources allowed for stable and in‐depth analyses of the incidence of CVST. Additionally, sample sizes from all three data sources allowed for assessment of the incidence of CVST with thrombocytopenia, and one of the data sources was sufficiently large to allow for more comprehensive analyses of both outcomes.
2. METHODS
2.1. Data sources
This study includes analyses from three large administrative databases, each representing a large share of the US population: the Agency for Healthcare Research and Quality’s Healthcare Cost and Utilization (HCUP) National Inpatient Sample (NIS), 19 the IBM MarketScan Commercial and Medicare Supplemental Claims Database, and the IBM MarketScan Multi‐state Medicaid Database. 20 The NIS is the largest publicly available all‐payer inpatient health care database designed to produce US regional and national estimates of inpatient use, covering >97% of the total US population who receive care in community hospitals, which exclude rehabilitation, long‐term care, substance abuse, and psychiatric facilities as well as federally owned facilities such as those of the Veterans Administration and Indian Health Service. 19 The NIS is a 20% stratified sample of discharge records from all HCUP‐participating hospitals. The annual sample of >7 million hospital stays can be weighted to generate nationally representative estimates for >35 million annual hospitalizations. 21 The MarketScan Commercial and Medicare Supplemental Claims Database is a multiyear, patient‐level database consisting of medical claims data from >85 million persons covered by employer‐sponsored commercial health plans throughout the United States. The MarketScan Multi‐state Medicaid Database is a multiyear, patient‐level database consisting of medical claims data from over 24 million Medicaid enrollees from 5 to 15 states, with the number of states contributing data varying by year. 20
2.2. Samples for current analyses
The current study NIS sample includes persons of all ages who had a 2018 hospitalization. The study MarketScan Commercial and Medicaid samples include persons aged 0 to 64 years who were continuously enrolled in a health plan for 12 months during 2019, the most recent full year of data available. The data for both samples are limited to persons aged <65 years because the MarketScan data sets include only a subset of Medicare enrollees. Medicare claims paid by Medicare programs for persons not enrolled in a Medicare supplemental plan are not included in the MarketScan data sets. The Commercial and Medicare Supplemental Claims (version 100% Sample 6 Plus Years 1/1/2013–7/31/2020) and Multi‐state Medicaid Claims (version 10 Plus Years 1/1/2009–12/31/2019) data sources were accessed through the IBM MarketScan Treatment Pathways platform. 20 , 22
Because 2018 is the most recent year of NIS data available, the time frame for the NIS does not align with the two MarketScan samples based on 2019 data. Nonetheless, we assume there is little variation in the profile of CVST events occurring in the United States in 2018 versus 2019. Of note, we empirically checked this assumption; CVST incidence estimates produced using 2018 MarketScan samples were similar to estimates produced using 2019 data (data not shown). Thus, any differences observed between estimates generated from the three samples are assumed to be primarily attributable to differences in the underlying populations covered by the samples rather than data year.
2.3. Definition of CVST
Analyses of all three samples examined events with one or more of the following International Classification of Diseases, Tenth Revision, Clinical Modification (ICD‐10‐CM) codes listed anywhere on the inpatient claims record: I63.6, G08.X, I67.6, O22.5X, or O87.3. This coding scheme has been previously validated in a large clinical sample 23 and has been used in a previous study of CVST incidence 13 in the United States. For both MarketScan samples, only the first CVST event in 2019 was counted. It is not possible to identify whether more than one CVST event was identified per person in the NIS. We found that ≈15% of persons with a CVST event in the MarketScan Commercial sample had a subsequent inpatient hospitalization claim with a CVST code within the same calendar year. Thus, given that the NIS is based on a 20% sample of hospitalizations and the vast majority of persons with CVST (85%) are estimated to have only one CVST hospitalization in a given year, the likelihood of identifying multiple events for the same person in the NIS is estimated to be low.
We examined CVST overall and CVST with co‐occurring thrombocytopenia. Thrombocytopenia was defined as one or more of the following ICD‐10‐CM codes listed anywhere on the inpatient claims record: D69.3, D69.41, D69.42, D69.49, D69.51, D69.59, D69.6, D75.82, or P61.0. Thrombocytopenia was defined solely based on ICD‐10‐CM codes for thrombocytopenia, as laboratory test results were not available. Because inpatient claims for different providers may be recorded separately, CVST with co‐occurring thrombocytopenia was defined as the presence of an inpatient claim with a thrombocytopenia code within 30 days of the index CVST inpatient claim for MarketScan samples.
2.4. Statistical analyses
2.4.1. Estimation of incidence rates
All three data sources allow for estimation of population‐specific rates.
The NIS datafile includes sampling weights to allow for generation of nationally representative estimates of cases. These case counts in conjunction with 2018 US Census data were used to generate incidence rates for the US population. Census data were obtained from 2010 to 2019 single‐race Vintage 2019 postcensal estimates of the July 1 resident population. 24 These estimates were prepared and released by the US Census Bureau on June 25, 2020.
MarketScan Commercial and Multi‐state Medicaid databases allow for direct generation of population rates. While MarketScan samples represent large numbers of persons receiving health care in the United States, rates generated from MarketScan are not nationally representative. The study populations are limited to persons covered under a contributing health care plan. Incidence rates were calculated using case counts and counts of plan enrollees meeting the inclusion criteria specified above.
Incidence rates and the corresponding 95% confidence intervals (CIs) for both CVST and CVST with thrombocytopenia were generated from all three samples, assuming a Poisson distribution. Rates stratified by demographic factors were also generated. However, in keeping with HCUP data reporting standards, rates with case sample sizes <10 are suppressed.
Sample sizes were sufficient to generate and present CVST total rates and rates for sex and age groups from all three samples. Age groups were 0 to 17 years, 18 to 29 years, 30 to 39 years, 40 to 49 years, 50 to 64 years, and 65+ years (NIS only). Rates were also generated for more refined age‐and‐sex groups; however, a few of these rates were suppressed for the Medicaid sample due to small numbers. For the NIS sample, race/ethnicity‐specific rates were also generated. Mutually exclusive race/ethnicity categories were non‐Hispanic White, non‐Hispanic Black, Hispanic, non‐Hispanic Asian/Pacific Islander, Non‐Hispanic Native American, and Other/Multiple Race. Although race/ethnicity data are also available for the Medicaid sample, small sample sizes preclude presentation. Race/ethnicity data were not available for the Commercial sample.
Sample sizes were sufficient to generate and present total population incidence rates for CVST with thrombocytopenia from all three samples. However, small sample sizes preclude presentation of rates within nearly all demographic subgroups for both MarketScan samples; thus, stratified rates are presented for the NIS sample only.
2.4.2. Assessment of illness course
We examined several measures related to illness severity of CVST and CVST with thrombocytopenia: length of hospital stay, admission to intensive care unit (ICU) during hospital stay, and death during hospitalization. Information on admission to the ICU was not available for the NIS. Information on death during hospitalization was not available for the Commercial sample. For the NIS, sample sizes were sufficient to additionally examine length of stay and death during hospitalization within age‐sex subgroups.
2.4.3. Assessment of comorbidities
From the NIS sample, the prevalence of select co‐occurring and comorbid conditions was estimated for persons with CVST and CVST with thrombocytopenia, some of which may cause thrombocytopenia. The conditions examined included hemorrhagic stroke; ischemic stroke; myocardial infarction; other arterial thrombosis; other venous thromboembolism (VTE); meningitis, encephalitis, or other central nervous system (CNS) infection; head or neck infection; prior VTE; thrombophilia; malignancy; head injury; thyroid disorder; cardiovascular disease; hypertension; obesity; type 2 diabetes; hemorrhagic disorder (includes platelet disorders, von Willebrand disease, and other coagulation deficiency); and systemic lupus erythematosus (SLE) or connective tissue disorder including arteritis, renal disease, liver disease, and thrombocytopenia‐inducing hemolytic or hemostatic conditions ([HIT], disseminated intravascular coagulation [DIC], antiphospholipid syndrome, paroxysmal nocturnal hemoglobinuria, thrombotic thrombocytopenic purpura, and hemolytic uremic syndrome). ICD‐10‐CM codes used to define each co‐occurring or comorbid condition are detailed in Table S1. Some ICD‐10‐CM codes were defined as more than one condition.
TABLE 1.
