Abstract
The purpose of the present investigation was to determine if the efficacy of amoxicillin-clavulanate against penicillin-resistant Streptococcus pneumoniae could be improved by increasing the pediatric amoxicillin unit dose (90 versus 45 mg/kg of body weight/day) while maintaining the clavulanate unit dose at 6.4 mg/kg/day. A rat pneumonia model was used. In that model approximately 6 log10 CFU of one of four strains of S. pneumoniae (amoxicillin MICs, 2 μg/ml [one strain], 4 μg/ml [two strains], and 8 μg/ml [one strain]) were instilled into the bronchi of rats. Amoxicillin-clavulanate was given by computer-controlled intravenous infusion to approximate the concentrations achieved in the plasma of children following the administration of oral doses of 45/6.4 mg/kg/day or 90/6.4 mg/kg/g/day divided every 12 h or saline as a control for a total of 3 days. Infusions continued for 3 days, and 2 h after the cessation of infusion, bacterial numbers in the lungs were significantly reduced by the 90/6.4-mg/kg/day equivalent dosage for strains for which amoxicillin MICs were 2 or 4 μg/ml. The 45/6.4-mg/kg/day equivalent dosage was fully effective only against the strain for which the amoxicillin MIC was 2 μg/ml and had marginal efficacy against one of the two strains for which amoxicillin MICs were 4 μg/ml. The bacterial load for the strain for which the amoxicillin MIC was 8 μg/ml was not reduced with either dosage. These data demonstrate that regimens which achieved concentrations in plasma above the MIC for at least 34% of a 24-h dosing period resulted in significant reductions in the number of viable bacteria, indicating that the efficacy of amoxicillin-clavulanate can be extended to include efficacy against less susceptible strains of S. pneumoniae by increasing the amoxicillin dose.
Amoxicillin-clavulanate is an extremely effective drug for the treatment of respiratory tract infections generally and otitis media specifically due to its spectrum of activity which includes Streptococcus pneumoniae and β-lactamase-producing Haemophilus influenzae and Moraxella catarrhalis. Although amoxicillin-clavulanate is still accepted as one of the most active oral agents against S. pneumoniae, the percentage of isolates that are showing either intermediate- or high-level resistance to penicillin has been increasing (2, 5, 10). A collaborative, international survey of the antimicrobial susceptibilities of bacterial pathogens from patients with community-acquired respiratory tract infections (The Alexander Project) has documented the extent of bacterial resistance in this setting (8). Of 1,856 isolates of S. pneumoniae, high-level and intermediate-level resistance was found in 12 and 12.3% of isolates, respectively. Of the variety of agents studied, only amoxicillin-clavulanate and ceftriaxone were found to achieve time above the MIC at which 90% of isolates are inhibited (T > MIC) for four major pathogens (S. pneumoniae, H. influenzae, M. catarrhalis, and Staphylococcus aureus) for at least 50% of the dosing interval (7). However, the potential for further increases in resistance may limit the utility of all β-lactams in the future, and for this reason a number of workers have suggested that the dose of amoxicillin be increased.
Clinically, intermediate-level penicillin resistance may be overcome by increasing the dose of the penicillin (9). Some investigators have therefore recommended the use of a combination of amoxicillin plus amoxicillin-clavulanate not only to improve efficacy against penicillin-intermediate and -resistant strains of S. pneumoniae but also to retain the activity against other, β-lactamase-producing pathogens of the respiratory tract, i.e., H. influenzae and M. catarrhalis (4). Recently, Lister et al. (12) demonstrated that high-dose amoxicillin has improved activity against penicillin-nonsusceptible strains of S. pneumoniae by using an in vitro model simulating pharmacokinetics in humans and suggested that dosages of amoxicillin of 70 to 90 mg/kg of body weight/day may be sufficient to provide adequate coverage against these strains.
