Abstract
Background:
We sought to determine if controlled, prospective clinical data validate the long-standing belief that intravenous (IV) antibiotic therapy is required for the full duration of treatment for three invasive bacterial infections: osteomyelitis, bacteremia, and infective endocarditis.
Methods:
We performed a systematic review of published, prospective, controlled trials that compared IV-only to oral stepdown regimens in the treatment of these diseases. Using the PubMed database, we identified 7 relevant randomized controlled trials (RCTs) of osteomyelitis, 9 of bacteremia, 1 including both osteomyelitis and bacteremia, and 3 of endocarditis, as well as one quasi-experimental endocarditis study. Study results were synthesized via forest plots and funnel charts (for risk of study bias), using RevMan 5.4.1 and Meta-Essentials freeware, respectively.
Results:
The 21 studies demonstrated either no difference in clinical efficacy, or superiority of oral vs. IV-only antimicrobial therapy, including for mortality; in no study was IV-only treatment superior in efficacy. The frequency of catheter-related adverse events and duration of inpatient hospitalization were both greater in IV-only groups.
Discussion:
Numerous prospective, controlled investigations demonstrate that oral antibiotics are at least as effective, safer, and lead to shorter hospitalizations than IV-only therapy; no contrary data were identified. Treatment guidelines should be modified to indicate that oral therapy is appropriate for reasonably selected patients with osteomyelitis, bacteremia, and endocarditis.
Keywords: oral antibiotics, osteomyelitis, bacteremia, endocarditis, meta-analysis
Introduction
For many years, clinicians have assumed that intravenous (IV) antibiotics are necessary to successfully treat osteomyelitis, bacteremia, and endocarditis. This presumption stems from uncontrolled case series from the 1940s and 1950s, and the limited bioavailability of the few oral antibiotics available at that time (sulfanilamide, erythromycin, tetracycline).1–3 More modern drugs were not subjected to rigorous testing until the 1980s, by which time the culture of medicine had already established a deep, more than 30 years’ old belief in IV-only therapy.2, 4, 5 The necessity of IV-only therapy for these diseases has been enshrined in clinical and professional society guidelines, reinforcing long-standing inertia.
However, such guidelines do not cite controlled investigations in which IV-only therapy was established to be superior in efficacy to oral therapy. More recently, a number of studies have tested the hypothesis that we can safely switch to oral antibiotics once patients with these infections have stabilized. Therefore, we sought to determine if prospective, controlled investigations substantiate the long-standing clinical belief that IV-only therapy is superior to oral therapy for such infections, and if populations of patients who are likely to benefit from oral step-down therapy may be identified from such studies.
Methods
Literature Search
In March of 2021, we conducted a systematic review of the literature for prospective, interventional studies comparing IV-only vs. oral antimicrobial therapy for serious, invasive bacterial infections. We searched PubMed for keywords: (oral, linezolid, ciprofloxacin, ofloxacin, levofloxacin, moxifloxacin, trimethoprim, or clindamycin), and (osteomyelitis, bacteremia, or endocarditis), and publication type “clinical trial.” References within these articles were also evaluated to identify other relevant publications.
Eligibility Criteria, Data Extraction, and Outcomes
We included only prospective, interventional studies, either randomized controlled trials (RCTs) or quasi-experimental. We excluded retrospective, observational, uncontrolled, and non-interventional studies (in which treatment was not assigned by study protocol), as well as studies of prophylaxis and infections caused by non-bacterial pathogens. All studies were reviewed for eligibility by three authors who were also responsible for abstracting results (NWD, RAL, and BS).
A standardized form was used to identify and extract relevant characteristics of included studies. The primary outcome was successful therapy as defined by the absence of the respective clinical infection at the last time point of long-term follow-up. Other outcomes included rates of adverse events, mortality, duration of hospitalization, and relapse rates where available. Random effects meta-analysis of the included studies were graphically illustrated by forest plots using RevMan 5.4.1 freeware, and funnel plots were generated using Meta-Essentials freeware and methods6 to assess for publication bias.
Results
Study Selection
Five hundred and fifty-five articles were identified from the initial search, of which 28 were prospective, interventional studies (Figure 1). Of these 27 RCTs and one quasi-experimental study, we excluded two RCTs of patients with bacteremia, one of which compared trimethoprim-sulfamethoxazole (TMP-SMX) vs. IV vancomycin for invasive Staphylococcus aureus infections, and the other of which compared amoxicillin/clavulanate vs. intramuscular ceftriaxone for febrile illness in small children.7, 8 The first study was excluded because the trial did not report that any patients actually received oral therapy (TMP-SMX was administered IV), and because there were concerns about external validity.7 The second study was excluded because of extensive cross-over of IV and oral therapy in both arms.8 An additional five excluded RCTs enrolled multiple different types of infections, in which only a small numbers of patients had bacteremia or osteomyelitis (i.e., ≤ 5 patients per treatment arm), or for the larger studies it was not possible to distinguish the outcomes of the subgroups of patients with bacteremia or osteomyelitis from those without.9–13 None of the excluded trials reported significantly different outcomes between patients who received oral vs. IV-only therapy. Ultimately, 20 RCTs and one quasi-experimental study were included in the analyses. Of the 20 RCTs, one was included in both the osteomyelitis and bacteremia analyses because clinical outcomes between groups were reported separately for both conditions in the same paper.14
Figure 1. Flow Chart for Study Inclusion.
