Abstract
In the era of immunotherapeutic control of cancers, many advances in biotechnology, especially in Ab engineering, have provided multiple new candidates as therapeutic immuno-oncology modalities. Bispecific Abs (BsAbs) that recognize 2 different antigens in one molecule are promising drug candidates and have inspired an upsurge in research in both academia and the pharmaceutical industry. Among several BsAbs, T cell engaging BsAb (TCEB), a new class of therapeutic agents designed to simultaneously bind to T cells and tumor cells via tumor cell specific antigens in immunotherapy, is the most promising BsAb. Herein, we are providing an overview of the current status of the development of TCEBs. The diverse formats and characteristics of TCEBs, in addition to the functional mechanisms of BsAbs are discussed. Several aspects of a new TCEB-Blinatumomab-are reviewed, including the current clinical data, challenges of patient treatment, drawbacks regarding toxicities, and resistance of TCEB therapy. Development of the next generation of TCEBs is also discussed in addition to the comparison of TCEB with current chimeric antigen receptor-T therapy.
Keywords: Bispecific antibodies, Cancer immunotherapy, T cell engager, Blinatumomab, Chimeric antigen receptor T cell therapy, Adverse effects
DEFINITION OF BISPECIFIC ANTIBODY (BsAb): HISTORICAL PERSPECTIVES
The original concept of BsAbs was first proposed by Alfred Nisonoff in the 1960s. He combined 2 different antigen binding sites in one molecule and obtained a F(ab’)2 molecule with dual specificity under mild reoxidation from a mixture of anti-bovine gamma globulin) and anti-ovalbumin monovalent fragments (1). He also proved the bispecificity through microscopy (2). In 1975, hybridoma technology was invented by Köhler and Milstein (3), which finally solved the problem of producing pure monoclonal Abs (mAbs) and opened a new era of mAb therapy. In 1983, hybrid-hybridoma (quadroma) technology was pioneered by Milstein and Cuello (4), which is based on the somatic fusion of 2 different hybridoma cell lines secreting a mixture of Abs, including a murine IgG-form BsAbs with dual specificities. However, the low yield of BsAbs (12.5% from random pairing of heavy and light chains) and the difficulty in purifying the desired Ab from the closely related mispaired by-products were significant problems (5). A major improvement to the conventional quadroma approach was the somatic fusion of a murine and a rat hybridoma cell line expressing mAbs with 2 IgG subclasses selected for their preferential pairing (6). Because this BsAb from ‘murine-rat’ quadroma cells contains a Fragment crystallizable (Fc) section, it can be considered ‘trispecific’ due to an additional interaction with Fcγ receptors. Bispecific (Fab’)2 fragments were prepared by removing Fc part through enzymatic digestion (7). Other approaches used chemical conjugation of 2 different mAbs or smaller Ab fragments (8). However, these chemical methods suffered from significant heterogeneity issues because the crosslinking reaction with the parental Abs was not site-specific.
In 1988, James Huston and his colleagues invented the single-chain variable fragment (scFv), which minimized the refolding problems, such as incorrect domain pairing or aggregation of 2-chain species (9). In 1996, knobs-into-holes technology was invented using recombinant DNA technology by scientists at Genentech, overcoming the limitations of quadroma technology; the mispairing of IgG heavy chains was reduced by mutating selected amino acids at the interface between CH3 domains of human IgG. This technology involves positions within the CH3 domain interface: an amino acid with a small side chain (threonine) is introduced, replacing a bulky amino acid to form the ‘hole,’ and an amino acid with a large side chain (tyrosine) is introduced on to the other chain to form the ‘knob.’ Thus, the more favorable protein interaction between ‘knob and hole’ chains leads to the formation of up to 90% of the correct bispecific heavy chain pairing from the transfected mammalian cells (10). Subsequently, with the progress in Ab engineering and biology, the diverse concept and constructs of BsAbs are evolving.
CLINICAL APPLICATIONS OF BsAb
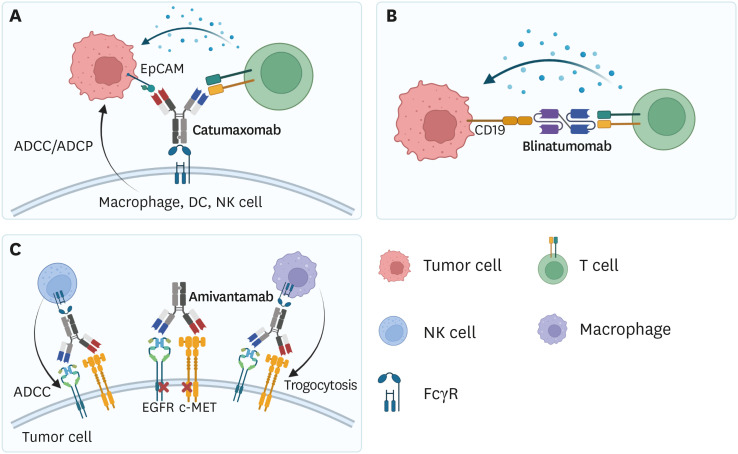
Numerous BsAbs have been developed as therapeutics for solid and hematologic cancers. As of December 2021, 3 types of the Food and Drug Administration (FDA)-approved BsAbs are used in clinics to treat cancer (Fig. 1).
Figure 1. Mode of actions of BsAbs used in clinics. (A) Catumaxomab binds to EpCAM-positive tumor cells and T cells, inducing T cell-mediated cytotoxicity. Fc region of Catumaxomab could bind to macrophages, DCs, and NK cells, resulting in ADCC or ADCP. (B) Blinatumomab engages CD19-positive tumor cells and T cells, inducing T cell-mediated tumor cell killing. (C) Amivantamab simultaneously targets EGFR and c-MET to downregulate the oncogenic signals by internalizing receptors.
MET, mesenchymal-epithelial transition factor.
Catumaxomab
Catumaxomab (Removab) was developed by Fresenius Biotech and Trion Pharma and approved by the European Medicines Agency (EMA) in 2009. Catumaxomab is a murine asymmetric full-length Ab that simultaneously targets epithelial cell adhesion molecule (EpCAM) and CD3 and treats malignant ascites with ovarian cancer. Catumaxomab engages EpCAM+ tumor cells and T cells, inducing T cell-mediated tumor cell killing. The Fc region of Catumaxomab binds to Fc receptors (FcRs) on macrophages, dendritic cells (DCs), or NK cells, inducing Ab-dependent cell cytotoxicity (ADCC) or Ab-dependent cell phagocytosis (ADCP) of tumor cells. However, catumaxomab generated anti-drug Abs, as it is a murine Ab form (11).
Blinatumomab
Blinatumomab (Blincyto) was developed by Amgen and was approved by the FDA in 2014 for the treatment of relapsed/refractory B-acute lymphoblastic leukemia (B-ALL) or Philadelphia chromosome-negative B-ALL. Blinatumomab was also approved to treat persistent minimal residual disease (MRD) of hematologic malignancies in 2018. Unlike Catumaxomab, blinatumomab is a bispecific T cell engager (BiTE) consisting of conjugated scFv targeting CD3 and CD19. Therefore, blinatumomab conjugates CD19-expressing tumor cells and T cells, resulting in tumor lysis. Blinatumomab treatment induced a complete response in 78% of adult patients with MRD-positive ALL who received chemotherapy. Also, blinatumomab therapy showed enhanced overall survival compared to that with standard chemotherapy (7.7 vs. 4.0 months) (12,13,14).
Amivantamab
Amivantamab (Rybrevant) was developed by Janssen and approved by the FDA in May 2021. Amivantamab is used to treat adult patients with locally advanced or metastatic non-small cell lung cancer (NSCLC) with epithelial growth factor (EGFR) exon 20 insertion mutations. Unlike other BsAbs that engage tumor-associated antigen (TAA) and T cells, amivantamab is an asymmetric IgG that simultaneously binds to EGFR and mesenchymal-epithelial transition factor on tumor cells. Binding of Abs induces heterodimerization and internalization of the 2 proteins, resulting downregulation of oncogenic signaling in tumor cells. Moreover, the Fc region binds to FcRs on NK cells to mediate ADCC, or on macrophages to mediate trogocytosis, a process which extracts receptor molecules from the tumor cell surfaces to the immune cell surfaces. In a clinical trial involving 81 patients with NSCLC, the overall response was 40%, with 3 complete and 29 partial responses. Total clinical benefit was 74%, with a median progression-free duration of 8.3 months and overall survival of 22.8 months, although longer follow-up data is required (15,16,17).
FUNCTIONAL MECHANISMS OF BsAb
BsAb can be categorized into 4 groups based on their functional mechanism: two epitope bindings with one antigen, cell-cell engagers, dual functional modulators, and BsAbs in cell therapy. Among these, the cell-cell engager is the most developed form in clinics and has been shown to induce tumor-specific immune cell activation. Therefore, optimal selection of the TAA is necessary for treatment efficacy and avoidance of adverse effects.
Two epitope bindings within one antigen
A biparatopic Ab that simultaneously targets 2 different epitopes within the same target antigen was developed (18,19). For example, BsAbs targeting carcinoembryonic antigen (CEA) or VEGF2 were developed to target cancer (20,21). Several BsAbs were also developed to neutralize viral antigens. For example, BsAbs targeting surface antigen of hepatitis B virus and HIV-1 envelop protein showed neutralizing activity against cognate antigens (22,23).
Cell-cell engagers
Cell-cell engagers link 2 different types of cells, mainly tumors and T/NK cells, to induce tumor cell lysis. Cell-cell engagers consist of a TAA targeting moiety and an effector cell recognizing moiety and are further divided into T cell engagers and NK cell engagers.
T cell engager
BiTEs, also known as BiTEs, employ TAA and TCR components (mainly CD3), which link tumor cells and T cells, bypassing TCR-MHC I interactions. Thus, BiTEs trigger T cell activation to produce cytotoxic molecules including perforin and granzyme, regardless of antigen specificity. BiTEs are further divided into conventional BiTEs and subset specific BiTEs (24,25).
1) Conventional BiTEs
Conventional BiTEs are a form of TAA×CD3, engaging tumor and T cells for T cell-mediated tumor cell killing. CD19-targeting blinatumomab is a typical example of BiTEs in clinics. More than 60 BiTEs are currently in phase I/II clinical trials. Pasotuxizumab (prostate-specific membrane antigen [PSMA]×CD3) simultaneously binds to PSMA expressed in prostate cancer and CD3. Pasotuxizumab treatment showed antitumor activity against metastatic castration-resistant prostate cancer. Also, pasotuxizumab induced complete regression of soft-tissue metastases and marked regression in bone metastases in a phase I trial. This was the first clinical study suggesting that BiTE immunotherapy could be effective in solid tumors (26,27). AMG 596 (EGFRvIII×CD3) engages EGFRvIII-positive tumor cells and T cells and exerts antitumor activity against glioblastoma. In a preclinical study, AMG 596 treatment effectively enhanced overall survival in EGFRvIII-expressing orthotopic tumors (28,29).
2) Subset specific BiTEs
A recent study revealed that γδ T cells, not only CD8+ cells, are involved in antitumor immune responses in glioblastoma. This study highlighted the importance of minor T cell subsets in cancer immunotherapy (30). γδ T cells can induce MHC-non-restricted tumor cell killing via recognition of pyrophosphate, which is overproduced in various tumors. Due to these advantages, γδ T cell-redirecting approaches are currently under development. Human epidermal growth factor receptor 2 (HER2)2×Vγ9 tribody redirects Vγ9 T cells to HER2-expressing tumor cells, inducing γδ T cell-mediated tumor cell killing (31). Anti-TRGV9/anti-CD123 simultaneously binds to the Vγ9 chain of Vγ9Vδ2+γδ TCR and acute myeloid leukemia (AML) target antigen CD123, recruiting Vγ9+ γδ T cells to AML blast cells. Recruited γδ T cells induce effective tumor cell killing via cell cytotoxicity, without inducing a cytokine storm, unlike pan-T cell redirection (32).
