Abstract
Background:
To evaluate urinary neopterin, a marker of pro-inflammatory state, as a potential biomarker of disease prognosis and progression in amyotrophic lateral sclerosis (ALS); and to compare its utility to urinary neurotrophin receptor p75 extracellular domain (p75ECD).
Methods:
Observational study including 21 healthy controls and 46 people with ALS, 29 of whom were sampled longitudinally. Neopterin and p75ECD were measured using enzyme-linked immunoassays. Baseline and longitudinal changes in clinical measures, neopterin and urinary p75ECD were examined, and prognostic utility explored by survival analysis.
Results:
At baseline, urinary neopterin was higher in ALS compared to controls (181.7 ± 78.9 μmol/mol creatinine vs 120.4 ± 60.8 μmol/mol creatinine, p= 0.002, Welch’s t-test) and correlated with ALSFRS-R (r= −0.36, p= 0.01). Combining previously published urinary p75ECD results from 22 ALS patients with a further 24 ALS patients, baseline urinary p75ECD was also higher compared to healthy controls (6.0 ± 2.7 vs 3.2 ± 1.0 ng/mg creatinine p<0.0001) and correlated with ALSFRS-R (r= −0.36, p= 0.01). Urinary neopterin and p75ECD correlated with each other at baseline (r= 0.38, p= 0.009). In longitudinal analysis, urinary neopterin increased on average (±SE) by 6.8 ± 1.1 μmol/mol creatinine per month (p<0.0001) and p75ECD by 0.19 ± 0.02 ng/mg creatinine per month (p<0.0001) from diagnosis in 29 ALS patients.
Conclusion:
Urinary neopterin holds promise as marker of disease progression in ALS and is worthy of future evaluation for its potential to predict response to anti-inflammatory therapies.
Keywords: ALS, Biomarker, Disease Progression, Pharmacodynamic, Proinflammatory
Graphical Abstract:
The pro-inflammatory marker urinary neopterin was investigated as a disease progression biomarker for ALS. It was also compared to urinary p75ECD. Urinary neopterin and p75ECD progressively increased from diagnosis. Urinary neopterin might serve as a biomarker of an underlying pro-inflammatory state in ALS.
INTRODUCTION
Biomarkers are widely believed to hold great potential for accelerating efforts to develop effective therapies for patients with amyotrophic lateral sclerosis (ALS). A critical issue is that potential biomarkers should be fit for purpose1,2. Prognostic markers, which aid in predicting the future course of disease, could be used to reduce patient heterogeneity and potentially to yield sample size savings in mid-phase clinical trials. Pharmacodynamic markers, a subset of which may also be markers of disease progression, have the potential to show that a biological response has occurred following administration of an experimental therapeutic; and thereby provide a rationale for advancing experimental compounds from phase 2 to phase 3 clinical trials. Significant progress has been made in developing generic biomarkers of neuronal degeneration that have utility as prognostic, disease progression and potential pharmacodynamic markers, with two notable examples being the urinary concentration of the extracellular domain of the common neurotrophin receptor p75 (p75ECD)3,4 and blood-based measurement of neurofilament light5. However, with the exception of genetic markers, which identify patients most likely to benefit from therapies targeting the underlying genetic cause of disease6, predictive biomarkers are lacking in other forms of ALS. This is not to say that biomarkers indicative of a particular biological mechanism (e.g CSF chitotriosidase-1 (Chit-1) as a marker of microglial activation 7 8, 9 10 11) have not been identified, but rather that none of these biomarkers predict response to a particular experimental (or approved) treatment. The development of such predictive biomarkers could transform how we identify subsets of ALS patients most likely to benefit from an experimental therapeutic with a particular mechanism of action.
Immune dysregulation has been identified as an important component of the underlying disease process in ALS, comprising both anti-inflammatory and pro-inflammatory phases12. It is hypothesised that the initial protective anti-inflammatory phase involves microglia in the central nervous system (CNS)13, as well as T-regulatory and T-helper type 2 (Th-2) cells in the periphery. Pre-clinical data suggest that this protective response emerges as motor neurons and neuromuscular junctions begin to degenerate14. Evidence from animal models15 suggest that this response shifts from anti- to pro-inflammatory as motor neurons continue to degenerate and accumulate ALS-associated protein aggregates. Microglia become pro-inflammatory15 14 and induce release of neurotoxic factors from astrocytes that can kill motor neurons16,17. In the periphery, the pro-inflammatory state is evidenced by a switch to T-helper cells types 1 and 17 (Th-1 and Th-17), as well as induction of cytotoxic CD8 cells, inflammatory monocytes and natural killer (NK) cells18. This cascade also results in Th-1 cell release of pro-inflammatory cytokines such as interleukins and interferon-γ (IFN-γ)12.
While neuroinflammation has been the target of several ALS clinical trials, including NP001, Tocilizumab, and Masitinib, results have been disappointing. This may, at least in part, reflect the limitations of existing markers of neuroinflammation, the extent to which they reflect a pro- vs. anti-inflammatory state, and the extent to which blood and CSF concentrations of these markers reflect inflammation in the CNS vs. the periphery. For example in the phase II placebo controlled trial of NP001 that aimed to inhibit the pro-inflammatory response19, baseline levels of plasma monocyte chemoattractant protein-1 (MCP-1) failed to identify the small group of responders in post-hoc analysis20; but the rationale for this analysis is unclear since MCP-1 has been defined as a Th-2 anti-inflammatory chemokine. Moreover, it is CSF levels of MCP-1 that are elevated in ALS21, 22, but this trial relied on plasma levels of MCP-123. A number of Th-1 cytokines (e.g. IL-18, IL-6) in CSF are also being investigated as markers of the pro-inflammatory state in the CNS, but there is sparse information on peripheral or systemic pro-inflammatory signals. The small molecule neopterin, released from macrophages and dendritic cells24 in the periphery in response to IFN-γ, and from microglia25 and neurons26 in the CNS, is a candidate marker of the pro-inflammatory state. Urinary neopterin has been used to assess the Th-1-type immune response in inflammatory conditions27,28 and previously suggested as a biomarker in ALS29,30, but has not been extensively studied. Here we have sought to understand the potential utility of urinary neopterin as a disease progression biomarker, with the knowledge that it might also serve as a biomarker of an underlying pro-inflammatory state. Secondarily, we compared urinary neopterin and p75ECD with respect to their potential utility as markers of disease progression.
Materials and Methods
Participant cohort and urine samples
Patients with ALS and healthy controls without underlying autoimmune disorder or immunosuppressive therapies, neurological disorders or untreated illness that affects kidney function were recruited between September 2011 and May 2021. All willing ALS patients and healthy controls were recruited without bias through the South Australian MND Clinic at Flinders Medical Centre and/or the ALS Research Program at the University of Miami; ALS patients were diagnosed according to the revised El Escorial criteria31. Written informed consent was obtained from all study participants following study approval from the Southern Adelaide Clinical Human Research Ethics Committee or the University of Miami IRB as appropriate. Study results were reported using the STROBE cohort reporting guidelines32.
