Abstract
Chimeric antigen receptor (CAR) T cells, which express a synthetic receptor engineered to target specific antigens, have demonstrated remarkable potential to treat haematological malignancies. However, their transition beyond haematological malignancy has so far been unsatisfactory. Here, we discuss recent challenges and improvements for CAR T cell therapy against solid tumors: Antigen heterogeneity which provides an effective escape mechanism against conventional mono-antigen-specific CAR T cells; and the immunosuppressive tumor microenvironment which provides physical and molecular barriers that respectively prevent T cell infiltration and drive T cell dysfunction and hypoproliferation. Further, we discuss the application of CAR T cells in infectious disease and autoimmunity.
Keywords: CAR-T cell, Immunotherapy, Cancer, Infectious disease, Autoimmunity
INTRODUCTION
Chimeric antigen receptor (CAR) T cell therapy has demonstrated remarkable potential to treat haematological malignancies (1). CARs are synthetic receptors that redirect immune cell specificity and reprogram their function. They are typically comprised of a single-chain variable fragment (scFv)-based antigen binding domain, a hinge domain, a transmembrane domain, and an intracellular signaling domain (2). First-generation CARs contain only the CD3ζ signaling domain and showed limited efficacy in vivo (3). Meanwhile, second-generation CARs incorporating the signaling domains of costimulatory molecules such as 4-1BB or CD28, which are fused with CD3ζ to enhance and sustain T cell activation signaling, have demonstrated more promising in vivo and clinical outcomes (4,5,6,7). Indeed, second-generation CAR-T cells that target the CD19 and BCMA antigens expressed in B cells and plasma cells, respectively, have shown significant responses in patients with relapsed or refractory B cell malignancies and multiple myeloma (7,8). This initial success has so far led to the approval of five CAR T cell products throughout several countries.
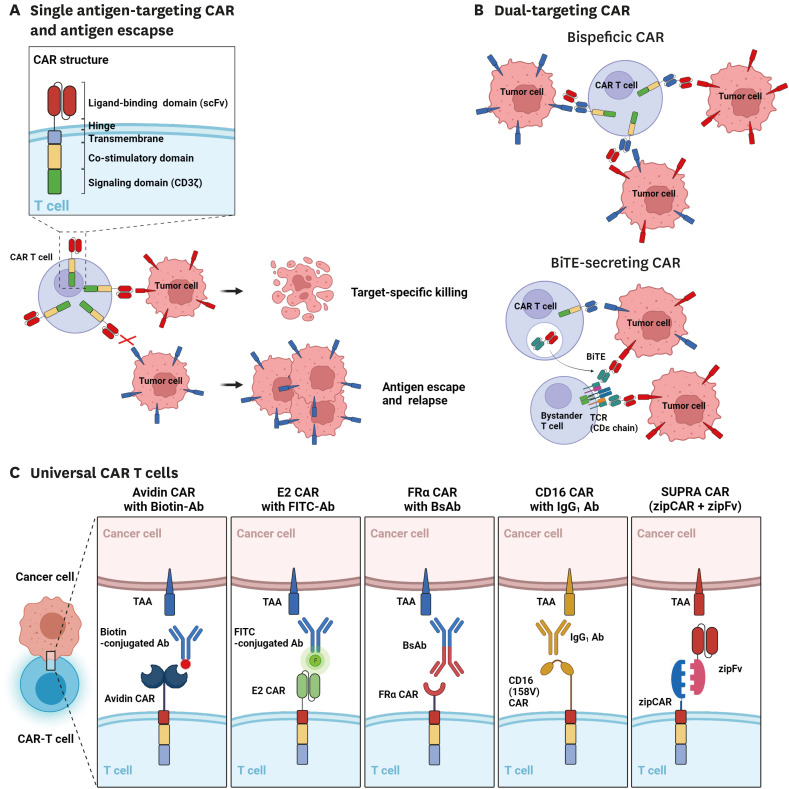
However, despite promising outcomes for haematological malignancies, the efficacy of CAR T cell therapy to treat solid tumors, which accounts for most cancers, remains unsatisfactory. Compared to haematological malignancies, solid tumors pose unique challenges such as antigen heterogeneity which provides an effective escape mechanism against conventional mono-antigen-specific CAR T cells (Fig. 1A); and the immunosuppressive tumor microenvironment (TME) which provides physical and molecular barriers that respectively prevent T cell infiltration and drive T cell dysfunction and hypoproliferation (9,10). Therefore, many groups are exploring next-generation CAR T cell designs to overcome these challenges.
Figure 1. Traditional and recent CAR T cell designs. (A) Traditional single antigen-binding CAR designs face antigen escape within haematological and solid tumors which can be alleviated by (B) dual-targeting CAR T cells. Top; Bispecific (Tandem) CAR T cells. Bottom; CAR T cells secreting BiTEs that target a different TAA. (C) universal CARs and their adapters.
Simultaneously, others are exploring the use of CAR T cells beyond cancer for major disease categories such as chronic infection and autoimmunity (11). Although both categories are still in the preliminary stages of clinical research, unlike cancer, it was quickly evident that each have unique challenges for CAR T therapy. In chronic infection, CAR T cells may face low antigen burden which limits optimal T cell engagement and high viral mutation rates which lead to antigen heterogeneity that requires the use of targeting domains with broad antigen specificity. In the case of autoimmunity, diseases are often complex with multiple or poorly defined antigens or are difficult to target without on-target off-site toxicity.
Here, we review novel CAR engineering strategies to address antigen heterogeneity in solid tumors and the challenges posed by the immunosuppressive TME. We also overview the pre-clinical applications of CAR T cells in infectious disease and autoimmunity and briefly discuss emerging perspectives that further broaden the scope of CAR T cell therapy.
CAR T CELL THERAPY AGAINST SOLID TUMORS
Overcoming antigen escape and heterogeneity
Despite a high initial response rate, the emergence of antigen escape variants is a major cause of relapse for 7%–25% of patients treated with CD19-targeting CAR T cells (12). Mechanisms of CD19 loss include mutations and splice variants of the CD19 gene, as well as the lineage switching from CD19+ lymphoid to CD19- myeloid malignancy (13). Further, antigen escape may be exacerbated in solid tumors that have inherently heterogeneous antigen expression (14,15). Most of the efforts around countering antigen escape were done to prevent the relapse of CD19-negative tumors in patients with haematological malignancies. For example, Fry et al. (16) sequentially administered CD22-targeting CAR T cells in B-cell acute lymphoblastic leukemia (ALL) patients who relapsed from CD19-targeting CAR T cell therapy and observed 73% complete remission for a median of 6 months but found diminished or variable expression of CD22 in leukemic cells which may have contributed to patient relapse. Pan et al. (17) conducted a similar study in patients with B-ALL and observed consistent efficacy with complete remission or incomplete count recovery in 80% of patients. They also found diminished CD22 expression in the leukemic cells from a patient who relapsed which was controllable by a second infusion of the same product.
Alternatively, bispecific (tandem) CAR T cells can be engineered by designing a single CAR molecule with two distinct binding domains (Fig. 1B; top). Shah et al. (18) developed CD19/CD20 bispecific CAR T cells in a phase 1 study for patients with B cell non-Hodgkin lymphoma or chronic lymphocytic leukemia and found a complete response in 64%–92% of patients, while also noting that CD19 antigen loss was not seen in patients who relapsed or failed treatment. Further, Spiegel et al. (19) tested CD19/CD22 bispecific CAR T cells in their phase 1 study for patients with large B cell lymphoma or B-ALL and found 29% and 88% complete remission, respectively. Interestingly, they found that a significant portion of patients relapsed with diminished expression of CD19 but not CD22.
Separately, bi-specific T cell engagers (BiTEs), which consist of two scFvs specific to CD3 and a tumor-associated antigen linked by a flexible linker, have been explored to extrinsically bridge cancer cells with T cells (20). Choi et al. (21) extended this method in combination with CAR T therapy by developing EGFRvIII-specific CAR T cells that also secrete EGFR-specific BiTEs, thus allowing for the engagement of both CAR T and endogenous bystander T cells (Fig. 1B; bottom). This combination proved feasible to eliminate heterogeneous glioblastomas in mice. Interestingly, EGFRvIII-targeting CAR T cells secreting EGFR-BiTEs showed no on-target off-tumor toxicity against human skin grafts compared to EGFR-targeting CAR T cells, which they suggest may be due to the lower expression of the EGFR-BiTEs.
Another approach to achieve simultaneous and customizable targeting of multiple tumor antigens without using complicated genetic engineering involves the design of a universal CAR recognizing adapter molecules containing molecular tags (Fig. 1C). Examples of molecular tags include Fc domains (22), small molecules such as biotin (23) and fluorescein isothiocyanate (FITC) (24,25), small peptides (26), and leucine zipper heterodimerization domains (SUPRA CAR) (27,28). For instance, Urbanska et al. (23) developed avidin-linked CARs that recognized biotinylated antibodies for customizable redirection of CAR T cells. They showed that these CAR T cells could be redirected for elimination of EpCAM-expressing tumors in mouse xenografts. Similarly, CARs recognizing the synthetic small molecule FITC have been used by our group and others in combination with FITC-conjugated antibodies (24,25) and small molecule ligands that target folate receptor (29) and prostate-specific membrane antigen (PSMA) (30). In addition, to overcome the complexity of generating FITC-conjugated targeting antibodies, our group generated CAR T cells that target a small peptide neo-epitope (PNE) derived from the yeast transcription factor GCN4 which can easily be fused genetically at various sites within the targeting antibody. Importantly, we showed that site-specific incorporation of both FITC and PNE is crucial for modulating the length of the immunological synapse, which may contribute to optimal kinetic segregation and T cell activation (25,26).