Number and population rates of hospitalized cerebral venous sinus thrombosis in the United States
| Healthcare Cost and Utilization 2018 National Inpatient Sample | 2019 MarketScan Commercial Sample b | 2019 MarketScan Medicaid Sample c | |||||
|---|---|---|---|---|---|---|---|
| Unweighted, N a | Weighted N | Cases per 100,000 population (95% CI) d | N a | Cases per 100,000 population (95% CI) e | N a | Cases per 100,000 population (95% CI) f | |
| Total | 1862 | 9310 | 2.85 (2.79‐2.91) | 418 | 2.45 (2.22‐2.69) | 235 | 3.16 (2.77‐3.59) |
| Age | |||||||
| 0‐17 y | 249 | 1245 | 1.70 (1.61‐1.80) | 50 | 1.23 (0.91‐1.62) | 73 | 1.67 (1.31‐2.10) |
| 18‐29 y | 303 | 1515 | 2.81 (2.67‐2.96) | 79 | 2.62 (2.07‐3.26) | 49 | 4.31 (3.19‐5.70) |
| 30‐39 y | 229 | 1145 | 2.62 (2.47‐2.78) | 72 | 2.76 (2.16‐3.48) | 40 | 4.82 (3.44‐6.56) |
| 40‐49 y | 265 | 1325 | 3.28 (3.11‐3.46) | 92 | 3.17 (2.56‐3.89) | 27 | 5.40 (3.56‐7.86) |
| 50‐64 y | 373 | 1865 | 2.96 (2.82‐3.09) | 125 | 2.78 (2.32‐3.31) | 46 | 7.60 (5.56‐10.13) |
| 65+ y | 443 | 2215 | 4.23 (4.06‐4.41) | NA | NA | NA | NA |
| Sex | |||||||
| Male | 845 | 4225 | 2.63 (2.55‐2.71) | 158 | 1.90 (1.61‐2.22) | 105 | 2.54 (2.08‐3.08) |
| Female | 1017 | 5085 | 3.07 (2.98‐3.15) | 260 | 2.97 (2.62‐3.35) | 130 | 3.93 (3.28‐4.67) |
| Age and sex | |||||||
| 0‐17 y | |||||||
| Male | 163 | 815 | 2.18 (2.03‐2.33) | 29 | 1.40 (0.93‐2.00) | 46 | 2.04 (1.49‐2.72) |
| Female | 86 | 430 | 1.20 (1.09‐1.32) | 21 | 1.05 (0.65‐1.61) | 27 | 1.28 (0.84‐1.86) |
| 18‐29 y | |||||||
| Male | 97 | 485 | 1.76 (1.61‐1.93) | 25 | 1.67 (1.08‐2.46) | 10 | 2.67 (1.28‐4.91) |
| Female | 206 | 1030 | 3.91 (3.67‐4.16) | 54 | 3.56 (2.68‐4.65) | 39 | 5.12 (3.64‐7.00) |
| 30‐39 y | |||||||
| Male | 68 | 340 | 1.55 (1.39‐1.72) | 14 | 1.13 (0.62‐1.90) | * | * |
| Female | 161 | 805 | 3.71 (3.46‐3.98) | 58 | 4.25 (3.22‐5.49) | * | * |
| 40‐49 y | |||||||
| Male | 99 | 495 | 2.47 (2.26‐2.70) | 28 | 2.03 (1.35‐2.93) | * | * |
| Female | 166 | 830 | 4.07 (3.80‐4.36) | 64 | 4.21 (3.24‐5.37) | * | * |
| 50‐64 y | |||||||
| Male | 207 | 1035 | 3.38 (3.18‐3.59) | 62 | 2.90 (2.23‐3.72) | 31 | 11.60 (7.88‐16.46) |
| Female | 166 | 830 | 2.56 (2.39‐2.74) | 63 | 2.67 (2.05‐3.42) | 15 | 4.43 (2.48‐7.31) |
| 65+ y | |||||||
| Male | 211 | 1055 | 4.53 (4.26‐4.81) | NA | NA | NA | NA |
| Female | 232 | 1160 | 3.99 (3.76‐4.22) | NA | NA | NA | NA |
| Race/ethnicity | |||||||
| White | 1132 | 5660 | 2.87 (2.79‐2.94) | NA | NA | 96 | 2.87 (2.32‐3.50) |
| Black | 263 | 1315 | 3.22 (3.05‐3.40) | NA | NA | 68 | 2.93 (2.28‐3.72) |
| Hispanic | 248 | 1240 | 2.08 (1.97‐2.20) | NA | NA | 17 | 2.30 (1.34‐2.68) |
| Asian/Pacific Islander | 54 | 270 | 1.41 (1.25‐1.59) | NA | NA | NA | NA |
| Native American | 12 | 60 | 2.48 (1.89‐3.19) | NA | NA | NA | NA |
| Other | 89 | 445 | 6.27 (5.70‐6.88) | NA | NA | 25 | 5.09 (3.29‐7.51) |
*Sparse data (cell size <10) precluded reporting data.
Abbreviations: CI, confidence interval; NA, not applicable (data not available for sample).
International Classification of Disease, tenth revision, clinical modification code for CVST listed anywhere on inpatient claim record.
MarketScan® Commercial sample sampled from the IBM Watson Health® MarketScan® Commercial and Medicare Supplemental Claims database (version 100% Sample 6 Plus Years 1/1/2013‐7/31/2020) and included persons aged 0 to 64 years who were continuously enrolled in an insurance plan for 12 months during 2019. Data were accessed through the IBM Watson Health® Treatment Pathways platform.
MarketScan Medicaid sample sampled from the IBM Watson Health MarketScan Multi‐state Medicaid Claims database (version 10 Plus Years 1/1/2009‐12/31/2019) and included persons aged 0 to 64 years who were continuously enrolled in an insurance plan for 12 months during 2019. Data were accessed through the IBM Watson Health® Treatment Pathways platform.
Rate calculated as number of cases divided by population estimate derived from 2018 US population estimates reported in the 2010‐2019 Single‐Race Vintage 2019 Postcensal Estimates of the July 1 Resident Population.
Rate calculated as number of cases divided by total number of persons who were continuously enrolled in an insurance plan for 12 months during 2019, as recorded in the MarketScan® Commercial and Medicare Supplemental Claims database.
Rate calculated as number of cases divided by total number of persons who were continuously enrolled in an insurance plan for 12 months during 2019, as recorded in the MarketScan® Multi‐state Medicaid Claims database.
Among persons with CVST and CVST with thrombocytopenia, prevalence of each co‐occurring or comorbid condition and the corresponding 95% CIs were estimated using the Wald method. 25 Prevalence and 95% CIs of the same conditions were also estimated for all inpatient admissions captured in the NIS for comparison. Prevalence estimates for comorbid conditions among persons with CVST and CVST with thrombocytopenia were compared with the prevalence estimates among persons in the general inpatient population using the prevalence odds ratio. 25
Prevalence of co‐occurring and comorbid conditions among persons with CVST and for all inpatient admissions were also assessed among women aged 18 to 49 years, men aged 18 to 49 years, women aged 50+ years, and men aged 50+ years. Small sample sizes precluded stratified analyses for CVST with thrombocytopenia.
Small sample sizes for many co‐occurring and comorbid conditions precluded analyses in both MarketScan samples. However, the Commercial sample allowed us to examine two sex‐specific risk factors: pregnancy‐related events and use of estrogen receptor modulator medications. Recent pregnancy was defined using ICD‐10‐CM codes indicating pregnancy or postpartum status (Table S1); women with two outpatient claims at least 30 days apart or one inpatient claim with a pregnancy‐related code were considered to be pregnant during the study period. Estrogen modulator medications, including oral contraceptives, hormonal replacement therapy, and other estrogen medication use, was defined on the basis of prescription claims data. Pregnancy‐related events were assessed for women aged 18 to 49 years and estrogen receptor modulator medications were assessed for women aged ≥18 years. Prevalence and 95% CIs of pregnancy and use of estrogen modulator medications were estimated among women with CVST, among women with CVST and thrombocytopenia, and among all women eligible for the Commercial analytic sample. Prevalence estimates among women with CVST and CVST with thrombocytopenia were compared with the prevalence estimates among all women in the analytic sample using the prevalence odds ratio.
3. RESULTS
3.1. Overview of CVST cases from each data source
The 2018 NIS sample included 1862 hospitalizations for CVST, of whom 137 (7.36%) also had thrombocytopenia. These cases represent 9310 hospitalizations for CVST in the United States in 2018 and 685 hospitalizations for CVST with thrombocytopenia. The 2019 Commercial sample included 418 cases of CVST among persons aged 0 to 64 years, of whom 37 (8.85%) also had thrombocytopenia. The 2019 Medicaid sample included 235 cases of CVST among persons 0 to 64 years, of whom 12 (5.11%) also had thrombocytopenia.