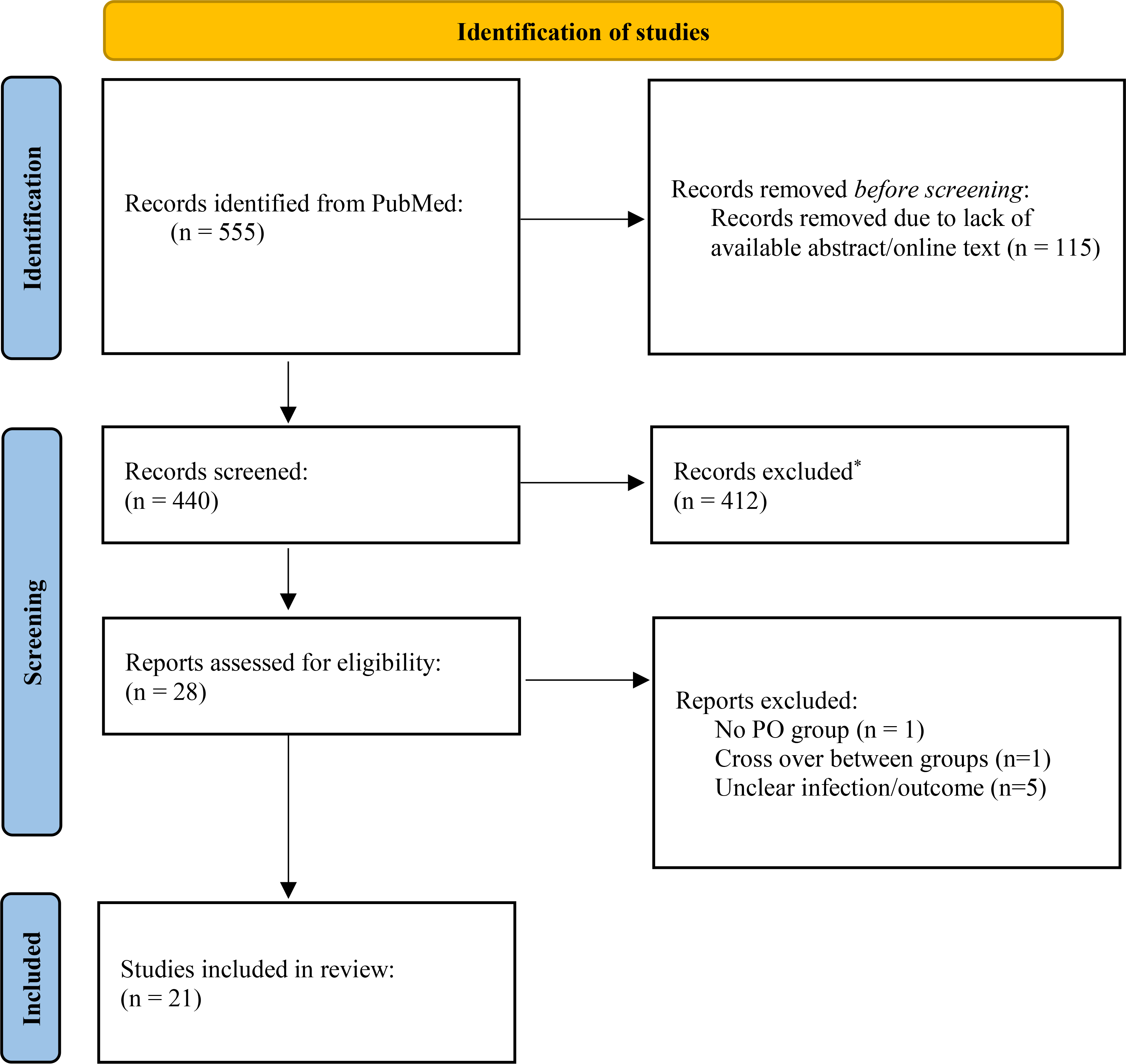
One RCT (Schrenzel 2004) was included in both the osteomyelitis and bacteremia sections as both patient populations were studied in the trial. *Excluded based on being retrospective or observational studies, non-interventional prospective studies (in which treatment with oral or IV was left to the discretion of the treating physician rather than being assigned by the protocol), studies of prophylaxis, studies of infections caused by non-bacterial pathogens, and single arm or non-controlled studies.
Osteomyelitis
Eight RCTs, totaling 1321 patients, compared IV-only vs. oral therapy for osteomyelitis (Table 1). All trials evaluated adult patients, and the majority excluded axial osteomyelitis, although the largest trial15 included 39 patients with surgery for vertebral osteomyelitis/diskitis evenly distributed between treatment arms. Four of the trials15–18 included patients with infected orthopedic hardware, all evenly distributed among oral and IV treatment groups. The largest trial of over 1000 patients included 678 patients with foreign material, including 125 patients with infected prosthetic joints implants.15 None of the trials included patients with osteomyelitis underlying a decubitus pressure ulcer, a condition for which antibiotics play little role.19
Table 1:
Prospective RCTs of Osteomyelitis
| Author | Year | N | Inclusion & Exclusion* | Regimen Oral vs IV | Success† Oral vs IV | Complications Oral vs IV, n (%) | |
|---|---|---|---|---|---|---|---|
| Greenberg22 | ‘87 | 30 |
Included: positive bacterial cultures (blood or bone) Excluded: malignant otitis externa, severity of disease requiring IV therapy |
Ciprofloxacin vs. standard IV | 50% (7/14) vs. 69% (11/16) | Relapse AEs |
4 (28%) vs. 1 (6%) 2 (14%) vs. 6 (38%) |
| Gentry17 | ‘90 | 59 |
Included: debrided OM Excluded: septicemia, MRSA |
Ciprofloxacin vs. βL+AG | 77% (24/31) vs. 79% (22/28) | Relapse AEs |
6 (19%) vs. 5 (18%) 1 (3%) vs. 4 (14%) |
| Mader16 | ’90 | 26 |
Included: extra-axial OM with debridement and culture Excluded: severe renal or hepatic disease, antibiotics within 3 days |
Ciprofloxacin vs. βL/clindamycin+AG | 79% (11/14) vs. 83% (10/12) | AEs | 7 (37%) vs. 4 (29%) |
| Gentry20 | ‘91 | 33 |
Included: biopsy confirmed OM Excluded: multiple sites of infection, retained prosthetic material, bacteremia |
Ofloxacin vs. cephalosporin | 74% (14/19) vs. 86% (12/14) | Relapse AEs |
6 (19%) vs. 5 (18%) 7 (37%) vs. 4 (29%) |
| Gomis21 | ‘99 | 32 | Included: debrided chronic OM (extra-axial, sacral), 1 PJI | Ofloxacin vs. imipenem | 69% (11/16) vs. 50% (8/16)‡ | Serious AEs | 0 (0%) vs. 3 (19%) |
| Schrenzel14 | ‘04 | 39 |
Included: S. aureus bone and joint infection Excluded: chronic OM without debridement, retained foreign bodies, antimicrobials given >72 hours before enrollment |
Fleroxacin + rifampin vs. βL/vancomycin | 82% (18/22) vs. 65% (11/17) | Death AEsϕ |
3 (4.4%) vs. 5 (8.5%) 15 (22%) vs. 5 (8%) |
| Euba18 | ‘09 | 48 |
Included: surgical debridement for chronic extra-axial OM with or without foreign body Excluded: PJI, polymicrobial |
TMP-SMX + rifampin vs. cloxacillin | 89% (24/27) vs. 91% (19/21)‡ | Relapse AEs |
3 (11%) vs. 2 (10%) 5 (18%) vs. 3 (14%) |
| Li15 | ’19 | 1054 | Included: extra-axial or vertebral OM, septic arthritis, PJI, fixation device infection | standard oral vs. standard IV | 87% (457/527) vs. 85% (450/527)‡ | Early discontinuation due to relapse Serious AE’s |
15 (3%) vs. 1 (0.1%) 138 (26%) vs. 147 (28%) |
| Totals (N=8 RCTs) | 1321 | Oral: 84% (566/670) vs. IV: 83% (543/651) | |||||
All studies excluded children, pregnancy, and patients with organisms resistant to study drug.
Success = absence of osteomyelitis at long term follow-up (most studies >1 year). AE: adverse events; AG: aminoglycoside; βL: beta-lactam; OM: osteomyelitis; PJI = prosthetic joint infection; standard: standard of care, within protocol specifications; TMP-SMX: trimethoprim-sulfamethoxazole;
Outcomes by intention-to-treat;
This study performed a subgroup analysis to determine treatment success, but AEs for the full study population regardless of site of infection, so the AE numbers are for the larger population from the study.
All trials reported microbiologic etiologies, with staphylococci followed by Pseudomonas aeruginosa as the most common monomicrobial organism. Six trials compared a fluoroquinolone (ciprofloxacin, ofloxacin, or fleroxacin), with or without an oral rifamycin, to various IV regimens. One additional study18 compared oral TMP-SMX plus rifampin to IV cloxacillin, while the largest osteomyelitis study15 compared standard IV regimens to varied oral regimens including fluoroquinolones (37%), oral combinations (17%), penicillins (16%), and macrolides/lincosamide (13%).
