Abstract
Two models of respiratory tract infection were used to investigate the pharmacodynamics of amoxicillin-clavulanate against Streptococcus pneumoniae. Eight strains of S. pneumoniae were used in a mouse model in which the animals were infected intranasally and were then treated with a range of doses and dose intervals. The time that the plasma amoxicillin concentration remained above the MIC (T>MIC) correlated well with bacterial killing, such that if T>MIC was below 20% there was no effect on bacterial numbers in the lungs. As T>MIC increased, the response, in terms of decreased bacterial load, improved and at T>MICs of greater than 35 to 40% of the dosing interval, bacteriological cure was maximal. On the basis of equivalent T>MICs, these data would suggest that in humans a dosage of 500 mg three times daily (t.i.d.) should have efficacy equal to that of a dosage of 875 mg twice daily (b.i.d.). This hypothesis was evaluated in a rat model in which amoxicillin-clavulanate was given by computer-controlled intravenous infusion to achieve concentrations that approximate the concentrations achieved in the plasma of humans following oral administration of 500/125 mg t.i.d. or 875/125 mg b.i.d. Infusions continued for 3 days and bacterial numbers in the lungs 2 h after the cessation of the infusion were significantly reduced (P < 0.01) by both treatments in strains of S. pneumoniae for which amoxicillin MICs were below 2 μg/ml. When tested against a strain of S. pneumoniae for which the amoxicillin MIC was 4 μg/ml, the simulated 500/125-mg dose was ineffective but the 875/125-mg dose demonstrated a small but significant (P < 0.01) reduction in bacterial numbers. These data confirm the findings in the mouse and indicate that amoxicillin-clavulanate administered at 875/125 mg b.i.d. would be as effective clinically as amoxicillin-clavulanate administered at 500/125 mg t.i.d.
Streptococcus pneumoniae is among the most common pathogens causing upper and lower respiratory tract infections (4, 8, 12). Although these bacteria have traditionally been susceptible to penicillin, the percentage of isolates that have either intermediate-level or high-level resistance has been increasing (2, 3, 10). However, penicillins, such as amoxicillin, still remain the most active oral β-lactam antibiotics against S. pneumoniae. The combination of amoxicillin-clavulanate should therefore provide adequate antimicrobial cover for respiratory infections caused not only by S. pneumoniae but also by β-lactamase-producing organisms such as Haemophilus influenzae and Moraxella catarrhalis. Craig and Andes (6) have recently reported that amoxicillin-clavulanate dosed three times daily (t.i.d.) and ceftriaxone dosed once daily appear to be the best agents against the full spectrum of bacterial pathogens causing otitis media, and amoxicillin-clavulanate has been recommended as the treatment of choice for community-acquired lower respiratory tract and inner-ear infections (11). However, it has been demonstrated that patient compliance with once-daily and twice-daily (b.i.d.) dosing regimens is significantly better than that with t.i.d. regimens, and this has led to the development of a b.i.d. dosage form of amoxicillin-clavulanate. In order to ensure that any proposed change in a dosage of antibiotic will be clinically effective, it is important to consider the interrelationship between intrinsic activity and pharmacokinetics, i.e., the pharmacodynamics of the drug, which should provide insight into potential clinical efficacy. Bacterial killing in vitro and in vivo by β-lactam antibiotics is not enhanced by increasing the drug concentrations above the MIC (7), and regrowth of bacteria occurs soon after the concentrations in serum or tissue fall below the MIC (for those compounds that have little or no postantibiotic effect), suggesting that the time that the concentrations exceed the MIC (T>MIC) should be the primary determinant of efficacy. Bacterial killing by β-lactam antimicrobial agents and efficacy in experimental models have been shown to be related to the duration of time that the concentration of the agent in plasma remains above the MIC (9). Thus, pharmacodynamic factors are clearly important in predicting drug efficacy and can suggest dosing methods to improve the outcome from treatment.
We have used two pharmacodynamic models to examine the effects that a decreased frequency of dosing of amoxicillin-clavulanate may have on efficacy. The first model, a murine pneumonia model, was used to confirm that T>MIC is an important parameter for the efficacy of amoxicillin against S. pneumoniae and to estimate the critical limits associated with efficacy. The second model, a rat respiratory infection, was used to examine the efficacy of proposed t.i.d. and b.i.d. regimens of amoxicillin-clavulanate by simulating in rat plasma the concentrations measured in human serum following the administration of a clinical dose to allow a more direct extrapolation of the experimental data to potential clinical efficacy.
