Abstract
Leigh syndrome (LS) is a neurodegenerative disease characterized by bilaterally symmetric brainstem or basal ganglia lesions. More than 80 genes, largely impacting mitochondrial energy metabolism, can underlie LS, and no approved medicines exist. Described 70 years ago, LS was initially diagnosed by the characteristic, necrotic lesions on autopsy. It has been broadly assumed that antemortem neuroimaging abnormalities in these regions correspond to end-stage histopathology. However, clinical observations and animal studies suggest that neuroimaging findings may represent an intermediate state, that is more dynamic than previously appreciated, and even reversible. We review this literature, discuss related conditions that are treatable, and present two new LS cases with radiographic improvement. We review studies in which hypoxia reverses advanced LS in a mouse model. The fluctuating and potentially reversible nature of radiographic LS lesions will be important in clinical trial design. Better understanding of this plasticity could lead to new therapies.
Introduction
Leigh syndrome (LS), also known as subacute necrotizing encephalomyelopathy, is the most common pediatric manifestation of inherited mitochondrial disorders [1]. In 1951, Dennis Leigh described a 5-month old male infant with subacute onset of progressive somnolence. He died at age 7 months, afebrile but diaphoretic with unreactive pupils, marked hypertonia, with upgoing toes but absent deep tendon reflexes. Autopsy revealed bilateral, symmetric brainstem, basal ganglia, and spinal cord gray matter lesions characterized by gliosis, vacuolation, capillary proliferation, and relative sparing of neurons within areas with severe necrosis [2].
As more cases of LS began to be recognized, there was growing appreciation that this is a metabolic disease. Acidosis, as indicated by low serum bicarbonate in patients in the 1950s and subsequently lactic acidosis found in patients in the 1960s, was the first salient biochemical observation [3]. Defects in pyruvate metabolism were subsequently documented in several patients with LS. Although defects in pyruvate metabolism were initially thought to be related to pyruvate carboxylase deficiency, this hypothesis was never proven, and many of these cases were ultimately linked to pyruvate dehydrogenase deficiency [4]. Cytochrome c oxidase deficiency became the first component of the electron transport chain (ETC) to be linked to LS in 1977 [5].
Our diagnostic approach and molecular genetic understanding of LS have dramatically improved over the past few decades. LS was diagnosed based on autopsy findings till the 1970s when the advent of brain contrast tomography (CT) and, subsequently, magnetic resonance imaging (MRI) enabled antemortem diagnosis [6–9]. With advances in biochemical and molecular genetic testing, scores of mitochondrial biochemical and genetic lesions were linked to LS. Today, LS is widely recognized as the most common pediatric manifestation of mitochondrial disease, with greater than 80 genes — mostly encoding proteins localized to mitochondria — underlying this syndrome [1].
Lake et al. have most recently defined LS as i) a characteristic clinical presentation including psychomotor retardation and/or regression with progressive neurologic decline, often in a stepwise fashion, with decompensations, frequently with illness, ii) radiologic evidence of LS lesions in the basal ganglia or brainstem nuclei, which appear hyperintense in T2-weighted MRI sequences, iii) biochemical evidence of abnormal energy metabolism, and iv) identification of a pathogenic variant in a characteristic gene [1]. ‘Leigh-like’ disease or “LS spectrum” is used to describe cases in which a subset of these features including the radiographic or histologic findings are fulfilled [1]. At present, the precise sequence of events linking mutations in disease genes to end brain disease is not known. A better understanding of this molecular pathogenesis is crucial as, at present, there are no proven therapies.
Here, we review the published literature, present two new clinical cases, and integrate recent animal studies that collectively suggest that the brain disease in LS may be more plastic than previously appreciated. In particular, we explore the question of whether the antemortem MRI abnormalities may not fully equate to end-stage pathology and that these radiographic findings might instead reflect intermediate — potentially reversible — stages of this disease.
Radiographic brain lesions in Leigh syndrome
Hyperintense signals on the T2-weighted MRI sequence can arise from multiple processes, making the origin of LS lesion signal abnormalities unclear. Tissues such as cerebrospinal fluid (CSF) that typically have high water content appear bright or white on T2-weighted sequences, whereas brain matter appears in shades of gray varying by composition. LS lesions are T2 hyperintense, as they represent an uncharacteristically bright signal in affected regions [10]. What processes typically produce T2 hyperintensity? Possibilities include hemorrhage, ischemia, neoplasm, infection, and inflammation [10]. Reduced diffusivity on isotropic diffusion mapping (thought to represent cytotoxic edema) [11] has occasionally been reported in LS lesions (e.g. the study reported by Bonfante et al. [9]). Notably, however, not all studies included this technique; and this abnormality can also represent an artifact of high T2 signal. While the contribution of hemorrhage, neoplasm, or infection to the T2 signal abnormalities in LS can be definitively excluded by autopsy and clinical studies, the relative contributions of inflammation, edema, loss of plasma membrane integrity, and/or frank necrosis to high T2 signals remain unknown in both the end-stage disease and antemortem intermediate states in humans.
Although LS lesions are quite distinctive, a small handful of hereditary conditions and a number of acquired conditions — ranging from toxins, insults, nutritional deficiencies, infections — can lead to lesions with MRI features resembling LS (Table 1) [1,12–35,54,56]. These mimetics are not only important considerations in the differential diagnosis, but also promise to provide insight into the etiopathogenesis of LS.
Table 1.
Radiographic differential diagnosis of Leigh syndrome brain lesions.
