Abstract
Objective:
Patient-centered studies have shown that several patients on thyroid hormone replacement therapy for hypothyroidism exhibit persistent symptoms, including “brain fog.” Here, we aimed to determine which of these specific symptoms are associated with brain fog, identify patient-reported factors that modify these symptoms, and identify patient concerns related to brain fog not included in thyroid-specific questionnaires.
Methods:
A survey on brain fog symptoms adapted from thyroid-specific patient-reported outcome was distributed online. Textual data analysis was performed to identify common areas of concern from open-ended survey responses.
Results:
A total of 5170 participants reporting brain fog while being treated for hypothyroidism were included in the analysis. Of these, 2409 (46.6%) participants reported symptom onset prior to the diagnosis of hypothyroidism, and 4096 (79.2%) participants experienced brain fog symptoms frequently. Of the symptoms listed, participants associated fatigue and forgetfulness most frequently with brain fog. More rest was the most common factor provided for improving symptoms. The textual data analysis identified areas of concern that are not often included in thyroid-specific quality of life questionnaires, including a focus on the diagnosis of hypothyroidism, the types and doses of medications, and the patient-doctor relationship.
Conclusion:
Brain fog in patients treated for hypothyroidism was associated most frequently with fatigue and cognitive symptoms. Several additional areas of patient concern were found to be associated with brain fog, which are not typically addressed in thyroid-specific questionnaires.
Keywords: hypothyroidism, brain fog, memory, fatigue
Introduction
It has become common in medical practice that many patients taking thyroid hormone complain of “brain fog,” which often refers to perceived cognitive impairment, physical fatigue, or mood disturbances. Brain fog has also been associated with several other disorders and patient conditions, including menopause syndrome, chemotherapy treatment, celiac disease, lupus, chronic fatigue syndrome, COVID-19 (most recently), and others.1–7 Due to the nonspecific nature of the term and the fact that the condition is not well characterized in the endocrine literature, brain fog represents a significant diagnostic and therapeutic challenge in patients with hypothyroidism.
The complaint of brain fog in patients with hypothyroidism may signify a state of persistent hypothyroidism despite treatment. In several cases, this may be due to undertreatment with thyroid hormone replacement, given that it has been observed that up to 35% of patients with hypothyroidism have elevated serum thyroid-stimulating hormone (TSH) levels.8,9 The presence of other conditions, for example, depression, sleep apnea, or vitamin B12 deficiency, may play a role, as well.
Alternatively, for some patients, brain fog may be a byproduct of the significant shift in the treatment of hypothyroidism that occurred in the early 1970s due to the development of the TSH assay and the discovery of substantial levels of triiodothyronine (T3) in patients taking only levothyroxine (LT4), providing evidence for the conversion of thyroxine (T4) to T3 outside the thyroid gland.10,11 Clinical practice shifted from thyroid extract to LT4 monotherapy as the predominant form of thyroid hormone replacement, with several patients experiencing a reduction in the overall daily dose of thyroid hormone.12 While this practice change has been accepted nearly universally, it has been known for over 50 years that a small group of patients transitioning to LT4 monotherapy from combination therapy with thyroid extract (containing T4 + T3) lacked energy and overall felt worse on LT4 alone.13
In general, there have been multiple reports of poor function and patient dissatisfaction with thyroid hormone replacement.14,15 A large community study from the United Kingdom observed that patients taking thyroid hormone experienced more thyroid-related symptoms and ranked their general health as poorer than controls, despite normalization of TSH levels.16 Several studies have evaluated psychologic and neurocognitive function in patients taking thyroid hormone and demonstrated significant functional deficits.17–19
Here, we studied hypothyroid individuals who complained of brain fog while being treated with replacement therapy for hypothyroidism to (1) determine which hypothyroid symptoms patients associate with brain fog, (2) aggregate lifestyle and other factors that patients identified that modified the severity of brain fog, and (3) gain a comprehensive view of the patient perspective by qualitatively analyzing patients’ free responses to an open-ended prompt: “what is brain fog?”
Materials and Methods
Survey Development and Distribution
The study was deemed exempt from review by the institutional review board at the University of Chicago. The English language survey consisted of 10 questions that were formulated to determine which symptoms participants perceived to be associated with brain fog and allow participants to provide lifestyle or pharmacologic factors that either improved or worsened those symptoms. The survey then concluded with an open-ended prompt: “Comment below if there is anything else researchers should know about ‘brain fog’ and hypothyroidism.” The survey was adapted from the validated thyroid symptom survey thyroidspecific patient-reported outcome measure (ThyPRO) and simplified for a projected completion time of <10 minutes.20 The survey was available online from September 15, 2020, through October 26, 2020. The survey was reviewed and approved for distribution by the American Thyroid Association, and the survey link was shared via email to those within the American Thyroid Association patient database. The survey was also distributed via hypothyroid support websites and social media groups, including Facebook and Twitter. No identifying or protected health information was collected from the participants. Submission details, including exact time of submission, were used to assist in elimination of duplicate submissions.
Survey Structure and Exclusion Criteria
Individuals with hypothyroidism were invited to participate in the study, and those who reported having brain fog despite treatment for hypothyroidism were asked to complete the survey. Those who answered that they “never” experienced brain fog despite treatment for hypothyroidism were excluded from the analysis. Participants included information on current age and sex. Participants who did not report having hypothyroidism or were <16 years of age were also excluded from the analysis.
Participants were asked to identify the cause of hypothyroidism, if known (“Hashimoto,” “thyroid surgery,” “treatment with radioactive iodine [RAI],” “a combination of thyroid surgery and RAI,” or “unknown”), and indicate the onset (“before I was diagnosed with hypothyroidism,” “within weeks/months of being diagnosed with hypothyroidism,” “years after I was diagnosed with hypothyroidism,” or “unknown”), frequency (“never,” “sometimes,” “frequently,” or “all the time”), and daily duration (“the whole day,” “mostly in the morning,” “mostly in the afternoon,” or “mostly in the evening/nighttime”) of brain fog. In this section of the survey, the term “my brain fog symptoms” was used to refer to any symptom that the participant associated with brain fog.
