Abstract
The approval of immunotherapies such as checkpoint inhibitors (CPIs), adoptive cell therapies and cancer vaccines has revolutionized the way cancer treatment is approached. While immunotherapies have improved clinical outcome in a variety of tumor types, some cancers have proven harder to combat using single agents, underscoring the need for multi-targeted immunotherapy approaches. Efficacy of CPIs and cancer vaccines requires patients to have a competent immune system with adequate cell numbers while the efficacy of adoptive cellular therapy is limited by the expansion and persistence of cells after infusion. A promising strategy to overcome these challenges is combination treatment with common gamma-chain cytokines. Gamma-chain cytokines play a critical role in the survival, proliferation, differentiation and function of multiple immune cell types, including CD8 T-cells and NK cells, which are at the center of the anti-tumor response. While the short half-life of recombinant cytokines initially limited their application in the clinic, advancements in protein engineering have led to the development of several next-generation drug candidates with dramatically increased half-life and bioactivity. When combining these cytokines with other immunotherapies, strong evidence of synergy has been observed in preclinical and clinical cancer settings. This promising data has led to the initiation of 70 ongoing clinical trials including IL-2, IL-7, IL-15 and IL-21. This review summarizes the recent advancements of common gamma-chain cytokines and their potential as a cancer immunotherapy.
Keywords: Cancer, Immunotherapy, Cytokines, IL-2, IL-7
INTRODUCTION
Ipilimumab (anti-CTLA-4) was the first checkpoint inhibitor (CPI) approved for the treatment of melanoma in 2011. In the years since, we have seen an unprecedented shift in how cancer treatment is approached. Checkpoint blockade, adoptive cell therapy (ACT) and cancer vaccines represent some of the major immunotherapy strategies being implemented and investigated for both hematological and solid tumors at various stages of disease.
While these immunotherapies have improved clinical outcomes for some cancer types, they fail in others, highlighting unique challenges for each strategy. CPIs largely rely on the presence of CPI-responsive immune cells to be effective, with low clinical efficacy in immunologically ‘cold’ tumors (1). ACT infuses educated or engineered immune cells into the patient, but the survival and persistence of these adoptively transferred cells is a major limitation (2). Cancer vaccines heavily rely on antigen immunogenicity and memory formation, with low efficacy in immunosuppressive tumor types (3). To overcome these limitations, the combination of these immunotherapies with members of the common gamma-chain family of cytokines represents a promising strategy. Common gamma-chain cytokines, whose biology and immunological effects have been thoroughly reviewed elsewhere (4,5), have strong effects on the survival, proliferation, differentiation and function of lymphocyte subsets capable of mounting an anti-tumor response (6). With current immunotherapies being limited by the number and function of lymphocytes upon treatment, common gamma-chain cytokines can act synergistically to improve clinical efficacies. While their early clinical development was limited due to rapid clearance, many strategies have since been developed to increase their half-life, making their application more practical and reliable. This review will highlight the recent advancements of gamma-chain cytokines being considered as drug candidates and their application as either a monotherapy or in combination with other leading immunotherapies for the treatment of cancer.
IL-2
IL-2 was the first common gamma-chain family cytokine to be discovered as a T-cell growth factor and later understood to function as an inducer of cytotoxic T-cells, NK cells and Treg cells (6). It is mainly produced by activated CD4 T-cells and its receptor is a heterotrimeric complex consisting of IL-2Rα, IL-2Rβ and IL-2Rγ. The IL-2Rβ/γ receptor is expressed on naïve CD8 and memory CD4 and CD8 T-cells. While all 3 subunits of the IL-2 receptor (IL-2Rα/β/γ) are found on activated CD4 and CD8 T-cells (7), high expression of IL-2Rα is a defining characteristic of Tregs (8). The presence of IL-2Rα significantly increases the binding affinity of IL-2 for its receptor. Consequently, low levels of IL-2 are likely to induce an anti-inflammatory response by expanding Tregs. In addition, an immunostimulatory effect requires high-dose (HD) IL-2 and is associated with serious toxicities. Next-generation IL-2 molecules are being developed to harness the immunostimulatory effect of IL-2 while avoiding Treg stimulation and toxicity.
IL-2 as a monotherapy
Proleukin® (Aldesleukin; Chiron, now Clinigen; also known as [aka] HD IL-2) was the first cytokine approved by the Food and Drug Administration (FDA, Silver Spring, MD, USA) in 1992 for metastatic renal cell carcinoma (mRCC) (9) and later (1998) for metastatic melanoma (mM) (10). Longitudinal follow-up studies established that approximately 7% mRCC (17/255) and 6% mM (17/270) patients had complete response (CR) without any further systemic therapy (10,11). However, the required repeated dosing due to short half-life (13–85 min), the black box warning for life-threatening toxicities such as vascular leak syndrome (VLS) and multiple contraindications (12) have spurred the development of next-generation IL-2-based cytokine therapies.
Next-generation IL-2 candidates have been developed to bypass IL-2Rα binding, a strategy that lowers the risk of toxicity by lowering the therapeutic dose. THOR-707 (SAR444245, Sanofi, Paris, France) is a recombinant IL-2 variant with a polyethylene glycol (PEG) moiety irreversibly bound to a novel amino acid via click chemistry (13), restraining the binding of the α chain while retaining native binding affinity of the βγ receptor (14). Functionally, THOR-707 elicits NK and CD8 T-cell-mediated anti-tumor activity while limiting proliferation of Tregs and other lymphoid cells that trigger life-threatening complications (15). In an open-label, multicenter phase I/II study (NCT04009681), THOR-707 was administered intravenously (i.v.) as a monotherapy every 2 wk (Q2W). Interim results reported from 28 patients with advanced or metastatic solid tumors showed no dose-limiting toxicity (DLT) or VLS. CD8 T-cells and NK cells increased by a median of 3.1-fold and 7.93-fold after cycle 1, respectively, and were sustained until the next cycle. Despite the modifications, there was a 1.89-fold increase in Tregs. Three patients had confirmed partial response (PR) while 2 had stable disease (SD) and continued for >5 cycles. THOR-707 half-life was about 10 h and there were no reported anti-drug antibodies (against IL-2 or PEG). Overall, these results demonstrate the safety and tolerability of an IL-2 variant that has bias for the IL-2Rβ/γ, leading to successful induction of CD8 T-cells and NK cells while avoiding significant Treg stimulation (16).
NKTR-214 (Bempegaldesleukin, Nektar Therapeutics, San Francisco, CA, USA) is a prodrug consisting of IL-2 conjugated to 6 resealable PEG chains. NKTR-214 retains the amino acid sequence of IL-2 while masking the IL-2Rα chain to mitigate engagement and proliferation of Tregs. In preclinical models, NKTR-214’s active conjugated IL-2 was 50-times higher when compared to aldesleukin, without evidence of VLS or pulmonary edema (17). Based on these promising results, a phase I/II trial was designed to study the safety, tolerability, pharmacokinetics (PK) and efficacy in patients with advanced or metastatic solid tumors (NCT02869295). NKTR-214 was administered i.v. at 0.003 to 0.012 mg/kg Q3W or 0.006 mg/kg Q2W until disease progression or unacceptable toxicities occurred (18). NKTR-214-related cytokine (NKTR-214-RC), which consists of various forms of IL-2 conjugated to PEG, remained detectable up to 8–11 days post administration while NKTR-214-active cytokine (NKTR-214-AC) concentrations increased gradually, with Tmax reaching between 24 and 48 h. Neither anti-NKTR-214 nor anti-IL-2 antibodies were detected. Pharmacodynamic analysis showed an increase in CD8 T-cell tumor infiltrating lymphocytes (TILs) within the tumor microenvironment (TME). These encouraging results have given way to combination therapies with CPIs, discussed below (Table 1).
Table 1. Summary of ongoing clinical trials involving next-generation gamma-chain cytokines.
| Drug | Company | Cytokine | Phases | Indications | Immunotherapy combinations | Testing as monotherapy* | Trial identifiers |
|---|---|---|---|---|---|---|---|
| ALKS 4230 | Alkermes, Inc. | IL-2 | I, II, III | Advanced solid tumors | Anti-PD-1 | Yes | NCT03861793; NCT04592653; NCT02799095; NCT04144517; NCT05092360; NCT04830124 |
| FAP-IL2v | Hoffmann-La Roche | IL-2 | I, II | Advanced solid tumors | Anti-PD-1, anti-PD-L1, CXCR4 antagonist, anti-CD40, adenosine receptor antagonist, anti-TIGIT, IL-6R inhibitor | No | NCT03386721; NCT03875079; NCT02627274; NCT03193190 |
| GI-101 | GI Innovation, Inc. | IL-2 | I, II | Advanced solid tumors | Anti-PD-1 | No | NCT04977453 |
| hu14.18-IL2 | Apeiron Biologics AG | IL-2 | I, II | Melanoma | Anti-PD-1, anti-CTLA-4 | No | NCT03958383 |
| L19-IL2 | Philogen S.p.A. | IL-2 | II, III | Advanced solid tumors | L19-TNFα | Yes | NCT03705403; NCT03567889; NCT04362722 |
| NKTR-214 | Nektar Therapeutics | IL-2 | I, II, III | Advanced solid tumors | Anti-PD-1, anti-CTLA-4, TLR7/8 agonist, DNA cancer vaccines, Flt3L, poly ICLC | No | NCT03785925; NCT04936841; NCT04730349; NCT03729245; NCT04410445; NCT02983045; NCT03138889; NCT03435640; NCT03548467; NCT03635983; NCT04209114; NCT04540705; NCT04969861; NCT03835533; NCT03282344 |
| RO7284755 | Hoffmann-La Roche | IL-2 | I | Advanced solid tumors | Anti-PD-L1 | No | NCT04303858 |
| THOR-707 | Sanofi | IL-2 | I, II | Advanced solid tumors | Anti-PD-1 | No | NCT05061420; NCT04914897; NCT04913220; NCT04009681; NCT05104567 |
| NT-I7/GX-I7 (efineptakin alfa) | NeoImmuneTech, Inc./Genexine, Inc. | IL-7 | I, II | Advanced solid tumors, lymphoma | Anti-PD-1, anti-PD-L1, CD19 CART cells | Yes | NCT04332653; NCT05075603; NCT03687957; NCT03901573; NCT04594811; NCT04984811; NCT04893018; NCT04588038; NCT02659800 |
| N-803 | ImmunityBio, Inc. | IL-15 | I, II, III | Advanced solid tumors, acute myeloid leukemia | Anti-PD-1, anti-PD-L1, anti-CTLA-4, NK cell adoptive transfers, ab-drug conjugate, anti-PD-L1/anti-TGFβ, DNA vaccines, autologous cancer vaccine, antigen vaccines, anti-VEGF, anti-VEGFR-2, anti-EGFR, anti-CD274 | Yes | NCT04290546; NCT03329248; NCT03563157; NCT03136406; NCT03228667; NCT03387111; NCT02138734; NCT03022825; NCT01898793; NCT05096663; NCT02465957; NCT02523469; NCT02890758; NCT03387111; NCT02989844; NCT04847466; NCT04247282; NCT03493945; NCT04927884; NCT04898543; NCT04390399; NCT03520686 |
| NKTR-255 | Nektar Therapeutics | IL-15 | I, II | Myeloma, lymphoma, HNSCC | Anti-CD38, anti-CD20 | No | NCT04136756; NCT04616196 |
HNSCC, head and neck squamous cell carcinoma.
*Whether or not the drug is being tested as the only immunotherapy in the trial.
