Abstract
Background:
HPV testing is the cornerstone of cervical cancer screening with outstanding sensitivity but only moderate specificity. We evaluated whether reflex testing for cancer biomarkers improves the sensitivity/specificity balance of screening.
Methods:
Cervical samples from women in Cape Town, South Africa, aged 30–65 years, were collected and tested with Xpert HPV and with real-time PCR to detect mRNA for Cyclin-Dependent Kinase Inhibitor 2A (CDKN2A), Topoisomerase 2 alpha (TOP2A) and Ki67 (MKi67). Women with histologically-confirmed cervical intraepithelial neoplasia grade 2 or worse (CIN2+) (85 women without and 166 with HIV) and women with no cervical disease (331 without and 257 with HIV) were included.
Results:
When used as reflex tests after a positive HPV result, biomarkers discriminated well between women with and without CIN2+. The inclusion of both CDKN2A and MKi67 had the best performance with area under the curve (AUC) of 0.9171 and 0.8734 in women without and with HIV, respectively. While excellent, these performance parameters did not improve on an approach utilizing only HPV testing with more stringent cycle threshold cut-offs and HPV genotype selection which achieved AUC of 0.9059 and 0.8705 in women without and with HIV, respectively.
Conclusion:
Biomarkers can be used as triage after positive HPV results but do not out-perform an approach utilizing higher viral load cut-offs on selected high-risk genotypes.
Impact:
A screening approach using HPV testing alone can be more easily implemented at the point-of-care.
Keywords: biomarkers, HPV DNA testing, cervical cancer, specificity, South Africa
Introduction
Human papillomavirus (HPV) testing is now considered the optimal, most efficient screening approach for cervical cancer in both low- and high-resource settings [1–6]. Compared to cytology, HPV testing has proven to be considerably more sensitive in detecting cervical intraepithelial neoplasia [7]. A single round of HPV testing provides greater and lengthier reassurance against future risk of developing pre-cancer/cancer and avoids the frequent, repeated testing associated with cytology [8, 9]. Furthermore, the availability of low-cost, point-of-care HPV tests allows for screening and treatment to occur in a single visit, which greatly reduces opportunities for loss-to-follow up [10].
The main disadvantage of HPV testing is its relatively low specificity arising from its inability to fully discriminate between transient and persistent infections [5, 6, 11]. It is well-established that persistent infection with one of the 14 high-risk HPV genotypes plays a central role in cervical carcinogenesis [12, 13]. However, only a subset of women with high-risk HPV progress to develop invasive cervical cancer since most infections will spontaneously clear [5, 14, 15]. This challenge is particularly marked in women living with HIV who have high rates of HPV infections [16]. To reduce overtreatment in the screen-and-treat model, there is a need to better identify HPV infections at highest risk of evolving into invasive cervical cancers and thereby improve risk stratification of women within screening programs [6, 17, 18]. For women living with and without HIV, we have previously evaluated two approaches to improving diagnostic accuracy of a single round of HPV testing. We have shown improved specificity with minimal reduction in sensitivity through: (1) cycle threshold optimization - to restrict to more stringent cycle threshold (Ct) cut-off values (higher viral loads); and (2) genotype selection - to select a subset of highest-risk HPV genotypes [19, 20].
In this analysis, we aimed to determine whether inclusion of cancer biomarkers as reflex tests following a positive HPV result can further improve the diagnostic accuracy of a round of cervical cancer screening. We selected the following three biomarkers from those that have shown promise in accurately identifying women with precancerous cervical lesions: Cyclin-Dependent Kinase Inhibitor 2A (CDKN2A), Topoisomerase 2 alpha (TOP2A), and proliferation marker Ki67 (Mki67) [18, 21–23]. CDKN2A is a tumor suppressor gene that encodes for p16 and prevents cell cycle progression through the retinoblastoma (pRB) pathway [23–26]. In normal functioning cells, p16 inactivates cyclin-dependent kinases that phosphorylate pRB and causes the cell to slow its progression from G1 phase to S phase. In transforming cells infected with high-risk HPV, the oncoprotein E7 degrades pRB causing the cell to proceed with division and p16 is overexpressed in attempt to inhibit the abnormal proliferation. Topoisomerase 2 alpha (TOP2A) encodes for an enzyme involved in chromosome condensing, chromatid separation and unwinding of DNA strands during S phase [23, 27]. While the role of proliferation marker Ki67 (Mki67) is less clear, it is required for cell division and present in all phases of the cell cycle except G0 [28]. TOP2A and MKi67 are also overexpressed when cells aberrantly enter cell division.
In a clinical study in Cape Town, South Africa, we investigated whether the addition of reflex testing for CDKN2A, TOP2A, and/or Mki67 following HPV testing can improve the diagnostic accuracy of a round of cervical cancer screening as compared to our previous approaches of genotype selection and cycle threshold optimization [19, 20].
Materials and Methods
Setting and study population
We recruited 1121 women aged 30–65 years from two sites in Cape Town, South Africa. A community-based clinic in Khayelitsha was used to recruit women seeking routine women’s health services and was considered representative of a healthy, screening population. A colposcopy clinic in Groote Schuur Hospital where women were referred for abnormal cervical cytology was used to obtain a referral population. Women were eligible to participate in the study if they had a documented HIV status, no history of treatment for cervical dysplasia, no previous hysterectomy, and no current pregnancy. An approximately equal number of women living with and without HIV were recruited by design. The study was approved by institutional review boards at the University of Cape Town and Columbia University. Written informed consent was obtained from each participant.
Sample collection and testing
HPV testing.
During pelvic examination, a physician collected two cervical samples from each woman in separate 20ml vials of PreservCyt. One sample was tested at point-of-care using Xpert HPV, which uses real-time PCR to detect 14 high-risk HPV genotypes. Xpert HPV reports cycle threshold (Ct) values for 5 separate channels: HPV16, HPV18,45, HPV 31,33,35,52,58, HPV51,59, and HPV39,56,66,68. For each channel, the manufacturer provides predetermined cutoff Ct values that define a positive HPV result (40 cycles for HPV channels 16 and 18,45; 38 cycles for the other 3 channels).
Biomarker testing.
For the first 1000 participants, the second cervical sample was sent to the Cepheid Research laboratory in Sweden to test for three biomarkers: Cyclin-Dependent Kinase Inhibitor 2A (CDKN2A), Topoisomerase 2 alpha (TOP2A) and the proliferation marker Ki67 (Mki67) using polymerase chain reaction (PCR).