Six trials14–18, 20 demonstrated similar success rates between the IV and oral groups. One trial21 actually showed significantly superior cure rates (69% vs 50%) for oral ofloxacin over IV imipenem/cilastin.
Severe drug reactions were either similar between treatment groups13, 15, 16, 18, 20 or more frequent17, 22 in the IV group. Three studies also described line-related complications, including local cellulitis, phlebitis, and deep vein thrombosis unique to the IV group, ranging in frequency from 7% to 13%.15, 18, 22 In by far the largest RCT conducted, patients in the IV arm had significantly higher adverse event rates, driven by line complications, as well as decreased patient satisfaction and longer durations of hospitalization.15
Meta-analysis of the 8 RCTs demonstrated a point estimate (95% confidence interval) of the difference in long-term treatment success of +1% (−3% to +5%) for oral vs. IV therapy (Figure 2). Funnel plot analysis revealed no evidence of publication bias (Supplemental Figure 1A).
Figure 2. Meta-Analysis Forest Plot of Osteomyelitis Treatment Success.
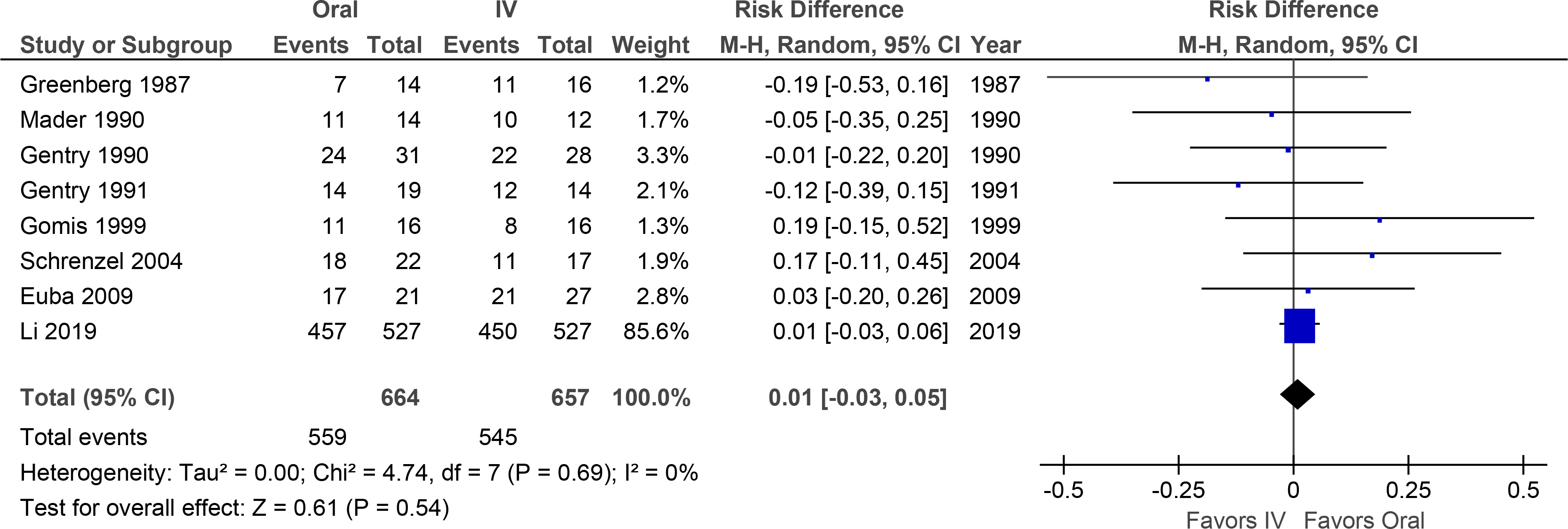
Overall treatment success was not significantly different.
In addition, 9 RCTs have been published in which oral antibiotics constituted the large majority of therapy in both arms for osteomyelitis, with excellent outcomes.23–31 These RCTs compared different durations or different oral antimicrobial agents, and included patients with vertebral osteomyelitis, diabetic foot infections, and prosthetic joint infections, with only short IV-lead in periods before patients were switched to oral therapy. Outcomes were favorable in these studies. Because these studies did not compare oral to IV therapy, they were not included in the meta-analysis, however they do add important context regarding the real-world efficacy of oral antibiotic therapy for a variety of types of osteomyelitis.
Bacteremia
Ten RCTs were identified totaling 705 patients randomized to either oral or IV therapy for non-endocarditis bacteremia (Table 2). Seven of the trials included only adults. Two trials enrolled only children22, 23 and one only neonates.24 Sources of bacteremia were diverse across trials, including urinary,32, 33 respiratory,32, 34–37 skin and soft tissue,32, 36–38 biliary,32, 39 catheter-related14, 32, 35, 36, 38, 40 and primary/unknown.14, 32, 35-37, 40 Microbiologic etiologies included both gram-positive14, 34–38, 40 and gram-negative32, 33, 39 bacteremias. Across studies, E. coli bacteremia was the most common among the gram-negative bacterial causes, followed by Klebsiella pneumoniae, whereas among the gram positive cocci there was more equal distribution among methicillin-sensitive and -resistant S. aureus (MSSA and MRSA), enterococci, coagulase-negative staphylococci (including methicillin-resistant strains), Streptococcus pneumoniae, and other streptococci.