ALKS 4230 (Nemvaleukin alfa, Alkermes Inc., Waltham, MA, USA) is a fusion protein of circularly-permuted IL-2 with IL-2Rα, designed to target cells expressing IL-2Rβ/γ and not IL-2Rα. In murine preclinical models the treatment was well tolerated with significantly less proinflammatory plasmatic cytokines compared to rhIL-2. After daily subcutaneous (s.c.) treatment for 5 days, there was a dose-dependent increase of NK and memory CD8 T-cells in the spleen without an increase of Tregs. ALKS 4230 also showed anti-tumor function, with i.v. and s.c. administration in a B16F10 melanoma lung model showing significantly fewer colonies in mice treated with ALKS 4230 compared to the vehicle control (19). In cynomolgus monkeys, 2 s.c. doses led to a 4-fold increase of NK cells, a 6-fold increase of CD8 T-cells and a 2-fold increase in Tregs, peaking between days 6–8 and returning to baseline by day 14 (20). Currently, ALKS 4230 monotherapy is being tested in a phase II trial in melanoma patients (NCT04830124) (Table 1).
Another strategy for next-generation IL-2 drug candidates is to target IL-2 to the tumor site. L19-IL2 (Darleukin, Philogen S.p.A, Siena, Italy) is a fully human antibody with the IL-2 moiety fused to the single-chain Fv antibody fragment targeting the alternatively spliced extra-domain B (EDB) of fibronectin. EDB preferentially accumulates around neo-vasculature structures and is usually excluded from healthy human tissues, except female reproductive tissues (21,22). In a first-in-human phase I/II clinical trial in advanced solid tumors, the maximum tolerated dose (MTD) was found to be 22.5 Mio IU and the half-life was 2–3 h, with manageable and reversible toxicities (23). Pharmacodynamic analysis showed an increase in CD8 T-cells and NK cells, while Tregs were markedly diminished. At the time of publication, 2 patients who had received 4 out of 6 total cycles of L19-IL2 remained progression-free for, approximately, 30 months. L19-IL2 is also currently being tested in phase II trials in combination with Rituximab (NCT02957019), radiation therapy (NCT02086721) and dacarbazine (NCT01055522), and in a phase III trial in combination with targeted TNF (NCT02938299). A recently developed novel format of L19-IL2, the L19L19-IL2 (antibody in a single-chain diabody), showed superior tumor-specific biodistribution, but it is still in preclinical development (24).
Hu14.18-IL2 (APN301, Apeiron Biologics AG, Wien, Austria) is a humanized immunocytokine (IC) comprised of human IL-2 linked to hu14.18 mAb, which recognizes the GD2 disialoganglioside expressed on melanoma, neuroblastoma, and certain sarcomas. The half-life of hu14.18-IL2 was reported to be 3.1 h (25) with daily dosing needed to maintain in vivo activity. Phase II trials with hu14.18-IL2 i.v. resulted in 1 PR in a melanoma patient (NCT00109863) (26) and 5 CRs in a pediatric oncology study of neuroblastoma (NCT00082758) (27). Most common DLTs included fever, chills, nausea, anaphylactoid reactions and neuropathic pain. Based on a superior clinical response in patients with low tumor burden (minimal residual disease) in the neuroblastoma cohort, a pilot phase II trial was conducted in 23 patients with completely resectable recurrent stage III or stage IV melanoma (NCT00590824). Out of 18 patients, 6 remained recurrence-free for a median of 5.6 months with a 24-month overall survival (OS) rate of 65% (28).
While initial studies testing aldesleukin revealed life-threatening toxicities, recent studies prove that next-generation IL-2 drug candidates have improved efficacy and are better tolerated. Accordingly, several trials are underway to test the safety and tolerability of these IL-2 drug candidates in combination with other immunotherapies.
IL-2 in combination with CPIs
Several trials investigating the combination of next-generation IL-2 drug candidates with CPIs are ongoing (Table 1). The PIVOT-10 study published preliminary results testing NKTR-214, the pro-IL-2 molecule that targets IL-2Rβ, in combination with nivolumab in patients with locally advanced or metastatic solid tumors. Results from this study showed that the combination of NKTR-214 and nivolumab was well tolerated and had encouraging response rates. Nivolumab monotherapy is approved for patients with urothelial cancer (UC) that have progressed following platinum-containing chemotherapy, after an overall response rate (ORR) of 19.6% observed in the CHECKMATE-275 trial (29). Of the 27 evaluable patients with UC, the combination treatment showed an investigator-assessed ORR of 48%, including 5 CR (30), regardless of baseline PD-L1 expression. These results highlight the potential of NKTR-214 to improve the ORR historically seen with nivolumab monotherapy and could represent a promising treatment option for cisplatin-ineligible UC patients.
A second next-generation IL-2 drug candidate tested in combination with a CPI is fibroblast activation protein (FAP)-IL2v (simlukafusp alfa, Hoffmann-La Roche, Basel, Swiss). This IL-2 variant has increased affinity for IL-2Rβγ and is conjugated to an antibody targeting FAPα. When combined with anti-muPD-L1 in a murine Panc02 pancreatic model, median survival was significantly longer when compared to either single agent therapy (31). Results from a phase II study combining FAP-IL2v with atezolizumab showed an ORR of 27% in patients with recurrent or metastatic cervical squamous cell carcinoma, with an acceptable safety profile (32).
RO72284755 (Hoffmann-La Roche) is a recombinant fusion protein with an antibody directed against PD-1 linked to IL-2v. This drug candidate is currently being tested in the clinic as a monotherapy or in combination with atezolizumab in patients with advanced and/or metastatic solid tumors (NCT04303858). GI-101 (GI Innovation, Inc., Seoul, Korea) is a bispecific CD80-IgG4-IL2 variant that targets CTLA-4-expressing cells with preferential binding for IL-2Rβ. Preclinical models showed significant proliferation of NK cells and CD8 T-cells and improved tumor control as a monotherapy and in combination with pembrolizumab (33). In a CT26 tumor model, the TME showed increased M1 macrophages and CD8 central memory cells, with no Treg cell proliferation (34). This data led to the KEYNOTE-B59 trial involving patients with advanced solid tumors to determine the safety, tolerability and the recommended phase 2 dose (NCT04977453) (35).
IL-2 in combination with ACT
While infusion of aldesleukin is common practice after ACT to improve T-cell activity in vivo, DLTs associated with HD IL-2 limit its use (36). Most recent clinical trials combining ACT with IL-2 have involved patients with advanced melanoma and have sought to improve toxicities by lowering the dose, administering subcutaneously or by using a modified IL-2 protein. Several trials have reported a favorable toxicity for aldesleukin given after TIL infusion as s.c. low-dose, (37,38), s.c. regular dose (39) or i.v. low-dose (39), with pharmacodynamic analysis showing some indirect evidence of TIL engraftment (38). However, to this date, these trials have failed to prove that combination treatment provides a clinical improvement over TIL infusion alone.
Regarding next-generation IL-2 drug candidates, while preclinical data from NKTR-214 suggests potential efficacy (40), the development has not reached clinical stages.
IL-2 in combination with cancer vaccines
IL-2 in combination with various cancer vaccines has shown little promise in completed clinical trials. Multiple trials have seen the expansion of Tregs, even with low-dose IL-2. For melanoma patients receiving a vaccine of HLA-class I peptides and low-dose cyclophosphamide (CTX), the depletion of Tregs was transient after CTX treatment and returned to baseline levels by 8 wk after vaccination. At 13 and 15 wk, the patients received low-dose IL-2 with the goal of boosting vaccine-induced immune responses. Pharmacodynamic analysis 1 wk after IL-2 treatment showed a significant increase of Tregs without impairing the expansion and functionality of peptide-specific CD8 T-cells (41). However, this CD8 T-cell increase was ultimately not associated with an improvement of disease-free or OS compared to the observation group (42). In a different approach involving patients with solid tumors, IL-2 was administered with GM-CSF and a vaccine containing mutant ras peptides. Patients receiving IL-2 alone or in combination with GM-CSF had lower vaccine-induced immune responses compared to patients that received GM-CSF alone, with no difference in progression-free survival (PFS) or OS observed (43). Low-dose IL-2 has also been tested in combination with a therapeutic lysate-loaded dendritic cells (DC) vaccine in a small cohort of 10 patients with ovarian cancer. After initial surgery and chemotherapy, patients received the DC vaccine followed by 2 wk of daily IL-2 injections. Pharmacodynamics analysis 8 wk post-vaccination showed that 5/10 patients had significantly increased NK cell activity and 3/10 patients had increased IFN-γ-secreting T-cells. While combining IL-2 with DC vaccination was safe, the increase of specific immunity observed in this small cohort was not associated with clinical outcomes (44). More recent studies combining IL-2 with cancer vaccines have uncovered new combinations with encouraging results. Combining low-dose IL-2 with a whole-tumor lysate-pulsed DC vaccine, CTX, bevacizumab and acetylsalicylic acid showed encouraging responses in a small cohort of ovarian cancer patients, with a significant increase in IFN-γ-secreting T-cells and prolonged survival (45).
IL-2 concluding remarks
In summary, considerable efforts are underway to develop next-generation IL-2 drug candidates. These drug candidates have a longer half-life, are well tolerated and have the ability to increase cytotoxic T-cells without stimulating Tregs. Several clinical trials are underway to test these candidates as monotherapies or in combination with other immunotherapies for the treatment of cancer (Table 1 and Fig. 1).
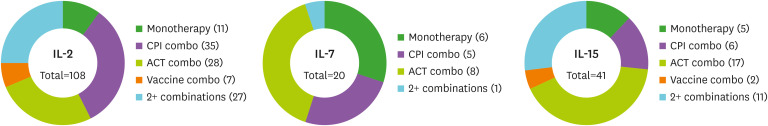
Figure 1. Combination strategies for gamma-chain cytokines in ongoing clinical trials. Trials involving IL-2, IL-7, IL-15 and IL-21 were identified on www.clinicaltrials.gov and organized by combination strategy. Only ongoing trials involving cancer patients were included. The number of trials for each strategy are indicated as (number). The 2+ combinations represents trials combining a gamma-chain cytokine with 2 or more immunotherapy strategies. Only one active trial was found for IL-21, which involves CART cells co-expressing IL-15 and IL-21 (NCT04715191).
IL-7
IL-7 is unique among the common gamma-chain family of cytokines because it is not produced by T-cells and, instead, is produced constitutively by non-immune stromal cells found in primary and secondary lymphoid structures. IL-7 is a non-redundant cytokine critical for the survival and differentiation of thymocytes and for the survival and proliferation of peripheral T-cells (46,47). IL-7 also has an important role in the formation of secondary and tertiary lymphoid structures (48). IL-7 has a heterodimeric receptor consisting of the IL-7Rα and the common gamma-chain (IL-2Rγ). In humans, IL-7R is expressed on all CD4 and CD8 T-cell subsets, with naïve and memory subsets having the highest expression (49). Importantly, Tregs have very low IL-7R expression, making IL-7 a straightforward candidate for cancer immunotherapy. IL-7 can boost naïve T-cells and support T-cell memory subsets important for the formation of durable anti-tumor immunity. In addition, the strong synergistic potential of IL-7 combined with current immunotherapies is being investigated in multiple ongoing clinical trials.
IL-7 as a monotherapy
There are currently 2 IL-7 drug candidates in the clinical stage: CYT107 (Cytheris, now RevImmune) and NT-I7 (efineptakin alfa, NeoImmuneTech, Inc., Rockville, MD, USA; aka GX-I7, Genexine, Inc., Seoul, Korea). In the first-in-human trial with CYT99 007 (unglycosylated version of CYT107), patients with refractory cancers were treated with doses ranging from 3–60 µg/kg Q1D for 14 days (NCT00062049). Frequent dosing was required because this unglycosylated form of IL-7 had a short half-life of 6-10 h (50). Pharmacodynamic data showed significant age-independent increases in circulating CD4 and CD8 T-cells, increased T-cell receptor diversity, decreased Treg frequency, and the dose was well tolerated. Following this, a randomized placebo-controlled phase IIa trial (ELYPSE, NCT01368107) was designed to study the effect of CYT107, a glycosylated rhIL-7 with a half-life of 8.7–34.6 h, in restoring CD4 lymphopenia in metastatic breast cancer patients before or during chemotherapy (51). Patients received 10 µg/kg Q1W of CYT107 or placebo for 3 wk. While there was no difference in PFS or OS between the placebo and the drug treatment cohorts (52), the treatment was safe and well tolerated and biomarker analysis revealed a significant increase of naive and memory CD4 and CD8 T-cell subsets compared to placebo, with a 2-fold increase of CD8 T-cells. Although there was a transient increase of Tregs at day 21, this coincided with a significant increase in all CD4 T-cells. Importantly, total CD4 T-cells remained higher through day 57, while Treg levels did not.