Participants were asked to report the frequency of association of specific symptoms adapted from ThyPRO with brain fog on a Likert scale from 1 to 4 (1, “never”; 4, “all the time”): “low energy/fatigue,” “feeling sleepy,” “forgetfulness,” “mental confusion,” “difficulty focusing,” “trouble making decisions,” “depressed mood,” and “anxiety.” Participants were also asked to report the degree to which brain fog had a “negative impact on life.”
The survey included 2 open-ended questions in which participants were allowed to respond in their own words (no word limit). In the first question, participants reported any factors (eg, behaviors and medications) that made brain fog symptoms better or worse. These factors were manually reviewed, and categories were created to group similar factors. All categories were framed in the context of improving brain fog symptoms (ie, a behavior that made symptoms worse would be framed as the absence of that behavior improving symptoms). Those categories that contained at least 50 individual responses were included in the final analysis. Participants were also asked to provide any additional comments “to help researchers better appreciate how you experience brain fog.” The complete survey is provided in Supplementary Fig. 1.
Analysis of Open-Ended Responses
Textual data analysis software (Alceste Software) was utilized to classify and synthesize the additional free text comments provided by the participants. The program performed a textual data analysis that determined significant co-occurrence of words, which has been used previously as computer-assisted method of discourse analysis.21 Significance was established using the χ2 test statistic. Each participant response was appropriately formatted for interpretation by the software, including grammatical adjustments and combining multiword terms by underscore (eg, “general_practitioner”). Synonyms were identified and switched to a single word, per the software guidelines. Medications with the same active ingredient were renamed to the generic name. The formatted text was then broken down into similarly sized elementary context units (ECUs) by grouping the occurrences of words and converting them into reduced root form (eg, “hypothyroid” and “hypothyroidism” into “hypothyroid+”). The frequencies of each root in each ECU were calculated. Using an algorithm based on descending hierarchical classification analysis, all ECUs were classified into subgroups by sequentially maximizing the difference of root frequencies between ECUs. As a result, the root vocabulary of each subgroup was maximally different from all other subgroups, with each subgroup comprising a unique set of ECUs with co-occurring words.22
Quantitative Data Analysis and Statistical Methods
Categorical variables of interest are presented as proportions, and numeric variables are presented as means and standard deviations. Due to the potential roles of age and menopausal status on the experience of brain fog symptoms, a subgroup analysis was performed in which the participants were divided into those above or below the age of 50 years old.23 In the subgroup analysis, categorical variables were compared using the χ2 test statistic to identify differences in proportions between subgroups. Numeric variables were compared using the t test statistic to identify differences in means. Multivariable logistic regression analysis was utilized to calculate the odds ratio (OR) of a participant aged ≥50 years associating brain fog with each symptom, adjusting for covariates including sex, etiology of hypothyroidism, and onset of symptoms. To create binary groups within symptom domains, “never” and “sometimes” responses were categorized as low frequency responses, and “frequently” and “all the time” were categorized as high frequency responses. Similarly, multivariable logistic regression was used in the analyses of factors modifying brain fog symptoms and classification of the open-ended responses.
Results
Demographic Characteristics
A total of 5635 individuals responded to the survey, of whom 5282 (93.7%) reported having brain fog symptoms despite treatment for hypothyroidism. An additional 112 participants were excluded due to missing age or age of <16 years (Fig. 1). The mean participant age was 50.6 years, and 95.9% of the participants were women (Table 1). Hashimoto disease without surgery or RAI therapy was the most common cause of hypothyroidism (45.3%), although 31.8% of participants reported their cause of hypothyroidism as unknown.
Fig. 1.
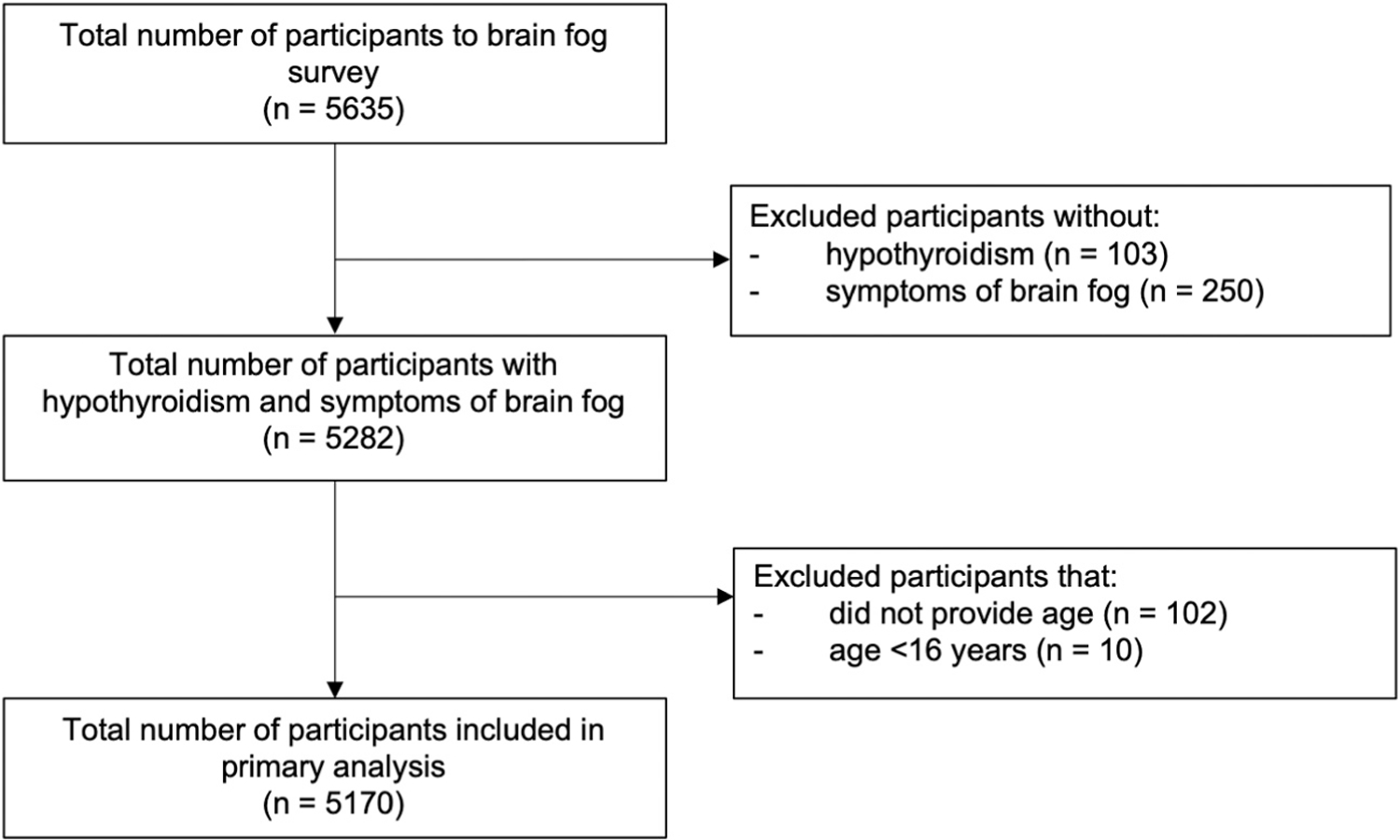
Flow diagram of survey participants.