TUMOR MICROENVIRONMENT
Boosting CAR-T cell infiltration
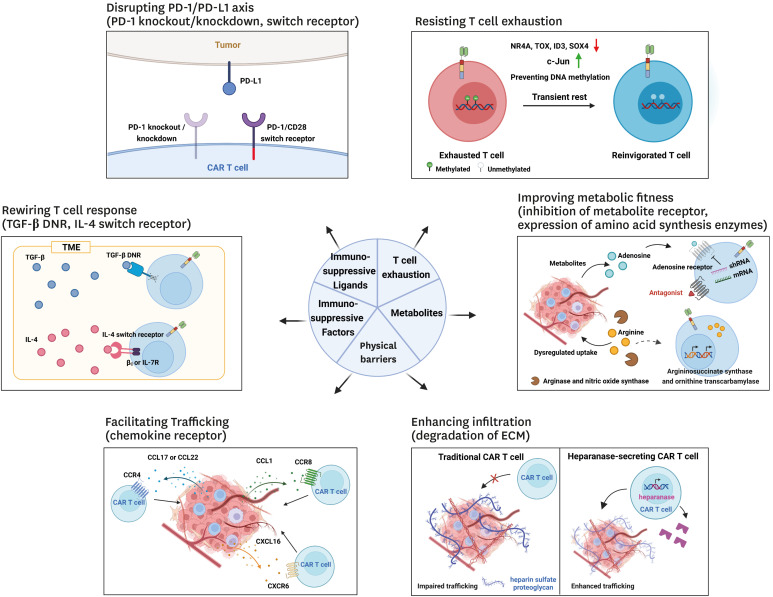
In contrast to haematological malignancies, solid tumors are comprised of an immunosuppressive TME and physical barriers that limit T cell infiltration and function (31) (Fig. 2). Indeed, tumors often secrete high levels of chemokines that preferentially recruit immunosuppressive cell types such as CD4+CD25+FoxP3+ regulatory T cells, tumor-associated macrophages, and myeloid-derived suppressor cells, and each of these cells have distinct roles in favor of tumor growth (32). Importantly, the receptors for these chemokines may not be highly expressed in effector T cells. Some groups have explored the development of CAR T cells co-expressing chemokine receptors such as CCR2b and CCR4, both of which have had success in mouse xenograft models of CCL-2 producing neuroblastoma (33) and malignant pleural mesothelioma (34); and CCL17/CCL22-expressing Hodgkin lymphoma (35). Importantly, in these designs, CAR T cells showed improved infiltration and control of tumors. Recently, Cadilha et al. (36) developed CAR T cells co-expressing CCR8 that showed greater in vivo efficacy due to increased migration into murine pancreatic tumor model. Further, since Treg cells that are abundant in the TME express higher levels of CCR8 compared to effector T cells and secrete large amounts of TGF-β, the authors reasoned that co-expression a TGF-β dominant-negative (DNR) receptor may enhance CAR T cell effector function. Indeed, CCR8 and DNR-co-expressing CAR T cells showed even better efficacy against murine and human pancreatic cancers in mouse models. Similarly, Lesch et al. (37) identified CXCL16 as an important chemokine secreted in murine pancreatic cancers, and their receptor CXCR6 was notably absent from cytotoxic T cells. Therefore, they engineered CAR T cells to co-express CXCR6 and demonstrated that these cells had both improved infiltration and killing of mouse subcutaneous pancreatic cancers, orthotopic pancreatic tumors, and patient-derived xenografts. Interestingly, they also showed that CXCL16-CXCR6 interaction directly enhanced the lytic function of CAR T cells.
Figure 2. Strategies to overcome limitations posed by the solid tumor microenvironment against CAR T cell therapy. CAR T therapy against solid tumors is challenged by the tumor microenvironment which includes immunosuppressive ligands and factors, physical barriers, and metabolites, which collectively impair T cell infiltration and function. Several strategies have been tested to overcome these challenges, such as disrupting the PD-1/PD-L1 axis, rewiring the T cell response through switch receptors, facilitating T cell trafficking through chemokine receptors and extracellular matrix degradation, improving metabolic fitness, and altering T cell function to overcome exhaustion.
Further, stromal cells, which include fibroblasts, mesenchymal stromal cells, osteoblasts, chondrocyte, and extracellular matrix (ECM), help form physical barriers that prevent T cell infiltration (9). Caruana et al. (38) developed GD2-targeting CAR T cells with exogenous co-expression of heparinase, which is normally absent in T cells, to degrade heparin sulfate proteoglycan which is one of the major components of the ECM. These cells had an improved capacity to degrade the ECM and showed enhanced tumor infiltration and prolonged mouse survival in a neuroblastoma xenograft model.
Resisting immunosuppression in the TME
As explained above, the TME contains many immunosuppressive cell types, and these secrete cytokines that favor tumor growth. For example, TGF-β, IL-4, and IL-10 inhibit the antitumor response directly while also promoting the differentiation of immune cells into immunosuppressive cells, thereby reinforcing the suppressive state (32).
Groups have engineered “switch receptors” which function to convert inhibitory signals to stimulatory signals by combining extracellular binding domains for immunosuppressive molecules with the intracellular signaling domains for stimulatory pathways. For example, Chang et al. (39) developed anti-TGF-β CAR T cells comprising a TGF-β-targeting scFv fused to CD28 and CD3z intracellular domains, and showed that these cells had drastically enhanced expression of cytokines such as IFN-γ and TNF-α and were able to proliferate extensively despite the presence of TGF-β in vitro. Leen et al. (40) also designed a switch receptor against IL-4, another major immunosuppressive cytokine expressed in the TME, by fusing the extracellular domain of the IL-4 receptor α with the intracellular domain of the IL-7 receptor α. Epstein-Barr virus (EBV)-specific T cells expressing this receptor showed improved in vitro proliferation and significantly reduced the size of EBV-transformed B cell tumors, thereby increasing mouse lifespan (41). Further, Wilkie et al. (42) developed CAR T cells that co-expressed a switch receptor comprising IL-4R linked with the βc subunit of the IL-2/IL-15 receptor and CARs specific for tumor-associated antigen MUC1 or PSMA. These showed enhanced antigen-specific cytotoxicity and had prolonged expansion in vitro.
Next, alongside cytokines, the TME contains an altered metabolic environment that influence T cell function both directly and indirectly. For example, adenosine is commonly found in the TME and acts as an immunosuppressive metabolite that inhibits T cell responses by inhibiting activation, proliferation, and pro-inflammatory cytokine secretion; alongside promoting FOXP3+ Treg cell generation (43,44). Beavis et al. (45) designed HER2-targeting CAR T cells with the pharmacological or shRNA-mediated downregulation of the adenosine 2A receptor and showed that these cells exhibited greater IFN-γ production and antitumor activity against murine fibrosarcoma and breast cancer models, particularly when combined with antibody-mediated PD-1 blockade. Further, as tumor cells proliferate aberrantly, they rapidly deplete nutrients such as glucose, amino acids, and fatty acids, thereby challenging CAR T cells with a harsh nutrient and oxygen-poor environment following TME infiltration. Therefore, metabolic reprogramming of CAR T cells may offer optimal therapeutic responses in the TME. For example, it is known that arginine enhances T cell function by promoting a shift from glycolysis to oxidative phosphorylation during T cell activation and leads to the generation of central memory cells with improved in vivo persistence (46). However, dysregulated uptake of amino acids by tumor cells and L-arginine depletion by myeloid suppressor cell-derived nitric oxide synthase and arginase in the TME is an important factor that may lead to T cell dysfunction (47). Fultang et al. (48) generated various CAR T cells overexpressing arginosuccinate synthase and/or ornithine transcarbamylase and showed that these cells had improved proliferation in vitro, regardless of the targeting scFv, and more efficient tumor clearance in murine leukemia and neuroblastoma xenografts.
Next, in addition to the soluble factors described above, inhibitory membrane ligands that are abundant in the TME such as PD-L1 are also important. Indeed, the PD-1/PD-L1 axis is known to directly disrupt T cell function through the recruitment of SHP-1/2 which dephosphorylate key signaling molecules following T cell activation (49). To overcome this, Jeong and Park (50) and Liu et al. (51) fused the CD28 co-stimulatory domain to a truncated PD-1 extracellular domain and showed that when co-expressed with a CD19-targeting CAR, this switch receptor led to enhanced antitumor effects in xenograft models of mesothelioma and prostate tumors. Some groups have opted to genetically disrupt PD-1 expression in CAR T cells; Rupp et al. (52) generated CD19-specific CAR T cells with CRISPR/Cas9-mediated knockout of PD-1 which led to enhanced clearance of PD-1+ tumor xenografts. Similar methods have been tested in mouse models of hepatocellular carcinoma (53), glioma (54), and breast cancer (55). However, since the complete ablation of PD-1 expression may have detrimental effects with respect to the persistence of T cells, likely owing to chronic overstimulation (56); and safety (57), our group and others developed a method for simultaneous and partial down-regulation of distinct immune checkpoint ligands via short-hairpin RNA (shRNA) (58,59). Against xenograft models of haematological and solid malignancies, we showed that the simultaneous downregulation of PD-1 and TIGIT led to distinct synergistic effects on short-term cell effector function and long-term persistence of CD19-targeting CAR T. Specifically, we found that downregulation of PD-1 was associated with an increase in transcripts related to effector and proliferation molecules, while TIGIT downregulation was associated with decreased expression of inhibitory receptors and chemokines and higher expression of genes related to naïve/central memory-phenotype and glucose metabolism, resulting in enhanced anti-tumor activity of CAR T in leukemia and lymphoma xenografts. In another strategy, Cherkassky et al. (60) co-expressed a PD-1 DNR in mesothelin-targeting CAR T cells which attenuated PD-1/PD-L1 axis-mediated inhibition, thus enhancing performance in vitro and in an orthotopic pleural mesothelioma tumor model.