NT-I7 is the only next-generation long-acting IL-7 drug candidate in the clinical stage. NT-I7 is a recombinant human IL-7-hyFc fusion protein consisting of the homodimeric human IL-7 built on the ‘hyFc’ (hybrid Fc) platform (Genexine, Inc.), where the Fc fusion fragment is composed of a hybridizing IgD/IgG4 immunoglobulin domain. This structural modification significantly increases stability and potency, generating a long-acting molecule that overcomes the major limitations encountered with early forms of unmodified recombinant gamma-chain cytokines. In a trial of 30 healthy individuals (NCT02860715), GX-I7 (aka NT-I7 in the Americas and Europe) was administered intramuscularly in doses up to 60 µg/kg. GX-I7 was well tolerated and showed an impressive half-life of 63.26 h. GX-I7 treatment significantly increased absolute lymphocyte counts (ALC) that peaked by day 21 (53). The administration of GX-I7 led to a dose-dependent increase in NK cells and all analyzed CD4 and CD8 T-cell subsets, but there was no dose-dependent increase in B-cells. Based on preclinical studies that demonstrated the ability of NT-I7 to significantly reduce tumor growth and increase CD8 TILs in murine solid tumor models (54), a clinical trial testing GX-I7 in patients with solid tumors was established to determine safety and tolerability (NCT03478995). GX-I7 was safely administered at dose levels up to 1,200 µg/kg with a notable half-life up to 147 h (55). The most common treatment-related adverse events (AEs) were injection site reactions, which were mild to moderate and resolved without intervention. Interim data showed an increase of ALC in both lymphopenic and non-lymphopenic patients, with a 3–4-fold increase of CD4 and CD8 T-cells.
NT-I7 preclinical data also showed the potential of combining NT-I7 with standard of care for high-grade gliomas (56). Based on these encouraging findings, NT-I7 is being tested as a monotherapy in patients with high-grade gliomas after concurrent radiation and temozolomide (NCT03687957; NCT02659800). Interim data has proven that NT-I7 is able to significantly boost ALC even in the presence of adjuvant chemotherapy. Interestingly, biomarker analysis showed that NT-I7 leads to the preferential increase of CD8 stem-cell memory T-cells (Tscm), a self-renewing subset with better anti-tumor activity compared to other memory CD8 T-cell subsets (57). In addition to testing NT-I7 monotherapy with standard of care in high-grade glioma patients, NT-I7 monotherapy is being tested in patients with locally recurrent squamous cell carcinoma of head and neck undergoing salvage surgery (NCT04588038) and Kaposi’s sarcoma with or without HIV (NCT04893018).
IL-7 in combination with CPIs
IL-7 reliably boosts T-cell numbers and therefore has great potential to synergize with CPIs to improve efficacy. Two combination trials involving CYT107 are in early stages and results are not available yet, but this synergy has already been highlighted by interim results from 2 ongoing clinical trials combining NT-I7 with pembrolizumab in patients with advanced solid tumors. In a trial testing GX-I7 in combination with pembrolizumab (NCT03752723) in patients with metastatic triple negative breast cancer who failed standard chemotherapy, GX-I7 was given Q12W and pembrolizumab Q3W. The treatment was well tolerated, with up to a 7-fold increase in peripheral ALC at all dose levels tested, regardless of concurrent CTX. Clinical outcome data is encouraging, with 4 out of 6 patients achieving SD at the 1,200 µg/kg dose level (58). A second trial testing NT-I7 1,200 µg/kg Q6W in combination with pembrolizumab in patients with advanced solid tumors (NCT04332653) has also published interim results for 2 arms of the study involving advanced recurrent/refractory pancreatic cancer and MSS-colorectal cancer. Preliminary data shows that NT-I7 significantly boosts peripheral T-cell counts, with a significant increase in the CD8:Treg ratio. Among the CD8 T-cell subsets, the most substantial fold-change increase occurred in the self-renewing Tscm subset, which increased 50-fold in peripheral blood compared to baseline after a single NT-I7 dose. Preliminary clinical outcome data from the ongoing trial is encouraging, with 4 PR (out of 34 evaluable patients) achieved at the time of analysis in a difficult to treat patient population (59,60). Following this encouraging data, NeoImmuneTech, Inc. has initiated clinical trials to test NT-I7 in combination with nivolumab (NCT04594811) and atezolizumab (NCT03901573 and NCT04984811). It is worth noting that, due to the significant increase in the next-generation cytokine half-life, all trials involving NT-I7 (and GX-I7) have a dosing schedule between Q3W and Q12W while increasing clinical efficacy.
IL-7 in combination with ACT
While combination of IL-7 with ACT is usually in the context of in vitro stimulation of CART cells prior to infusion (61), there has been increasing interest in adding IL-7 expression to the CART design. This strategy has rendered encouraging results in both preclinical (62,63,64,65) and clinical settings (NCT03198546). In this clinical study tumors were determined to express either GPC3 or MSLN and subsequently treated with anti-GPC3 or anti-MSLN-7 × 19-CART cells. No grade 2–4 AEs occurred, and clinical outcomes included 1 CR, 1 PR and 2 SD out of 6 patients. Clinical trials testing the coexpression of IL-7 and CCL19 in CART cells are also underway (NCT03929107, NCT04381741).
Preclinical studies combining ACT with IL-7 administration have also produced encouraging data. In mice inoculated with a B-cell lymphoma cell line, rhIL-7 significantly increased the expansion and persistence of antigen-specific CD4 T-cells and significantly prolonged mouse survival, preventing the relapse typically seen with ACT alone in this model (66). In addition, preclinical data using next-generation long-acting IL-7 has shown that NT-I7 enhances CART expansion, persistence and anti-tumor activity in vivo (67). After these encouraging results, NeoImmuneTech, Inc. has started a Phase 1b trial (NCT05075603) to evaluate the safety, tolerability and efficacy of NT-I7 post-Kymriah® in subjects with relapsed/refractory large B-cell lymphoma (Table 1).
IL-7 in combination with cancer vaccines
While preclinical data supports the combination of IL-7 with cancer vaccines (68,69), clinical data is still scarce. CYT107 has been combined with a tumor lysate-pulsed DC vaccine in pediatric patients with metastatic and recurrent sarcoma. Following standard therapy, patients received the DC vaccination and an autologous lymphocyte infusion, with or without administration of CYT107 Q2W. While standard of care includes alkylators that induce prolonged lymphopenia, patients receiving CYT107 had significantly increased lymphocyte recovery with increased CD4 and CD8 T-cells at 6-wk post treatment. Notably, CYT107 led to decreased Treg frequencies compared to baseline. While CYT107 increased the number of circulating lymphocytes, patients treated with CYT107 did not have a significantly different OS compared to patients in the control arm (70). Additionally, CYT107 has been tested in combination with sipuleucel-T (sip-T), an autologous cellular vaccine that is the only FDA-approved immunotherapy for castration-resistant metastatic prostate cancer. After administration of sip-T, patients either received CYT107 weekly for a total of 4 doses or were assigned to an observation group. The treatment was well tolerated and led to a significant increase of peripheral T-cells and NK cells, with increased intracellular pro-inflammatory cytokines. Encouraging data showed that CYT107 treatment increased the PSA doubling time compared to the observation group. Due to production issues of CYT107, patient enrollment was ended prematurely, and the small sample size did not allow for proper assessment of the clinical efficacy of sip-T in combination with IL-7 (71).
IL-7 concluding remarks
In summary, preclinical studies testing IL-7 as a monotherapy or in combination with CPIs, ACT and cancer vaccines have yielded consistently positive results. The ability to increase T-cell numbers safely and significantly in the blood, lymphoid tissues and tumor, together with a safe and well tolerated profile, makes IL-7 a particularly attractive cytokine for cancer immunotherapy. When unmodified, the clinical efficacy of IL-7 remained uncertain, likely due to the short half-life. By significantly increasing the half-life of IL-7, NT-I7 (aka GX-I7) has allowed for more infrequent dosing (up to 12 wk) with consistent bioactivity, generating encouraging preliminary clinical efficacy in a range of tumor types and in combination with a CPI (Table 1 and Fig. 1).
IL-15
IL-15 is important for NK, NKT and CD8 memory T-cell function and homeostasis (72). It is expressed as a heterodimer with IL-15Rα, mainly by monocytes, macrophages and DCs which then present it to neighboring cells that express IL-15Rβ/γ (73). While usually presented in trans, the IL-15Rα can also be cleaved to form a soluble heterodimer superagonist (hetIL-15) with increased stability and bioactivity compared to the IL-15 monomer (74). The IL-15Rβ/γ is expressed on NK cells and CD8 T-cells and receptor binding with IL-15/IL-15Rα has been shown to increase the proliferation and cytotoxic function of these cells (72,73). IL-15 is often compared to IL-2 as they share the same IL-2/15Rβ and gamma-chain receptor complex and both expand activated T-cells and NK cells. But unlike IL-2, IL-15 is dispensable for Treg generation and is more important for the maintenance of CD8 memory T-cells (73). However, it should be noted that trans-presentation of the IL-15/IL-15Rα complex, but not IL-15 alone, can influence CD4 T-cell function and differentiation (75). Given its ability to expand and enhance the function of effector lymphocytes important for the anti-tumor response, IL-15 is actively being investigated for cancer immunotherapy, with 2 next-generation IL-15 candidates being tested in the clinic (Table 1).
IL-15 as a monotherapy
A first-in-human phase I study was designed to investigate the safety and tolerability of recombinant human IL-15 (rhIL-15, National Cancer Institute, Bethesda, MD, USA) in patients with malignant melanoma or metastatic renal cell cancer (NCT01021059). rhIL-15 was administered i.v. at 0.3–3 µg/kg per day for 12 consecutive days. Correlative analysis showed a substantial increase in NK and γδ T-cells, followed by CD8 memory T-cells, with limited expansion of Tregs (76). The average half-life across the doses was approximately 2.5 h with a non-linear Cmax suggesting clearance by the high-affinity IL-15 receptor and, possibly, IL-15R-independent mechanisms, and an MTD of 0.3 µg/kg per day. The most common AEs were fevers, rigors, and hypotension related to the marked elevation in IFNα, IL-6, IL-8, and IL-2Rα associated with i.v. administration, and clinically, there were no responses. To circumvent these limitations, daily s.c. rhIL-15 treatment was investigated in patients with solid tumors (NCT01727076). The regimen was well tolerated and achieved robust NK cell expansion while there was less effect on CD8 T-cells (77). Out of 18 evaluable patients, 7 patients had SD while 11 patients stopped treatment because of disease progression. Although this route of administration was better tolerated, PK analysis showed that the plasmatic concentration of rhIL-15 significantly decreased after 24 h with daily injections required.
In addition to a short half-life, native rhIL-15 has limited immunomodulatory effects because it is usually trans-presented to CD8 T- and NK cells in a complex with the IL-15Rα. N-803 (ALT-803, ImmunityBio, Inc., Culver City, CA, USA) contains 2 molecules of an optimized amino acid–substituted (N72D) IL-15 “superagonist” and 2 molecules of the IL-15α receptor “sushi” domain fused to a dimeric human IgG1 Fc (IL-15N72D:IL-15RαSu/IgG1 Fc complex), with a half-life of 29.3 h (78,79). Following pre-clinical studies using N-803 in murine models of solid tumors (80) that showed immunomodulatory and anti-tumor activity, a first-in-human trial was initiated to test the safety, tolerability, PK and immunologic effects in a dose escalating schedule via i.v. and s.c. administration (NCT01946789). Subcutaneous weekly administration of N-803 at 20 µg/kg showed low Cmax values between 8–24 h, robust NK but moderate CD8 T-cell expansion, no indication of neutralizing antibodies and was well tolerated in patients (81). These encouraging findings have bolstered some of the current trials of N-803 in combination with CPIs, adoptive NK and T-cell therapies, and antitumor antibodies among others (Table 1). Nevertheless, recent findings suggest that engagement of the IL-2Rβ expressing lymphocyte subsets by the precomplexed rhIL-15/IL-15Rα molecules may lead to diminished biological responses after repeated dosing (82).