5 mL of each PreservCyt sample was used for RNA preparation. The samples were centrifuged at 300g for 5 min, the cell pellets were mixed with 700 μL QIAzol (Qiagen, Hilden, Germany) lysis reagent and stored in −80°C until extraction. Total RNA was extracted using RNeasy RNA extraction kit (Qiagen, Hilden, Germany), according to manufacturer’s protocol. RNA concentration was measured with a NanoDrop instrument (Thermo Scientific, Wilmington, DE). Reverse transcription was performed starting with 10 μL of total RNA in a 20 μL reaction volume using random hexamers and the High Capacity cDNA RT-kit from Applied Biosystems Inc (Foster City, CA) according to the manufacturer’s protocol.
All PCR reactions were performed in duplicate in a reaction volume of 25 μL containing 5 μL of cDNA, diluted 1:10, mixed with Taqman Universal PCR MasterMix (Applied Biosystems Inc, Foster City, CA). The following protocol was used for all assays: denaturation (95°C for 10 min) and amplification (95°C for 20 seconds8, 60°C for 1 min) repeated for 40 cycles. The expression of CDKN2A, TOP2A, and MKI67 were analyzed. Beta glucuronidase (GUSB) and cGMP-dependent protein kinase 1 (PRKG1) were used as reference genes for normalization, these genes have previously been demonstrated to have a high stability in expression between groups of normal samples vs. HSIL samples [18]. Signal cutoff for target genes was set at 40 cycles, whereas for the reference gene PKG1, a Ct value above 30 cycles indicates poor RNA quality and was therefore considered invalid and excluded from the analysis. Primers and probes used in the PCR are listed in Supplementary Table 1. All probes were FAM-labeled and all reactions were run in single-plex.
Histology.
After collection of cervical samples, the physician conducted colposcopy to obtain histological samples by biopsy or loop electrosurgical excision procedure for women with visible abnormal-appearing areas of the cervix or by endocervical curettage for women with no visible lesions. Second and third colposcopies were conducted for women with positive HPV results at the screening clinic who had not had cervical cancer precursor lesions detected by histological sampling at the first colposcopy. Random four quadrant biopsies were done at the repeat colposcopies to reduce the likelihood of missing disease. Histological samples were reviewed initially by a pathology laboratory in Cape Town and then sent to the US for blind re-review to determine cervical intraepithelial neoplasia grade 2 or worse (CIN2+) and grade 3 or worse (CIN3+). If needed, a third US pathologist was used to resolve any discrepancies.
Statistical Analysis
All statistical analyses were conducted using SAS software version 9.4.
Biomarker distributions.
Biomarkers were treated as continuous variables with the intensity of the expression of the biomarker quantified as delta cycle threshold values (ΔCt = Ct biomarker − Ct reference gene). Since higher Ct values indicate lower expression of the biomarker, higher ΔCt values indicate lower expression of the biomarker relative to the reference. We used the following parameters to characterize the distributions of each biomarker by HIV status and cervical disease status: median, 25th percentile, 75th percentile, and range. To assess each biomarker’s ability to discriminate women with and without cervical disease, we used Kruskal Wallis tests to compare biomarker distributions between women with <CIN2 and CIN2+ by HIV status. We further conducted logistic regression analysis to determine if the 3 biomarkers, alone or in combination, predicted CIN2+ or CIN3+. Logistic regression models were run separately for women living with and without HIV. Of note, women from both the screening and referral populations were combined to create the disease endpoint group of CIN2+ or CIN3+. Only women from the screening population were included in the no disease endpoint group (<CIN2 or <CIN3).
Primary HPV testing.
We defined HPV positivity in two different ways: (1) positive for any of the five channels detecting the 14 high-risk HPV types and (2) positive only for three select channels detecting HPV16, 18, 45, 31, 33, 35, 52, and 58. Both definitions of HPV positivity utilize the manufacturer-defined Ct values. We calculated sensitivity and specificity of HPV testing using conventional methods. Sensitivity was calculated using the screening and referral populations, while specificity was calculated with the screening population only.
Biomarkers as Reflex tests.
We used multivariable logistic regression models to evaluate the utility of biomarkers as reflex tests after HPV testing. Each of the three biomarkers as reflex tests were entered individually into the regression models, as well as together, and those that remained statistically significant (p<0.05) were retained in the final multivariable model. When the HPV test was positive, we reversed the direction of the delta Ct values for each biomarker (12 minus ΔCt). When the HPV test was negative, we coded the biomarker as 0.
Furthermore, we compared logistic regression models for biomarkers as reflex tests to the alternative triage strategy of cycle threshold optimization. As previously described in Kuhn et al, Ct values from the three informative channels on the Xpert assay were utilized in logistic regression models [19]. In brief, channel-specific reverse cycle threshold (rCt) values (45 minus Ct) for the three channels detecting HPV types 16, 18, 45, 31, 33, 35, 52, and 58 were used when manufacturer’s cut-off defined the test result as positive. If the result was defined as HPV-negative based on the manufacturer’s cut off, then the rCt was equal to 0.
From each of the logistic regression models, we generated receiver operating characteristic (ROC) curves and the area under the curves (AUC): 0.7–0.8 acceptable, 0.8–0.9 excellent, 0.9–1.0 outstanding [29]. 95% Confidence Intervals (CI) were calculated for the AUC estimates. We selected a sensitivity of 80% as the benchmark for the cut-offs on biomarkers and the HPV viral load measures, which we reasoned was adequate for a round of screening. We derived corresponding specificities at 80% sensitivity using the ROC curves from each logistic regression model. In addition, we calculated the positive and negative predictive values and the proportion who would test positive at the identified sensitivity/specificity combinations. For these parameters, calculations were based on the observed prevalence of CIN2+ or CIN3+ in the screening population and standard algebraic formulae derived from the 2×2 table. Delta Ct cut-off values for the biomarkers were calculated using the probability derived from the ROC curve at sensitivity of 80% and the beta and intercept parameters from the logistic regression model. Formulae are available in supplementary materials and methods.
Results
Study population
One thousand of the 1121 women recruited in the original study were additionally tested for biomarkers CDKN2A, TOP2A, and MKi67. Demographic characteristics of this study population have been described elsewhere [19]. Six-hundred sixty-eight women (355 without HIV, 313 with HIV) were from the screening population while the remaining 332 women (130 without HIV, 202 with HIV) were from the referral population. After exclusions for missing HPV results (n=1), invalid biomarker data (n=8), missing and inadequate pathology results (n=12), there were 251 women with CIN2+ (85 without HIV and 166 with HIV) from the screening and referral populations combined. For the non-disease group only women in the screening population were included. There were 588 women with <CIN2 (331 without HIV and 257 with HIV) (Supplementary Figure 1).