Table 2:
Prospective RCTs of Bacteremia
| Author | Year | N | Inclusion & Exclusion | Regimen Oral vs IV | Success* Oral vs IV | Complications Oral vs IV, n (%) | |
|---|---|---|---|---|---|---|---|
| Gram positive bacteria | |||||||
| San Pedro34 | ’02 | 51 | Included: age ≥13 years, suspected CAP with S. pneumoniae bacteremia | Linezolid vs. ceftriaxone/cefpodoxime | 93% (27/29) vs. 68% (15/22) | AEs† | 218 (57%) vs. 200 (55%) |
| Deville40 | ’03 | 36 |
Included: neonates up to 90 days old with gram positive bacteremia Excluded: device that could not be removed, condition not appropriate for drug regimen |
Linezolid vs. vancomycin | 80% (20/25) vs. 64% (7/11)‡ | AEs† | 5 (12%) vs. 6 (32%) |
| Jantausch35 | ’03 | 103 |
Included: age ≤12 years with bacteremia, including Enterococcus, S. aureus, CoNS Excluded: device that could not be removed, condition not appropriate for regimen |
Linezolid vs vancomycin | 72% (54/75) vs. 64% (18/28)‡ | AEs† | 20 (19%) vs. 13 (28%) |
| Kaplan36 | ’03 | 80 |
Included: age ≤ 12 years with CRBSI, bacteremia caused by drug-resistant gram positives Excluded: treated with active antibiotic >24 hours |
Linezolid vs vancomycin | 82% (47/57) vs. 74% (17/23) | AEs† | 40 (19%) vs. 34 (34%) |
| Schrenzel14 | ’04 | 67 |
Included: adults with S. aureus or CoNS primary bacteremia or CRBSI Excluded: Excluded infections with foreign bodies retained |
Fleroxacin + rifampin vs. βL/vancomycin | 87% (34/39) vs 89% (25/28) | Microbiologic failure rates for S. aureus | 4 (14%) vs. 2 (13%) |
| Wilcox37 | ’04 | 56 |
Included: age ≥13 years, gram positive bacteremia Excluded: effective antibiotic therapy within 48 hours of study entry, infections requiring >28 days therapy |
Linezolid Vs. teicoplanin | 89% (23/26) vs . 57% (17/30) | AEs† | 121 (56%) vs. 110 (51%) |
| Wilcox38 | ’09 | 166 |
Included: age ≥13 years, gram positive CRBSI Excluded: catheter could not be removed, endovascular or other infection, antibiotic within 72 h before study entry |
Linezolid vs. vancomycin | 75% (70/93) vs. 81% (59/73) | AEs† | 244 (67%) vs. 230 (63%) |
| Gram negative bacteria | |||||||
| Amodio-Groton32 | ’96 | 50 |
Included: adults with gram negative bacteremia Excluded: severe renal impairment, strict anaerobes, non-biliary abdominal source, critically ill, neutropenia, AIDS |
Ciprofloxacin vs. ciprofloxacin (72 hours after any upfront IV agent) | 83% (20/24) vs. 77% (20/26) | AEs† | 1 (4%) vs. 2 (8%) |
| Monmaturpoj33 | ‘12 | 17 |
Included: 82 adults with pyelonephritis (17 bacteremic) Excluded: severe hepatic or renal disease, immune-compromised hosts |
Cefditoren vs. ceftriaxone | 100% (6/6) vs. 91% (10/11) | AEs† | 4 (10%) vs. 2 (5%) |
| Park39 | ‘14 | 59 |
Included: adults with bacteremic, obstructive acute cholangitis with successful biliary decompression Excluded: critical illness, biliary drainage in prior 2 weeks, need for surgery, immune-compromise, bacteremia complications of liver abscess or endocarditis |
Ciprofloxacin vs. std IV | 93% (27/29) vs. 93% (28/30) | Relapse 30-day mortality |
1 (3%) vs. 0 (0%) 0 (0%) vs. 0 (0%) |
| Totals (N=10 RCTs) | 685 | Oral: 81% (328/403) vs. IV: 77% (216/282) | |||||
Success classified as clinical resolution of infection. CAP: community-acquired pneumonia; CoNS: coagulase negative staphylococci; CRBSI: catheter-related bloodstream infection; std: standard of care, within protocol specifications; LOS: Length of stay; AE = adverse events;
These studies performed a subgroup analysis to determine success of antimicrobials for bacteremia, but AEs for the full study population regardless of site of infection, so the AE numbers are for the larger population from the study;
Analysis by intention-to-treat
Six bacteremia trials showed equivalent results for clinical success between IV-only and oral arms. However, two trials35, 36 showed non-statistically significantly higher rates of success with oral therapy, and the remaining two studies demonstrated statistically significantly higher cure rates for oral over IV-only therapy.34, 37 No studies reported significantly higher cure rates of IV-only therapy.
Three studies14, 32, 39 reported shorter hospital length of stay for oral therapy recipients compared to IV-only therapy, ranging from 1.5 to 11 days shorter.
Five RCTs reported similar overall rates of drug-related adverse events in both arms.32, 33, 35, 37, 38 Two trials showed higher rates of vancomycin-related adverse events compared to oral linezolid, including rash, infusion reactions and oral candidiasis.36, 40 Two trials reported IV therapy-only adverse effects directly related to IV drug infusion.33, 36
Central nervous system side effects, hallucinations and insomnia, were more common in the fleroxacin arm compared to IV therapy recipients in one trial.14 Notably, rates of cytopenias were similar in all trials of oral linezolid versus a comparator IV agent. Serious adverse events, including mortality, were similar between study arms in almost all of the studies, although one trial of patients who were intended to be enrolled with gram-positive bacteremia had unexpectedly higher mortality among linezolid recipients compared to IV vancomycin recipients.38 On further analysis, excess mortality in that study was attributable to underlying gram-negative co-infection.
Meta-analysis of the 10 RCTs demonstrated a difference in long-term treatment success (95% confidence interval) for oral vs. IV therapy of +7% (−1% to +15%) (Figure 3). Funnel plot demonstrated slight asymmetry; however imputation to adjust for that asymmetry did not substantively alter the resulting treatment effect (Supplemental Figure 1B).
Figure 3. Meta-Analysis Forest Plot of Bacteremia Treatment Success.
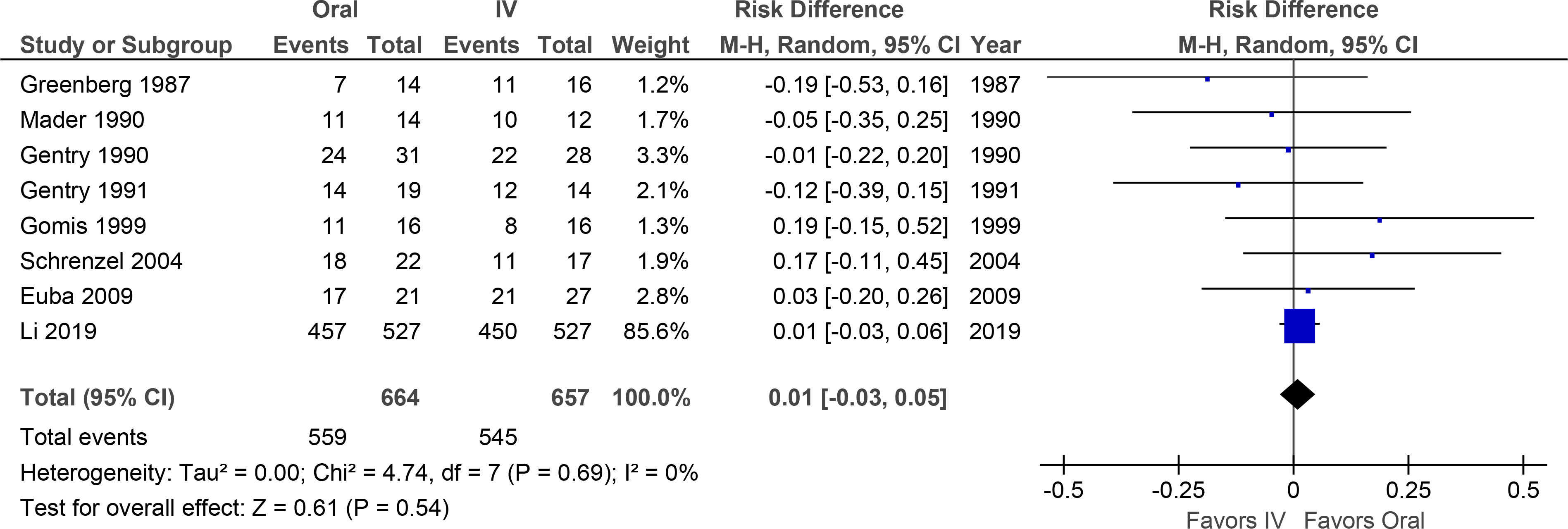
Overall treatment success was not significantly different, although the confidence interval favored oral therapy.