Overcoming T cell exhaustion
Exhaustion of T cells occurs when T cells are exposed persistently to antigens in situations like chronic infection and cancer (10). Exhausted T cells gradually lose effector function (e.g. cytokine secretion and cytotoxicity), proliferative capacity, and elevate the surface expression of multiple inhibitory receptors such as PD-1, TIM-3, and TIGIT. It is well established that CAR T cells also acquire exhausted phenotype in the TME, which may in part account for their limited efficacy in solid tumors. As such, several research groups have engineered exhaustion-resistant CAR-T cells with through approaches.
Chen et al. (61) identified nuclear receptor 4A (NR4A) as a possible transcriptional effector of T cell exhaustion. They developed CD19-targeting CAR T cells deficient in NR4A1, NR4A2, and NR4A3 and found that these cells exhibited significantly enhanced activity against CD19-expressing B16-OVA tumors in mice. Next, another TF candidate that may play an important role in T cell exhaustion is TOX, which is highly expressed in dysfunctional T cells in mouse models of cancer (62) and chronic viral infection (63) as well as in human cancer patient samples (64). Seo et al. (65) generated CD19-targeting CAR T cells doubly deficient in TOX and TOX2 and showed that these cells exerted superior tumor control and had prolonged survival compared with wild type and TOX or TOX2 single-deficient cells. Of note, they found a potential positive feedback regulation between TOX and NR4A, both of which are induced by NFAT which is a major downstream TF of the TCR- or CAR-mediated calcium-calcineurin pathway.
More recently, Good et al. (66) identified ID3 and SOX4 as key regulator or CAR T cell exhaustion an in vitro system of continuous antigen exposure. In addition, transcriptomic and phenotypic analysis revealed that the exhausted CAR T cells acquire an NK-like phenotype with expression of KLRB1 and/or CD56. Similar to the exhausted T cells observed in vivo, mesothelin-targeting CAR T cells gradually lost proliferative potential and lytic activity upon persistent antigen exposure, a phenotype that was reversible through the CRISPR-Cas9-mediated knockout of both TFs. Indeed, despite continuous antigen exposure, these cells demonstrated a significant reduction in the frequency of NK-like T cells, an improved dysfunction score, and enhanced in vitro lytic activity compared with wild type CAR T cells.
Another study showed that exhaustion can be also induced in vitro in some CAR designs because of tonic signaling mediated by antigen-independent aggregation of CAR (67). Lynn et al. (68) utilized this model and showed that these CAR T cells recapitulate various hallmarks of T cell exhaustion including epigenetic changes, with the most significantly enriched TF binding motifs of AP-1-bZIP and bZIP-IRF. From RNA-seq and immunoblotting analysis, they also found significantly increased level of JunB, IRF4, and BATF, compared with the canonical AP-1 factor c-Jun. Thus, they postulated that overexpression of c-Jun may disrupt the immunoregulatory AP-1-IRF transcriptional complexes. Indeed, overexpression of c-Jun enhanced the effector function and long-term proliferative capacity of the exhausted CAR T cells in vitro and increased the in vivo activity of freshly prepared CAR T cells in mouse leukemia and solid tumor xenograft models.
Epigenetic modifications, in particular de novo DNA methylation, has been shown to promote T cell exhaustion, and treatment of DNA demethylating agents such as decitabine synergizes with PD-1 blockade therapy by enhancing reinvigoration of exhausted T cells (69). Wang et al. (70) found that treatment of ex vivo-cultured human CAR T cells with very low doses of decitabine induces less differentiation and enhances memory phenotypes. Further, even after repeated exposure to antigens in vitro, these CAR T cells showed sustained expression of T cell proliferation and memory-related genes, but reduced expression of exhaustion-related genes, resulting in enhanced anti-tumor activity in mouse leukemia and lymphoma models. To achieve sustained blocking of de novo DNA methylation, Prinzing et al. (71) employed CRISPR-Cas9-mediated knockout of Dnmt3a in CAR T cells targeting various tumor-associated antigens and showed that these cells retain proliferative capacity and effector function during repeated antigen stimulation in vitro. The enhanced proliferation of these cells was coupled to decreased methylation of promoter regions of TCF7 and LEF1, TFs that are associated with the stem-like phenotype of T cells, as well as to the increased expression of IL-10. DNMT3 knockout also enhanced anti-tumor activity of the CAR T cells in various types of mouse blood and solid tumor xenografts.
Next, since exhaustion of CAR T cells is induced through chronic antigen stimulation or antigen-independent tonic signaling by CARs (10,67), Weber et al. (72) hypothesized that transient inhibition of CAR signaling, termed rest, may affect the development and maintenance of exhaustion. To test this, they employed two approaches: 1) Modifying the CAR with a destabilizing domain that enables drug-dependent, tunable control of CAR protein levels and 2) pharmacologic inhibition of CAR signaling with the Src kinase inhibitor dasatinib. Using CAR designs that induce tonic signaling, they demonstrate that transient rest of pre-exhausted CAR T cells, unlike PD-1 blockade, resulted in the reversal of exhaustion phenotypes and reprogrammed transcriptional and epigenetic signatures to resemble functional T cells. Further, transient inhibition of CAR signaling with dasatinib in vivo augmented anti-tumor activity of both tonically and non-tonically signaling CAR T cells against mouse xenografts. Importantly, the results from this study challenge the putative irreversibility of epigenetic modification in exhausted T cells (73), thus opening new avenues to improve the therapeutic outcomes of cancer immunotherapy by achieving deep, sustained reinvigoration of T cells.
CAR T CELLS BEYOND ONCOLOGY
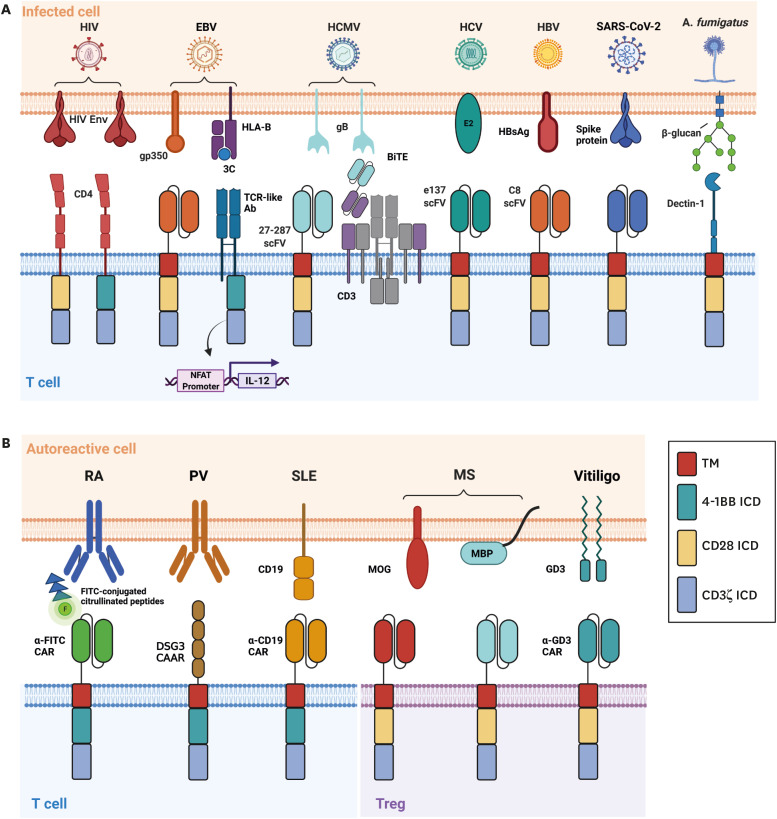
Despite the large research efforts performed for the implementation of CAR T cells against haematological and solid malignancies, their use is not limited to cancer. Here, we review how many groups have explored the implementation of CAR T therapy against infectious disease (Fig. 3A) and autoimmunity (Fig. 3B). We further separate designs for autoimmunity into traditional CAR T cells and CAR Treg cells.
Figure 3. CAR T cell designs against infectious disease and autoimmunity. CAR T cells can be expanded beyond haematological and solid malignancies for (A) infectious disease and (B) autoimmunity.
Infectious disease
HIV is the causative agent of acute immunodeficiency syndrome which is characterized by the depletion of CD4+ T cells (74). Despite the large success of combined antiretroviral therapy (ART), a definite cure for HIV has not yet been achieved through drug intervention. Maldini et al. (75) developed dual CAR T cells that co-express CD28 and 4-1BB-based second generation CARs, which contain the CD4 extracellular domain, alongside the C34-CXCR4 fusion inhibitor to prevent infection of the CAR T cells. These CAR T cells had greater effector functions compared to conventional second- and third-generation CARs in vivo. However, this study failed to achieve a sustained decrease in viremia in the absence of ART In humanized mice. Rust et al. (76) published another study showing that a modified CAR T treatment regimen incorporating infusion of K562-env expressing cells to allow for robust CAR T cell expansion and engraftment in SHIV-infected macaque secondary lymphoid organs. Following ART removal, they showed a delay in viral rebound and overall reduction in viremia in some animals. Further, Zhen et al. (77) showed that HSPC-derived CD46CD4CD3z CAR T engrafted and persisted in peripheral tissues for over 2 years without measurable toxicity. These cells contracted during periods of low antigen presence (during cART administration) and rapidly expanded following cART removal, mimicking a functional memory response.