The approved pediatric dosage recommendation for amoxicillin-clavulanate is 25/3.2 mg/kg/day divided every 12 h for less severe infections and 45/6.4 mg/kg/day divided every 12 h for otitis media and other more severe infections. The purpose of this investigation was to determine if the efficacy of amoxicillin-clavulanate against S. pneumoniae could be extended to include more resistant strains by the use of a higher amoxicillin dosage (90 versus 45 mg of amoxicillin per kg/day). This would be difficult to assess clinically because, although resistance rates appear to be increasing, the actual number of isolates that may be collected in a limited clinical trial would be relatively small. Thus, we have used a rat pneumonia model to assess the efficacies of doses of amoxicillin-clavulanate that result in levels in the sera of rats simulating those obtained in the sera of pediatric patients following the administration of either 45/6.4 or 90/6.4 mg/kg divided into two daily doses. The pharmacodynamics of amoxicillin-clavulanate were examined against four strains of S. pneumoniae, all of which were penicillin and amoxicillin resistant on the basis of current susceptibility criteria of the National Committee for Clinical Laboratory Standards.
MATERIALS AND METHODS
Animals.
Specific-pathogen-free Sprague-Dawley rats (Charles River, UK, Ltd.) weighing approximately 200 g were used throughout the study. Animals were housed two to a cage; however, individual animals were separated by a partition. The use of animals for this study was approved by the SmithKline Beecham Animal Care and Usage Committee.
Cannulae implantation.
Animals were anesthetized (with N2O-O2 [1:1] and 3% isoflurane) and were prepared for surgery. The carotid artery and jugular vein were cannulated for blood sampling and antibiotic administration, respectively, with polythene tubing (internal diameter, 0.4 mm; outside diameter, 0.8 mm; Portex, Kent, United Kingdom). Both cannulae were exteriorized dorsally and were extended to the top of the cage through a flexible metal sheath. A polytetrafluoroethylene flange attached to one end of the metal sheath was implanted subcutaneously on the back of the rat, and the distal end was affixed to a brass ferrule and swivel joint. The device allowed full movement of the animal but prevented the sheath from being pulled into the cage. Local anesthetic (Xylocaine; Astra, Kings Langley, United Kingdom) was applied after wound closure. The animals were allowed to recover for at least 2 days prior to the establishment of infection. The cannulae were kept patent by regular flushing with 0.9% sodium chloride with heparin at 50 U/ml. A filter (Minisart; Sartorius, Epsom, United Kingdom) was affixed to the swivel joint to ensure the sterility of the infusion fluid.
Respiratory tract infection model.
Four strains of S. pneumoniae were selected from a collection of clinical isolates. Susceptibilities to amoxicillin-clavulanate, amoxicillin, and penicillin G (Britannia Pharm Ltd., Redhill Surrey, United Kingdom) are presented in Table 1. Organisms were grown overnight on nutrient agar plates containing 7% defibrinated horse blood. Inocula were prepared by harvesting the growth from three plates and suspending this in 2 ml of Todd-Hewitt broth, which then contained approximately 8 log10 CFU/ml. Immediately prior to infection the culture was diluted 1:10 in molten nutrient agar maintained at 40°C to give a final bacterial inoculum of approximately 6 log10 CFU in 50 μl of molten agar.
TABLE 1.
Susceptibilities of isolates used in the experimental infection model
| Strain | MIC (μg/ml)
|
||
|---|---|---|---|
| Amoxicillin | Amoxicillin-clavulanate | Penicillin G | |
| N1387 | 2 | 2 | 2 |
| 14319 | 4 | 4 | 8 |
| 410101 | 4 | 4 | 4 |
| RS1 | 8 | 8 | 16 |
The rats were anesthetized by separate intramuscular injections of fentanyl fluanisone (Hypnorm; Janssen Pharmaceuticals, Ltd., Grove, United Kingdom) and diazepam (Valium; Roche Products, Ltd., Welwyn Garden City, United Kingdom). The anesthetized rats were then infected by intrabronchial instillation of the 50-μl inoculum, in cooled molten agar, containing S. pneumoniae by means of nonsurgical intubation (14).
Antimicrobial administration.