NKTR-255 (Nektar Therapeutics) is a PEG-conjugated rhIL-15 that retains the same receptor binding affinity of the native IL-15 molecule. Preclinical studies in cynomolgus monkeys testing a single dose of NKTR-255 at 0.1 mg/kg showed a half-life of 30.5 h, with significant expansion of both NK and effector CD8 T-cells with equal efficacy. Interestingly, no expansion of CD4 T- or B-cells was observed (83). NKTR-255 is currently being investigated in a phase I open-label study for patients with relapsed or refractory multiple myeloma and non-Hodgkin’s lymphoma, either alone or in combination with Rituximab or Daratumumab (NCT04136756).
IL-15 in combination with CPIs
A clinical trial is currently underway to test the combination of nivolumab with N-803 for the treatment of non-small cell lung cancer. Data from the phase 1b trial demonstrated the combination is safe and well tolerated, with no reported DLTs. Two out of 21 participants experienced grade 3 lymphocytopenia that spontaneously resolved. Pharmacodynamic analysis showed a 7-fold increase of NK cells 7 days after starting treatment. Post-hoc analysis of clinical responses showed 29% (6/21) of patients had an objective response. Notably, 10/11 patients that progressed after previous anti-PD-1 therapy achieved disease control (84). Results from the phase Ib trial demonstrate combination of N-803 with nivolumab is safe and tolerable, with the phase II study currently underway (NCT02523469).
Based on this success, ImmunityBio, Inc. is developing N-809, a first-in-class molecule where N-803 is fused to 2 anti-PD-L1 domains. N-809 showed superior tumor control in murine models and surviving mice were protected from rechallenge (85). Other promising next-generation molecules, like the IL-15 superagonist receptor linker IL-15 (RLI), also showed increased survival and tumor control in murine models of cancer (86). However, while preclinical studies continue to highlight the potential of this combination, these molecules have not yet reached the clinical stage.
IL-15 in combination with ACT
IL-15, like IL-7, has been mostly tested for the preconditioning of cells before adoptive transfer. While results have not been very promising for NK cell therapy (87,88), preclinical data for the preconditioning of CART cells with IL-15 has been more positive. In a murine cancer model it was shown that IL-7/IL-15-generated CART cells showed increased tumor control and significantly prolonged survival when combined with anti-PD-1 therapy compared to IL-2/IL-15-generated CART cells (89). Similar to IL-7 studies, engineering cells to express IL-15 in vivo is also being tested. In a study involving patients with CD19-positive cancers, NK cells engineered to co-express CD19-targeting CAR and IL-15 were infused after lymphodepleting chemotherapy. The therapy was well tolerated with no cytokine release syndrome, neurotoxicity or GvHD developed after infusion of the CAR-NK cells. Clinical efficacy data from the 11 patients treated was encouraging, with 7 patients in complete remission and responses seen within 30 days of treatment (90). IL-15 expression has also been added to NKT cells expressing a GD2-targeting CAR. Preliminary data from a study involving pediatric patients with relapsed or resistant neuroblastoma showed infusion of the autologous NKT cells was well tolerated with no DLTs. Although the reported data was only for 3 patients, pharmacodynamic analysis showed elevated NKT cells compared to baseline and one patient experienced an objective response with lesion regression (91). Although study sizes are small, this clinical data supports further investigation of IL-15 expressing adoptively transferred cells for cancer immunotherapy.
Multiple preclinical studies testing the combination of IL-15 with ACT have been conducted by ImmunityBio, Inc. They have demonstrated the ability of N-803 to enhance the anti-tumor function of PD-L1 t-haNK cells, a novel NK cell line engineered to express CD16 and a PD-L1-targeting CAR (92). N-820, a fusion protein of N-803 and 4 single-chains of rituximab, when combined with ex vivo expanded NK cells significantly increased the anti-tumor response against CD20+ Burkitt lymphoma (93). Multiple clinical trials testing N-803 in combination with several immunotherapies, including t-haNK cell infusion, are ongoing (Table 1).
IL-15 in combination with cancer vaccines
Results from a phase Ib trial combining N-803 with BCG vaccination in bladder cancer were recently published (94). The combination was well tolerated for all doses tested, with MTD not reached and no treatment-related AEs at or exceeding grade 3. Although this single-arm study was small, with the enrollment of only 9 patients, no patients experienced disease progression 6 years after treatment. Pharmacodynamic analysis showed elevated IL-6 in urine and serum after the combination therapy, but this did not correlate with dose levels of N-803. Two trials combining N-803 with BCG vaccination are ongoing, testing in both BCG-naïve and BCG-unresponsive bladder cancer patients (NCT02138734; NCT03022825). Additional strategies, still in preclinical development, include an autologous cell-based cancer vaccine secreting IL-15 in complex with IL-15Rα. Mice receiving inactivated cancer cells secreting IL-15:IL-15Rα had significant tumor control in both prophylactic and therapeutic cancer models (95), highlighting the promising potential of IL-15 to improve the anti-tumor response elicited by cancer vaccines.
IL-15 concluding remarks
Next-generation IL-15 drug candidates have produced promising results in both clinical and preclinical settings. Complexing IL-15 with the IL-15Rα significantly extends the half-life and promotes NK cell expansion and function. Combining N-803 with nivolumab achieved clinical responses in patients that had previously progressed with anti-PD-1 therapy. In addition, the ability of IL-15 to expand NK cells has led to its application in the setting of NK cell adoptive transfers, with IL-15 expressing NK cells increasing in number after infusion. Boosting CD8 memory T-cells has also prompted the combination of IL-15 with cancer vaccines, with preclinical studies showing increased antigen-specific CD8 T-cells. These data have prompted intense investigation of these combinations in the clinic (Table 1 and Fig. 1).
IL-21
IL-21 is produced mainly by CD4 T-cells but is also secreted by NKT cells and CD8 T-cells. IL-21 can affect the homeostasis and function of several immune cells, including B-cells, T-cells, NK cells, macrophages, DCs and mast cells, all of which express the heterodimeric receptor composed of IL-21Rα and the common gamma-chain. IL-21 is well known for enhancing the survival, proliferation and differentiation of B-cells, germinal center functions and plasma cell differentiation. Interestingly, IL-21 can have a pro-apoptotic effect on B-cells in the absence of co-stimulation, making IL-21 important for the prevention of autoreactive antibody production. IL-21 can further promote the expansion of CD8 memory T-cells in the presence of IL-7 or IL-15, improves NK cell viability and function, memory T-cell persistence and cytotoxic CD8 T-cell function (6,96). Notably, IL-21 does not promote Treg differentiation, although the presence of Tregs can mitigate the IL-21-induced expansion of CD8 T-cells (97). With the ability to improve the function of multiple immune cells important for the anti-tumor response, IL-21 is a relevant cytokine to be considered for cancer immunotherapy.
IL-21 as a monotherapy
Phase I and phase IIa clinical trials were initiated to study the safety and efficacy of rIL-21 (98,99,100). A dosing regimen of rIL-21 at 30 µg/kg/dose (i.v. bolus injection in 8-wk cycles) was established as the recommended dose. DLTs included hyponatremia, hypophosphatemia and increased liver transaminases, although there was no evidence of VLS (NCT00095108). The half-life of rIL-21 was 3.09 h and biomarker analysis showed significant increases in CD8 T-cells and soluble CD25 with a concomitant increase in IFN-γ, perforin, and granzyme B mRNA expression. About 10% of patients developed anti-drug antibodies, although these were not causatively associated with any AEs. Anti-tumor activity was observed in the mM cohort of 48 patients as 1 CR, 1 PR and 20 SD, while in the renal cancer cohort, out of 19 patients 4 PR and 13 SD were recorded. Despite these promising Phase I/II results, a recent report identified comparable efficacy between rIL-21 and dacarbazine in refractory or mM patients (99).
Other clinical strategies include IL-21 in combination with sorafenib in mRCC (NCT00389285) and with anti-CD20 mAb rituximab for non-Hodgkin’s lymphoma (NCT00347971). Preclinical studies have attempted to address the short half-life of IL-21 using fusion cytokines in the form of IL-21-αHSA, αCD20-IL-21 and GIFT-21 (101,102,103,104). Also in preclinical development is an Erbitux-based IL-21 fusion protein (Erb-IL21, IL-21 fused to the Fc portion of IgG1) that specifically targets murine tumors expressing chimeric EGFR (105). However, none of these next-generation IL-21 drug candidates are in clinical stages yet.
IL-21 in combination with CPIs
While there are currently no clinical trials testing IL-21 in combination with CPIs, there are multiple preclinical studies supporting its combination. Erb-IL21 combined with anti-CTLA-4 or anti-PD-L1 improved tumor control and mouse survival in CPI-resistant tumors (105). Murine IL-21 administered in combination with anti-CTLA-4 or anti-PD-1 in multiple cancer models, has rendered mixed results (106). Additional preclinical studies have investigated the effect of IL-21 on NK cells and its ability to enhance CPI therapy through an NK-cell dependent mechanism. By treating mice bearing MHCI-deficient MC38 cells with CD8-depleting antibodies and a combination of intratumoral anti-PD-1, anti-Tim-3 and rIL-21, the triple combination significantly improved tumor control and survival (107). Lastly, PD-1Ab21 is an IC that combines IL-21 with a single-chain anti-PD-1 antibody that directs IL-21 to PD-1+ T-cells. PD-1Ab21 elicited significant tumor control and increased expansion of tumor-specific memory T-cells (108). The existing preclinical work demonstrates the ability of IL-21 to synergize with CPIs to increase the number and function of NK and CD8 T-cells in the tumor, supporting further investigation of this combination.
IL-21 in combination with ACT
While there have not been many attempts to combine IL-21 with ACT, there is evidence to support the culturing of cytotoxic lymphocytes (CTLs) with IL-21 prior to infusion. Chapuis et al. (109) demonstrated that culturing donor-derived WTI-specific CTLs with IL-21 led to increased persistence and CTLs cultured with IL-21 had a less differentiated phenotype and enhanced survival in vivo. Although all 3 treated patients had a high risk of relapse, all continued to be in complete remission when the study was published. A similar strategy was then applied to a patient with mM that was refractory to monoclonal CTL and anti-CTLA-4 applied as monotherapies. Infusion of donor-derived IL-21-primed MART1-specific T-cells and administration of anti-CTLA-4 led to a CR and the patient remained disease free for at least 5 years (110).
IL-21 in combination with cancer vaccines
While there is a strong rationale to combine IL-21 with cancer vaccines, evidence to support its combination remains limited to preclinical studies. Recently, a preclinical study involving a therapeutic bladder cancer vaccine revealed that mice treated with irradiated MB49 bladder cancer cells expressing IL-21 had significantly decreased tumor growth and significantly more CD4 and CD8 T-cells in the spleen and tumor. Interestingly, they found that if the cancer vaccine expressed both IL-21 and GM-CSF, the anti-tumor response was even greater (111). In addition, incorporating IL-21 expression into a B16F10-ESTAT-6-gpi tumor vaccine has shown decreased tumor growth in a murine melanoma model (112). There is also evidence that the co-expression of IL-21 with IL-7 in a whole cell cancer vaccine has synergistic effects in a melanoma model (69).