MATERIALS AND METHODS
Organisms.
Eight clinical strains of S. pneumoniae were used. S. pneumoniae 1629 was penicillin susceptible; S. pneumoniae 1320, 27783, and APS1 were penicillin intermediate; and the remaining four S. pneumoniae strains, N1387, 14319, 410101, and RS1, were penicillin resistant. Strains 1629, 27783, 410101, and RS1 were not used in the rat pharmacodynamic studies.
Antimicrobial susceptibility.
The susceptibilities of the S. pneumoniae strains used in this study were measured by a broth microdilution assay in accordance with the procedures recommended by the National Committee for Clinical Laboratory Standards (15). The MICs for these strains are presented in Table 1.
TABLE 1.
Susceptibilities of isolates used in the experimental infection models
| Strain | MIC (μg/ml)
|
||
|---|---|---|---|
| Amoxicillin | Amoxicillin-clavulanate | Penicillin G | |
| 1629 | 0.01 | 0.01 | 0.01 |
| 1320 | 0.5 | 0.5 | 1 |
| 27783 | 1 | 1 | 1 |
| APS1 | 1 | 1 | 1 |
| N1387 | 2 | 2 | 2 |
| 14319 | 4 | 4 | 8 |
| 410101 | 4 | 4 | 4 |
| RS1 | 8 | 8 | 16 |
Preparation of inocula.
Organisms were grown overnight on nutrient agar plates containing 7% defibrinated horse blood, and inocula were prepared by harvesting the growth from three plates and suspending it in 2 ml of Todd-Hewitt broth immediately before infection.
Murine pharmacodynamic model. (i) Animals.
Female CD-1 mice (weight, 18 to 20 g) were supplied by Charles River, UK, Ltd.
(ii) Experimental pneumonia.
Mice were rendered neutropenic by administration of cyclophosphamide (150 mg/kg of body weight administered intraperitoneally 4 days before infection, followed by 100 mg/kg administered intraperitoneally 1 day before infection). On the day of infection the mice were lightly anesthetized (2% isoflurane in a 1:1 mixture of oxygen and nitrous oxide) and infected by intranasal instillation of 50 μl of the pneumococcal strain being tested (106 to 107 CFU/mouse). The animals were allowed to recover and were then divided into groups of four animals according to the dosing schedule to be followed.
(iii) Administration of compounds.
Amoxicillin trihydrate was obtained from SmithKline Beecham Pharmaceuticals, Worthing, United Kingdom. Amoxicillin was prepared as a fine acacia suspension in sterile distilled water, and animals received total dosages ranging between 2.5 and 1,280 mg/kg/day given orally as divided doses every 4, 6, 8, or 12 h for 2 days.
(iv) Assessment of therapy.
To estimate the bacterial load at the start of dosing, one control group of animals was killed and the lungs were aseptically removed and then homogenized (Colworth Stomacher) in 2 ml of Todd-Hewitt broth. Homogenates were diluted serially in broth and were then plated in triplicate (0.02 ml/drop) onto 5% blood agar by a modified Miles-Misra technique. The plates were incubated overnight at 37°C to determine the numbers of viable bacteria in the lungs, and the lower limit of detection was 1.7 log10 CFU per lung. All remaining groups (48 in total) were killed 48 h after the start of therapy, and the bacterial numbers in the lungs were estimated as described above. Data were expressed as the change in bacterial numbers compared with the numbers in the starting control group.
Rat pharmacodynamic model. (i) Animals.
Specific-pathogen-free Sprague-Dawley rats (Charles River, UK, Ltd.) weighing approximately 200 g were used throughout. Animals were housed two to a cage; however, individual animals were separated by a partition.
(ii) Cannula implantation.
Animals were anesthetized (with N2O-O2 [1:1] and 3% isoflurane) and prepared for surgery. The carotid artery and jugular vein were cannulated for blood sampling and antibiotic administration, respectively, with polythene tubing (internal diameter, 0.4 mm; outside diameter, 0.8 mm; Portex, Kent, United Kingdom). Both cannulae were exteriorized dorsally and were extended to the top of the cage through a flexible metal sheath. A polytetrafluoroethylene flange attached to one end of the metal sheath was implanted subcutaneously on the back of the rat, and the distal end was affixed to a brass ferrule and swivel joint. The device allowed full movement of the animal but prevented the sheath from being pulled into the cage. Local anesthetic (Xylocaine; Astra, Kings Langley, United Kingdom) was applied after wound closure. The animals were allowed to recover for at least 2 days prior to the establishment of infection. The cannulae were kept patent by regular flushing with 0.9% sodium chloride with heparin at 50 U/ml. A filter (Minisart; Sartorius, Epsom, United Kingdom) was affixed to the swivel joint to ensure the sterility of the infusion fluid.