The prevalence of confirmed cervical intraepithelial neoplasia grade 2 or worse (CIN2+) was 5.16% (95% CI: 2.84 – 7.48) and 16.29% (95% CI: 12.16 – 20.42); and the prevalence of high-risk HPV 16.62% (95% CI: 12.71 – 20.52) and 48.21% (95% CI: 42.62 – 53.80) among women without and with HIV, respectively, in the screening population (Table 1).
Table 1.
Prevalence of HPV, cervical intraepithelial neoplasia grade 2 or worse (CIN2+) and sensitivity and specificity of HPV testing by HIV status
| Women without HIV | Women living with HIV | |
|---|---|---|
| Mean age in years (standard deviation) | 44.01 (9.24) | 40.30 (7.08) |
| N in screening population | 349 | 307 |
| Prevalence of any high-risk HPV | 16.62 (12.71 – 20.52) | 48.21 (42.62 – 53.80) |
| Prevalence of HPV 3 channels detecting HPV 16, 18, 45, 31, 33, 35, 52, 58 | 13.75 (10.14 – 17.37) | 42.02 (36.50 – 47.54) |
| Prevalence of CIN2+ | 5.16 (2.84 – 7.48) | 16.29 (12.16 – 20.42) |
| N without CIN2+ in the screening population | 331 | 257 |
| Specificity in screening population of any high-risk HPV | 86.40 (82.71 – 90.10) | 59.92 (53.93 – 65.91) |
| Specificity of HPV 3 channels detecting HPV 16, 18, 45, 31, 33, 35, 52, 58 | 89.43 (86.11 – 92.74) | 66.93 (61.17 – 72.68) |
| N with CIN2+ screening and referral population | 85 | 166 |
| Sensitivity of any high-risk HPV | 88.24 (81.39 – 95.08) | 93.37 (89.59 – 97.16) |
| Sensitivity of HPV 3 channels detecting HPV 16, 18, 45, 31, 33, 35, 52, 58 | 87.06 (79.92 – 94.19) | 91.57 (87.34 – 95.79) |
HPV Testing
Table 1 shows the conventional sensitivity and specificity of HPV testing. Sensitivity of Xpert HPV to detect CIN2+ was 88.24% (95% CI: 81.39 – 95.08) and 93.37% (95% CI: 89.59 – 97.16) among women living without and with HIV, respectively, when defining screen-positive as detection of any the 14 high-risk HPV types (positive on any of the five channels). Specificity of HPV testing, with the same definition of screen-positive, was 86.40% (95% CI: 82.71 – 90.10) and 59.92% (95% CI: 53.93 – 65.91) among women living without and with HIV, respectively.
Shifting to defining screen-positive as detection of HPV16, 18, 45, 31, 33, 35, 52, and/or 58 (positive on only three of the channels) had little reduction in sensitivity. However, specificity improved to 89.43% (86.11 – 92.74) and 66.93% (61.17 – 72.68) in women living without and with HIV, respectively (Table 1). The high sensitivity but relatively low specificity of HPV testing, particularly in women living with HIV, was the prime justification for investigating additional approaches. In a round of screening, our goal was to determine how much improvement we could achieve in specificity when allowing sensitivity to decline to a threshold of 80%.
Distributions of the biomarkers