Clinical information was collected by investigators blinded to urinary neopterin and p75ECD results and included ALSFRS-R scores; medications; timing of symptom onset, diagnosis and sample collection use of non-invasive ventilation, tracheostomy, and death. Baseline ΔFRS, the estimated monthly change in ALSFRS-R prior to enrolment, was calculated as (48 - ALSFRS-R at baseline) / number of months between symptom onset and baseline33. To determine the prognostic value of urinary biomarkers, we analyzed the association between baseline predictors and future survival in a time to event (death or censorship) analysis.
Urine samples were collected and stored as per Urine & Kidney Proteome Project Standards 34 and were coded to ensure participant anonymity. Samples were tested by urinalysis (Siemens Multistix) and samples with signs of infection (blood and high pH) or diabetes (e.g high glucose) were excluded. Samples collected in Miami were shipped on dry ice. All samples were stored in 200 μl aliquots at −80°C until analysis.
Urinary neopterin measurement
Urinary neopterin was quantified using the IBL International/Tecan competitive enzyme-linked immunoassay (RE59321) as per manufacturer’s instructions24. Briefly, urine samples were diluted 1:100 in buffer, and samples, standards and controls were added to plates, followed by enzyme conjugate and neopterin antiserum. Following incubation, plates were washed, and neopterin quantity visualised using a tetramethylbenzidine (TMB) reaction, with optical density measured at 450nm. Neopterin concentration was determined as per the manufacturer’s instructions using a four-parameter nonlinear inhibitor vs response standard curve using GraphPad Prism 9. Storage of urine at −80C was sufficient to prevent degradation of neopterin post storage, when measured by this ELISA35. The average coefficient of variation over 3 separate assays per sample in this study was 12.23 ± 5.61 % (range 0.6-19.34).
Urinary p75ECD measurement
Urinary p75ECD was quantified using a sandwich ELISA modified from that previously described 3. Our previously published ELISA used a monoclonal capture antibody (MLR1) and a polyclonal detection antibody3. The modified ELISA used the MLR1 capture antibody, and a monoclonal detection antibody (NGFR536, 2.0 μg/mL) that was biotinylated as per manufacturer’s instructions (Thermo Fisher Scientific Australia, #UG283022). The blocking and sample buffers were also changed from 2% bovine serum albumin in PBS to BlockAce (BioRad, BUF-029). The enzyme reaction was achieved as previously described3 using streptavidin horse radish peroxidase (Jackson ImmunoResearch Laboratories, #JIO16030084) diluted to 1.0 μg/mL and colour developed using TMB (A:B; BioRad Australia, #1721067). This assay was transferred to a Hamilton Starlet, integrated with an MD reader and Biotek 405 washer, to increase testing capacity. The average coefficient of variation over 3 separate assays per sample was 11.1 ± 5.3 % (range 1.6-18.2). We verified the new method by quantifying p75ECD in 69 urine samples using the modified assay, and comparing to previously published results3. There was a 97% association between the new method and our published results in quantified p75ECD levels (n=69 samples, CI: 0.96 to 0.98; see Passing-Bablok equation and correlation in Supplementary figure e-1). This also indicates there was no degradation of p75ECD measured post long-term (up to 10 years) storage of urine at −80C.
Urinary creatinine measurement
Urinary neopterin and p75ECD measurements were corrected for urine dilution using urinary creatinine3, expressing values as μmol neopterin/mol creatinine or ng p75ECD/ mg creatinine. Urinary creatinine was measured using Enzo Life Sciences Creatinine kits (ADI-907-030A) as per manufacturer’s instructions3. Samples with urinary creatinine below 0.3± 0.03 mg/ml, or above 3.0± 0.3 mg/ml were rejected as per WHO guidelines37. These indicate that urine samples with extremely low creatinine concentrations are too dilute and may distort detection of low levels of analyte measurement while extremely high creatinine concentrations indicate dehydration, which could have changed the kidney’s processing of the analyte.
Statistical analysis
Statistical analysis of correlation, logistic regression, Cox proportional-hazard models and longitudinal change (linear mixed model analysis, taking into account repeated measures) in urinary neopterin, p75ECD and ALSFRS-R, were examined using SPSS, R, and GraphPad Prism 9. Sensitivity and specificity were examined using receiver operating characteristic (ROC) analysis using the area under the curve (AUC) in GraphPad Prism 9. The cut-off levels for sensitivity and specificity of the ROC curves was determined using the Youden Index 38. All statistical analysis was done by B.B and M.LR.
Results
Study population
The study population included 21 healthy controls and 46 people with ALS, with longitudinal data available from n=29 (total of 103 person-visits). Most ALS (n=44) was sporadic, with no documented family history; genetic mutations were identified in two patients – one with a C9orf72 repeat expansion and one with an E101G (E100G) mutation in SOD1. Disease characteristics of the ALS population are similar to previously reported studies (Table-1)3 5.
Table 1:
Study participant characteristics
| Controls | ALS | |||
|---|---|---|---|---|
| Baseline | Longitudinal | |||
| N | 21 | 46 | 29 | |
| Age at baseline, years | Mean ± SD (range) | 60.6 ± 6.9 (49-71) | 66.0 ± 11.7 (42-85) | 65.7 ± 12.9 (42-84) |
| Age at diagnosis, years | Mean ± SD (range) | -- | 66.1 ± 11.7 (42-85) | 64.4 ± 11.4 (42-84) |
| Sex, male | N (%) | 10 (48%) | 33 (72%) | 22 (76%) |
| Familial disease | N (%) | -- | 2 (4.3%) | 0 |
| Bulbar onset | N (%) | -- | 17 (36%) | 11 (38%) |
| Months from onset to diagnosis | Mean ± SD (range) | -- | 10.4 ± 8.1 (0.4-41) | 10.4 ± 8.9 (0.4-41) |
| Months from diagnosis to baseline | Mean ± SD (range) | -- | 5.2 ± 6.8 (0.3-37) | 5.27 ± 5.6 (0.5-21) |
| Baseline ALSFRS-R | Mean ± SD (range) | -- | 39.8 ± 4.6 (27-47) | 41.4 ± 3.9 (31-47) |
| Baseline ΔFRS | Mean ± SD (range) | -- | 0.68 ± 0.5 (0.02-2.7) | 0.54 ± 0.3 (0.02-1.17) |
| Death | N (%) | -- | 44 (96%) | 27 (93%) |
| Disease duration, months a | Median (IQR) | -- | 19.5 (11.34-38.6) | 22.14 (18.3-41.9) |
| Sampling time points | Median (range) | -- | -- | 3 (2-6) |
ΔFRS = estimated rate of progression (change in ALSFRS-R per month from onset of weakness to baseline)
IQR = interquartile range (25th-75th percentile)
From diagnosis to death
Cross Sectional Analysis
Baseline urinary neopterin levels were elevated in people with ALS (n=46, 181.7 ± 78.5 μmol/mol creatinine, p= 0.002, Welch’s t-test) compared to controls (n= 21, 120.4 ± 60.8 μmol/mol creatinine; Fig 1A). Combining previously published urinary p75ECD results from 22 ALS patients (in red symbols)3 with data from an additional 24 ALS patients, urinary p75ECD was higher among the ALS patients compared to healthy individuals (6.0 ± 2.7 ng/mg creatinine vs. 3.2 ± 1.0 ng/mg creatinine, p<0.0001 (red symbols from 10 previously published data 3, Welch’s t-test, Fig 1B). The type of p75ECD assay (new or old) did not contribute to the significance (logistic regression; p= 0.729 for assay type and p75ECD x assay type [p= 0.437]).