| Leigh and Leigh-like syndrome | ||
| Mutations in >80 disease genes related to mitochondrial energy metabolism (e.g., | T2 hyperintense bilaterally symmetric brainstem nuclei or basal ganglia lesions; lesions are frequently also found in the cerebellum and spinal cord. | [1] |
| PDH, OXPHOS) | ||
| Genetic defects in thiamine transport/ processing (SLC19A3 and TPK1) | T2 hyperintensities of bilateral basal ganglia, deep gray nuclei; SLC19A3- related disease also involves cortical and subcortical white matter | [54,56] |
| Organic acidurias, GM1 and GM2 gangliosidoses, guanidinoacetate methyltransferase deficiency | May at times present with T2 hyperintense bilaterally symmetric brainstem nuclei or basal ganglia lesions, often in addition to more canonical white matter or other intracranial disease | [26–28] |
| Acquired neurologic insult | ||
| Hypoxic ischemic injury (HII) | MRI may present with T2 hyperintense lesions of the bilateral putamina and thalami ± bilateral globi pallidi lesions and white matter lesions. HII after the neonatal period tends to spare the thalami. | [12,23] |
| Hypoglycemic ischemic encephalopathy | Classically involves the occipital cortex in addition to basal ganglia, thalami. T2 lesions preceded by diffusion restriction may evolve into encephalomalacia. | [29] |
| Refractory status epilepticus | Typically involves pulvinar (+/- cortex), accompanied by diffusion restriction, histopathology similar to Wernicke-Korsakoff lesions. | [30] |
| Toxic/metabolic | ||
| Wernicke-Korsakoff Syndrome (thiamine deficiency) | Uniformly involves mammillary bodies in addition to structures affected in LS; basal ganglia involvement is rare in classical (alcohol use disorder- associated) disease but can occur in pediatric and nonalcohol use disorder- associated adult cases; histology differs from LS in that petechial hemorrhage is often observed. | [31] |
| Kernicterus (CNS bilirubin toxicity) | T2 hyperintense basal ganglia lesions have been reported as late as 1 year; some reports involve T1 hyperintense lesions. | [32] |
| Osmotic myelinolysis | Most commonly occurs in individuals with comorbid alcohol use disorder, cases with thalamic and striatal T2 signal changes have been reported. | [33] |
| Carbon monoxide poisoning | Bilateral globi pallidi are uniformly T2 hyperintense; cerebral cortex, cerebral white matter, cerebellum, midbrain may also be involved. In rare cases, T2 and T1 hyperintensity of the globi pallidi have been observed. Lesions may regress with time. | [34] |
| Metronidazole encephalopathy | Lesions mostly commonly occur in the brainstem and/or cerebellar dentate nuclei, although basal ganglia are rarely involved. Metronidazole encephalopathy is more common in individuals with comorbid alcohol use disorder. | [35] |
| Fedratinib toxicity | Reported as rare side effect in individuals being treated for myeloproliferative disorders. T2 hyperintense lesions were observed in the bilateral caudate nuclei, lenticular nuclei, and thalami but not in the mammillary bodies. Clinical and radiographic improvements were reported after thiamine supplementation. | [13] |
| Disulfiram toxicity | Bilateral globi pallidi and putamina are affected. Most occurrences in individuals with alcohol use disorder being treated with disulfiram; however, one case reported in a child after accidental ingestion. | [14] |
| Carbon disulfide toxicity | This industrial solvent is metabolized by cytochrome P450 enzymes to thiocarbamide, 2-mercapto-2-thiazolinone-5, and 2-thiothiazolidine-4- carboxylic acid, the latter of which conjugates to glutathione. Chronic exposure leads to encephalopathy, parkinsonism, and neuropathy. Imaging features may be suggestive of a microangiopathy. | [15] |
| Vigabatrin exposure | Lesions occur in ~30% of patients with epilepsy treated with vigabatrin (a GABA transaminase inhibitor) and are diffusion restricting as well as T2 hyperintense. Lesions resolve with discontinuation of therapy. | [16] |
| Infection-related | ||
| Hemolytic-uremic syndrome (HUS) | Lesions often involve splenium of the corpus callosum in addition to structures typically affected in LS, were diffusion-restricting and T2 hyperintense, and resolve in most cases. Edema, necrosis, spongiosis, gliosis, hemorrhages, and thrombotic microangiopathy have all been reported on histology. | [17] |
| Flavivirus (West Nile, Murray Valley Encephalitis) | Lesions occur in a significant proportion of patients and may be diffusion restricting and T2 hyperintense or diffusion restricting (and resolving) only, with the latter seeming to confer a better prognosis. | [18] |
| Variant Creutzfeldt-Jakob disease (bovine spongiform encephalopathy) | Pulvinar most commonly affected, dorsomedial thalamic nuclei, caudate head, and periaqueductal gray matter may also be involved. Lesions may regress. Histology is characterized by spongiform change, neuronal loss, astrocytosis, and deposition of partially protease-resistant prion protein. | [19] |
| Influenza A, B associated encephalopathy/Reye syndrome | Involvement of the splenium of the corpus callosum is often reported in addition to the gray matter structures affected by LS. | [20] |
| Cerebral malaria | Lesions of gray and white matter have been reported with some authors interpreting the gray matter lesions as resulting from vasogenic edema. | [21] |
| Other genetic disorders | ||
| Wilson disease (ATP7B) | The MRI brain may be normal, but the most common abnormality is a T2 hyperintense signal in the lateral rim of the bilateral putamina. T2 hyperintensity of the caudate, globi pallidi, and thalami were seen only when putaminal lesions were absent. Rarely, T1 hyperintensity may be seen in the thalami. Response of brain lesions to copper chelation remains unclear. | [22] |
| Menkes disease (ATP7A) | T2 hyperintense lesions of the caudate nuclei, lenticular nuclei, and globi pallidi have been reported. Isolated T2 hyperintensities of the parieto-occipital white matter and isolated cerebral atrophy have also been reported. | [24] |
| Juvenile Huntington disease (HTT) | Caudate atrophy, similar to adult patients, was observed in addition to T2 signal abnormalities of the caudate, which is not a feature of adult-onset disease. Interestingly, juvenile onset HD mostly typically presents with Parkinsonism, although adult-onset disease is characterized by chorea. | [25] |
| Dentatorubral-pallidoluysian atrophy (ATN1) | T2 hyperintensity of the globi pallidi and thalami are present with atrophy of the tegmentum, other deep gray nuclei, and cerebellum. Histopathology demonstrates characteristic neuronal intranuclear inclusions. | [26] |
MRI, magnetic resonance imaging; OXPHOS, oxidative phosphorylation; GABA, Gamma-Aminobutyric Acid; HD, Huntington Disease; CNS, central nervous system.
The rare LS cases reporting both neuroimaging and subsequent autopsy findings are instructive and reveal histologic evidence of edema and capillary proliferation, gliosis, and — to a lesser extent — inflammation. Kissel et al. [36] reported an apparent adult-onset LS case with progressive T2 prolongation signal changes of the cerebral peduncles, periaqueductal gray, and basal ganglia with initial expansion, followed by contraction of the latter. Microscopic evaluation on autopsy reviewed capillary proliferation and gliosis in regions with relative neuronal preservation, as well as lipid-laden macrophages in areas of complete neuronal replacement by cavitation and necrosis. All areas affected on histopathologic examination were also detected on MRI [36]. Koch et al. [7] reported a case of twin infants with LS where CT scan lesions observed in late stages also correlated with loss of cellularity, vascular engorgement, and gliosis on autopsy.
Resolution of radiographic brain lesions in patients with Leigh syndrome
Serial imaging has at times demonstrated regression of some radiographic LS lesions. Indeed, as early as 1985, Koch et al. reported the aforementioned case of twins diagnosed with LS on head CT. In that study, some of the lesions appeared to regress on repeat imaging. Initial CT demonstrated hypodensities of the bilateral basal ganglia and midbrain tegmentum at age 10 months. The basal ganglia lesions were not, however, apparent on repeat imaging at 17 months despite persistent decline in respiratory function. Autopsy performed on one twin showed that the basal ganglia lesions that had resolved on repeat imaging demonstrated no gross necrosis, only mild neuronal loss, capillary proliferation, and reactive astrocytosis. However, in the brainstem, where imaging abnormalities had been persistent, there was ‘significant’ loss of cells, vascular ‘engorgement,’ and ‘marked’ reactive astrocytosis [7]. These findings led the authors to hypothesize that ‘active lesions with vascular proliferation’ but without frank necrosis might appear and subsequently resolve [7,8]. Intriguingly, recent reports of arterial spin labeling in patients with LS indicate increased blood flow to LS regions during acute symptomatic crises [37]. Others have similarly observed radiologic improvement of T2 signal abnormalities without evidence of necrosis in various cohorts [9,38,39] and in case report format (Table 2). These findings suggest that LS lesions that regress on repeat imaging potentially correspond to intermediate pathologic states rather than end-stage necrotic lesions.
Table 2.
