Abstract
In the past few decades, biological drugs and small molecule inhibitors targeting inflammatory cytokines, immune cells, and intracellular kinases have become the standard-of-care to treat autoimmune diseases. Inhibition of TNF, IL-6, IL-17, and IL-23 has revolutionized the treatment of autoimmune diseases, such as rheumatoid arthritis, ankylosing spondylitis, and psoriasis. B cell depletion therapy using anti-CD20 mAbs has shown promising results in patients with neuroinflammatory diseases, and inhibition of B cell survival factors is approved for treatment of systemic lupus erythematosus. Targeting co-stimulatory molecules expressed on Ag-presenting cells and T cells is also expected to have therapeutic potential in autoimmune diseases by modulating T cell function. Recently, small molecule kinase inhibitors targeting the JAK family, which is responsible for signal transduction from multiple receptors, have garnered great interest in the field of autoimmune and hematologic diseases. However, there are still unmet medical needs in terms of therapeutic efficacy and safety profiles. Emerging therapies aim to induce immune tolerance without compromising immune function, using advanced molecular engineering techniques.
Keywords: Autoimmune disease, Molecular targeted therapy, Biologic therapy, Protein kinase inhibitors, Investigational drugs
INTRODUCTION
Autoimmune diseases are pathologic conditions characterized by dysregulated inflammation against autoantigens and affect 3%–10% of the general population (1). Conventional treatments for autoimmune diseases have suppressed general immune function to modulate uncontrolled inflammation. However, those therapeutic approaches have not been completely successful in heterogeneous patient populations, and their efficacy comes at the expense of side effects, particularly increased risk of infection, usually from non-selective immune suppression. To overcome the limitations of conventional therapies, current treatments aim to more selectively inhibit inflammatory signals while causing minimal disruption to homeostatic immune functions.
Recent advances in understanding disease pathogenesis and new drug manufacturing techniques have led to the widespread use of targeted immunotherapy to treat autoimmune disease. Moreover, advanced molecular engineering has enabled the emergence of recombinant protein therapeutics such as mAbs and receptor-Ab fusion proteins that target soluble mediators or cell surface markers (2). Since selective protein therapeutics targeting TNF were first approved for rheumatoid arthritis (RA) in the 1990s, targeted immunotherapies have been a game changer for treatment of autoimmune diseases. According to the Global Pharmaceuticals Market Report, adalimumab has been the top selling drug worldwide for several years, followed by other targeted immunotherapies, such as pembrolizumab, ibrutinib, and ustekinumab (3).
As knowledge about the pathogenesis of disease is rapidly increasing, numerous biological drugs targeting inflammatory signaling pathways are being developed to treat intractable inflammatory diseases. Following successful introduction of biologic therapies to treat autoimmune diseases, the molecular targets have expanded to intracellular kinases. Blockade of convergent signals by small molecule kinase inhibitors is of great interest in terms of therapeutic efficacy and long-term safety (4,5).
This review summarizes current therapeutic approaches that target signaling pathways involved in the pathogenesis of autoimmune diseases and presents emerging immunotherapies intended to induce immune tolerance. Because the market for targeted immunotherapy is growing rapidly, we focus on drugs that have received clinical approval to treat autoimmune diseases.
INFLAMMATION IN AUTOIMMUNE DISEASES
Inflammation is a natural process by which living organisms repair tissue damage and protect against foreign substances. However, dysregulated immune reactions against self-Ags lead to loss of immune tolerance and development of autoimmune disease. Autoimmunity arises from central and peripheral defects in tolerance checkpoints and activation of nontolerant immune cells. Autoantigens can be induced by release of self-Ags from immune-privileged sites, generation of neo-self Ag, and molecular mimicry of self-proteins with foreign substances (6).
Clinical manifestations of autoimmunity can be diverse, ranging from asymptomatic conditions in the presence of autoantibodies to fulminant autoimmune diseases that cause life-threatening organ damage. Development of autoimmune disease can be triggered by environmental factors in genetically susceptible individuals. Environmental triggers, including stress, smoking, and infection, induce the pro-inflammatory functions of innate immunity, and promotes the pathologic response of adaptive immunity (7).
Although the conventional concept of autoimmunity was dysregulation of the adaptive immune system, growing evidence indicates that the innate immune system is also critical to initiation and progression of autoimmune diseases. As the key players in innate immunity, macrophages and dendritic cells (DCs) are essential to Ag presentation and production of pro-inflammatory cytokines such as TNF, IL-1β, IL-6, IL-23, B cell-activating factor (BAFF, also known as Blys or TNFSF13B), and a proliferation-inducing ligand (APRIL, also known as TNFSF13A) (8,9). Type 1 IFN, critically implicated in the pathogenesis of systemic lupus erythematosus (SLE) and its related diseases, is primarily produced by plasmacytoid DCs (pDCs), a specialized subset of DCs (8,10). The interaction between macrophages/DCs and T cells/B cells further promotes autoimmune inflammation.
Naïve CD4+ Th cells differentiate into distinct T cell subsets depending on the cytokine milieu (11). T cells play a key role in the pathogenesis of autoimmune diseases through autoantigen recognition, cytokine production, and enhanced cytotoxicity (6). In recent decades, Th17 cells producing IL-17 and FOXP3+ Tregs have been highlighted as therapeutic targets for autoimmune diseases.
Autoreactive B cells, another major component of adaptive immunity, produce pathologic autoantibodies and activate T cells through Ag presentation and cytokine production (6,7). Autoantibody production is a hallmark of various autoimmune diseases, including RA and SLE. Anti-citrullinated peptide Ab in RA and anti-dsDNA Ab in SLE are representative pathogenic autoantibodies responsible for clinical presentation and disease activity. Due to the important role of B cells in autoimmunity, B cell surface molecules are therapeutic targets for various autoimmune diseases.
Soluble mediators from activated immune cells transduce inflammatory signals by binding to their cognate receptors. Once engaged by inflammatory cytokines, the receptors activate the JAK family to induce phosphorylation, dimerization, and nuclear translocation of STATs (4). Gene transcription by STATs promotes cell proliferation and differentiation and production of a variety of inflammatory mediators, further exacerbating autoimmune inflammation.
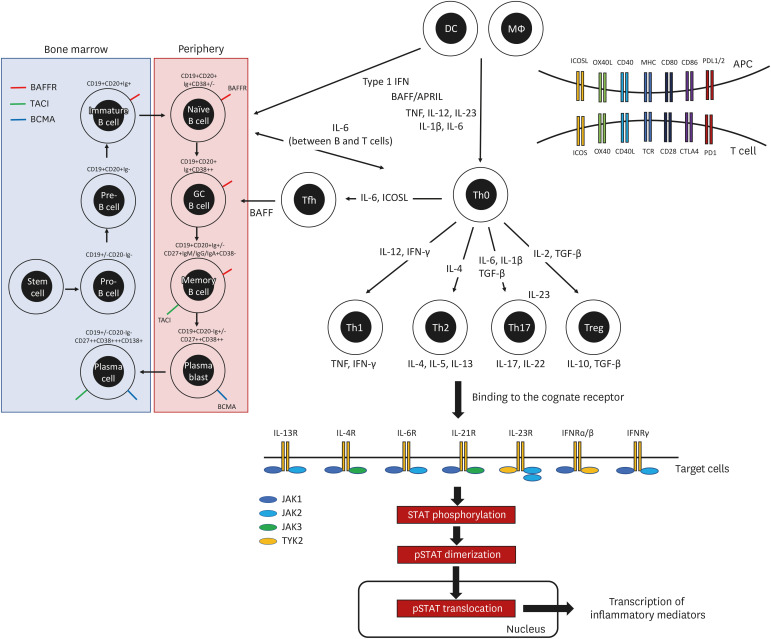
Although the pathophysiologic mechanisms differ for each autoimmune disease, several common inflammatory pathways could be therapeutic targets for immunotherapy. The key therapeutic targets for the treatment of autoimmune diseases based on our current understanding of the pathogenesis of autoimmune inflammation are depicted in Fig. 1. Tables 1, 2, 3 list targeted immunotherapies approved for autoimmune diseases or under clinical development based on the promising results, detailed below. This review focuses on the clinical application of targeted immunotherapies in the area of immune-mediated inflammatory diseases.