The distributions of CDKN2A, TOP2A, and MKi67 for women with CIN2+ were distinct from that of women without (Figure 1). For each biomarker, women with CIN2+ had higher expression of the biomarkers (lower ΔCt values) than women without. For women without HIV, the median ΔCt values were 0.80 vs. 2.38 with CDKN2A, 2.60 vs. 5.43 with MKi67, and 3.13 vs. 5.99 with TOP2A for CIN2+ and <CIN2, respectively. For women with HIV, median ΔCt values were 1.12 vs. 2.69 with CDKN2A, 3.61 vs. 5.40 with MKi67, and 3.94 vs. 5.60 with TOP2A for CIN2+ and <CIN2, respectively (Figure 1, p<0.001 for all comparisons). Among women without CIN2+, the distribution of the biomarkers did not differ by HPV status. Among women with CIN2+, the distribution of the biomarkers was shifted towards less expression in women with HPV among women without HIV only (Supplementary Table 2).
Figure 1:
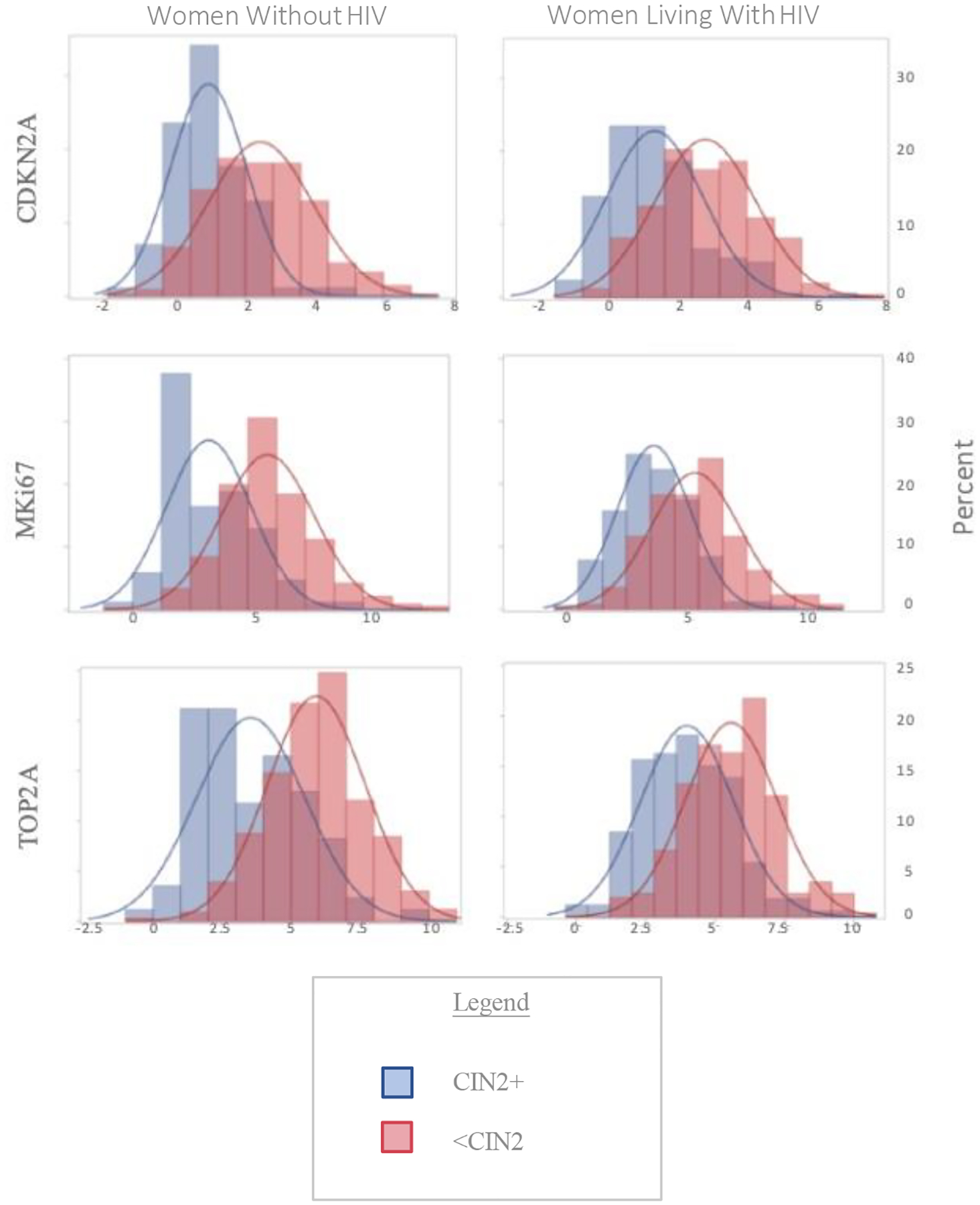
Distribution of Biomarkers by disease status and HIV status
Histograms of Delta Cycle Threshold (ΔCt) values for Cyclin-Dependent Kinase Inhibitor 2A (CDKN2A), Topoisomerase 2 alpha (TOP2A) and the proliferation marker Ki67 (MKi67) by cervical intraepithelial neoplasia grade 2 or worse (CIN2+) status and HIV status
Diagnostic Performance of the Biomarkers as Reflex Tests
With reflex testing, biomarkers are used to further triage women who have already tested positive for HPV. First, we considered using the HPV cut-offs defined by the manufacturer to detect all 14 high-risk types on all five channels as the basis to prompt a biomarker reflex test; and second, considered the results if the definition of a positive result was restricted to the three channels that detect HPV types 16, 18, 45, 31, 33, 35, 52 and 58. In univariate regression models, each biomarker was significantly associated with CIN2+ among women who tested HPV-positive using either definition. When included together in multivariable regression models, TOP2A was no longer significant. Both CDKN2A and MKi67 remained significantly associated with CIN2+.
Best results were obtained for models that included ΔCt values for both CDKN2A and MKi67. Among those who tested positive for any of the 14 high-risk types, the AUC using the two biomarkers together to predict CIN2+ was 0.9013 (95% CI: 0.8494 – 0.9533) for women without HIV and 0.7993 (95% CI: 0.7447 – 0.8539) for women living with HIV. Results were largely similar among women with the genotype-restricted definition of a positive HPV test where the AUC using the two biomarkers together to predict CIN2+ was 0.8988 (95% CI: 0.8427 – 0.9549) for women without HIV and 0.7949 (95% CI: 0.7358 – 0.8540) for women living with HIV. Performance of biomarkers were consistently better (higher AUC values) in women living without HIV compared to women living with HIV (Figure 2).
Figure 2:
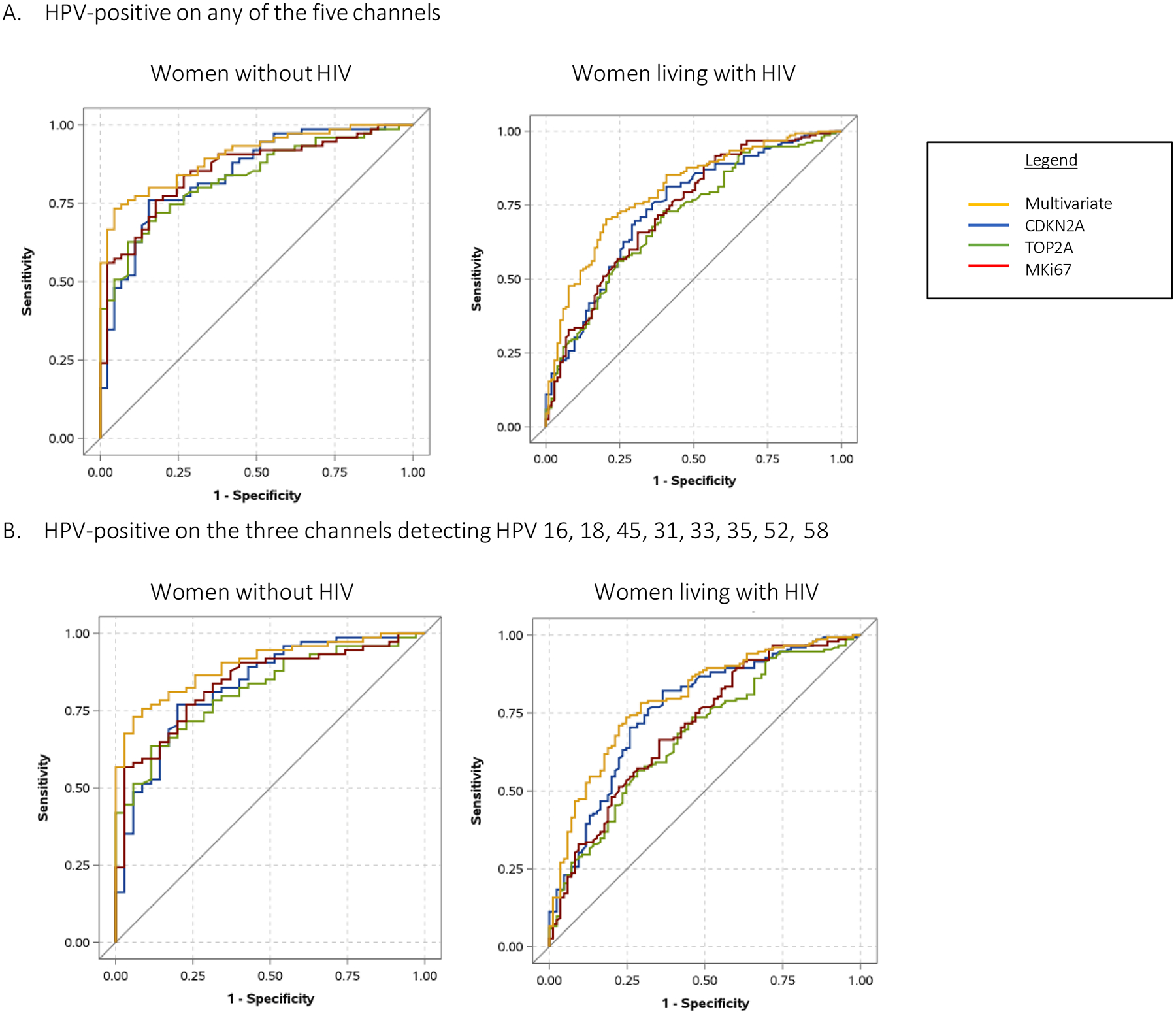
Receiver operating characteristic (ROC) curves of biomarkers
ROC curves based on logistic regression models using the biomarkers to predict presence or absence of cervical intraepithelial neoplasia grade 2 or worse (CIN2+) by HIV status. Top panel display women who were HPV-positive on any of the channels for high risk HPV. Bottom panels display women who were positive only on the three channels detecting HPV 16, 18, 45, 31, 33, 35, 52, 58.