IL-21 concluding remarks
As IL-21 was the most recently discovered common gamma-chain family cytokine, there is less clinical development. Like other unmodified recombinant cytokines, IL-21 half-life is short. However, several preclinical studies have demonstrated IL-21 can increase the function of NK and CD8 T-cells in the tumor and often works in synergy with other immunotherapies. While there are currently no next-generation IL-21 drug candidates in clinical trials, the development of candidates with increased half-life continues with promising results.
CONCLUSION
When considering the broad importance of gamma-chain cytokines on immune cell development, survival and function, administering these cytokines in clinical settings where enhanced anti-tumor immune responses is desired is a reasonable and promising approach. Early application of these cytokines in the clinic revealed rapid clearance in vivo, requiring frequent injections and with some showing toxic side effects at HDs. Fortunately, several next-generation drug candidates have been developed that are safer, have an increased half-life or are able to target the cytokine to specific cells (Table 2). In addition, the advancement of engineering cells to express various gamma-chain cytokines has proven promising for ACT and cancer vaccine settings. An aspect of common gamma-chain cytokine biology that is outside the scope of this review, but could have important implications in cancer immunotherapy, is the soluble form of each cytokine receptor. The generation of soluble cytokine receptors and their ability to regulate the immune response has been comprehensively reviewed elsewhere (113). It is worth noting that while all cytokines reviewed in this manuscript are currently being tested in ongoing clinical trials (Table 1), IL-4 and IL-9 also utilize the gamma-chain receptor subunit. However, their role in the anti-tumor response is less understood and concerns about potential pro-tumorigenic effects have hindered their clinical development (114,115,116).
Table 2. Comparison of unaltered and next-generation gamma-chain cytokines.
| Variables | Half-life | Dosing | Safety | CD8 T-cell/NK cell increase | |
|---|---|---|---|---|---|
| IL-2 | |||||
| Unaltered (Aldesleukin) | 13–85 min | Daily | Low-dose expands Tregs | N/A | |
| HD has life-threatening toxicities | |||||
| Next-Gen (ALKS 4230; FAP-IL-2v; GI-101; hu14.18-IL2; L19-IL2; NKTR-214; RO72284755; THOR-707) | Up to 10–17 h | Q2W–Q3W | Decreased Treg engagement and increased potency allow for lower doses and a better safety profile | Up to 3X CD8 T-cells | |
| Up to 8X NK cells | |||||
| IL-7 | |||||
| Unaltered (CYT-107) | 6–10 h (unglycosylated) | Q1W–Q2W | Good safety profile (no MTD reached) | Up to 2X CD8 T-cells | |
| 9–35 h (glycosylated) | |||||
| Next-Gen (NT-I7/GX-I7 (efineptakin alfa)) | 33–147 h | Q3W–Q12W | Good safety profile (no MTD reached) | Up to 4X CD8 T-cells (preferential TSCM expansion) | |
| IL-15 | |||||
| Unaltered (rhIL-15) | 2.5 h | Daily | Marked elevation of inflammatory cytokines with i.v. injection, better safety profile when administered s.c. | Up to 1.5–2X CD8 T-cells | |
| Up to 2–3X NK cells | |||||
| Next-Gen (N-803; NKTR-255) | Up to 30 h | Q1W–Q4W | Well tolerated | Up to 2.5X NK cells | |
| IL-21 | |||||
| Unaltered (rIL-21) | 3 h | Daily | Serious dose-limiting toxicities | Up to 2.5X NK cells | |
| Next-Gen | N/A | N/A | N/A | N/A | |
N/A, not applicable.
As various cancer immunotherapies continue to be tested in the clinic, disappointing clinical efficacy following monotherapy approaches has revealed that many tumor types will require a combination of strategies to completely eliminate the tumor. With the backing of strong preclinical and clinical data, next generation gamma-chain cytokines have earned the distinction of being a critical component in future combination immunotherapy strategies.
Abbreviations
- ACT
adoptive cell therapy
- AE
adverse event
- aka
also known as
- ALC
absolute lymphocyte counts
- CPI
checkpoint inhibitor
- CR
complete response
- CTL
cytotoxic lymphocyte
- CTX
cyclophosphamide
- DC
dendritic cells
- DLT
dose-limiting toxicity
- EDB
extra-domain B
- FAP
fibroblast activation protein
- HD
high-dose
- i.v.
intravenously
- IC
immunocytokine
- mM
metastatic melanoma
- mRCC
metastatic renal cell carcinoma
- MTD
maximum tolerated dose
- ORR
overall response rate
- OS
overall survival
- PEG
polyethylene glycol
- PFS
progression-free survival
- PK
pharmacokinetics
- PR
partial response
- Q2W
every 2 weeks
- s.c.
subcutaneous
- SD
stable disease
- sip-T
sipuleucel-T
- TIL
tumor infiltrating lymphocyte
- TME
tumor microenvironment
- Tscm
stem-cell memory T-cells
- UC
urothelial cancer
- VLS
vascular leak syndrome
Footnotes
Conflict of Interest: The authors declare no potential conflicts of interest.
- Conceptualization: Wolfarth AA, Dhar S, Ferrando-Martínez S.
- First Author: Wolfarth AA.
- Supervision: Lee BH.
- Validation: Wolfarth AA.
- Writing - original draft: Wolfarth AA, Dhar S, Goon JB, Ezeanya U, Ferrando-Martínez S.
- Writing - review & editing: Wolfarth AA, Lee BH.
References
- 1.Meyers DE, Banerji S. Biomarkers of immune checkpoint inhibitor efficacy in cancer. Curr Oncol. 2020;27:S106–S114. doi: 10.3747/co.27.5549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McLellan AD, Ali Hosseini Rad SM. Chimeric antigen receptor T cell persistence and memory cell formation. Immunol Cell Biol. 2019;97:664–674. doi: 10.1111/imcb.12254. [DOI] [PubMed] [Google Scholar]
- 3.Bowen WS, Svrivastava AK, Batra L, Barsoumian H, Shirwan H. Current challenges for cancer vaccine adjuvant development. Expert Rev Vaccines. 2018;17:207–215. doi: 10.1080/14760584.2018.1434000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lin JX, Leonard WJ. The common cytokine receptor γ chain family of cytokines. Cold Spring Harb Perspect Biol. 2018;10:a028449. doi: 10.1101/cshperspect.a028449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Raeber ME, Zurbuchen Y, Impellizzieri D, Boyman O. The role of cytokines in T-cell memory in health and disease. Immunol Rev. 2018;283:176–193. doi: 10.1111/imr.12644. [DOI] [PubMed] [Google Scholar]
- 6.Leonard WJ, Lin JX, O’Shea JJ. The γc family of cytokines: basic biology to therapeutic ramifications. Immunity. 2019;50:832–850. doi: 10.1016/j.immuni.2019.03.028. [DOI] [PubMed] [Google Scholar]
- 7.Waldmann TA. The multi-subunit interleukin-2 receptor. Annu Rev Biochem. 1989;58:875–911. doi: 10.1146/annurev.bi.58.070189.004303. [DOI] [PubMed] [Google Scholar]
- 8.Xue D, Hsu E, Fu YX, Peng H. Next-generation cytokines for cancer immunotherapy. Antib Ther. 2021;4:123–133. doi: 10.1093/abt/tbab014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fyfe G, Fisher RI, Rosenberg SA, Sznol M, Parkinson DR, Louie AC. Results of treatment of 255 patients with metastatic renal cell carcinoma who received high-dose recombinant interleukin-2 therapy. J Clin Oncol. 1995;13:688–696. doi: 10.1200/JCO.1995.13.3.688. [DOI] [PubMed] [Google Scholar]
- 10.Atkins MB, Lotze MT, Dutcher JP, Fisher RI, Weiss G, Margolin K, Abrams J, Sznol M, Parkinson D, Hawkins M, et al. High-dose recombinant interleukin 2 therapy for patients with metastatic melanoma: analysis of 270 patients treated between 1985 and 1993. J Clin Oncol. 1999;17:2105–2116. doi: 10.1200/JCO.1999.17.7.2105. [DOI] [PubMed] [Google Scholar]
- 11.Fisher RI, Rosenberg SA, Fyfe G. Long-term survival update for high-dose recombinant interleukin-2 in patients with renal cell carcinoma. Cancer J Sci Am. 2000;6(Suppl 1):S55–S57. [PubMed] [Google Scholar]
- 12.Clinigen. Proleukin® (aldesleukin) [Internet] [accessed on 30 December 2021]. Available at https://proleukin.com/
- 13.Kolb HC, Finn MG, Sharpless KB. Click chemistry: diverse chemical function from a few good reactions. Angew Chem Int Ed Engl. 2001;40:2004–2021. doi: 10.1002/1521-3773(20010601)40:11<2004::AID-ANIE2004>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 14.Ptacin JL, Caffaro CE, Ma L, San Jose Gall KM, Aerni HR, Acuff NV, Herman RW, Pavlova Y, Pena MJ, Chen DB, et al. An engineered IL-2 reprogrammed for anti-tumor therapy using a semi-synthetic organism. Nat Commun. 2021;12:4785. doi: 10.1038/s41467-021-24987-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Milla ME, Ptacin JL, Ma L, Caffar CE, Aerni HR, San Jose KM, Pena MJ, Herman RW, Pavlova Y, Chen DB, et al. 1225P - THOR-707, a novel not-alpha IL-2, promotes all key immune system anti-tumoral actions of IL-2 without eliciting vascular leak syndrome (VLS) Ann Oncol. 2019;30:v501. [Google Scholar]
- 16.Filip Janku RA, Abdul-Karim R, Azad A, Bendell J, Falchook G, Gan HK, Tan T, Wang JS, Chee CE, Ma L, et al. Abstract LB041: THOR-707 (SAR444245), a novel not-alpha IL-2 as monotherapy and in combination with pembrolizumab in advanced/metastatic solid tumors: interim results from HAMMER, an open-label, multicenter phase 1/2 study. Cancer Res. 2021;81:LB041. [Google Scholar]
- 17.Charych DH, Hoch U, Langowski JL, Lee SR, Addepalli MK, Kirk PB, Sheng D, Liu X, Sims PW, VanderVeen LA, et al. NKTR-214, an engineered cytokine with biased IL2 receptor binding, increased tumor exposure, and marked efficacy in mouse tumor models. Clin Cancer Res. 2016;22:680–690. doi: 10.1158/1078-0432.CCR-15-1631. [DOI] [PubMed] [Google Scholar]
- 18.Bentebibel SE, Hurwitz ME, Bernatchez C, Haymaker C, Hudgens CW, Kluger HM, Tetzlaff MT, Tagliaferri MA, Zalevsky J, Hoch U, et al. A first-in-human study and biomarker analysis of NKTR-214, a novel IL2Rβγ-biased cytokine, in patients with advanced or metastatic solid tumors. Cancer Discov. 2019;9:711–721. doi: 10.1158/2159-8290.CD-18-1495. [DOI] [PubMed] [Google Scholar]
- 19.Lopes JE, Fisher JL, Flick HL, Wang C, Sun L, Ernstoff MS, Alvarez JC, Losey HC. ALKS 4230: a novel engineered IL-2 fusion protein with an improved cellular selectivity profile for cancer immunotherapy. J Immunother Cancer. 2020;8:e000673. doi: 10.1136/jitc-2020-000673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lopes JE, Sun L, Flick HL, Murphy EA, Losey HC. Pharmacokinetics and pharmacodynamic effects of nemvaleukin alfa, a selective agonist of the intermediate-affinity IL-2 receptor, in cynomolgus monkeys. J Pharmacol Exp Ther. 2021;379:203–210. doi: 10.1124/jpet.121.000612. [DOI] [PubMed] [Google Scholar]