(iii) Respiratory tract infection model.
Immediately prior to infection the inocula were diluted 1:10 in molten nutrient agar that had been maintained at 40°C to give a final bacterial inoculum of approximately 6 log10 CFU in 50 μl of molten agar.
The rats were anesthetized by separate intramuscular injections of fentanyl fluanisone (Hypnorm; Janssen Pharmaceuticals, Ltd., Grove, Oxfordshire, United Kingdom) and diazepam (Valium; Roche Products, Ltd., Welwyn Garden City, United Kingdom). The anesthetized rats were then infected by intrabronchial instillation of the 50-μl inoculum, in cooled molten agar, containing S. pneumoniae by means of nonsurgical intubation (16).
(iv) Antimicrobial administration.
Amoxicillin sodium and potassium clavulanate were obtained from SmithKline Beecham Pharmaceuticals. The drugs were prepared as sterile solutions in 0.9% sodium chloride at concentrations of 2.5 and 4.4 mg/ml for amoxicillin (for the 500- and 875-mg simulations, respectively) and 0.8 mg/ml for clavulanate.
All antimicrobial agents were administered by continuous intravenous infusions that were rate adjusted to simulate the concentrations of amoxicillin-clavulanate achieved in the plasma of adults when administered orally at either 500/125 mg t.i.d. or 875/125 mg b.i.d. Dosing commenced 24 h postinfection.
There were three experimental groups, as follows: (i) animals receiving amoxicillin-clavulanate at a simulated dosage of 500/125 mg every 8 h; (ii) animals receiving amoxicillin-clavulanate at a simulated dosage of 875/125 mg every 12 h; and (iii) untreated controls, which were infused with 0.9% sodium chloride at a rate similar to those at which the other groups were infused. Seven or eight rats were included in each group. Infusions continued for 3 days (a total of six doses were simulated for the b.i.d. group and nine doses were simulated for the t.i.d. group).
(v) Simulation of concentrations in serum.
The desired concentrations in plasma were determined by application of a linear, one-compartment absorption model, C(t) = A (e−kelt − e−kat), where C is the concentration in plasma at time t, A is the zero-time intercept assuming instantaneous absorption, e is the base of the natural log, kel is the elimination rate constant, and ka is the absorption rate constant. The values for the required pharmacokinetic parameters in humans were obtained from the literature (12) and are given in Table 2. The data on the pharmacokinetics of amoxicillin and potassium clavulanate in rats (Table 2) that were used to determine dosing parameters were determined from concentrations measured in uninfected animals after the intravenous administration of a bolus dose of 100 mg of amoxicillin per kg of body weight and 20 mg of potassium clavulanate per kg.
TABLE 2.
Pharmacokinetic parameters in adult humansa and rats used to determine infusion rates for simulation studies with rats
| Species, pharmaco-kinetic model | Parameter | Amoxicillin | Clavulanate |
|---|---|---|---|
| Human, C(t) = A(exp−kelt − exp−kat) | A (μg/ml) | 28 | 4.5 |
| kel (h−1) | 0.65 | 0.61 | |
| ka (h−1) | 1.65 | 1.56 | |
| Rat, C = a · exp−λt b | a (μg/ml) | 240 | 27 |
| λ (h−1) | 2.1 | 2.4 | |
| Dose (mg/kg) | 44 | 24 |
Data for humans have been published previously (12).
C is concentration, a is the zero-time intercept, and λ is the elimination rate constant.
Flow rates were controlled with infusion pumps (Pump 22; Harvard Instruments, Edenbridge, United Kingdom) linked serially to a microcomputer. The infusion flow rates were changed individually every 60 s, and the system was programmed to administer the calculated rates in 8-h (t.i.d. simulations) or 12-h (b.i.d. simulations) cycles over 3 days.
(vi) Assessment of therapy.
At approximately 2 h after the cessation of infusion, the animals were killed by inhalation of rising concentrations of CO2 and the lungs were removed aseptically and homogenized in 1 ml of isotonic saline for the enumeration of viable bacteria. Tenfold serial dilutions were prepared in phosphate-buffered saline (0.1 M; pH 7.4), and the appropriate dilutions were plated in triplicate (0.02 ml per drop) onto blood agar plates by a modified Miles-Misra technique. The colonies were counted after overnight incubation at 37°C. The lower limit of detection was 1.7 log10 CFU per pair of lungs.