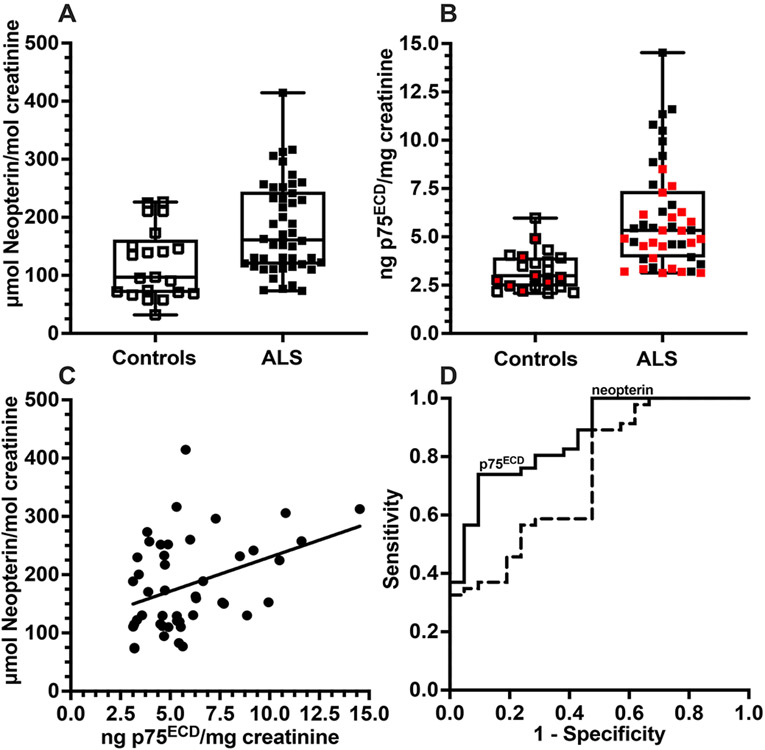
Figure 1:
Urinary neopterin (A, p= 0.0017) and p75ECD (B, p<0.0001) levels are higher in patients with ALS (n=46) than in controls (n=21) at baseline (2-sample t test), with previously published p75ECD data in red. Neopterin levels correlate with p75ECD (C) sampled in the same patients (n=46) at baseline (Pearson’s r= 0.38; p= 0.009). ROC analysis of urinary neopterin and p75ECD, illustrating discrimination between ALS patients and controls, with an AUC of 0.74 (p= 0.0024) and 0.87 (p<0.0001) respectively (D) (Wilson-Brown method). Analysis: SPSS and Prism 9.
Interestingly, among the n=46 ALS patients, there was a correlation between baseline levels of urinary neopterin and urinary p75ECD (Pearson’s correlation, r= 0.38, p= 0.009; Fig 1C). Receiver operating characteristic analysis using the area under the curve (AUC) indicated neopterin distinguished ALS from controls 74% of time (p= 0.002; 95% CI: 0.61-0.87) with 56% sensitivity whereas p75ECD distinguished ALS from controls 87% of time (p<0.0001; CI: 0.78-0.96), with 76% sensitivity (Fig 1D). Both neopterin and p75ECD were inversely correlated with ALSFRS-R at baseline: r= −0.36, p= 0.01 (Fig 2A) and r = −0.36, p= 0.01 (Fig 2B) respectively. Among controls (n=21, median age 61.3; range 48-72), there was no correlation between age and neopterin, or between age and p75ECD (Fig 3A and 3C). Among the 46 ALS patients (median age 66.7; range 42-85), baseline age did correlate with neopterin (r = 0.5, p= 0.001) and p75ECD (r= 0.37, p= 0.01). After excluding those over 72 and below 48 years of age, such that both control (n= 21) and ALS (n= 26) had a similar age range (median 63.5, range: 48.6.-71.5 years Fig 3B and Fig 3D), there was no correlation. Baseline urinary neopterin levels in these 26 with ALS, however, remains elevated (167.1 ± 66.36 μmol/mol creatinine), compared to 21 controls (120.4 ± 60.8 μmol/mol creatinine p= 0.01, Welch’s t-test). Urinary p75ECD was also higher in n= 26 people with ALS in the 48-72 years old age-range at baseline compared to healthy controls (5.49 ± 2.52 ng/mg creatinine vs. 3.2 ± 1.0 ng/mg creatinine, p<0.0001).
Figure 2:
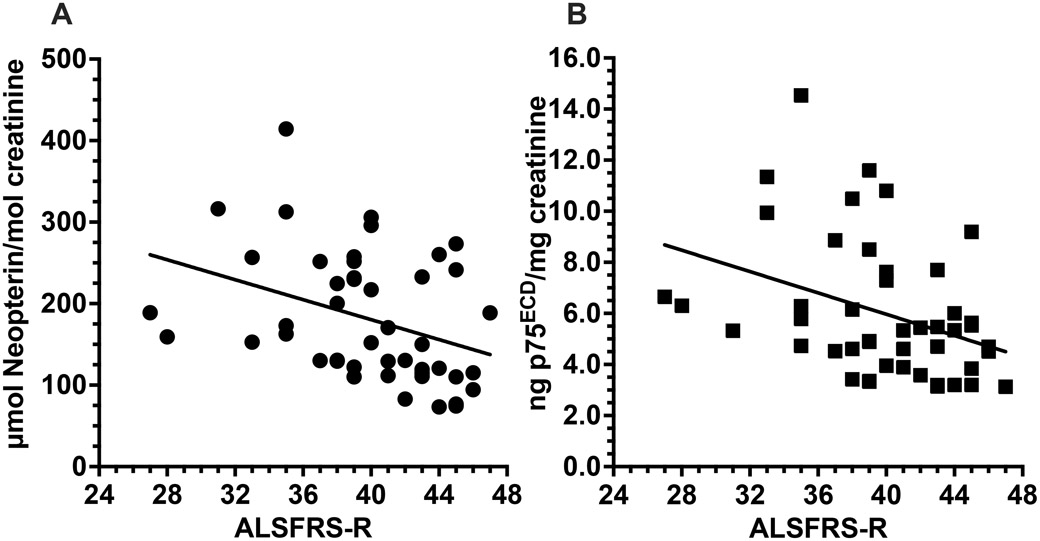
Urinary neopterin (A) is correlated with ALSFRS-R at baseline for each of the 46 patients with ALS in the study (r= −0.36, p= 0.013). As previously reported, urinary p75ECD also correlates with ALSFRS-R (r= −0.36, p= 0.013) at baseline (B). Pearson’s Tests in SPSS/ Prism 9.