Published cases of Leigh syndrome reporting radiographic improvement.
| Gene or biochemical defect | Age initial imaging | Age repeat imaging | Reversing lesion(s) | References |
|---|---|---|---|---|
| n/r | 10mo | 17mo | Basal ganglia | [7] |
| n/r | 10mo | 17mo | Basal ganglia | [7] |
| n/r | 22mo | 30mo | Right caudate, midbrain | [7] |
| n/r | 10yr 11mo | 11yr 7mo | Left caudate | [8] |
| n/r | 3yr | 3.5yr | Basal ganglia | [43] |
| PDHC deficiency | 1yr | 2yr | Globus pallidus, cerebellum | [44] |
| Complex I deficiency | n/r | n/r | Basal ganglia | [42] |
| PDHC deficiency | 4yr 7mo | 4yr 9mo | Basal ganglia | [42] |
| n/r | n/r | n/r | Upper brainstem | [39] |
| Partial COX deficiency | 22yr | 22yr | Basal ganglia, thalami | [45] |
| n/r | 14mo | 27mo | Globi pallidi | [46] |
| PDHA1 | 3yr | 7yr | Basal ganglia, inferior olivary nuclei | [48] |
| PDHA1 | n/r | n/r | Basal ganglia, cerebellum | [41] |
| MT - ND5 | n/r | n/r | Midbrain | [41] |
| MT-ATP6 | n/r | n/r | Basal ganglia | [41] |
| HIBCH (c.287C > A) | 14mo | 24mo | Basal ganglia | [47] |
| MT-ND3 (m.10197G > A) | 16yr | 17yr | Thalamus, cerebral peduncle, pons, medulla oblongata | [49] |
| PDHA1 | 1yr 8mo | 6yr 8mo (multiple) | Basal ganglia (initially improved, then progressed) | [40] |
| SLC19A3 | 2.5yr | 3yr | Basal ganglia | [54] |
| MT-ATP6 (m.8689G > A) | n/r | n/r | Putamen | [9] |
| MT-ND3 (m.10158T > C) | n/r | n/r | regression reported, lesion location not detailed | [9] |
| MT-ATP6 (m.9176 T> C) | n/r | n/r | regression reported, lesion location not detailed | [9] |
| TPK1 | 21mo | 29mo, 3yr 2mo, 3yr 5mo, 6yr 1mo |
Basal ganglia, dentate nuclei | [56] |
| SUOX (c.1096C > T; c.1376G > A) | 1yr | 2yr, 6.5yr | Globi pallidi, substantia nigra | [53] |
| SUOX (c.1096C > T; c.1376G > A | 1yr 2mo | 2yr 8mo | Globi pallidi, substantia nigra | [53] |
| SUOX (c.1096C > T; c.1376G > A) | 1yr 4mo | 3yr 10mo | Globi pallidi, substantia nigra | [53] |
| NDUFS4 (c.355G > C) | 1yr | 2yr 5mo 3yr 9mo | Thalami, brainstem (f/u MRI showing new abnormal signal in medulla) | [52] |
| MT-ATP6 (m.8993T > G) | n/r | 32mo later | Putamen | [50] |
| MT-ND4 (m.11778G > A) | n/r | n/r | Brainstem and basal ganglia lesions at | [50] |
| MT - ND6 (m.14484T > C) | baseline; only oculomotor nuclei in the f/u study | |||
| MECR | n/r | n/r | Brainstem and basal ganglia at baseline; mildly evident basal ganglia in the f/u study | [50] |
| PDHA1 (c.1132C > T) | 16mo | 28mo | Globi pallidi | [38] |
| PDHA1 (c.615C > G) | 1yr 9mo | 4yr 1mo | Globi pallidi | [38] |
| NDUFAF3 | 9mo | 5yr | Caudate, putamen, thalami, red nuclei | Case 1 |
| PDHA1 | 16mo | 4yr | Dentate nuclei | Case 2 |
n/r, not reported; f/u, follow up; PDHC, pyruvate dehydrogenase complex; COX, cytochrome C oxidase; MRI, magnetic resonance imaging.
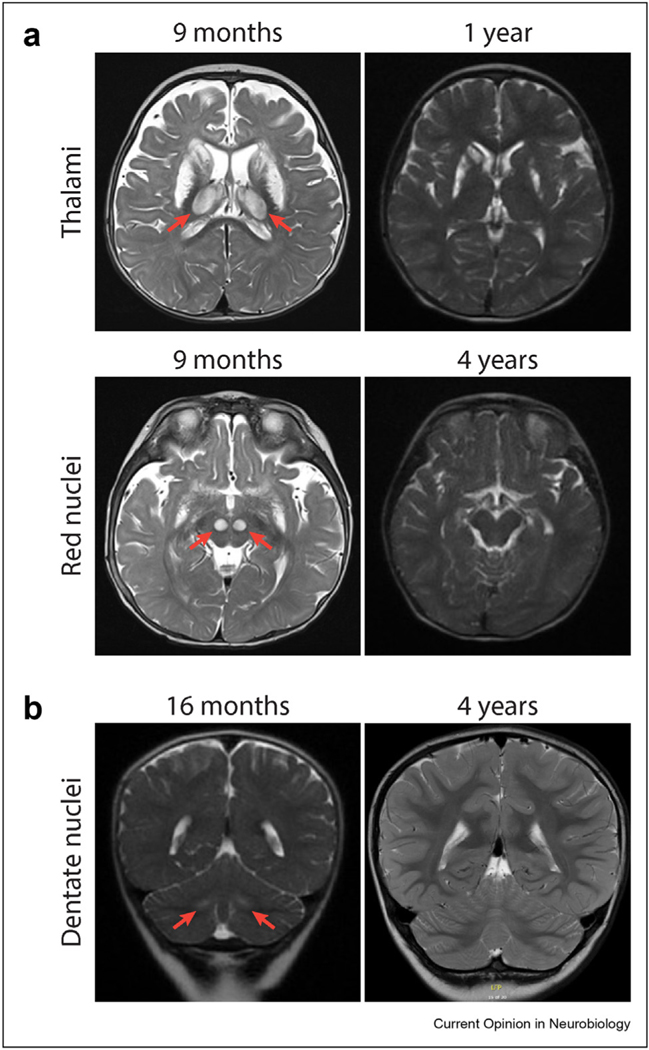
We report two new cases of transient LS brain lesions. Case 1 is a 10-year-old girl who first presented at age 9 months with failure to thrive and stridor. The MRI brain obtained at that time revealed T2 hyperintense bilateral lesions of the caudate nuclei and putamina, thalami, and red nuclei. CSF lactate was elevated at ∼5 mM. DNA sequencing revealed compound heterozygous mutations in mitochondrial complex I (CI) assembly factor NDUFAF3 (c.489_490delTG and p.Y11D). She began taking α-Tocotrienol quinone in a clinical trial as a toddler and continues on this medication. At age 4 years, she was nonambulatory but had made significant developmental progress, with 2–3 spoken words and the ability to point to multiple people or body parts on command. The T2 half-Fourier single-shot turbo spin-echo (HASTE) brain sequence demonstrated the decreased total area of T2 hyperintensity of the basal ganglia lesions and apparent regression of basal ganglia, thalami, and red nuclei signal abnormalities (Figure 1A). Case 2 is a 9-year-old boy who initially presented at age 16 months with ataxia, decreased arousal, and seizures. The MRI brain at that time demonstrated bilateral, symmetric T2 hyperintensities of the dentate nuclei, and lactate peaks on magnetic resonance spectroscopy. Enzyme and genetic testing confirmed defects in the pyruvate dehydrogenase complex subunit PDHA1 (hemizygous p.L5P), and ketogenic diet was initiated. The repeat MRI brain at age 4 years showed interval decrease in T2 hyperintense lesions (Figure 1B). At age 8 years, he speaks in full sentences and ambulates independently.
Figure 1.
T2-weighted MRI showing resolution of lesions. (a) Patient 1 basal ganglia and thalamic lesions at age 9 months with resolution of thalamic lesions at age 4 years (top) and red nuclei lesions at 9 months with resolution at age 4 years (bottom). (b) Patient 2 demonstrates improvement of dentate nuclei hyperintensities at age 16 months with marked improvement at age 4 years. MRI, magnetic resonance imaging.