Amoxicillin sodium and potassium clavulanate were obtained from SmithKline Beecham Pharmaceuticals (Worthing, United Kingdom). The drugs were prepared as sterile solutions in 0.9% sodium chloride at concentrations of 2.5 and 5 mg/ml for amoxicillin (for the 45- and 90-mg/kg/day simulations, respectively) and 0.8 mg/ml for clavulanate.
All antimicrobial agents were administered by continuous intravenous infusions rate adjusted to simulate the concentrations of amoxicillin-clavulanate achieved in the plasma of children when administered orally at either 45/6.4 mg/kg/day (amoxicillin/clavulanate) or 90/6.4 mg/kg/day in divided doses every 12 h. Dosing commenced at 24 h postinfection.
There were three experimental groups, as follows: (i) animals receiving amoxicillin-clavulanate at a simulated dosage of 45/6.4 mg/kg/day given in divided doses every 12 h, (ii) animals receiving amoxicillin-clavulanate at a simulated dosage of 90/6.4 mg/kg/day given in divided doses every 12 h, and (iii) untreated control animals, which were infused with 0.9% sodium chloride at a rate similar to those at which the other groups were infused. Seven or eight rats were included in each group. Infusions continued for 3 days, with a total of six doses being simulated.
Simulation of concentrations in serum.
The desired concentrations in plasma were determined by application of a linear, one-compartment absorption model, C(t) = A(e−kelt − e−kat), where C is the concentration in plasma at time t, A is the zero-time intercept assuming instantaneous absorption, e is the base of the natural log, kel is the elimination rate constant, and ka is the absorption rate constant. The values for the required pharmacokinetic parameters in children were obtained from the literature (13) and are given in Table 2. Data on the pharmacokinetics of amoxicillin and potassium clavulanate in rats (Table 2) used to determine dosing parameters were determined from concentrations measured in uninfected animals after intravenous administration of a bolus dose of 100 mg of amoxicillin per kg of body weight and 20 mg of potassium clavulanate per kg.
TABLE 2.
Pharmacokinetic parameters in childrena and in rats used to determine infusion rates for simulation studies with rats
| Species, pharmaco-kinetic model | Parameter | Amoxicillin | Clavulanate |
|---|---|---|---|
| Human, C(t) = A(exp−ket −exp−kat) | A (μg/ml) | 22 | 4.5 |
| kel (H−1) | 0.56 | 0.61 | |
| Ka (h−1) | 1.56 | 1.57 | |
| Rat, C = a · exp−λtb | a (μg/ml) | 240 | 27 |
| λ (h−1) | 2.1 | 2.4 | |
| Dose (mg/kg) | 44 | 24 |
Data for children have been published previously (13).
C is concentration, a is the zero-time intercept, and λ is the elimination rate constant.
Flow rates were controlled with infusion pumps (Pump 22; Harvard Instruments, Edenbridge, United Kingdom) linked serially to a microcomputer. The infusion flow rates were changed individually every 60 s, and the system was programmed to administer the calculated rates in 12-h cycles over 3 days.
Sampling and assay.
Blood samples were taken from the carotid artery cannulae at seven intervals during antimicrobial infusion for determination of antimicrobial concentrations in plasma. The concentrations of amoxicillin and clavulanate were determined by a large-plate agar diffusion assay with Micrococcus luteus NCTC 8340 for amoxicillin and an enzyme inhibition assay with Klebsiella pneumoniae NCTC 11228 for clavulanate (11). Samples were assayed in duplicate against standards over the concentration range of 0.015 to 1 μg/ml for amoxicillin and 0.078 to 5 μg/ml for clavulanate. The lowest concentration was taken as the limit of detection for the assay. The correlation coefficients for the regression lines were not less than 0.997. The within-day coefficients of variation were less than 5% for amoxicillin and 6.1 to 9.4% for clavulanate, and the between-day coefficients of variation were 3.7 to 6.2% for amoxicillin and 6.3 to 9.8% for clavulanate.