Figure 3:
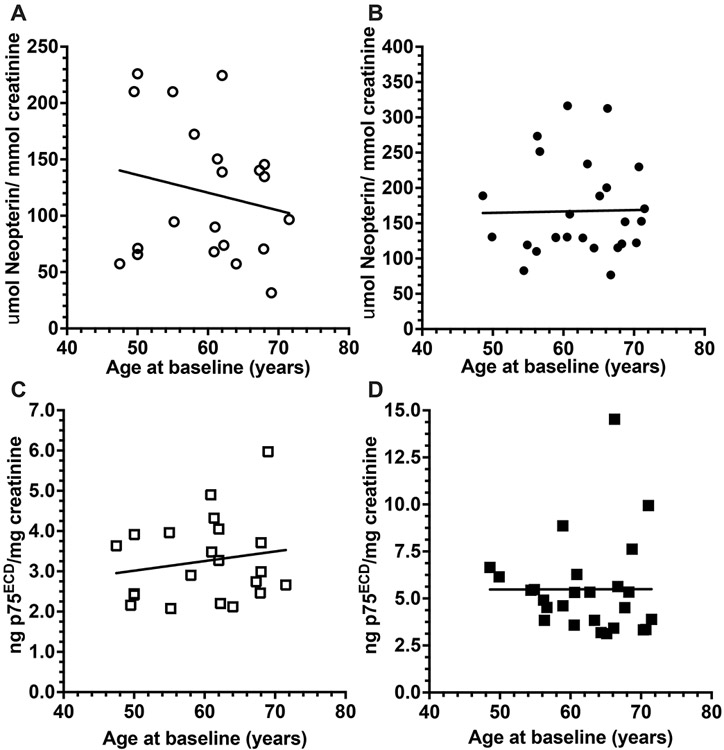
There is no correlation between urinary neopterin and age (A, r = −0.19, p= 0.44) or between urinary p75ECD and age (C; r= −0.17. p= 0.45) among 21 healthy individuals aged 49-72 years. Similarly, there was no correlation between neopterin levels and age (B; r= 0.02, p= 0.93) or between urinary p75ECD and age (D; r = 0.002, p = 0.99) among the n= 26 ALS patients aged 49-72 years. Pearson’s Tests in SPSS/ Prism 9.
Prognostic Biomarkers
To investigate the potential utility of baseline urinary neopterin and p75ECD as predictors of prognosis, we performed survival analyses on 46 ALS patients, 44 of whom have died during follow-up. In univariate analysis, age at diagnosis, sex, and site of onset were not associated with survival, but consistent with previously published data3 higher p75ECD (HR= 1.13, 95% CI: 1.02-1.25, p= 0.019) and faster estimated progression rate (ΔFRS) predicted shorter survival (HR= 3.66, 95% CI: 1.9-6.85, p<0.0001); with p75ECD levels having only modest predictive value. Higher neopterin levels at baseline, while statistically significant, did not meaningfully predict survival (HR = 1.01, 95% CI: 1.001-1.01, p= 0.01). Urinary neopterin and p75ECD were dichotomized above or below their median baseline values, and their Kaplan Meier survival plots are shown in Fig 4A and 4B. ALS patients with lower than the median neopterin (161 μmol/mol creatinine) survived 19.8 months versus 10.7 months for those with neopterin above the median value (p= 0.046). Longer median survival from baseline was also observed for ALS with urinary p75ECD levels below versus above the median value of 5.33 (20.5 vs. 8.7 months p= 0.005 (Wilcoxon test, SPSS and Prism 9).
Figure 4:
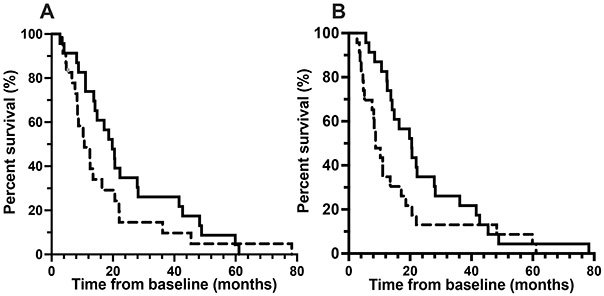
Kaplan-Meier survival estimates of 46 amyotrophic lateral sclerosis patients, comparing 22 ALS patients who had baseline urinary neopterin (A) and p75ECD (B) above (dashed line) and 22 with values below (black line) the median (161.1 μmol neopterin/mol creatinine; 5.33 ng p75ECD /mg creatinine), illustrates the longer median survival in those with lower compared to higher urinary neopterin (p= 0.046; 10.7 versus 19.8 months) and p75ECD levels (p= 0.005; 8.7 versus 20.5 months). Kaplan-Meier analysis (Wilcoxon test) was in SPSS and Prism 9.
In multivariate Cox regression analysis, only ΔFRS (HR= 3.61, CI: 1.62-8.05, p=0.002) predicted survival; but not urinary neopterin, urinary p75ECD, sex, site of onset or baseline age. This. indicates that neither neopterin nor p75ECD add prognostic value beyond what can be determined based on ΔFRS.