We identified more than 32 published cases of LS with radiographic resolution of some or all lesions [7–9,38–50,52–54] (Table 2). Age of onset ranged from birth to 22 years. Twenty-one patients carried a genetically confirmed diagnosis. Eight patients carried mitochondrial DNA (mtDNA) mutations, 13 had nuclear gene defects, with only two of these occurring in genes functioning outside of the mitochondrion (SLC19A3 [54,55] and TPK1 [56]). Transient lesions were located either in the basal ganglia (18/30), the brainstem (1/30), or in multiple brain regions (basal ganglia and the brainstem [4/30], basal ganglia and thalami [1/30], basal ganglia and the cerebellum [4/30], the brainstem and thalami [2/30]). Although most cases describe bilateral regression of lesions, asymmetric resolution of caudate lesions was observed in two patients. Transient lesions often recurred in the setting of illness or metabolic crises, which are well known to be preciptants of disease progression [40,41,48]. Among published cases, 16 patients’ improved MRIs coincided with clinical improvement at the time of publication [8,38,40,42–46,48,49,52–54]. In some cases, this improvement was coincident with a treatment including ketogenic diet [38,44], rapamycin/steroids/n-acetylcysteine [52], coenzyme Q10 [49], or biotin and thiamine supplementation [54,57]. Critically, no contemporaneous histology has been reported for these intermediate states in which T2 hyperintensity had been observed but had later regressed, and the availability of associated clinical data is variable making it difficult to correlate imaging findings with clinical course.
Inherited or acquired thiamine deficiency: a special case of treatable lesions in humans
Owing to shared features in the disease mechanism (Table 1), clinical presentation, radiographic, and histological findings, comparison of LS to Wernicke Korsakoff syndrome (WKS) — which is often reversible with treatment and comparatively well-understood — merits special attention. WKS results from dietary deficiency of and is treated with supplementation of the essential vitamin thiamine (B1), which is a coenzyme component for several mitochondrial enzymes, including the pyruvate dehydrogenase complex, a known genetic cause of LS [1]. It is increasingly recognized that WKS has two phenotypes: one arising purely from nutritional deficiency; and a nutritional deficiency in the setting of alcohol use disorder [31]. The former presents in the acute phase (Wernicke encephalopathy) with hearing loss in addition to the classic triad of confusion, oculomotor dysfunction, and ataxia, although hearing impairment is not a feature of alcohol use disorder-related disease [58]. In WKS, T2 hyperintensities are found in the bilateral mammillary bodies, thalami, tectal plate, and periaqueductal gray matter. Notably, nonalcohol use disorder patients — who are more frequently pediatric — may additionally have signal abnormalities of the basal ganglia, particularly the putamina [31]. The mammillary bodies are uniformly affected in WKS but never to our knowledge in LS (Table 1). The radiographic appearance and postmortem histology of WKS and LS are similar, with both characterized by relative neuronal sparing and striking gliosis, but unlike LS, WKS often also demonstrates evidence of prior hemorrhage [59]. With prompt thiamine supplementation [31], most patients with WKS will experience significant clinical recovery, and resolution of brain lesions after treatment has been reported [60].
Intriguingly, although most LS disease genes encode mitochondrial proteins involved in energy metabolism, two of the genetic causes of LS involve perturbations of thiamine metabolism (Table 1), raising the possibility of shared pathogenesis between WKS and LS. LS-linked genes SLC19A3 and TPK1 encode the plasma membrane and cytosolic proteins required for thiamine transport into cells and its processing, respectively. In contrast to WKS, mutations in these genes lead to lesions in brain regions entirely consistent with LS and comprise the very small subset of treatable forms of LS that exhibit reversibility with high doses of thiamine [56,61].
Improvement of Leigh syndrome brain disease in a mouse model
The best-characterized animal model of LS is the mitochondrial CI accessory subunit Ndufs4 knockout (Ndufs4−/−) mouse developed by the Palmiter laboratory [62]. CI deficiency is a common cause of LS, and mutations in NDUFS4 can, rarely, cause LS in humans [63]. Ndufs4−/− mice are born at term and show little or no apparent disease till about 5 weeks when they develop progressive encephalopathy and die around 7 weeks [64]. The Ndufs4−/− have reduced body weight and temperature, ataxia, seizures, lethargy, blindness, and irregular breathing [64,64,65]. These mice typically develop bilateral symmetric gray matter lesions in the brainstem (vestibular nuclei), cerebellum, and olfactory bulb. Although the neuroanatomy of these lesions is consistent with the aforementioned definition of LS [1], vestibular nuclei lesions have also specifically been reported in humans [68], and cerebellar lesions are a common feature in patients with LS, it is notable that these mice do not exhibit lesions in the basal ganglia [64]. Similar to humans, the Ndufs4−/− lesions are characterized by gliosis with prominent microglial activation, vascular proliferation, neuronal loss, and vacuolation [64]. Furthermore, the lesions identified by histopathology correlate with T2-weighted MRI hyperintensities [65]. Other murine mitochondrial encephalopathy models have been reported [69–72], but the correlation of the histopathology with radiologic findings has only been firmly established for the Ndufs4−/−.
Multiple therapeutic approaches have been tested in the Ndufs4−/− mouse. Recent gene therapy strategies to re-express mouse Ndufs4 systemically restored lifespan and healthspan in the Ndufs4−/− [73]. Repletion of nicotinamide adenine dinucleotide (NAD+) provided moderate extension of the the Ndufs4−/− lifespan from 60 to 100 days [74,75]. Interestingly, ectopic expression of the yeast NADH dehydrogenase NDI1 in the brain rescued the lifespan of the brain-specific Ndufs4 knockout, but the mice still had severe ataxia [76]. It is unclear whether the beneficial effect of NDI1 is specific to restoring NAD+ redox balance or of other downstream effects of electron transport chain inhibition (i.e. redox of the coenzyme Q pool, oxygen consumption, proton pumping by downstream complexes, or mitochondrial ATP). Surprisingly, targeting reactive oxygen species with a potent antioxidant (KH176) [77] or transgenic expression of metallothionein 1 [78] had no effect on lifespan. Rapamycin and doxycycline improved healthspan and doubled the lifespan of Ndufs4−/− [79–82].
The most powerful intervention to date in this model, comparable to gene therapy, has been chronic, continuous exposure to mild hypoxia (11% oxygen), which led to a dramatic extension of lifespan and healthspan, with these mice now living for more than 270 days. Moreover, radiologic (T2 MRI signal abnormalities) and histologic staining of neuroinflammatory microglial marker ionized calcium binder adaptor moleculare-1 (IBA1) show no evidence of the lesions in hypoxia-treated mice [83,84]. Although rapamycin, doxycycline, and hypoxia prevent neuroinflammation, anti-inflammatory drug tacrolimus had no beneficial effect [79] suggesting that the mechanism(s) of action of successful interventions go beyond blocking inflammation. To our knowledge, spontaneous recovery or reversal — partial or complete, by imaging or histology — has never been documented in this mouse model.
Treatment of Ndufs4−/− with hypoxia (11% oxygen) initiated at advanced disease (7 weeks), when the mice present advanced symptoms including radiologic abnormalities and are close to fulfilling humane euthanasia criteria, clinically reverses disease and rescues the mice by improving their overall body weight and motor function, as well as substantially extending their lifespan. Given the uniformly progressive nature of the disease in this mouse model and that neurodegeneration has been reported at this timepoint [64], this dramatic improvement was surprising. Remarkably, in addition to clinical improvement, T2 MRI hyperintensities progressively decrease till they become almost undetectable after one month of hypoxia treatment, which correlates to a substantial reduction of neuroinflammation in histopathology at this age [84] (Figure 2).
Figure 2.
Radiologic and histopathologic reversal of lesions in the Ndufs4−/− mouse after hypoxia treatment. Top: T2 MRI; red arrows indicate hyperintensities in vestibular nuclei. Bottom: immunohistochemistry of coronal section labeling microglia with IBA1 and the nuclear counterstain DAPI (4′,6-diamidino-2-phenylindole). MRI adapted from Ferrari et al., 2017.