Figure 1. Key therapeutic targets in the pathogenesis of autoimmune diseases.
Autoimmune inflammation involves the dysregulated activation of immune cells, inflammatory cytokines, and intracellular signaling molecules. Key molecules in the inflammatory pathway are therapeutic targets for the treatment of autoimmune diseases.
Table 1. Cytokine-targeted therapies for treatment of autoimmune diseases.
| Target cytokine | Structure | Drug | Clinical application | Under investigation (phase IIb or III) |
|---|---|---|---|---|
| TNF-ɑ | sTNFR2-IgG1 Fc | Etanercept | RA, pJIA, AS, Psoriasis, PsA | |
| Anti-TNF mAb | Infliximab | RA, AS, Psoriasis, PsA, UC, CD | ||
| Off-label use: BD, sarcoidosis | ||||
| Adalimumab | RA, pJIA, AS, psoriasis, PsA, UC, CD, hiradenitis suppurativa, uveitis | |||
| Off-label use: BD | ||||
| Golimumab | RA, AS, PsA, UC | |||
| Certolizumab | RA, AS, psoriasis, PsA, CD | |||
| IL-1 | IL-1R antagonist | Anakinra | RA, CAPS | Kawasaki disease (NCT04656184) |
| Off-label use: AOSD, sJIA, gout, recurrent pericarditis, and many others | ||||
| IL-1R1-IgG Fc | Rilonacept | CAPS, DIRA, recurrent pericarditis | ||
| Off-label use: AOSD, gout, and many others | ||||
| Anti-IL-1β mAb | Canakinumab | AOSD, sJIA, CAPS, TRAPS, HIDS/MKD, FMF | COVID19-associated CRS (12) | |
| Off-label use: gout and many others | ||||
| IL-6 | Anti-IL-6 mAb | Sirukumab | RA (13,14)* | |
| Olokizumab | RA (15) (NCT02760407, NCT02760433, NCT03120949) | |||
| Clazakizumab | RA (NCT02015520) | |||
| PsA (16) | ||||
| COVID19-associated CRS (NCT04343989) | ||||
| Siltuximab | CAR-T-associated CRS (NCT04975555) | |||
| Anti-IL-6R mAb | Tocilizumab | RA, sJIA, pJIA, SSc-associated ILD, Giant cell arteritis, CRS | PMR (NCT02908217) | |
| Off-label use: AOSD, Takayasu arteritis | NMOSD (17) | |||
| COVID19 pneumonia (18,19) (NCT04409262) | ||||
| Sarilumab | RA | COVID19-associated CRS (20) (NCT04315298) | ||
| Vobarilizumab | RA (NCT02518620) | |||
| IL-17 | Anti-IL-17 mAb | Ixekizumab | Psoriasis, PsA, axial SpA | |
| Secukinumab | AS, Psoriasis, PsA | SLE (NCT04181762) | ||
| Axial SpA (21) (NCT04156620, NCT04732117) | ||||
| Hiradenitis supprativa (NCT03713619, NCT03713632, NCT04179175) | ||||
| Giant cell arteritis (NCT04930094) | ||||
| Grave’s ophthalmopathy (NCT03713619) | ||||
| Anti-IL-17R mAb | Brodalumab | Psoriasis | Axial SpA (22) | |
| PsA (23) | ||||
| SSc (NCT03957681) | ||||
| Anti-IL17A/F mAb | Bimekizumab | AS (NCT03928743) | ||
| Axial SpA (NCT03928704, NCT04436640) | ||||
| Psoriasis (24,25) (NCT03598790, NCT03766685) | ||||
| PsA (NCT03895203, NCT03896581, NCT04009499, NCT04109976) | ||||
| Hiradenitis suppruativa (NCT04242446, NCT04242498, NCT04901195) | ||||
| IL-23 | Anti-p40 mAb | Ustekinumab | Psoriasis, PsA, UC, CD | Idiopathic inflammatory myositis (NCT03981744) |
| Takayasu arteritis (NCT04882072) | ||||
| Anti-p19 mAb | Guselkumab | Psoriasis, PsA | UC (NCT04033445) | |
| CD (NCT03466411, NCT04397263) | ||||
| Risankizumab | Psoriasis | PsA (26,27) | ||
| UC (NCT03398135, NCT03398148) | ||||
| CD (NCT03104413, NCT03105102, NCT03105128, NCT04524611) | ||||
| Tildrakizumab | Psoriasis | PsA (NCT03552276, NCT04314544, NCT04314531, NCT04991116) | ||
| Mirikizumab | Psoriasis (NCT03482011, NCT03535194, NCT03556202) | |||
| UC (NCT03518086, NCT03519945, NCT03524092) | ||||
| CD (NCT03926130, NCT04232553) | ||||
| Type 1 IFN | Anti-IFNR1 mAb | Anifrolumab | SLE |
This table includes drugs with one or more approved indications for autoimmune diseases. The indication covers only the area of autoimmune diseases.
pJIA, polyarticular juvenile idiopathic arthritis; UC, ulcerative colitis; CD, Crohn’s disease; BD, Behcet’s disease; sJIA, systemic juvenile idiopathic arthritis; DIRA, deficiency of IL-1 receptor antagonist; TRAPS, TNF receptor associated periodic syndrome; HIDS/MKD, hyperimmunoglobulin D syndrome/Mevalonate kinase deficiency; FMF, familial Mediterranean fever; CRS, cytokine release syndrome; CAR-T, chimeric Ag receptor-T cell; SSc-associated ILD, systemic sclerosis-associated interstitial lung disease, PMR, polymyalgia rheumatica, NMOSD, neuromyelitis optica spectrum disorder; axial SpA, axial spondyloarthritis.
*Despite positive results in phase III clinical trials, FDA did not recommend sirukumab for treatment of RA due to safety issues.
Table 2. Cell-targeted therapies for treatment of autoimmune diseases.
| Target cell | Structure | Drug | Clinical application | Under investigation (phase IIb or III) |
|---|---|---|---|---|
| B cell | Anti-CD20 mAb | Rituximab | RA | Pemphigus vulgaris (28) |
| GPA, MPA | ||||
| Off-label use: MS, immune thrombocytopenia | ||||
| Ocrelizumab | MS | |||
| Ofatumumab | MS | |||
| Ublituximab | MS (NCT03277261, NCT03277248, NCT04130997) | |||
| Anti-CD19 mAb | Inebilizumab | NMOSD | IgG4RD (NCT04540497) | |
| Myasthenia gravis (NCT04524273) | ||||
| Anti-BAFF mAb | Belimumab | SLE | ||
| Anti-BAFF-R mAb | Ianalumab | SLE (NCT05126277) | ||
| pSS (29) | ||||
| T cell | CTLA4-IgG1 Fc | Abatacept | RA, pJIA | pSS (NCT02067910, NCT02915159) |
| PsA (30) | ||||
| Idiopathic inflammatory myositis (NCT02971683) | ||||
| GPA (NCT02108860) | ||||
| Anti-CD40 mAb | Iscalimab | pSS (NCT03905525) |
This table includes drugs approved for autoimmune diseases or under clinical development based on positive results from phase II trials.