EBV is an opportunistic pathogen that causes life-long asymptomatic infection in up to 95% of the population (78). It poses a significant risk for the development of EBV-related B cell cancers, epithelial tumors, and post-transplant lymphoproliferative disease. Dragon et al. (79) developed TCR-like CAR TRUCKs, which are modified second-and-third-generation CAR T cells with inducible expression of IL-12, recognizing EBV antigen 3C peptide when loaded on HLA-B*35:01. They showed that these cells could upregulate 4-1BB and CD69 and secrete TNF-α, IFN-γ, and IL-2 in response to target cells. They also showed release of perforin and granzyme B and specific lysis. Despite this, EBV-mediated downregulation of HLA poses a concern for the efficacy of these cells in vivo (78). As such, Slabik et al. (80) developed CAR T cells targeting gp350 which is more uniformly expressed in lytic EBV-expressing cells and sporadically in latently infected cells. These CAR T cells secreted IFN-γ and specifically lysed target cells in vitro. In humanized mice, the cells delayed EBV progression, decreased viral load, and decreased tumor development. Importantly, minimal side effects and changes in body weight for the mice were seen.
Human cytomegalovirus (HCMV) is a herpesvirus that causes opportunistic infections in hosts with compromised immune system (81). It is one of the most important causes of opportunistic infection following solid organ and hematopoietic stem cell transplantation and can lead to serious complications in pregnancy and may cause congenital infections. CAR T cells targeting gB or other HCMV glycoproteins showed specific cytokine secretion and lysis of target cells in vitro, albeit with only weak efficacy (82,83). In a later study, Brey et al. used bispecific antibodies targeting gB and CD3 and showed that although the T cells still had poor cytotoxic function, likely influenced by HCMV-encoded UL36 and UL37x1 which inhibit apoptosis, the cytokines secreted by the T cells halted viral replication (84). Further studies are required to assess their effectiveness in vivo.
HCV is one of the leading causes of chronic liver disease and transplantation (85). Despite the availability of broad and direct-acting antivirals, some patients are still not functionally cured from HCV. Since HCV has a quasispecies distribution during infection, resistance mechanisms against targeted therapies may quickly develop. Sautto et al. (86) developed an the broadly neutralizing scFv-based CAR T cell targeting the HCV envelope glycoprotein E2 and showed that they could exert cytotoxic effects alongside secrete IFN-γ when cocultured with E2-expressing HEK-293 cells and HepG2 cells, and with genotype 2 HCV (JFH-1) strain-infected HuH-7.5 cells.
Hepatitis B virus (HBV) can cause chronic liver infection and is a significant factor for the development of liver cirrhosis and hepatocellular carcinoma (87). Despite available vaccines and antiretroviral or interferon therapies, as many as 3.5% of the global population is suspected of being chronically infected with HBV. Bohne et al. (88) designed CAR T cells against the HBV surface antigens and showed that they were capable of secreting IFN-γ and IL-2 and lysing HBV-infected hepatocytes and eliminate cccDNA-positive cells in vitro. These cells could also engraft within transgenic HBV-expressing immunocompetent mice and demonstrated better viral control while causing only minor liver damage (89). However, in this study, the authors failed to achieve complete viral control and HBV core antigen titers in the serum and liver rose again 34 days after treatment. They further saw that by this time, their CAR T cells were almost completely absent from the liver, likely due to the eventual immune rejection of the human scFv-based CAR T cells. In a more recent study, the same group showed that sublethal body irradiation and injection of signaling-defective CAR T cells prior to injection of signaling-capable CAR T cells tolerized mice and allowed for more persistent viremia control while preventing the mounting of an adaptive immune response against the therapy (90).
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is the etiologic agent of coronavirus disease 2019 (COVID-19) and the cause of significant human and economic harm since the latter months of 2019 (91). Parallel to vaccine and drug development, many groups are investigating the use of immunotherapies against COVID-19, as the immune system was shown to be severely dysregulated in severe disease. Guo et al. (92) developed CAR T cells targeting the SARS-CoV-2 spike receptor. They showed that these cells could secrete IFN-γ, and upregulate CD69, granzyme B, and perforin in co-culture with HEK293 cells expressing the spike receptor binding domain. In vivo, these cells decreased the abundance of S1-expressing NIH/3T3 cells. However, it is worth noting that since COVID19 is an acute infection unlike the previously mentioned chronic infections, applying traditional CAR T therapy would be challenging due to the long manufacturing time for CAR T products, which may be overcome by off-the-shelf CAR approaches (93).
Aspergillus fumigatus is an environmental fungus that can cause life-threatening opportunistic infections in immunocompromised hosts such as those receiving solid organ or hematopoietic stem cell transplantation (94). Kumaresan et al. (95) designed a CAR T cell expressing Dectin-1, which recognizes β-glycans on the cell wall of A. fumigatus, could upregulate IFN-γ, perforin, and granzyme B. These T cells could further damage hyphae and inhibit fungal growth in vitro and diminished infection in NSG mice.
Autoimmunity
Traditional CAR T cells
Rheumatoid arthritis (RA) has two major classifications, the most aggressive and common of which involves the presence of antibodies targeting one or many citrullinated proteins (CP) that are generated as a result of post-translational modification (96). Zhang et al. (97) identified common citrullinated protein peptides in RA used the universal anti-FITC CAR design to link CAR T cells to chosen peptides. They demonstrated that the T cells could specifically lyse anti-CP hybridoma cells, B cells from collagen-induced arthritis mice, and B cells extracted from RA patient sera.
Pemphigus Vulgaris is a skin-blistering autoimmune disease mainly caused by the autoantibody-mediated destruction of keratinocyte cell adhesion through the targeting of desmosomal cadherins, particularly desmoglein 3 (Dsg3) (98). Ellebrecht et al. (99) developed chimeric auto-antigen receptor (CAAR) T cells expressing extracellular Dsg3 domains. They found that their Dsg3 CAAR T cells lysed polyclonal hybridoma xenografts leading to decreased anti-Dsg3 serum IgG levels and blistering of oral mucosa in a PV mouse model. In a subsequent CD19+Dsg3+ Nalm-6 xenograft model, cytotoxic activity was comparable to the clinically approved CD19 CAR T cells and showed successful tumor control. Importantly, Dsg3 CAAR T cells did not cross-react with keratinocytes expressing known ligands for Dsg3 and did not promote cell death of Fcγ+ cell types through antibody binding of the CAAR. The same group later showed that these CAAR T cells could also reduce mucocutaneous blistering and anti-DSG3 autoantibody concentrations in RAG-/- mice that had adoptively transferred splenocytes from DSG3-/- mice immunized with recombinant human DSG3 ectodomain (100).
Systemic lupus erythematosus (SLE) is an autoimmune condition characterized primarily by the presence of pathogenic antibodies targeting nucleic acids alongside additional genetic or environmental factors (101). In a mouse model of SLE, Kansal et al. (102) showed that CD19-CAR T cells could prevent the development of SLE characteristics. They found that the CAR-treated group showed decreased total IgM and IgG, alongside specific decreases in anti-DNA IgM and IgG. They also found improved clinical characteristics, alleviated pathology scores, and a greatly increased lifespan of the mice. Similarly, Jin et al. (103) more recently found similar results in a therapeutic model of SLE treatment with CD19 CD28 and 4-1BB-based CAR T cells. Further, a case report was recently published in which a 20-year old woman with severe and refractory SLE was treated with CD19 CAR T cells (104). The patient experienced serologic and clinical remission characterized by a large decrease in dsDNA autoantibodies, C3, C4, and proteinuria. These results show that CD19 CAR T cell therapy, and potentially those with other targets, may be a convincing strategy for B cell-related autoimmune diseases such as SLE.
CAR Treg cells
Multiple sclerosis is a demyelinating disease characterized by the infiltration of various immune cells and subsequent proinflammatory cytokine production in the central nervous system – ultimately causing significant damage to neuronal myelin sheaths and leading to significant neurodegeneration (105). Fransson et al. (106) transduced polyclonal activated CD4+ T cells with an anti-MOG CAR fused to 2A and FoxP3 for differentiation into Treg cells. In vitro, these cells suppressed polyclonally-stimulated T cell proliferation in the presence of MOG+ cells and activated macrophages. In vivo, the CAR-MOG Tregs migrated to the brain and showed significant reductions of clinical disease symptoms and even complete recovery in mouse models of experimental autoimmune encephalomyelitis (EAE). In a similar approach, De Paula Pohl et al. (107) transduced CD4+CD25highCD127low Treg cells with second-generation anti-MBP or anti-MOG CARs. Consistently, they found that these CAR Tregs ameliorated mean EAE scores in mice when compared to mock or PBS treatment.
Vitiligo is an autoimmune disease of the skin that causes depigmentation primarily through the CD8-mediated killing of melanocytes (108). Mukhatayev et al. (109) found that Ganglioside D3 (GD3) is highly expressed in skin that is undergoing active depigmentation in mice and humans. Thus, they designed an anti-GD3 CD28-based second generation CAR construct and transduced in vitro-derived FoxP3+CD4+ regulatory T cells. They found that these T cells protected melanocytes and secreted immune-suppressive cytokines IL-4 and IL-10 when cocultured with CD8+ T cells. In vivo, the anti-GD3 CAR regulatory T cells led to decreased depigmentation of the dorsal and ventral skin of mice which may be attributed to greater antigen-specific Treg cell homing and protection of melanocytes.