Although the mechanism of LS lesion improvement in the Ndufs4−/− remains unknown, partial reversibility in WKS disease rodent models has been reported. In a thiamine-deficient mouse model, thiamine treatment prevented further neuroinflammation and neuronal death only when administered before extensive neuronal loss occurs [85], indicating that treatment halts disease progression but does not fully reverse it, suggesting that inflammation preceding necrosis may be amenable to intervention. Differences in the ‘lesion age’ may explain why thiamine treatment in the analogous rat model normalized radiologic hyperintensities in the thalamus and colliculi but not in mammillary nuclei and lateral ventricles [86]. Unlike thiamine rescue in WKS models, pathologic phenotype, imaging abnormalities, and histology are all ameliorated by hypoxia even when it is implemented at very advanced disease stages and does not restore the primary CI biochemical defect in the Ndufs4−/− [83].
Radiographic improvement in lesions in the Ndufs4−/− has also been achieved experimentally with other interventions that reduce brain oxygen delivery including anemia and administration of sublethal carbon monoxide but not with constitutive activation of the hypoxia-inducible factor (HIF) pathway [87]. Ndufs4−/− mice exhibit a high partial pressure of brain oxygen, and hyperoxia worsens disease, potentially reflecting reduced oxygen extraction by brain mitochondria [87], a finding reminiscent of the decreased oxygen utilization observed in patients with mitochondrial myopathies [88].
Future outlook
In the 70 years since the initial description of LS, the field has made tremendous progress both in antemortem diagnosis enabled by MRI imaging and in defining more than 80 genetic causes for this disease. Although we typically think about LS as being a uniformly progressive and lethal disease, as we have discussed, there are a growing number of clinical case reports and now mouse models that support the notion that some of the antemortem imaging abnormalities observed in LS may not represent end-stage disease but may in fact represent an intermediate state in the disease that is far more dynamic and even reversible. In particular, mouse studies suggest that neuroinflammation is one of the features that is reversible and correlates with phenotypic and radiographic improvement.
At present, we do not have a clear understanding of how inherited mutations in any of greater than 80 different genes can lead to radiographically supported LS. Key open questions are as follows: (1) which are the dynamic neuropathological features of LS lesions evidenced by T2 MRI (e.g. neuroinflammation, edema)? (2) in addition to neuroinflammation, which aspects of intermediate disease are reversible? (3) why do infections or illness precipitate these lesions on imaging? (4) is there a critical treatment time window before irreversible tissue damage sets in? (5) is improvement caused by removal of pathogenic drivers (e.g. excess oxygen), by halting neuropathological effects of mitochondrial dysfunction (e.g. neuroinflammation), or instead by activating brain repair pathways?
The answers to these important questions remain unknown, but a detailed study of the human and mouse model literature yields a handful of common themes. As expected, repletion of cofactor deficiencies such as thiamine correlates temporally with brain lesion improvement in humans and rodents. It has also been shown that interventions to either buffer consequences of oxidative phosphorylation defects (e.g. redox imbalances) or reduce energetic demands (e.g. via mechanistic target of rapamycin (mTOR) inhibition) can delay brain disease and extend life in the mouse. Finally, capillary proliferation on histopathology and hyperperfusion of lesions during crises in humans supports a role for brain microvasculature in LS irrespective of genetic etiology. Strikingly, the radiographic differential diagnosis for LS lesions (Table 1) includes multiple processes in which pathogenic vasogenic edema — which may be associated with inflammation — has been implicated. These findings are of course insufficient to determine if hyperperfusion or any of its resultant effects — notable excess oxygenation — is causal or merely secondary findings in LS. However, bench research has produced exciting evidence that the presence of local hyperoxia — presumably unused oxygen substrate in the setting of deficient oxidative phosphorylation — may be driving disease progression and that removing it by various mechanisms improves disease phenotypes, neuroimaging, and even histology after disease onset. Having mouse models that share biochemical, histopathological, and radiographic features of LS combined with interventions that can tune the dynamics of the lesion will be a valuable resource to start addressing the key questions to understand and hopefully harness the dynamic nature of LS lesions for treatment.
Finally, it is critical to appreciate the dynamic nature of LS brain lesions in the context of therapeutic discovery and approval. If the underlying biology of the intermediate states of disease and their reversibility can be understood, it could imply that we can identify risk factors to be avoided and hopefully new medicines that directly target this biology for treating the scores of patients. Although such interventions may not fix the proximal genetic lesion, they could be very powerful in preventing disease progression. Mitochondrial diseases are extremely rare and heterogeneous, and although interventions in the past have been coincident with recovery in LS, we emphasize that most of the cases of radiographic improvement that we have reviewed here (Table 2) occurred spontaneously (e.g. studies reported by Koch et al. [7], Koch et al. [8], Arii and Tanabe [39], Sofou et al. [41], Roig et al. [43], and Alves et al. [50]). This underscores the need for proper natural history studies and rigorous control arms in clinical trials [89].
Patient cases
Written consent to publish case information was obtained from patients.
Acknowledgements
The authors thank Darryl DeVivo, Mel B. Feany, and Lance Rodan for valuable feedback.
Funding
The authors acknowledge support from the NIH K08NS117889 (MAW), Deutsche Forschungsgemeinschaft 431313887 (MM), the Marriott Foundation (VKM), the Howard Hughes Medical Institute (VKM), and the MacCurtain family (VKM).
Footnotes
Conflict of interest statement
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: VKM is listed as a coinventor on a patent on the therapeutic uses of hypoxia for mitochondrial diseases. VKM is on the Scientific Advisory Board of 5am Ventures and Janssen Pharmaceuticals.
References
Papers of particular interest, published within the period of review, have been highlighted as:
* of special interest
** of outstanding interest
- 1. Lake NJ, Compton AG, Rahman S, Thorburn DR: Leigh syn drome: one disorder, more than 75 monogenic causes. Ann Neurol 2016, 10.1002/ana.24551. * Outstanding review of the clinical definition and genetic basis of Leigh syndrome.
- 2. Leigh D: Subacute necrotizing encephalomyelopathy in an infant. J Neurol Neurosurg Psychiatry 1951, 10.1136/jnnp.14.3.216. **The first and classical description of subacute necrotizing encephalomyelopathy authored by the British neuropathologist Dennis Leigh after whom the disease is named. Presents a detailed description of the disease that remains the definitive reference for histopathology of LS.
- 3.Hommes FA, Polman HA, Reerink JD: Leigh’s encephalomyelopathy: an inborn error of gluconeogenesis. Arch Dis Child 1968,43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Devivo DC, Haymond MW, Obert KA, Nelson JS, Pagliara AS: Defective activation of the pyruvate dehydrogenase complex in subacute necrotizing encephalomyelopathy (Leigh disease). Ann Neurol 1979,6. [DOI] [PubMed] [Google Scholar]
- 5.Willems JL, Monnens LA, Trijbels JM, Veerkamp JH, Meyer AE, van Dam K, van Haelst U:Leigh’s encephalomyelopathy in a patient with cytochrome c oxidase deficiency in muscle tissue. Pediatrics 1977, 60:850–857. [PubMed] [Google Scholar]
- 6.Hall K, Gardner-Medwin D: CT scan appearances in Leigh’s disease (subacute necrotizing encephalomyelopathy). Neuroradiology 1978, 10.1007/BF00395200. [DOI] [PubMed] [Google Scholar]
- 7.Koch TK, Lo WD, Berg BO:Variability of serial CT scans in subacute necrotizing encephalomyelopathy (Leigh disease). Pediatr Neurol 1985, 1:48–51. [DOI] [PubMed] [Google Scholar]
- 8.Koch TK, Yee MHC, Hutchinson HT, Berg BO: Magnetic resonance imaging in subacute necrotizing encephalomyelopathy (Leigh’s disease). Ann Neurol 1986, 10.1002/ana.410190617. [DOI] [PubMed] [Google Scholar]
- 9. Bonfante E, Koenig MK, Adejumo RB, Perinjelil V, Riascos RF: The neuroimaging of Leigh syndrome: case series and review of the literature. Pediatr Radiol 2016, 10.1007/s00247-015-3523-5. **A case series of 17 patients with MR imaging and genetic findings.