NK cell engager
NK cell engagers redirect CD16A+ NK cells to tumor cells, inducing NK cell activation. NK cell engagers show fewer adverse effects, such as cytokine release syndrome (CRS) and neurotoxicity, than BiTEs (33). Multiple cytotoxic receptors can activate NK cells: CD16 (FcγRIII), natural cytotoxicity receptors (NCRs; NKp30, NKp44, NKp46), C-type lectin-like receptors NKG2D (CD314), and CD94/NKG2C. Among these, CD16A is an activating receptor expressed on NK cells and macrophages, and binding of the Fc region induces ADCC of target cells (34). NK cell engagers are divided into tandem scFv-based bispecific killer cell engagers (BiKEs) and TAA-specific scFv fused to anti-CD16A, also referred to as trispecific killer cell engager (TriKE).
1) BiKE
BiKE activates NK cells by linking TAA and NK cells. CD16×CD133 is a fully humanized scFv-based BsAb, recognizing CD16 on NK cells and CD133 on cancer stem cell (CSC) of colorectal carcinoma. CD16×CD133 showed cytotoxicity against the NK-resistant human Burkitt’s lymphoma Daudi cell line (35). CD16×CD33 simultaneously binds to CD16 and CD33 expressed on pediatric AML and biphenotypic ALL. CD16×CD33 could induce the effector function in the CD33lo cell line, increasing the production of cytotoxic receptor CD107a and cytokines IFN-γ and TNF-α in a dose-dependent manner (36).
2) TriKE
TriKE is a BiKE molecule, which is crosslinked with IL-15. IL-15 moiety improves the proliferation and survival of NK cells by inducing superior NK cell cytotoxicity and degranulation (37). The 161519 (CD16×IL-15×CD19) TriKE recruits CD19+ tumor cells to NK cells, promoting tumor cell killing. “Armed” NK cells show stronger cytolytic activity than unarmed NK cells. In a preclinical B-cell lymphoma model of human peripheral blood mononuclear cell-reconstituted xenograft mice, 161519 TriKE treatment increased tumor growth inhibition and overall survival compared to CD16×CD19 BiKE treatment (38). Moreover, 1615133 (CD16×IL-15×CD133) TriKE provides IL-15 signaling, which supports the proliferation and survival of NK cells, inducing selective elimination of CSC of 16133 BiKE (39). HER2 TriKE showed antitumor efficacy against human ovarian cancer in a mouse xenograft model (40).
Dual functional modulators
Dual functional modulators simultaneously bind to 2 different immune co-stimulatory or co-inhibitory molecules, inducing functional changes in target cells. Functional mechanisms are further divided into 3 types based on the binding molecule.
Double functional inhibition
Double functional inhibitors induce inhibition in target cells by binding to 2 different immune checkpoint molecules. Most of them target PD-1 or PD-L1 proteins and other immune inhibitory molecules, such as CTLA-4 (CD152), T cell immunoglobulin, mucin domain-containing protein 3 (TIM-3), lymphocyte activation gene-3 (LAG-3), or TGF-β. MEDI5752 (PD-1×CTLA-4) inhibits the function of CTLA-4 in PD-1+ activated T cells, inducing rapid internalization and degradation of PD-1. Also, MEDI5752 showed enhanced activity compared to that of the combination of PD-1 and CTLA-4 mAb treatment (41,42). Functional inhibitors targeting other checkpoint molecules, including LAG-3 and TIM-3, are under development (43,44).
Double functional stimulation
Double functional stimulators provide immune co-stimulation signals in target cells by simultaneously binding to different co-stimulatory molecules. FS120 (CD137×OX40) targets CD137 (4-1BB) and OX40 (CD134), which belong to the tumor-necrosis factor receptor superfamily. Moreover, FS120 initiates CD4+ and CD8+ T cell activation by FcγR in an independent manner. FS120 treatment showed antitumor efficacy in a syngeneic mouse tumor model with low liver T cell infiltration compared to that of CD137 agonistic mAb treatment (45). GEN1042 (Duobody®-CD40×4-1BB) simultaneously binds to CD40 and 4-1BB, enhancing the priming and (re)activation of tumor-specific immune cells. Thus, GEN1042 treatment resulted in disease control in 51% (25/49) patients with advanced solid tumors and showed a favorable safety profile in phase I/II clinical trials. A clinical trial of combination therapy of GEN1042 and PD-1 blockade is in progress (46), in addition to various other dual co-stimulators.
Dual inhibition and stimulation
BsAbs that simultaneously target co-stimulation and co-inhibition pathways are also under development. ATOR-1015 (CTLA-4×OX40) is an agonistic IgG1 Ab of OX40 with an Ig-like V-type domain of CD86. ATOR-1015 showed T cell activation and Treg-depleting activity in vitro. In a syngeneic model of bladder, colon, and pancreatic cancer, ATOR-1015 treatment reduced tumor growth and increased survival rate by increasing CD8+ T cell activation and number with reduced frequency of Tregs (47). ABL503 (PD-L1×4-1BB) binds to 4-1BB and PD-L1, inducing 4-1BB signaling in the context of PD-L1. ABL503 invigorates tumor-infiltrated CD8+ T cells by blocking PD-1/PD-L1 signaling and showed antitumor responses in humanized PD-L1/4-1BB transgenic mice model without liver toxicity (48).
BsAbs in adoptive cell therapy
While chimeric antigen receptor (CAR)-T cell therapy shows efficacy in hematological malignancies, side effects, including CRS, neurotoxicity, and on-target off-tumor effects, are also observed. To minimize adverse effects, studies to prime T cells ex vivo with BsAbs are in progress. Therapy with T cells armed (primed) ex vivo by BsAbs induced robust antitumor responses with effective infiltration into tumor site and reduced cytokine release compared to that by separate BsAb and T cell infusion, thereby minimizing systemic adverse effects (49). OKT3×hu3F8 BsAb armed T cells (GD2BATs) induced specific killing of GD2-positive neuroblastoma and osteosarcoma cell line in vitro. In phase I trials for GD2-positive tumor patients, GD2BATs showed responses in some patients without significant side effects (50). Recent studies focused on combining the BsAb arming strategy to induce non-MHC-restricted cytotoxicity in headless CAR-T (hCART) cells, which only contain the transmembrane, intracellular domain of co-stimulatory receptors, in addition to TCR signaling CD3ζ domains, without extracellular scFv CAR domains. Targeting ability of hCART cells is determined by arming them with BsAbs, and they could be armed with one or more BsAbs to target multiple tumor antigens (51).
DIVERSE FORMATS OF BsAb
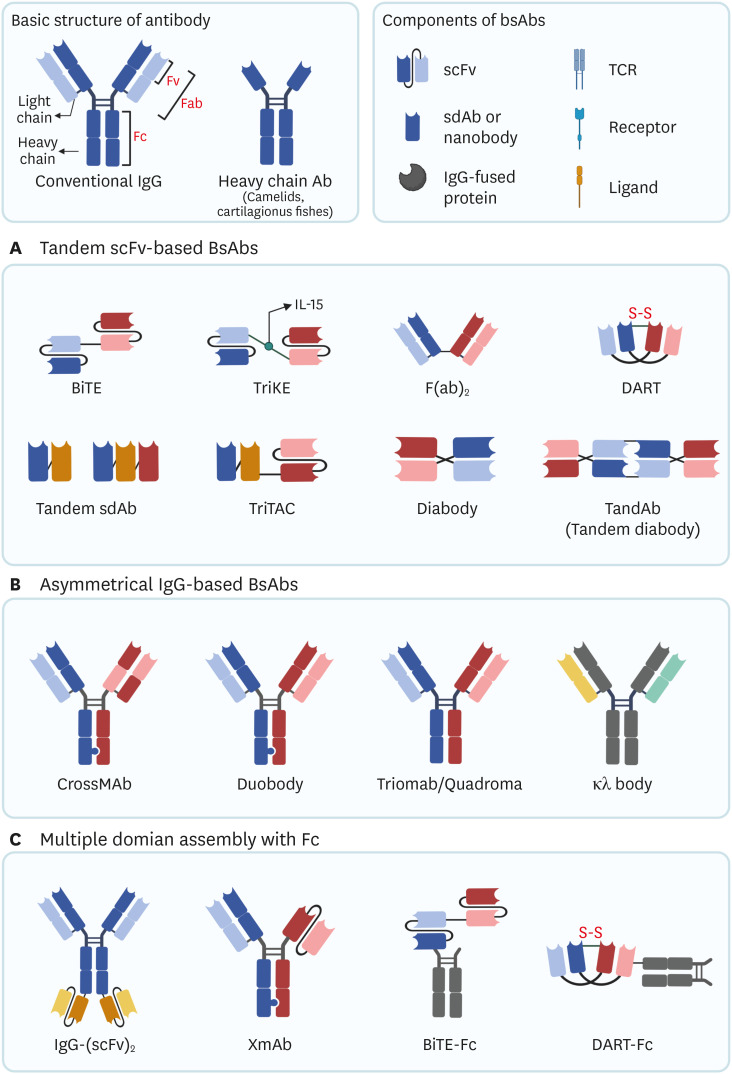
Available formats of BsAbs are shown in Fig. 2. BsAbs are grouped into 3 categories based on the components.
Figure 2. Available formats of BsAbs. Conventional IgG consists of 2 heavy chains and 2 light chains linked by 4 disulfide bonds. Heavy chain Ab is only comprised of heavy chains. Most bsAbs consist of Ab-based fragments, such as scFv, sdAb (nanobody), Fv, Fab, and Fc. Some non-Ab-based proteins, such as TCR, receptor, and ligand can be components of bsAbs. (A) Examples of tandem scFv-based bsAbs. (B) Examples of asymmetric IgG-based bsAbs. These bsAbs are constructed by paring 2 different heavy chains and lights chains (heterodimeric bsAbs). (C) Examples of multiple domains assembled with Fc. Fc domain is conjugated with scFvs, BiTE, or DART.
DART, dual affinity retargeting.
Tandem scFv-based
Tandem scFv-based BsAbs consist of 2 or more scFvs to form tandem scFv. scFvs could be fused with another scFv to form immunological synapses. BiTE, DART, and Diabody belong to this class. Because molecular weight of tandem-scFv-based BsAbs is low, serum half-life is generally short. Single domain Abs (sdAbs) of heavy chain Abs could be engineered to form tandem sdAbs.
Asymmetrical IgG-based
Asymmetrical IgG-based Abs mainly have a full-length Ab structure, which have a longer half-life than tandem scFv-based BsAbs. Each Ab binding fragment of asymmetrical IgG simultaneously targets 2 different epitopes. For generation of heterodimeric Abs, proper paring of different Ab chains is necessary. Knobs-into-holes technology significantly enhanced the development of asymmetric BsAbs (52). Other heterodimerization technologies, such as Charge Repulsion Improved Bispecific (CRIB™) platform enables correct paring of the Ab chains (53).