GPA, granulomatosis with polyangiitis; MPA, microscopic polyangiitis; NMOSD, neuromyelitis optica spectrum disorder; IgG4RD, immunoglobulin G4 related disease; pJIA, polyarticular juvenile idiopathic arthritis.
Table 3. Kinase-targeted therapies for treatment of autoimmune diseases.
| Target kinases | Structure | Drug | Clinical application | Under investigation (phase IIb or III) |
|---|---|---|---|---|
| JAK | JAK1/3 inhibitor | Tofacitinib | RA | JIA (31) (NCT01500551, NCT03000439) |
| PsA | AS (32) | |||
| UC | ||||
| JAK 1/2 inhibitor | Baricitinib | RA | SLE (NCT03843125, NCT03616912, NCT03616964) | |
| JIA (NCT03773965, NCT03773978) | ||||
| sJIA (NCT04088396) | ||||
| Atopic dermatitis (33,34) | ||||
| Uveitis (NCT04088409) | ||||
| JAK1 selective inhibitor | Upadacitinib | RA | CD (NCT03345823, NCT03345836, NCT03345849) | |
| PsA | UC (35) (NCT03006068, NCT03653026) | |||
| Axial SpA (NCT04169373) | ||||
| Atopic dermatitis (36,37) (NCT03661138, NCT04195698) | ||||
| Takayasu arteritis (NCT04161898) | ||||
| Giant cell arteritis (NCT03725202) | ||||
| Filgotinib | RA (by EMA and Japan) | UC (38) (NCT02914535) | ||
| CD (NCT02914561, NCT02914600) | ||||
| TYK | TYK2 selective inhibitor | Deucravacitinib | Psoriasis (NCT03624127, NCT03611751) | |
| PsA (NCT04908189, NCT04908202) | ||||
| BTK | BTK inhibitor | Evobrutinib | MS (NCT04338022, NCT04338061) | |
| Tolebrutinib | MS (NCT04410978, NCT04410991, NCT04411641, NCT04458051) | |||
| Myasthenia gravis (NCT05132569) | ||||
| Fenebrutinib | MS (NCT04544449, NCT04586010, NCT04586023) | |||
| Rilzabrutinib | Pemphigus vulgaris (NCT03762265) | |||
| Immune thrombocytopenia (NCT04562766) |
This table includes drugs approved for autoimmune diseases or under clinical development based on positive results from phase II trials.
UC, ulcerative colitis; sJIA, systemic juvenile idiopathic arthritis; CD, Crohn’s disease; axial SpA, axial spondyloarthritis.
CYTOKINE-TARGETED THERAPY
TNF
TNF is a proinflammatory cytokine mainly produced by myeloid cells and activated T cells (39). The pathogenic role of TNF in chronic inflammation was suggested by increased expression of TNF in RA synovium and development of arthritis in TNF-transgenic mice (40,41). The success of anti-TNF therapy has revolutionized the treatment strategy for RA patients. Since the first approval of infliximab and etanercept in 1998, 4 mAbs (infliximab, adalimumab, golimumab, and certolizumab) and one receptor-Fc fusion protein (etanercept) have been approved and are currently available for treatment of chronic immune-mediated diseases, including RA, juvenile idiopathic arthritis (JIA), ankylosing spondylitis (AS), psoriasis, and psoriatic arthritis (PsA). Such mAbs are also indicated for treatment of inflammatory bowel disease and noninfectious uveitis.
Although TNF is well known to have a pro-inflammatory role, it is a pleiotropic cytokine that depends on the binding of TNF receptor (TNFR) (42,43). TNFR1, constitutively expressed on almost all nucleated cells, is mostly responsible for the inflammatory function of TNF, whereas TNFR2, which is expressed only on specific cell types, such as myeloid-derived suppressor cells, Treg cells, and monocytes, is associated with the regulatory function of TNF. The distinct signaling pathways derived from TNFR1 and TNFR2 have been reviewed elsewhere (42,43). Currently available anti-TNF therapy inhibits both TNFR1 and TNFR2. Due to the regulatory aspect of TNF, TNF blockades may paradoxically induce expansion of Th1/Th17 cells and dysregulated IFN response, which could explain treatment failure, autoantibody generation, and paradoxical psoriasis during anti-TNF therapy (44,45). Therefore, more selective treatments to inhibit TNFR1 and enhance TNFR2 are under investigation (43,46).
IL-1
IL-1α and IL-1β, members of the IL-1 family, are pro-inflammatory cytokines that are closely associated with innate immune responses. Although IL-1α and IL-1β share biological functions by binding with IL-1 receptor 1 (IL-1R1), several characteristics distinguish IL-1α from IL-1β (47). Pro-IL-1α, constitutively expressed in mesenchymal cells, is biologically active, whereas pro-IL-1β produced by macrophages requires cleavage by caspase-1 to become active IL-1β. Caspase-1-dependent cleavage of pro-IL-1β is mediated by activation of an inflammasome containing either a member of nucleotide binding domain and leucine-rich-repeat-containing [NLR] protein family (e.g. NLR family pyrin domain-containing [NLRP] 1, NLRP3, NLR family CARD domain-containing 4) or a member of PYRIN-HIN-200 domain-containing protein family (e.g. absent in melanoma 2) (48). In addition, IL-1α is not detected in systemic circulation, in contrast to IL-1β, which suggests that the pathogenic role of IL-1α in autoimmune diseases is local rather than systemic. At this time, IL-1β is considered to be more closely associated with diverse rheumatic diseases than IL-1α, including systemic JIA, adult onset-Still’s disease (AOSD), and gout, and with hereditary autoinflammatory diseases, such as cryopyrin-associated periodic syndromes (CAPS) and familial Mediterranean fever (49).
Upon occupation by IL-1α or IL-1β, IL-1R1 forms a heterotrimeric complex with IL-1R3 to recruit myeloid differentiation primary response gene 88 (MYD88), which triggers a subsequent kinase cascade (IL-1R-associated kinases [IRAKs], IκB kinase, IκB, and NF-κB) that promotes a pro-inflammatory state (47,50). This inflammatory activity of IL-1 is regulated by the naturally occurring IL-1R antagonist (IL-1Ra) from the same IL-1 family. Occupation of IL-1R1 by IL-1Ra leads to a conformational change that hinders formation of the heterotrimeric complex with IL-1R3, thereby hampering the IL-1-mediated inflammatory process.
Currently, 3 protein therapeutics have been approved as anti-IL-1 therapies: canakinumab, an anti-IL-1β mAb; anakinra, a recombinant IL-1 receptor antagonist; and rilonacept, an IL-1R1-Fc fusion protein. Based on their molecular structures, all 3 drugs block IL-1β, and anakinra and rilonacept also inhibit IL-1α (47). As a new therapeutic approach to inhibit IL-1 signaling, oral NLRP3 inhibitors, including dapansutrile (OLT177), are under investigation (51).