At approximately 2 h after the cessation of infusion, the animals were killed by CO2 inhalation and the lungs were removed aseptically and homogenized in 1 ml of isotonic saline for the enumeration of viable bacteria. Tenfold serial dilutions were prepared in phosphate-buffered saline (0.1 M; pH 7.4), and the appropriate dilutions were plated in triplicate (0.02 ml per drop) onto blood agar plates by a modified Miles-Misra technique. Colonies were counted after overnight incubation at 37°C. The lower limit of detection was 1.7 log10 CFU per pair of lungs.
Data analysis.
Data on the concentration in serum were fitted with an iterative least-squares modeling program, MK-MODEL. The outcome measure for comparison of treatments was the number of bacteria in the lungs at the conclusion of the study. All results are presented as group means with standard deviations. Differences among groups were tested by analysis of variance with Scheffe’s test for multiple contrasts (StatisticA for Windows; Statsoft Inc., Tulsa, Okla.). A P value of ≤0.05 was considered significant.
RESULTS
Concentrations achieved in plasma.
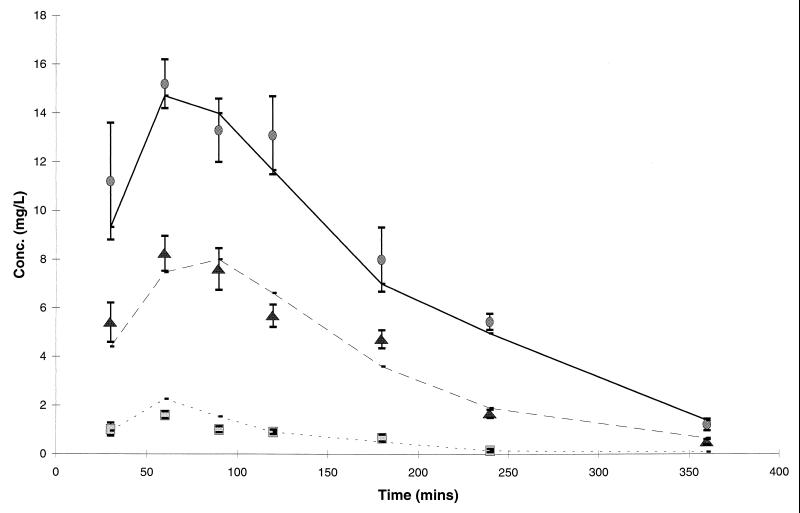
The concentrations of amoxicillin and clavulanate achieved in the plasma of rats are presented in Fig. 1 and are compared with the target concentrations determined from pharmacokinetic parameters in children.
FIG. 1.
Concentrations of amoxicillin ( ; 45 mg/kg/day equivalent;
; 45 mg/kg/day equivalent;  , 90 mg/kg/day equivalent) and clavulanate (
, 90 mg/kg/day equivalent) and clavulanate ( ; 6.4 mg/kg/day) in the plasma of rats in comparison with target levels in humans (—). Data for rats are presented as the means and standard deviations for eight animals.
; 6.4 mg/kg/day) in the plasma of rats in comparison with target levels in humans (—). Data for rats are presented as the means and standard deviations for eight animals.
Efficacy data.