Urinary biomarkers of disease progression
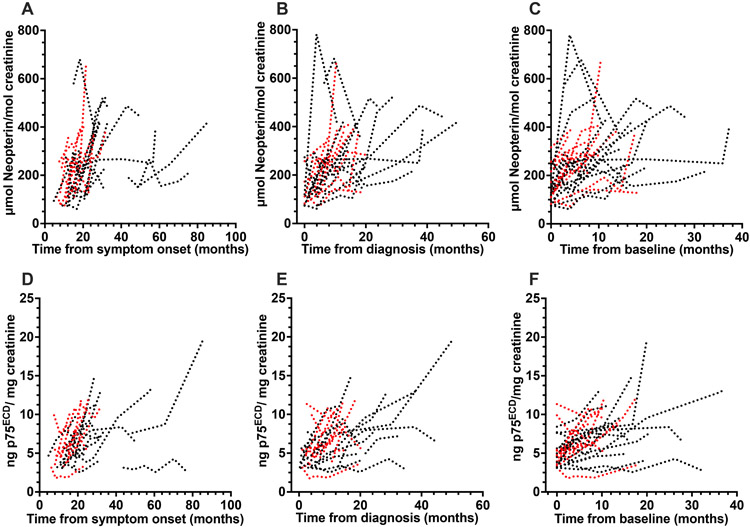
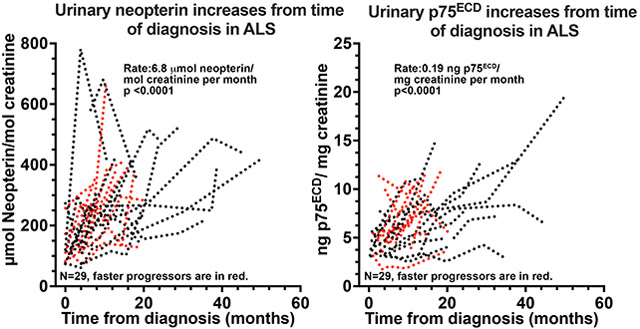
The potential utility of urinary neopterin as a biomarker of disease progression was investigated in 29 patients with ALS in whom longitudinal clinical data and urine samples were available. All 29 had more than one sample post baseline (median of 3 time points, 3.0 (range 2- 6); Table 1). The median duration between first and second sample was 3.9 months (range: 2- 14.7 months). Fourteen of these patients were included in our prior publication focused on p75ECD 3, but eight of these had additional samples collected at later time points. To illustrate the consistency of urinary neopterin and p75ECD as markers of disease progression, we have examined changes in these biomarkers using time from symptom onset, time from baseline and time from diagnosis. Since the dates of diagnosis and baseline are reliably obtained from clinical notes3, we have chosen to use time from diagnosis and baseline to each sample collection for our primary analyses (Fig 5B, 5C, 5E and 5F). The urinary concentration of neopterin increased from diagnosis by 6.8 μmol/mol creatinine each month (95% CI: 4.6-9.1; p<0.0001; Fig 5B) and from baseline by an average of 6.8 μmol (CI: 4.1 to 9.5, p<0.0001; Fig 5C), with a similar magnitude increase from symptom onset (Fig 5A). Urinary p75ECD increased by an average of 0.19 ng/mg creatinine per month from diagnosis (Fig 5E 95% CI: 0.14-0.23, p<0.0001), which is the same as our previously published study3 and by 0.19 ng/mg creatinine from baseline (CI 0.14–0.24, p<0.0001 Fig 5F) and similarly from onset (Fig 5D).
Figure 5:
Individual longitudinal trajectories of neopterin (A-C) and p75ECD (D-F) for 29 ALS patients. Neopterin increased from symptom onset at a rate of 4.5 μmol neopterin/mol creatinine per month (A; CI: 2.2-6.8, p<0.0001); from time of diagnosis (B) at a rate of 6.8 μmol neopterin/ mol creatinine per month (95% confidence interval (CI) 4.6-9.1; p <0.0001) and baseline (C) at a rate of 6.8 μmol neopterin/ mol creatinine per month (CI: 4.1-9.5, p<0.0001). p75ECD also increased from time of symptom onset (D, 0.15 ng p75ECD/mg creatinine/ month, CI 0.1-0.2, p<0.0001); diagnosis (E, 0.19 ng/mg creatinine/ month, CI: 0.14-0.23, p<0.0001) and baseline (F, 0.19 ng/mg creatinine/ month, CI 0.14–0.24, p<0.0001). Each patient was sampled at least twice. Faster progressors (>0.49 ΔFRS) are in red.
Among the 29 patients with longitudinal data, the slope of the ALSFRS-R declined by an average of −0.61 points/month from diagnosis (CI −0.49 to −0.73, p<0.0001) and by −0.66 points/month (CI −0.54 to −0.79, p <0.0001) from baseline, similar to that reported in a recent study examining neurofilament light (NfL) in a larger cohort5. Comparing the rate of change in ALSFRS-R and the urinary biomarkers, the monthly increase in neopterin (−0.015; CI: −0.02 to −0.004; p= 0.009) and p75ECD (−1.475; CI: −1.95 to −0.995; p< 0.0001) are significantly associated with a monthly decrease in ALSFRS-R.
To illustrate differences in the trajectories of neopterin and p75ECD among faster and slower progressors, the data were split around the median ΔFRS at baseline (−0.49 ALSFRS points/month) into slower (<0.49 points/month; n= 13) and faster (>0.49; n= 16), progressors. In the slower progressors, neopterin increased by 4.7 μmol/mol creatinine per month (95% CI: 1.4-8.0; p <0.0001) from diagnosis and in the faster progressing group neopterin increased by 16.6 μmol/mol creatinine per month (95% CI: 13-20; p <0.0001; red lines in Fig 5B). The difference between groups was significant (p= 0.0005). p75ECD increased by 0.17 ng/mg creatinine per month from diagnosis (CI 0.12-0.22, p <0.0001) in the slower and 0.36 ng/mg creatinine per month in the faster progressors (CI 0.26-0.45, p <0.0001; red lines in Fig 5E). This difference was significant (p= 0.002).
Discussion
Our results show that urinary neopterin is elevated in ALS patients and increases as disease progresses. Previous work has shown neopterin levels in healthy individuals are not impacted by age between the ages of 18 and 75 years 39. p75 expression is also known to be increased in older individuals 40. Consistent with these published results, we found an association between age and neopterin only amongst those older than 72 years of age. Neopterin and p75ECD remain elevated in ALS compared to control in those aged 48-72. Age, therefore, does not appear to confound our observation that neopterin increases measurably over time as ALS advances – i.e. that it is a marker of disease progression. Neopterin, therefore, together with p75ECD 3, might have potential utility as pharmacodynamic biomarkers in clinical trials of ALS treatments, where a decrease in rate of change of these markers post treatment might indicate a biological effect of an experimental therapeutic.
Urinary neopterin and urinary p75ECD correlate with the ALSFRS-R at baseline. While univariate analysis suggests that that p75ECD has modest prognostic value, multivariate analysis suggests that neither neopterin nor p75ECD add prognostic value to what can be discerned from the ΔFRS3. We found no relationship between sex or site of onset with ALS prognosis, even though some other studies have reported such an association5. By contrast, serum NfL has been verified in large cohorts as prognostic of future ALSFRS-R decline and survival duration, providing information that is not captured by readily available clinical predictions such as ΔFRS and site of onset5.