3.2. Estimation of incidence rates
Incidence estimates for CVST from NIS, Commercial, and Medicaid samples were 2.85, 2.45, and 3.16 (all per 100,000 population), respectively (Table 1). However, it should be acknowledged that the Commercial and Medicaid samples did not include persons aged ≥65 years. For each of the age categories for adults aged 18 to 64 years, estimates from the NIS and Commercial samples were similar, while estimates from the Medicaid sample were higher than both NIS and Commercial samples. The overall CVST incidence estimates for persons aged 18 to 64 years from NIS, Commercial, and Medicaid samples were 2.91 (95% CI, 2.83‐2.98), 2.83 (95% CI, 2.54‐3.13), and 5.27 (95% CI, 4.49‐6.15), respectively.
In all samples, incidence estimates were higher for females than males and also generally increased with age. Moreover, the pattern of results by age varied by sex (Table 1 and Figure 1). In all three samples, males under 18 years of age had higher incidence estimates than comparably aged females (46%‐81% higher estimates in males). Conversely, in each sample, the estimate for women aged 18 to 29 years was decidedly higher than (i) the estimate for men aged 18 to 29 years and (ii) the estimates for both males and females aged <18 years. Incidence estimates for women aged 18–29 years from the three samples ranged from 3.56 to 5.12 per 100,000. Despite some slight fluctuations, in each sample, incidence estimates were similarly high for women across all adult age groups. In men, incidence estimates remained low at younger ages and began to increase at age 40 to 49 years. At ages ≥50, estimates for men were higher than those for women in all samples, albeit with overlapping CIs in the Commercial sample.
FIGURE 1.
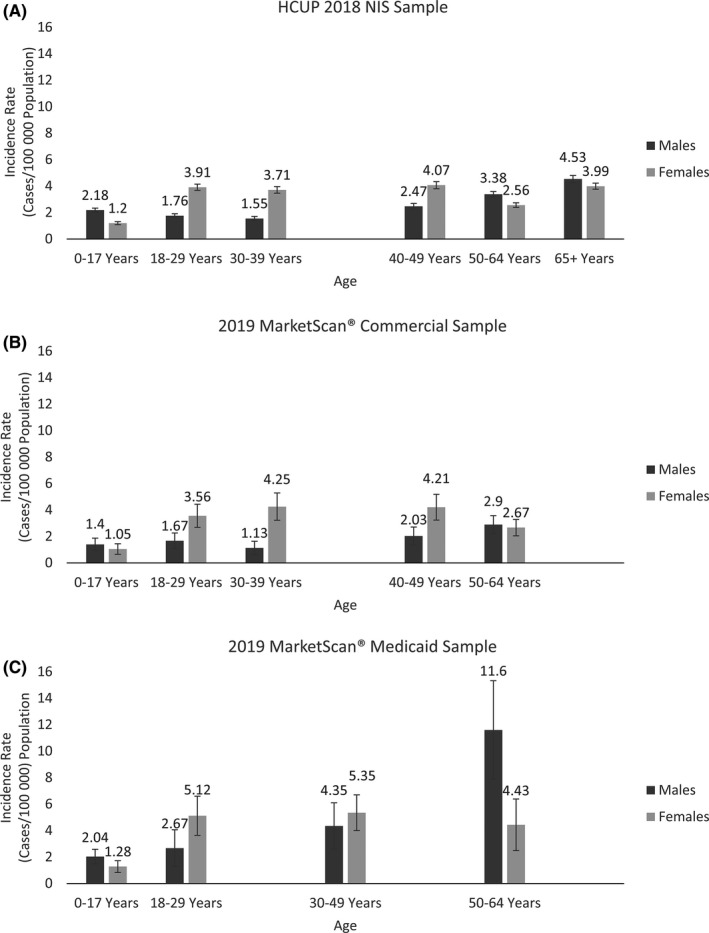
Age‐ and sex‐specific incidence rates of cerebral venous sinus thrombosis (CVST) in the United States. (A) Age‐ and sex‐specific incidence rates of CVSTa in the United States using data from the Healthcare Cost and Utilization (HCUP), 2018 National Inpatient Sample (NIS), Agency for Healthcare Research and Quality. (B) Age‐ and sex‐specific incidence rates of CVSTa among persons aged 0 to 64 years continually enrolled in an insurance plan in 2019, as recorded in the IBM Watson Health MarketScan Commercial and Medicare Supplemental Claims database (version 100% Sample 6 Plus Years 1/1/2013‐7/31/2020). (C) Age‐ and sex‐specific incidence ratesb of CVSTa among persons aged 0 to 64 years continually enrolled in an insurance plan in 2019, as recorded in the MarketScan® Medicaid sample sampled from the IBM Watson Health MarketScan Multi‐state Medicaid Claims database (version 10 Plus Years 1/1/2009‐12/31/2019). aInternational Classification of Diseases, Tenth Revision, Clinical Modification codes for CVST listed anywhere on inpatient claim record. bDue to sparse data (cell size <10), age groups 30 to 39 years and 40 to 49 years were combined
In the NIS sample, CVST incidence estimates were significantly lower in persons who were Hispanic and Asian/Pacific Islander than in persons who were non‐Hispanic White. Estimates were significantly higher in persons classified as non‐Hispanic Black or other/mixed race than in persons who were non‐Hispanic White.
Incidence estimates from NIS, Commercial, and Medicaid samples for CVST with thrombocytopenia were 0.21, 0.22, and 0.16 (all per 100,000), respectively; the 95% CIs around these estimates overlapped (Table 2). Estimates from NIS did not vary by sex but did increase with age and were significantly higher in persons who were non‐Hispanic Black than non‐Hispanic White. Sample size was insufficient to assess age‐sex categories for this outcome.
TABLE 2.
Number and population rates of hospitalized cerebral venous sinus thrombosis with thrombocytopenia in the United States
|
Healthcare Cost and Utilization 2018 National Inpatient Sample |
2019 MarketScan Commercial Sample b | 2019 MarketScan Medicaid Sample c | |||||
|---|---|---|---|---|---|---|---|
| Unweighted, N a | Weighted N | Cases per 100,000 Population (95% CI) d | N a | Cases per 100,000 Population (95% CI) e | N a | Cases per 100, 000 Population (95% CI) f | |
| Total | 137 | 685 | 0.21 (0.19‐0.23) | 37 | 0.22 (0.15‐0.30) | 12 | 0.16 (0.08‐0.28) |
| Sex | |||||||
| Male | 70 | 350 | 0.22 (0.20‐0.24) | 19 | 0.23 (0.14‐0.36) | * | * |
| Female | 67 | 335 | 0.2 (0.18‐0.22) | 18 | 0.21 (0.12‐0.32) | * | * |
| Age | * | ||||||
| 0‐17 y | 31 | 155 | 0.21 (0.18‐0.25) | * | * | * | * |
| 18‐29 y | 15 | 75 | 0.14 (0.11‐0.17) | * | * | * | * |
| 30‐39 y | 13 | 65 | 0.15 (0.11‐0.19) | * | * | * | * |
| 40‐49 y | 23 | 115 | 0.28 (0.24‐0.34) | * | * | * | * |
| 50‐64 y | 27 | 135 | 0.21 (0.18‐0.25) | * | * | * | * |
| 65+ y | 28 | 140 | 0.27 (0.22‐0.32) | NA | NA | NA | NA |
| Race/ethnicity | |||||||
| White | 64 | 320 | 0.16 (0.14‐0.18) | NA | NA | * | * |
| Black | 27 | 135 | 0.33 (0.28‐0.39) | NA | NA | * | * |
| Hispanic | 26 | 130 | 0.22 (0.18‐0.26) | NA | NA | * | * |
| Asian/Pacific Islander | * | * | * | NA | NA | NA | NA |
| Native American | * | * | * | NA | NA | NA | NA |
| Other | * | * | * | NA | NA | * | * |
*Sparse data (cell size <10) precluded reporting data.
Abbreviations: CI, confidence interval; NA, not applicable (data not available for sample).
International Classification of Diseases, Tenth Revision, Clinical Modification codes for CVST and thrombocytopenia listed anywhere on inpatient claim record.
MarketScan Commercial sample sampled from the IBM Watson Health MarketScan Commercial and Medicare Supplemental Claims database (version 100% Sample 6 Plus Years 1/1/2013‐7/31/2020) and included persons aged 0 to 64 years who were continuously enrolled in an insurance plan for 12 months during 2019. Data were accessed through the IBM Watson Health® Treatment Pathways platform.
MarketScan® Medicaid sample sampled from the IBM Watson Health® MarketScan® Multi‐state Medicaid Claims database (version 10 Plus Years 1/1/2009‐12/31/2019) and included persons aged 0 to 64 years who were continuously enrolled in an insurance plan for 12 months during 2019. Data were accessed through the IBM Watson Health® Treatment Pathways platform.