- 10.Harrison M: The Massachusetts general hospital handbook of neurology. 2nd ed. Lippincott Williams & Wilkins; 2008. [Google Scholar]
- 11.Warach S, Gaa J, Siewert B, Wielopolski P, Edelman RR: Acute human stroke studied by whole brain echo planar diffusion-weighted magnetic resonance imaging. Ann Neurol 1995, 37. [DOI] [PubMed] [Google Scholar]
- 12.Barkovich AJ, Westmark K, Partridge C, Sola A, Ferriero DM: Perinatal asphyxia: MR findings in the first 10 days. Am J Neuroradiol 1995, 16. [PMC free article] [PubMed] [Google Scholar]
- 13.Rodríguez-Pardo J, Puertas-Muñoz I, Martínez-Sánchez P, De Terán JD, Pulido-Valdeolivas I, Fuentes B:Putaminainvolvement in Wernicke encephalopathy induced by Janus Kinase2 inhibitor. Clin Neuropharmacol 2015, 38. [DOI] [PubMed] [Google Scholar]
- 14.Krauss JK, Mohadjer M, Wakhloo AK, Mundinger F: Dystonia and akinesia due to pallidoputaminal lesions after disulfiram intoxication. Mov Disord 1991, 6. [DOI] [PubMed] [Google Scholar]
- 15.Ku MC, Huang CC, Kuo HC, Yen TC, Chen CJ, Shih TS, Chang HY: Diffuse white matter lesions in carbon disulfide intoxication: microangiopathy or demyelination. Eur Neurol 2003, 50. [DOI] [PubMed] [Google Scholar]
- 16.Pearl PL, Vezina LG, Saneto RP, McCarter R, Molloy-Wells E, Heffron A, Trzcinski S, McClintock WM, Conry JA, Elling NJ, et al. : Cerebral MRI abnormalities associated with vigabatrin therapy. Epilepsia 2009, 50. [DOI] [PubMed] [Google Scholar]
- 17.Löbel U, Eckert B, Simova O, Meier-Cillien M, Kluge S, Gerloff C, Röther J, Magnus T, Fiehler J: Cerebral magnetic resonance imaging findings in adults with haemolytic uraemic syndrome following an infection with Escherichia coli, subtype O104:H4. Clin Neuroradiol 2014, 24. [DOI] [PubMed] [Google Scholar]
- 18.Handique SK: Viral infections of the central nervous system. Neuroimaging Clin 2011,21. [DOI] [PubMed] [Google Scholar]
- 19.Collie DA, Summers DM, Sellar RJ, Ironside JW, Cooper S, Zeidler M, Knight R, Will RG: Diagnosing variant CreutzfeldtJakob disease with the pulvinar sign: MR imaging findings in 86neuropathologically confirmed cases. Am J Neuroradiol 2003, 24. [PMC free article] [PubMed] [Google Scholar]
- 20.Singh P, Goraya JS, Gupta K, Saggar K, Ahluwalia A:Magnetic resonance imaging findings in reye syndrome: case report and review of the literature. J Child Neurol 2011,26. [DOI] [PubMed] [Google Scholar]
- 21.Schiess N, Villabona-Rueda A, Cottier KE, Huether K, Chipeta J, Stins MF: Pathophysiology and neurologic sequelae of cerebral malaria. Malar J 2020, 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.King AD, Walshe JM, Kendall BE, Chinn RJS, Paley MNJ, Wilkinson ID, Halligan S, Hall-Craggs MA: Cranial MR imaging in Wilson’s disease. Am J Roentgenol 1996, 167. [DOI] [PubMed] [Google Scholar]
- 23.Huang BY, Castillo M: Hypoxic-Ischemic brain injury: imaging findings from birth to adulthood. Radiographics 2008, 28. [DOI] [PubMed] [Google Scholar]
- 24.Ahmed MI, Hussain N: Neuroimaging in menkes disease. J Pediatr Neurosci 2017, 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Arraj P, Robbins K, Dengle Sanchez L, Veltkamp DL, Pfeifer CM: MRI findings in juvenile Huntington’s disease. Radiol Case Rep 2021, 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vanderver A:Genetic leukoencephalopathies in adults. Contin Lifelong Learn Neurol 2016, 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wajner M: Neurological manifestations of organic acidurias. Nat Rev Neurol 2019, 15. [DOI] [PubMed] [Google Scholar]
- 28.Leuzzi V, Bianchi MC, Tosetti M, Carducci C, Cerquiglini A, Cioni G, Antonozzi I:Brain creatine depletion: guanidinoacetate methyltransferase deficiency (improving with creatine supplementation). Neurology 2000, 55. [DOI] [PubMed] [Google Scholar]
- 29.Burns CM, Rutherford MA, Boardman JP, Cowan FM: Patterns of cerebral injury and neurodevelopmental outcomes after symptomatic neonatal hypoglycemia. Pediatrics 2008, 122. [DOI] [PubMed] [Google Scholar]
- 30.Hernandez-Lain A, Hedley-Whyte ET, Hariri LP, Molyneaux B, Nagle KJ, Cole AJ, Kilbride R: Pathology of bilateral pulvinar degeneration following long duration status epilepticus. Seizure 2013, 22. [DOI] [PubMed] [Google Scholar]
- 31.Manzo G, De Gennaro A, Cozzolino A, Serino A, Fenza G, Manto A: MR imaging findings in alcoholic and nonalcoholic acute Wernicke’s encephalopathy: a review. BioMed Res Int 2014, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Martich-Kriss V, Kollias SS, Ball WS:MR findings in kernicterus. Am J Neuroradiol 1995, 16. [PMC free article] [PubMed] [Google Scholar]
- 33.Brown WD, Caruso JM: Extrapontine myelinolysis with involvement of the hippocampus in three children with severe hypernatremia. J Child Neurol 1999, 14. [DOI] [PubMed] [Google Scholar]
- 34.Kim DM, Lee IH, Park JY, Hwang SB, Yoo DS, Song CJ: Acute carbon monoxide poisoning: MR imaging findings with clinical correlation. Diagn Interv Imag 2017, 98. [DOI] [PubMed] [Google Scholar]
- 35.Kim E, Na DG, Kim EY, Kim JH, Son KR, Chang KH:MR imaging of metronidazole-induced encephalopathy: lesion distribution and diffusion-weighted imaging findings. Am J Neuroradiol 2007, 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kissel JT, Kolkin S, Chakeres D, Boesel C, Weiss K: Magnetic resonance imaging in a case of autopsy-proved adult subacute necrotizing encephalomyelopathy (Leigh’s Disease). Arch Neurol 1987, 10.1001/archneur.1987.00520170089030. [DOI] [PubMed] [Google Scholar]
- 37.Loiselet K, Ruzzenente B, Roux CJ, Barcia G, Pennisi A, Desguerre I, Rötig A, Munnich A, Boddaert N, Levy R, et al. : Cerebral blood flow and acute episodes of Leigh syndrome in neurometabolic disorders. Dev Med Child Neurol 2021, 10.1111/dmcn.14814. [DOI] [PubMed] [Google Scholar]
- 38.Shelkowitz E, Ficicioglu C, Stence N, Van Hove J, Larson A: Serial Magnetic Resonance Imaging (MRI) in Pyruvate Dehydrogenase Complex Deficiency. J Child Neurol 2020, 35: 137–145. [DOI] [PubMed] [Google Scholar]
- 39. Arii J, Tanabe Y:Leigh syndrome: Serial MR imaging clinical follow-up. Am J Neuroradiol 2000, 21. ** The first case series reporting fluctuating MRI findings in a Leigh Syndrome cohort.