Multiple domains assembled with Fc
Fc domain of the Ab binds to FcγR and induces effector function, such as ADCC or ADCP (54). Fc domain assembly could provide additional effector function or increase the short half-life of scFv-based Abs (55). The Fc domain of an Ab not only binds to scFv-based BsAbs, but also conjugates with other proteins. B7-H3×4-1BB BsAb has a 4-1BB binding scFv conjugated with Fc region of B7-H3 targeting Ab. Because B7-H3 is overexpressed on various tumor cells, it induces tumor antigen-dependent 4-1BB crosslinking, reducing severe liver toxicity due to 4-1BB mAbs (56).
T CELL ENGAGING BsAb (TCEB) IN CLINICAL DEVELOPMENT
Immune cells engaging BsAbs
One of the major applications of BsAbs has been the redirection of cytotoxic immune cells toward disease-related target cells that have a key function in disease processes. Virus-infected cells or cancer cells are the major targets during the development of BsAbs, wherein one Ab component recognizes cancer-associated surface antigens, such as CD19, CD20, HER2, CEA, and EpCAM, or viral antigens, and the other recognizes the surface protein of immune cells. With respect to cytotoxic immune cells, a variety of Abs against specific molecules on cytotoxic effector cells, such as CD64, CD89, CD16 and CD3, have been tested for the development of immune cell engaging BsAbs, of which TCEB has been the most successful application.
BsAbs targeting T cells
CD3 is a key component of the TCR complex and consists of 3 chains (CD3ε, CD3δ, CD3γ) which trigger T cell activation signals when TCR binds to MHC-peptide complex on the surface of antigen-presenting cells. Moreover, it is well-known that tumor-specific T cells represent a crucial element of the body’s immune surveillance against cancer (57). Significant positive correlations between the presence and number of CD8+ T cells in tumors and long-term survival have been observed for non-Hodgkin lymphoma (NHL), ovarian cancer, and colorectal cancer patients (58,59,60). Therefore, CD3 has been chosen as the key triggering molecule in most BsAb-related approaches aimed at redirecting cytotoxic T cell activity toward tumor cells. CD3-engaging BsAbs were able to mediate TCR activation without specific clonotypic interaction between TCR and tumor antigens, but they needed the activation of other costimulatory molecules, such as CD28, to achieve full T cell activation. Indeed, most CD3-directed BsAbs require co-stimulation or pre-stimulation of T cells to elicit cytotoxic activity against target (tumor) cells in in vitro and in vivo models. This dependency applies to various BsAb formats from the conventional bispecific F(ab’)2 format to various recombinant diabodies (61,62,63,64,65,66,67). Further, most T cell-engaging BsAbs require a high number of effector cells for significant tumor cell lysis (E:T ratio of at least 2:1), and a broad range of BsAb concentrations is reported for efficient induction of redirected T cell cytotoxicity (68,69,70).
BiTEs are recombinant bispecific single-chain Abs consisting of 2 sequential scFv Ab fragments directed against a surface target antigen on disease cells (cancers) and CD3 on T cells. BiTEs can be efficiently produced recombinantly as fully functional molecules by mammalian cells with high productivity. In contrast to other CD3-directed BsAbs, BiTEs can efficiently induce the cytotoxic activities of T cells against different target cells without any requirement for pre- or co-stimulation of effector T cells (71,72,73,74); this can be explained by the fact that BiTEs can induce immunological cytolytic synapses between target and cytotoxic T cells that are indistinguishable from synapses induced by conventional T cell activation in terms of composition, subdomain arrangement, and size. The engagement of only a few TCR molecules per T cell is sufficient to form an immune synapse to induce efficient T cell cytotoxicity (75), similarly only a few BiTE molecules binding to target and T cells are sufficient to kill the target cells. This is in line with the observation that BiTE concentrations as low as 10–100 pg/ml (up to 0.2–2 pM) are usually sufficient for half-maximal target-cell lysis.
Clinical usage of Blinatumomab
Blinatumomab, a bispecific CD19-directed CD3 T-cell engager (BiTE) mAb, was originally developed from German biotech, Micromet, Inc. in late 1990s, which was acquired by Amgen in 2012 when blinatumomab was in phase 2 trial on B-ALL.
It consists of 2 scFvs that target CD3 and CD19 which are covalently connected by small linker peptides (GGGGS). Its small size (up to 55kDa) and highly flexible linker allowed the close interaction between immune effector and cancer cells, resulting the formation of a lytic immune synapse between them. The omission of Fc domains of Ab in BiTEs has pharmacokinetic implications, which might hamper the advantages of long half-life in serum but avoid the FcR-mediated toxicities (72).
Because of its small size and the omission of Fc domain, blinatumomab is given as an intravenous (IV) infusion through a central venous catheter or implantable port using bags with mixed solution of the medication containing tiny amounts of the drug (28 μg/day or less) and usually given continuously (24 h a day) for 4 wk followed by 2-wk break without infusion. It may be given at home using a portable infusion pump too. Total number of treatment cycle (4 wk IV + 2 wk off) is determined through reviewing the tumor responses (76). Despite of such inconvenience on the patient treatment, blinatumomab has demonstrated remarkable clinical efficacies and approved by FDA for the treatment of both children and adults with R/R B-ALL in 2014 and 2017. In 2018, blinatumomab gained accelerated approval to treat B-ALL patients with MRD, the status of persistent leukemia cell detection after complete remission by anti-cancer treatment, which has been the most important risk factor for hematologic relapse in T- and B-ALL.
Adverse effects of blinatumomab treatment
In a phase II study for R/R B-ALL patients, the common adverse events during blinatumomab treatment included infection, pyrexia, fatigue, headache, tremor, and leukopenia, neurological adverse events and CRS. Most of the adverse events occurred during the first cycle of administration (77). However, a comparison of the adverse effects observed in blinatumomab group with those in the chemotherapy group in a clinical trial for advanced ALL patients revealed that although the blinatumomab-treated patients suffered from more adverse effects, the incidence of severe adverse effects in the blinatumomab group was lower than that in the chemotherapy group (78).
Severe CRS and neurological adverse events are the main reasons for the interruption of blinatumomab therapy. CRS is caused by the release of large amounts of cytokines and the subsequent systemic inflammation, with clinical manifestations that include high fever, skin rash, vomiting, and nausea (79). These problems are considered to originate as a result of abnormal activation of effector T cells and macrophages by blinatumomab treatment, wherein the activated T cells release high levels of IFN-γ and other cytokines, and induce macrophages to release high levels of IL-6 and IL-10. Patients with severe CRS even suffer from hemophagocytic lymphohistiocytosis (HLH) (80). IL-6 is at the center of this pathological process. Other cytokines, including TNF, IL-2, GM-CSF, and IL-5, also participate in this process (81,82). Severe CRS can be prevented by the administration of dexamethasone prior to blinatumomab treatment and stepwise dosing (77,83,84). Tocilizumab, an anti-IL-6 receptor mAb, is effective in patients with HLH (80).
A neurotoxicity syndrome, now termed immune effector cell-associated neurotoxicity syndrome (ICANS), is another major adverse effect associated with T cell-engaging therapies. ICANS presents with a broad variety of symptoms. Serious neurological adverse events occur in 9%–26% of patients treated with blinatumomab (13,85,86,87,88,89,90) in a dose-dependent manner (91). The neurological adverse events associated with BiTE treatment are attributed to the redistribution of activated T cells across the blood–brain barrier. However, the underlying mechanism of ICANS is not well understood. Activated T cells are hypothesized to interact with the blood–brain barrier, consequently disrupting the protective barrier to induce local inflammation in the CNS and finally leading to a clinically apparent toxic encephalopathy-like syndrome (92). Patients with diminished B/T cell ratios are more likely to suffer neurological adverse events (77). Although neurological adverse events can occur in patients with CRS, there is still no evidence for their association with the release of cytokines. (90). The neurological symptoms can be controlled after the withdrawal of blinatumomab treatment (93). Severe neurological events can be prevented by the administration of steroids in advance and close clinical monitoring (94).
Some patients receiving blinatumomab have a mild to severe allergic reaction to the medicine. Signs of mild allergic response include skin rashes and itching, high temperature, shivering, redness of the face, dizziness, or headache and those of a severe response include any of the above as well as shortness of breath.
CAR-T CELL THERAPY vs. BiTE THERAPY
Both BiTEs and CARs can manifest T cell-mediated killing of malignant cells by redirecting autologous T lymphocytes to surface antigens on cancer cells and have demonstrated dramatic cancer-killing effects in patients with hematologic cancers. A CAR is a synthetic receptor comprising an extracellular tumor antigen targeting the scFv, which is fused to the CD3ζ chain for intracellular signaling and contains one or more costimulatory domains from CD28 or 4-1BB. Binding of CAR to tumor antigens triggers the activation of TCR intracellular domains and leads to T cell responses against antigen-expressing cells. Various molecular formats of CAR, such as the diverse formats for BiTEs, differing in their extracellular transmembrane and cytoplasmic domains have been developed. Five CAR-T therapies have been approved by the FDA: Kymriah for ALL and B-cell lymphoma, Yescarta for B-cell lymphoma and follicular lymphoma, Tecartus for mantle cell lymphoma, Abecma for myeloma, and Breyanzi for B-cell lymphoma. Recent advances and positive clinical results of BiTE and CAR-T therapies have generated new hope. However, both therapies are facing similar dilemmas, such as toxicity concerns in hematologic cancers and insufficient responses in solid cancers. Characteristics of both therapies are summarized in Table 1.
Table 1. Comparison of characteristics between CAR-T cells and BiTE therapy.
| Characteristics | CAR-T cell | BiTE |
|---|---|---|
| Structure | • A synthetic gene construct encoding an scFv against tumor antigen linked to activation and costimulatory motifs. | • A recombinant protein composed of 2 linked scFvs; one binds to CD3 on T cells and the other to target a tumor antigen on tumor cells. |
| Effector cell types | • Engineered CD8+ and CD4+ T cells. Less-differentiated subsets displaying better antitumor activity in vivo (TSCM and TCM). | • Endogenous CD8+ and CD4+ T cells. Antigen-experienced TEM but not TN effective. |
| Immune synapse | • Atypical. | • Typical. |
| Serial killing | • Yes. | • Yes. |
| Killing mechanisms | • Perforin and granzyme B, Fas/Fas-L, or TNF/TNF-R. | • Perforin and granzyme B. |
| Trafficking | • Active. Trafficking of CAR-T cells involves comprehensive interactions between various molecules and cell-cell interactions. | • Passive. Biodistribution depends on factors related to rates of diffusion through vascular endothelium, fluid flow rates, and interaction with target. |
| Toxicity | • CRS, neurotoxicity, B-cell aplasia. | • CRS, neurotoxicity, B-cell aplasia. |
| Clinical applications | • Pretreatment lymphodepleting regime using cyclophosphamide and fludarabine. | • No lymphodepletion regime required. Premedicate with dexamethasone. Repeat administration necessary, including continuous IV infusion regimens. |
| • Premedicate with acetaminophen and an H1-antihistamine. One infusion. | ||
| FDA approval | • Yescarta was approved to treat adult patents with relapsed/refractory large B-cell lymphoma in 2017. | • Blinatumomab was approved to treat relapsed/refractory B-ALL in 2014 and 2017. |
| • Kymriah was approved to treat patients up to 25 years of age with refractory/relapsed B-ALL in 2017. | ||
| • Tecartus was approved for the treatment of adult patients diagnosed with MCL in 2020. | ||
| • Abecma was approved to treat multiple myeloma targeting BCMA in 2021. | ||
| • Breyanzi was approved for the treatment of adult patients with relapsed or refractory LBCL in 2021. | ||
| Other characteristics | • Individually produced for each patient. | • “Off the shelf” reagents. |
MCL, mantle cell lymphoma; BCMA, B-cell maturation antigen; LBCL, large B-cell lymphoma.