In early clinical development, anakinra was tested as a treatment for RA. Despite its proven therapeutic efficacy in patients with RA, anakinra is not recommended as the first-line biologic therapy due to its low cost-effectiveness. Anti-IL-1 therapy is more widely used in autoinflammatory diseases such as systemic JIA, AOSD, and CAPS (52). Other conditions with high inflammatory burdens, such as gout and recurrent pericarditis, are also managed by IL-1-targeted therapy. Due to the broad role of IL-1 in inflammatory diseases, IL-1 blockades are expected to offer clinical benefit in treating intractable autoimmune diseases, including SLE and systemic sclerosis, and in controlling excessive pro-inflammatory responses, such as cytokine releasing syndrome and macrophage activation syndrome (47,53).
IL-6
IL-6 is a pleiotropic cytokine produced by various cell types in the context of infection, inflammation, and malignancy. IL-6 was initially identified as a ‘B cell differentiation factor’ or ‘B cell stimulation factor’ secreted by T cells (54). Despite its crucial role in B cell maturation, anti-IL-6 therapy failed to show therapeutic efficacy in patients with multiple myeloma (55). However, IL-6-targeted therapy with tocilizumab, the first anti-IL-6R-blocking mAb, was shown to have significant clinical benefits in RA and was even superior to adalimumab in efficacy (56). Tocilizumab is currently approved for the treatment of RA, JIA, AOSD, giant cell arteritis, and cytokine releasing syndrome based on its ability to regulate systemic hyperinflammation (54). Recently, therapeutic application of anti-IL-6 therapy has been investigated in SLE, neuromyelitis optica, and systemic sclerosis (17,57,58).
IL-6 has multiple biological functions in physiological and pathological conditions. In physiological states, IL-6 is responsible for macrophage differentiation to the M2 state, osteoclastogenesis through the increased expression of the RANK ligand on osteoblasts, and the acute phase response via the JAK-STAT pathway in the liver. In pathological (inflammatory) conditions, IL-6 is critically involved in Th17 differentiation by regulating FOXP3, RORC, and IL-23R expression (59). IL-6 is also important for development of follicular helper T cells and maturation of B cells (60).
IL-6 signaling is transduced through a complex of IL-6R and glycoprotein 130 (gp130). Dimerization of gp130 sequentially activates multiple signaling pathways including JAK-STAT, MAPK, PI3K, and YES-associated protein 1, which translocates to the nucleus and controls the transcription of genes associated with cell growth, proliferation, and inflammation (61,62). To interfere with the IL-6 signaling pathway, anti-IL6 therapy can target IL-6, IL-6R, gp130, JAK, and STAT3. However, concerns about side effects limit the potential of gp130 and STAT3 as therapeutic targets (63,64). Thus, anti-IL6 mAbs, anti-IL-6R mAbs, and JAK inhibitors have been used to block IL-6 signaling, and the potential for using soluble gp130 to inhibit the IL-6/IL-6R complex is under investigation (60).
IL-17
IL-17A, commonly known as IL-17, is the signature cytokine of Th17 subsets of CD4+ T cells, but it is also produced by CD8+ T cells (Tc17), γδ T cells, natural killer T cells, group 3 innate lymphoid cells, and neutrophils (65). In the physiological state, Th17 cells contribute to innate immunity by recruiting neutrophils, host defense against bacteria and fungi, and tissue repair at skin and mucosal sites (66,67). In pathological conditions, IL-17A promotes autoimmunity, synergistically with TNF and other inflammatory chemokines (68). Animal models of inflammatory arthritis and neuroinflammation suggest the predominant role of Th17 cells rather than Th1 cells in the development of these diseases (69).
Th17 cells are derived from naïve CD4+ naïve T cells through repression of FOXP3 and activation of STAT3 and RORC in the presence of IL-1β, IL-6 and TGF-β (59). Subsequently, IL-23 stabilizes pathogenic Th17 cells for proliferation and survival (70). Upon binding with IL-17A, a unique intracellular cytoplasmic domain termed SEF/IL-17R of IL-17R subunit A (IL-17RA) and subunit C (IL-17RC) recruit the adaptor protein Act1, which triggers ubiquitination of TNF-receptor associated factor (TRAF) 6 and subsequent activation of the NF-κB, MAPK, and activator protein 1 (AP1) pathways and C/EBP transcription factors (71,72).
A growing body of evidence from animal and human studies suggests that IL-23/IL-17 axis is an excellent therapeutic target for several autoimmune diseases, such as AS, PsA, and RA (68,73). Current anti-IL-17 therapies include mAbs to IL-17A (secukinumab and ixekizumab) and IL-17RA (brodalumab). As expected, anti-IL-17 therapeutics have produced a dramatic response in psoriasis, PsA, and AS (73,74). Unexpectedly, this approach failed to show remarkable clinical efficacy in patients with RA, a Th17-dependent disease (75). This unsatisfactory result could be explained by heterogeneity in the disease itself or differential pathogenic role of IL-17A depending on RA stage (e.g. early versus advanced).
IL-23
IL-23 is a proinflammatory cytokine secreted by DCs and activated macrophages (76). As a member of the IL-12 family, IL-23 is a heterodimer composed of the p40 subunit, which is shared by IL-12 (IL-12/IL-23p40), and the p19 subunit unique to IL-23 (IL-23p19). Although IL-12 and IL-23 share a structural subunit, IL-23 is more critically and widely involved in the pathogenesis of autoimmune diseases. Most importantly, IL-23 stabilizes the pathogenic features of Th17 cells through maintenance of Th17 signature genes, suppression of repressive factors, upregulation of IL-23R expression, and induction of effector genes (77,78). It also promotes the pro-inflammatory functions of DCs and macrophages as an autocrine factor (79).
IL-23 interacts with a receptor complex composed of IL-12Rβ1 (binding with IL-12/IL-23p40) and IL-23R (binding with IL-23p19), which are linked with tyrosine kinase (TYK) 2 and JAK2, respectively (78). Activation of TYK2 and JAK2 leads to the phosphorylation and nuclear translocation of STATs, predominantly STAT3. Blockade of IL-23 signaling can be achieved by the targeted inhibition of IL-12/IL-23p40 or IL-23p19. As an anti-IL-12/IL-23p40 mAb, ustekinumab blocks both IL-12 and IL-23, whereas the anti-IL-23p19 mAbs (guselkumab, tildrakizumab, risankizumab, and mirikizumab) inhibit only IL-23 (80).
Similar to anti-IL-17 therapy, IL-23 inhibition is effective in the treatment of psoriasis and PsA (80). However, both mAbs targeting the p40 and p19 subunits failed to improve the clinical course of AS, in contrast to anti-IL17 therapy. Despite the clear pathogenic role of IL-23 in preclinical models of AS, such unexpected result suggests that IL-23 plays a different role in a tissue- and time-dependent context in human autoimmune diseases (73). In Crohn’s disease, ustekinumab successfully improved clinical outcomes, and clinical trials of anti-IL-23p19 mAbs are ongoing (Table 1).
Type 1 IFN
Type 1 IFNs, including IFN-α, IFN-β, IFN-ε, IFN-κ, and IFN-ω, were initially identified as soluble antiviral factors (10). Subsequent studies revealed that IFN-α has an essential role in the pathogenesis of autoimmune diseases, especially SLE. Clinical data from patients with SLE showed significantly increased expression of type 1 IFN signatures (81). The development of SLE-like syndrome after recombinant IFN-α therapy also suggests a pathologic role of type 1 IFN in autoimmune disease (82).