Table 2 displays the performance characteristics of the biomarkers when undertaken as reflex tests (1) among women who tested positive for any of the 14 high-risk types or (2) among women who tested positive for the selected high-risk HPV types. Utilizing the ROC curves from the logistic regression models, we determined the specificity that would be attained if sensitivity of a round of reflex testing was set at 80%. We also determined the ΔCt values for each biomarker that would be needed for a woman to be considered screen-positive and achieve a sensitivity of 80%. We further calculated positive and negative predictive values and percent of women who would be screen positive at these cut-offs for ΔCt values.
Table 2:
Performance indicators for detection of cervical intraepithelial neoplasia grade 2 or worse (CIN2+) for reflex testing for Cyclin-Dependent Kinase Inhibitor 2A (CDKN2A), Topoisomerase 2 alpha (TOP2A), and/or proliferation marker Ki67 (MKi67) following a) an HPV test positive on any of the five channels detecting high-risk types or b) an HPV test positive on one or more of the three channels by HIV status. Area Under the Curve and 95% confidence interval is shown as well as the specificity, positive and negative predictive values, screen positive rate, and delta cycle threshold (ΔCt) cut-offs needed to achieve 80% sensitivity.
| Area Under the Curve | Sensitivity | Specificity | Positive Predictive Value | Negative Predictive Value | Screen Positive | ΔCt Cut-point |
|
|---|---|---|---|---|---|---|---|
| a) Reflex test if HPV-positive any high risk type | |||||||
| CDKN2A | |||||||
| Without HIV | 0.9145 (0.8752 – 0.9538) | 80.00 | 93.35 | 39.57 | 98.85 | 10.43 | 2.41 |
| Living with HIV | 0.8561 (0.8198 – 0.8925) | 80.00 | 79.77 | 43.48 | 95.35 | 30.00 | 2.57 |
| TOP2A | |||||||
| Without HIV | 0.9130 (0.8737 – 0.9522) | 80.00 | 92.75 | 37.51 | 98.84 | 11.00 | 5.82 |
| Living with HIV | 0.8446 (0.8072 – 0.8820) | 80.00 | 75.87 | 39.22 | 95.12 | 33.23 | 5.82 |
| MKi67 | |||||||
| Without HIV | 0.9156 (0.8764 – 0.9549) | 80.00 | 94.86 | 45.87 | 98.87 | 9.00 | 5.08 |
| Living with HIV | 0.8543 (0.8177 – 0.8909) | 80.00 | 78.60 | 42.11 | 95.28 | 30.95 | 5.15 |
| CDKN2A and MKi67 | |||||||
| Without HIV | 0.9179 (0.8779 – 0.9563) | 80.00 | 95.17 | 47.39 | 98.87 | 8.71 | N/A |
| Living with HIV | 0.8746 (0.8389 – 0.9079) | 80.00 | 81.32 | 45.46 | 95.43 | 28.67 | N/A |
| b) Reflex test if HPV-positive for selected high risk types only | |||||||
| CDKN2A | |||||||
| Without HIV | 0.9130 (0.8732 – 0.9527) | 80.00 | 94.56 | 44.46 | 98.86 | 9.29 | 2.41 |
| Living with HIV | 0.8711 (0.8360 – 0.9062) | 80.00 | 82.88 | 47.63 | 95.51 | 27.36 | 2.77 |
| TOP2A | |||||||
| Without HIV | 0.9115 (0.8718 – 0.9512) | 80.00 | 94.26 | 43.13 | 98.86 | 9.57 | 5.85 |
| Living with HIV | 0.8465 (0.8092 – 0.8838) | 80.00 | 77.43 | 40.82 | 95.21 | 31.94 | 5.95 |
| MKi67 | |||||||
| Without HIV | 0.9135 (0.8739 – 0.9532) | 80.00 | 94.86 | 45.87 | 98.87 | 9.00 | 5.42 |
| Living with HIV | 0.8547 (0.8180 – 0.8913) | 80.00 | 80.54 | 44.45 | 95.39 | 29.32 | 5.24 |
| CDKN2A and MKi67 | |||||||
| Without HIV | 0.9160 (0.8761 – 0.9554) | 80.00 | 95.77 | 50.72 | 98.88 | 8.14 | N/A |
| Living with HIV | 0.8810 (0.8472 – 0.9149) | 80.00 | 84.05 | 49.39 | 95.57 | 26.39 | N/A |
Each biomarker alone was an effective triage after a positive HPV test. However, the combination of CDKN2A and MKi67 yielded the best results for women living with and without HIV. Benchmarking sensitivity at 80%, specificity for a round of reflex testing with CDKN2A and MKi67 after a positive HPV test was 95.17% in women without HIV and 81.32% in women with HIV. Slightly better specificity for a round of reflex testing with the biomarkers were observed in women with HIV if the definition of a positive HPV test was confined to the subset of high risk types. Results also favored using the selective HPV types as the initial screen as this reduced the proportions who would need a second test and thus the overall proportions defined as screen-positive (Table 2).
Comparative Performance of Primary HPV Testing vs. Biomarkers as Reflex Tests
We have previously shown that the sensitivity/specificity trade-off of HPV testing can be improved by shifting thresholds for the cut-off valuess on the three Xpert channels detecting HPV16, 18,45, and 31,33,35,52,58 [19]. As shown in Figure 3, AUC was excellent for women without HIV (AUC=0.9059) and women living with HIV (AUC=0.8705) when HPV results were treated quantitatively using the reverse Ct values on the three informative HPV channels. When sensitivity was set at 80%, utilizing the HPV Ct values alone led to specificity rates of 93.96% and 83.27% in women living without and with HIV, respectively. These performance characteristics were near identical to that achieved with reflex testing with the biomarkers. Results using CIN3+ as the endpoint produced essentially similar results (Figure 3).