Pharmacokinetic studies. (i) Mice.
Amoxicillin was administered orally to uninfected mice at 20 mg/kg. Groups of five animals were killed at intervals to up to 4.0 h after dosing, and blood was obtained from the cut axillary vein.
(ii) Rats.
Blood samples were taken from the carotid artery cannula at intervals to up to 6 h after the start of infusion.
Blood samples were centrifuged to isolate the serum, and the samples were assayed within 2 h of collection. The concentrations of amoxicillin and clavulanate were determined by a large-plate agar diffusion assay with Micrococcus luteus NCTC 8340 for amoxicillin and an enzyme inhibition assay with Klebsiella pneumoniae NCTC 11228 for clavulanate (12). The samples were assayed in duplicate against standards over the concentration range of 0.015 to 1 μg/ml for amoxicillin and the concentration range of 0.078 to 5 μg/ml for clavulanate. The lowest concentration was taken as the limit of detection for the assay. The correlation coefficients for the regression lines of the standard solutions were not less than 0.997. The within-day coefficients of variation were less than 5% for amoxicillin and 6.1 to 9.4% for clavulanate, and the between-day coefficients of variation were 3.7 to 6.2% for amoxicillin and 6.3 to 9.8% for clavulanate.
Data analysis.
The correlation between T>MIC and efficacy in the murine pneumonia model was evaluated with a sigmoid dose-effect model (Emax model). Data on the concentration in serum were fitted with an iterative least-squares modeling program, MK-MODEL. The area under the concentration-time curve (AUC) from time zero to infinity was calculated by noncompartmental analysis by using the trapezoidal rule for data up to the last concentration-time point, with the remaining area to infinity calculated by dividing this point by kel.
In the efficacy studies the outcome measure for comparison of treatments was the number of bacteria in the lungs at the conclusion of the study. All results are presented as group means with standard deviations. Differences among groups were tested by analysis of variance with Scheffe’s test for multiple contrasts (StatisticA for Windows; Statsoft Inc., Tulsa, Okla.). A P value of ≤0.05 was considered significant.
RESULTS
Murine pharmacokinetics.
Peak concentrations of amoxicillin (8.2 ± 2.2 μg/ml) were achieved within 15 min of administration at 20 mg/kg. The elimination half-life was 0.22 h, with an AUC of 4.88 μg · h/ml.
Data generated for doses of amoxicillin of up to 400 mg/kg demonstrated linearity with the dose and indicated that there would be no accumulation in serum even with dosing intervals as short as every 4 h.
Mouse pneumonitis.
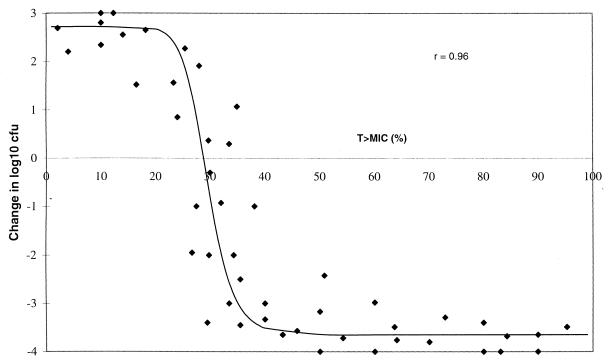
Efficacy data from the mouse pneumonia model are presented in Fig. 1 and are presented as the change in bacterial numbers in the lungs in relation to the numbers in the lungs of the untreated controls as a function of T>MIC (in 24 h) for each dosage regimen. For all of the strains of S. pneumoniae tested, the numbers in the lungs of untreated animals increased by approximately 2 to 3 log10 CFU during the study period. It is evident that when the T>MIC was approximately 20% or less of the 24-h dosing period, there was no significant decrease in bacterial numbers in the lungs of infected animals (P > 0.05). However, as T>MIC increased above this value there was a progressive reduction in the number of bacteria present in the lungs compared with the numbers present in the lungs of the untreated animals. The efficacy of amoxicillin was maximal (decrease in bacterial load of >3 log10 CFU/pair of lungs) when T>MIC was 35 to 40%, and a further increase in the time that the amoxicillin concentration exceeded the MIC had no increased effect. In general, efficacy correlated well with T>MIC, irrespective of the dosing interval. The fitted sigmoid dose-effect model resulted in an Emax of 6.37 ± 0.36 log10 CFU/pair of lungs. The T > MIC necessary to achieve stasis was calculated as 28.8%, and that required to achieve 95% of the maximal response was 37.3%.