Complications in CAR-T and BiTE therapies
Even though CAR-T and BiTE therapies showed profound anti-tumor activities in several cancers, their efficacies are limited and need further improvements for broader applications. There are several factors that hamper the activities of CAR-T- and BiTE-mediated interactions of activated T cells with tumors and tumor microenvironments (TMEs).
Major adverse effect of CAR-T and BiTE therapies
Two main toxicities associated with CAR-T therapy, CRS and neurological adverse effects, are similar to those observed in BiTE therapy. However, their clinical aspects showed slight incidence dissimilarity, the detailed mechanisms of which are not fully understood. In general, CAR-T therapy shows higher level of CRS events. In the clinical trials for patients with ALL or NHL, the incidence of grade ≥3 CRS with T cell engager, blinatumomab, ranges from 0% to 6% across various settings (13,77,85,89,90,91), while the incidence with CD19-directed CAR-T therapy is relatively high, ranging from as low as 2% in R/R large B-cell lymphoma studies with JCAR017 from Juno Therapeutics to approximately 47% in R/R ALL studies with Kymriah from Novartis (95,96,97,98,99).
With respect to the rates of grade ≥3 neurological adverse events in clinical trials, blinatumomab showed higher rates of grade ≥3 neurological adverse events with a dose-dependent increase in adverse events from 22% to 26% in the NHL trials when administered at much higher doses (up to 112 μg/day), whereas the rate of grade ≥3 NE was only 9% in the TOWER trial for ALL patients, in which the patients received up to 28 μg/day blinatumomab (85,89,91). In the clinical trials for CAR-T therapy, the rates of grade ≥3 NE were 5%–13% for ALL patients and 10%–28% for NHL (13,100,101,102,103).
On-target/off-tumor-related toxicities
A primary requirement for successful BiTE therapy is the identification of appropriate TAAs expressed on target cells other than those expressed on normal cells to avoid on-target/off-tumor toxicity. However, many tumor antigens found in solid tumors lack perfect specificity and often show low level expression on normal tissues, for which BiTEs can bind and deliver cytotoxic T cells with off-tumor toxicity (104). Such limitations have been seen with BiTEs such as solitomab (MT110, AMG 110), a BsAb targeting EpCAM, an antigen frequently overexpressed in solid tumors but also expressed at lower levels in normal tissue, particularly the gastrointestinal tract. The phase 1 study for solitomab could not determine an adequate dose due to the occurrence of dose-limiting toxicities, such as transaminitis and diarrhea (105).
Sporadic efficacy in solid tumor indications
Unfortunately, the potent killing activities of BiTEs against hematologic cancers have not been replicated in the clinical studies for solid tumors, and this remains a challenge (106). The first evidence for the clinical activity of BiTEs in solid tumors came with the approval of catumaxomab for the intraperitoneal treatment of malignant ascites in adult patients with EpCAM+ carcinoma by the EMA in 2009, where large phase II/III studies showed positive results in terms of time to next paracentesis and signs and symptoms of ascites (107,108). However, when catumaxomab was tested for systemic administration, the results were not encouraging. A phase I study revealed dose-dependent hepatotoxicity of different grades, in which one patient experienced fatal acute liver failure due to the off-target binding of catumaxomab to FcR expressed by Kupffer cells in the liver, inducing local cytokine release and T cell-mediated hepatotoxicity (109). Ertumaxomab, an HER2×CD3 BsAb produced by quadroma, showed encouraging phase I study results for patients with HER2-positive metastatic breast cancer, and strong immunogenic response to tumors were induced in a third of patients (110). Pasotuxizumab (PSMA×CD3; AMG 212) showed an acceptable safety profile and dose-dependent clinical activity in a phase I study involving patients with metastatic castration-resistant prostate cancer, which was the first clinical study to show that BiTEs therapy can be efficacious in solid tumors. However, no subsequent clinical trials have been done (111).
Stimulation of immunosuppressive cells in TME
Upregulation of inhibitory immune checkpoint molecules, such as PD-1/PD-L1, CTLA-4, LAG-3, TIM-3, T cell immunoglobulin and ITIM domain, and V-domain Ig suppressor of T cell activation, on tumor and immune suppressor cells represents another hurdle for T cell engagers. These immune suppressor cells include Tregs, myeloid-derived suppressor cells (MDSCs), and tumor-associated macrophages (TAMs), whose immune inhibitory functions are attributed to either direct interaction with activated T effectors or the release of inhibitory factors, such as TGF-β, IL-10 (112), IL-35 (113), PGE2 (114), ARG1 (115), inducible nitric oxide synthase (116), and cyclooxygenase 2 (117). TAMs also release tumor-supporting factors (e.g., EGF, IL-6, and TNF), extracellular matrix-degrading enzymes (e.g., matrix metalloproteinases and cysteine cathepsins), and proangiogenic molecules that enable nutrient and oxygen delivery to tumors (e.g., vascular endothelial growth factor A, a chemotactic factor that guides macrophages to avascular tumor sites) (118). Therefore, the combination of BiTE therapy with either inhibition of immune checkpoint signals or direct attack of TME immunosuppressive cells, such as MDSCs (e.g., targeting CD33 overexpressed on MDSCs), was shown to enhance the antitumor efficacy of BiTEs in preclinical models (119,120).
DEVELOPMENT OF NEXT GENERATION T CELL ENGAGERS
Modulation of T cell activation with CD3-binding Ab with specific epitopes
Because most T cell engagers are constructed based on historical mouse-derived CD3 Abs, such as OKT3, SP34, and UCHT1, they might exhibit similar T cell activation patterns and detrimental effects, such as CRS and neurological adverse events. However, sophisticated Ab binding on CD3ε, CD3δ, and CD3γ can modulate T cell activation (121). Using a sequence-based discovery platform, 12 different anti-CD3 Abs were developed; these showed equivalent levels of tumor cell lysis by primary T cells but with a thousand-fold difference in potency levels. Of these, F2B binds preferentially to CD3εδ heterodimer with intermediate affinity but does not bind to CD3εγ dimer. Moreover, it showed very low levels of cytokine release but drove robust tumor antigen-specific killing in vitro and in a mouse xenograft model; thus, it may provide a unique CD3-binding T cell engager platform without serious CRS toxicity in vivo. Such novel CD3 Ab-based therapeutics were under preclinical development at the US biotech company, Teneobio Inc., which was acquired by Amgen Inc. in October 2021.
Decoupling of tumor-killing cytotoxicity from cytokine release by T cells
Recent studies by Zuch de Zafra et al. (122) and Li et al. (123) demonstrated that T cell-mediated cytotoxicity can be decoupled from cytokine release during T cell activation by T cell engagers. Li et al. (123) further showed that T cell-generated TNF-α is the primary agent mediating monocyte activation and systemic cytokine release after CD3-based T cell engager treatment, which generally appears post initial treatment with CD3-targeting BiTEs but not after subsequent doses. Therefore, the prevention of TNF-α release after the first infusion of T cell engager is sufficient to decouple the systemic release of toxic cytokines from beneficial anti-tumor T cell activities.
Modulation of the affinity of CD3 Ab
Bortoletto et al. (124) reported the effect of modulating the CD3 binding affinity of an EpCAM×CD3 BsAb, wherein decreasing the affinity still resulted in efficient T cell activation and cytotoxicity, even at low Ab concentrations. Similarly, Bonvini et al. (125) reported the characterization of a series of CD123×CD3 DART molecules with varied CD3 binding affinities and showed that the low affinity variants required higher treatment concentration to induce T cell proliferation in vitro. In a mouse efficacy model, higher doses of DART molecules were required to achieve efficacious tumor growth inhibition, but reduced cytokine release was observed irrespective of the dose level. In a study on cynomolgus monkey, the lower-affinity CD3 variant showed much improved safety profile, which was well tolerated up to 20 mg/kg with a higher and longer duration of exposure, compared to that of the higher-affinity CD3 variant that demonstrated cytokine release and poor tolerability at 3 mg/kg. Haber et al. (126) reported another aspect of T cell engager with affinity tuning, where low-affinity CD3-binding molecules were less potent (higher EC50 values) than the high-affinity CD3 binders. In contrast, in vivo tumor models showed that CD3-based BsAbs with different CD3 affinities exhibited similar potencies and that the levels of T cell-induced cytokine accumulation in serum were lower for the weaker CD3-binding molecules. These reports provide several approaches to develop less toxic BiTEs by modulating the affinity of the CD3 binding Ab.
Development of ‘Half-life-extended (HLE) BiTE’
As blinatumomab has a very short half-life in the blood stream of patients, it is administered as a continuous infusion for several weeks to extend its serum half-life, which will potentially improve patient compliance and broaden its clinical usage. Accordingly, HLE BiTEs (a canonical BiTE fused to an Fc domain) have been developed at Amgen. Comparative studies in nonhuman primates indicate that HLE BiTEs exhibit in vivo and in vitro activity similar to those of standard BiTEs (127,128) and have a longer serum half-life of 210 h after a single IV dose, potentially allowing a single weekly dosing (129). There are several HLE BiTEs under development, including AMG 160 (anti-PSMA), AMG 199 (anti-mucin17), AMG 562 (anti-CD19), AMG 673 (anti-CD33), AMG 701 (anti-BCMA), AMG 910 (anti-CLDN18.2), and AMG 757 (anti-DLL3) (129). However, there are concerns that prolonged retention in circulation at high concentration may be associated with increased toxicity compared to that of canonical BiTEs. In fact, a few BiTE clinical pipeline programs, which include AMG 701 (anti-BCMA) and AMG 427 (anti-FLT3), have been terminated or placed on hold because of increased toxicity.
CONCLUSION
T cell-engaging BsAbs have shown unprecedented anti-tumor activities in hematologic malignant tumors along with CAR-T therapy and have been approved in several major countries with a breakthrough therapy designation. Together with the well-proven immune checkpoint inhibitory drugs, such as pembrolizumab and nivolumab, T cell-engaging BsAbs can modulate our intrinsic immune activities via the interaction between effector T cells and tumors. Despite the toxicity issues associated with BiTE treatment, such as CRS and neurological adverse events, as well as patient compliance issues, further advances in the development of novel immune-cell binders, dosing strategies, and combination therapies, through continuous innovation and trials, will improve the efficacy and safety of BiTEs for cancer patients. Currently, glimpses of results from such innovative approaches employed in several preclinical studies show positive outcomes. Furthermore, application of BsAbs in targeting other immune cells, such as NK cells or DCs, may open new avenues for boosting the immune surveillance functions of the human body. We believe that the application of BiTEs will further broaden in the future, even for solid tumors, and thus provide highly efficacious anti-tumor strategies in clinical settings.
ACKNOWLEDGEMENTS
This work was supported by the Bio & Medical Technology Development Program (No. 2017M3A9C8033570 & No. 2020M3H1A1075314) and the Regional Leading Research Center program (No. 2020R1A5A8019180) of the National Research Foundation (NRF) funded by the Korean government (MSIT) and by Korea Basic Science Institute (National Research Facilities and Equipment Center) grant funded by the Ministry of Education (No. 2021R1A6C101A390).