Endocarditis
Three RCTs and one quasi-experimental trial were identified comparing oral stepdown vs. IV-only therapy for infective endocarditis, including native and prosthetic valves, left and right-sided, and cardiac devices endocarditis.41–45 In all cases, appropriate valvular surgical intervention or device removal was performed equally in both groups. While three trials focused only on specific etiologic organisms like S. aureus42, 45 or streptococci,44 the largest trial41 included a variety of causal bacterial organisms including S. aureus, streptococci, enterococci and coagulase-negative staphylococci.
In the two smaller studies, oral step-down and IV-only therapy resulted in similar outcomes, including no differences in mortality (no deaths in the former study, and none in the evaluable population in the latter study).44, 45 In the two larger studies, which included by far the largest RCT conducted, oral therapy was superior in efficacy, resulting in significantly lower long term mortality and infectious relapse than IV-only therapy.41–43 In no identified study was IV-only therapy superior in efficacy.
Adverse events were similar in most trials with a few exceptions. Slightly higher rates of acute kidney injury (AKI) associated with TMP-SMX + clindamycin (5% vs <1%) compared to IV standard were reported in one trial,42 although another45 showed significantly higher rates of liver toxicity (mostly oxacillin-related) and AKI with IV therapy. All four studies demonstrated shorter lengths of inpatient hospitalization in their oral therapy arms.
By meta-analysis, oral therapy was significantly more likely to result in treatment success and mortality, with a treatment difference (95% confidence interval) of +8% (+3% to +14%) (Figure 4). Funnel plot revealed no evidence of publication bias (Supplemental Figure 1C).
Figure 4. Meta-Analysis Forest Plot of Endocarditis Treatment Success.
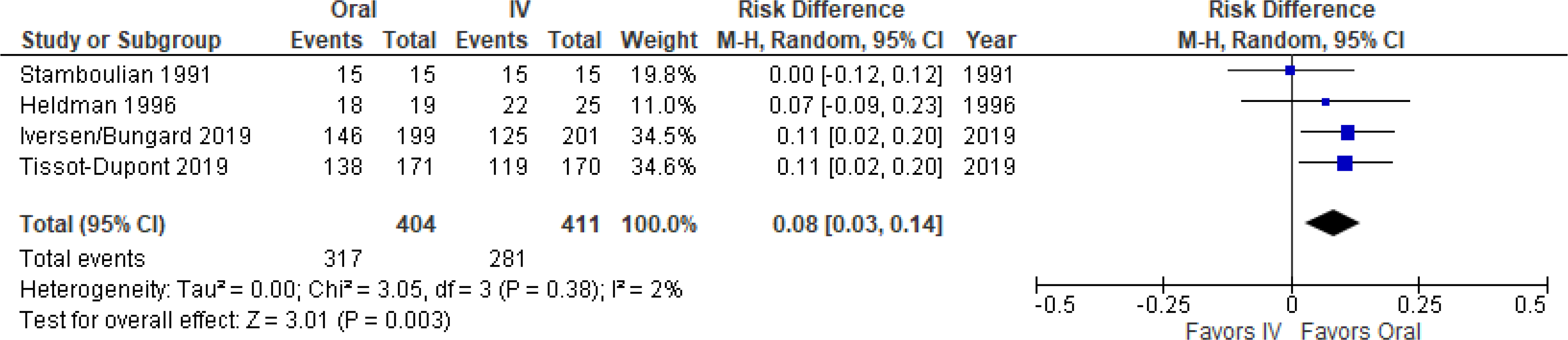
Oral therapy was significantly more effective.
Discussion
All 20 RCTs, and a single quasi-experimental study, found that oral antibiotics were at least as effective as IV therapy for the treatment of osteomyelitis, bacteremia, and endocarditis. Indeed, multiple of the studies found that oral was more effective than IV therapy for bacteremia and endocarditis, including for mortality. No contrary data were identified.
The evaluated trials used a wide array of antimicrobial therapy, offering reassurance that oral efficacy is not limited to only 1 or 2 classes of drugs. Nevertheless, not all oral antimicrobial options are likely to be effective for treating these diseases, and clinicians should use oral regimens that have been demonstrated to have favorable efficacy in published studies.
For osteomyelitis, most of the published efficacy data were with fluoroquinolones or TMP-SMX, with or without adjunctive rifampin.1, 18 The addition of rifampin is important when treating S. aureus infections with fluoroquinolones due to the high rate of quinolone resistance emerging with monotherapy.1, 46 Furthermore, the majority of adverse events from oral therapy in published RCTs were due to fluoroquinolones, and there have been rising concerns about fluoroquinolone toxicity in general.47, 48 Thus, if alternative agents are available (e.g., TMP-SMX, linezolid for < 4 weeks, amoxicillin for Streptococcus, etc), these may be preferrable to minimize toxicities. Nevertheless, clinicians may underestimate the dangers of prolonged IV catheterization, and the data demonstrate that IV catheter complications are at least as frequent, and potentially more dangerous, than fluoroquinolone complications.
The typical published dose of TMP-SMX for osteomyelitis has been 7.5 mg/kg/d (2 double strength tablets twice daily for a 70 kg patient).1 Clindamycin has also been studied extensively in pediatric osteomyelitis,49–52 and both clindamycin (600 mg thrice daily or 450 mg four times daily) and linezolid (600 mg twice daily) achieved high cure rates in numerous observational studies of adult osteomyelitis,1, 24, 53 and were options in the largest RCT for osteomyelitis.15 Caution should be used when administering linezolid for more than 2–3 weeks due to toxicities such as cytopenias, which are reversible, and neuropathy which may be irreversible with prolonged dosing. All these drug options have excellent oral bioavailability and bone penetration. In contrast, oral β-lactams and doxycycline achieve low blood levels and have relatively poor bone penetration compared to these other options.1 Caution may be warranted in selecting these drugs for treating osteomyelitis, although 10–15% of patients in the largest RCT for osteomyelitis did receive them.15
Treatment options for bacteremia and endocarditis are similar to osteomyelitis.2 One major exception is the need to avoid using up front TMP-SMX monotherapy to treat S. aureus bloodstream or endocarditis infections, in contrast to osteomyelitis.7,54 However, Tissot-Dupont et al. demonstrated that a combination of TMP-SMX plus clindamycin, followed by step-down oral therapy with TMP-SMX was effective for treating endocarditis (indeed it was superior to the IV control group).42 Thus TMP-SMX may be reasonable to use as an oral step-down option after initial IV therapy has stabilized the patient and cleared their bacteremia.
The amount of IV antimicrobial therapy that was administered prior to initiation of oral therapy varied dramatically across the trials. Some studies administered no IV therapy per protocol prior to initiation of oral therapy.14, 45 Other studies ranged between 7 and 14 days of IV therapy prior to oral therapy. Thus, there is no specific duration of required IV lead-in defined by the identified studies.
Why oral therapy might be superior in efficacy to IV therapy for bacteremia/endocarditis remains uncertain. Harm from the long-term IV catheter could contribute to treatment failures. Furthermore, retaining plastic catheters could make clearance of bacteremia more difficult and serve as a nidus for relapse. Further study is warranted to determine if there is a pathophysiological basis for clinical superiority of oral regimens, generally. Such research is in line with recent trends challenging other aspects of traditional management of these patients, such as the increasing movement to short course therapy,26, 31, 55–59 and the necessity or not of follow up blood cultures.