Rate calculated as number of cases divided by population estimate derived from 2018 US population estimates reported in the 2010‐2019 Single‐Race Vintage 2019 Postcensal Estimates of the July 1 Resident Population.
Rate calculated as number of cases divided by total number of persons who were continuously enrolled in an insurance plan for 12 months during 2019, as recorded in the MarketScan Commercial and Medicare Supplemental Claims database.
Rate calculated as number of cases divided by total number of persons who were continuously enrolled in an insurance plan for 12 months during 2019, as recorded in the MarketScan Multi‐state Medicaid Claims database.
3.3. Assessment of illness course
Median length of hospital stay for persons with CVST ranged from 4 to 6 days, depending on the sample (Table 3). Within each sample, length of stay was higher among the subset of persons with CVST with thrombocytopenia. Length of stay was longest for children aged <18 years in the NIS and MarketScan Medicaid samples. There were no significant age‐ or sex‐specific differences in length of stay among adults. Across all strata with sufficient data on age and sex, length of stay was longer for persons with CVST with thrombocytopenia compared with CVST overall, although interquartile ranges overlap. Percentage of deaths during hospitalization, which was available and sufficiently stable only for the NIS sample, was more than twice as high for CVST with thrombocytopenia (10.2%) than for CVST overall (4.4%). ICU data were available only for the MarketScan samples, and the Medicaid sample had limited sample size. Data from the MarketScan Commercial sample indicated high percentages of ICU admission for both persons with CVST and CVST with thrombocytopenia (40.6% and 56.8%, respectively).
TABLE 3.
Health outcome measures among persons hospitalized with cerebral venous sinus thrombosis (CVST) recorded in the Healthcare Cost and Utilization, 2018 National Inpatient Sample
|
Healthcare Cost and Utilization 2018 National Inpatient Sample |
2019 MarketScan Commercial Sample c | 2019 MarketScan Medicaid Sample d | ||||
|---|---|---|---|---|---|---|
| CVST a | CVST with thrombocytopenia b | CVST a | CVST with thrombocytopenia b | CVST a | CVST with thrombocytopenia b | |
| Median length of stay in days (interquartile range) | ||||||
| Overall | 5.2 (2.3‐10.9) | 9.9 (4.7‐20.8) | 4 (2‐9) | 7 (4‐11) | 6 (3‐12) | 10.5 (7‐35.5) |
| Males and females <18 y | 8.9 (3.9‐18.4) | 18.5 (8.3‐46.5) | 5 (3‐9) | * | 9 (4‐20) | * |
| Males 18‐49 y | 4.9 (3.4‐10.5) | 7.5 (3.8‐10.9) | 6 (3‐9) | * | 5 (2‐12.5) | * |
| Females 18‐49 y | 4.1 (1.8‐8.0) | 7.3 (3.5‐10.5) | 3.5 (2‐7) | 5.5 (3‐9) | 5.5 (3‐8) | * |
| Males 50+ y | 5.0 (2.2‐11.7) | 9.5 (4.8‐17.1) | 6 (3‐14) | 7 (4‐16) | 5 (3‐11) | * |
| Females 50+y | 5.7 (2.6‐10.8) | 13.0 (4.3‐23.0) | 4 (2‐10) | * | 7 (4‐14) | * |
| Percentage admitted to intensive care unit | NA | NA | 40.6 | 56.8 | 48.5 | * |
| Percentage of deathsbefore discharge | 4.4 | 10.2 | NA | NA | * | * |
*Sparse data (cell size <10) precluded reporting data.
Abbreviation: NA, not applicable (data not available for sample).
International Classification of Diseases, Tenth Revision, Clinical Modification codes for CVST listed anywhere on inpatient claim record.
International Classification of Diseases, Tenth Revision, Clinical Modification codes for CVST and thrombocytopenia listed anywhere on inpatient claim record.
MarketScan Commercial sample sampled from the IBM Watson Health MarketScan Commercial and Medicare Supplemental Claims database (version 100% Sample 6 Plus Years 1/1/2013‐‐7/31/2020) and included persons aged 0 to 64 years who were continuously enrolled in an insurance plan for 12 months during 2019. Data were accessed through the IBM Watson Health® Treatment Pathways platform.
MarketScan® Medicaid sample sampled from the IBM Watson Health MarketScan Multi‐state Medicaid Claims database (version 10 Plus Years 1/1/2009‐12/31/2019) and included persons aged 0 to 64 years who were continuously enrolled in an insurance plan for 12 months during 2019. Data were accessed through the IBM Watson Health Treatment Pathways platform.
3.4. Assessment of co‐occurring and comorbid conditions
As expected, prevalence of co‐occurring hemorrhagic stroke, ischemic stroke, and other VTE at another location was substantially higher in persons with CVST overall and CVST with thrombocytopenia compared with the general inpatient population (Table 4, Figure 2). Additionally, prevalence of hemorrhagic stroke among persons with CVST with thrombocytopenia was higher than the prevalence among persons with CVST overall. For all subgroups, prevalence estimates of hemorrhagic stroke, ischemic stroke, and other VTE were significantly higher among persons with CVST compared with the general inpatient population (Table S2). However, the prevalence of these conditions was notably higher for women aged 18 to 49 years in comparison to other subgroups.
TABLE 4.
Prevalence of co‐occurring and comorbid conditions among inpatient admissions, overall and among persons hospitalized for cerebral venous sinus thrombosis (CVST) or CVST and thrombocytopenia, in the Healthcare Cost and Utilization, 2018 National Inpatient Sample
|
General inpatient population Weighted N=35,527,481 Weighted % (95% CI) |
CVST b Weighted N=9310 Weighted % (95% CI) |
CVST with thrombocytopenia c Weighted N=685 Weighted % (95% CI) |
|
|---|---|---|---|
| Co‐occurring conditions a | |||
| Thrombocytopenia | 4.3 (4.2‐4.4) | 7.4 (6.1‐8.6) | 100 |
| Hemorrhagic stroke | 0.8 (0.8‐0.8) | 19.3 (17.4‐21.2) | 32.1 (24.4‐39.9) |
| Ischemic stroke | 5.3 (5.2‐5.4) | 25.0 (23.0‐27.1) | 26.3 (19.0‐33.5) |
| Myocardial infarction | 3.3 (3.2‐3.4) | 1.2 (0.7‐1.7) | * |
| Other arterial thrombosis | 0.3 (0.3‐0.3) | 0.8 (0.3‐1.2) | * |
| Other venous thromboembolism | 2.3 (2.2‐2.3) | 17.8 (15.9‐19.6) | 25.6 (18.2‐32.9) |
| Comorbid conditions a | |||
| Meningitis, encephalitis, or other CNS infection | 0.4 (0.4‐0.4) | 8.1 (6.8‐9.4) | 12.4 (7.1‐17.8) |
| Head or neck infection | 1.5 (1.4‐1.5) | 6.7 (5.5‐7.9) | * |
| Prior venous thromboembolism | 5.1 (5.0‐5.2) | 10.2 (8.9‐11.6) | 8.8 (4.2‐13.3) |
| Thrombophilia | 0.6 (0.6‐0.7) | 10.6 (9.2‐12.0) | 11.0 (5.1‐16.9) |
| Malignancy | 8.7 (8.5‐9.0) | 14.5 (12.6‐16.4) | 13.1 (7.5‐18.8) |
| Head injury | 1.6 (1.5‐1.6) | 10.4 (8.7‐12.0) | * |
| Thyroid disorder | 12.0 (11.8‐12.1) | 11.7 (10.2‐13.2) | 16.1 (9.9‐22.2) |
| Cardiovascular disease | 32.6 (32.2‐33.0) | 20.6 (18.7‐22.5) | 31.4 (23.3‐39.4) |
| Hypertension | 49.9 (49.4‐50.4) | 42.5 (40.0‐45.1) | 33.6 (25.9‐41.3) |
| Obesity | 14.9 (14.7‐15.2) | 15.0 (13.3‐16.8) | 13.9 (7.8‐20.0) |
| Type 2 diabetes | 22.2 (22.0‐22.5) | 15.0 (13.3‐16.7) | 15.3 (8.8‐21.8) |
| Hemorrhagic disorder d | 1.3 (1.3‐1.3) | 3.5 (2.7‐4.4) | 9.5 (4.3‐14.7) |
| Systemic lupus erythematosus (SLE) or other connective tissue disorder e | 1.2 (1.2‐1.2) | 2.6 (1.9‐3.3) | * |
| Renal disease | 24.5 (24.2‐24.7) | 14.7 (13.1‐16.3) | 26.3 (19.0‐33.5) |
| Liver disease | 7.1 (7.0‐7.2) | 5.1 (4.0‐6.1) | 11.7 (6.2‐17.1) |
| Thrombocytopenia‐inducing hemolytic or hemostatic conditions f | 0.4 (0.4‐0.5) | 3.1 (2.3‐3.9) | 25.6 (17.8‐33.3) |
| None g | 30.4 (29.9‐30.9) | 15.4 (13.8‐17.0) | 11.0 (5.3‐16.6) |
Note: *Sparse data (cell size <10) precluded reporting data.