FIG. 1.
Correlation between efficacy in a murine pneumonia model and T>MIC for amoxicillin against strains of S. pneumoniae for which in vitro MICs are different. Each point represents the mean for four animals.
Rat pneumonia. (i) Concentrations achieved in plasma.
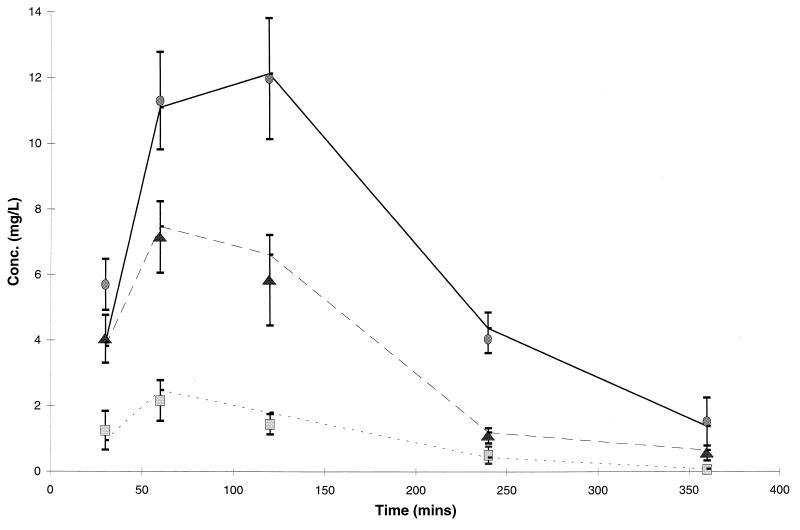
The plasma amoxicillin and clavulanate concentrations achieved in rats are presented in Fig. 2 and are compared with the target concentrations determined from pharmacokinetic parameters in adults.
FIG. 2.
Concentrations of amoxicillin (▴; 500-mg equivalent; •, 875-mg equivalent) and clavulanate (■, 125-mg equivalent) in the plasma of rats in comparison with target levels in humans (—). Data for rats are presented as the mean and standard deviation for eight animals.
(ii) Efficacy data.
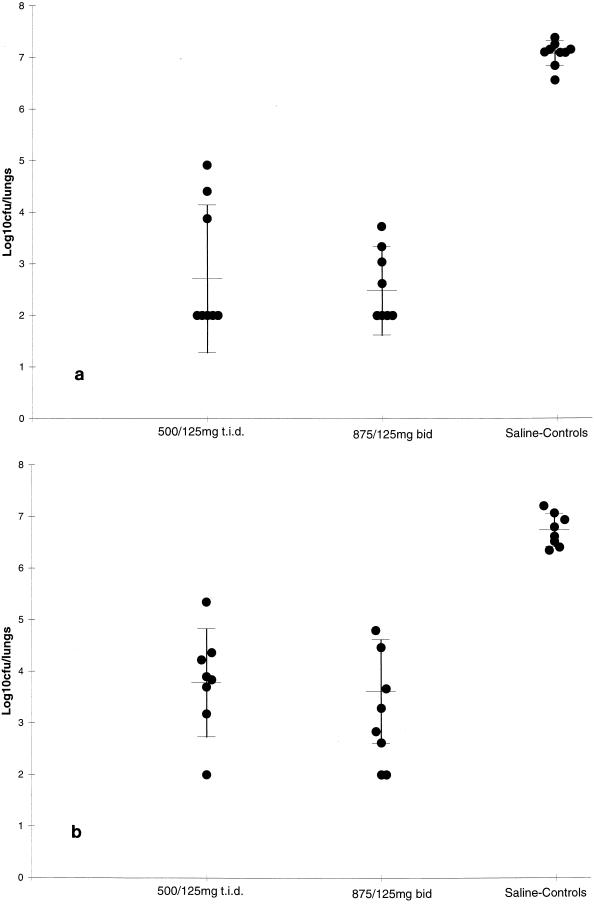
The efficacy of amoxicillin-clavulanate against S. pneumoniae 1320 and N1387 is indicated in Fig. 3. Bacterial numbers from the lungs of animals treated with saline were 7.07 ± 0.24 and 6.74 ± 0.32 log10 CFU/pair of lungs for strains 1320 and N1387, respectively. Against S. pneumoniae 1320 amoxicillin-clavulanate at a simulated dosage of 500/125 mg t.i.d. reduced the bacterial numbers in the lungs to 2.71 ± 1.43 log10 CFU/pair of lungs, which was significantly lower than that for control animals (P < 0.01). Amoxicillin-clavulanate at the simulated b.i.d. dosage produced a similar response. Against the more resistant strain of S. pneumoniae, both simulated dosages of amoxicillin-clavulanate were significantly more effective (P < 0.01) than saline, and the efficacy of the simulated b.i.d. dose (3.61 ± 1.01 log10 CFU/pair of lungs) was similar to the efficacy of the t.i.d. dose (3.78 ± 1.05 log10 CFU/pair of lungs; P > 0.05). Data for the remaining two strains tested are presented in Table 3. For the second penicillin-intermediate strain (S. pneumoniae APS1) used in these studies, the results were similar to those obtained for S. pneumoniae 1320. When amoxicillin-clavulanate was dosed to simulate the 500/125-mg t.i.d. dosage used in humans, it was ineffective (P > 0.05) against respiratory infections caused by the penicillin-resistant strain, S. pneumoniae 14319. However, the simulated 875/125-mg dose reduced the bacterial numbers in the lungs significantly compared to those in the lungs of saline-treated control animals (P < 0.01), although the reduction in bacterial numbers was less than that measured for the more susceptible strains.
FIG. 3.