Table 1.
Participant Characteristics and Burden of Brain Fog
| Characteristics | All participants n = 5170 |
Age <50 years n = 2334 |
Age ≥50 years n = 2836 |
P value |
|---|---|---|---|---|
| Age (y) (mean, SD) | 50.6 (12.1) | 39.80 (7.0) | 59.55 (7.1) | <.001 |
| Sex (n, %) | … | … | … | .082 |
| Male | 202 (3.9) | 76 (3.3) | 126 (4.4) | … |
| Female | 4960 (95.9) | 2255 (96.6) | 2705 (95.4) | … |
| Did not respond | 8 (0.2) | 3 (0.1) | 5 (0.2) | … |
| Etiology of hypothyroidism (n, %) | … | … | … | <.001 |
| Hashimoto/autoimmune | 2342 (45.3) | 1133 (48.5) | 1209 (42.6) | … |
| Hashimoto and surgery and/or RAI | 515 (10.0) | 246 (10.5) | 269 (9.5) | … |
| Thyroid surgery | 325 (6.3) | 104 (4.5) | 221 (7.8) | … |
| RAI | 304 (5.9) | 121 (5.2) | 183 (6.5) | … |
| Thyroid surgery and RAI | 39 (0.8) | 16 (0.7) | 23 (0.8) | … |
| Unknown | 1645 (31.8) | 714 (30.6) | 931 (32.8) | … |
| Frequency of having brain fog (n, %) | … | … | … | <.001 |
| Never/rarely | 59 (1.1) | 20 (0.9) | 39 (1.4) | … |
| Sometimes | 1015 (19.6) | 383 (16.4) | 632 (22.3) | … |
| Frequently | 2440 (47.2) | 1112 (47.6) | 1328 (46.8) | … |
| All the time | 1656 (32.0) | 819 (35.1) | 837 (29.5) | … |
| Onset of symptoms (n, %) | … | … | … | <.001 |
| Before diagnosis | 2409 (46.6) | 1206 (51.7) | 1203 (42.4) | … |
| Weeks/months after diagnosis | 887 (17.2) | 399 (17.1) | 488 (17.2) | … |
| 1 year or more after diagnosis | 847 (16.4) | 319 (13.7) | 528 (18.6) | … |
| Unknown | 1027 (19.9) | 410 (17.6) | 617 (21.8) | … |
| Timing of worst symptoms (n, %) | … | … | … | .002 |
| All day | 2914 (56.4) | 1349 (57.8) | 1565 (55.2) | … |
| Morning | 828 (16.0) | 327 (14.0) | 501 (17.7) | … |
| Midday | 1079 (20.9) | 508 (21.8) | 571 (20.1) | … |
| Evening/night | 349 (6.8) | 150 (6.4) | 199 (7.0) | … |
| Negative impact scores (mean, SD) | 3.1 (0.9) | 3.2 (0.8) | 3.0 (0.9) | <.001 |
Abbreviations: n = number of participants; RAI = radioactive iodine therapy; SD = standard deviation; y = years.
Numeric variables are presented as means and standard deviations. Categorical variables are presented as numbers and proportions. Negative impact scores represent the frequency at which each participant rated brain fog negatively affects their life, where 1 indicated never and 4 indicated all the time. The Student t test and chi-square test were performed as appropriate to assess statistical differences between age groups.
Frequency, Onset, and Duration of Brain Fog Symptoms
Participants reported experiencing brain fog often, with 79.2% having brain fog “frequently” or “all the time.” Over half of all participants (56.4%) reported brain fog lasting all day. Having brain fog just in the evening time was the least common. Nearly half of all participants (46.6%) reported experiencing brain fog prior to the diagnosis of hypothyroidism.
Analysis of Symptoms Associated with Brain Fog
The frequencies of association scores between brain fog and each symptom are presented in Figure 2. Regarding individual symptoms scores, most participants associated all 8 symptoms with brain fog at least sometimes. In particular, (1) low energy/fatigue, (2) forgetfulness, (3) feeling sleepy, and (4) difficulty focusing emerged as more prominent symptoms. These symptoms were associated with brain fog by >95% of participants. The mean negative impact score was 3.1, indicating that it was common for brain fog symptoms to negatively impact participants’ lives.
Fig. 2.