International Classification of Diseases, Tenth Revision, Clinical Modification codes indicating co‐occurring or comorbid condition listed anywhere on inpatient claim record.
Among individuals with International Classification of Diseases, Tenth Revision, Clinical Modification code for CVST listed anywhere on inpatient claim record.
Among individuals with International Classification of Diseases, Tenth Revision, Clinical Modification codes for CVST and thrombocytopenia listed anywhere on inpatient claim record.
Includes platelet disorders and other coagulation deficiencies.
Includes arteritis.
Includes heparin‐induced thrombocytopenia, disseminated intravascular coagulation, antiphospholipid syndrome, paroxysmal nocturnal hemoglobinuria, thrombotic thrombocytopenia purpura, and hemolytic uremic syndrome.
None of the comorbid conditions investigated.
FIGURE 2.
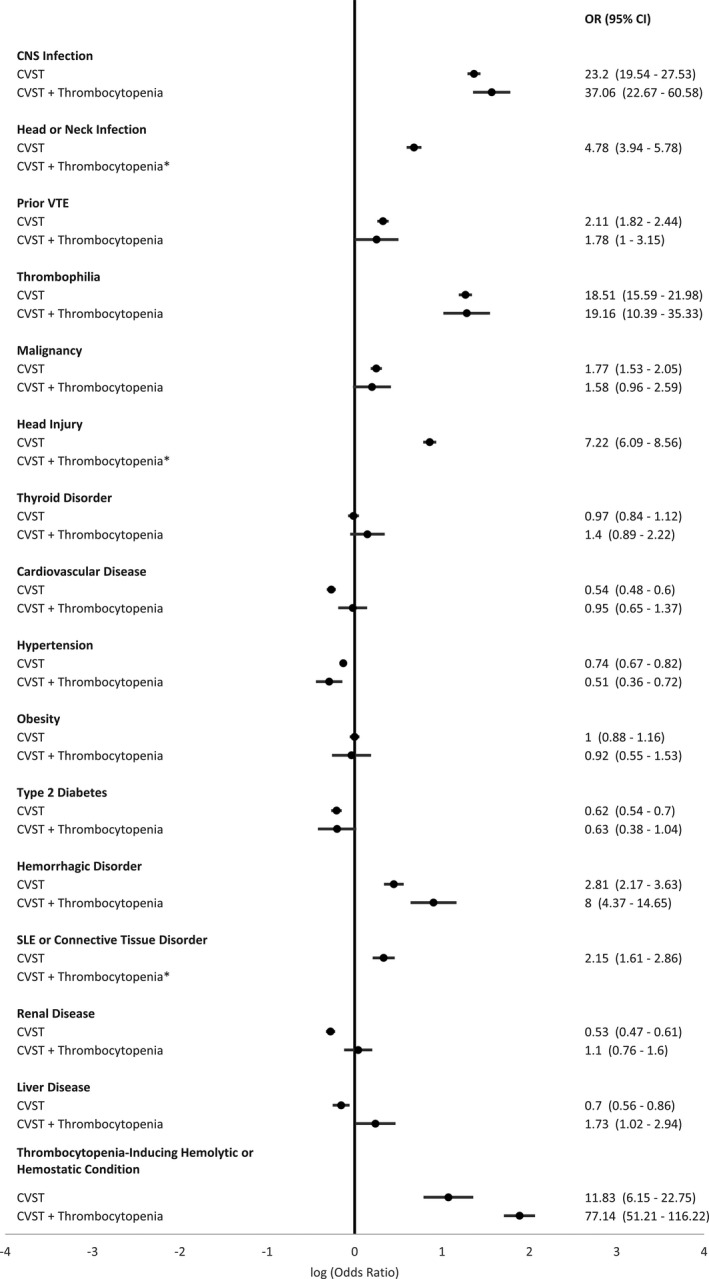
Prevalence odds ratio estimates comparing prevalence of comorbid conditions among hospitalized persons with cerebral venous sinus thrombosis (CVST) or CVST with thrombocytopenia to the general inpatient population, in the Healthcare Cost and Utilization (HCUP), 2018 National Inpatient Sample (NIS). * Sparse data (cell size <10) precluded reporting data
Prevalence of meningitis, encephalitis, or other CNS infection; head or neck infection; prior VTE; thrombophilia; malignancy; head injury; hemorrhagic disorder; SLE or other connective tissue disorder; and thrombocytopenia‐inducing hemolytic or hemostatic conditions were also notably higher in persons with CVST than in the general inpatient population; and for those conditions with sufficient sample size for assessment (meningitis, encephalitis, or other CNS infection, prior VTE, thrombophilia, malignancy, hemorrhagic disorder, and thrombocytopenia‐inducing hemolytic or hemostatic conditions), prevalence was also higher for persons with CVST with thrombocytopenia in comparison to the general population. There was little to no difference in prevalence of thyroid disorder or obesity in persons with CVST than persons in the general inpatient population. Prevalence of cardiovascular disease, hypertension, type 2 diabetes, and renal and liver disease were lower in persons with CVST than in the general inpatient population; however, with the exception of hypertension, estimates for these conditions among the subset of persons with CVST with thrombocytopenia were comparable to estimates for the general inpatient population. We found that a significantly smaller proportion of persons with CVST and CVST with thrombocytopenia had no evidence of any of the comorbidities investigated than the general inpatient population (15.4% [95% CI, 13.8%‐17.0%] and 11.0% [95% CI, 5.3%‐16.6%] vs 30.4% [95% CI, 29.9%‐30.9%]).
Sex‐ and age‐stratified analyses for comorbidity prevalence among persons with CVST compared with the general inpatient population are presented in Figure 3. For all subgroups, prevalence estimates of meningitis, encephalitis, or other CNS infection; head or neck infection; prior VTE; thrombophilia; malignancy; and head injury were significantly higher among persons with CVST compared with the general inpatient population. However, the prevalence odds ratio for prior VTE was notably higher for women aged 18 to 49 years in comparison to other sex‐age groups. Prevalence estimates for hemorrhagic disorders, SLE or other connective tissue disorders, and thrombocytopenia‐inducing hemolytic or hemostatic conditions were higher among women aged 18 to 49 years with CVST compared with the general inpatient population, but, due to sparse data, we were unable to estimate prevalence of these conditions for other subgroups. Among males of all ages and women aged >50 years, cardiovascular disease, hypertension, obesity, and type 2 diabetes were less common among persons with CVST than the general inpatient population. In contrast, these conditions were more common among women aged 18 to 49 years with CVST than the female inpatient population aged 18 to 49 years; however, this difference was statistically significant only for obesity. For all subgroups, prevalence estimates for renal and liver disease were lower among persons with CVST compared with the general inpatient population for all subgroups.
FIGURE 3.