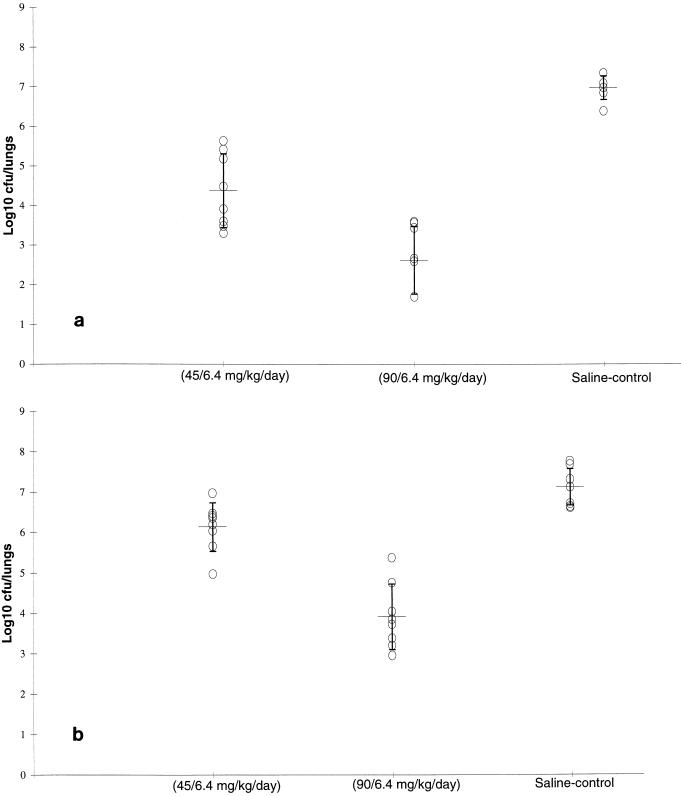
The efficacies of both dosages of amoxicillin-clavulanate against S. pneumoniae N1387 (MIC, 2 μg/ml) are indicated in Fig. 2a. The mean number of bacteria in the lungs of saline-treated animals was 6.97 ± 0.3 log10 CFU/pair of lungs. Both simulated dosages of amoxicillin-clavulanate significantly reduced the numbers of viable bacteria in the lungs compared with the numbers in the lungs of the control animals (4.37 ± 0.93 and 2.62 ± 0.85 log10 CFU/pair of lungs for the low- and high-dosage simulations, respectively). However, amoxicillin-clavulanate at the higher simulated dosage (90/6.4 mg/kg/day equivalent) was significantly more effective than at the lower simulated dosage (45/6.4 mg/kg/day equivalent; P < 0.01).
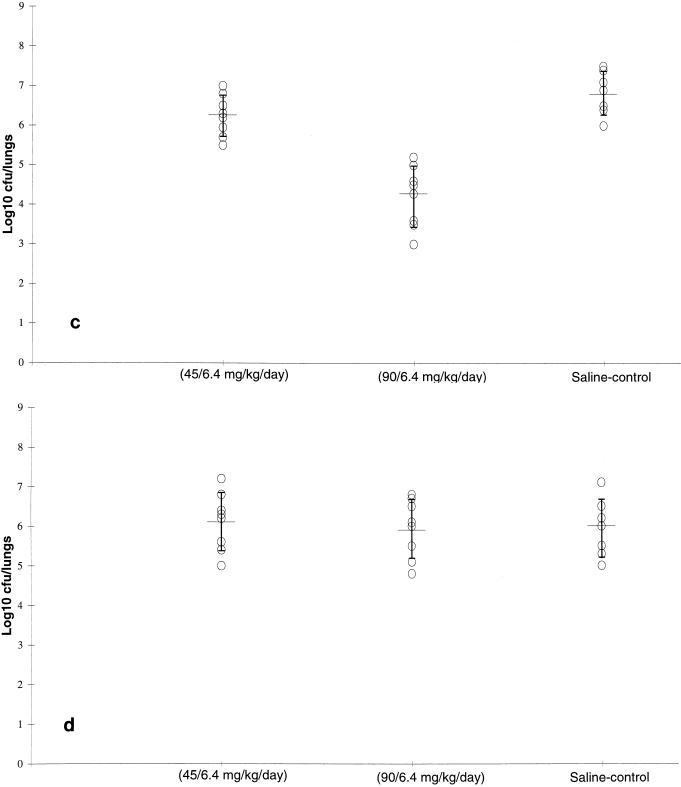
FIG. 2.
Efficacy of doses of amoxicillin plus potassium clavulanate resulting in levels in rat plasma simulating those achieved in the plasma of humans against S. pneumoniae N1387 (a), S. pneumoniae 410101 (b), S. pneumoniae 14319 (c), and S. pneumoniae RS1 (d) in an experimental respiratory infection in rats. Data are represented as mean and standard deviation bacterial numbers in the lungs of individual animals.