Although most nucleated cells can produce type 1 IFNs, the main cellular source is pDCs (8). Nucleic acid-containing extracellular stimuli, such as RNAs and DNAs complexed with anti-RNA and DNA autoantibodies, respectively, interact with Toll-like receptor 7 (TLR7) and TLR9 in pDCs to recruit MYD88 and activate IRAKs and TRAFs, which lead to translocation of IFN regulatory family (IRF) 7 and resultant transcription of IFN-α (10,83). Type 1 IFNs bind with the IFN-α/β receptor (IFNAR) which consists of IFNAR1 and IFNAR2. Upon engagement by type 1 IFNs, IFNAR1 and IFNAR2 activate JAK1 and TYK2, respectively, which leads to phosphorylation and nuclear translocation of STAT1 and STAT2. In the nucleus, along with IRF9, STAT1/STAT2 induce the expression of IFN-stimulated genes (10). In SLE, IFN-α promotes the pro-inflammatory response by stimulating myeloid DCs, Th1 cells, and B cells and by suppressing Tregs (83).
The pathophysiologic significance of IFN-α in SLE has led to the development of type 1 IFN-targeted immunotherapy. Rontalizumab and sifalimumab, mAbs that neutralize IFN-α, were tested in SLE but failed to control disease activity (84,85). Although the role of IFNs other than IFN-α is less clear, suppressing only IFN-α while leaving IFN-β and IFN-κ active might explain the inadequate therapeutic effect in SLE. Recently, anifrolumab, a human mAb targeting IFNAR1 to block the biological function of all type 1 IFNs, has been approved for moderate to severe SLE (86). Anifrolumab showed a consistent clinical benefit in terms of reduced disease activity, oral corticosteroid use, and annualized flares. However, patients with lupus nephritis and neuropsychiatric lupus were excluded from the clinical trials.
Several new therapeutic approaches to block type 1 IFNs are under investigation. To reduce the production of type 1 IFNs, drugs are targeting pDCs and signaling molecules, such as TLR, MyD88, and IRAK4 (83). Kinase inhibitors also are under clinical development for various autoimmune diseases based on their ability to inhibit the IFNAR downstream signaling pathways.
CELL-TARGETED THERAPY
B cells
As our understanding of B cell function has expanded, B cells are considered an active participant in autoimmune diseases. Interestingly, the pathologic significance of B cells in autoimmunity was highlighted by the success of B cell depletion therapies in RA and multiple sclerosis (MS). Beyond their role in Ab production, B cells actively participate in autoimmunity through Ag-presentation, formation of tertiary lymphoid tissues, and production of cytokines, such as IL-6, TNF, IFN-γ, and GM-CSF (87). Ab-independent functions of B cells explain the treatment efficacy of B cell depletion therapies in RA and MS despite the absence of autoantibody reduction (88,89).
B cells are generated in the bone marrow and sequentially differentiate into Ag-specific B cells in peripheral tissues. B cell differentiation proceeds toward increasing affinity for an Ag through somatic hypermutation and the class-switch recombination of immunoglobulins, accompanied by changes in B cell surface markers (90). CD20 is expressed exclusively in the B cell lineage from pre-B cells to memory B cells, but not in plasma cells (Fig. 1). During the past several decades, anti-CD20 therapy has been the main tool for B cell depletion. Rituximab, a chimeric anti-CD20 mAb, redistributes CD20 into lipid rafts, and then activates complement-dependent cytotoxicity and Ab-dependent cellular cytotoxicity (ADCC) (90). Effective elimination of CD20+ B cells by rituximab exhausts the cellular source of Ab-secreting plasmablasts and prevents the Ab-independent B cell function, resulting in attenuation of autoimmune inflammation (91). Following the clinical success of rituximab in RA and MS, diverse anti-CD20 therapeutics with distinct binding epitopes, different route of administration, and advanced therapeutic efficacy have been developed. Ocrelizumab and ofatumumab, humanized anti-CD20 mAbs, have been approved for relapsing MS, and clinical trials of other anti-CD20 mAbs, obinutuzumab and ublituximab, are ongoing (Table 2).
Unfortunately, rituximab was not fully successful in the treatment of SLE, one of the stereotypical B-cell-mediated diseases. Aside from heterogeneity in patients with SLE, treatment failure with rituximab could arise from the presence of autoreactive CD20− (but CD19+) long-lived plasma cells, incomplete depletion of B cells in peripheral blood and tissues, or disease flares during B-cell reconstitution (91). For broad inhibition of B cells resistant to anti-CD20 therapy, B cell depletion therapy has targeted CD19, which is expressed on CD20+ B cells as well as CD20- plasmablasts and some plasma cells. A humanized anti-CD19 mAb, inebilizumab, was approved for neuromyelitis optica spectrum disorder (92) and is undergoing clinical trials to treat other inflammatory neurological disorders (Table 2).
Another approach to B cell inhibition is to block B cell survival factors, specifically BAFF and APRIL. BAFF is critically involved in B-cell maturation and survival after ligation with its 3 distinct receptors: 1) BAFF receptor (BAFF-R, known as TNFRSF13C), which is expressed on most B-cell subsets except plasma cells; 2) transmembrane activator, calcium modulator and cyclophilin ligand interactor (TACI; known as TNFRSF13B), which is expressed on marginal zone B cells, memory B cells, and plasma cells; 3) B cell maturation Ag (BCMA; known as TNFRSF17) expressed on plasmablasts and plasma cells (87). Unlike BAFF-R, TACI and BCMA also bind with APRIL, which is important for Ab class switching and plasma cell survival.
Given the essential role of B cell maturation, proliferation, and survival, the BAFF/APRIL system has been considered a promising therapeutic target in SLE and its related autoimmune diseases. Belimumab, a humanized anti-BAFF mAb, is the first targeted immunotherapy approved for SLE by FDA.; however, belimumab does not always have universal therapeutic benefits in all patients with SLE (93). Ianalumab, a mAb against BAFF-R, has shown promising results in a phase II trial in patients with primary Sjögren syndrome (pSS) (29). As a B cell depletion therapy, ianalumab has 2 modes of action: B-cell lysis by enhanced ADCC and blockade of BAFF signaling. Telitacicept, a TACI-Ig fusion protein, is also under clinical development based on its inhibitory effect on the BAFF/APRIL system (94).
T cells
As key regulators in adaptive immunity, T cells play a critical role in the development and progression of autoimmunity. T cell effector functions require Ag recognition by TCRs and additional engagement of co-stimulatory receptors. Thus, modulation of those co-stimulatory molecules has been investigated for T cell-targeted therapy in autoimmune diseases and malignancies (95,96).
CD28 signaling is a well-known co-stimulatory pathway for T cell activation and differentiation through activation of the MAPK, AKT, and NF-κB pathways (97). CD28 expressed on CD4+ T cells binds to CD80 or CD86 on Ag-presenting cells, which are shared by CTLA-4 as an inhibitory counterpart of CD28 (98). Based on counter-regulation of CD28 and CTLA-4, a fusion protein composed of extracellular domain of CTLA4 and an IgG Fc region (CTLA4-Ig) was developed to inhibit CD28 signaling by occupying CD80 and CD86. The first CTLA4-Ig, abatacept, was approved to treat RA and JIA with proven efficacy in the control of inflammatory arthritis (99). Subsequently, another CTLA4-Igs with improved binding to CD80 and CD86, including belatacept and MEDI5256, have been investigated for clinical applications (100,101). Although belatacept was approved for kidney transplant recipients in 2011, the additional clinical benefit of belatacept beyond conventional calcineurin inhibitor-based regimen is controversial (102).