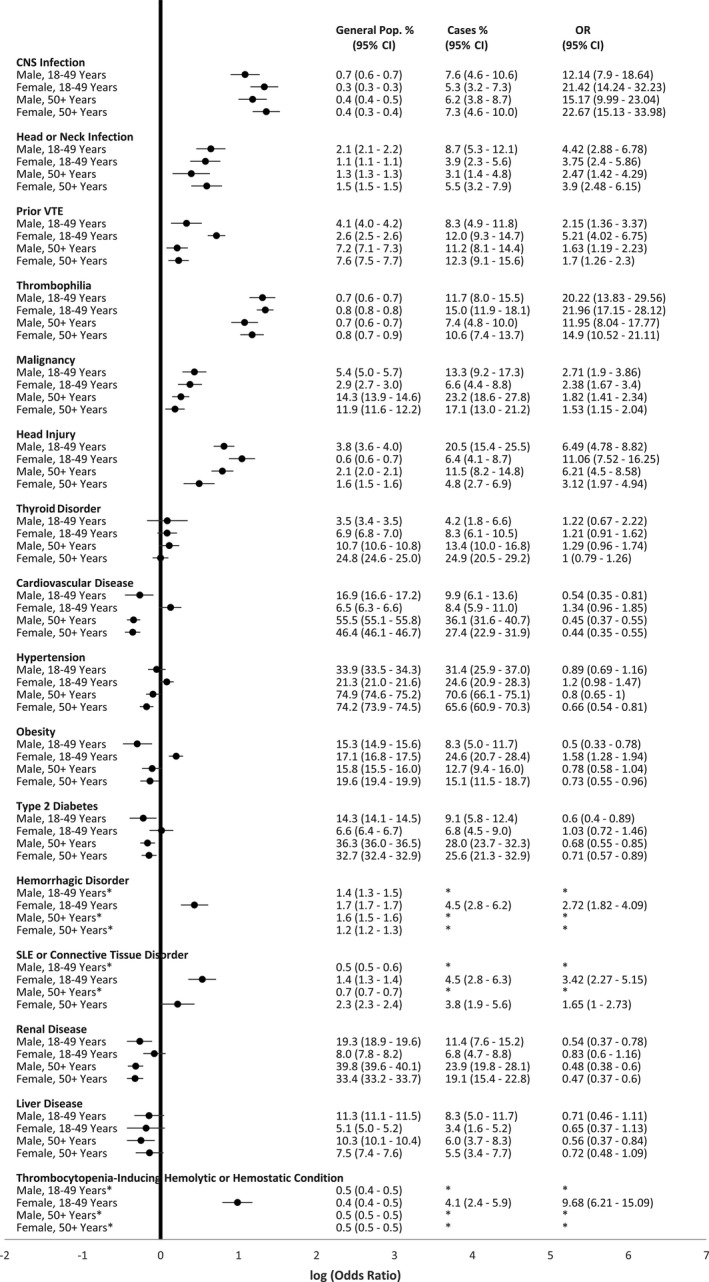
Age‐ and sex‐specific prevalence and prevalence odds ratio estimates comparing prevalence of comorbid conditions among hospitalized persons with cerebral venous sinus thrombosis (CVST) to the general inpatient population, in the Healthcare Cost and Utilization (HCUP), 2018 National Inpatient Sample (NIS). * Sparse data (cell size <10) precluded reporting data
Regarding gender‐specific risk factors assessed in the MarketScan Commercial sample, prevalence of pregnancy among women aged 18 to 49 years was higher among women with CVST compared with the general population (Table 5). While 5.6% (95% CI, 5.6%‐5.7%) of women in the general MarketScan Commercial claims sample had evidence of pregnancy during the study period, 12.5% (95% CI, 7.6%‐17.4%) of women with CVST had evidence of pregnancy during the study period. Among women aged 18 to 64 years, prevalence of estrogen‐modulating medication use was also higher among women with CVST (24.3% [95% CI, 18.8%‐29.7%]) compared with women in the general population (19.8% [95% CI, 19.8%‐19.8%]). This difference was primarily driven by women aged >49 years.
TABLE 5.
Prevalence of pregnancy and estrogen therapy use among all women and among women hospitalized for cerebral venous sinus thrombosis (CVST), in the MarketScan Commercial sample
| All Women | CVST a | |||
|---|---|---|---|---|
| N | % (95% CI) | N | % (95% CI) | |
| Pregnancy b | 247,934 | 5.6 (5.6‐5.7) | 22 | 12.5 (7.6‐17.4) |
| Estrogen modulator therapy c | ||||
| Women 18‐64 y | 1,339,075 | 19.8 (19.8‐19.8) | 58 | 24.3 (18.8‐29.7) |
| Women 18‐49 y | 1,058,127 | 24.0 (24.0‐24.1) | 45 | 25.6 (19.1‐32.0) |
| Women 50‐64 y | 280,948 | 11.9 (11.9‐12.0) | 13 | 20.6 (10.6‐30.6) |
Abbreviation: CI, confidence interval.
Among individuals with International Classification of Diseases, Tenth Revision, Clinical Modification code for CVST listed anywhere on inpatient claim record.
Includes during pregnancy or postpartum. Pregnancy‐related International Classification of Diseases, Tenth Revision, Clinical Modification code for CVST listed anywhere on inpatient claim record or on two outpatient claim records 30 days apart. Estimates limited to women aged 18‐49 years.
Pharmacy‐related claim for estrogen modulator medications. Estrogen modulator medications include oral contraceptives and other estrogen medications. Estimates limited to women aged ≥18 years.
4. DISCUSSION
Our study of CVST and CVST with thrombocytopenia in three large US population‐based data systems provides recent incidence estimates and comorbidity profiles for the total population and for key demographic subgroups. To our knowledge, this study provides the most in‐depth assessment of the epidemiology of CVST, particularly CVST with thrombocytopenia, to date.
We report CVST incidence estimates ranging from 2.45 to 3.16 per 100,000 for the total samples examined in this study. Among persons aged 18 to 64 years, CVST incidence estimates ranged from 2.83 to 5.27 per 100,000. This is similar to estimated incidence of CVST based on hospitalized persons with CVST in the United States in 2016 (2.62 per 100,000 among persons aged >18 years). 13 Furthermore, we confirm that incidence rates among females are generally higher than males; however, in‐depth subgroup analyses indicates this is driven by higher rates among young adult women in comparison to young adult men. 13 In fact, the highest incidence rate we report is among men aged ≥65 years. We found a significantly higher incidence of CVST in the MarketScan Medicaid sample in comparison with the MarketScan Commercial and NIS samples; further assessment of demographic subgroups indicates that this increase is driven by high rates among Medicaid enrollees aged 50 to 64 years (both men and women). This is possibly reflective of an increased burden of comorbidity in this age group, as >40% of the Medicaid sample is eligible for Medicaid on the basis of disability. Our study also confirms previous reports of lower CVST incidence among persons who are Hispanic or Asian/Pacific Islander and higher CVST incidence rates among persons who are non‐Hispanic Black compared with persons who are non‐Hispanic White. 13 Additional studies regarding sex‐specific incidence rates and differences in incidence rates by race/ethnicity may help to identify factors that could be underlying these observations.
Outcomes among persons with CVST have improved over time due to early detection and improved treatment. 26 Due to data reporting limitations, we were not able to assess all outcome measures of interest in all three data sources. Nevertheless, we report the acute‐phase mortality rate among persons with CVST to be >4% in the NIS sample and that >40% of persons with CVST were admitted to the ICU in the MarketScan Commercial sample, indicating that CVST may be associated with poor outcomes. Furthermore, we report that over 20% of persons hospitalized with CVST had a hemorrhagic stroke reported for the same hospitalization and >25% had an ischemic stroke reported. While it is likely that many of these strokes were clinical manifestations of the patient’s CVST, we were not able to definitively determine whether these strokes preceded or succeeded the diagnosis of CVST from the data available. While our study was not able to assess long‐term outcomes, previous studies have indicated poorer outcomes among persons with CVST manifesting as hemorrhagic stroke. 27 , 28
CVST is reported to have multiple etiologies, including medical and surgical interventions predisposing one to VTE, genetic and acquired prothrombotic disorders and other hematologic conditions, cancer, systemic inflammatory disorders, and infection. 14 , 17 , 18 However, many historical studies of CVST were based on case series that reported the proportion of CVST cases with a given comorbid condition, without comparison to expected rates based on the general inpatient population. We were able to address this limitation in the current study, thus allowing a better understanding of which comorbidities are elevated among persons with CVST and thus possibly associated with development of thrombosis, rather than being equally common among persons with CVST and inpatients generally. We found prevalence of the following comorbid conditions were significantly increased among persons with CVST: meningitis, encephalitis, or other CNS infection; head or neck infection; prior VTE; thrombophilia; malignancy; head injury; hemorrhagic disorder; SLE or other connective tissue disorder; and thrombocytopenia‐inducing hemolytic or hemostatic conditions. Conversely, we found that the prevalence of several comorbid conditions, which were common among both persons with CVST and in the general inpatient population, were actually significantly decreased among persons with CVST compared with the general inpatient population; these conditions were cardiovascular disease, hypertension, type 2 diabetes, and renal and liver disease. There was little to no difference in prevalence of thyroid disorder or obesity among persons with CVST compared with the general inpatient population.
While the general patterns of comorbidities in the total population of persons with CVST were similar for most sex‐age subgroups we examined, we observed some divergence among women aged 18 to 49 years. Obesity diagnosis codes were significantly more common among women aged 18 to 49 years with CVST compared with similarly aged females in the general inpatient population. Obesity has previously been reported as a risk factor for CVST among women with other underlying comorbidities. 29 We also observed higher percentages of cardiovascular disease and hypertension in women aged 18 to 49 years with CVST versus in the general population; these differences approached statistical significance.