The mean number of bacteria in the lungs of saline-treated animals infected with S. pneumoniae 410101 (MIC, 4 μg/ml; Fig. 2b) was 7.11 ± 0.45 log10 CFU/pair of lungs.
Amoxicillin-clavulanate administered at a dosage to simulate a dosage of 45/6.4 mg/kg/day reduced the number of viable bacteria in the lungs to a limited but significant extent (6.14 ± 0.6 log10 CFU/pair of lungs; P < 0.05). In animals in which the higher dosage of amoxicillin-clavulanate was simulated, the mean number of bacteria in the lungs was further reduced to 3.91 + 0.81 log10 CFU/pair of lungs and this dosage was significantly more effective than amoxicillin-clavulanate at the lower simulated dosage (P < 0.01).
Against S. pneumoniae 14319 (MIC, 4 μg/ml; Fig. 2c), the effect of treatment with the 45/6.4-mg/kg/day amoxicillin-clavulanate equivalent dosage (6.26 ± 0.47 log10 CFU/pair of lungs) was no different from the effect of the control treatment (6.8 ± 0.62 log10 CFU/pair of lungs). In contrast, treatment with the 90/6.4-mg/kg/day amoxicillin-clavulanate equivalent dosage was more effective (4.28 ± 0.82 log10 CFU/pair of lungs) than no treatment (control) and treatment with the 45/6.4-mg/kg/day equivalent dosage (P < 0.01). Neither dosage of amoxicillin-clavulanate reduced the numbers of viable bacteria in the lungs compared with the numbers in the lungs of the control animals (P > 0.05) when the drug was tested against S. pneumoniae RS1 (MIC, 8 μg/ml; Fig. 2d).
T > MIC.
The T > MIC for each strain was calculated for both amoxicillin-clavulanate dosage regimens. The T > MIC and the percentages of the dosing intervals that the concentrations in plasma were above the MICs are presented in Table 3. Regimens that achieved concentrations in plasma above the MIC for at least 34% of the 12-h dosing interval resulted in significant reductions in viable bacterial counts.
TABLE 3.
Duration of time and percentage of dosing interval above the amoxicillin-clavulanate MIC at the doses resulting in levels in rat plasma simulating those achieved in the plasma of pediatric patients
| MIC (μg/ml) |
T > MIC (h) (% of 24 h dosing period)
|
|
|---|---|---|
| Amoxicillin-clavulanate (45/6.4 mg/kg/day) | Amoxicillin-clavulanate (90/6.4 mg/kg/day) | |
| 8 | 0 (0) | 6.5 (27.1) |
| 4 | 5.5 (23) | 8.8 (36.7) |
| 2 | 8.1 (34) | 11 (45.8) |
| 1 | 10.8 (45) | 13.3 (55.6) |
DISCUSSION
The controlled, adjusted-rate intravenous infusion model in rats used in the studies described here simulated accurately the concentrations of amoxicillin-clavulanate that would be obtained in the plasma of children treated with amoxicillin-clavulanate at dosages of 45/6.4 and 90/6.4 mg/kg/day divided every 12 h. The data obtained with this model may therefore be expected to more accurately reflect the efficacy that might be seen clinically with these doses of amoxicillin-clavulanate.
Pharmacodynamic factors are clearly important in predicting drug efficacy and can suggest the dosing methods that can be used to improve outcome from treatment. Bacterial killing by β-lactam antimicrobial agents and efficacy in experimental models are related to the duration of time that the concentration of the agent in plasma remains above the MIC (6). T > MIC was found to be the only significant parameter predictive of the efficacy of penicillin treatment against S. pneumoniae (15) in a thigh infection model with neutropenic mice. Therefore, dosage regimens that increase T > MIC beyond a critical minimum value may positively influence outcome from infection.