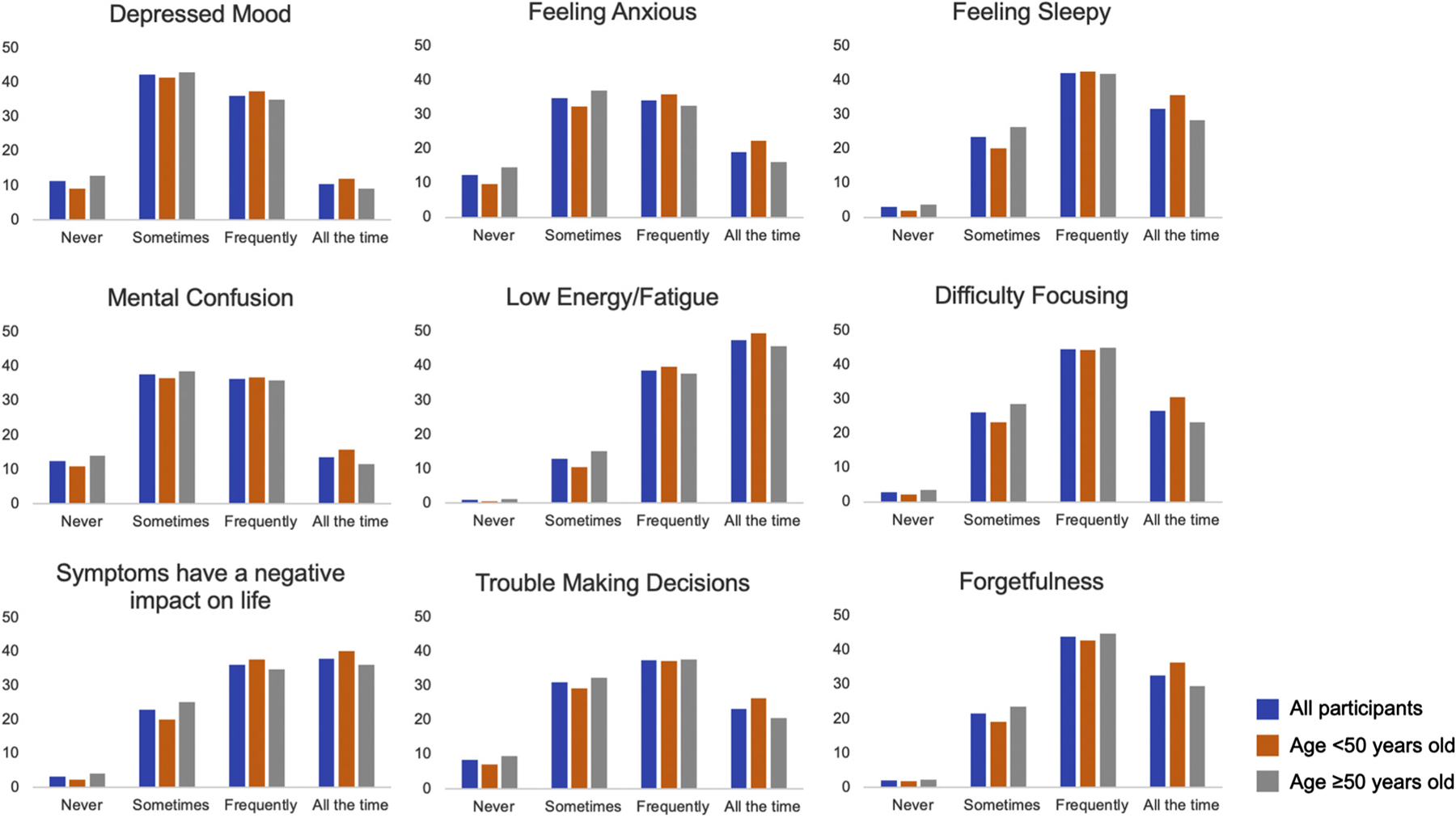
The frequency of association between brain fog and each survey symptom. Participant responses to Likert-style questions associating 8 symptoms with brain fog. Participants rated each symptom based on how frequently they associated that symptom with brain fog. The results are presented as 3 groups: (1) all participants (blue), (2) those aged <50 years (orange), and (3) those aged ≥ 50 years (gray).
Participant-Reported Lifestyle and Thyroid Hormone Factors That Modified the Symptoms of Brain Fog
Most participants provided at least 1 lifestyle or pharmacologic factor that affected brain fog symptoms (Table 2). Getting more rest and more exercise were the most commonly reported lifestyle factors to improve symptoms (51.7% and 10.4%, respectively). Several dietary factors were reported as well but less frequently. A total of 28.3% of participants reported that adjustment of thyroid hormone helped their symptoms. Liothyronine (LT3) was reported to help symptoms of brain fog more frequently than desiccated thyroid extract (8.3% vs 4.0%).
Table 2.
Lifestyle and Thyroid Hormone Factors Found to Improve Symptoms of Brain Fog
| Factors that improve symptoms | All participants n (%) |
Age <50 years n (%) |
Age ≥50 years n (%) |
P value |
|---|---|---|---|---|
| Provided comment (%) | 3879 (75.0) | 1755 (75.2) | 2124 (74.9) | .83 |
| Lifestyle factors | … | … | … | … |
| More rest | 2673 (51.7) | 1285 (55.1) | 1388 (48.9) | <.001 |
| More exercise | 539 (10.4) | 253 (10.8) | 286 (10.1) | .402 |
| Less exercise | 98 (1.9) | 49 (2.1) | 47 (1.7) | .285 |
| Better nutrition in diet | 333 (6.4) | 192 (8.2) | 141 (5.0) | <.001 |
| Less work stress | 281 (5.4) | 139 (6.0) | 142 (5.0) | .151 |
| More caffeine | 202 (3.9) | 96 (4.1) | 106 (3.7) | .534 |
| Less sugar in diet | 185 (3.6) | 92 (3.9) | 93 (3.3) | .23 |
| Less loud noise | 102 (2.0) | 43 (1.8) | 59 (2.1) | .609 |
| Avoid gluten | 86 (1.7) | 49 (2.1) | 37 (1.3) | .034 |
| Less alcohol | 87 (1.7) | 48 (2.1) | 39 (1.4) | .074 |
| Stay hydrated | 69 (1.3) | 43 (1.8) | 26 (0.9) | .006 |
| Thyroid hormone-related factors | … | … | … | … |
| Adjust thyroid hormone (unspecified) | 654 (12.6) | 319 (13.7) | 335 (11.8) | .051 |
| LT4 alone worsened symptoms | 248 (4.8) | 91 (3.9) | 157 (5.5) | .007 |
| LT3 improved symptoms | 428 (8.3) | 167 (7.2) | 261 (9.2) | .009 |
| DTE improved symptoms | 206 (4.0) | 80 (3.4) | 126 (4.4) | .074 |
| LT4 improved symptoms | 178 (3.4) | 89 (3.8) | 89 (3.1) | .212 |
Abbreviations: DTE = desiccated thyroid extract; LT3 = liothyronine; LT4 = levothyroxine; n = number of participants.
Categorical variables are presented as numbers and proportions. Due to some participants offering more than 1 factor, the proportions were calculated from the total number of factors provided from all participants. Statistical differences between age groups were determined using the chi-square test. Factors included in the analysis were mentioned by at least 50 participants.