In our study, prevalence of evidence of pregnancy during the study period among women aged 18 to 49 years was higher for those with CVST compared with expectations based on the general MarketScan Commercial sample. Moreover, our estimate of pregnancy‐related events in women with CVST (12.5%) is in line with previous reports, such as the International Study of Cerebral Venous Thrombosis (ISCVT), which found that 17% of CVST events among women were pregnancy related. 30 We also report a moderately higher use of estrogen‐modifying therapies in adult women with CVST than the expected rate based on females in the general population sample, particularly among women aged 50 to 64 years. Nonetheless, the use of estrogen‐modifying therapies among adult women with CVST in our sample (24.3%) was notably lower than that reported by the ISCVT (47%). Because our study defined estrogen‐modifying therapy use based on claims records, it is possible the proportion of women using estrogen‐modifying therapy is underestimated. Further study of this potential risk factor is needed. 30
As expected, CVST with thrombocytopenia was rare in our study, with 5% to 9% of persons with CVST also having thrombocytopenia, depending on the data source. This equates to an incidence rate of around 0.2 per 100,000. CVST with thrombocytopenia likely has multiple etiologies, some with CVST and thrombocytopenia sharing the same mechanism of disease (eg, HIT or DIC) and others with CVST and thrombocytopenia having different mechanisms (eg, malignancy). In this study, we were unable to fully explore the mechanisms by which CVST and thrombocytopenia arose, as we were unable disentangle thrombocytopenia that was concomitant with CVST from thrombocytopenia that was due to other causes and may have preceded the CVST. Thus, our data must be interpreted in the context of this limitation. We were able to determine a link with a thrombocytopenia‐inducing hemolytic or hemostatic condition known to be highly associated with thrombosis for 26% of the cases of CVST with thrombocytopenia. However, this may be an underestimate of the proportion of cases caused by such conditions, especially if these conditions preceded the CVST event. Of note, ≈11% of persons with CVST identified in our study did not have evidence of any of the comorbid conditions we investigated. In general, the prevalence of other comorbid conditions among persons with CVST with thrombocytopenia was similar to that among all persons with CVST. One exception was comorbid liver disease. Prevalence of comorbid liver disease was more common among persons with CVST with thrombocytopenia (12%) compared with all persons with CVST (5%) and the general inpatient population (7%). This finding was not unexpected, as thrombocytopenia is a known complication of advanced liver disease. 31 In keeping with expectations, persons with CVST with thrombocytopenia typically had longer hospitalization stays in our study and higher mortality rates than persons with CVST overall.
4.1. Limitations
Our study has some limitations. We identified persons with CVST based on inpatient admissions data with an ICD‐10‐CM code for CVST. This may be an underestimation of total CVST events, as some patients may have been treated in the outpatient setting and some patients may have died before admission to the hospital. However, due to the complex nature of treating persons with CVST, outpatient treatment alone is not likely. It is also possible that we slightly overestimated CVST in the NIS sample, where the unit of observation is the hospitalization and not the patient. However, given that the NIS is based on a random sample of inpatient admissions at participating hospitals, and we found that multiple CVST hospitalizations for the same person were uncommon in our two MarketScan samples, we estimate that the level of overestimation was small. CVST with thrombocytopenia may be underestimated because we lacked laboratory data and thus relied solely on ICD‐10‐CM codes. Additionally, it is possible we slightly overestimated CVST with thrombocytopenia, in the MarketScan samples, where it was not always possible to disentangle claims submitted by more than one provider for the same inpatient admission; thus, we defined CVST as being associated with thrombocytopenia if there was an inpatient claim for thrombocytopenia within 30 days of the inpatient claim for CVST. However, our data suggest this was not a major issue, as CVST with thrombocytopenia incidence estimates from the MarketScan samples were similar to (and not statistically different from) the NIS estimate. Use of inpatient ICD‐10‐CM codes, without availability of more in‐depth clinical or laboratory data may have also resulted in underestimation of some comorbidity percentages, particularly those for preexisting comorbidities. We used the general inpatient population as our comparison group for comorbidity analyses; while this is an appropriate comparison group, it is important to bear in mind that comorbidity percentages in the general inpatient population are not necessarily reflective of comorbidity percentages for the population at large (ie, the inpatient comorbidity percentages are likely higher). Thus, some relative comparisons reported here of comorbidities in persons with CVST versus in the general population are likely conservative. Because MarketScan is not an all‐payer system, estimates are not generalizable to the national population. Also, we limited the MarketScan samples to enrollees aged <65 years; thus, we could not examine the oldest age group in two of the three samples. Additionally, small sample sizes precluded several stratum‐specific and comorbidity assessments in the MarketScan samples. Finally, our findings suggest possible differences in incidence of CVST by race/ethnicity. These data must be interpreted cautiously given that race/ethnicity data in administrative databases are not based on self‐report and may be incomplete. 32 , 33 Thus, the underlying reason of the race/ethnicity differences reported here requires further investigation. To better understand and address differences in CVST incidence, collection of complete and representative race/ethnicity data in administrative databases is needed.
4.2. Strengths
Our study leveraged three large population‐based data systems to report updated information on the incidence of CVST and CVST with thrombocytopenia in the United States. Further, because these systems were sufficiently large, we were able to report age‐ and sex‐specific rates, providing a more in‐depth analysis of CVST. Indeed, the comparability of age‐sex–specific CVST incidence rates across the three samples speaks to the robustness of our findings. Additionally, we were able to report age‐ and sex‐specific rates of CVST with thrombocytopenia using data from the NIS sample, findings that to our knowledge have not been previously reported.
In addition to an in‐depth assessment of CVST incidence rates, the large NIS sample allowed for a comprehensive assessment of comorbid conditions among persons with both CVST and CVST with thrombocytopenia. Moreover, this study is novel in that we were able to compare prevalence estimates of comorbid conditions in persons with CVST to those for the general inpatient population, including detailed assessments for age and sex subgroups. This study thus greatly advances the state of knowledge on the unique comorbidity risk factors in persons with CVST and CVST with thrombocytopenia.
5. CONCLUSIONS
Our study used data from three large population‐based data systems to estimate incidence rates of CVST and CVST with thrombocytopenia. We observed a slightly higher incidence rate of CVST in comparison to estimates published in 2016 in all three data systems, continuing a reported increasing incidence trend over time. 13 Incidence of CVST with thrombocytopenia was much lower than incidence of CVST overall in all three data systems, with <10% of CVST cases having thrombocytopenia. Additionally, we highlight specific risk factors for CVST and identify sex‐specific differences in effect of risk factors. The impetus for these analyses was to provide contextual data to inform the recent VITT/TTS events associated with COVID‐19 vaccines. However, our findings have broader applicability in understanding the epidemiology of rare but often severe thrombotic events, CVST, and CVST with thrombocytopenia.
RELATIONSHIP DISCLOSURE
The authors have no relevant conflicts of interest to declare.
AUTHOR CONTRIBUTIONS
ABP and AA, developed the study design; performed the data analysis; and drafted, reviewed, and edited the manuscript. KA, KLR, LCR, and WCH developed the study design and reviewed and edited the manuscript. LAS developed the study design and drafted, reviewed, and edited the manuscript.
ETHICAL APPROVAL
The National Inpatient Sample database is consisted with the defintion of limited data sets under the Health Insurance Portability and Accountability Act (HIPAA) Privacy Rule and contains no direct patient identifiers. Further, use of the National Inpatient Sample prohibits identification of individual patients. Similarly, MarketScan is comprised of de‐identified enrollment, claims, and other administrative data. These data are a by‐product of a patient's clinical encounters and captured as the point‐of‐care by a health care provider via EHR and associated medical billing systems. IBM Watson health, Truven Health Analytics, receives these data from their data contributors and processes them into a format that is suitable for analyses. The Centers for Disease Control and Prevention is a tertiary user of these data and not involved in patient consent, mechanisms of data capture, or processing at the point of care. Analyses conducted on these de‐identified data are not considered human subjects research.