- 21.Castellani P, Viale G, Dorcaratto A, Nicolo G, Kaczmarek J, Querze G, Zardi L. The fibronectin isoform containing the ED-B oncofetal domain: a marker of angiogenesis. Int J Cancer. 1994;59:612–618. doi: 10.1002/ijc.2910590507. [DOI] [PubMed] [Google Scholar]
- 22.Lieverse RI, Marcus D, van der Wiel AM, Van Limbergen EJ, Theys J, Yaromina A, Lambin P, Dubois LJ. Human fibronectin extra domain B as a biomarker for targeted therapy in cancer. Mol Oncol. 2020;14:1555–1568. doi: 10.1002/1878-0261.12705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Johannsen M, Spitaleri G, Curigliano G, Roigas J, Weikert S, Kempkensteffen C, Roemer A, Kloeters C, Rogalla P, Pecher G, et al. The tumour-targeting human L19-IL2 immunocytokine: preclinical safety studies, phase I clinical trial in patients with solid tumours and expansion into patients with advanced renal cell carcinoma. Eur J Cancer. 2010;46:2926–2935. doi: 10.1016/j.ejca.2010.07.033. [DOI] [PubMed] [Google Scholar]
- 24.Ongaro T, Gouyou B, Stringhini M, Corbellari R, Neri D, Villa A. A novel format for recombinant antibody-interleukin-2 fusion proteins exhibits superior tumor-targeting properties in vivo . Oncotarget. 2020;11:3698–3711. doi: 10.18632/oncotarget.27726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Osenga KL, Hank JA, Albertini MR, Gan J, Sternberg AG, Eickhoff J, Seeger RC, Matthay KK, Reynolds CP, Twist C, et al. A phase I clinical trial of the hu14.18-IL2 (EMD 273063) as a treatment for children with refractory or recurrent neuroblastoma and melanoma: a study of the Children’s Oncology Group. Clin Cancer Res. 2006;12:1750–1759. doi: 10.1158/1078-0432.CCR-05-2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Albertini MR, Hank JA, Gadbaw B, Kostlevy J, Haldeman J, Schalch H, Gan J, Kim K, Eickhoff J, Gillies SD, et al. Phase II trial of hu14.18-IL2 for patients with metastatic melanoma. Cancer Immunol Immunother. 2012;61:2261–2271. doi: 10.1007/s00262-012-1286-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shusterman S, London WB, Gillies SD, Hank JA, Voss SD, Seeger RC, Reynolds CP, Kimball J, Albertini MR, Wagner B, et al. Antitumor activity of hu14.18-IL2 in patients with relapsed/refractory neuroblastoma: a Children’s Oncology Group (COG) phase II study. J Clin Oncol. 2010;28:4969–4975. doi: 10.1200/JCO.2009.27.8861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Albertini MR, Yang RK, Ranheim EA, Hank JA, Zuleger CL, Weber S, Neuman H, Hartig G, Weigel T, Mahvi D, et al. Pilot trial of the hu14.18-IL2 immunocytokine in patients with completely resectable recurrent stage III or stage IV melanoma. Cancer Immunol Immunother. 2018;67:1647–1658. doi: 10.1007/s00262-018-2223-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sharma P, Retz M, Siefker-Radtke A, Baron A, Necchi A, Bedke J, Plimack ER, Vaena D, Grimm MO, Bracarda S, et al. Nivolumab in metastatic urothelial carcinoma after platinum therapy (CheckMate 275): a multicentre, single-arm, phase 2 trial. Lancet Oncol. 2017;18:312–322. doi: 10.1016/S1470-2045(17)30065-7. [DOI] [PubMed] [Google Scholar]
- 30.Huddart RA, Siefker-Radtke AO, Balar AV, Bilen MA, Powles T, Bamias A, Castellano D, Khalil MF, Van Der Heijden MS, Koshkin VS, et al. PIVOT-10: Phase II study of bempegaldesleukin plus nivolumab in cisplatin-ineligible advanced urothelial cancer. Future Oncol. 2021;17:137–149. doi: 10.2217/fon-2020-0795. [DOI] [PubMed] [Google Scholar]
- 31.Waldhauer I, Gonzalez-Nicolini V, Freimoser-Grundschober A, Nayak TK, Fahrni L, Hosse RJ, Gerrits D, Geven EJ, Sam J, Lang S, et al. Simlukafusp alfa (FAP-IL2v) immunocytokine is a versatile combination partner for cancer immunotherapy. MAbs. 2021;13:1913791. doi: 10.1080/19420862.2021.1913791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Italiano A, Verlingue L, Prenen H, Guerra EM, Tosi D, Perets R, Lugowska I, Moiseenko V, Gumus M, Arslan C, et al. Clinical activity and safety of simlukafusp alfa, an engineered interleukin-2 variant targeted to fibroblast activation protein-α, combined with atezolizumab in patients with recurrent or metastatic cervical cancer. J Clin Oncol. 2021;39(15_suppl):5510. [Google Scholar]
- 33.Pyo KH, Koh YJ, Synn CB, Kim JH, Byeon Y, Jo HN, Kim YS, Lee W, Kim DH, Lee S, et al. Abstract 1826: Comprehensive preclinical study on GI-101, a novel CD80-IgG4-IL2 variant protein, as a therapeutic antibody candidate with bispecific immuno-oncology target. Cancer Res. 2021;81:1826. [Google Scholar]
- 34.Pyo KH, Koh YJ, Synn CB, Park JC, Kim JH, Byeon Y, Kim SE, Lee JM, Jo HN, Lee W, et al. Abstract 6529: GI101, A novel CD80-IgG4-IL2 variant bispecific protein, inhibits tumor growth and induces anti-tumor immune response in multiple preclinical models. Cancer Res. 2020;80:6529. [Google Scholar]
- 35.Cho BC, Shin SJ, Lee JL, Shim BY, Park HS, Yun N, Ham M, Koh YJ, Jang MH. 470 A phase 1/2, open-label, dose escalation and expansion study of GI-101 as a single agent and in combination with a pembrolizumab, lenvatinib or local RT in advanced solid tumors (KEYNOTE-B59) J Immunother Cancer. 2021;9(Suppl 2):A499. [Google Scholar]
- 36.Mahmoudpour SH, Jankowski M, Valerio L, Becker C, Espinola-Klein C, Konstantinides S, Quitzau K, Barco S. Safety of low-dose subcutaneous recombinant interleukin-2: systematic review and meta-analysis of randomized controlled trials. Sci Rep. 2019;9:7145. doi: 10.1038/s41598-019-43530-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Khammari A, Nguyen JM, Leccia MT, Guillot B, Saiagh S, Pandolfino MC, Knol AC, Quéreux G, Chiffolettau A, Labarrière N, et al. Tumor infiltrating lymphocytes as adjuvant treatment in stage III melanoma patients with only one invaded lymph node after complete resection: results from a multicentre, randomized clinical phase III trial. Cancer Immunol Immunother. 2020;69:1663–1672. doi: 10.1007/s00262-020-02572-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nguyen LT, Saibil SD, Sotov V, Le MX, Khoja L, Ghazarian D, Bonilla L, Majeed H, Hogg D, Joshua AM, et al. Phase II clinical trial of adoptive cell therapy for patients with metastatic melanoma with autologous tumor-infiltrating lymphocytes and low-dose interleukin-2. Cancer Immunol Immunother. 2019;68:773–785. doi: 10.1007/s00262-019-02307-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dréno B, Khammari A, Fortun A, Vignard V, Saiagh S, Beauvais T, Jouand N, Bercegay S, Simon S, Lang F, et al. Phase I/II clinical trial of adoptive cell transfer of sorted specific T cells for metastatic melanoma patients. Cancer Immunol Immunother. 2021;70:3015–3030. doi: 10.1007/s00262-021-02961-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Parisi G, Saco JD, Salazar FB, Tsoi J, Krystofinski P, Puig-Saus C, Zhang R, Zhou J, Cheung-Lau GC, Garcia AJ, et al. Persistence of adoptively transferred T cells with a kinetically engineered IL-2 receptor agonist. Nat Commun. 2020;11:660. doi: 10.1038/s41467-019-12901-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Camisaschi C, Filipazzi P, Tazzari M, Casati C, Beretta V, Pilla L, Patuzzo R, Maurichi A, Cova A, Maio M, et al. Effects of cyclophosphamide and IL-2 on regulatory CD4+ T cell frequency and function in melanoma patients vaccinated with HLA-class I peptides: impact on the antigen-specific T cell response. Cancer Immunol Immunother. 2013;62:897–908. doi: 10.1007/s00262-013-1397-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Filipazzi P, Pilla L, Mariani L, Patuzzo R, Castelli C, Camisaschi C, Maurichi A, Cova A, Rigamonti G, Giardino F, et al. Limited induction of tumor cross-reactive T cells without a measurable clinical benefit in early melanoma patients vaccinated with human leukocyte antigen class I-modified peptides. Clin Cancer Res. 2012;18:6485–6496. doi: 10.1158/1078-0432.CCR-12-1516. [DOI] [PubMed] [Google Scholar]
- 43.Rahma OE, Hamilton JM, Wojtowicz M, Dakheel O, Bernstein S, Liewehr DJ, Steinberg SM, Khleif SN. The immunological and clinical effects of mutated ras peptide vaccine in combination with IL-2, GM-CSF, or both in patients with solid tumors. J Transl Med. 2014;12:55. doi: 10.1186/1479-5876-12-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Baek S, Kim YM, Kim SB, Kim CS, Kwon SW, Kim Y, Kim H, Lee H. Therapeutic DC vaccination with IL-2 as a consolidation therapy for ovarian cancer patients: a phase I/II trial. Cell Mol Immunol. 2015;12:87–95. doi: 10.1038/cmi.2014.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tanyi JL, Chiang CL, Chiffelle J, Thierry AC, Baumgartener P, Huber F, Goepfert C, Tarussio D, Tissot S, Torigian DA, et al. Personalized cancer vaccine strategy elicits polyfunctional T cells and demonstrates clinical benefits in ovarian cancer. NPJ Vaccines. 2021;6:36. doi: 10.1038/s41541-021-00297-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mazzucchelli R, Durum SK. Interleukin-7 receptor expression: intelligent design. Nat Rev Immunol. 2007;7:144–154. doi: 10.1038/nri2023. [DOI] [PubMed] [Google Scholar]
- 47.Pulliam SR, Uzhachenko RV, Adunyah SE, Shanker A. Common gamma chain cytokines in combinatorial immune strategies against cancer. Immunol Lett. 2016;169:61–72. doi: 10.1016/j.imlet.2015.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Onder L, Narang P, Scandella E, Chai Q, Iolyeva M, Hoorweg K, Halin C, Richie E, Kaye P, Westermann J, et al. IL-7-producing stromal cells are critical for lymph node remodeling. Blood. 2012;120:4675–4683. doi: 10.1182/blood-2012-03-416859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zaunders JJ, Lévy Y, Seddiki N. Exploiting differential expression of the IL-7 receptor on memory T cells to modulate immune responses. Cytokine Growth Factor Rev. 2014;25:391–401. doi: 10.1016/j.cytogfr.2014.07.012. [DOI] [PubMed] [Google Scholar]
- 50.Sportès C, Babb RR, Krumlauf MC, Hakim FT, Steinberg SM, Chow CK, Brown MR, Fleisher TA, Noel P, Maric I, et al. Phase I study of recombinant human interleukin-7 administration in subjects with refractory malignancy. Clin Cancer Res. 2010;16:727–735. doi: 10.1158/1078-0432.CCR-09-1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Perales MA, Goldberg JD, Yuan J, Koehne G, Lechner L, Papadopoulos EB, Young JW, Jakubowski AA, Zaidi B, Gallardo H, et al. Recombinant human interleukin-7 (CYT107) promotes T-cell recovery after allogeneic stem cell transplantation. Blood. 2012;120:4882–4891. doi: 10.1182/blood-2012-06-437236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Trédan O, Ménétrier-Caux C, Ray-Coquard I, Garin G, Cropet C, Verronèse E, Bachelot T, Rebattu P, Heudel PE, Cassier P, et al. ELYPSE-7: a randomized placebo-controlled phase IIa trial with CYT107 exploring the restoration of CD4+ lymphocyte count in lymphopenic metastatic breast cancer patients. Ann Oncol. 2015;26:1353–1362. doi: 10.1093/annonc/mdv173. [DOI] [PubMed] [Google Scholar]