Textual Data Analysis of Open-Ended Comments
In total, 2031 participants provided additional free text responses to the open-ended prompt, from which 2113 textual units were derived. This portion of the survey allowed participants to include other symptoms and areas of concern regarding brain fog that were not addressed in the survey. Table 3 contains several notable excerpts from participant responses. Descending hierarchical classification analysis of the ECUs identified 2 major groups of responses: (1) symptom-centric (12.0%) and (2) medical-centric (15.3%). Two subgroups emerged within the symptom-centric group: (A) language/memory and (B) sleep/time. Three subgroups emerged within the medical-centric group: (C) disease/diagnosis, (D) patient/doctor, and (E) medication. Figure 3 shows the classification flowchart, including the word roots with the strongest association that defined each subgroup. Table 4 contains the proportions of all classifiable responses that make up each subgroup. The memory/language subgroup was the largest subgroup, containing 28.8% of all classifiable responses. Example textual units representative of each subgroup were identified (Supplementary Fig. 2).
Table 3.
Notable Excerpts from Open-Ended Responses
| Cognitive concerns | “[Brain fog] hinders my ability to find the right word” “I cannot articulate what I want to say” “Watching things on a screen or reading was such an uphill battle to accomplish” |
| Physical concerns | “Feels like I am walking through sludge” “A constant feeling of wired and tired” |
| Negative impact on career | “I have had to give up work as a social worker” “I had to quit my job because I could not think straight” |
| Interpersonal challenges | “It is not fun […] to have medical professionals dismiss you” “People ridicule me when I try to explain how I feel” |
Fig. 3.
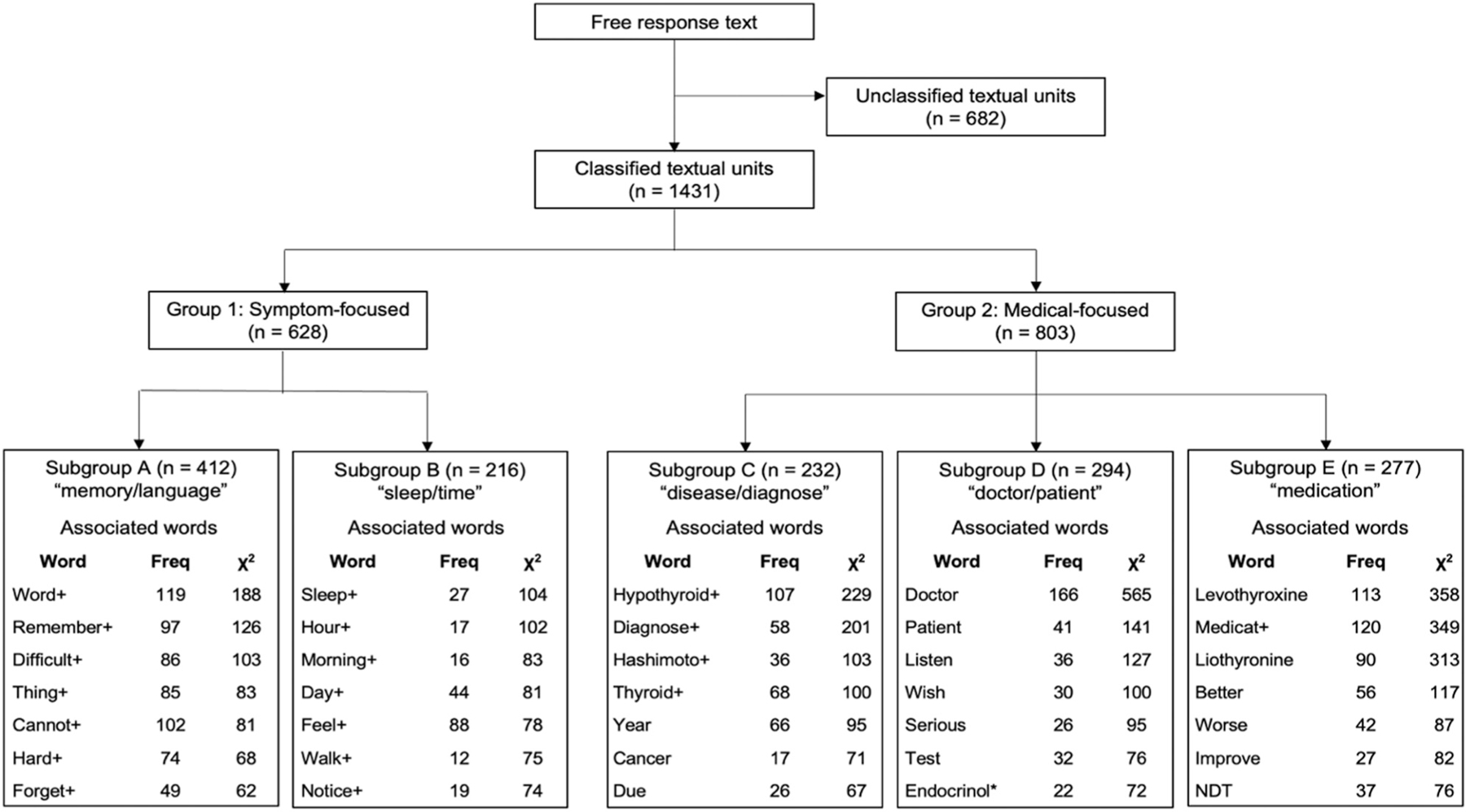
Flowchart of textual data analysis with associated words by subgroup. Textual data analysis identified 2 main groups and 5 subgroups among all classifiable textual units. Associated words are ordered from the highest to lowest strength of association. Strength of association was determined using the chi-square test. Plus symbols denote the reduced root word form. Freq = frequency; NDT = natural desiccated thyroid. *Endocrinol represents endocrinologist.
Table 4.