CONCLUSION
In this review, we focused on CAR T cell therapy beyond haematological malignancy such as against solid tumors. Cell-based immunotherapies against solid tumors have been challenged by antigen heterogeneity which allows for antigen escape, the TME which prevents T cell infiltration and provides immunomodulatory metabolites and cytokines, and T cell exhaustion which contributes to disease relapse and treatment failure. We also describe how CAR T cells have been used in pre-clinical studies against infectious disease and autoimmunity, alongside their challenges such as low antigen burden and the lack of clear target antigens. Despite the limitations that CAR T therapy beyond haematological malignancy faces, continuous genetic and protein engineering efforts are in progress. CAR T therapy is a rapidly evolving field that is expanding even beyond these categories, such as cardiac fibrosis (110), and further careful research into its improvements as well as expansion to other immune effector cell types (28) may yet achieve a functional cure for a plethora of previously incurable and debilitating diseases.
ACKNOWLEDGEMENTS
This work was supported by KAIST END-run program.
Abbreviations
- ALL
acute lymphoblastic leukemia
- ART
antiretroviral therapy
- BiTEs
bi-specific T cell engagers
- CAAR
chimeric auto-antigen receptor
- CAR
chimeric antigen receptor
- COVID-19
coronavirus disease 2019
- CP
citrullinated proteins
- DNR
dominant-negative
- EAE
experimental autoimmune encephalomyelitis
- EBV
Epstein-Barr virus
- ECM
extracellular matrix
- FITC
fluorescein isothiocyanate
- HBV
Hepatitis B virus
- HCMV
Human cytomegalovirus
- NR4A
nuclear receptor 4A
- PNE
peptide neo-epitope
- PSMA
prostate-specific membrane antigen
- RA
rheumatoid arthritis
- SARS-CoV-2
severe acute respiratory syndrome coronavirus 2
- scFv
single-chain variable fragment
- shRNA
short-hairpin RNA
- SLE
systemic lupus erythematosus
- TAA
tumor-associated antigen
- TME
tumor microenvironment
Footnotes
Conflict of Interest: The authors declare no potential conflicts of interest.
- Conceptualization: Hupperetz C, Lah S, Kim H.
- Writing - original draft: Hupperetz C, Lah S, Kim H, Kim CH.
- Writing - review & editing: Hupperetz C, Lah S, Kim H, Kim CH.
References
- 1.June CH, Riddell SR, Schumacher TN. Adoptive cellular therapy: a race to the finish line. Sci Transl Med. 2015;7:280ps7. doi: 10.1126/scitranslmed.aaa3643. [DOI] [PubMed] [Google Scholar]
- 2.Sadelain M, Brentjens R, Rivière I. The basic principles of chimeric antigen receptor design. Cancer Discov. 2013;3:388–398. doi: 10.1158/2159-8290.CD-12-0548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tokarew N, Ogonek J, Endres S, von Bergwelt-Baildon M, Kobold S. Teaching an old dog new tricks: next-generation CAR T cells. Br J Cancer. 2019;120:26–37. doi: 10.1038/s41416-018-0325-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Finney HM, Lawson AD, Bebbington CR, Weir AN. Chimeric receptors providing both primary and costimulatory signaling in T cells from a single gene product. J Immunol. 1998;161:2791–2797. [PubMed] [Google Scholar]
- 5.Maher J, Brentjens RJ, Gunset G, Rivière I, Sadelain M. Human T-lymphocyte cytotoxicity and proliferation directed by a single chimeric TCRzeta /CD28 receptor. Nat Biotechnol. 2002;20:70–75. doi: 10.1038/nbt0102-70. [DOI] [PubMed] [Google Scholar]
- 6.Imai C, Mihara K, Andreansky M, Nicholson IC, Pui CH, Geiger TL, Campana D. Chimeric receptors with 4-1BB signaling capacity provoke potent cytotoxicity against acute lymphoblastic leukemia. Leukemia. 2004;18:676–684. doi: 10.1038/sj.leu.2403302. [DOI] [PubMed] [Google Scholar]
- 7.Maude SL, Frey N, Shaw PA, Aplenc R, Barrett DM, Bunin NJ, Chew A, Gonzalez VE, Zheng Z, Lacey SF, et al. Chimeric antigen receptor T cells for sustained remissions in leukemia. N Engl J Med. 2014;371:1507–1517. doi: 10.1056/NEJMoa1407222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ali SA, Shi V, Maric I, Wang M, Stroncek DF, Rose JJ, Brudno JN, Stetler-Stevenson M, Feldman SA, Hansen BG, et al. T cells expressing an anti-B-cell maturation antigen chimeric antigen receptor cause remissions of multiple myeloma. Blood. 2016;128:1688–1700. doi: 10.1182/blood-2016-04-711903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Valkenburg KC, de Groot AE, Pienta KJ. Targeting the tumour stroma to improve cancer therapy. Nat Rev Clin Oncol. 2018;15:366–381. doi: 10.1038/s41571-018-0007-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wherry EJ. T cell exhaustion. Nat Immunol. 2011;12:492–499. doi: 10.1038/ni.2035. [DOI] [PubMed] [Google Scholar]
- 11.Maldini CR, Ellis GI, Riley JL. CAR T cells for infection, autoimmunity and allotransplantation. Nat Rev Immunol. 2018;18:605–616. doi: 10.1038/s41577-018-0042-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Majzner RG, Mackall CL. Tumor antigen escape from CAR T-cell therapy. Cancer Discov. 2018;8:1219–1226. doi: 10.1158/2159-8290.CD-18-0442. [DOI] [PubMed] [Google Scholar]
- 13.Rafiq S, Brentjens RJ. Tumors evading CARs-the chase is on. Nat Med. 2018;24:1492–1493. doi: 10.1038/s41591-018-0212-6. [DOI] [PubMed] [Google Scholar]
- 14.O’Rourke DM, Nasrallah MP, Desai A, Melenhorst JJ, Mansfield K, Morrissette JJ, Martinez-Lage M, Brem S, Maloney E, Shen A, et al. A single dose of peripherally infused EGFRvIII-directed CAR T cells mediates antigen loss and induces adaptive resistance in patients with recurrent glioblastoma. Sci Transl Med. 2017;9:eaaa0984. doi: 10.1126/scitranslmed.aaa0984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rafiq S, Hackett CS, Brentjens RJ. Engineering strategies to overcome the current roadblocks in CAR T cell therapy. Nat Rev Clin Oncol. 2020;17:147–167. doi: 10.1038/s41571-019-0297-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fry TJ, Shah NN, Orentas RJ, Stetler-Stevenson M, Yuan CM, Ramakrishna S, Wolters P, Martin S, Delbrook C, Yates B, et al. CD22-targeted CAR T cells induce remission in B-ALL that is naive or resistant to CD19-targeted CAR immunotherapy. Nat Med. 2018;24:20–28. doi: 10.1038/nm.4441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pan J, Niu Q, Deng B, Liu S, Wu T, Gao Z, Liu Z, Zhang Y, Qu X, Zhang Y, et al. CD22 CAR T-cell therapy in refractory or relapsed B acute lymphoblastic leukemia. Leukemia. 2019;33:2854–2866. doi: 10.1038/s41375-019-0488-7. [DOI] [PubMed] [Google Scholar]
- 18.Shah NN, Johnson BD, Schneider D, Zhu F, Szabo A, Keever-Taylor CA, Krueger W, Worden AA, Kadan MJ, Yim S, et al. Bispecific anti-CD20, anti-CD19 CAR T cells for relapsed B cell malignancies: a phase 1 dose escalation and expansion trial. Nat Med. 2020;26:1569–1575. doi: 10.1038/s41591-020-1081-3. [DOI] [PubMed] [Google Scholar]
- 19.Spiegel JY, Patel S, Muffly L, Hossain NM, Oak J, Baird JH, Frank MJ, Shiraz P, Sahaf B, Craig J, et al. CAR T cells with dual targeting of CD19 and CD22 in adult patients with recurrent or refractory B cell malignancies: a phase 1 trial. Nat Med. 2021;27:1419–1431. doi: 10.1038/s41591-021-01436-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goebeler ME, Bargou RC. T cell-engaging therapies - BiTEs and beyond. Nat Rev Clin Oncol. 2020;17:418–434. doi: 10.1038/s41571-020-0347-5. [DOI] [PubMed] [Google Scholar]
- 21.Choi BD, Yu X, Castano AP, Bouffard AA, Schmidts A, Larson RC, Bailey SR, Boroughs AC, Frigault MJ, Leick MB, et al. CAR-T cells secreting BiTEs circumvent antigen escape without detectable toxicity. Nat Biotechnol. 2019;37:1049–1058. doi: 10.1038/s41587-019-0192-1. [DOI] [PubMed] [Google Scholar]
- 22.Kudo K, Imai C, Lorenzini P, Kamiya T, Kono K, Davidoff AM, Chng WJ, Campana D. T lymphocytes expressing a CD16 signaling receptor exert antibody-dependent cancer cell killing. Cancer Res. 2014;74:93–103. doi: 10.1158/0008-5472.CAN-13-1365. [DOI] [PubMed] [Google Scholar]
- 23.Urbanska K, Lanitis E, Poussin M, Lynn RC, Gavin BP, Kelderman S, Yu J, Scholler N, Powell DJ., Jr A universal strategy for adoptive immunotherapy of cancer through use of a novel T-cell antigen receptor. Cancer Res. 2012;72:1844–1852. doi: 10.1158/0008-5472.CAN-11-3890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tamada K, Geng D, Sakoda Y, Bansal N, Srivastava R, Li Z, Davila E. Redirecting gene-modified T cells toward various cancer types using tagged antibodies. Clin Cancer Res. 2012;18:6436–6445. doi: 10.1158/1078-0432.CCR-12-1449. [DOI] [PubMed] [Google Scholar]