Abbreviations
- ADCC
antibody-dependent cell cytotoxicity
- ADCP
antibody-dependent cell phagocytosis
- AML
acute myeloid leukemia
- B-ALL
B-acute lymphoblastic leukemia
- BCMA
B-cell maturation antigen
- BiKE
bispecific killer cell engager
- BiTE
bispecific T cell engager
- BsAb
bispecific antibody
- CAR
chimeric antigen receptor
- CEA
carcinoembryonic antigen
- CRS
cytokine release syndrome
- CSC
cancer stem cell
- DART
dual affinity retargeting
- DC
dendritic cell
- EGFR
epithelial growth factor
- EMA
European Medicines Agency
- EpCAM
epithelial cell adhesion molecule
- FcR
Fc receptor
- FDA
Food and Drug Administration
- hCART
headless chimeric antigen receptor-T
- HER2
human epidermal growth factor receptor 2
- HLE
half-life-extended
- HLH
hemophagocytic lymphohistiocytosis
- ICANS
immune effector cell-associated neurotoxicity syndrome
- IV
intravenous
- LAG-3
lymphocyte activation gene-3
- mAb
monoclonal antibody
- MDSC
myeloid-derived suppressor cell
- MRD
minimal residual disease
- NCR
natural cytotoxicity receptor
- NHL
non-Hodgkin lymphoma
- NSCLC
non-small cell lung cancer
- PSMA
prostate-specific membrane antigen
- scFv
single-chain variable fragment
- sdAb
single domain antibody
- TAA
tumor-associated antigen
- TAM
tumor-associated macrophage
- TCEB
T cell engaging bispecific antibody
- TIM-3
T cell immunoglobulin, mucin domain-containing protein 3
- TME
tumor microenvironment
- TriKE
trispecific killer cell engager
Footnotes
Conflict of Interest: The authors declare no potential conflicts of interest.
- Conceptualization: Lee SW, Kim DH.
- Writing - original draft: Moon D, Tae N, Park Y, Lee SW, Kim DH.
- Writing - review & editing: Park Y, Lee SW, Kim DH.
References
- 1.Nisonoff A, Rivers MM. Recombination of a mixture of univalent antibody fragments of different specificity. Arch Biochem Biophys. 1961;93:460–462. doi: 10.1016/0003-9861(61)90296-x. [DOI] [PubMed] [Google Scholar]
- 2.Fudenberg HH, Drews G, Nisonoff A. Serologic demonstration of dual specificity of rabbit bivalent hybrid antibody. J Exp Med. 1964;119:151–166. doi: 10.1084/jem.119.1.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Köhler G, Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature. 1975;256:495–497. doi: 10.1038/256495a0. [DOI] [PubMed] [Google Scholar]
- 4.Milstein C, Cuello AC. Hybrid hybridomas and their use in immunohistochemistry. Nature. 1983;305:537–540. doi: 10.1038/305537a0. [DOI] [PubMed] [Google Scholar]
- 5.Suresh MR, Cuello AC, Milstein C. Advantages of bispecific hybridomas in one-step immunocytochemistry and immunoassays. Proc Natl Acad Sci U S A. 1986;83:7989–7993. doi: 10.1073/pnas.83.20.7989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lindhofer H, Mocikat R, Steipe B, Thierfelder S. Preferential species-restricted heavy/light chain pairing in rat/mouse quadromas. Implications for a single-step purification of bispecific antibodies. J Immunol. 1995;155:219–225. [PubMed] [Google Scholar]
- 7.Mezzanzanica D, Canevari S, Ménard S, Pupa SM, Tagliabue E, Lanzavecchia A, Colnaghi MI. Human ovarian carcinoma lysis by cytotoxic T cells targeted by bispecific monoclonal antibodies: analysis of the antibody components. Int J Cancer. 1988;41:609–615. doi: 10.1002/ijc.2910410422. [DOI] [PubMed] [Google Scholar]
- 8.Brennan M, Davison PF, Paulus H. Preparation of bispecific antibodies by chemical recombination of monoclonal immunoglobulin G1 fragments. Science. 1985;229:81–83. doi: 10.1126/science.3925553. [DOI] [PubMed] [Google Scholar]
- 9.Huston JS, Levinson D, Mudgett-Hunter M, Tai MS, Novotný J, Margolies MN, Ridge RJ, Bruccoleri RE, Haber E, Crea R. Protein engineering of antibody binding sites: recovery of specific activity in an anti-digoxin single-chain Fv analogue produced in Escherichia coli . Proc Natl Acad Sci U S A. 1988;85:5879–5883. doi: 10.1073/pnas.85.16.5879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ridgway JB, Presta LG, Carter P. ‘Knobs-into-holes’ engineering of antibody CH3 domains for heavy chain heterodimerization. Protein Eng. 1996;9:617–621. doi: 10.1093/protein/9.7.617. [DOI] [PubMed] [Google Scholar]
- 11.Linke R, Klein A, Seimetz D. Catumaxomab: clinical development and future directions. MAbs. 2010;2:129–136. doi: 10.4161/mabs.2.2.11221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brown P. Blinatumomab for MRD+ B-ALL: the evidence strengthens. Blood. 2018;131:1497–1498. doi: 10.1182/blood-2018-02-830364. [DOI] [PubMed] [Google Scholar]
- 13.Gökbuget N, Dombret H, Bonifacio M, Reichle A, Graux C, Faul C, Diedrich H, Topp MS, Brüggemann M, Horst HA, et al. Blinatumomab for minimal residual disease in adults with B-cell precursor acute lymphoblastic leukemia. Blood. 2018;131:1522–1531. doi: 10.1182/blood-2017-08-798322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Halford Z, Coalter C, Gresham V, Brown T. A systematic review of blinatumomab in the treatment of acute lymphoblastic leukemia: engaging an old problem with new solutions. Ann Pharmacother. 2021;55:1236–1253. doi: 10.1177/1060028020988411. [DOI] [PubMed] [Google Scholar]
- 15.Neijssen J, Cardoso RM, Chevalier KM, Wiegman L, Valerius T, Anderson GM, Moores SL, Schuurman J, Parren PW, Strohl WR, et al. Discovery of amivantamab (JNJ-61186372), a bispecific antibody targeting EGFR and MET. J Biol Chem. 2021;296:100641. doi: 10.1016/j.jbc.2021.100641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Park K, Haura EB, Leighl NB, Mitchell P, Shu CA, Girard N, Viteri S, Han JY, Kim SW, Lee CK, et al. Amivantamab in EGFR exon 20 insertion–mutated non–small-cell lung cancer progressing on platinum chemotherapy: initial results from the CHRYSALIS phase I study. J Clin Oncol. 2021;39:3391–3402. doi: 10.1200/JCO.21.00662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Romero D. Amivantamab is effective in NSCLC harbouring EGFR exon 20 insertions. Nat Rev Clin Oncol. 2021;18:604. doi: 10.1038/s41571-021-00551-8. [DOI] [PubMed] [Google Scholar]
- 18.Cheong HS, Chang JS, Park JM, Byun SM. Affinity enhancement of bispecific antibody against two different epitopes in the same antigen. Biochem Biophys Res Commun. 1990;173:795–800. doi: 10.1016/s0006-291x(05)80857-5. [DOI] [PubMed] [Google Scholar]
- 19.Kontermann RE. Dual targeting strategies with bispecific antibodies. MAbs. 2012;4:182–197. doi: 10.4161/mabs.4.2.19000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Robert B, Dorvillius M, Buchegger F, Garambois V, Mani JC, Pugnières M, Mach JP, Pèlegrin A. Tumor targeting with newly designed biparatopic antibodies directed against two different epitopes of the carcinoembryonic antigen (CEA) Int J Cancer. 1999;81:285–291. doi: 10.1002/(sici)1097-0215(19990412)81:2<285::aid-ijc19>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 21.Lu D, Kotanides H, Jimenez X, Zhou Q, Persaud K, Bohlen P, Witte L, Zhu Z. Acquired antagonistic activity of a bispecific diabody directed against two different epitopes on vascular endothelial growth factor receptor 2. J Immunol Methods. 1999;230:159–171. doi: 10.1016/s0022-1759(99)00135-0. [DOI] [PubMed] [Google Scholar]
- 22.Asokan M, Rudicell RS, Louder M, McKee K, O’Dell S, Stewart-Jones G, Wang K, Xu L, Chen X, Choe M, et al. Bispecific antibodies targeting different epitopes on the HIV-1 envelope exhibit broad and potent neutralization. J Virol. 2015;89:12501–12512. doi: 10.1128/JVI.02097-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tan W, Meng Y, Li H, Chen Y, Han S, Zeng J, Huang A, Li B, Zhang Y, Guo Y. A bispecific antibody against two different epitopes on hepatitis B surface antigen has potent hepatitis B virus neutralizing activity. MAbs. 2013;5:946–955. doi: 10.4161/mabs.26390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.You G, Won J, Lee Y, Moon D, Park Y, Lee SH, Lee SW. Bispecific antibodies: a smart arsenal for cancer immunotherapies. Vaccines (Basel) 2021;9:724. doi: 10.3390/vaccines9070724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Blanco B, Domínguez-Alonso C, Alvarez-Vallina L. Bispecific immunomodulatory antibodies for cancer immunotherapy. Clin Cancer Res. 2021;27:5457–5464. doi: 10.1158/1078-0432.CCR-20-3770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hummel HD, Kufer P, Grüllich C, Deschler-Baier B, Chatterjee M, Goebeler ME, Miller K, De Santis M, Loidl WC, Buck A, et al. Phase I study of pasotuxizumab (AMG 212/BAY 2010112), a PSMA-targeting BiTE (bispecific T-cell engager) immune therapy for metastatic castration-resistant prostate cancer (mCRPC) J Clin Oncol. 2020;38(6_suppl):124. [Google Scholar]
- 27.Tran B, Horvath L, Dorff T, Rettig M, Lolkema MP, Machiels JP, Rottey S, Autio K, Greil R, Adra N, et al. 609O results from a phase I study of AMG 160, a half-life extended (HLE), PSMA-targeted, bispecific T-cell engager (BiTE®) immune therapy for metastatic castration-resistant prostate cancer (mCRPC) Ann Oncol. 2020;31:S507. [Google Scholar]
- 28.Rosenthal MA, Balana C, van Linde ME, Sayehli C, Fiedler WM, Wermke M, Massard C, Mellinghoff IK, Khasraw M, Ang A, et al. ATIM-49 (LTBK-01). AMG 596, a novel anti-EGFRvIII bispecific T cell engager (BiTE®) molecule for the treatment of glioblastoma (GBM): planned interim analysis in recurrent GBM (rGBM) Neuro Oncol. 2019;21(Supplement_6):vi283. [Google Scholar]
- 29.Sternjak A, Lee F, Thomas O, Balazs M, Wahl J, Lorenczewski G, Ullrich I, Muenz M, Rattel B, Bailis JM, et al. Preclinical assessment of AMG 596, a bispecific T-cell engager (BiTE) immunotherapy targeting the tumor-specific antigen EGFRvIII. Mol Cancer Ther. 2021;20:925–933. doi: 10.1158/1535-7163.MCT-20-0508. [DOI] [PubMed] [Google Scholar]
- 30.Park JH, Kim HJ, Kim CW, Kim HC, Jung Y, Lee HS, Lee Y, Ju YS, Oh JE, Park SH, et al. Tumor hypoxia represses γδ T cell-mediated antitumor immunity against brain tumors. Nat Immunol. 2021;22:336–346. doi: 10.1038/s41590-020-00860-7. [DOI] [PubMed] [Google Scholar]
- 31.Oberg HH, Kellner C, Gonnermann D, Peipp M, Peters C, Sebens S, Kabelitz D, Wesch D. γδ T cell activation by bispecific antibodies. Cell Immunol. 2015;296:41–49. doi: 10.1016/j.cellimm.2015.04.009. [DOI] [PubMed] [Google Scholar]