Textual Data Analysis in All Responses and by Age Group
| Classification | All responsesa n (%) |
Responses in those aged <50 years n (%) |
Responses in those aged ≥50 years n (%) |
P value |
|---|---|---|---|---|
| Group classification | … | … | … | <.001 |
| Symptom-centric | 628 (12.0) | 292 (12.3) | 336 (11.7) | … |
| Medical-centric | 803 (15.3) | 295 (12.4) | 508 (17.6) | … |
| Unclassified | 682 (13.0) | 280 (11.8) | 402 (14.0) | … |
| No response provided | 3137 (59.8) | 1504 (63.4) | 1633 (56.7) | … |
| Subgroup classification | … | … | … | .001 |
| Memory/language (A) | 412 (28.8) | 179 (30.5) | 233 (27.6) | … |
| Sleep/time (B) | 216 (15.1) | 113 (19.3) | 103 (12.2) | … |
| Disease/diagnose (C) | 232 (16.2) | 84 (14.3) | 148 (17.5) | … |
| Doctor/patient (D) | 294 (20.5) | 107 (18.2) | 187 (22.2) | … |
| Medication (E) | 277 (19.4) | 104 (17.7) | 173 (20.5) | … |
Abbreviations: n = number of participants; SD = standard deviation.
Categorical variables are presented as numbers and proportions. The chi-square test was used as appropriate to assess statistical differences between the groups.
A total of 74 responses were classified into 2 or more subgroups, for a total of 5250 responses.
Subgroup Analysis by Age
Each analysis was repeated in those aged <50 years and those aged ≥50 years. Table 1 shows the demographic and general symptom burden differences between the age groups. Of note, the ≥50 years old age group had a relatively higher number of participants who underwent thyroid surgery and RAI therapy. Regarding symptom domain scores, the ≥50 years old age group associated energy, cognitive, and mood symptoms significantly less frequently with brain fog compared with the <50 years old age group, but the actual differences were small. These findings are highlighted in Figure 2, with a smaller proportion of the older age group associating brain fog with each symptom domain “all the time” compared with the younger age group. Findings were similar in the multivariable analysis after adjusting for sex, etiology of hypothyroidism, and onset of brain fog symptoms (Supplementary Table 1).
There were several small but significant differences in frequencies of lifestyle factors that emerged between the age groups (Table 2). The ≥50 years old age group reported more frequently that LT3 improved symptoms (9.2% vs 7.2%, P =.009) and that LT4 alone worsened symptoms (5.5% vs 3.9%, P =.007) (Table 4). The results were similar in the multivariable analysis, with the addition of those aged ≥50 years being significantly more likely to mention desiccated thyroid extract as helping symptoms (OR, 1.42; 95% confidence interval [CI], 1.07–1.90; P =.018) (Table 5).
Table 5.
Multivariable Logistic Regression Analysis of the Association Between Age Group and Symptom Modifying Factors
| OR | 95% CI | P value | |
|---|---|---|---|
| Lifestyle factors | |||
| More rest | 0.81 | (0.72–0.90) | <.001 |
| Better nutrition in diet | 0.61 | (0.49–0.77) | <.001 |
| Stay hydrated | 0.49 | (0.30–0.80) | .005 |
| Thyroid hormone-related | … | ||
| LT4 alone worsened symptoms | 1.47 | (1.13–1.93) | .005 |
| LT3 improved symptoms | 1.40 | (1.14–1.72) | .001 |
| DTE improved symptoms | 1.42 | (1.07–1.90) | .018 |
Abbreviations: CI = confidence interval; DTE = desiccated thyroid extract; LT3 = liothyronine; LT4 = levothyroxine; OR = odds ratio.
Odds ratios were adjusted for sex, etiology of hypothyroidism, and onset of symptoms. Only factors that met a statistical significance of P <.05 were included.
Regarding the textual data age group analysis, 50.2% of textual units from the <50 years old age group were medical-centric compared with 60.3% of textual units of the ≥50 years old age group (P <.001) (Table 4). In the multivariable logistic regression analysis of classifiable responses, the results were similar with the ≥50 years old age group more likely to provide a medical-centric response (OR, 1.45; 95% CI, 1.17–1.81; P =.001).
Discussion
By surveying individuals who reported having hypothyroidism and brain fog, we found that brain fog is a multisymptom condition characterized by frequent fatigue, sleepiness, and forgetfulness and that the overall combined symptom burden negatively impacts life. More rest was the most frequently reported factor to mitigate brain fog. The textual data analysis revealed 2 prominent areas of concern (symptom-centric and medical-centric) that were not addressed among the symptom domains included in the survey. Patients used words related to memory, language, medication, and the patient-doctor relationship to indicate important aspects of brain fog. Small differences between the age groups were identified in the subgroup analysis, most notably that participants aged ≥50 years were more likely to report improvement in brain fog symptoms with T3-containing therapies and were more likely to have medical-centric concerns about brain fog outside of symptoms addressed in the survey.
It is notable that fatigue was found to be the main symptom associated with brain fog. One hypothesis is that the fatigue associated with brain fog is driven by the sustained level of attention necessary to complete tasks throughout the day. Patients with brain fog may struggle with multiple competing tasks or thoughts, and the additional mental attention required to prioritize and complete task results in fatigue.
It is also noteworthy that for nearly half of all participants, brain fog was present prior to the diagnosis of hypothyroidism. One explanation is that brain fog for these participants was not primarily caused by hypothyroidism. The term brain fog has been associated with a variety of illnesses, medical therapies (eg, chemotherapy), aging, and even environmental factors.24 The primary descriptors of brain fog in the present analysis appear to overlap to a certain degree with those of other conditions. For example, patients with postural orthostatic tachycardia syndrome associate brain fog with forgetfulness, difficulty focusing, and mental fatigue.25 Those with chronic fatigue syndrome described it as “the conscious perception of cognitive impairment.”5 A recent survey of COVID-19 “long haulers” found that the 2 most prevalent symptoms were fatigue (85%) and brain fog (81%).26
In addition, given the association of hypothyroidism with increasing age and female sex, there is a probable overlap of symptoms with hypothyroidism, menopause, and normal aging. There is a well-recognized pattern of normal cognitive decline associated with aging that tends to affect memory and language processing in particular, which may overlap with brain fog.27 As a result, it may be difficult not only for the clinician but also for the patient to tease apart cognitive symptoms related to normal aging versus hypothyroidism. This may explain in the subgroup analysis why the >50 years old age group tended to associate brain fog to a lesser degree with hypothyroid symptoms.