A primary limitation of the meta-analysis is that many of the RCTs included were small. However, for each disease, at least one large RCT completed within the last decade anchored the meta-analyses. Furthermore, concerns raised about the quality of the 20 individual RCTs must be tempered by the fact that not a single prospective study was identified demonstrating superiority of IV therapy. Finally, the relative paucity of MRSA infections in these published RCTs may be of concern. While that is a limitation, and caution should always be taken in treating invasive MRSA infections, there is a logical fallacy to the argument that clinicians should be comfortable treating MSSA but not MRSA infections with oral therapy. The IV options for MSSA (β-lactams) are considerably more effective than the primary IV option for MRSA infections (vancomycin).60, 61 The mecA methicillin-resistance mechanism does not alter the antimicrobial activity of fluoroquinolones, TMP-SMX, clindamycin, linezolid, or rifampin. Thus, if these oral options are at least as effective as the IV therapy for MSSA infections, they logically must be at least as effective as the less effective IV therapy for susceptible MRSA infections.
Building off a previously proposed algorithm,2 we suggest it is reasonable to consider oral therapy for osteomyelitis, bacteremia, and endocarditis when all of the following criteria are met:
The patient is clinically and hemodynamically stable
Surgical or procedural source control has been achieved if possible, with no persistent bacteremia
The patient is likely to be able to tolerate and absorb oral medications
A published regimen is available with clinical outcomes data for targeted pathogens
There are no psychosocial or logistical reasons to prefer IV therapy
In summary, there are now 20 RCTs and a quasi-experimental study that unanimously demonstrate that oral therapy is at least as effective as IV-only therapy for osteomyelitis, bacteremia, and endocarditis. Furthermore, oral therapy is safer, results in superior patient satisfaction, and markedly decreases length of hospital stay and cost.15 It is time for evidence to overcome anchor bias and inertia in medicine. These findings should be incorporated into treatment guidelines to help drive change to clinical practice, indicating that oral therapy is a reasonable option for these diseases in reasonably selected patients.
Supplementary Material
Table 3.
Prospective Controlled Studies of Infective Endocarditis
| Author | Year | N | Inclusion and Exclusion Criteria | Regimen Oral vs IV | Success Oral vs IV | Reported Complications Oral vs IV, n (%) | |
|---|---|---|---|---|---|---|---|
|
| |||||||
| Stamboulian44 | ’91 | 30 |
Included: native valve IE due to penicillin-susceptible streptococci Exclusion: cardiovascular risk factors, prosthetic valves |
2 weeks ceftriaxone then 2 weeks amoxicillin vs. 4 weeks ceftriaxone | 100% (15/15) vs 100% (15/15) | Relapse AE’s |
1 (7%) vs. 0 (0%) 1 (7%) vs. 1 (7%) |
|
| |||||||
| Heldman45 | ’96 | 44 |
Included: adult injection drug users with right-sided staphylococcal IE (95% MSSA) Excluded: left sided IE, prosthetic device, pregnant, intubated |
Ciprofloxacin + rifampin vs. standard IV | 95% (18/19) vs. 88% (22/25) | AE’s | 1 (3%) vs. 24 (62%) |
|
| |||||||
| Iversen41/Bungaard43* | ’19 | 400 |
Included: IE of any valve, including prosthetic valves and pacemakers due to streptoccci, E. faecalis, S. aureus or CoNS Excluded: unstable patients |
Standard oral vs. standard IV | 73% (146/199) vs. 62% (125/201) | AE’s | 10 (5%) vs. 12 (6%) |
|
| |||||||
| Tissot-Dupont42† | ’19 | 341 | Included: IE of any valve, including prosthetic value due to S. aureus (including MRSA) | IV TMP-SMX + clindamycin for 7 days transitioned to oral vs. standard IV | 81% (138/171) vs. 70% (119/170) | Relapse AEs |
7 (4%) vs. 10 (6%) 27 (16%) vs. 16 (9%) |
|
| |||||||
| Totals (N=3 RCTs) + 1 quasi-experimental | 474 | Oral 77% (179/233) vs IV 67% (162/241) | |||||
| 815 | Oral 78% (317/404) vs IV 68% (281/411) | ||||||
Iversen et al. reported 6-month follow up, and Bungaard et al. reported median 3 year follow up of the same study patients. Outcomes shown are from the longer term follow up.
This was a quasi-experimental, pre-post study. CoNS: coagulase-negative staphylococci; IE: infective endocarditis; MRSA: methicillin-resistant S. aureus; MSSA: methicillin-sensitive S. aureus. AE = adverse events.
Clinical Significance.
All 20 published randomized controlled trials comparing oral to IV therapy for osteomyelitis, bacteremia, and endocarditis demonstrated oral antibiotic therapy was at least as effective as IV.
In no published studies was IV superior in efficacy.
The data are overwhelmingly clear regarding the relative efficacy of oral to IV-only therapy for these diseases; it is time to change how we practice.
Acknowledgments
Funding
This work was funded by National Institute of Allergy and Infectious Diseases at the United States National Institutes of Health [Grant Numbers R01 AI130060 and AI1117211, R21 AI127954, and R42 AI106375 to BS].