Efficacies of doses of amoxicillin-clavulanate resulting in levels in rat serum simulating those in humans against experimental respiratory infections in the rat caused by S. pneumoniae 1320 (a) and S. pneumoniae N1387 (b). Data are represented as mean and standard deviation bacterial numbers in the lungs of individual animals.
TABLE 3.
Efficacy of amoxicillin-clavulanate at doses resulting in levels in rat serum simulating levels in human serum obtained following oral administration of either 500/125 mg t.i.d. or 875/125 mg b.i.d. against a respiratory tract infection caused by strains of S. pneumoniae with different susceptibilities
| Strain | Bacteriological counts (mean ± SD log10CFU/pair of lungs)
|
||
|---|---|---|---|
| Amoxicillin-clavulanate (500/125 mg t.i.d.) | Amoxicillin-clavulanate (875/125 mg b.i.d.) | Untreated controls | |
| 1320 | 2.71 ± 1.43a | 2.48 ± 0.86a | 7.07 ± 0.24 |
| APS1 | 3.42 ± 0.93a | 2.88 ± 0.99a | 6.73 ± 0.67 |
| N1387 | 3.78 ± 1.05a | 3.61 ± 1.01a | 6.74 ± 0.32 |
| 14319 | 6.01 ± 0.52 | 4.95 ± 0.58b | 6.61 ± 0.49 |
Significantly different from untreated controls (P < 0.01).
Significantly different from results for untreated controls and animals treated with amoxicillin-clavulanate at a dose resulting in levels of 500/125 mg in humans (P < 0.01).
DISCUSSION
The results obtained from the murine pneumonia model demonstrate clearly that, for S. pneumoniae infections, the time that the concentrations of amoxicillin in serum exceed the MICs for the bacteria correlates well with bacteriological efficacy. This has been demonstrated previously for β-lactam antibiotics against both gram-negative and gram-positive organisms in a number of infection models (18). In these reported studies the T>MIC required for maximal bacterial efficacy for penicillins appeared to be in the region of 30 to 40% in experimental models. Our finding that a T>MIC of approximately 35 to 40% was required for maximal efficacy against S. pneumoniae strains is in full agreement with the published reports, as are the data demonstrating that there is significant bacterial killing even when the MIC is exceeded for only 25 to 30% of the total dosing period. This finding has also been confirmed recently in a review of the pharmacokinetics and pharmacodynamics of penicillins and cephalosporins in otitis media in humans (6). Importantly, data on the pharmacodynamics of antibiotics are not dependent upon the species tested, unlike pharmacokinetics per se, and therefore should allow the estimation of, for example, clinical breakpoints and the prediction of optimal dosing regimens (5).