Alternatively, the presence of brain fog before and after the diagnosis and treatment of hypothyroidism may signify inadequacy of thyroid hormone replacement to restore euthyroidism. As mentioned earlier, this can be due to undertreatment, coinciding with an elevated TSH level. In a patient treated for hypothyroidism presenting with hypothyroid symptoms, such as brain fog, the standard of care would be to assess thyroid function (TSH, free T4) and adjust treatment according to the thyroid function abnormality. However, the presence of symptoms with normal thyroid function tests (and no other obvious cause for symptoms) can be a challenging situation for both the patient and physician. In fact, we believe that it is likely that the study population was enriched with patients with persistent brain fog despite regular medical evaluations (and regular assessments of thyroid function) given the survey targeted people in hypothyroid support groups. Although more controversial, some advocate for the use of a therapeutic trial of LT4 and LT3 for persistent hypothyroid symptoms. A recent consensus statement from the American, British, and European Thyroid Associations on the treatment of patients with persistent symptoms of hypothyroidism has addressed the role of a therapeutic trial with LT3 and LT4 in clinical practice, calling for additional clinical trials to help evaluate the effectiveness of combination therapy.28
It is clear that many patients with hypothyroidism have made behavioral changes to manage brain fog, although the role of these changes in the standard care of hypothyroidism is not clear. Residual symptoms of brain fog despite normal TSH levels may push patients to seek alternate treatment methods. Patients may seek lifestyle advice on social media and other internet forums.29,30 A recent survey by the British Thyroid Foundation found that over one third of hypothyroid patients found the internet and other media sources to be a better source of information than their primary doctors.15 It is difficult to speculate the underlying mechanisms through which certain behavioral changes, such as more exercise or changes in diet, would directly or indirectly modify symptoms of hypothyroidism, including brain fog. In this regard, we note that in mouse studies, treadmill exercise eliminated the phenotype of anxiety-depressive-like behavior observed in mice with brain-specific hypothyroidism.31 In mice, treadmill exercise accelerates type 2 deiodinase activity in the skeletal muscle, which activates T4, leading to increased levels of active thyroid hormone.32
The textual analysis opens a new window into the concerns exhibited by some patients with hypothyroidism and brain fog. These concerns have been missed because they are not addressed in commonly used questionnaires that focus mostly on residual thyroid-related symptoms. For example, the common thyroid-related questionnaire ThyPRO probes symptoms of tiredness, vitality, cognition, nervousness and tension, psychologic well-being, difficulty coping or having mood swings, relationships with other people, daily activities, sex life, appearance, and overall impact on quality of life.20 These areas are important and would address those symptom-centric patients. However, the available questionnaires do not address all concerns exhibited by the medical-centric patients. Of note, the patient-doctor relationship does not appear as a relevant issue in the thyroid-related quality of life issue identification study that served in the development of ThyPRO.33 This issue and others have been identified in the textual analysis as important areas of concern for patients with hypothyroidism and brain fog.
This study has several important limitations. First, the study population was a nonrandom, self-selected sample of survey participants who reported having hypothyroidism with brain fog symptoms and had regular access to hypothyroidism-related websites where the survey was posted, which is a form of selection bias.34 As such, the results of this study should only be generalized to patients with hypothyroidism that report having brain fog despite treatment and not the population with hypothyroidism at large. Due to the study design, neither prevalence of brain fog nor the degree to which hypothyroidism increases (or decreases) the likelihood of having brain fog could be determined. The textual data analysis is subject to further selection bias as participants were free to provide additional comments or not. Because the survey link could be shared via email and social media platforms to engage as many patients as possible, we cannot estimate a survey response rate. In addition, the survey was designed to be minimally burdensome to encourage participation and to not restrict the study to a specific location. As a result, survey data did not include medical record information to confirm the diagnosis of hypothyroidism or identify comorbid conditions. Further research is needed to determine the roles of both thyroid function and comorbidities in the character and severity of brain fog in hypothyroidism.
Conclusion
The present study characterizes brain fog from the perspective of patients with hypothyroidism as an array of symptoms that represent a significant cognitive and functional burden on daily life. Fatigue, cognitive, and, to a lesser extent, mood symptoms were frequently associated with brain fog in the survey participants. To manage brain fog, patients may seek both behavioral and thyroid hormone changes to alleviate symptoms. Several participants used language that centered on symptoms, diagnosis, and treatment of hypothyroidism or on the patient-doctor relationship. Exploring these new areas of patient concern may provide a new pathway through which to approach the experience of patients with hypothyroidism, in addition to the traditional thyroid-specific symptom approach.
Supplementary Material
Acknowledgment
We thank the American Thyroid Association for its participation in survey development and distribution. We are grateful to the patients who participated in this study, many of whom provided open, honest responses on their experience with brain fog. The study was supported by the National Institute of Diabetes and Digestive and Kidney Disease of the National Institutes of Health under award 5T32DK007011–46 (M.D.E.).
Abbreviations:
- ECUs
elementary context units
- LT3
liothyronine
- LT4
levothyroxine
- OR
odds ratio
- TSH
thyroid-stimulating hormone
- T3
triiodothyronine
- T4
thyroxine
Footnotes
Disclosure
M.D.E., A.R., A.B., S.P.B., E.M., M.C.T.V.T., J.J., N.L., and M.O.R. have no multiplicity of interest to disclose. A.C.B. reports consulting fees from Synthonics, Allergan, and BLA Technology. These are not relevant to the content of this manuscript.