The CD40 pathway is the primary activation signal for T cell effector function. CD80 and CD86 expression is upregulated after ligation of CD40L to CD40 (103). When CD40L on activated T cells binds to CD40 on target cells, multiple kinase cascades activate transcription factors, such as NF-κB and AP1, to induce cell survival, proliferation and differentiation (104). CD40 signaling is particularly important for the B cell-T cell interactions involved in T cell-dependent Ab responses, germinal center formation, and memory B cell differentiation (105). Due to its critical role in B cell function, the therapeutic potential of targeting CD40 signaling has been investigated in preclinical and clinical studies. A recent phase II trial of iscalimab, a humanized anti-CD40 mAb, showed clinical improvement in patients with pSS (106), and a subsequent phase III trial is ongoing (Table 2).
Immunotherapies that target other co-stimulatory signaling, such as the inducible T cell co-stimulator (ICOS) and OX40 pathways, are also being developed to inhibit the T cell effector function in autoimmune diseases (96). As co-stimulatory molecules expressed on activated CD4+ T cells, ICOS and OX40 are involved in T cell survival, differentiation, and activation.
KINASE-TARGETED THERAPY
As previously stated, binding soluble inflammatory mediators to their cognate receptors transduce signals through activation of intracellular kinases, typically the JAK family. The JAK family, which consists of JAK1, JAK2, JAK3, and TYK2, is associated with cytokine receptors (including IL-2R, IL-4R, IL-5R, IL-6R, IL-13R, and type 1 IFNs), growth hormones and erythropoietin (4). Activation of the JAK family leads to phosphorylation, dimerization, and nuclear transformation of STATs. In the nucleus, STATs contribute to the gene transcription involved in cell differentiation, proliferation, and survival, cytokine production, and angiogenesis (107,108).
Due to the pivotal role of JAK in hematopoiesis, JAK inhibitors were first evaluated for treatment of hematologic diseases, especially myeloproliferative neoplasm (108). Since ruxolitinib (a JAK1 and JAK2 inhibitor) was first approved for myelofibrosis in 2011, numerous kinase inhibitors have been approved or investigated for treatment of hematologic malignancies and inflammatory diseases (5). Several JAK inhibitors have been approved for RA, PsA, and ulcerative colitis based on their proven clinical efficacy (108). Selectivity for JAK differs by each JAK inhibitor, although differences in therapeutic efficacy have not been demonstrated between JAK inhibitors. Tofacitinib and baricitinib mainly target JAK1/3 and JAK1/2, respectively. Upadacitinib and filgotinib are second-generation JAK inhibitors that selectively inhibit JAK1.
In contrast to the aforementioned immunotherapies, which regulate cellular activation signals in the extracellular space, JAK inhibitors target signaling pathways in the intracellular space. JAK-targeted therapy inhibits the convergent signals from multiple cytokine receptors and disrupts the feedback loop (4). As a result, even a partial blockade of select kinases can efficiently downregulate multiple inflammatory signaling pathways, attenuating autoimmune inflammation. It should be noted that in a head-to-head, randomized controlled trial, baricitinib showed a better clinical response than the anti-TNF mAb adalimumab (109).
In addition to JAK inhibitors, a selective TYK2 inhibitor, deucravacitinib, is awaiting clinical approval as the first oral targeted therapy in psoriasis (110). TYK2 mediates the intracellular inflammatory signals originating from IL-12, IL-23, and type 1 IFNs. The therapeutic potential of deucravacitinib is currently being investigated in patients with PsA, inflammatory bowel diseases, and SLE based on mechanistic insights into the pathogenesis of those diseases (Table 3).
Bruton tyrosine kinase (BTK) inhibitors are also expected to ameliorate autoimmune inflammation by modulating B cell function. BTK inhibitors are currently being investigated in various autoimmune disease, including RA, MS, pSS, and SLE (4). Some recent phase II trials with BTK inhibitors showed promising results in patients with relapsing MS (111,112).
The future direction of kinase inhibition is development of more selective inhibitors with minimal off-target effects and improved organ specificity based on advanced molecular techniques. Furthermore, on-target effects caused by unregulated cellular behaviors other than amelioration of inflammation should be investigated extensively. Recent evidence suggests that patients treated with tofacitinib face an increased risk of cardiac events, malignancies, thrombosis, and death, and FDA has decided to revise the boxed warnings of JAK inhibitors (113). The chronic course of autoimmune diseases raises safety concerns for long-term drug use, which can substantially influence drug selection by patients and physicians.
EMERGING IMMUNOTHERAPY
With better understandings of the immuno-pathogenesis of autoimmune diseases and continuing advances in biotechnology, numerous drugs with novel therapeutic targets and advanced efficacy continue to be developed, showing promisingly efficacious results. Notwithstanding all of this, there are still unmet medical needs, such as heterogeneous therapeutic effects and adverse events caused by immunosuppression. To overcome the limitations of current treatments, therapeutic approaches for inflammatory diseases have been diversified and individualized. Here, we briefly introduce a new perspective of targeted immunotherapy enabled by advanced molecular engineering techniques.
Chimeric Ag receptor (CAR) T cell therapy
The basic concept of CAR T cell therapy is administration of autologous T cells containing genetically engineered TCRs to capture tumor-specific Ags and eliminate tumor cells by increasing cytotoxicity. Following therapeutic success in a patient with diffuse large B cell lymphoma, CD19 CAR T cell therapy was approved for treatment of B-cell lymphoma and acute lymphoblastic leukemia (114).
CARs are engineered receptors composed of an extracellular Ag recognition domain, a transmembrane domain, and a intracellular T cell activation domain (115). The extracellular domain, also known as single-chain Fv, is designed as the variable heavy and light chains of a monoclonal Ab. Ag recognition by extracellular domains activates the immunoreceptor tyrosine-based activation motifs on intracellular domains, leading to cytokine production and lysis of target cells. To improve therapeutic efficacy, the intracellular domain consists of an activation domain, CD3ζ, and a co-stimulatory domain, such as CD28.
Although CAR T cells were first introduced in the field of oncology, their therapeutic potential to eliminate pathologic cells extends to the treatment of autoimmune inflammatory diseases. Recently, Mougiakakos et al. (116) reported a rapid clinical remission with autologous CD19 CAR T cell therapy in a patients with SLE refractory to conventional treatment. In that patient, infused CD19 CAR T cells were detectable in the peripheral blood for 7 weeks after a single dose with a rapid and sustained decrease in anti-dsDNA Ab. That is a promising result in that current B cell depletion with anti-CD20 mAbs requires regular injections and attention to immunogenicity to maintain the initial therapeutic effect.
With the development of engineered T cell therapy, chimeric autoantigen receptor (CAAR) T cell and CAR Treg therapies have drawn attention as novel therapeutic strategies for autoimmune diseases (117). The extracellular domain of CAAR T cells expresses an autoantigen that can interact with Ag-specific autoreactive B cells and T cells. CAAR T cell therapy could be effective in autoimmune diseases with known autoantigens. CAR Treg cells are also being designed with the expectation of an Ag-specific immune-regulatory response that cannot be adequately achieved by infusion of polyclonal Treg cells. However, such engineered T cell therapies for autoimmune diseases have not yet been widely studied. Moreover, despite the hypothetical therapeutic effect of engineered T cell therapies, cytokine releasing syndrome and neurotoxicity could limit enthusiasm for the use of cell therapies in autoimmune diseases, particularly in high inflammation microenvironments (118).
Low dose IL-2 therapy
IL-2 is a key cytokine for differentiation, activation, and survival of Treg cells and is mostly produced by activated CD4+ T cells (119). Tregs, defined by CD4+FOXP3+CD25+CD127low expression, modulate autoreactive immune cells through secretion of anti-inflammatory cytokines (IL-10, tumor growth factor-β, and IL-35), induction of immunosuppressive enzyme (indoleamine 2,3-dioxygenase) by DCs, inactivation of effector T cells and NK cells, and direct cytotoxicity against CD8+ T cells and NK cells (119,120). Treg cell deficiency and/or dysfunction are frequently reported in various autoimmune diseases, such as RA, SLE, pSS, and AS (120).