There have been many clinical trials of experimental therapeutics that target the inflammatory system, (e.g. COX2 inhibitor; glatiramer acetate)20 and their negative results have highlighted the need to better understand the immune response in ALS, and to identify biomarkers that might identify potential responders at trial enrolment. Previous work on immune dysfunction in ALS12 has led us to investigate neopterin as a potential biomarker. Low levels of urinary neopterin in healthy individuals indicate that Th-1 lymphocytes are supressed in the absence of an inflammatory response. However, progressively increasing neopterin in ALS patients suggests the emergence of a pro-inflammatory state in which Th-1 and Th-17 lymphocytes release IFN-γ and tumour necrosis factor (TNF)-α, which in turn induce neopterin release from monocytic cells such as proinflammatory macrophages, dendritic cells, microglia and astrocytes18 41 42, with eventual removal from the circulation by the kidney24. It will be valuable, in future studies, to determine if high baseline neopterin levels might identify the subset of ALS patients most likely to benefit from anti-inflammatory drug in trials. Neopterin might also be used as a pharmacodynamic biomarker in clinical trials that target the immune system, where a decrease in the monthly change in neopterin levels with an experimental treatment might suggest a beneficial reduction in the pro-inflammatory response.
Chitotriosidase-1 (CHIT-1), and monocyte chemoattractant protein-1 (MCP-1) have also been investigated43 as inflammatory biomarkers of ALS. CHIT-1 is released from microglia, and in five studies7 8, 9 10 11 found to be significantly elevated in the cerebrospinal fluid (CSF) of patients with ALS compared to controls, but in those that examined progression, CHIT-1 was relatively stable as disease progressed9 11 and did not always correlate with the ALSFRS-R8. In serum and plasma, there are conflicting reports as to the significance of CHIT-144 9 11, with only one study reporting a significant amount of CHIT-1 in serum44 and there was no correlation between serum and CSF CHIT-1. MCP-1 levels in CSF appear to be related to faster progression22 21 but are fairly stable over progression45 22. A meta-analysis of 8 studies46, found serum MCP-1 is not significantly increased in ALS compared to controls. MCP-1 is a Th-2 anti-inflammatory chemokine, however, other proinflammatory cytokines including IL-6, IL-18, and TNF-α that are associated with Th-1 phenotypes are also upregulated in the CSF in ALS12. For example, serum IL-6 was not prognostic for ALS, but was found to be robustly increased, especially at later stages of disease47. However, it has not been clearly demonstrated that the serum/plasma levels of these markers increase (or decrease) from baseline at a measurable rate as ALS progresses9 11 48. By contrast, we have found urinary neopterin is correlated to ALSFRS-R and changes over time as disease progresses, suggesting measurement of immune markers may be useful in the less complex proteome of human urine, compared to serum. The levels of neopterin are understood to reflect the Th-1 pro-inflammatory IFN-γ, and found at similar levels in serum and urine49. This suggests urinary neopterin may be a marker that reflects pro-inflammation and disease progression. Urinary neopterin measurement does not require stringent collection protocols49 whereas for reliable IFN-γ measurement, fresh serum or plasma sample (within 1 hour) must be used, or measured from samples that were processed and frozen no more than 1h post collection50 51. Since it is difficult to ensure such stringent collection protocols, and ALS patients are willing to provide urine, urinary neopterin is thus an easily accessible marker of systemic proinflammation and ALS progression.
In ALS, prior small cross-sectional studies of neopterin have been inconclusive, variably showing an increase in serum and CSF concentrations29,52. Our results are consistent with a recent study by Lunetta et al, that examined urinary neopterin in ALS, using non-specific high-performance liquid chromatography (HPLC) with neopterin measured at 355 nm30; this study reported a significantly higher level of urinary neopterin in 81 individuals with ALS (mean= 263.9 μmol neopterin/mol creatinine) compared to 68 healthy controls (169.6 μmol neopterin/mol creatinine). While these levels were higher than we observed (mean 181.7 μmol versus control at 120.4 μmol neopterin/mol creatinine), the apparent discordance may reflect the fact that we analysed baseline levels of neopterin at an average of 16.9 ±8.8 months following onset, compared to an average of 26.4 months in the prior study30 i.e., that patients were later in their disease course may account for the higher neopterin levels. In addition, the non-specific UV detection at 355 nm used in the prior study30 may yield apparently higher levels of neopterin than the ELISA used in our study, which has higher specificity24. More recently, HPLC-mass spectrometry provides a means to detect neopterin more accurately. This technique has been shown to be robust in measuring urinary neopterin53 and will be pursued in the future.
The limitations of the study include the relatively small number of samples. Future directions would be to analyse larger cohorts over progression, refining neopterin quantification using the HPLC-mass spectrometry method. We will also determine the usefulness of neopterin in ALS clinical trials that target neuroinflammation.
Supplementary Material
Figure e1: Correlation between published urinary p75ECD from ALS patients (n=69) measured by published sandwich ELISA method4 and automated method (r= 0.97, p<0.0001). The Passing-Bablok equation was y= −0.05 + 1.06 (*old values), correlation, r= 0.97. The automated method has the same capture antibody to p75ECD, but different detection antibody. Analysis in Prism 9.
Acknowledgments
We acknowledge Professor Mark Bothwell (University of Washington) for gifting the NGFR5 hybridoma clone. We extend thanks to Flinders Medical Centre MND SA staff and Vyoma Modi and Alex Colella at Flinders University for technical and mass spectrometry expertise, respectively. We are also grateful to all the individuals who participated in this study.
Study funding.
The study was supported by the NIH RDCRN CReATe Consortium (U54 NS092091, U01 NS107027), Motor Neurone Disease Research Association, Australia (# IG 2048; BG 2106) and FightMND Australia (11_IMPACT_2020_Rogers and Flinders University MND Drug Efficacy Testing Facility). CReATe is part of the Rare Disease Clinical Research Network (RDCRN), which is funded by the National Institutes of Health (NIH) and led by the National Center for Advancing Translational Sciences (NCATS) through its Office of Rare Diseases Research (ORDR). CReATe is funded through a collaboration between NCATS and the National Institute of Neurological Disorders and Stroke (NINDS).
Footnotes
The data that support the findings of this study are available from the corresponding author(s) upon reasonable request.
Conflicts of Interest:
Research Assoc. Professor J. Wuu reports grants from the National Institutes of Health and Target ALS during the conduct of the study
Professor M. Benatar reports grants from National Institutes of Health, the ALS Association, the Muscular Dystrophy Association, the Centers for Disease Control and Prevention, the Department of Defense, and Target ALS during the conduct of the study; personal fees from Roche, Biogen, Jazz Pharmaceuticals, and AveXis outside the submitted work. In addition, Dr. Benatar has a provisional patent entitled ‘Determining Onset of Amyotrophic Lateral Sclerosis’. Dr. Benatar also serves as a site investigator on clinical trials funded by Biogen and Orphazyme, and as the global coordinating investigator for Orphazyme’s trial of Arimoclomol in ALS.