Supporting information
Table S1‐S2
ACKNOWLEDGMENTS
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Payne AB, Adamski A, Abe K, et al. Epidemiology of cerebral venous sinus thrombosis and cerebral venous sinus thrombosis with thrombocytopenia in the United States, 2018 and 2019. Res Pract Thromb Haemost. 2022;6:e12682. doi: 10.1002/rth2.12682
Handling Editor: Dr Cihan Ay
REFERENCES
- 1. Greinacher A, Thiele T, Warkentin TE, Weisser K, Kyrle PA, Eichinger S. Thrombotic thrombocytopenia after ChAdOx1 nCov‐19 vaccination. N Engl J Med. 2021;384:2092‐2101. doi: 10.1056/NEJMoa2104840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Schultz NH, Sørvoll IH, Michelsen AE, et al. Thrombosis and thrombocytopenia after ChAdOx1 nCoV‐19 vaccination. N Engl J Med. 2021;384:2124‐2130. doi: 10.1056/NEJMoa2104882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Scully M, Singh D, Lown R, et al. Pathologic antibodies to platelet factor 4 after ChAdOx1 nCoV‐19 vaccination. N Engl J Med. 2021;384:2202‐2211. doi: 10.1056/NEJMoa2105385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Shay DK, Gee J, Su JR, et al. Safety monitoring of the Janssen (Johnson & Johnson) COVID‐19 vaccine ‐ United States, March‐April 2021. MMWR Morb Mortal Wkly Rep. 2021;70:680‐684. doi: 10.15585/mmwr.mm7018e2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. See I, Su JR, Lale A, Woo EJ, Guh AY, Shimabukuro TT, Streiff MB, Rao AK, Wheeler AP, Beavers SF, Durbin AP. US case reports of cerebral venous sinus thrombosis with thrombocytopenia after Ad26.COV2.S vaccination, March 2 to April 21, 2021. JAMA. 2021;325(24):2448. doi: 10.1001/jama.2021.7517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Abdalkader M, Shaikh SP, Siegler JE, et al. Cerebral venous sinus thrombosis in COVID‐19 patients: a multicenter study and review of literature. J Stroke Cerebrovasc Dis. 2021;30:105733. doi: 10.1016/j.jstrokecerebrovasdis.2021.105733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Khazaei M, Karimi K, Sedighi P, Khazaei S. Cerebral sinus thrombosis secondary to SARS‐CoV‐2 infection. Case Rep Neurol Med. 2021;2021:6640368. doi: 10.1155/2021/6640368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ostovan VR, Foroughi R, Rostami M, et al. Cerebral venous sinus thrombosis associated with COVID‐19: a case series and literature review. J Neurol. 2021;268(10):3549–3560. doi: 10.1007/s00415-021-10450-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Taquet M, Husain M, Geddes JR, Luciano S, Harrison PJ. Cerebral venous thrombosis and portal vein thrombosis: a retrospective cohort study of 537,913 COVID‐19 cases. EClinicalMedicine. 2021;39:101061. doi: 10.1016/j.eclinm.2021.101061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Coutinho JM, Zuurbier SM, Aramideh M, Stam J. The incidence of cerebral venous thrombosis: a cross‐sectional study. Stroke. 2012;43:3375‐3377. doi: 10.1161/strokeaha.112.671453 [DOI] [PubMed] [Google Scholar]
- 11. Devasagayam S, Wyatt B, Leyden J, Kleinig T. Cerebral venous sinus thrombosis incidence is higher than previously thought: a retrospective population‐based study. Stroke. 2016;47:2180‐2182. doi: 10.1161/strokeaha.116.013617 [DOI] [PubMed] [Google Scholar]
- 12. Kristoffersen ES, Harper CE, Vetvik KG, Zarnovicky S, Hansen JM, Faiz KW. Incidence and mortality of cerebral venous thrombosis in a Norwegian population. Stroke. 2020;51:3023‐3029. doi: 10.1161/strokeaha.120.030800 [DOI] [PubMed] [Google Scholar]
- 13. Otite FO, Patel S, Sharma R, et al. Trends in incidence and epidemiologic characteristics of cerebral venous thrombosis in the United States. Neurology. 2020;95:e2200‐e2213. doi: 10.1212/wnl.0000000000010598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Stam J. Thrombosis of the cerebral veins and sinuses. N Engl J Med. 2005;352:1791‐1798. doi: 10.1056/NEJMra042354 [DOI] [PubMed] [Google Scholar]
- 15. Wasay M, Dai AI, Ansari M, Shaikh Z, Roach ES. Cerebral venous sinus thrombosis in children: a multicenter cohort from the United States. J Child Neurol. 2008;23:26‐31. doi: 10.1177/0883073807307976 [DOI] [PubMed] [Google Scholar]
- 16. Sébire G, Tabarki B, Saunders DE, et al. Cerebral venous sinus thrombosis in children: risk factors, presentation, diagnosis and outcome. Brain. 2005;128:477‐489. doi: 10.1093/brain/awh412 [DOI] [PubMed] [Google Scholar]
- 17. Silvis SM, de Sousa DA, Ferro JM, Coutinho JM. Cerebral venous thrombosis. Nat Rev Neurol. 2017;13:555‐565. doi: 10.1038/nrneurol.2017.104 [DOI] [PubMed] [Google Scholar]
- 18. Silvis SM, Middeldorp S, Zuurbier SM, Cannegieter SC, Coutinho JM. Risk factors for cerebral venous thrombosis. Semin Thromb Hemost. 2016;42:622‐631. doi: 10.1055/s-0036-1584132 [DOI] [PubMed] [Google Scholar]
- 19. HCUP National Inpatient Sample (NIS) . Healthcare Cost and Utilization Project (HCUP). Agency for Healthcare Research and Quality; 2018. www.hcup‐us.ahrq.gov/nisoverview.jsp [Google Scholar]
- 20. IBM MarketScan Research Databases for Health Services Researchers . IBM Watson Health. Somers; 2019. https://www.ibm.com/downloads/cas/6KNYVVQ2 [Google Scholar]
- 21. Producing National HCUP Estimates. Healthcare Cost and Utilization Project (HCUP) . Agency for Healthcare Research and Quality. Rockville; 2018. https://www.hcup‐us.ahrq.gov/tech_assist/nationalestimates/508_course/508course_2018.jsp [Google Scholar]
- 22. IBM MarketScan Treatment Pathways User Guide . IBM Watson Health. Somers; 2020. https://marketscan2.truvenhealth.com/treatmentpathways4/TxP4%20User%20Guide.pdf [Google Scholar]
- 23. Handley JD, Emsley HC. Validation of ICD‐10 codes shows intracranial venous thrombosis incidence to be higher than previously reported. Health Inf Manag. 2020;49:58‐61. doi: 10.1177/1833358318819105 [DOI] [PubMed] [Google Scholar]
- 24. Single‐race Population Estimates . United States, 2010‐2019. July 1st Resident Population by State, Age, Sex, Single‐Race, and Hispanic origin, on CDC WONDER Online Database. Vintage 2019 Estimates Released by U.S. Census Bureau on June 25, 2020. Accessed at http://wonder.cdc.gov/single‐race‐single‐year‐v2019.html
- 25. SAS Institute Inc . SAS/STAT® 13.1 User’s guide. SAS Institute Inc.; 2013. [Google Scholar]
- 26. Coutinho JM, Zuurbier SM, Stam J. Declining mortality in cerebral venous thrombosis: a systematic review. Stroke. 2014;45:1338‐1341. doi: 10.1161/STROKEAHA.113.004666 [DOI] [PubMed] [Google Scholar]
- 27. Gosk‐Bierska I, Wysokinski W, Brown RD Jr, et al. Cerebral venous sinus thrombosis: incidence of venous thrombosis recurrence and survival. Neurology. 2006;67:814‐819. doi: 10.1212/01.wnl.0000233887.17638.d0 [DOI] [PubMed] [Google Scholar]
- 28. Borhani Haghighi A, Edgell RC, Cruz‐Flores S, et al. Mortality of cerebral venous‐sinus thrombosis in a large national sample. Stroke. 2012;43:262‐264. doi: 10.1161/STROKEAHA.111.635664 [DOI] [PubMed] [Google Scholar]
- 29. Zuurbier SM, Arnold M, Middeldorp S, et al. Risk of cerebral venous thrombosis in obese women. JAMA Neurol. 2016;73:579‐584. doi: 10.1001/jamaneurol.2016.0001 [DOI] [PubMed] [Google Scholar]
- 30. Coutinho JM, Ferro JM, Canhao P, et al. Cerebral venous and sinus thrombosis in women. Stroke. 2009;40:2356‐2361. doi: 10.1161/STROKEAHA.108.543884 [DOI] [PubMed] [Google Scholar]
- 31. Lisman T, Hernandez‐Gea V, Magnusson M, et al. The concept of rebalanced hemostasis in patients with liver disease: communication from the ISTH SSC Working Group on Hemostatic Management of Patients with Liver Disease. J Thromb Haemost. 2021;19:1116‐1122. doi: 10.1111/jth.15239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Haozous EA, Strickland CJ, Palacios JF, Solomon TG. Blood politics, ethnic identity, and racial misclassification among American Indians and Alaska Natives. J Environ Public Health. 2014;2014:321604. doi: 10.1155/2014/321604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Smith N, Iyer RL, Langer‐Gould A, et al. Health plan administrative records versus birth certificate records: quality of race and ethnicity information in children. BMC Health Serv Res. 2010;10:316. doi: 10.1186/1472-6963-10-316 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1‐S2