Figure 3:
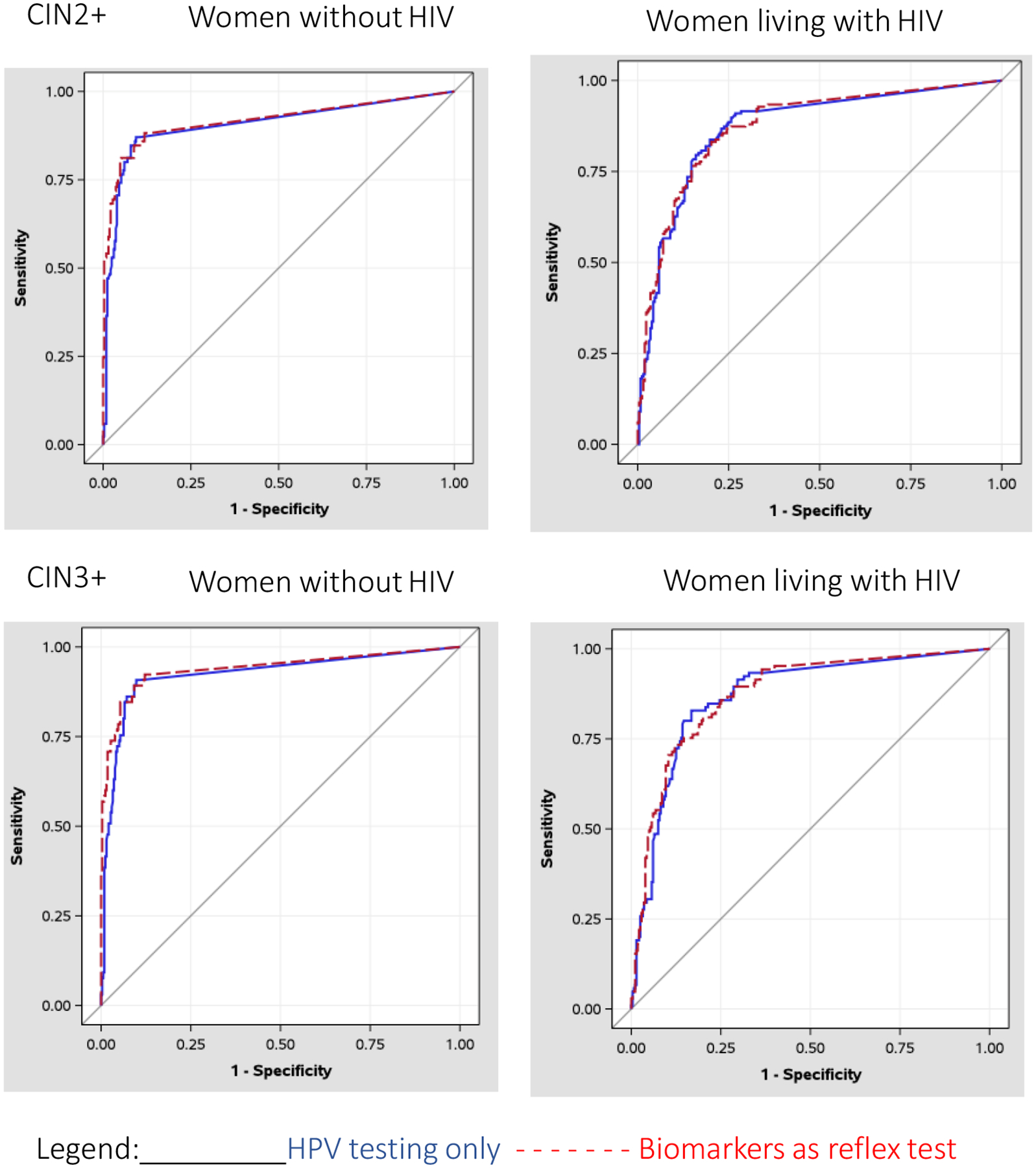
Receiver Operating Characteristic (ROC) Curves comparing reflex testing with biomarkers to HPV testing alone.
ROC curves for detection of cervical intraepithelial neoplasia grade 2 or worse (CIN2+) or grade 3 or worse (CIN3+) using (1) cycle threshold values on the three channels detecting HPV 16, 18, 45, 31, 33, 35, 52, 58 (HPV testing alone) or (2) delta cycle threshold values for CDKN2A and MKi67 measured if the HPV test was positive for any high risk types (reflex testing) by HIV status.
Discussion
HPV testing is the optimal, most efficient screening approach for both low- and high-resource settings and has the highest sensitivity and negative predictive values of any known approach to cervical cancer screening [1–6]. Despite these strengths, there has been some reluctance to embrace HPV testing for screening in low-resource settings because of its relatively low specificity, particularly for women living with HIV. However, the very high sensitivity of HPV testing, as it is currently configured, is arguably not necessary for most screening programs. From a public health standpoint, a degree of compromise in sensitivity, if the concomitant gain in specificity is substantial enough, would lead to more effective screening programs. This logic has prompted much interest in cancer biomarkers that could be used in conjunction with HPV testing to help better triage women who test positive on HPV tests [18, 26].
In our study, each of the three biomarkers we evaluated, CDKN2A, TOP2A and MKi67, strongly discriminated between women with and without CIN2+. If used as triage tests following HPV testing, cut-offs for the expression of these biomarkers can be identified that would result in favorable performance parameters for a round of screening. Consistent with prior studies [18], CDKN2A was the most specific biomarker, likely given its expression as a direct consequence of transforming HPV viral oncoproteins E6 and E7 [26]. In contrast, TOP2A and MKi67 are non-specific markers of cell proliferation [26]. In our study, the combined expression of CDKN2A and MKi67 led to the best differentiation between women with and without CIN2+. One of the strengths of our study methodology is the use of molecular testing on liquid-based samples to detect mRNA through PCR [18]. Several prior biomarker analyses have been done with immunohistochemistry, which risks intra-observer subjectivity when conducting microscopic analysis of histologic samples.
From the same study[19], we have previously reported our results pertaining to the improvements in the sensitivity/specificity balance that can be achieved by shifting the cycle-threshold values on the three informative channels of Xpert HPV that detect HPV types HPV16,18,45,31,33,35,52, and/or 58. Here we observed that the magnitude of the benefit achieved with the inclusion of the cancer biomarkers did not exceed that achieved with the changes to the interpretation of the output of the Xpert HPV assay that we have previously evaluated. Several groups are evaluating biomarkers as triage tests for women who have positive HPV results. Our results provide proof of concept that this is a viable approach, should appropriate tests become commercially-available and affordable. Nevertheless, inclusion of biomarkers as reflex tests has several disadvantages, particularly for low resource settings. Notably, the costs can be prohibitive for an additional test in those who require it as well as additional logistic steps and delay before reaching a clinical decision required in this approach. Xpert HPV is currently formulated to be easily used at the point-of-care allowing for single-visit screen-and-treat approaches to be implemented.