- 40.van Dongen S, Brown RM, Brown GK, Thorburn DR, Boneh A: Thiamine-responsive and non-responsive patients with PDHC-E1 deficiency: A retrospective assessment. In JIMD Reports; 2015:13–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sofou K, Steneryd K, Wiklund LM, Tulinius M, Darin N:MRI of the brain in childhood-onset mitochondrial disorders with central nervous system involvement. Mitochondrion 2013, 13. [DOI] [PubMed] [Google Scholar]
- 42.Kimura S, Osaka H, Saitou K, Ohtuki N, Kobayashi T, Nezu A: Improvement of lesions shown on MRI and CT scan by administration of dichloroacetate in patients with Leigh syndrome. J Neurol Sci 1995, 134. [DOI] [PubMed] [Google Scholar]
- 43.Roig M, Macaya A, Munell F, Capdevila A:Acute neurologic dysfunction associated with destructive lesions of the basal ganglia: A benign form of infantile bilateral striatal necrosis. J Pediatr 1990, 117. [DOI] [PubMed] [Google Scholar]
- 44.Wijburg FA, Barth PG, Bindoff LA, Birch-Machin MA, Van der Blij JF, Ruitenbeek W, Turnbull DM, Schutgens RBH: Leigh syndrome associated with a deficiency of the pyruvate dehydrogenase complex: Results of treatment with a ketogenic diet. Neuropediatrics 1992, 23. [DOI] [PubMed] [Google Scholar]
- 45.Goldenberg PC, Steiner RD, Merkens LS, Dunaway T, Egan RA, Zimmerman EA, Nesbit G, Robinson B, Kennaway NG: Remarkable improvement in adult Leigh syndrome with partial cytochrome c oxidase deficiency. Neurology 2003, 60. [DOI] [PubMed] [Google Scholar]
- 46.El-Hajj TI, Karam PE, Mikati MA: Biotin-responsive basal ganglia disease: Case report and review of the literature. Neuropediatrics 2008, 39. [DOI] [PubMed] [Google Scholar]
- 47.Yamada K, Naiki M, Hoshino S, Kitaura Y, Kondo Y, Nomura N, Kimura R, Fukushi D, Yamada Y, Shimozawa N, et al. : Clinical and biochemical characterization of 3-hydroxyisobutyryl-CoA hydrolase (HIBCH) deficiency that causes Leigh-like disease and ketoacidosis. Mol Genet Metabol Rep 2014, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Giribaldi G, Doria-Lamba L, Biancheri R, Severino M, Rossi A, Santorelli FM, Schiaffino C, Caruso U, Piemonte F, Bruno C: Intermittent-relapsing pyruvate dehydrogenase complex deficiency: A case with clinical, biochemical, and neuroradiological reversibility. Dev Med Child Neurol 2012, 54. [DOI] [PubMed] [Google Scholar]
- 49.Chen Z, Zhao Z, Ye Q, Chen Y, Pan X, Sun B, Huang H, Zheng A:Mild clinical manifestation and unusual recovery upon coenzyme Q10 treatment in the first Chinese Leigh syndrome pedigree with mutation m.10197 G>A. Mol Med Rep 2015, 11. [DOI] [PubMed] [Google Scholar]
- 50. Alves CAPF, Teixeira SR, Martin-Saavedra JS, Guimares Gonçalves F, Lo Russo F, Muraresku C, McCormick EM, Falk MJ, Zolkipli-Cunningham Z, Ganetzky R, et al. : Pediatric Leigh Syndrome: Neuroimaging Features and Genetic Correlations. Ann Neurol 2020, 88. **A series of 53 patients with Leigh Syndrome with baseline and follow up MRI studies, laboratory studies, clinical and genetic findings.
- 52.Sage-Schwaede A, Engelstad K, Salazar R, Curcio A, Khandji A, Garvin JH, De Vivo DC: Exploring mTOR inhibition as treatment for mitochondrial disease. Ann Clin Transl Neurol 2019, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tian M, Qu Y, Huang L, Su X, Li S, Ying J, Zhao F, Mu D:Stable clinical course in three siblings with late-onset isolated sulfite oxidase deficiency: A case series and literature review. BMC Pediatr 2019, 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Aljabri MF, Kamal NM, Arif M, AlQaedi AM, Santali EYM: A case report of biotin-thiamine-responsive basal ganglia disease in a Saudi child: Is extended genetic family study recommended? Med (United States) 2016, 95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yamada K, Miura K, Hara K, Suzuki M, Nakanishi K, Kumagai T, Ishihara N, Yamada Y, Kuwano R, Tsuji S, et al. : A wide spectrum of clinical and brain MRI findings in patients with SLC19A3 mutations. BMC Med Genet 2010, 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Invernizzi F, Panteghini C, Chiapparini L, Moroni I, Nardocci N, Garavaglia B, Tonduti D: Thiamine-responsive disease due to mutation of tpk1: Importance of avoiding misdiagnosis. Neurology 2017, 89. [DOI] [PubMed] [Google Scholar]
- 57.Gerards M, Kamps R, Van Oevelen J, Boesten I, Jongen E, De Koning B, Scholte HR, De Angst I, Schoonderwoerd K, Sefiani A, et al. : Exome sequencing reveals a novel Moroccan founder mutation in SLC19A3 as a new cause of early-childhood fatal Leigh syndrome. Brain 2013, 136. [DOI] [PubMed] [Google Scholar]
- 58.Walker MA, Zepeda R, Afari HA, Cohen AB: Hearing loss in Wernicke encephalopathy. Neurol Clin Pract 2014, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Victor M, Adams RD, Collins G:The Wernicke-Korsakoff Syndrome and related Neurological Disorders of Alcoholism and Malnutrition. In The Wernicke-Korsakoff syndrome and related neurological disorders of alcoholism and Malnutrition. FA Davis Co.; 1989. [Google Scholar]
- 60.Sparacia G, Anastasi A, Speciale C, Agnello F, Banco A:Magnetic resonance imaging in the assessment of brain involvement in alcoholic and nonalcoholic Wernicke’s encephalopathy. World J Radiol 2017, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kassem H, Wafaie A, Alsuhibani S, Farid T:Biotin-responsive basal ganglia disease: Neuroimaging features before and after treatment. Am J Neuroradiol 2014, 35: 1990–1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kruse SE, Watt WC, Marcinek DJ, Kapur RP, Schenkman KA, Palmiter RD: Mice with Mitochondrial Complex I Deficiency Develop a Fatal Encephalomyopathy. Cell Metabol 2008, 7: 312–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lake NJ, Bird MJ, Isohanni P, Paetau A:Leigh Syndrome: Neuropathology and Pathogenesis. J Neuropathol Exp Neurol 2015, 74: 482–492. [DOI] [PubMed] [Google Scholar]
- 64. Quintana A, Kruse SE, Kapur RP, Sanz E, Palmiter RD: Complex I deficiency due to loss of Ndufs4 in the brain results in progressive encephalopathy resembling Leigh syndrome. Proc Natl Acad Sci U S A 2010:107. **The authors present the neuropathology of the Ndufs4−/− mouse model, previously generated by the same group, establishing it as the first mouse model of LS.