The data from the murine pneumonia model described in these studies indicate that, on the basis of T>MIC, amoxicillin-clavulanate at a dosage of 500/125 mg t.i.d. should be effective against respiratory infections caused by S. pneumoniae strains for which MICs are up to and including 2 μg/ml (T>MIC, >40%; Table 3). This is confirmed by the data from the rat respiratory tract infection model in which concentrations simulating those achieved with clinical doses were used. In these studies amoxicillin-clavulanate dosed to simulate a dosage of 500/125 mg t.i.d. resulted in a significant reduction in bacterial numbers in the lungs of rats infected with strains of S. pneumoniae for which MICs are ≤2 μg/ml. Using a neutropenic murine thigh infection model in which human kinetics were approximated by administration of uranyl nitrate to render the animals renally impaired, Andes et al. (1) demonstrated that a dose of 7 mg of amoxicillin per kg administered subcutaneously to mice (approximately equivalent to a 250-mg oral dose in humans) produced a significant reduction in bacterial numbers in the thigh and reduced mortality to 0% compared with 100% mortality for untreated animals from infections caused by organisms for which the MIC was 2 μg/ml or less. Furthermore, Soriano et al. (17), using a murine model of pneumonia, showed that amoxicillin administered subcutaneously was effective in the treatment of infections caused by S. pneumoniae strains for which amoxicillin-clavulanate MICs were up to 1 μg/ml. The doses used in this study (50 mg/kg) were approximately equivalent to a 250-mg oral dose in humans. Although the numbers of patients were limited, data from human trials in which the efficacy of amoxicillin-clavulanate for the treatment of otitis media was examined have shown that clinical cure was seen with S. pneumoniae isolates for which the MICs were as high as 4 μg/ml (14). However, it is noteworthy that a good clinical response may occur without a maximal bacteriological response, which may explain the discrepancy in the results obtained in studies of animal infections and with humans. In 1995 the National Committee for Clinical Laboratory Standards (15) agreed to amoxicillin and amoxicillin-clavulanate breakpoints of ≤0.5 μg/ml for fully susceptible strains, 1.0 μg/ml for strains with intermediate resistance, and ≥2 μg/ml for resistant strains. These breakpoints would be considered relatively conservative on the basis of the available data from studies with animals.
Thus, examination of the T>MIC data for a 500/125-mg dose of amoxicillin-clavulanate in humans given t.i.d. shows that the proportion of T>MIC would be approximately 40% for organisms for which the MIC was 2 μg/ml or less. In order to provide the same T>MIC data for a reduced frequency of dosing, i.e., b.i.d. it would be necessary to increase the amoxicillin-clavulanate dose to 875/125 mg (Table 4). In the studies in which the concentrations measured in human adults following the oral administration of doses of either 500/125 or 875/125 mg of amoxicillin-clavulanate were simulated, the efficacies of both dosing regimens were found to be similar against strains for which the MICs were 2 μg/ml or less. Against the single organism for which the MIC was 4 μg/ml, the simulated dose of 875/125 mg was significantly better than the simulated lower dose, which may reflect the greater proportion of time that the concentrations of amoxicillin were above the MIC. These findings therefore validate the hypothesis suggested by the T>MIC data.
TABLE 4.
Duration of time and percentage of dosing interval above the MIC of amoxicillin-clavulanate at the doses resulting in levels in rat serum simulating those achieved in humans
| MIC (μg/ml) |
T>MIC (h) (% of 24-h dosing period)
|
|
|---|---|---|
| Amoxicillin-clavulanate (500/125 mg t.i.d.) | Amoxicillin-clavulanate (875/125 mg b.i.d.) | |
| 8 | 0 (0) | 3.8 (16) |
| 4 | 6.2 (26) | 7.2 (30) |
| 2 | 10.3 (43) | 9.6 (40) |
| 1 | 15.4 (64) | 12.5 (52) |
In conclusion, the experiments described here have demonstrated that it is necessary to increase the concentration of the amoxicillin component of amoxicillin-clavulanate with a reduction of the dosing frequency. This produces efficacy equivalent to that achieved with an increased dosing frequency and would suggest the adoption of the same National Committee for Clinical Laboratory Standards breakpoint for both formulations. The data also demonstrate that the use of experimental animal models linked with a fundamental understanding of the pharmacodynamics of an antibiotic can not only provide an estimation of clinical endpoints but can also provide a rational basis for the determination of the optimal dosing regimens for clinical use.
ACKNOWLEDGMENT
We thank Joanna Bryant for technical assistance.