- 25.Ma JS, Kim JY, Kazane SA, Choi SH, Yun HY, Kim MS, Rodgers DT, Pugh HM, Singer O, Sun SB, et al. Versatile strategy for controlling the specificity and activity of engineered T cells. Proc Natl Acad Sci U S A. 2016;113:E450–E458. doi: 10.1073/pnas.1524193113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rodgers DT, Mazagova M, Hampton EN, Cao Y, Ramadoss NS, Hardy IR, Schulman A, Du J, Wang F, Singer O, et al. Switch-mediated activation and retargeting of CAR-T cells for B-cell malignancies. Proc Natl Acad Sci U S A. 2016;113:E459–E468. doi: 10.1073/pnas.1524155113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cho JH, Collins JJ, Wong WW. Universal chimeric antigen receptors for multiplexed and logical control of T cell responses. Cell. 2018;173:1426–1438.e1411. doi: 10.1016/j.cell.2018.03.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cho JH, Okuma A, Sofjan K, Lee S, Collins JJ, Wong WW. Engineering advanced logic and distributed computing in human CAR immune cells. Nat Commun. 2021;12:792. doi: 10.1038/s41467-021-21078-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim MS, Ma JS, Yun H, Cao Y, Kim JY, Chi V, Wang D, Woods A, Sherwood L, Caballero D, et al. Redirection of genetically engineered CAR-T cells using bifunctional small molecules. J Am Chem Soc. 2015;137:2832–2835. doi: 10.1021/jacs.5b00106. [DOI] [PubMed] [Google Scholar]
- 30.Lee YG, Marks I, Srinivasarao M, Kanduluru AK, Mahalingam SM, Liu X, Chu H, Low PS. Use of a single CAR T cell and several bispecific adapters facilitates eradication of multiple antigenically different solid tumors. Cancer Res. 2019;79:387–396. doi: 10.1158/0008-5472.CAN-18-1834. [DOI] [PubMed] [Google Scholar]
- 31.Newick K, O’Brien S, Moon E, Albelda SM. CAR T cell therapy for solid tumors. Annu Rev Med. 2017;68:139–152. doi: 10.1146/annurev-med-062315-120245. [DOI] [PubMed] [Google Scholar]
- 32.Jin MZ, Jin WL. The updated landscape of tumor microenvironment and drug repurposing. Signal Transduct Target Ther. 2020;5:166. doi: 10.1038/s41392-020-00280-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Craddock JA, Lu A, Bear A, Pule M, Brenner MK, Rooney CM, Foster AE. Enhanced tumor trafficking of GD2 chimeric antigen receptor T cells by expression of the chemokine receptor CCR2b. J Immunother. 2010;33:780–788. doi: 10.1097/CJI.0b013e3181ee6675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moon EK, Carpenito C, Sun J, Wang LC, Kapoor V, Predina J, Powell DJ, Jr, Riley JL, June CH, Albelda SM. Expression of a functional CCR2 receptor enhances tumor localization and tumor eradication by retargeted human T cells expressing a mesothelin-specific chimeric antibody receptor. Clin Cancer Res. 2011;17:4719–4730. doi: 10.1158/1078-0432.CCR-11-0351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Di Stasi A, De Angelis B, Rooney CM, Zhang L, Mahendravada A, Foster AE, Heslop HE, Brenner MK, Dotti G, Savoldo B. T lymphocytes coexpressing CCR4 and a chimeric antigen receptor targeting CD30 have improved homing and antitumor activity in a Hodgkin tumor model. Blood. 2009;113:6392–6402. doi: 10.1182/blood-2009-03-209650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cadilha BL, Benmebarek MR, Dorman K, Oner A, Lorenzini T, Obeck H, Vänttinen M, Di Pilato M, Pruessmann JN, Stoiber S, et al. Combined tumor-directed recruitment and protection from immune suppression enable CAR T cell efficacy in solid tumors. Sci Adv. 2021;7:eabi5781. doi: 10.1126/sciadv.abi5781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lesch S, Blumenberg V, Stoiber S, Gottschlich A, Ogonek J, Cadilha BL, Dantes Z, Rataj F, Dorman K, Lutz J, et al. T cells armed with C-X-C chemokine receptor type 6 enhance adoptive cell therapy for pancreatic tumours. Nat Biomed Eng. 2021;5:1246–1260. doi: 10.1038/s41551-021-00737-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Caruana I, Savoldo B, Hoyos V, Weber G, Liu H, Kim ES, Ittmann MM, Marchetti D, Dotti G. Heparanase promotes tumor infiltration and antitumor activity of CAR-redirected T lymphocytes. Nat Med. 2015;21:524–529. doi: 10.1038/nm.3833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chang ZL, Lorenzini MH, Chen X, Tran U, Bangayan NJ, Chen YY. Rewiring T-cell responses to soluble factors with chimeric antigen receptors. Nat Chem Biol. 2018;14:317–324. doi: 10.1038/nchembio.2565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tormoen GW, Crittenden MR, Gough MJ. Role of the immunosuppressive microenvironment in immunotherapy. Adv Radiat Oncol. 2018;3:520–526. doi: 10.1016/j.adro.2018.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Leen AM, Sukumaran S, Watanabe N, Mohammed S, Keirnan J, Yanagisawa R, Anurathapan U, Rendon D, Heslop HE, Rooney CM, et al. Reversal of tumor immune inhibition using a chimeric cytokine receptor. Mol Ther. 2014;22:1211–1220. doi: 10.1038/mt.2014.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wilkie S, Burbridge SE, Chiapero-Stanke L, Pereira AC, Cleary S, van der Stegen SJ, Spicer JF, Davies DM, Maher J. Selective expansion of chimeric antigen receptor-targeted T-cells with potent effector function using interleukin-4. J Biol Chem. 2010;285:25538–25544. doi: 10.1074/jbc.M110.127951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ohta A, Gorelik E, Prasad SJ, Ronchese F, Lukashev D, Wong MK, Huang X, Caldwell S, Liu K, Smith P, et al. A2A adenosine receptor protects tumors from antitumor T cells. Proc Natl Acad Sci U S A. 2006;103:13132–13137. doi: 10.1073/pnas.0605251103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zarek PE, Huang CT, Lutz ER, Kowalski J, Horton MR, Linden J, Drake CG, Powell JD. A2A receptor signaling promotes peripheral tolerance by inducing T-cell anergy and the generation of adaptive regulatory T cells. Blood. 2008;111:251–259. doi: 10.1182/blood-2007-03-081646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Beavis PA, Henderson MA, Giuffrida L, Mills JK, Sek K, Cross RS, Davenport AJ, John LB, Mardiana S, Slaney CY, et al. Targeting the adenosine 2A receptor enhances chimeric antigen receptor T cell efficacy. J Clin Invest. 2017;127:929–941. doi: 10.1172/JCI89455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Geiger R, Rieckmann JC, Wolf T, Basso C, Feng Y, Fuhrer T, Kogadeeva M, Picotti P, Meissner F, Mann M, et al. L-arginine modulates T cell metabolism and enhances survival and anti-tumor activity. Cell. 2016;167:829–842.e13. doi: 10.1016/j.cell.2016.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bronte V, Zanovello P. Regulation of immune responses by L-arginine metabolism. Nat Rev Immunol. 2005;5:641–654. doi: 10.1038/nri1668. [DOI] [PubMed] [Google Scholar]
- 48.Fultang L, Booth S, Yogev O, Martins da Costa B, Tubb V, Panetti S, Stavrou V, Scarpa U, Jankevics A, Lloyd G, et al. Metabolic engineering against the arginine microenvironment enhances CAR-T cell proliferation and therapeutic activity. Blood. 2020;136:1155–1160. doi: 10.1182/blood.2019004500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Marasco M, Berteotti A, Weyershaeuser J, Thorausch N, Sikorska J, Krausze J, Brandt HJ, Kirkpatrick J, Rios P, Schamel WW, et al. Molecular mechanism of SHP2 activation by PD-1 stimulation. Sci Adv. 2020;6:eaay4458. doi: 10.1126/sciadv.aay4458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jeong S, Park SH. Co-Stimulatory Receptors in Cancers and Their Implications for Cancer Immunotherapy. Immune Netw. 2020;20:e3. doi: 10.4110/in.2020.20.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Liu X, Ranganathan R, Jiang S, Fang C, Sun J, Kim S, Newick K, Lo A, June CH, Zhao Y, et al. A chimeric switch-receptor targeting PD1 augments the efficacy of second-generation CAR T cells in advanced solid tumors. Cancer Res. 2016;76:1578–1590. doi: 10.1158/0008-5472.CAN-15-2524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rupp LJ, Schumann K, Roybal KT, Gate RE, Ye CJ, Lim WA, Marson A. CRISPR/Cas9-mediated PD-1 disruption enhances anti-tumor efficacy of human chimeric antigen receptor T cells. Sci Rep. 2017;7:737. doi: 10.1038/s41598-017-00462-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Guo X, Jiang H, Shi B, Zhou M, Zhang H, Shi Z, Du G, Luo H, Wu X, Wang Y, et al. Disruption of PD-1 enhanced the anti-tumor activity of chimeric antigen receptor T cells against hepatocellular carcinoma. Front Pharmacol. 2018;9:1118. doi: 10.3389/fphar.2018.01118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hu B, Zou Y, Zhang L, Tang J, Niedermann G, Firat E, Huang X, Zhu X. Nucleofection with plasmid DNA for CRISPR/Cas9-mediated inactivation of programmed cell death protein 1 in CD133-specific CAR T cells. Hum Gene Ther. 2019;30:446–458. doi: 10.1089/hum.2017.234. [DOI] [PubMed] [Google Scholar]