- 32.Ganesan R, Chennupati V, Ramachandran B, Hansen MR, Singh S, Grewal IS. Selective recruitment of γδ T cells by a bispecific antibody for the treatment of acute myeloid leukemia. Leukemia. 2021;35:2274–2284. doi: 10.1038/s41375-021-01122-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xie G, Dong H, Liang Y, Ham JD, Rizwan R, Chen J. CAR-NK cells: a promising cellular immunotherapy for cancer. EBioMedicine. 2020;59:102975. doi: 10.1016/j.ebiom.2020.102975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Quatrini L, Della Chiesa M, Sivori S, Mingari MC, Pende D, Moretta L. Human NK cells, their receptors and function. Eur J Immunol. 2021;51:1566–1579. doi: 10.1002/eji.202049028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schmohl JU, Gleason MK, Dougherty PR, Miller JS, Vallera DA. Heterodimeric bispecific single chain variable fragments (scFv) killer engagers (BiKEs) enhance NK-cell activity against CD133+ colorectal cancer cells. Target Oncol. 2016;11:353–361. doi: 10.1007/s11523-015-0391-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Reusing SB, Vallera DA, Manser AR, Vatrin T, Bhatia S, Felices M, Miller JS, Uhrberg M, Babor F. CD16xCD33 bispecific killer cell engager (BiKE) as potential immunotherapeutic in pediatric patients with AML and biphenotypic ALL. Cancer Immunol Immunother. 2021;70:3701–3708. doi: 10.1007/s00262-021-03008-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bogen JP, Carrara SC, Fiebig D, Grzeschik J, Hock B, Kolmar H. Design of a trispecific checkpoint inhibitor and natural killer cell engager based on a 2 + 1 common light chain antibody architecture. Front Immunol. 2021;12:669496. doi: 10.3389/fimmu.2021.669496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cheng Y, Zheng X, Wang X, Chen Y, Wei H, Sun R, Tian Z, Sun H. Trispecific killer engager 161519 enhances natural killer cell function and provides anti-tumor activity against CD19-positive cancers. Cancer Biol Med. 2020;17:1026–1038. doi: 10.20892/j.issn.2095-3941.2020.0399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schmohl JU, Felices M, Oh F, Lenvik AJ, Lebeau AM, Panyam J, Miller JS, Vallera DA. Engineering of anti-CD133 trispecific molecule capable of inducing NK expansion and driving antibody-dependent cell-mediated cytotoxicity. Cancer Res Treat. 2017;49:1140–1152. doi: 10.4143/crt.2016.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vallera DA, Oh F, Kodal B, Hinderlie P, Geller MA, Miller JS, Felices M. A HER2 tri-specific NK cell engager mediates efficient targeting of human ovarian cancer. Cancers (Basel) 2021;13:3994. doi: 10.3390/cancers13163994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dovedi SJ, Elder MJ, Yang C, Sitnikova SI, Irving L, Hansen A, Hair J, Jones DC, Hasani S, Wang B, et al. Design and efficacy of a monovalent bispecific PD-1/CTLA4 antibody that enhances CTLA4 blockade on PD-1+ activated T cells. Cancer Discov. 2021;11:1100–1117. doi: 10.1158/2159-8290.CD-20-1445. [DOI] [PubMed] [Google Scholar]
- 42.Dovedi SJ, Mazor Y, Elder M, Hasani S, Wang B, Mosely S, Jones D, Hansen A, Yang C, Wu Y, et al. Abstract 2776: MEDI5752: a novel bispecific antibody that preferentially targets CTLA-4 on PD-1 expressing T-cells. Cancer Res. 2018;78:2776. [Google Scholar]
- 43.Anderson AC, Joller N, Kuchroo VK. Lag-3, Tim-3, and TIGIT: co-inhibitory receptors with specialized functions in immune regulation. Immunity. 2016;44:989–1004. doi: 10.1016/j.immuni.2016.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Deak LL, Seeber S, Perro M, Weber P, Lauener L, Chen S, Offner S, Dengl S, Hesse F, Zwick A, et al. Abstract 2270: RG7769 (PD1-TIM3), a novel heterodimeric avidity-driven T cell specific PD-1/TIM-3 bispecific antibody lacking Fc-mediated effector functions for dual checkpoint inhibition to reactivate dysfunctional T cells. Cancer Res. 2020;80:2270. [Google Scholar]
- 45.Gaspar M, Pravin J, Rodrigues L, Uhlenbroich S, Everett KL, Wollerton F, Morrow M, Tuna M, Brewis N. CD137/OX40 bispecific antibody induces potent antitumor activity that is dependent on target coengagement. Cancer Immunol Res. 2020;8:781–793. doi: 10.1158/2326-6066.CIR-19-0798. [DOI] [PubMed] [Google Scholar]
- 46.Johnson M, Lopez J, LoRusso P, Bauman J, Haggstrom D, Lagkadinou E, Bajaj G, Türeci Ö, Adams H, Şahin U, et al. 493 first-in-human phase 1/2 trial to evaluate the safety and initial clinical activity of DuoBody®-CD40×4–1bb (GEN1042) in patients with advanced solid tumors. J Immunother Cancer. 2021;9(Suppl 2):A525. [Google Scholar]
- 47.Kvarnhammar AM, Veitonmäki N, Hägerbrand K, Dahlman A, Smith KE, Fritzell S, von Schantz L, Thagesson M, Werchau D, Smedenfors K, et al. The CTLA-4 x OX40 bispecific antibody ATOR-1015 induces anti-tumor effects through tumor-directed immune activation. J Immunother Cancer. 2019;7:103. doi: 10.1186/s40425-019-0570-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jeong S, Park E, Kim HD, Sung E, Kim H, Jeon J, Kim Y, Jung UJ, Son YG, Hong Y, et al. Novel anti-4-1BB×PD-L1 bispecific antibody augments anti-tumor immunity through tumor-directed T-cell activation and checkpoint blockade. J Immunother Cancer. 2021;9:e002428. doi: 10.1136/jitc-2021-002428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Park JA, Santich BH, Xu H, Lum LG, Cheung NV. Potent ex vivo armed T cells using recombinant bispecific antibodies for adoptive immunotherapy with reduced cytokine release. J Immunother Cancer. 2021;9:e002222. doi: 10.1136/jitc-2020-002222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yankelevich M, Modak S, Chu R, Lee DW, Thakur A, Cheung NK, Lum LG. Phase I study of OKT3 x hu3F8 bispecific antibody (GD2Bi) armed T cells (GD2BATs) in GD2-positive tumors. J Clin Oncol. 2019;37(15_suppl):2533. [Google Scholar]
- 51.Thakur A, Scholler J, Kubicka E, Bliemeister ET, Schalk DL, June CH, Lum LG. Bispecific antibody armed metabolically enhanced headless car T cells. Front Immunol. 2021;12:690437. doi: 10.3389/fimmu.2021.690437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Liu H, Saxena A, Sidhu SS, Wu D. Fc engineering for developing therapeutic bispecific antibodies and novel scaffolds. Front Immunol. 2017;8:38. doi: 10.3389/fimmu.2017.00038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhang J, Yi J, Zhou P. Development of bispecific antibodies in China: overview and prospects. Antib Ther. 2020;3:126–145. doi: 10.1093/abt/tbaa011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Stewart R, Hammond SA, Oberst M, Wilkinson RW. The role of Fc gamma receptors in the activity of immunomodulatory antibodies for cancer. J Immunother Cancer. 2014;2:29. [Google Scholar]
- 55.Saunders KO. Conceptual approaches to modulating antibody effector functions and circulation half-life. Front Immunol. 2019;10:1296. doi: 10.3389/fimmu.2019.01296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.You G, Lee Y, Kang YW, Park HW, Park K, Kim H, Kim YM, Kim S, Kim JH, Moon D, et al. B7-H3×4-1BB bispecific antibody augments antitumor immunity by enhancing terminally differentiated CD8+ tumor-infiltrating lymphocytes. Sci Adv. 2021;7:eaax3160. doi: 10.1126/sciadv.aax3160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mlecnik B, Bindea G, Pagès F, Galon J. Tumor immunosurveillance in human cancers. Cancer Metastasis Rev. 2011;30:5–12. doi: 10.1007/s10555-011-9270-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhang L, Conejo-Garcia JR, Katsaros D, Gimotty PA, Massobrio M, Regnani G, Makrigiannakis A, Gray H, Schlienger K, Liebman MN, et al. Intratumoral T cells, recurrence, and survival in epithelial ovarian cancer. N Engl J Med. 2003;348:203–213. doi: 10.1056/NEJMoa020177. [DOI] [PubMed] [Google Scholar]
- 59.Galon J, Costes A, Sanchez-Cabo F, Kirilovsky A, Mlecnik B, Lagorce-Pagès C, Tosolini M, Camus M, Berger A, Wind P, et al. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science. 2006;313:1960–1964. doi: 10.1126/science.1129139. [DOI] [PubMed] [Google Scholar]
- 60.Wahlin BE, Sander B, Christensson B, Kimby E. CD8+ T-cell content in diagnostic lymph nodes measured by flow cytometry is a predictor of survival in follicular lymphoma. Clin Cancer Res. 2007;13:388–397. doi: 10.1158/1078-0432.CCR-06-1734. [DOI] [PubMed] [Google Scholar]
- 61.Zhu Z, Zapata G, Shalaby R, Snedecor B, Chen H, Carter P. High level secretion of a humanized bispecific diabody from Escherichia coli . Biotechnology (N Y) 1996;14:192–196. doi: 10.1038/nbt0296-192. [DOI] [PubMed] [Google Scholar]
- 62.Titus JA, Garrido MA, Hecht TT, Winkler DF, Wunderlich JR, Segal DM. Human T cells targeted with anti-T3 cross-linked to antitumor antibody prevent tumor growth in nude mice. J Immunol. 1987;138:4018–4022. [PubMed] [Google Scholar]
- 63.Weiner GJ, Hillstrom JR. Bispecific anti-idiotype/anti-CD3 antibody therapy of murine B cell lymphoma. J Immunol. 1991;147:4035–4044. [PubMed] [Google Scholar]
- 64.Demanet C, Brissinck J, De Jonge J, Thielemans K. Bispecific antibody-mediated immunotherapy of the BCL1 lymphoma: increased efficacy with multiple injections and CD28-induced costimulation. Blood. 1996;87:4390–4398. [PubMed] [Google Scholar]
- 65.Renner C, Jung W, Sahin U, Denfeld R, Pohl C, Trümper L, Hartmann F, Diehl V, van Lier R, Pfreundschuh M. Cure of xenografted human tumors by bispecific monoclonal antibodies and human T cells. Science. 1994;264:833–835. doi: 10.1126/science.8171337. [DOI] [PubMed] [Google Scholar]
- 66.Kipriyanov SM, Moldenhauer G, Strauss G, Little M. Bispecific CD3 x CD19 diabody for T cell-mediated lysis of malignant human B cells. Int J Cancer. 1998;77:763–772. doi: 10.1002/(sici)1097-0215(19980831)77:5<763::aid-ijc16>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 67.Cochlovius B, Kipriyanov SM, Stassar MJ, Schuhmacher J, Benner A, Moldenhauer G, Little M. Cure of Burkitt’s lymphoma in severe combined immunodeficiency mice by T cells, tetravalent CD3 x CD19 tandem diabody, and CD28 costimulation. Cancer Res. 2000;60:4336–4341. [PubMed] [Google Scholar]