Professor A. Malaspina reports grants from the ALS Association, MND Association UK, Wellcome Trust UK, NIHR UK, Barts and The London Charities UK. He has acted as consultant and received personal fees from Roche and Pfizer. He has a joint provisional patent entitled ‘Determining Onset of Amyotrophic Lateral Sclerosis’ with Dr Benatar.
Assoc Professor M-L Rogers reports grants from MND Research Australia, FightMND, the ALS Association, Target ALS and National Institutes of Health. In addition, Assoc Prof Rogers, Dr Shepheard and Assoc Prof Chataway have a provisional patent entitled “Antibody against ALS Biomarker”.
References
- 1.Benatar M, Boylan K, Jeromin A, et al. ALS biomarkers for therapy development: State of the field and future directions. Muscle & Nerve 2016;53:169–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Goyal NA, Berry JD, Windebank A, et al. Addressing heterogeneity in amyotrophic lateral sclerosis Clinical Trials. Muscle Nerve 2020;62:156–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shepheard SR, Wuu J, Cardoso M, et al. Urinary p75(ECD): A prognostic, disease progression, and pharmacodynamic biomarker in ALS. Neurology 2017;88:1137–1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gold J, Rowe DB, Kiernan MC, et al. Safety and tolerability of Triumeq in amyotrophic lateral sclerosis: the Lighthouse trial. Amyotrophic lateral sclerosis and frontotemporal degeneration 2019;63:1–10. [DOI] [PubMed] [Google Scholar]
- 5.Benatar M, Zhang L, Wang L, et al. Validation of serum neurofilaments as prognostic and potential pharmacodynamic biomarkers for ALS. Neurology 2020;95:e59–e69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Miller T, Cudkowicz M, Shaw PJ, et al. Phase 1-2 Trial of Antisense Oligonucleotide Tofersen for SOD1 ALS. N Engl J Med 2020;383:109–119. [DOI] [PubMed] [Google Scholar]
- 7.Varghese AM, Sharma A, Mishra P, et al. Chitotriosidase - a putative biomarker for sporadic amyotrophic lateral sclerosis. Clinical proteomics 2013;10:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thompson AG, Gray E, Thezenas ML, et al. Cerebrospinal fluid macrophage biomarkers in amyotrophic lateral sclerosis. Ann Neurol 2018;83:258–268. [DOI] [PubMed] [Google Scholar]
- 9.Steinacker P, Verde F, Fang L, et al. Chitotriosidase (CHIT1) is increased in microglia and macrophages in spinal cord of amyotrophic lateral sclerosis and cerebrospinal fluid levels correlate with disease severity and progression. Journal of neurology, neurosurgery, and psychiatry 2018;89:239–247. [DOI] [PubMed] [Google Scholar]
- 10.Varghese AM, Ghosh M, Bhagat SK, et al. Chitotriosidase, a biomarker of amyotrophic lateral sclerosis, accentuates neurodegeneration in spinal motor neurons through neuroinflammation. J Neuroinflammation 2020;17:232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vu L, An J, Kovalik T, Gendron T, Petrucelli L, Bowser R. Cross-sectional and longitudinal measures of chitinase proteins in amyotrophic lateral sclerosis and expression of CHI3L1 in activated astrocytes. J Neurol Neurosurg Psychiatry 2020;91:350–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Malaspina A, Puentes F, Amor S. Disease origin and progression in amyotrophic lateral sclerosis: an immunology perspective. International Immunology 2015;27:117–129. [DOI] [PubMed] [Google Scholar]
- 13.Alami NO, Schurr C, Heuvel FO, et al. NF-κB activation in astrocytes drives a stage-specific beneficial neuroimmunological response in ALS. The EMBO Journal 2018;37 (e98697):https://www.embopress.org/doi/full/10.15252/embj.201798697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liao B, Zhao W, Beers DR, Henkel JS, Appel SH. Transformation from a neuroprotective to a neurotoxic microglial phenotype in a mouse model of ALS. Exp Neurol 2012;237:147–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhao W, Beers DR, Henkel JS, et al. Extracellular mutant SOD1 induces microglial-mediated motoneuron injury. Glia 2010;58:231–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nagai M, Re DB, Nagata T, et al. Astrocytes expressing ALS-linked mutated SOD1 release factors selectively toxic to motor neurons. Nat Neurosci 2007;10:615–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brettschneider J, Toledo JB, Van Deerlin VM, et al. Microglial activation correlates with disease progression and upper motor neuron clinical symptoms in amyotrophic lateral sclerosis. Plos One 2012;7:e39216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhao W, Beers DR, Hooten KG, et al. Characterization of Gene Expression Phenotype in Amyotrophic Lateral Sclerosis Monocytes. JAMA Neurol 2017;74:677–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Miller RG, Block G, Katz JS, et al. Randomized phase 2 trial of NP001-a novel immune regulator: Safety and early efficacy in ALS. Neurol Neuroimmunol Neuroinflamm 2015;2:e100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wosiski-Kuhn M, Lyon MS, Caress J, Milligan C. Inflammation, immunity, and amyotrophic lateral sclerosis: II. immune-modulating therapies. Muscle Nerve 2019;59:23–33. [DOI] [PubMed] [Google Scholar]
- 21.Gille B, De Schaepdryver M, Dedeene L, et al. Inflammatory markers in cerebrospinal fluid: independent prognostic biomarkers in amyotrophic lateral sclerosis? J Neurol Neurosurg Psychiatry 2019;90:1338–1346. [DOI] [PubMed] [Google Scholar]
- 22.Huang F, Zhu Y, Hsiao-Nakamoto J, et al. Longitudinal biomarkers in amyotrophic lateral sclerosis. Ann Clin Transl Neurol 2020;7:1103–1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gschwandtner M, Derler R, Midwood KS. More Than Just Attractive: How CCL2 Influences Myeloid Cell Behavior Beyond Chemotaxis. Front Immunol 2019;10:2759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Berdowska A, Zwirska-Korczala K. Neopterin measurement in clinical diagnosis. Journal of clinical pharmacy and therapeutics 2001;26:319–329. [DOI] [PubMed] [Google Scholar]
- 25.Molero-Luis M, Fernandez-Urena S, Jordan I, et al. Cerebrospinal fluid neopterin analysis in neuropediatric patients: establishment of a new cut off-value for the identification of inflammatory-immune mediated processes. Plos One 2013;8:e83237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Martins RdP, Ghisoni K, Lim CK, Aguiar AS, Guillemin GJ, Latini A. Neopterin preconditioning prevents inflammasome activation in mammalian astrocytes. Free Radical Biology and Medicine 2018;115:371 – 382. [DOI] [PubMed] [Google Scholar]