Footnotes
Declaration of Competing Interest
All authors declare no relevant conflicts of interest
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Spellberg B, Lipsky BA. Systemic antibiotic therapy for chronic osteomyelitis in adults. Clin Infect Dis. 2012;54:393–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Spellberg B, Chambers HF, Musher DM, Walsh TL, Bayer AS. Evaluation of a Paradigm Shift From Intravenous Antibiotics to Oral Step-Down Therapy for the Treatment of Infective Endocarditis: A Narrative Review. JAMA internal medicine. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Waldvogel FA, Medoff G, Swartz MN. Osteomyelitis: a review of clinical features, therapeutic considerations and unusual aspects. N Engl J Med. 1970;282:198–206. [DOI] [PubMed] [Google Scholar]
- 4.Finland M. Treatment of bacterial endocarditis. N Engl J Med. 1954;250:419–428; concl. [DOI] [PubMed] [Google Scholar]
- 5.Finland M. Treatment of bacterial endocarditis. N Engl J Med. 1954;250:372–383; contd. [DOI] [PubMed] [Google Scholar]
- 6.Suurmond R, van Rhee H, Hak T. Introduction, comparison, and validation of Meta-Essentials: A free and simple tool for meta-analysis. Res Synth Methods. 2017;8:537–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Paul M, Bishara J, Yahav D, et al. Trimethoprim-sulfamethoxazole versus vancomycin for severe infections caused by meticillin resistant Staphylococcus aureus: randomised controlled trial. Bmj. 2015;350:h2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bass JW, Steele RW, Wittler RR, et al. Antimicrobial treatment of occult bacteremia: a multicenter cooperative study. The Pediatric infectious disease journal. 1993;12:466–473. [DOI] [PubMed] [Google Scholar]
- 9.Fass RJ, Plouffe JF, Russell JA. Intravenous/oral ciprofloxacin versus ceftazidime in the treatment of serious infections. Am J Med. 1989;87:164S–168S. [DOI] [PubMed] [Google Scholar]
- 10.Paladino JA, Sperry HE, Backes JM, et al. Clinical and economic evaluation of oral ciprofloxacin after an abbreviated course of intravenous antibiotics. Am J Med. 1991;91:462–470. [DOI] [PubMed] [Google Scholar]
- 11.Geddes A, Thaler M, Schonwald S, Harkonen M, Jacobs F, Nowotny I. Levofloxacin in the empirical treatment of patients with suspected bacteraemia/sepsis: comparison with imipenem/cilastatin in an open, randomized trial. J Antimicrob Chemother. 1999;44:799–810. [DOI] [PubMed] [Google Scholar]
- 12.Mombelli G, Pezzoli R, Pinoja-Lutz G, Monotti R, Marone C, Franciolli M. Oral vs intravenous ciprofloxacin in the initial empirical management of severe pyelonephritis or complicated urinary tract infections: a prospective randomized clinical trial. Arch Intern Med. 1999;159:53–58. [DOI] [PubMed] [Google Scholar]
- 13.Peacock JE Jr., Pegram PS, Weber SF, Leone PA. Prospective, randomized comparison of sequential intravenous followed by oral ciprofloxacin with intravenous ceftazidime in the treatment of serious infections. Am J Med. 1989;87:185S–190S. [DOI] [PubMed] [Google Scholar]
- 14.Schrenzel J, Harbarth S, Schockmel G, et al. A randomized clinical trial to compare fleroxacin-rifampicin with flucloxacillin or vancomycin for the treatment of staphylococcal infection. Clin Infect Dis. 2004;39:1285–1292. [DOI] [PubMed] [Google Scholar]
- 15.Li HK, Rombach I, Zambellas R, et al. Oral versus Intravenous Antibiotics for Bone and Joint Infection. N Engl J Med. 2019;380:425–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mader JT, Cantrell JS, Calhoun J. Oral ciprofloxacin compared with standard parenteral antibiotic therapy for chronic osteomyelitis in adults. J Bone Joint Surg Am. 1990;72:104–110. [PubMed] [Google Scholar]
- 17.Gentry LO, Rodriguez GG. Oral ciprofloxacin compared with parenteral antibiotics in the treatment of osteomyelitis. Antimicrob Agents Chemother. 1990;34:40–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Euba G, Murillo O, Fernandez-Sabe N, et al. Long-term follow-up trial of oral rifampin-cotrimoxazole combination versus intravenous cloxacillin in treatment of chronic staphylococcal osteomyelitis. Antimicrob Agents Chemother. 2009;53:2672–2676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wong D, Holtom P, Spellberg B. Osteomyelitis Complicating Sacral Pressure Ulcers: Whether or Not to Treat With Antibiotic Therapy. Clin Infect Dis. 2019;68:338–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gentry LO, Rodriguez-Gomez G. Ofloxacin versus parenteral therapy for chronic osteomyelitis. Antimicrob Agents Chemother. 1991;35:538–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gomis M, Barberan J, Sanchez B, Khorrami S, Borja J, Garcia-Barbal J. Oral ofloxacin versus parenteral imipenem-cilastatin in the treatment of osteomyelitis. Rev Esp Quimioter. 1999;12:244–249. [PubMed] [Google Scholar]
- 22.Greenberg RN, Tice AD, Marsh PK, et al. Randomized trial of ciprofloxacin compared with other antimicrobial therapy in the treatment of osteomyelitis. Am J Med. 1987;82:266–269. [PubMed] [Google Scholar]
- 23.Lipsky BA, Baker PD, Landon GC, Fernau R. Antibiotic therapy for diabetic foot infections: comparison of two parenteral-to-oral regimens. Clin Infect Dis. 1997;24:643–648. [DOI] [PubMed] [Google Scholar]
- 24.Lipsky BA, Itani K, Norden C. Treating foot infections in diabetic patients: a randomized, multicenter, open-label trial of linezolid versus ampicillin-sulbactam/amoxicillin-clavulanate. Clin Infect Dis. 2004;38:17–24. [DOI] [PubMed] [Google Scholar]
- 25.Lazaro-Martinez JL, Aragon-Sanchez J, Garcia-Morales E. Antibiotics versus conservative surgery for treating diabetic foot osteomyelitis: a randomized comparative trial. Diabetes care. 2014;37:789–795. [DOI] [PubMed] [Google Scholar]
- 26.Tone A, Nguyen S, Devemy F, et al. Six-week versus twelve-week antibiotic therapy for nonsurgically treated diabetic foot osteomyelitis: a multicenter open-label controlled randomized study. Diabetes care. 2015;38:302–307. [DOI] [PubMed] [Google Scholar]
- 27.Gariani K, Pham TT, Kressmann B, et al. Three versus six weeks of antibiotic therapy for diabetic foot osteomyelitis: A prospective, randomized, non-inferiority pilot trial. Clin Infect Dis. 2020. [DOI] [PubMed] [Google Scholar]
- 28.Lora-Tamayo J, Euba G, Cobo J, et al. Short- versus long-duration levofloxacin plus rifampicin for acute staphylococcal prosthetic joint infection managed with implant retention: a randomised clinical trial. Int J Antimicrob Agents. 2016;48:310–316. [DOI] [PubMed] [Google Scholar]
- 29.Benkabouche M, Racloz G, Spechbach H, Lipsky BA, Gaspoz JM, Uckay I. Four versus six weeks of antibiotic therapy for osteoarticular infections after implant removal: a randomized trial. J Antimicrob Chemother. 2019;74:2394–2399. [DOI] [PubMed] [Google Scholar]
- 30.Bernard L, Arvieux C, Brunschweiler B, et al. Antibiotic Therapy for 6 or 12 Weeks for Prosthetic Joint Infection. N Engl J Med. 2021;384:1991–2001. [DOI] [PubMed] [Google Scholar]
- 31.Bernard L, Dinh A, Ghout I, et al. Antibiotic treatment for 6 weeks versus 12 weeks in patients with pyogenic vertebral osteomyelitis: an open-label, non-inferiority, randomised, controlled trial. Lancet. 2015;385:875–882. [DOI] [PubMed] [Google Scholar]
- 32.Amodio-Groton M, Madu A, Madu CN, et al. Sequential parenteral and oral ciprofloxacin regimen versus parenteral therapy for bacteremia: a pharmacoeconomic analysis. Ann Pharmacother. 1996;30:596–602. [DOI] [PubMed] [Google Scholar]