In addition to activating Treg cells, IL-2 induces survival and proliferation of effector T cells and inhibits Th17 cell differentiation (119). Based on its stimulatory effect on conventional T cells, high-dose IL-2 therapy was first approved for metastatic renal cell cancer and metastatic melanoma. However, later studies have shown that the main role of IL-2 is mediated through the high-affinity IL-2R expressed on Treg cells. IL-2R is expressed in monomeric, dimeric, and trimeric variants, and trimeric IL-2R has higher affinity than the monomeric and dimeric forms (121). Because Treg cells constitutively express trimeric IL-2R at high level, Treg cells are highly sensitive to IL-2 than effector memory CD4+ T cells. Thus, low-dose IL-2 therapy (0.3 to 3 million international unit/day) has been evaluated in autoimmune diseases in anticipation that it will induce immune tolerance by means of Treg stimulation while having minimal effects on effector T cells (120).
In patients with autoimmune diseases, including SLE, PsA, and hepatitis C virus-induced cryoglobulinemic vasculitis, low dose IL-2 therapy improved clinical outcomes, which was consistent with an increase in Treg cells (120). In clinical trials, low dose IL-2 had a tolerable safety profile and was not associated with an increased risk of infection. Moreover, in mice, immune responses to vaccination, infection, and cancer were not impaired by long-term treatment with low dose IL-2 (122).
The favorable clinical trial results have led to the development of engineered IL-2 proteins with altered amino acid sequences (IL-2 muteins) to improve selectivity and reduce adverse effects (120). Combinations of IL-2 and biologic therapies such as TNF- and IL-6 inhibition are also under investigation.
Immune tolerance induction
Inducing immune tolerance to autoantigens without disrupting the rest of the immune system is an ideal approach to autoimmune diseases. In this regard, oral tolerance that can induce Ag-specific immune tolerance has been investigated for more than a century (123). Gut-associated lymphoid tissue (GALT) is the largest immune organ that maintains tolerance to large amounts of food and commensal microorganisms (124). Given the role of GALT in intestinal homeostasis and systemic regulation, the tolerogenic effects of fed Ags on systemic autoimmune diseases have been studied extensively in preclinical and clinical trials. In animal models, oral tolerance induction was found to effectively prevent and even treat various inflammatory diseases, including inflammatory arthritis, experimental autoimmune encephalitis, diabetes, and uveitis (123). Depending on the dose of Ag, low doses of Ag promote the generation of Treg cells secreting IL-4, IL-10, and TGF-β, whereas high doses of Ag promote the clonal deletion or anergy of specific T cells (125). However, such mechanisms of immune tolerance are not exclusive and can overlap.
Early pilot trials and phase II trials of oral tolerance induction also showed promising results in patients with RA, MS, and uveitis without treatment-related toxicity. However, phase III trials of Ag feeding in patients with RA and MS showed a suboptimal therapeutic effects (125). Despite the experimental efficacy and acceptable safety of Ag feeding, unsolved issues need to be addressed to enable successful induction of immune tolerance: 1) protocol for Ag feeding (dose and type of Ag, use of mucosal adjuvant, and frequency of administration); 2) combined treatment with conventional immunosuppressive drugs; 3) biological and immunological markers that reflect the effectiveness of oral tolerance development; and 4) selection of suitable patients amenable to induced oral tolerance (age, disease onset, and history of desensitization).
Another approach to Ag-specific tolerance is tolerogenic vaccines that deliver autoantigens. Tolerogenic vaccines have successfully induced Ag-specific Treg cells, and promoted autoreactive T cell anergy and apoptosis in animal models of autoimmune diseases (126,127). Several platforms of tolerogenic vaccines, including protein/peptide-, nanoparticle-, cell-, and DNA/RNA-based vaccines, have been investigated to enhance immunogenicity in preclinical and clinical research of MS (128). Although a phase III trial using a peptide vaccine failed to show clinical benefit (129), the tolerogenic approach is still being studies using several vaccine platforms in patients with MS.
Recently, Krienke et al. (130) reported successful induction of immune tolerance with a noninflammatory mRNA-based vaccine in mice model of experimental autoimmune encephalomyelitis induced by myelin oligodendrocyte glycoprotein (MOG35-55). Although double-stranded RNA molecules are inherently proinflammatory in the extracellular environment, their proinflammatory nature was reduced by replacing uridine with 1-methylpseudouridine (m1Ψ). The MOG35-55-encoding m1Ψ-modified single-stranded mRNA vaccine prevented disease development and progression in mice. The tolerogenic vaccine induced MOG35-55-specific FOXP3+ Treg cells and suppressed MOG33-35-specific Th1 and Th17 cells. Expression of inhibitory molecules such as PD1 and CTLA4 was upregulated on Ag-specific cells, and the protective effect of the vaccine was abrogated by PD1- and CTLA-4-targeted checkpoint inhibitors.
Nonetheless, development of tolerogenic vaccines is challenging because it is inconclusive and controversial which autoantigens are specific for autoimmune diseases, and multiple autoantigens could be involved in most autoimmune diseases.
CONCLUSION
Advances in targeted immunotherapy during the past few decades have revolutionized the treatment and clinical outcomes of patients with autoimmune diseases. Current targeted immunotherapies lead to suppression of major pro-inflammatory signaling pathways by blocking inflammatory cytokines, cell surface molecules, and intracellular kinases. Despite the tremendous success of targeted therapies in autoimmune disease, unmet medical needs remain in terms of drug efficacy and long-term safety. Beyond blocking inflammatory signaling pathways, future therapies aim to induce long-standing immune tolerance while maintaining protective immune functions. Hopefully, advances in biotechnology and knowledge of diseases will provide opportunities to develop new drugs with improved therapeutic efficacy and minimal adverse effects. Additionally, considering the multifaceted roles of inflammatory mediators and immune cells, the development of more selective and specific drugs will be required together with a more precise understanding of disease pathogenesis.
ACKNOWLEDGEMENTS
This work was supported by a grant from the National Research Foundation of Korea (NRF), funded by the Ministry of Education, Science and Technology (NRF-2015R1A3A2032927).
Abbreviations
- ADCC
Ab-dependent cellular cytotoxicity
- AIM2
absent in melanoma 2
- AOSD
adult onset-Still’s disease
- AP1
activator protein 1
- APRIL
a proliferation-inducing ligand
- AS
ankylosing spondylitis
- BAFF
B cell-activating factor
- BCMA
B cell maturation Ag
- BTK
bruton tyrosine kinase
- CAAR
chimeric autoantigen receptor
- CAPS
cryopyrin-associated periodic syndromes
- CAR
chimeric Ag receptor
- CTLA4-Ig
a fusion protein composed of extracellular domain of CTLA4 and an IgG Fc region
- DC
dendritic cell
- GALT
gut-associated lymphoid tissue
- ICOS
inducible T cell co-stimulator
- IFNAR
IFN-α/β receptor
- IL-1R
IL-1 receptor
- IL-1Ra
IL-1R antagonist
- IRAK
IL-1R-associated kinase
- IRF
IFN regulatory family
- JIA
juvenile idiopathic arthritis MS, multiple sclerosis
- MYD88
myeloid differentiation primary response gene 88
- NLR
nucleotide binding domain and leucine-rich-repeat-containing
- NLRP
NLR family pyrin domain-containing
- pDC
plasmacytoid dendritic cell
- PsA
psoriatic arthritis
- pSS
primary Sjögren syndrome
- RA
rheumatoid arthritis
- SLE
systemic lupus erythematosus
- TACI
transmembrane activator, calcium modulator and cyclophilin ligand interactor
- TNFR
TNF receptor
- TRAF
TNF-receptor associated factor
- TYK
tyrosine kinase
Footnotes
Conflict of Interest: The authors declare no potential conflicts of interest.