- 27.Huber C, Batchelor JR, Fuchs D, et al. Immune response-associated production of neopterin. Release from macrophages primarily under control of interferon-gamma. J Exp Med 1984;160:310–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Molero-Luis M, Casas-Alba D, Orellana G, et al. Cerebrospinal fluid neopterin as a biomarker of neuroinflammatory diseases. Sci Rep 2020;10:18291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Westarp ME, Fuchs D, Bartmann P, et al. Amyotrophic lateral sclerosis an enigmatic disease with B-cellular and anti-retroviral immune responses. The European journal of medicine 1993;2:327–332. [PubMed] [Google Scholar]
- 30.Lunetta C, Lizio A, Gerardi F, et al. Urinary neopterin, a new marker of the neuroinflammatory status in amyotrophic lateral sclerosis. Journal of neurology 2020;7:639. [DOI] [PubMed] [Google Scholar]
- 31.Brooks BR, Miller RG, Swash M, Munsat TL; World Federation of Neurology Research Group on Motor Neuron Diseases. El Escorial revisited: revised criteria for the diagnosis of amyotrophic lateral sclerosis. Amyotroph Lateral Scler Other Motor Neuron Disord. 2000. Dec;1(5):293–9. doi: 10.1080/146608200300079536. PMID: 11464847 [DOI] [PubMed] [Google Scholar]
- 32.von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. PLoS Med 2007;4:e296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kimura F, Fujimura C, Ishida S, et al. Progression rate of ALSFRS-R at time of diagnosis predicts survival time in ALS. Neurology 2006;66:265–267. [DOI] [PubMed] [Google Scholar]
- 34.Mischak H, Kolch W, Aivaliotis M, et al. Comprehensive human urine standards for comparability and standardization in clinical proteome analysis. Proteomics Clin Appl 2010;4:464–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Westermann J, Thiemann F, Gerstner L, et al. Evaluation of a new simple and rapid enzyme-linked immunosorbent assay kit for neopterin determination. Clin Chem Lab Med 2000;38:345–353. [DOI] [PubMed] [Google Scholar]
- 36.Thompson SJ, Schatteman GC, Gown AM, Bothwell M. A monoclonal antibody against nerve growth factor receptor. Immunohistochemical analysis of normal and neoplastic human tissue. Am J Clin Pathol 1989;92:415–423. [DOI] [PubMed] [Google Scholar]
- 37.Mikheev MI, Lowry LK. WHO global project on biological monitoring of chemical exposure at the workplace. Int Arch Occup Environ Health 1996;68:387–388. [DOI] [PubMed] [Google Scholar]
- 38.Baker SG, Krame BS. Peirce, Youden, and Receiver Operating Characteristic Curves. The American Statistician 2007;61:343–346. [Google Scholar]
- 39.Werner ER, Bichler A, Daxenbichler G, et al. Determination of neopterin in serum and urine. Clinical chemistry 1987;33:62–66. [PubMed] [Google Scholar]
- 40.Mufson EJ, Kordower JH. Cortical neurons express nerve growth factor receptors in advanced age and Alzheimer disease. P Natl Acad Sci USA 1992;89:569–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Du Y, Zhao W, Thonhoff JR, Wang J, Wen S, Appel SH. Increased activation ability of monocytes from ALS patients. Exp Neurol 2020;328:113259. [DOI] [PubMed] [Google Scholar]
- 42.Roberts K, Zeineddine R, Corcoran L, Li W, Campbell IL, Yerbury JJ. Extracellular aggregated Cu/Zn superoxide dismutase activates microglia to give a cytotoxic phenotype. Glia 2013;61:409–419. [DOI] [PubMed] [Google Scholar]
- 43.Kuhle J, Lindberg RL, Regeniter A, et al. Increased levels of inflammatory chemokines in amyotrophic lateral sclerosis. Eur J Neurol 2009;16:771–774. [DOI] [PubMed] [Google Scholar]
- 44.Pagliardini V, Pagliardini S, Corrado L, et al. Chitotriosidase and lysosomal enzymes as potential biomarkers of disease progression in amyotrophic lateral sclerosis: a survey clinic-based study. J Neurol Sci 2015;348:245–250. [DOI] [PubMed] [Google Scholar]
- 45.Guo J, Yang X, Gao L, Zang D. Evaluating the levels of CSF and serum factors in ALS. Brain Behav 2017;7:e00637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hu Y, Cao C, Qin XY, et al. Increased peripheral blood inflammatory cytokine levels in amyotrophic lateral sclerosis: a meta-analysis study. Sci Rep 2017;7:9094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lu CH, Allen K, Oei F, et al. Systemic inflammatory response and neuromuscular involvement in amyotrophic lateral sclerosis. Neurol Neuroimmunol Neuroinflamm 2016;3:e244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vahsen BF, Gray E, Thompson AG, et al. Non-neuronal cells in amyotrophic lateral sclerosis - from pathogenesis to biomarkers. Nat Rev Neurol 2021;17:333–348. [DOI] [PubMed] [Google Scholar]
- 49.Murr C, Widner B, Wirleitner B, Fuchs D. Neopterin as a marker for immune system activation. Curr Drug Metab 2002;3:175–187. [DOI] [PubMed] [Google Scholar]
- 50.Jackman RP, Utter GH, Heitman JW, et al. Effects of blood sample age at time of separation on measured cytokine concentrations in human plasma. Clin Vaccine Immunol 2011;18:318–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Valaperti A, Bezel P, Vonow-Eisenring M, Franzen D, Steiner UC. Variability of cytokine concentration in whole blood serum and bronchoalveolar lavage over time. Cytokine 2019;123:154768. [DOI] [PubMed] [Google Scholar]
- 52.Yoshida Y, Une F, Utatsu Y, et al. Adenosine and neopterin levels in cerebrospinal fluid of patients with neurological disorders. Internal medicine (Tokyo, Japan) 1999;38:133–139. [DOI] [PubMed] [Google Scholar]
- 53.Burton C, Shi H, Ma Y. Development of a high-performance liquid chromatography - Tandem mass spectrometry urinary pterinomics workflow. Analytica chimica acta 2016;927:72–81. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure e1: Correlation between published urinary p75ECD from ALS patients (n=69) measured by published sandwich ELISA method4 and automated method (r= 0.97, p<0.0001). The Passing-Bablok equation was y= −0.05 + 1.06 (*old values), correlation, r= 0.97. The automated method has the same capture antibody to p75ECD, but different detection antibody. Analysis in Prism 9.