- 33.Monmaturapoj T, Montakantikul P, Mootsikapun P, Tragulpiankit P. A prospective, randomized, double dummy, placebo-controlled trial of oral cefditoren pivoxil 400mg once daily as switch therapy after intravenous ceftriaxone in the treatment of acute pyelonephritis. International journal of infectious diseases : IJID : official publication of the International Society for Infectious Diseases. 2012;16:e843–849. [DOI] [PubMed] [Google Scholar]
- 34.San Pedro GS, Cammarata SK, Oliphant TH, Todisco T, Linezolid Community-Acquired Pneumonia Study G. Linezolid versus ceftriaxone/cefpodoxime in patients hospitalized for the treatment of Streptococcus pneumoniae pneumonia. Scandinavian journal of infectious diseases. 2002;34:720–728. [DOI] [PubMed] [Google Scholar]
- 35.Jantausch BA, Deville J, Adler S, et al. Linezolid for the treatment of children with bacteremia or nosocomial pneumonia caused by resistant gram-positive bacterial pathogens. The Pediatric infectious disease journal. 2003;22:S164–171. [DOI] [PubMed] [Google Scholar]
- 36.Kaplan SL, Deville JG, Yogev R, et al. Linezolid versus vancomycin for treatment of resistant Gram-positive infections in children. The Pediatric infectious disease journal. 2003;22:677–686. [DOI] [PubMed] [Google Scholar]
- 37.Wilcox M, Nathwani D, Dryden M. Linezolid compared with teicoplanin for the treatment of suspected or proven Gram-positive infections. J Antimicrob Chemother. 2004;53:335–344. [DOI] [PubMed] [Google Scholar]
- 38.Wilcox MH, Tack KJ, Bouza E, et al. Complicated skin and skin-structure infections and catheter-related bloodstream infections: noninferiority of linezolid in a phase 3 study. Clin Infect Dis. 2009;48:203–212. [DOI] [PubMed] [Google Scholar]
- 39.Park TY, Choi JS, Song TJ, Do JH, Choi SH, Oh HC. Early oral antibiotic switch compared with conventional intravenous antibiotic therapy for acute cholangitis with bacteremia. Dig Dis Sci. 2014;59:2790–2796. [DOI] [PubMed] [Google Scholar]
- 40.Deville JG, Adler S, Azimi PH, et al. Linezolid versus vancomycin in the treatment of known or suspected resistant gram-positive infections in neonates. The Pediatric infectious disease journal. 2003;22:S158–163. [DOI] [PubMed] [Google Scholar]
- 41.Iversen K, Ihlemann N, Gill SU, et al. Partial Oral versus Intravenous Antibiotic Treatment of Endocarditis. N Engl J Med. 2019;380:415–424. [DOI] [PubMed] [Google Scholar]
- 42.Tissot-Dupont H, Gouriet F, Oliver L, et al. High dose trimethoprim-sulfamethoxazole and clindamycin for Staphylococcus aureus endocarditis. Int J Antimicrob Agents. 2019. [DOI] [PubMed] [Google Scholar]
- 43.Bundgaard H, Ihlemann N, Gill SU, et al. Long-Term Outcomes of Partial Oral Treatment of Endocarditis. N Engl J Med. 2019;380:1373–1374. [DOI] [PubMed] [Google Scholar]
- 44.Stamboulian D, Bonvehi P, Arevalo C, et al. Antibiotic management of outpatients with endocarditis due to penicillin-susceptible streptococci. Reviews of infectious diseases. 1991;13 Suppl 2:S160–163. [DOI] [PubMed] [Google Scholar]
- 45.Heldman AW, Hartert TV, Ray SC, et al. Oral antibiotic treatment of right-sided staphylococcal endocarditis in injection drug users: prospective randomized comparison with parenteral therapy. Am J Med. 1996;101:68–76. [DOI] [PubMed] [Google Scholar]
- 46.Zimmerli W, Widmer AF, Blatter M, Frei R, Ochsner PE. Role of rifampin for treatment of orthopedic implant-related staphylococcal infections: a randomized controlled trial. Foreign-Body Infection (FBI) Study Group. JAMA : the journal of the American Medical Association. 1998;279:1537–1541. [DOI] [PubMed] [Google Scholar]
- 47.FDA warns about increased risk of ruptures or tears in the aorta blood vessel with fluoroquinolone antibiotics in certain patients: FDA Drug Safety Communication: US Food and Drug Administration; 2019. [Google Scholar]
- 48.Kuula LSM, Viljemaa KM, Backman JT, Blom M. Fluoroquinolone-related adverse events resulting in health service use and costs: A systematic review. PLoS One. 2019;14:e0216029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kaplan SL, Mason EO Jr., Feigin RD. Clindamycin versus nafcillin or methicillin in the treatment of Staphylococcus aureus osteomyelitis in children. South Med J. 1982;75:138–142. [DOI] [PubMed] [Google Scholar]
- 50.Frank AL, Marcinak JF, Mangat PD, et al. Clindamycin treatment of methicillin-resistant Staphylococcus aureus infections in children. The Pediatric infectious disease journal. 2002;21:530–534. [DOI] [PubMed] [Google Scholar]
- 51.Peltola H, Unkila-Kallio L, Kallio MJ. Simplified treatment of acute staphylococcal osteomyelitis of childhood. The Finnish Study Group. Pediatrics. 1997;99:846–850. [DOI] [PubMed] [Google Scholar]
- 52.Peltola H, Paakkonen M, Kallio P, Kallio MJ, Group O-SS. Clindamycin vs. first-generation cephalosporins for acute osteoarticular infections of childhood--a prospective quasi-randomized controlled trial. Clin Microbiol Infect. 2012;18:582–589. [DOI] [PubMed] [Google Scholar]
- 53.Leijtens B, Elbers JBW, Sturm PD, Kullberg BJ, Schreurs BW. Clindamycin-rifampin combination therapy for staphylococcal periprosthetic joint infections: a retrospective observational study. BMC Infect Dis. 2017;17:321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Markowitz N, Quinn EL, Saravolatz LD. Trimethoprim-sulfamethoxazole compared with vancomycin for the treatment of Staphylococcus aureus infection. Annals of internal medicine. 1992;117:390–398. [DOI] [PubMed] [Google Scholar]
- 55.Wald-Dickler N, Spellberg B. Short Course Antibiotic Therapy-Replacing Constantine Units with “Shorter Is Better”. Clin Infect Dis. 2019;ePub. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Spellberg B. The New Antibiotic Mantra-”Shorter Is Better”. JAMA internal medicine. 2016;176:1254–1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Spellberg B, Rice LB. Duration of Antibiotic Therapy: Shorter Is Better. Annals of internal medicine. 2019;171:210–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yahav D, Franceschini E, Koppel F, et al. Seven Versus 14 Days of Antibiotic Therapy for Uncomplicated Gram-negative Bacteremia: A Noninferiority Randomized Controlled Trial. Clin Infect Dis. 2019;69:1091–1098. [DOI] [PubMed] [Google Scholar]
- 59.von Dach E, Albrich WC, Brunel AS, et al. Effect of C-Reactive Protein-Guided Antibiotic Treatment Duration, 7-Day Treatment, or 14-Day Treatment on 30-Day Clinical Failure Rate in Patients With Uncomplicated Gram-Negative Bacteremia: A Randomized Clinical Trial. JAMA : the journal of the American Medical Association. 2020;323:2160–2169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.McDanel JS, Perencevich EN, Diekema DJ, et al. Comparative effectiveness of beta-lactams versus vancomycin for treatment of methicillin-susceptible Staphylococcus aureus bloodstream infections among 122 hospitals. Clin Infect Dis. 2015;61:361–367. [DOI] [PubMed] [Google Scholar]
- 61.Mitaka H, Miyashita S, Yamada T, Satoi S, Chernyavsky S. Effectiveness of Beta-Lactams Versus Vancomycin for Treatment of Methicillin-Susceptible Staphylococcus aureus Bacteremia: A Systematic Review and Meta-Analysis. J Scientific Innovation Med. 2019;2:8. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