References
- 1.Gava G, Orsili I, Alvisi S, Mancini I, Seracchioli R, Meriggiola MC. Cognition, mood and sleep in menopausal transition: the role of menopause hormone therapy. Medicina (Kaunas) 2019;55(10):668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kovalchuk A, Kolb B. Chemo brain: from discerning mechanisms to lifting the brain fog—an aging connection. Cell Cycle 2017;16(14):1345–1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yelland GW. Gluten-induced cognitive impairment (“brain fog”) in coeliac disease. J Gastroenterol Hepatol 2017;32(Suppl 1):90–93. [DOI] [PubMed] [Google Scholar]
- 4.Mackay M. Lupus brain fog: a biologic perspective on cognitive impairment, depression, and fatigue in systemic lupus erythematosus. Immunol Res 2015;63(1–3):26–37. [DOI] [PubMed] [Google Scholar]
- 5.Ocon AJ. Caught in the thickness of brain fog: exploring the cognitive symptoms of chronic fatigue syndrome. Front Physiol 2013;4:63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yelin D, Wirtheim E, Vetter P, et al. Long-term consequences of COVID-19: research needs. Lancet Infect Dis 2020;20(10):1115–1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Raj V, Opie M, Arnold AC. Cognitive and psychological issues in postural tachycardia syndrome. Auton Neurosci 2018;215:46–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Canaris GJ, Manowitz NR, Mayor G, Ridgway EC. The Colorado thyroid disease prevalence study. Arch Intern Med 2000;160(4):526–534. [DOI] [PubMed] [Google Scholar]
- 9.Okosieme OE, Belludi G, Spittle K, Kadiyala R, Richards J. Adequacy of thyroid hormone replacement in a general population. QJM 2011;104(5):395–401. [DOI] [PubMed] [Google Scholar]
- 10.Utiger RD. Radioimmunoassay of human plasma thyrotropin. J Clin Invest 1965;44(8):1277–1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Braverman LE, Ingbar SH, Sterling K. Conversion of thyroxine (T4) to triiodothyronine (T3) in athyreotic human subjects. J Clin Invest 1970;49(5):855–864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ettleson MD, Bianco AC. Individualized therapy for hypothyroidism: is T4 enough for everyone? J Clin Endocrinol Metab 2020;105(9):e3090e–3104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Taylor S, Kapur M, Adie R. Combined thyroxine and triiodothyronine for thyroid replacement therapy. Br Med J 1970;2(5704):270–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Peterson SJ, Cappola AR, Castro MR, et al. An online survey of hypothyroid patients demonstrates prominent dissatisfaction. Thyroid 2018;28(6): 707–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mitchell AL, Hegedüs L, Žarkovíc M, Hickey JL, Perros P. Patient satisfaction and quality of life in hypothyroidism: an online survey by the british thyroid foundation. Clin Endocrinol (Oxf) 2021;94(3):513–520. [DOI] [PubMed] [Google Scholar]
- 16.Saravanan P, Chau WF, Roberts N, Vedhara K, Greenwood R, Dayan CM. Psychological well-being in patients on ‘adequate’ doses of l-thyroxine: results of a large, controlled community-based questionnaire study. Clin Endocrinol (Oxf) 2002;57(5):577–585. [DOI] [PubMed] [Google Scholar]
- 17.Wekking EM, Appelhof BC, Fliers E, et al. Cognitive functioning and well-being in euthyroid patients on thyroxine replacement therapy for primary hypothyroidism. Eur J Endocrinol 2005;153(6):747–753. [DOI] [PubMed] [Google Scholar]
- 18.Djurovic M, Pereira AM, Smit JWA, et al. Cognitive functioning and quality of life in patients with Hashimoto thyroiditis on long-term levothyroxine replacement. Endocrine 2018;62(1):136–143. [DOI] [PubMed] [Google Scholar]
- 19.Samuels MH, Schuff KG, Carlson NE, Carello P, Janowsky JS. Health status, psychological symptoms, mood, and cognition in L-thyroxine-treated hypothyroid subjects. Thyroid 2007;17(3):249–258. [DOI] [PubMed] [Google Scholar]
- 20.Watt T, Bjorner JB, Groenvold M, et al. Establishing construct validity for the thyroid-specific patient reported outcome measure (ThyPRO): an initial examination. Qual Life Res 2009;18(4):483–496. [DOI] [PubMed] [Google Scholar]
- 21.Noël-Jorand MC, Reinert M, Giudicelli S, Dassa D. A new approach to discourse analysis in psychiatry, applied to a schizophrenic patient’s speech. Schizophr Res 1997;25(3):183–198. [DOI] [PubMed] [Google Scholar]
- 22.De Looze MA, Roy A, Coronini R, Reinert M, Jouve O. Two measures for identifying the perception of risk associated with the introduction of transgenic plants. Scientometrics 1999;44(3):401–426. [Google Scholar]
- 23.McKinlay SM, Brambilla DJ, Posner JG. The normal menopause transition. Maturitas 2008;61(1–2):4–16. [DOI] [PubMed] [Google Scholar]
- 24.Schmidt S. Brain fog: does air pollution make us less productive? Environ Health Perspect 2019;127(5):52001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ross AJ, Medow MS, Rowe PC, Stewart JM. What is brain fog? An evaluation of the symptom in postural tachycardia syndrome. Clin Auton Res 2013;23(6): 305–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Graham EL, Clark JR, Orban ZS, et al. Persistent neurologic symptoms and cognitive dysfunction in non-hospitalized Covid-19 “long haulers”. Ann Clin Transl Neurol 2021;8(5):1073–1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Harada CN, Natelson Love MC, Triebel KL. Normal cognitive aging. Clin Geriatr Med 2013;29(4):737–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jonklaas J, Bianco AC, Cappola AR, et al. Evidence-based use of levothyroxine/liothyronine combinations in treating hypothyroidism: a consensus document. Thyroid 2021;31(2):156–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ramachandran D, Kite J, Vassallo AJ, et al. Food trends and popular nutrition advice online - implications for public health. Online J Public Health Inform 2018;10(2):e213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rounsefell K, Gibson S, McLean S, et al. Social media, body image and food choices in healthy young adults: a mixed methods systematic review. Nutr Diet 2020;77(1):19–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bocco BM, Werneck-de-Castro JP, Oliveira KC, et al. Type 2 deiodinase disruption in astrocytes results in anxiety-depressive-like behavior in male mice. Endocrinology 2016;157(9):3682–3695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bocco BM, Louzada RA, Silvestre DH, et al. Thyroid hormone activation by type 2 deiodinase mediates exercise-induced peroxisome proliferator-activated receptor-γ coactivator-1α expression in skeletal muscle. J Physiol 2016;594(18):5255–5269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Watt T, Hegedüs L, Rasmussen AK, et al. Which domains of thyroid-related quality of life are most relevant? Patients and clinicians provide complementary perspectives. Thyroid 2007;17(7):647–654. [DOI] [PubMed] [Google Scholar]
- 34.Tripepi G, Jager KJ, Dekker FW, Zoccali C. Selection bias and information bias in clinical research. Nephron Clin Pract 2010;115(2):c94–c99. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