- 65.Quintana A, Zanella S, Koch H, Kruse SE, Lee D, Ramirez JM, Palmiter RD: Fatal breathing dysfunction in a mouse model of Leigh syndrome. J Clin Invest 2012, 122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Quintana A, Zanella S, Koch H, Kruse SE, Lee D, Ramirez JM, Palmiter RD: Fatal breathing dysfunction in a mouse model of Leigh syndrome. J Clin Invest 2012, 122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Quintana A, Zanella S, Koch H, Kruse SE, Lee D, Ramirez JM, Palmiter RD: Fatal breathing dysfunction in a mouse model of Leigh syndrome. J Clin Invest 2012, 122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Montpetit VJA, Andermann F, Carpenter S, Fawcett JS, Zborowskasluis D, Giberson HR: Subacute necrotizing encephalomyelopathy: A review and a study of two families. Brain 1971, 94. [DOI] [PubMed] [Google Scholar]
- 69.Al Khazal F, Holte MN, Bolon B, White TA, LeBrasseur N, Maher LJ:A conditional mouse model of complex II deficiency manifesting as Leigh-like syndrome. Faseb J 2019, 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Salama M, El-Desouky S, Alsayed A, El-Hussiny M, Moustafa A, Taalab Y, Mohamed W:FOXRED1 silencing in mice: a possible animal model for Leigh syndrome. Metab Brain Dis 2019, 34. [DOI] [PubMed] [Google Scholar]
- 71.Spinazzi M, Radaelli E, Horré K, Arranz AM, Gounko NV, Agostinis P, Maia TM, Impens F, Morais VA, Lopez-Lluch G, et al. : PARL deficiency in mouse causes Complex III defects, coenzyme Q depletion, and Leigh-like syndrome. Proc Natl Acad Sci U S A 2019, 116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.García-Corzo L, Luna-Sánchez M, Doerrier C, García JA, Guarás A, Acín-Pérez R, Bullejos-Peregrín J, López A, Escames G, Enríquez JA, et al. : Dysfunctional coq9 protein causes predominant encephalomyopathy associated with CoQ deficiency. Hum Mol Genet 2013, 22. [DOI] [PubMed] [Google Scholar]
- 73.Reynaud-Dulaurier R, Benegiamo G, Marrocco E, Al-Tannir R, Surace EM, Auwerx J, Decressac M: Gene replacement therapy provides benefit in an adult mouse model of Leigh syndrome. Brain 2020, 143. [DOI] [PubMed] [Google Scholar]
- 74.Lee CF, Caudal A, Abell L, Nagana Gowda GA, Tian R:Targeting NAD + Metabolism as Interventions for Mitochondrial Disease. Sci Rep 2019, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Grange RMH, Sharma R, Shah H, Reinstadler B, Goldberger O, Cooper MK, Nakagawa A, Miyazaki Y, Hindle AG, Batten AJ, et al. : Hypoxia ameliorates brain hyperoxia and NAD+ deficiency in a murine model of Leigh syndrome. Mol Genet Metabol 2021, 10.1016/j.ymgme.2021.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.McElroy GS, Reczek CR, Reyfman PA, Mithal DS, Horbinski CM, Chandel NS: NAD+ Regeneration Rescues Lifespan, but Not Ataxia, in a Mouse Model of Brain Mitochondrial ComplexI Dysfunction. Cell Metabol 2020, 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.De Haas R, Das D, Garanto A, Renkema HG, Greupink R, Van Den Broek P, Pertijs J, Collin RWJ, Willems P, Beyrath J, et al. : Therapeutic effects of the mitochondrial ROS-redox modulator KH176 in a mammalian model of Leigh Disease. Sci Rep 2017, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Miller HC, Louw R, Mereis M, Venter G, Boshoff JD, Mienie L, van Reenen M, Venter M, Lindeque JZ, Domínguez-Martínez A, et al. : Metallothionein 1 Overexpression Does Not Protect Against Mitochondrial Disease Pathology in Ndufs4 Knockout Mice. Mol Neurobiol 2021,58. [DOI] [PubMed] [Google Scholar]
- 79.Johnson SC, Yanos ME, Kayser EB, Quintana A, Sangesland M, Castanza A, Uhde L, Hui J, Wall VZ, Gagnidze A, et al. : MTOR inhibition alleviates mitochondrial disease in a mouse model of Leigh syndrome. Science (80-) 2013,342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Felici R, Cavone L, Lapucci A, Guasti D, Bani D, Chiarugi A: PARP Inhibition Delays Progression of Mitochondrial Encephalopathy in Mice. Neurotherapeutics 2014, 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Martin-Perez M, Grillo AS, Ito TK, Valente AS, Han J, Entwisle SW, Huang HZ, Kim D, Yajima M, Kaeberlein M, et al. : PKC downregulation upon rapamycin treatment attenuates mitochondrial disease. Nat Metabol 2020, 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Perry EA, Bennett CF, Luo C, Balsa E, Jedrychowski M, O’Malley KE, Latorre-Muro P, Ladley RP, Reda K, Wright PM, et al. : Tetracyclines promote survival and fitness in mitochondrial disease models. Nat Metabol 2021, 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Jain IH, Zazzeron L, Goli R, Alexa K, Schatzman-Bone S, Dhillon H, Goldberger O, Peng J, Shalem O, Sanjana NE, et al. : Hypoxia as a therapy for mitochondrial disease. Science (80-) 2016, 10.1126/science.aad9642. **Starting from a CRISPR screen for suppressors of mitochondrial disfunction, theauthors present for the first time the discovery that hypoxia can prevent mitochondrial disease in the Ndufs4−/− mouse model of LS.
- 84. Ferrari M, Jain IH, Goldberger O, Rezoagli E, Thoonen R, Chen KH, Sosnovik DE, Scherrer-Crosbie M, Mootha VK, Zapol WM: Hypoxia treatment reverses neurodegenerative disease in a mouse model of Leigh syndrome. Proc Natl Acad Sci U S A 2017,114: E4241–E4250. **This study shows that ambient hypoxia can reverse disease in the Ndufs4−/− mouse model of LS.
- 85.Ke ZJ, DeGiorgio LA, Volpe BT, Gibson GE: Reversal of thiamine deficiency-induced neurodegeneration. J Neuropathol Exp Neurol 2003, 62. [DOI] [PubMed] [Google Scholar]
- 86.Pfefferbaum A, Adalsteinsson E, Bell RL, Sullivan EV: Development and resolution of brain lesions caused by pyrithiamine- and dietary-induced thiamine deficiency and alcohol exposure in the alcohol-preferring rat: A longitudinal magnetic resonance imaging and spectroscopy study. Neuropsychopharmacology 2007, 32. [DOI] [PubMed] [Google Scholar]
- 87.Jain IH, Zazzeron L, Goldberger O, Marutani E, Wojtkiewicz GR, Ast T, Wang H, Schleifer G, Stepanova A, Brepoels K, et al. : Leigh Syndrome Mouse Model Can Be Rescued by Interventions that Normalize Brain Hyperoxia, but Not HIF Activation. Cell Metabol 2019, 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Taivassalo T, Ayyad K, Haller RG: Increased capillaries in mitochondrial myopathy: Implications for the regulation of oxygen delivery. Brain 2012,135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Pfeffer G, Horvath R, Klopstock T, Mootha VK, Suomalainen A, Koene S, Hirano M, Zeviani M, Bindoff LA, Yu-Wai-Man P, et al. : New treatments for mitochondrial disease - No time to drop our standards. Nat Rev Neurol 2013, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]