- 55.Hu W, Zi Z, Jin Y, Li G, Shao K, Cai Q, Ma X, Wei F. CRISPR/Cas9-mediated PD-1 disruption enhances human mesothelin-targeted CAR T cell effector functions. Cancer Immunol Immunother. 2019;68:365–377. doi: 10.1007/s00262-018-2281-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Odorizzi PM, Pauken KE, Paley MA, Sharpe A, Wherry EJ. Genetic absence of PD-1 promotes accumulation of terminally differentiated exhausted CD8+ T cells. J Exp Med. 2015;212:1125–1137. doi: 10.1084/jem.20142237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wartewig T, Kurgyis Z, Keppler S, Pechloff K, Hameister E, Öllinger R, Maresch R, Buch T, Steiger K, Winter C, et al. PD-1 is a haploinsufficient suppressor of T cell lymphomagenesis. Nature. 2017;552:121–125. doi: 10.1038/nature24649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lee YH, Lee HJ, Kim HC, Lee Y, Nam SK, Hupperetz C, Ma JSY, Wang X, Singer O, Kim WS, et al. PD-1 and TIGIT downregulation distinctly affect the effector and early memory phenotypes of CD19-targeting CAR T cells. Mol Ther. 2022;30:579–592. doi: 10.1016/j.ymthe.2021.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zou F, Lu L, Liu J, Xia B, Zhang W, Hu Q, Liu W, Zhang Y, Lin Y, Jing S, et al. Engineered triple inhibitory receptor resistance improves anti-tumor CAR-T cell performance via CD56. Nat Commun. 2019;10:4109. doi: 10.1038/s41467-019-11893-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cherkassky L, Morello A, Villena-Vargas J, Feng Y, Dimitrov DS, Jones DR, Sadelain M, Adusumilli PS. Human CAR T cells with cell-intrinsic PD-1 checkpoint blockade resist tumor-mediated inhibition. J Clin Invest. 2016;126:3130–3144. doi: 10.1172/JCI83092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chen J, López-Moyado IF, Seo H, Lio CJ, Hempleman LJ, Sekiya T, Yoshimura A, Scott-Browne JP, Rao A. NR4A transcription factors limit CAR T cell function in solid tumours. Nature. 2019;567:530–534. doi: 10.1038/s41586-019-0985-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Scott AC, Dündar F, Zumbo P, Chandran SS, Klebanoff CA, Shakiba M, Trivedi P, Menocal L, Appleby H, Camara S, et al. TOX is a critical regulator of tumour-specific T cell differentiation. Nature. 2019;571:270–274. doi: 10.1038/s41586-019-1324-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Khan O, Giles JR, McDonald S, Manne S, Ngiow SF, Patel KP, Werner MT, Huang AC, Alexander KA, Wu JE, et al. TOX transcriptionally and epigenetically programs CD8(+) T cell exhaustion. Nature. 2019;571:211–218. doi: 10.1038/s41586-019-1325-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kim K, Park S, Park SY, Kim G, Park SM, Cho JW, Kim DH, Park YM, Koh YW, Kim HR, et al. Single-cell transcriptome analysis reveals TOX as a promoting factor for T cell exhaustion and a predictor for anti-PD-1 responses in human cancer. Genome Med. 2020;12:22. doi: 10.1186/s13073-020-00722-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Seo H, Chen J, González-Avalos E, Samaniego-Castruita D, Das A, Wang YH, López-Moyado IF, Georges RO, Zhang W, Onodera A, et al. TOX and TOX2 transcription factors cooperate with NR4A transcription factors to impose CD8(+) T cell exhaustion (vol 116, pg 12410, 2019) Proc Natl Acad Sci U S A. 2019;116:19761–19761. doi: 10.1073/pnas.1905675116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Good CR, Kuramitsu S, Samareh P, Donahue G, Ishiyama K, Ma Y, Wellhausen N, Tian L, Agarwal S, Guedan S, et al. Induction of T cell dysfunction and NK-like T cell differentiation in vitro and in patients after CAR T cell treatment. Cancer Res. 2021;81(Suppl):A60. [Google Scholar]
- 67.Long AH, Haso WM, Shern JF, Wanhainen KM, Murgai M, Ingaramo M, Smith JP, Walker AJ, Kohler ME, Venkateshwara VR, et al. 4-1BB costimulation ameliorates T cell exhaustion induced by tonic signaling of chimeric antigen receptors. Nat Med. 2015;21:581–590. doi: 10.1038/nm.3838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Murphy TL, Tussiwand R, Murphy KM. Specificity through cooperation: BATF-IRF interactions control immune-regulatory networks. Nat Rev Immunol. 2013;13:499–509. doi: 10.1038/nri3470. [DOI] [PubMed] [Google Scholar]
- 69.Ghoneim HE, Fan Y, Moustaki A, Abdelsamed HA, Dash P, Dogra P, Carter R, Awad W, Neale G, Thomas PG, et al. De novo epigenetic programs inhibit PD-1 Blockade-mediated T cell rejuvenation. Cell. 2017;170:142–157.e19. doi: 10.1016/j.cell.2017.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wang Y, Tong C, Dai H, Wu Z, Han X, Guo Y, Chen D, Wei J, Ti D, Liu Z, et al. Low-dose decitabine priming endows CAR T cells with enhanced and persistent antitumour potential via epigenetic reprogramming. Nat Commun. 2021;12:409. doi: 10.1038/s41467-020-20696-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Prinzing B, Zebley CC, Petersen CT, Fan Y, Anido AA, Yi Z, Nguyen P, Houke H, Bell M, Haydar D, et al. Deleting DNMT3A in CAR T cells prevents exhaustion and enhances antitumor activity. Sci Transl Med. 2021;13:eabh0272. doi: 10.1126/scitranslmed.abh0272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Weber EW, Parker KR, Sotillo E, Lynn RC, Anbunathan H, Lattin J, Good Z, Belk JA, Daniel B, Klysz D, et al. Transient rest restores functionality in exhausted CAR-T cells through epigenetic remodeling. Science. 2021;372:eaba1786. doi: 10.1126/science.aba1786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Pauken KE, Sammons MA, Odorizzi PM, Manne S, Godec J, Khan O, Drake AM, Chen Z, Sen DR, Kurachi M, et al. Epigenetic stability of exhausted T cells limits durability of reinvigoration by PD-1 blockade. Science. 2016;354:1160–1165. doi: 10.1126/science.aaf2807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Deeks SG, Overbaugh J, Phillips A, Buchbinder S. HIV infection. Nat Rev Dis Primers. 2015;1:15035. doi: 10.1038/nrdp.2015.35. [DOI] [PubMed] [Google Scholar]
- 75.Maldini CR, Claiborne DT, Okawa K, Chen T, Dopkin DL, Shan X, Power KA, Trifonova RT, Krupp K, Phelps M, et al. Dual CD4-based CAR T cells with distinct costimulatory domains mitigate HIV pathogenesis in vivo . Nat Med. 2020;26:1776–1787. doi: 10.1038/s41591-020-1039-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Rust BJ, Kean LS, Colonna L, Brandenstein KE, Poole NH, Obenza W, Enstrom MR, Maldini CR, Ellis GI, Fennessey CM, et al. Robust expansion of HIV CAR T cells following antigen boosting in ART-suppressed nonhuman primates. Blood. 2020;136:1722–1734. doi: 10.1182/blood.2020006372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zhen A, Peterson CW, Carrillo MA, Reddy SS, Youn CS, Lam BB, Chang NY, Martin HA, Rick JW, Kim J, et al. Long-term persistence and function of hematopoietic stem cell-derived chimeric antigen receptor T cells in a nonhuman primate model of HIV/AIDS. PLoS Pathog. 2017;13:e1006753. doi: 10.1371/journal.ppat.1006753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Young LS, Yap LF, Murray PG. Epstein-Barr virus: more than 50 years old and still providing surprises. Nat Rev Cancer. 2016;16:789–802. doi: 10.1038/nrc.2016.92. [DOI] [PubMed] [Google Scholar]