- 68.Nitta T, Sato K, Yagita H, Okumura K, Ishii S. Preliminary trial of specific targeting therapy against malignant glioma. Lancet. 1990;335:368–371. doi: 10.1016/0140-6736(90)90205-j. [DOI] [PubMed] [Google Scholar]
- 69.Canevari S, Stoter G, Arienti F, Bolis G, Colnaghi MI, Di Re EM, Eggermont AM, Goey SH, Gratama JW, Lamers CH, et al. Regression of advanced ovarian carcinoma by intraperitoneal treatment with autologous T lymphocytes retargeted by a bispecific monoclonal antibody. J Natl Cancer Inst. 1995;87:1463–1469. doi: 10.1093/jnci/87.19.1463. [DOI] [PubMed] [Google Scholar]
- 70.Manzke O, Tesch H, Borchmann P, Wolf J, Lackner K, Gossmann A, Diehl V, Bohlen H. Locoregional treatment of low-grade B-cell lymphoma with CD3xCD19 bispecific antibodies and CD28 costimulation. I. Clinical phase I evaluation. Int J Cancer. 2001;91:508–515. doi: 10.1002/1097-0215(200002)9999:9999<::aid-ijc1068>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 71.Mack M, Riethmüller G, Kufer P. A small bispecific antibody construct expressed as a functional single-chain molecule with high tumor cell cytotoxicity. Proc Natl Acad Sci U S A. 1995;92:7021–7025. doi: 10.1073/pnas.92.15.7021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Löffler A, Kufer P, Lutterbüse R, Zettl F, Daniel PT, Schwenkenbecher JM, Riethmüller G, Dörken B, Bargou RC. A recombinant bispecific single-chain antibody, CD19 x CD3, induces rapid and high lymphoma-directed cytotoxicity by unstimulated T lymphocytes. Blood. 2000;95:2098–2103. [PubMed] [Google Scholar]
- 73.Kufer P, Mack M, Gruber R, Lutterbüse R, Zettl F, Riethmüller G. Construction and biological activity of a recombinant bispecific single-chain antibody designed for therapy of minimal residual colorectal cancer. Cancer Immunol Immunother. 1997;45:193–197. doi: 10.1007/s002620050431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mack M, Gruber R, Schmidt S, Riethmüller G, Kufer P. Biologic properties of a bispecific single-chain antibody directed against 17-1A (EpCAM) and CD3: tumor cell-dependent T cell stimulation and cytotoxic activity. J Immunol. 1997;158:3965–3970. [PubMed] [Google Scholar]
- 75.Irvine DJ. Function-specific variations in the immunological synapses formed by cytotoxic T cells. Proc Natl Acad Sci U S A. 2003;100:13739–13740. doi: 10.1073/pnas.2536626100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Portell CA, Wenzell CM, Advani AS. Clinical and pharmacologic aspects of blinatumomab in the treatment of B-cell acute lymphoblastic leukemia. Clin Pharmacol. 2013;5:5–11. doi: 10.2147/CPAA.S42689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Topp MS, Gökbuget N, Zugmaier G, Klappers P, Stelljes M, Neumann S, Viardot A, Marks R, Diedrich H, Faul C, et al. Phase II trial of the anti-CD19 bispecific T cell-engager blinatumomab shows hematologic and molecular remissions in patients with relapsed or refractory B-precursor acute lymphoblastic leukemia. J Clin Oncol. 2014;32:4134–4140. doi: 10.1200/JCO.2014.56.3247. [DOI] [PubMed] [Google Scholar]
- 78.Duell J, Lukic DS, Karg M, Reusch U, Koch J, Zhukovsky EA, Rajkovic E, Treder M, Rasche L, Eisele F, et al. Functionally defective T cells after chemotherapy of B-cell malignancies can be activated by the tetravalent bispecific CD19/CD3 antibody AFM11. J Immunother. 2019;42:180–188. doi: 10.1097/CJI.0000000000000267. [DOI] [PubMed] [Google Scholar]
- 79.Frey NV, Porter DL. Cytokine release syndrome with novel therapeutics for acute lymphoblastic leukemia. Hematology Am Soc Hematol Educ Program. 2016;2016:567–572. doi: 10.1182/asheducation-2016.1.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Teachey DT, Rheingold SR, Maude SL, Zugmaier G, Barrett DM, Seif AE, Nichols KE, Suppa EK, Kalos M, Berg RA, et al. Cytokine release syndrome after blinatumomab treatment related to abnormal macrophage activation and ameliorated with cytokine-directed therapy. Blood. 2013;121:5154–5157. doi: 10.1182/blood-2013-02-485623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Aldoss I, Khaled SK, Budde E, Stein AS. Cytokine release syndrome with the novel treatments of acute lymphoblastic leukemia: pathophysiology, prevention, and treatment. Curr Oncol Rep. 2019;21:4. doi: 10.1007/s11912-019-0753-y. [DOI] [PubMed] [Google Scholar]
- 82.Barker CA, Kim SK, Budhu S, Matsoukas K, Daniyan AF, D’Angelo SP. Cytokine release syndrome after radiation therapy: case report and review of the literature. J Immunother Cancer. 2018;6:1. doi: 10.1186/s40425-017-0311-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Jung SH, Lee SR, Yang DH, Lee S, Yoon JH, Lee H, Bang SM, Koh Y, Park S, Kim DS, et al. Efficacy and safety of blinatumomab treatment in adult Korean patients with relapsed/refractory acute lymphoblastic leukemia on behalf of the Korean Society of Hematology ALL Working Party. Ann Hematol. 2019;98:151–158. doi: 10.1007/s00277-018-3495-2. [DOI] [PubMed] [Google Scholar]
- 84.Nägele V, Kratzer A, Zugmaier G, Holland C, Hijazi Y, Topp MS, Gökbuget N, Baeuerle PA, Kufer P, Wolf A, et al. Changes in clinical laboratory parameters and pharmacodynamic markers in response to blinatumomab treatment of patients with relapsed/refractory ALL. Exp Hematol Oncol. 2017;6:14. doi: 10.1186/s40164-017-0074-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Viardot A, Goebeler ME, Hess G, Neumann S, Pfreundschuh M, Adrian N, Zettl F, Libicher M, Sayehli C, Stieglmaier J, et al. Phase 2 study of the bispecific T-cell engager (BiTE) antibody blinatumomab in relapsed/refractory diffuse large B-cell lymphoma. Blood. 2016;127:1410–1416. doi: 10.1182/blood-2015-06-651380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Topp MS, Kufer P, Gökbuget N, Goebeler M, Klinger M, Neumann S, Horst HA, Raff T, Viardot A, Schmid M, et al. Targeted therapy with the T-cell-engaging antibody blinatumomab of chemotherapy-refractory minimal residual disease in B-lineage acute lymphoblastic leukemia patients results in high response rate and prolonged leukemia-free survival. J Clin Oncol. 2011;29:2493–2498. doi: 10.1200/JCO.2010.32.7270. [DOI] [PubMed] [Google Scholar]
- 87.Gökbuget N, Zugmaier G, Klinger M, Kufer P, Stelljes M, Viardot A, Horst HA, Neumann S, Brüggemann M, Ottmann OG, et al. Long-term relapse-free survival in a phase 2 study of blinatumomab for the treatment of patients with minimal residual disease in B-lineage acute lymphoblastic leukemia. Haematologica. 2017;102:e132–e135. doi: 10.3324/haematol.2016.153957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.von Stackelberg A, Locatelli F, Zugmaier G, Handgretinger R, Trippett TM, Rizzari C, Bader P, O’Brien MM, Brethon B, Bhojwani D, et al. Phase I/phase II study of blinatumomab in pediatric patients with relapsed/refractory acute lymphoblastic leukemia. J Clin Oncol. 2016;34:4381–4389. doi: 10.1200/JCO.2016.67.3301. [DOI] [PubMed] [Google Scholar]
- 89.Kantarjian H, Stein A, Gökbuget N, Fielding AK, Schuh AC, Ribera JM, Wei A, Dombret H, Foà R, Bassan R, et al. Blinatumomab versus chemotherapy for advanced acute lymphoblastic leukemia. N Engl J Med. 2017;376:836–847. doi: 10.1056/NEJMoa1609783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Topp MS, Gökbuget N, Stein AS, Zugmaier G, O’Brien S, Bargou RC, Dombret H, Fielding AK, Heffner L, Larson RA, et al. Safety and activity of blinatumomab for adult patients with relapsed or refractory B-precursor acute lymphoblastic leukaemia: a multicentre, single-arm, phase 2 study. Lancet Oncol. 2015;16:57–66. doi: 10.1016/S1470-2045(14)71170-2. [DOI] [PubMed] [Google Scholar]
- 91.Goebeler ME, Knop S, Viardot A, Kufer P, Topp MS, Einsele H, Noppeney R, Hess G, Kallert S, Mackensen A, et al. Bispecific T-cell engager (BiTE) antibody construct blinatumomab for the treatment of patients with relapsed/refractory non-Hodgkin lymphoma: final results from a phase I study. J Clin Oncol. 2016;34:1104–1111. doi: 10.1200/JCO.2014.59.1586. [DOI] [PubMed] [Google Scholar]
- 92.Goebeler ME, Bargou RC. T cell-engaging therapies - BiTEs and beyond. Nat Rev Clin Oncol. 2020;17:418–434. doi: 10.1038/s41571-020-0347-5. [DOI] [PubMed] [Google Scholar]
- 93.Nagorsen D, Bargou R, Ruttinger D, Kufer P, Baeuerle PA, Zugmaier G. Immunotherapy of lymphoma and leukemia with T-cell engaging BiTE antibody blinatumomab. Leuk Lymphoma. 2009;50:886–891. doi: 10.1080/10428190902943077. [DOI] [PubMed] [Google Scholar]
- 94.Martinelli G, Boissel N, Chevallier P, Ottmann O, Gökbuget N, Topp MS, Fielding AK, Rambaldi A, Ritchie EK, Papayannidis C, et al. Complete hematologic and molecular response in adult patients with relapsed/refractory Philadelphia chromosome-positive B-precursor acute lymphoblastic leukemia following treatment with blinatumomab: results from a phase II, single-arm, multicenter study. J Clin Oncol. 2017;35:1795–1802. doi: 10.1200/JCO.2016.69.3531. [DOI] [PubMed] [Google Scholar]
- 95.Maude SL, Laetsch TW, Buechner J, Rives S, Boyer M, Bittencourt H, Bader P, Verneris MR, Stefanski HE, Myers GD, et al. Tisagenlecleucel in children and young adults with B-cell lymphoblastic leukemia. N Engl J Med. 2018;378:439–448. doi: 10.1056/NEJMoa1709866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lee DW, Kochenderfer JN, Stetler-Stevenson M, Cui YK, Delbrook C, Feldman SA, Fry TJ, Orentas R, Sabatino M, Shah NN, et al. T cells expressing CD19 chimeric antigen receptors for acute lymphoblastic leukaemia in children and young adults: a phase 1 dose-escalation trial. Lancet. 2015;385:517–528. doi: 10.1016/S0140-6736(14)61403-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Abramson JS, Palomba ML, Gordon LI, Lunning MA, Wang M, Arnason J, Mehta A, Purev E, Maloney DG, Andreadis C, et al. Lisocabtagene maraleucel for patients with relapsed or refractory large B-cell lymphomas (TRANSCEND NHL 001): a multicentre seamless design study. Lancet. 2020;396:839–852. doi: 10.1016/S0140-6736(20)31366-0. [DOI] [PubMed] [Google Scholar]