- 53.Lee SW, Choi D, Heo M, Shin EC, Park SH, Kim SJ, Oh YK, Lee BH, Yang SH, Sung YC, et al. hIL-7-hyFc, a long-acting IL-7, increased absolute lymphocyte count in healthy subjects. Clin Transl Sci. 2020;13:1161–1169. doi: 10.1111/cts.12800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kim JH, Kim YM, Choi D, Jo SB, Park HW, Hong SW, Park S, Kim S, Moon S, You G, et al. Hybrid Fc-fused interleukin-7 induces an inflamed tumor microenvironment and improves the efficacy of cancer immunotherapy. Clin Transl Immunology. 2020;9:e1168. doi: 10.1002/cti2.1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Heo M, Kim TW. P425 Phase 1b study of GX-I7, a long-acting interleukin-7, evaluating the safety, pharmacokinetics and pharmacodynamics profiles in patients with advanced solid cancers; Proceedings of the Society for Immunotherapy of Cancer (SITC) 2019; 6–10 November 2019; National Harbor, MD, USA. Milwaukee, WI: SITC; 2019. p. 282. [Google Scholar]
- 56.Campian JL, Ghosh S, Kapoor V, Yan R, Thotala S, Jash A, Hu T, Mahadevan A, Rifai K, Page L, et al. Long-acting recombinant human interleukin-7, NT-I7, increases cytotoxic CD8+ T cells and enhances survival in mouse glioma models. Clin Cancer Res. 2022:clincanres.0947.2021. doi: 10.1158/1078-0432.CCR-21-0947. [DOI] [PubMed] [Google Scholar]
- 57.Zhou A, Rettig M, Foltz J, Luo J, Butt O, Avvaru C, Katumba R, Kim A, Dunn G, Abraham C, et al. 396 NT-I7, a long-acting interleukin-7, promotes expansion of CD8 T cells and NK cells and immune activation in patients with newly diagnosed high-grade gliomas after chemoradiation. J Immunother Cancer. 2021;9(Suppl 2):A428. [Google Scholar]
- 58.Sohn J, Im YH. 322 Efficacy and safety of GX-I7 plus pembrolizumab for heavily pretreated patients with metastatic triple negative breast cancer: The Phase 1b/2 KEYNOTE-899 Study. J Immunother Cancer. 2020;8(Suppl 3):A197–A198. [Google Scholar]
- 59.Kim R, Barve M, Mamdani H, Johnson M, Lee BH, Ferrando-Martinez S, Chaney M, Fan J, Le N, Naing A. 404 Initial biomarker and clinical data of a phase 2a study of NT-I7, a long-acting interleukin-7, plus pembrolizumab: cohort of subjects with checkpoint inhibitor-naïve advanced MSS-colorectal cancer. J Immunother Cancer. 2021;9(Suppl 2):A435. [Google Scholar]
- 60.Naing A, Kim R, Barve M, Johnson M, Lee BH, Ferrando-Martinez S, Pant S, Wolff R, Haymaker C, Chaney M, et al. 408 Preliminary biomarker and clinical ata of a phase 2a study of NT-I7, a long-acting interleukin-7, plus pembrolizumab: cohort of subjects with checkpoint inhibitor-naïve advanced pancreatic cancer. J Immunother Cancer. 2021;9(Suppl 2):A439. [Google Scholar]
- 61.Stock S, Schmitt M, Sellner L. Optimizing manufacturing protocols of chimeric antigen receptor T cells for improved anticancer immunotherapy. Int J Mol Sci. 2019;20:6223. doi: 10.3390/ijms20246223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Adachi K, Kano Y, Nagai T, Okuyama N, Sakoda Y, Tamada K. IL-7 and CCL19 expression in CAR-T cells improves immune cell infiltration and CAR-T cell survival in the tumor. Nat Biotechnol. 2018;36:346–351. doi: 10.1038/nbt.4086. [DOI] [PubMed] [Google Scholar]
- 63.He C, Zhou Y, Li Z, Farooq MA, Ajmal I, Zhang H, Zhang L, Tao L, Yao J, Du B, et al. Co-expression of IL-7 improves NKG2D-based CAR T cell therapy on prostate cancer by enhancing the expansion and inhibiting the apoptosis and exhaustion. Cancers (Basel) 2020;12:1969. doi: 10.3390/cancers12071969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Luo H, Su J, Sun R, Sun Y, Wang Y, Dong Y, Shi B, Jiang H, Li Z. Coexpression of IL7 and CCL21 increases efficacy of CAR-T cells in solid tumors without requiring preconditioned lymphodepletion. Clin Cancer Res. 2020;26:5494–5505. doi: 10.1158/1078-0432.CCR-20-0777. [DOI] [PubMed] [Google Scholar]
- 65.Pang N, Shi J, Qin L, Chen A, Tang Y, Yang H, Huang Y, Wu Q, Li X, He B, et al. IL-7 and CCL19-secreting CAR-T cell therapy for tumors with positive glypican-3 or mesothelin. J Hematol Oncol. 2021;14:118. doi: 10.1186/s13045-021-01128-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ding ZC, Habtetsion T, Cao Y, Li T, Liu C, Kuczma M, Chen T, Hao Z, Bryan L, Munn DH, et al. Adjuvant IL-7 potentiates adoptive T cell therapy by amplifying and sustaining polyfunctional antitumor CD4+ T cells. Sci Rep. 2017;7:12168. doi: 10.1038/s41598-017-12488-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kim MY, Jayasinghe R, Staser KW, Devenport JM, O’Neal J, Kennerly KM, Carter AJ, Ritchey JK, Gao F, Lee BH, et al. A long-acting interleukin-7, rhIL-7-hyFc, enhances CAR T cell expansion, persistence and anti-tumor activity. Nat Commun. 2022 doi: 10.1038/s41467-022-30860-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Choi YW, Kang MC, Seo YB, Namkoong H, Park Y, Choi DH, Suh YS, Lee SW, Sung YC, Jin HT. Intravaginal administration of Fc-fused IL7 suppresses the cervicovaginal tumor by recruiting HPV DNA vaccine-induced CD8 T cells. Clin Cancer Res. 2016;22:5898–5908. doi: 10.1158/1078-0432.CCR-16-0423. [DOI] [PubMed] [Google Scholar]
- 69.Gu YZ, Fan CW, Lu R, Shao B, Sang YX, Huang QR, Li X, Meng WT, Mo XM, Wei YQ. Forced co-expression of IL-21 and IL-7 in whole-cell cancer vaccines promotes antitumor immunity. Sci Rep. 2016;6:32351. doi: 10.1038/srep32351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Merchant MS, Bernstein D, Amoako M, Baird K, Fleisher TA, Morre M, Steinberg SM, Sabatino M, Stroncek DF, Venkatasan AM, et al. Adjuvant immunotherapy to improve outcome in high-risk pediatric sarcomas. Clin Cancer Res. 2016;22:3182–3191. doi: 10.1158/1078-0432.CCR-15-2550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Pachynski RK, Morishima C, Szmulewitz R, Harshman L, Appleman L, Monk P, Bitting RL, Kucuk O, Millard F, Seigne JD, et al. IL-7 expands lymphocyte populations and enhances immune responses to sipuleucel-T in patients with metastatic castration-resistant prostate cancer (mCRPC) J Immunother Cancer. 2021;9:e002903. doi: 10.1136/jitc-2021-002903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Isvoranu G, Surcel M, Munteanu AN, Bratu OG, Ionita-Radu F, Neagu MT, Chiritoiu-Butnaru M. Therapeutic potential of interleukin-15 in cancer (review) Exp Ther Med. 2021;22:675. doi: 10.3892/etm.2021.10107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Waldmann TA. The biology of interleukin-2 and interleukin-15: implications for cancer therapy and vaccine design. Nat Rev Immunol. 2006;6:595–601. doi: 10.1038/nri1901. [DOI] [PubMed] [Google Scholar]
- 74.Bergamaschi C, Rosati M, Jalah R, Valentin A, Kulkarni V, Alicea C, Zhang GM, Patel V, Felber BK, Pavlakis GN. Intracellular interaction of interleukin-15 with its receptor alpha during production leads to mutual stabilization and increased bioactivity. J Biol Chem. 2008;283:4189–4199. doi: 10.1074/jbc.M705725200. [DOI] [PubMed] [Google Scholar]
- 75.Waickman AT, Ligons DL, Hwang S, Park JY, Lazarevic V, Sato N, Hong C, Park JH. CD4 effector T cell differentiation is controlled by IL-15 that is expressed and presented in trans. Cytokine. 2017;99:266–274. doi: 10.1016/j.cyto.2017.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Conlon KC, Lugli E, Welles HC, Rosenberg SA, Fojo AT, Morris JC, Fleisher TA, Dubois SP, Perera LP, Stewart DM, et al. Redistribution, hyperproliferation, activation of natural killer cells and CD8 T cells, and cytokine production during first-in-human clinical trial of recombinant human interleukin-15 in patients with cancer. J Clin Oncol. 2015;33:74–82. doi: 10.1200/JCO.2014.57.3329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Miller JS, Morishima C, McNeel DG, Patel MR, Kohrt HE, Thompson JA, Sondel PM, Wakelee HA, Disis ML, Kaiser JC, et al. A first-in-human phase I study of subcutaneous outpatient recombinant human IL15 (rhIL15) in adults with advanced solid tumors. Clin Cancer Res. 2018;24:1525–1535. doi: 10.1158/1078-0432.CCR-17-2451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Liu B, Kong L, Han K, Hong H, Marcus WD, Chen X, Jeng EK, Alter S, Zhu X, Rubinstein MP, et al. A novel fusion of ALT-803 (interleukin (IL)-15 superagonist) with an antibody demonstrates antigen-specific antitumor responses. J Biol Chem. 2016;291:23869–23881. doi: 10.1074/jbc.M116.733600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Romee R, Cooley S, Berrien-Elliott MM, Westervelt P, Verneris MR, Wagner JE, Weisdorf DJ, Blazar BR, Ustun C, DeFor TE, et al. First-in-human phase 1 clinical study of the IL-15 superagonist complex ALT-803 to treat relapse after transplantation. Blood. 2018;131:2515–2527. doi: 10.1182/blood-2017-12-823757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kim PS, Kwilas AR, Xu W, Alter S, Jeng EK, Wong HC, Schlom J, Hodge JW. IL-15 superagonist/IL-15RαSushi-Fc fusion complex (IL-15SA/IL-15RαSu-Fc; ALT-803) markedly enhances specific subpopulations of NK and memory CD8+ T cells, and mediates potent anti-tumor activity against murine breast and colon carcinomas. Oncotarget. 2016;7:16130–16145. doi: 10.18632/oncotarget.7470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Margolin K, Morishima C, Velcheti V, Miller JS, Lee SM, Silk AW, Holtan SG, Lacroix AM, Fling SP, Kaiser JC, et al. Phase I trial of ALT-803, a novel recombinant IL15 complex, in patients with advanced solid tumors. Clin Cancer Res. 2018;24:5552–5561. doi: 10.1158/1078-0432.CCR-18-0945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Knudson KM, Hodge JW, Schlom J, Gameiro SR. Rationale for IL-15 superagonists in cancer immunotherapy. Expert Opin Biol Ther. 2020;20:705–709. doi: 10.1080/14712598.2020.1738379. [DOI] [PubMed] [Google Scholar]
- 83.Miyazaki T, Kuo P, Maiti M, Obalapur P, Addepalli M, Rubas W, Sims P, Zhang P, Marcondes MQ, Madakamutil L, et al. Pharmacokinetic and pharmacodynamic study of NKTR-255, a polymer-conjugated human IL-15, in cynomolgus monkey. Blood. 2018;132:2952. [Google Scholar]