Andes et al. (1) and Woodnutt et al. (16) used two pharmacodynamic models and have reported that for amoxicillin-clavulanate, maximal bacterial killing occurs when the concentrations in serum exceed the MIC for 35 to 40% of the dosing interval, although significant reductions in bacterial numbers occur with proportions of T > MICs of as little as 25% of the dosing interval (1). These data would therefore suggest that for a dose of amoxicillin-clavulanate of 45/6.4 mg/kg/day, organisms for which MICs are 2 μg/ml should prove to be fully susceptible (proportion of T > MIC = 34%). In the studies described here concentrations that simulate those obtained in children following administration of this standard clinical dose of amoxicillin-clavulanate were significantly effective against S. pneumoniae N1387, for which the MIC was 2 μg/ml. However, against strains for which the MICs were 4 or 8 μg/ml, this dose had a slight (approximately 1 log reduction in bacterial numbers) but significant effect against only one of the three strains tested. An increase in the amoxicillin dosage to 90 mg/kg/day resulted in significant efficacy against those strains for which the MICs were 4 μg/ml (proportion of T > MIC = 37%), but this dosage was also not effective against the single organism for which the MIC was 8 μg/ml.
Barry et al. (3) demonstrated with a gerbil model of otitis media that effective treatment of highly penicillin-resistant strains of S. pneumoniae (amoxicillin MICs, 2 to 4 μg/ml) could be achieved by elevation of the dose from 2.5 mg/kg (for sensitive strains) to 25 mg/kg for the resistant strain tested. These doses would provide proportions of T > MICs in serum for the intermediate and resistant strains of less than 20%. However, the levels of amoxicillin in the middle ear, which were similar to those obtained in children following the oral administration of a dose of 15 mg/kg, were significantly prolonged compared with those in serum and would have provided proportions of T > MICs of approximately 33 to 42%. Recently, Lister et al. (12), using an in vitro model in which concentrations in the human middle ear were simulated, reported that increased concentrations of amoxicillin proved to be effective against resistant strains of S. pneumoniae for which amoxicillin MICs were as high as 4 μg/ml. These data demonstrated that concentrations of approximately 6 to 9 μg/ml were necessary for efficacy. The proportions of T > MIC from these simulated data against strains for which the MICs were either 2 or 4 μg/ml were between approximately 33 and 41%, assuming a twice-daily dosing regimen. The simulated concentrations were estimated to be achievable in the middle ears of children receiving dosages approximating 70 to 90 mg/kg/day.
In conclusion, assuming that T > MIC is a valid indicator of the efficacies of β-lactam antimicrobial agents, then amoxicillin doses approximating 45 mg/kg/day in divided doses given every 12 h (with 6.4 mg of clavulanate per kg per day) are likely to provide drug for a sufficient duration of time above the MIC to be effective against strains of S. pneumoniae that are susceptible or that have intermediate resistance on the basis of the current breakpoints of the National Committee for Clinical Laboratory Standards. An increase in the amoxicillin dosage to 90 mg/kg/day (with 6.4 mg of clavulanate per kg per day) should be sufficient to eradicate S. pneumoniae strains for which MICs are 4 μg/ml or less. These findings support the use of the higher-dosage (90/6.4 mg/kg/day divided every 12 h) amoxicillin-clavulanate regimen for the treatment of infections caused by penicillin-intermediate and even penicillin-resistant S. pneumoniae strains.
ACKNOWLEDGMENTS
The study was supported by SmithKline Beecham Pharmaceuticals.
We thank Joanna Bryant for technical assistance.