- 98.Schuster SJ, Bishop MR, Tam CS, Waller EK, Borchmann P, McGuirk JP, Jäger U, Jaglowski S, Andreadis C, Westin JR, et al. Tisagenlecleucel in adult relapsed or refractory diffuse large B-cell lymphoma. N Engl J Med. 2019;380:45–56. doi: 10.1056/NEJMoa1804980. [DOI] [PubMed] [Google Scholar]
- 99.Neelapu SS, Locke FL, Bartlett NL, Lekakis LJ, Miklos DB, Jacobson CA, Braunschweig I, Oluwole OO, Siddiqi T, Lin Y, et al. Axicabtagene ciloleucel CAR T-cell therapy in refractory large B-cell lymphoma. N Engl J Med. 2017;377:2531–2544. doi: 10.1056/NEJMoa1707447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Hoffmann P, Hofmeister R, Brischwein K, Brandl C, Crommer S, Bargou R, Itin C, Prang N, Baeuerle PA. Serial killing of tumor cells by cytotoxic T cells redirected with a CD19-/CD3-bispecific single-chain antibody construct. Int J Cancer. 2005;115:98–104. doi: 10.1002/ijc.20908. [DOI] [PubMed] [Google Scholar]
- 101.Raponi S, De Propris MS, Intoppa S, Milani ML, Vitale A, Elia L, Perbellini O, Pizzolo G, Foá R, Guarini A. Flow cytometric study of potential target antigens (CD19, CD20, CD22, CD33) for antibody-based immunotherapy in acute lymphoblastic leukemia: analysis of 552 cases. Leuk Lymphoma. 2011;52:1098–1107. doi: 10.3109/10428194.2011.559668. [DOI] [PubMed] [Google Scholar]
- 102.Dombret H, Topp MS, Schuh AC, Wei AH, Durrant S, Bacon CL, Tran Q, Zimmerman Z, Kantarjian H. Blinatumomab versus chemotherapy in first salvage or in later salvage for B-cell precursor acute lymphoblastic leukemia. Leuk Lymphoma. 2019;60:2214–2222. doi: 10.1080/10428194.2019.1576872. [DOI] [PubMed] [Google Scholar]
- 103.Berry DA, Zhou S, Higley H, Mukundan L, Fu S, Reaman GH, Wood BL, Kelloff GJ, Jessup JM, Radich JP. Association of minimal residual disease with clinical outcome in pediatric and adult acute lymphoblastic leukemia: a meta-analysis. JAMA Oncol. 2017;3:e170580. doi: 10.1001/jamaoncol.2017.0580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Edeline J, Houot R, Marabelle A, Alcantara M. CAR-T cells and BiTEs in solid tumors: challenges and perspectives. J Hematol Oncol. 2021;14:65. doi: 10.1186/s13045-021-01067-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kebenko M, Goebeler ME, Wolf M, Hasenburg A, Seggewiss-Bernhardt R, Ritter B, Rautenberg B, Atanackovic D, Kratzer A, Rottman JB, et al. A multicenter phase 1 study of solitomab (MT110, AMG 110), a bispecific EpCAM/CD3 T-cell engager (BiTE®) antibody construct, in patients with refractory solid tumors. Oncoimmunology. 2018;7:e1450710. doi: 10.1080/2162402X.2018.1450710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Yu WL, Hua ZC. Chimeric antigen receptor T-cell (CAR T) therapy for hematologic and solid malignancies: efficacy and safety-a systematic review with meta-analysis. Cancers (Basel) 2019;11:47. doi: 10.3390/cancers11010047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Sebastian M, Passlick B, Friccius-Quecke H, Jäger M, Lindhofer H, Kanniess F, Wiewrodt R, Thiel E, Buhl R, Schmittel A. Treatment of non-small cell lung cancer patients with the trifunctional monoclonal antibody catumaxomab (anti-EpCAM x anti-CD3): a phase I study. Cancer Immunol Immunother. 2007;56:1637–1644. doi: 10.1007/s00262-007-0310-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Heiss MM, Murawa P, Koralewski P, Kutarska E, Kolesnik OO, Ivanchenko VV, Dudnichenko AS, Aleknaviciene B, Razbadauskas A, Gore M, et al. The trifunctional antibody catumaxomab for the treatment of malignant ascites due to epithelial cancer: results of a prospective randomized phase II/III trial. Int J Cancer. 2010;127:2209–2221. doi: 10.1002/ijc.25423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Borlak J, Länger F, Spanel R, Schöndorfer G, Dittrich C. Immune-mediated liver injury of the cancer therapeutic antibody catumaxomab targeting EpCAM, CD3 and Fcγ receptors. Oncotarget. 2016;7:28059–28074. doi: 10.18632/oncotarget.8574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Haense N, Atmaca A, Pauligk C, Steinmetz K, Marmé F, Haag GM, Rieger M, Ottmann OG, Ruf P, Lindhofer H, et al. A phase I trial of the trifunctional anti Her2 × anti CD3 antibody ertumaxomab in patients with advanced solid tumors. BMC Cancer. 2016;16:420. doi: 10.1186/s12885-016-2449-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Hummel HD, Kufer P, Grüllich C, Deschler-Baier B, Chatterjee M, Goebeler ME, Miller K, De Santis M, Loidl WC, Buck A. Phase 1 study of pasotuxizumab (BAY 2010112), a PSMA-targeting bispecific T cell engager (BiTE) immunotherapy for metastatic castration-resistant prostate cancer (mCRPC) J Clin Oncol. 2019;37(15_suppl):5034. [Google Scholar]
- 112.Asseman C, Mauze S, Leach MW, Coffman RL, Powrie F. An essential role for interleukin 10 in the function of regulatory T cells that inhibit intestinal inflammation. J Exp Med. 1999;190:995–1004. doi: 10.1084/jem.190.7.995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Collison LW, Workman CJ, Kuo TT, Boyd K, Wang Y, Vignali KM, Cross R, Sehy D, Blumberg RS, Vignali DA. The inhibitory cytokine IL-35 contributes to regulatory T-cell function. Nature. 2007;450:566–569. doi: 10.1038/nature06306. [DOI] [PubMed] [Google Scholar]
- 114.Baratelli F, Lin Y, Zhu L, Yang SC, Heuzé-Vourc’h N, Zeng G, Reckamp K, Dohadwala M, Sharma S, Dubinett SM. Prostaglandin E2 induces FOXP3 gene expression and T regulatory cell function in human CD4+ T cells. J Immunol. 2005;175:1483–1490. doi: 10.4049/jimmunol.175.3.1483. [DOI] [PubMed] [Google Scholar]
- 115.Bronte V, Serafini P, De Santo C, Marigo I, Tosello V, Mazzoni A, Segal DM, Staib C, Lowel M, Sutter G, et al. IL-4-induced arginase 1 suppresses alloreactive T cells in tumor-bearing mice. J Immunol. 2003;170:270–278. doi: 10.4049/jimmunol.170.1.270. [DOI] [PubMed] [Google Scholar]
- 116.Youn JI, Nagaraj S, Collazo M, Gabrilovich DI. Subsets of myeloid-derived suppressor cells in tumor-bearing mice. J Immunol. 2008;181:5791–5802. doi: 10.4049/jimmunol.181.8.5791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Nagaraj S, Nelson A, Youn JI, Cheng P, Quiceno D, Gabrilovich DI. Antigen-specific CD4+ T cells regulate function of myeloid-derived suppressor cells in cancer via retrograde MHC class II signaling. Cancer Res. 2012;72:928–938. doi: 10.1158/0008-5472.CAN-11-2863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Lewis JS, Landers RJ, Underwood JC, Harris AL, Lewis CE. Expression of vascular endothelial growth factor by macrophages is up-regulated in poorly vascularized areas of breast carcinomas. J Pathol. 2000;192:150–158. doi: 10.1002/1096-9896(2000)9999:9999<::AID-PATH687>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 119.Franklin RA, Liao W, Sarkar A, Kim MV, Bivona MR, Liu K, Pamer EG, Li MO. The cellular and molecular origin of tumor-associated macrophages. Science. 2014;344:921–925. doi: 10.1126/science.1252510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Cortez-Retamozo V, Etzrodt M, Newton A, Rauch PJ, Chudnovskiy A, Berger C, Ryan RJ, Iwamoto Y, Marinelli B, Gorbatov R, et al. Origins of tumor-associated macrophages and neutrophils. Proc Natl Acad Sci U S A. 2012;109:2491–2496. doi: 10.1073/pnas.1113744109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Trinklein ND, Pham D, Schellenberger U, Buelow B, Boudreau A, Choudhry P, Clarke SC, Dang K, Harris KE, Iyer S, et al. Efficient tumor killing and minimal cytokine release with novel T-cell agonist bispecific antibodies. MAbs. 2019;11:639–652. doi: 10.1080/19420862.2019.1574521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Zuch de Zafra CL, Fajardo F, Zhong W, Bernett MJ, Muchhal US, Moore GL, Stevens J, Case R, Pearson JT, Liu S, et al. Targeting multiple myeloma with AMG 424, a novel anti-CD38/CD3 bispecific T-cell–recruiting antibody optimized for cytotoxicity and cytokine release. Clin Cancer Res. 2019;25:3921–3933. doi: 10.1158/1078-0432.CCR-18-2752. [DOI] [PubMed] [Google Scholar]
- 123.Li J, Piskol R, Ybarra R, Chen YJ, Li J, Slaga D, Hristopoulos M, Clark R, Modrusan Z, Totpal K, et al. CD3 bispecific antibody-induced cytokine release is dispensable for cytotoxic T cell activity. Sci Transl Med. 2019;11:eaax8861. doi: 10.1126/scitranslmed.aax8861. [DOI] [PubMed] [Google Scholar]
- 124.Bortoletto N, Scotet E, Myamoto Y, D’Oro U, Lanzavecchia A. Optimizing anti-CD3 affinity for effective T cell targeting against tumor cells. Eur J Immunol. 2002;32:3102–3107. doi: 10.1002/1521-4141(200211)32:11<3102::AID-IMMU3102>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 125.Bonvini E, La Motte-Mohs R, Huang L, Lam CY, Kaufman T, Liu L, Alderson RF, Stahl K, Brown JG, Li H, et al. A next-generation Fc-bearing CD3-engaging bispecific dart® platform with extended pharmacokinetic and expanded pharmacologic window: characterization as CD123 X CD3 AND CD19 X CD3 dart molecules. Blood. 2018;132:5230. [Google Scholar]
- 126.Haber L, Olson K, Kelly MP, Crawford A, DiLillo DJ, Tavaré R, Ullman E, Mao S, Canova L, Sineshchekova O, et al. Generation of T-cell-redirecting bispecific antibodies with differentiated profiles of cytokine release and biodistribution by CD3 affinity tuning. Sci Rep. 2021;11:14397. doi: 10.1038/s41598-021-93842-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Arvedson TL, Balazs M, Bogner P, Black K, Graham K, Henn A, Friedrich M, Hoffmann P, Kischel R, Kufer P, et al. Abstract 55: Generation of half-life extended anti-CD33 BiTE® antibody constructs compatible with once-weekly dosing. Cancer Res. 2017;77:55. [Google Scholar]
- 128.Lorenczewski G, Friedrich M, Kischel R, Dahlhoff C, Anlahr J, Balázs M, Rock D, Boyle M, Goldstein R, Coxon A, et al. Generation of a half-life extended anti-CD19 BiTE® antibody construct compatible with once-weekly dosing for treatment of CD19-positive malignancies. Blood. 2017;130:2815. [Google Scholar]
- 129.Einsele H, Borghaei H, Orlowski RZ, Subklewe M, Roboz GJ, Zugmaier G, Kufer P, Iskander K, Kantarjian HM. The BiTE (bispecific T-cell engager) platform: development and future potential of a targeted immuno-oncology therapy across tumor types. Cancer. 2020;126:3192–3201. doi: 10.1002/cncr.32909. [DOI] [PubMed] [Google Scholar]