- 84.Wrangle JM, Velcheti V, Patel MR, Garrett-Mayer E, Hill EG, Ravenel JG, Miller JS, Farhad M, Anderton K, Lindsey K, et al. ALT-803, an IL-15 superagonist, in combination with nivolumab in patients with metastatic non-small cell lung cancer: a non-randomised, open-label, phase 1b trial. Lancet Oncol. 2018;19:694–704. doi: 10.1016/S1470-2045(18)30148-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Knudson KM, Hicks KC, Ozawa Y, Schlom J, Gameiro SR. Functional and mechanistic advantage of the use of a bifunctional anti-PD-L1/IL-15 superagonist. J Immunother Cancer. 2020;8:e000493. doi: 10.1136/jitc-2019-000493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Desbois M, Le Vu P, Coutzac C, Marcheteau E, Béal C, Terme M, Gey A, Morisseau S, Teppaz G, Boselli L, et al. IL-15 trans-signaling with the superagonist RLI promotes effector/memory CD8+ T cell responses and enhances antitumor activity of PD-1 antagonists. J Immunol. 2016;197:168–178. doi: 10.4049/jimmunol.1600019. [DOI] [PubMed] [Google Scholar]
- 87.Pérez-Martínez A, Fernández L, Valentín J, Martínez-Romera I, Corral MD, Ramírez M, Abad L, Santamaría S, González-Vicent M, Sirvent S, et al. A phase I/II trial of interleukin-15--stimulated natural killer cell infusion after haplo-identical stem cell transplantation for pediatric refractory solid tumors. Cytotherapy. 2015;17:1594–1603. doi: 10.1016/j.jcyt.2015.07.011. [DOI] [PubMed] [Google Scholar]
- 88.Shah NN, Baird K, Delbrook CP, Fleisher TA, Kohler ME, Rampertaap S, Lemberg K, Hurley CK, Kleiner DE, Merchant MS, et al. Acute GVHD in patients receiving IL-15/4-1BBL activated NK cells following T-cell-depleted stem cell transplantation. Blood. 2015;125:784–792. doi: 10.1182/blood-2014-07-592881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Giuffrida L, Sek K, Henderson MA, House IG, Lai J, Chen AX, Todd KL, Petley EV, Mardiana S, Todorovski I, et al. IL-15 preconditioning augments CAR T Cell responses to checkpoint blockade for improved treatment of solid tumors. Mol Ther. 2020;28:2379–2393. doi: 10.1016/j.ymthe.2020.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Liu E, Marin D, Banerjee P, Macapinlac HA, Thompson P, Basar R, Nassif Kerbauy L, Overman B, Thall P, Kaplan M, et al. Use of CAR-transduced natural killer cells in CD19-positive lymphoid tumors. N Engl J Med. 2020;382:545–553. doi: 10.1056/NEJMoa1910607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Heczey A, Courtney AN, Montalbano A, Robinson S, Liu K, Li M, Ghatwai N, Dakhova O, Liu B, Raveh-Sadka T, et al. Anti-GD2 CAR-NKT cells in patients with relapsed or refractory neuroblastoma: an interim analysis. Nat Med. 2020;26:1686–1690. doi: 10.1038/s41591-020-1074-2. [DOI] [PubMed] [Google Scholar]
- 92.Fabian KP, Padget MR, Donahue RN, Solocinski K, Robbins Y, Allen CT, Lee JH, Rabizadeh S, Soon-Shiong P, Schlom J, et al. PD-L1 targeting high-affinity NK (t-haNK) cells induce direct antitumor effects and target suppressive MDSC populations. J Immunother Cancer. 2020;8:e000450. doi: 10.1136/jitc-2019-000450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Chu Y, Nayyar G, Kham Su N, Rosenblum JM, Soon-Shiong P, Lee J, Safrit JT, Barth M, Lee D, Cairo MS. Novel cytokine-antibody fusion protein, N-820, to enhance the functions of ex vivo expanded natural killer cells against Burkitt lymphoma. J Immunother Cancer. 2020;8:e001238. doi: 10.1136/jitc-2020-001238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Rosser CJ, Tikhonenkov S, Nix JW, Chan OT, Ianculescu I, Reddy S, Soon-Shiong P. Safety, tolerability, and long-term clinical outcomes of an IL-15 analogue (N-803) admixed with Bacillus Calmette-Guérin (BCG) for the treatment of bladder cancer. OncoImmunology. 2021;10:1912885. doi: 10.1080/2162402X.2021.1912885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Do-Thi VA, Lee H, Jeong HJ, Lee JO, Kim YS. Protective and therapeutic effects of an IL-15:IL-15Rα-secreting cell-based cancer vaccine using a baculovirus system. Cancers (Basel) 2021;13:4039. doi: 10.3390/cancers13164039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kim DH, Kim HY, Lee WW. Induction of unique STAT heterodimers by IL-21 provokes IL-1RI expression on CD8+ T cells, resulting in enhanced IL-1β dependent effector function. Immune Netw. 2021;21:e33. doi: 10.4110/in.2021.21.e33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Davis MR, Zhu Z, Hansen DM, Bai Q, Fang Y. The role of IL-21 in immunity and cancer. Cancer Lett. 2015;358:107–114. doi: 10.1016/j.canlet.2014.12.047. [DOI] [PubMed] [Google Scholar]
- 98.Davis ID, Skrumsager BK, Cebon J, Nicholaou T, Barlow JW, Moller NP, Skak K, Lundsgaard D, Frederiksen KS, Thygesen P, et al. An open-label, two-arm, phase I trial of recombinant human interleukin-21 in patients with metastatic melanoma. Clin Cancer Res. 2007;13:3630–3636. doi: 10.1158/1078-0432.CCR-07-0410. [DOI] [PubMed] [Google Scholar]
- 99.Petrella TM, Mihalcioiu C, Monzon J, McWhirter E, Belanger K, Savage KJ, Song X, Hamid O, Cheng T, Davis M, et al. Final efficacy results of a randomized phase II study of recombinant interleukin-21 compared to dacarbazine in patients with recurrent or metastatic melanoma. J Oncol Res Treat. 2019;4:10001322019 [Google Scholar]
- 100.Thompson JA, Curti BD, Redman BG, Bhatia S, Weber JS, Agarwala SS, Sievers EL, Hughes SD, DeVries TA, Hausman DF. Phase I study of recombinant interleukin-21 in patients with metastatic melanoma and renal cell carcinoma. J Clin Oncol. 2008;26:2034–2039. doi: 10.1200/JCO.2007.14.5193. [DOI] [PubMed] [Google Scholar]
- 101.Bhatt S, Parvin S, Zhang Y, Cho HM, Kunkalla K, Vega F, Timmerman JM, Shin SU, Rosenblatt JD, Lossos IS. Anti-CD20-interleukin-21 fusokine targets malignant B cells via direct apoptosis and NK-cell-dependent cytotoxicity. Blood. 2017;129:2246–2256. doi: 10.1182/blood-2016-09-738211. [DOI] [PubMed] [Google Scholar]
- 102.Liu H, Wang R, An D, Liu H, Ye F, Li B, Zhang J, Liu P, Zhang X, Yao S, et al. An engineered IL-21 with half-life extension enhances anti-tumor immunity as a monotherapy or in combination with PD-1 or TIGIT blockade. Int Immunopharmacol. 2021;101:108307. doi: 10.1016/j.intimp.2021.108307. [DOI] [PubMed] [Google Scholar]
- 103.Williams P, Rafei M, Bouchentouf M, Raven J, Yuan S, Cuerquis J, Forner KA, Birman E, Galipeau J. A fusion of GMCSF and IL-21 initiates hypersignaling through the IL-21Ralpha chain with immune activating and tumoricidal effects in vivo . Mol Ther. 2010;18:1293–1301. doi: 10.1038/mt.2010.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Uricoli B, Birnbaum LA, Do P, Kelvin JM, Jain J, Costanza E, Chyong A, Porter CC, Rafiq S, Dreaden EC. Engineered cytokines for cancer and autoimmune disease immunotherapy. Adv Healthc Mater. 2021;10:e2002214. doi: 10.1002/adhm.202002214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Deng S, Sun Z, Qiao J, Liang Y, Liu L, Dong C, Shen A, Wang Y, Tang H, Fu YX, et al. Targeting tumors with IL-21 reshapes the tumor microenvironment by proliferating PD-1intTim-3-CD8+ T cells. JCI Insight. 2020;5:e132000. doi: 10.1172/jci.insight.132000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Lewis KE, Selby MJ, Masters G, Valle J, Dito G, Curtis WR, Garcia R, Mink KA, Waggie KS, Holdren MS, et al. Interleukin-21 combined with PD-1 or CTLA-4 blockade enhances antitumor immunity in mouse tumor models. Oncoimmunology. 2017;7:e1377873. doi: 10.1080/2162402X.2017.1377873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Seo H, Kim BS, Bae EA, Min BS, Han YD, Shin SJ, Kang CY. IL21 therapy combined with PD-1 and Tim-3 blockade provides enhanced NK cell antitumor activity against MHC class I-deficient tumors. Cancer Immunol Res. 2018;6:685–695. doi: 10.1158/2326-6066.CIR-17-0708. [DOI] [PubMed] [Google Scholar]
- 108.Li Y, Cong Y, Jia M, He Q, Zhong H, Zhao Y, Li H, Yan M, You J, Liu J, et al. Targeting IL-21 to tumor-reactive T cells enhances memory T cell responses and anti-PD-1 antibody therapy. Nat Commun. 2021;12:951. doi: 10.1038/s41467-021-21241-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Chapuis AG, Ragnarsson GB, Nguyen HN, Chaney CN, Pufnock JS, Schmitt TM, Duerkopp N, Roberts IM, Pogosov GL, Ho WY, et al. Transferred WT1-reactive CD8+ T cells can mediate antileukemic activity and persist in post-transplant patients. Sci Transl Med. 2013;5:174ra27. doi: 10.1126/scitranslmed.3004916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Chapuis AG, Lee SM, Thompson JA, Roberts IM, Margolin KA, Bhatia S, Sloan HL, Lai I, Wagener F, Shibuya K, et al. Combined IL-21-primed polyclonal CTL plus CTLA4 blockade controls refractory metastatic melanoma in a patient. J Exp Med. 2016;213:1133–1139. doi: 10.1084/jem.20152021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Peng J, Ye L, Li T, Zhu Q, Guo J, Xiao K, Wei Y. Irradiated bladder cancer cells expressing both GM-CSF and IL-21 versus either GM-CSF or IL-21 alone as tumor vaccine in a mouse xenograft model. BioMed Res Int. 2019;2019:8262989. doi: 10.1155/2019/8262989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.He X, Wang J, Zhao F, Chen D, Chen J, Zhang H, Yang C, Liu Y, Dou J. ESAT-6-gpi DNA vaccine augmented the specific antitumour efficacy induced by the tumour vaccine B16F10-ESAT-6-gpi/IL-21 in a mouse model. Scand J Immunol. 2013;78:69–78. doi: 10.1111/sji.12074. [DOI] [PubMed] [Google Scholar]
- 113.Kefaloyianni E. Soluble forms of cytokine and growth factor receptors: mechanisms of generation and modes of action in the regulation of local and systemic inflammation. FEBS Lett. 2022 doi: 10.1002/1873-3468.14305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Do-Thi VA, Lee JO, Lee H, Kim YS. Crosstalk between the producers and immune targets of IL-9. Immune Netw. 2020;20:e45. doi: 10.4110/in.2020.20.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Li Z, Chen L, Qin Z. Paradoxical roles of IL-4 in tumor immunity. Cell Mol Immunol. 2009;6:415–422. doi: 10.1038/cmi.2009.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Wan J, Wu Y, Ji X, Huang L, Cai W, Su Z, Wang S, Xu H. IL-9 and IL-9-producing cells in tumor immunity. Cell Commun Signal. 2020;18:50. doi: 10.1186/s12964-020-00538-5. [DOI] [PMC free article] [PubMed] [Google Scholar]