New World Health Organization guidelines propose the need for triage tests after HPV testing for primary screening, particularly for women living with HIV who have high HPV prevalence rates. Our study was designed to consider the performance of screening modalities in women both living with and without HIV. This is particularly relevant for a setting like South Africa with extraordinarily high rates of HIV infection among women. Our study was also designed for a setting where resources are scarce and where screening programs have failed to be effective due to attrition inherent in the multi-step cascade of conventional screening algorithms. Given the high prevalence of HPV among women living with HIV, our approach has marked benefit for these women and would substantially reduce over-treatment in the screen-and -treat model. For women without HIV, HPV testing without any further triage has adequate performance parameters but the adaptations to the interpretation of the output of the HPV test that we have evaluated optimizes this further..
Although rigorously designed, there are several limitations to our study. Due to limited resources, we used an ‘enrichment method’ to recruit women from both a screening population and a referral population to attain sufficient numbers of women with cervical disease. Therefore, the study sample may not be representative of disease detected in a true screening population and the sample size was too limited to investigate directly the generalizability here. This enrichment method was utilized and supported in the VALGENT HPV test validation [30].
In conclusion, our results provide proof of principle that biomarkers can be effectively used as a triage after positive HPV results to improve the overall sensitivity/specificity balance in a round of cervical cancer screening. Excellent performance parameters can be attained even for women living with HIV. However, addition of reflex testing for biomarkers does not further improve on the modifications to interpretation of the Xpert HPV assay that we have previously evaluated: cycle threshold optimization and genotype restriction [19]. For settings without access to HPV assays that have characteristics similar to Xpert HPV, namely the capacity to obtain partial typing and markers of viral quantitation, biomarker assays will have clinical utility. For settings with access to Xpert HPV or similar assays, use of a single HPV assay that can be done at the point-of-care is more desirable from a programmatic standpoint.
Supplementary Material
Table 3:
Performance indicators for detection of cervical intraepithelial neoplasia grade 2 or worse (CIN2+) or grade 3 or worse (CIN3+) using (1) cycle threshold values on the three channels detecting HPV 16, 18, 45, 31, 33, 35, 52, 58 (HPV test only) or (2) delta cycle threshold values for CDKN2A and MKi67 measured if the HPV test was positive for any high risk types (reflex testing) by HIV status.
| Area Under the Curve (95% confidence interval) | Sensitivity | Specificity | Positive Predictive Value | Negative Predictive Value | Screen Positive | |
|---|---|---|---|---|---|---|
| CIN2+ | ||||||
| 1) HPV testing only: Cycle threshold values on channels detecting HPV 16, 18, 45, 31, 33, 35, 52, 58 | ||||||
| Without HIV | 0.9059 (0.8658 – 0.9460) | 80.00 | 93.96 | 41.87 | 98.86 | 9.86 |
| Living with HIV | 0.8705 (0.8354 – 0.9056) | 80.00 | 83.27 | 48.20 | 95.53 | 27.04 |
| 2) Reflex testing: CDKN2A and MKi67 if HPV-positive (any high risk) | ||||||
| Without HIV | 0.9171 (0.8799 – 0.9563) | 80.00 | 95.17 | 47.39 | 98.87 | 8.71 |
| Living with HIV | 0.8734 (0.8389 – 0.9079) | 80.00 | 81.32 | 45.46 | 95.43 | 28.67 |
| CIN3+ | ||||||
| 1) HPV testing only: Cycle threshold values on channels detecting HPV 16, 18, 45, 31, 33, 35, 52, 58 | ||||||
| Without HIV | 0.9235 (0.8836 – 0.9633) | 80.00 | 93.77 | 31.38 | 99.25 | 6.71 |
| Living with HIV | 0.8701 (0.8304 – 0.9097) | 80.00 | 85.36 | 34.49 | 97.79 | 20.39 |
| 2) Reflex testing: CDKN2A and MKi67 if HPV-positive (any high risk) | ||||||
| Without HIV | 0.9398 (0.9019 – 0.9769) | 80.00 | 94.96 | 36.10 | 99.26 | 5.56 |
| Living with HIV | 0.8756 (0.8368 – 0.9137) | 80.00 | 81.07 | 28.94 | 97.68 | 24.30 |
Acknowledgements
We would like to thank Dr. Gwynn Stevens, Ms. Dipti Lallubhai and Ms. Tessa Visser of Cepheid–South Africa, Ms. Jill Birkmeier and the Cepheid Clinical Affairs and Data Management and Analytics team for study record capture, database construction, and cleaning, and the facility managers and senior leadership of the Department of Health, Western Cape Provincial Government for support with the study.
Financial support:
The study was supported in part by the National Cancer Institute UH2/3 CA189908. LGJ is supported by the National Cancer Institute T32 Postdoctoral Fellowship in Cancer Epidemiology at Columbia University Mailman School of Public Health (T32CA094061).
Footnotes
Conflict of interest: LJ, RS, RB, AT, JM, WYT, LD, and LK have no conflicts to declare. DP, CSB and SC declare that they receive salary, benefits and equity from Danaher Corp. Cepheid is a wholly owned entity of Danaher Corporation. TW declares that he serves as a consultant in clinical trial design and as an expert pathologist for HPV vaccine and/or diagnostic trials for Becton, Dickinson and Company, BD Life Sciences – Diagnostic Systems, Roche, Q-squared Solutions and Inovio Pharmaceuticals and as a speaker for Roche and Becton, Dickinson and Company, BD Life Sciences – Diagnostic Systems.