REFERENCES
- 1.Andes D, Urban A, Craig W A. Program and abstracts of the 35th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1995. In-vivo activity of amoxicillin and amoxicillin/clavulanate against penicillin-resistant pneumococci, abstr; p. A82, 16. [Google Scholar]
- 2.Applebaum P C. Antimicrobial resistance in Streptococcus pneumoniae: an overview. Clin Infect Dis. 1992;15:77–83. doi: 10.1093/clinids/15.1.77. [DOI] [PubMed] [Google Scholar]
- 3.Barry B, Muffat-Joly M, Gehanno P, Pocidalo J. Effect of increased dosages of amoxicillin in treatment of experimental middle ear otitis due to penicillin-resistant Streptococcus pneumoniae. Antimicrob Agents Chemother. 1993;37:1599–1603. doi: 10.1128/aac.37.8.1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Block S L, Harrison C J, Hedrick J A, Tyler R D, Smith R A, Keegan E, Chartrand S A. Penicillin-resistant Streptococcus pneumoniae in acute otitis media: risk factors, susceptibility patterns and antimicrobial management. Pediatr Infect Dis. 1995;15:751–759. doi: 10.1097/00006454-199509000-00005. [DOI] [PubMed] [Google Scholar]
- 5.Caputo G M, Applebaum P C, Liu H H. Infections due to penicillin-resistant pneumococci. Clinical, epidemiologic, and microbiologic features. Arch Intern Med. 1993;153:1301–1310. [PubMed] [Google Scholar]
- 6.Drusano G L. Role of pharmacokinetics in outcome of infections. Antimicrob Agents Chemother. 1988;32:289–297. doi: 10.1128/aac.32.3.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Drusano, G. L., and F. W. Goldstein. 1996. Relevance of the Alexander Project: pharmacodynamic considerations. J. Antimicrob. Chemother. 38(Suppl. A):141–154. [DOI] [PubMed]
- 8.Felmingham, D., and R. N. Gruneberg. 1996. A multicentre collaborative study of the antimicrobial susceptibility of community-acquired, lower respiratory tract pathogens 1992–1993: the Alexander Project. J. Antimicrob. Chemother. 38(Suppl. A):1–57. [DOI] [PubMed]
- 9.Friedland I R, McCracken G H. Management of infections caused by antibiotic-resistant Streptococcus pneumoniae. N Engl J Med. 1994;331:377–382. doi: 10.1056/NEJM199408113310607. [DOI] [PubMed] [Google Scholar]
- 10.Gold H S, Moellering R C. Antimicrobial-drug resistance. N Engl J Med. 1996;335:1445–1453. doi: 10.1056/NEJM199611073351907. [DOI] [PubMed] [Google Scholar]
- 11.Jackson D, Cooper D L, Horton R, Langley P F, Staniforth D S, Sutton A J. Absorption, pharmacokinetic and metabolic studies with Augmentin. In: Croydon E A P, Michael M F, editors. Augmentin: clavulanate potentiated amoxycillin. Proceedings of the European Symposium. Scheveningen, The Netherlands. Amsterdam, The Netherlands: Excerpta Medica; 1982. [Google Scholar]
- 12.Lister P D, Pong A, Chartrand S A, Sanders C C. Rationale behind high-dose amoxicillin therapy for acute otitis media due to penicillin-nonsusceptible pneumococci: support from in vitro pharmacodynamic studies. Antimicrob Agents Chemother. 1997;41:1926–1932. doi: 10.1128/aac.41.9.1926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nelson J D, Kusmiesz H, Shelton S. Pharmacokinetics of potassium clavulanate in combination with amoxicillin in pediatric patients. Antimicrob Agents Chemother. 1982;21:681–682. doi: 10.1128/aac.21.4.681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Smith G M, Abbott K H. Development of experimental respiratory infections in neutropenic rats with either penicillin-resistant Streptococcus pneumoniae or β-lactamase-producing Haemophilus influenzae. Antimicrob Agents Chemother. 1994;38:608–610. doi: 10.1128/aac.38.3.608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vogelman B, Gundmundsson S, Leggett J, Turnidge J, Ebert S, Craig W A. Correlation of antimicrobial pharmacokinetic parameters with therapeutic efficacy in an animal model. J Infect Dis. 1988;158:831–847. doi: 10.1093/infdis/158.4.831. [DOI] [PubMed] [Google Scholar]
- 16.Woodnutt G, Berry V, Bryant J. Program and abstracts of the 36th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1996. Evaluation of reduced dosing schedule of amoxicillin (AMX) using two pharmacodynamic models, abstr. A40; p. 8. [Google Scholar]