- 79.Dragon AC, Zimmermann K, Nerreter T, Sandfort D, Lahrberg J, Klöß S, Kloth C, Mangare C, Bonifacius A, Tischer-Zimmermann S, et al. CAR-T cells and TRUCKs that recognize an EBNA-3C-derived epitope presented on HLA-B*35 control Epstein-Barr virus-associated lymphoproliferation. J Immunother Cancer. 2020;8:e000736. doi: 10.1136/jitc-2020-000736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Slabik C, Kalbarczyk M, Danisch S, Zeidler R, Klawonn F, Volk V, Krönke N, Feuerhake F, Ferreira de Figueiredo C, Blasczyk R, et al. CAR-T cells targeting Epstein-Barr virus gp350 validated in a humanized mouse model of EBV infection and lymphoproliferative disease. Mol Ther Oncolytics. 2020;18:504–524. doi: 10.1016/j.omto.2020.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Griffiths P, Reeves M. Pathogenesis of human cytomegalovirus in the immunocompromised host. Nat Rev Microbiol. 2021;19:759–773. doi: 10.1038/s41579-021-00582-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Full F, Lehner M, Thonn V, Goetz G, Scholz B, Kaufmann KB, Mach M, Abken H, Holter W, Ensser A. T cells engineered with a cytomegalovirus-specific chimeric immunoreceptor. J Virol. 2010;84:4083–4088. doi: 10.1128/JVI.02117-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ali A, Chiuppesi F, Nguyen M, Hausner MA, Nguyen J, Kha M, Iniguez A, Wussow F, Diamond DJ, Yang OO. Chimeric antigen receptors targeting human cytomegalovirus. J Infect Dis. 2020;222:853–862. doi: 10.1093/infdis/jiaa171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Brey CU, Proff J, Teufert N, Salzer B, Brozy J, Münz M, Pendzialek J, Ensser A, Holter W, Lehner M. A gB/CD3 bispecific BiTE antibody construct for targeting Human Cytomegalovirus-infected cells. Sci Rep. 2018;8:17453. doi: 10.1038/s41598-018-36055-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Manns MP, Buti M, Gane E, Pawlotsky JM, Razavi H, Terrault N, Younossi Z. Hepatitis C virus infection. Nat Rev Dis Primers. 2017;3:17006. doi: 10.1038/nrdp.2017.6. [DOI] [PubMed] [Google Scholar]
- 86.Sautto GA, Wisskirchen K, Clementi N, Castelli M, Diotti RA, Graf J, Clementi M, Burioni R, Protzer U, Mancini N. Chimeric antigen receptor (CAR)-engineered T cells redirected against hepatitis C virus (HCV) E2 glycoprotein. Gut. 2016;65:512–523. doi: 10.1136/gutjnl-2014-308316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Yuen MF, Chen DS, Dusheiko GM, Janssen HL, Lau DT, Locarnini SA, Peters MG, Lai CL. Hepatitis B virus infection. Nat Rev Dis Primers. 2018;4:18035. doi: 10.1038/nrdp.2018.35. [DOI] [PubMed] [Google Scholar]
- 88.Bohne F, Chmielewski M, Ebert G, Wiegmann K, Kürschner T, Schulze A, Urban S, Krönke M, Abken H, Protzer U. T cells redirected against hepatitis B virus surface proteins eliminate infected hepatocytes. Gastroenterology. 2008;134:239–247. doi: 10.1053/j.gastro.2007.11.002. [DOI] [PubMed] [Google Scholar]
- 89.Krebs K, Böttinger N, Huang LR, Chmielewski M, Arzberger S, Gasteiger G, Jäger C, Schmitt E, Bohne F, Aichler M, et al. T cells expressing a chimeric antigen receptor that binds hepatitis B virus envelope proteins control virus replication in mice. Gastroenterology. 2013;145:456–465. doi: 10.1053/j.gastro.2013.04.047. [DOI] [PubMed] [Google Scholar]
- 90.Festag MM, Festag J, Fräßle SP, Asen T, Sacherl J, Schreiber S, Mück-Häusl MA, Busch DH, Wisskirchen K, Protzer U. Evaluation of a fully human, hepatitis B virus-specific chimeric antigen receptor in an immunocompetent mouse model. Mol Ther. 2019;27:947–959. doi: 10.1016/j.ymthe.2019.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Sette A, Crotty S. Adaptive immunity to SARS-CoV-2 and COVID-19. Cell. 2021;184:861–880. doi: 10.1016/j.cell.2021.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Guo X, Kazanova A, Thurmond S, Saragovi HU, Rudd CE. Effective chimeric antigen receptor T cells against SARS-CoV-2. iScience. 2021;24:103295. doi: 10.1016/j.isci.2021.103295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Depil S, Duchateau P, Grupp SA, Mufti G, Poirot L. ‘Off-the-shelf’ allogeneic CAR T cells: development and challenges. Nat Rev Drug Discov. 2020;19:185–199. doi: 10.1038/s41573-019-0051-2. [DOI] [PubMed] [Google Scholar]
- 94.van de Veerdonk FL, Gresnigt MS, Romani L, Netea MG, Latgé JP. Aspergillus fumigatus morphology and dynamic host interactions. Nat Rev Microbiol. 2017;15:661–674. doi: 10.1038/nrmicro.2017.90. [DOI] [PubMed] [Google Scholar]
- 95.Kumaresan PR, Manuri PR, Albert ND, Maiti S, Singh H, Mi T, Roszik J, Rabinovich B, Olivares S, Krishnamurthy J, et al. Bioengineering T cells to target carbohydrate to treat opportunistic fungal infection. Proc Natl Acad Sci U S A. 2014;111:10660–10665. doi: 10.1073/pnas.1312789111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Guo Q, Wang Y, Xu D, Nossent J, Pavlos NJ, Xu J. Rheumatoid arthritis: pathological mechanisms and modern pharmacologic therapies. Bone Res. 2018;6:15. doi: 10.1038/s41413-018-0016-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zhang B, Wang Y, Yuan Y, Sun J, Liu L, Huang D, Hu J, Wang M, Li S, Song W, et al. In vitro elimination of autoreactive B cells from rheumatoid arthritis patients by universal chimeric antigen receptor T cells. Ann Rheum Dis. 2021;80:176–184. doi: 10.1136/annrheumdis-2020-217844. [DOI] [PubMed] [Google Scholar]
- 98.Hammers CM, Stanley JR. Mechanisms of disease: pemphigus and bullous pemphigoid. Annu Rev Pathol. 2016;11:175–197. doi: 10.1146/annurev-pathol-012615-044313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ellebrecht CT, Bhoj VG, Nace A, Choi EJ, Mao X, Cho MJ, Di Zenzo G, Lanzavecchia A, Seykora JT, Cotsarelis G, et al. Reengineering chimeric antigen receptor T cells for targeted therapy of autoimmune disease. Science. 2016;353:179–184. doi: 10.1126/science.aaf6756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lee J, Lundgren DK, Mao X, Manfredo-Vieira S, Nunez-Cruz S, Williams EF, Assenmacher CA, Radaelli E, Oh S, Wang B, et al. Antigen-specific B cell depletion for precision therapy of mucosal pemphigus vulgaris. J Clin Invest. 2020;130:6317–6324. doi: 10.1172/JCI138416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kaul A, Gordon C, Crow MK, Touma Z, Urowitz MB, van Vollenhoven R, Ruiz-Irastorza G, Hughes G. Systemic lupus erythematosus. Nat Rev Dis Primers. 2016;2:16039. doi: 10.1038/nrdp.2016.39. [DOI] [PubMed] [Google Scholar]
- 102.Kansal R, Richardson N, Neeli I, Khawaja S, Chamberlain D, Ghani M, Ghani QU, Balazs L, Beranova-Giorgianni S, Giorgianni F, et al. Sustained B cell depletion by CD19-targeted CAR T cells is a highly effective treatment for murine lupus. Sci Transl Med. 2019;11:eaav1648. doi: 10.1126/scitranslmed.aav1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Jin X, Xu Q, Pu C, Zhu K, Lu C, Jiang Y, Xiao L, Han Y, Lu L. Therapeutic efficacy of anti-CD19 CAR-T cells in a mouse model of systemic lupus erythematosus. Cell Mol Immunol. 2021;18:1896–1903. doi: 10.1038/s41423-020-0472-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Mougiakakos D, Krönke G, Völkl S, Kretschmann S, Aigner M, Kharboutli S, Böltz S, Manger B, Mackensen A, Schett G. CD19-Targeted CAR T Cells in Refractory Systemic Lupus Erythematosus. N Engl J Med. 2021;385:567–569. doi: 10.1056/NEJMc2107725. [DOI] [PubMed] [Google Scholar]
- 105.Filippi M, Bar-Or A, Piehl F, Preziosa P, Solari A, Vukusic S, Rocca MA. Multiple sclerosis. Nat Rev Dis Primers. 2018;4:43. doi: 10.1038/s41572-018-0041-4. [DOI] [PubMed] [Google Scholar]
- 106.Fransson M, Piras E, Burman J, Nilsson B, Essand M, Lu B, Harris RA, Magnusson PU, Brittebo E, Loskog AS. CAR/FoxP3-engineered T regulatory cells target the CNS and suppress EAE upon intranasal delivery. J Neuroinflammation. 2012;9:112. doi: 10.1186/1742-2094-9-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.De Paula Pohl A, Schmidt A, Zhang AH, Maldonado T, Königs C, Scott DW. Engineered regulatory T cells expressing myelin-specific chimeric antigen receptors suppress EAE progression. Cell Immunol. 2020;358:104222. doi: 10.1016/j.cellimm.2020.104222. [DOI] [PubMed] [Google Scholar]
- 108.Frisoli ML, Essien K, Harris JE. Vitiligo: mechanisms of pathogenesis and treatment. Annu Rev Immunol. 2020;38:621–648. doi: 10.1146/annurev-immunol-100919-023531. [DOI] [PubMed] [Google Scholar]
- 109.Mukhatayev Z, Dellacecca ER, Cosgrove C, Shivde R, Jaishankar D, Pontarolo-Maag K, Eby JM, Henning SW, Ostapchuk YO, Cedercreutz K, et al. Antigen specificity enhances disease control by tregs in vitiligo. Front Immunol. 2020;11:581433. doi: 10.3389/fimmu.2020.581433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Aghajanian H, Kimura T, Rurik JG, Hancock AS, Leibowitz MS, Li L, Scholler J, Monslow J, Lo A, Han W, et al. Targeting cardiac fibrosis with engineered T cells. Nature. 2019;573:430–433. doi: 10.1038/s41586-019-1546-z. [DOI] [PMC free article] [PubMed] [Google Scholar]