References
- 1.Catarino R, Petignat P, Dongui G, Vassilakos P. Cervical cancer screening in developing countries at a crossroad: Emerging technologies and policy choices. World J Clin Oncol. 2015;6(6):281–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ogilvie G, Nakisige C, Huh WK, Mehrotra R, Franco EL, Jeronimo J. Optimizing secondary prevention of cervical cancer: Recent advances and future challenges. Int J Gynaecol Obstet. 2017;138 Suppl 1:15–9. [DOI] [PubMed] [Google Scholar]
- 3.Tota JE, Bentley J, Blake J, Coutlee F, Duggan MA, Ferenczy A, et al. Introduction of molecular HPV testing as the primary technology in cervical cancer screening: Acting on evidence to change the current paradigm. Prev Med. 2017;98:5–14. [DOI] [PubMed] [Google Scholar]
- 4.Kuhn L, Denny L. The time is now to implement HPV testing for primary screening in low resource settings. Prev Med. 2017;98:42–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schiffman M, Wentzensen N, Wacholder S, Kinney W, Gage JC, Castle PE. Human papillomavirus testing in the prevention of cervical cancer. J Natl Cancer Inst. 2011;103(5):368–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ebisch RM, Siebers AG, Bosgraaf RP, Massuger LF, Bekkers RL, Melchers WJ. Triage of high-risk HPV positive women in cervical cancer screening. Expert Rev Anticancer Ther. 2016;16(10):1073–85. [DOI] [PubMed] [Google Scholar]
- 7.Arbyn M, Ronco G, Anttila A, Meijer CJ, Poljak M, Ogilvie G, et al. Evidence regarding human papillomavirus testing in secondary prevention of cervical cancer. Vaccine. 2012;30 Suppl 5:F88–99. [DOI] [PubMed] [Google Scholar]
- 8.Gage JC, Schiffman M, Katki HA, Castle PE, Fetterman B, Wentzensen N, et al. Reassurance against future risk of precancer and cancer conferred by a negative human papillomavirus test. J Natl Cancer Inst. 2014;106(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sahasrabuddhe VV, Luhn P, Wentzensen N. Human papillomavirus and cervical cancer: biomarkers for improved prevention efforts. Future Microbiol. 2011;6(9):1083–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kelly H, Mayaud P, Segondy M, Pant Pai N, Peeling RW. A systematic review and meta-analysis of studies evaluating the performance of point-of-care tests for human papillomavirus screening. Sex Transm Infect. 2017;93(S4):S36–S45. [DOI] [PubMed] [Google Scholar]
- 11.Tornesello ML, Buonaguro L, Giorgi-Rossi P, Buonaguro FM. Viral and cellular biomarkers in the diagnosis of cervical intraepithelial neoplasia and cancer. Biomed Res Int. 2013;2013:519619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Walboomers JM, Jacobs MV, Manos MM, Bosch FX, Kummer JA, Shah KV, et al. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol. 1999;189(1):12–9. [DOI] [PubMed] [Google Scholar]
- 13.Bosch FX, Lorincz A, Munoz N, Meijer CJ, Shah KV. The causal relation between human papillomavirus and cervical cancer. J Clin Pathol. 2002;55(4):244–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schiffman M, Wentzensen N. Human papillomavirus infection and the multistage carcinogenesis of cervical cancer. Cancer Epidemiol Biomarkers Prev. 2013;22(4):553–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rodriguez AC, Schiffman M, Herrero R, Wacholder S, Hildesheim A, Castle PE, et al. Rapid clearance of human papillomavirus and implications for clinical focus on persistent infections. J Natl Cancer Inst. 2008;100(7):513–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jeronimo J, Castle PE, Temin S, Denny L, Gupta V, Kim JJ, et al. Secondary Prevention of Cervical Cancer: ASCO Resource-Stratified Clinical Practice Guideline. Journal of global oncology. 2017;3(5):635–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.van Dam PA, Rolfo C, Ruiz R, Pauwels P, Van Berckelaer C, Trinh XB, et al. Potential new biomarkers for squamous carcinoma of the uterine cervix. ESMO Open. 2018;3(4):e000352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Del Pino M, Svanholm-Barrie C, Torne A, Marimon L, Gaber J, Sagasta A, et al. mRNA biomarker detection in liquid-based cytology: a new approach in the prevention of cervical cancer. Mod Pathol. 2015;28(2):312–20. [DOI] [PubMed] [Google Scholar]
- 19.Kuhn L, Saidu R, Boa R, Tergas A, Moodley J, Persing D, et al. Clinical evaluation of modifications to a human papillomavirus assay to optimise its utility for cervical cancer screening in low-resource settings: a diagnostic accuracy study. Lancet Glob Health. 2020;8(2):e296–e304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Johnson LG, Saidu R, Mbulawa Z, Williamson AL, Boa R, Tergas A, et al. Selecting human papillomavirus genotypes to optimize the performance of screening tests among South African women. Cancer Med. 2020;9(18):6813–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Peres AL, Paz ESKM, de Araujo RF, de Lima Filho JL, de Melo Junior MR, Martins DB, et al. Immunocytochemical study of TOP2A and Ki-67 in cervical smears from women under routine gynecological care. J Biomed Sci. 2016;23(1):42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Van Ostade X, Dom M, Tjalma W, Van Raemdonck G. Candidate biomarkers in the cervical vaginal fluid for the (self-)diagnosis of cervical precancer. Arch Gynecol Obstet. 2018;297(2):295–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang QC, Zhu Y, Liou HB, Zhang XJ, Shen Y, Ji XH. A cocktail of MCM2 and TOP2A, p16INK4a and Ki-67 as biomarkers for the improved diagnosis of cervical intraepithelial lesion. Pol J Pathol. 2013;64(1):21–7. [DOI] [PubMed] [Google Scholar]
- 24.Romagosa C, Simonetti S, Lopez-Vicente L, Mazo A, Lleonart ME, Castellvi J, et al. p16(Ink4a) overexpression in cancer: a tumor suppressor gene associated with senescence and high-grade tumors. Oncogene. 2011;30(18):2087–97. [DOI] [PubMed] [Google Scholar]
- 25.CDKN2A gene Bethesda, Maryland: U.S. National Library of Medicine; [Available from: https://ghr.nlm.nih.gov/gene/CDKN2A. [Google Scholar]
- 26.von Knebel Doeberitz M, Reuschenbach M, Schmidt D, Bergeron C. Biomarkers for cervical cancer screening: the role of p16(INK4a) to highlight transforming HPV infections. Expert Rev Proteomics. 2012;9(2):149–63. [DOI] [PubMed] [Google Scholar]
- 27.TOP2A DNA topoisomerase II alpha: National Center for Biotechnology Information; [Available from: https://www.ncbi.nlm.nih.gov/gene/7153.
- 28.Brown CA, Bogers J, Sahebali S, Depuydt CE, De Prins F, Malinowski DP. Role of protein biomarkers in the detection of high-grade disease in cervical cancer screening programs. J Oncol. 2012;2012:289315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mandrekar JN. Receiver operating characteristic curve in diagnostic test assessment. J Thorac Oncol. 2010;5(9):1315–6. [DOI] [PubMed] [Google Scholar]
- 30.Arbyn M, Depuydt C, Benoy I, Bogers J, Cuschieri K, Schmitt M, et al. VALGENT: A protocol for clinical validation of human papillomavirus assays. J Clin Virol. 2016;76 Suppl 1:S14–S21. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