REFERENCES
- 1.Andes D, Urban A, Craig W A. Program and abstracts of the 35th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1995. In-vivo activity of amoxicillin and amoxicillin-clavulanate against penicillin-resistant pneumococci, abstr. A82; p. 16. [Google Scholar]
- 2.Applebaum P C. Antimicrobial resistance in Streptococcus pneumoniae: an overview. Clin Infect Dis. 1992;15:77–83. doi: 10.1093/clinids/15.1.77. [DOI] [PubMed] [Google Scholar]
- 3.Caputo G M, Applebaum P C, Liu H H. Infections due to penicillin-resistant pneumococci. Clinical, epidemiologic, and microbiologic features. Arch Intern Med. 1993;153:1301–1310. [PubMed] [Google Scholar]
- 4.Celin S E, Bluestone C D, Stephenson J, Yilmaz H M, Collins J J. Bacteriology of acute otitis media in adults. JAMA. 1991;266:2249–2252. [PubMed] [Google Scholar]
- 5.Craig, W. A. 1993. Pharmacodynamics of antimicrobial agents as a basis for determining dosage regimens. Eur. J. Clin. Microbiol. Infect. Dis. 12(Suppl. 1):6–8. [DOI] [PubMed]
- 6.Craig W A, Andes D. Pharmacokinetics and pharmacodynamics of antibiotics in otitis media. Pediatr Infect Dis J. 1996;15:255–259. doi: 10.1097/00006454-199603000-00015. [DOI] [PubMed] [Google Scholar]
- 7.Craig W A, Ebert S. Killing and regrowth of bacteria in vitro: a review. Scand J Infect Dis. 1991;74:S63–S70. [PubMed] [Google Scholar]
- 8.Del Beccaro M A, Mendelman P M, Inglis A F, Richardson M A, Duncan N O, Clausen C R, Stull T L. Bacteriology of acute otitis media: a new perspective. J Pediatr. 1992;120:81–84. doi: 10.1016/s0022-3476(05)80605-5. [DOI] [PubMed] [Google Scholar]
- 9.Drusano G L. Role of pharmacokinetics in outcome of infections. Antimicrob Agents Chemother. 1988;32:289–297. doi: 10.1128/aac.32.3.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gold H S, Moellering R C. Antimicrobial-drug resistance. N Engl J Med. 1996;335:1445–1453. doi: 10.1056/NEJM199611073351907. [DOI] [PubMed] [Google Scholar]
- 11.Gruneberg, R. N. 1996. The Alexander Project: using in-vitro susceptibility data for choosing empirical therapy in LRTI. J. Antimicrob. Chemother. 38(Suppl. A):155–170. [DOI] [PubMed]
- 12.Gwaltney J M. Acute community acquired sinusitis. Clin Infect Dis. 1996;23:1209–1223. doi: 10.1093/clinids/23.6.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jackson D, Cooper D L, Horton R, Langley P F, Staniforth D S, Sutton A J. Absorption, pharmacokinetic and metabolic studies with Augmentin. In: Croydon E A P, Michael M F, editors. Augmentin: clavulanate potentiated amoxycillin. Proceedings of the European Symposium, Scheveningen, The Netherlands. Amsterdam, The Netherlands: Excerpta Medica; 1982. [Google Scholar]
- 14.Jacobs M R, Bajaksouzian S, Burch D, Poupard J, Appelbaum P C. Program and abstracts of the 35th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1995. Activity of amoxicillin ± clavulanate against Streptococcus pneumoniae strains from patients with acute otitis media in eastern Europe, Israel and USA, abstr. E29; p. 90. [Google Scholar]
- 15.National Committee for Clinical Laboratory Standards. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. Approved standard M7-A4. Wayne, Pa: National Committee for Clinical Laboratory Standards; 1993. [Google Scholar]
- 16.Smith G M, Abbott K H. Development of experimental respiratory infections in neutropenic rats with either penicillin-resistant Streptococcus pneumoniae or β-lactamase-producing Haemophilus influenzae. Antimicrob Agents Chemother. 1994;38:608–610. doi: 10.1128/aac.38.3.608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Soriano F, Ponte C, Nieto E, Parra A. Correlation of in-vitro activity and pharmacokinetic parameters with in-vivo effect of amoxycillin, co-amoxiclav and cefotaxime in a murine model of pneumococcal pneumoniae. J Antimicrob Chemother. 1996;38:227–236. doi: 10.1093/jac/38.2.227. [DOI] [PubMed] [Google Scholar]
- 18.Vogelman B, Gundmundsson S, Leggett J, Turnidge J, Ebert S, Craig W A. Correlation of antimicrobial pharmacokinetic parameters with therapeutic efficacy in an animal model. J Infect Dis. 1988;158:831–847. doi: 10.1093/infdis/158.4.831. [DOI] [PubMed] [Google Scholar]