- Conceptualization: Jung SM, Kim WU.
- Funding acquisition: Kim WU.
- Investigation: Jung SM.
- Visualization: Jung SM.
- Supervision: Kim WU.
- Wiring - original draft: Jung SM, Kim WU.
- Writing - reviewing & editing: Jung SM, Kim WU.
References
- 1.Cooper GS, Bynum ML, Somers EC. Recent insights in the epidemiology of autoimmune diseases: improved prevalence estimates and understanding of clustering of diseases. J Autoimmun. 2009;33:197–207. doi: 10.1016/j.jaut.2009.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lagassé HA, Alexaki A, Simhadri VL, Katagiri NH, Jankowski W, Sauna ZE, Kimchi-Sarfaty C. Recent advances in (therapeutic protein) drug development. F1000 Res. 2017;6:113. doi: 10.12688/f1000research.9970.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Top drugs and pharma companies by sales in 2020 [Internet] [accessed on 21 January 2022]. Available at https://www.pharmacompass.com/radio-compass-blog/top-drugs-and-pharma-companies-by-sales-in-2020.
- 4.Zarrin AA, Bao K, Lupardus P, Vucic D. Kinase inhibition in autoimmunity and inflammation. Nat Rev Drug Discov. 2021;20:39–63. doi: 10.1038/s41573-020-0082-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Attwood MM, Fabbro D, Sokolov AV, Knapp S, Schioth HB. Trends in kinase drug discovery: targets, indications and inhibitor design. Nat Rev Drug Discov. 2021;20:839–861. doi: 10.1038/s41573-021-00252-y. [DOI] [PubMed] [Google Scholar]
- 6.Theofilopoulos AN, Kono DH, Baccala R. The multiple pathways to autoimmunity. Nat Immunol. 2017;18:716–724. doi: 10.1038/ni.3731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wahren-Herlenius M, Dörner T. Immunopathogenic mechanisms of systemic autoimmune disease. Lancet. 2013;382:819–831. doi: 10.1016/S0140-6736(13)60954-X. [DOI] [PubMed] [Google Scholar]
- 8.Swiecki M, Colonna M. The multifaceted biology of plasmacytoid dendritic cells. Nat Rev Immunol. 2015;15:471–485. doi: 10.1038/nri3865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ma WT, Gao F, Gu K, Chen DK. The role of monocytes and macrophages in autoimmune diseases: a comprehensive review. Front Immunol. 2019;10:1140. doi: 10.3389/fimmu.2019.01140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jiang J, Zhao M, Chang C, Wu H, Lu Q. Type I interferons in the pathogenesis and treatment of autoimmune diseases. Clin Rev Allergy Immunol. 2020;59:248–272. doi: 10.1007/s12016-020-08798-2. [DOI] [PubMed] [Google Scholar]
- 11.Zhu J, Paul WE. Peripheral CD4+ T-cell differentiation regulated by networks of cytokines and transcription factors. Immunol Rev. 2010;238:247–262. doi: 10.1111/j.1600-065X.2010.00951.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Caricchio R, Abbate A, Gordeev I, Meng J, Hsue PY, Neogi T, Arduino R, Fomina D, Bogdanov R, Stepanenko T, et al. Effect of canakinumab vs placebo on survival without invasive mechanical ventilation in patients hospitalized with severe COVID-19: a randomized clinical trial. JAMA. 2021;326:230–239. doi: 10.1001/jama.2021.9508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aletaha D, Bingham CO, 3rd, Tanaka Y, Agarwal P, Kurrasch R, Tak PP, Popik S. Efficacy and safety of sirukumab in patients with active rheumatoid arthritis refractory to anti-TNF therapy (SIRROUND-T): a randomised, double-blind, placebo-controlled, parallel-group, multinational, phase 3 study. Lancet. 2017;389:1206–1217. doi: 10.1016/S0140-6736(17)30401-4. [DOI] [PubMed] [Google Scholar]
- 14.Takeuchi T, Thorne C, Karpouzas G, Sheng S, Xu W, Rao R, Fei K, Hsu B, Tak PP. Sirukumab for rheumatoid arthritis: the phase III SIRROUND-D study. Ann Rheum Dis. 2017;76:2001–2008. doi: 10.1136/annrheumdis-2017-211328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nasonov E, Fatenejad S, Feist E, Ivanova M, Korneva E, Krechikova DG, Maslyanskiy AL, Samsonov M, Stoilov R, Zonova EV, et al. Olokizumab, a monoclonal antibody against interleukin 6, in combination with methotrexate in patients with rheumatoid arthritis inadequately controlled by methotrexate: efficacy and safety results of a randomised controlled phase III study. Ann Rheum Dis. 2021 doi: 10.1136/annrheumdis-2021-219876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mease PJ, Gottlieb AB, Berman A, Drescher E, Xing J, Wong R, Banerjee S. The efficacy and safety of clazakizumab, an anti-interleukin-6 monoclonal antibody, in a phase IIb study of adults with active psoriatic arthritis. Arthritis Rheumatol. 2016;68:2163–2173. doi: 10.1002/art.39700. [DOI] [PubMed] [Google Scholar]
- 17.Zhang C, Zhang M, Qiu W, Ma H, Zhang X, Zhu Z, Yang CS, Jia D, Zhang TX, Yuan M, et al. Safety and efficacy of tocilizumab versus azathioprine in highly relapsing neuromyelitis optica spectrum disorder (TANGO): an open-label, multicentre, randomised, phase 2 trial. Lancet Neurol. 2020;19:391–401. doi: 10.1016/S1474-4422(20)30070-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Salama C, Han J, Yau L, Reiss WG, Kramer B, Neidhart JD, Criner GJ, Kaplan-Lewis E, Baden R, Pandit L, et al. Tocilizumab in patients hospitalized with COVID-19 pneumonia. N Engl J Med. 2021;384:20–30. doi: 10.1056/NEJMoa2030340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rosas IO, Bräu N, Waters M, Go RC, Hunter BD, Bhagani S, Skiest D, Aziz MS, Cooper N, Douglas IS, et al. Tocilizumab in hospitalized patients with severe COVID-19 pneumonia. N Engl J Med. 2021;384:1503–1516. doi: 10.1056/NEJMoa2028700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lescure FX, Honda H, Fowler RA, Lazar JS, Shi G, Wung P, Patel N, Hagino O, Bazzalo IJ, Casas MM, et al. Sarilumab in patients admitted to hospital with severe or critical COVID-19: a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Respir Med. 2021;9:522–532. doi: 10.1016/S2213-2600(21)00099-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Deodhar A, Blanco R, Dokoupilová E, Hall S, Kameda H, Kivitz AJ, Poddubnyy D, van de Sande M, Wiksten AS, Porter BO, et al. Improvement of signs and symptoms of nonradiographic axial spondyloarthritis in patients treated with secukinumab: primary results of a randomized, placebo-controlled phase iii study. Arthritis Rheumatol. 2021;73:110–120. doi: 10.1002/art.41477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wei JC, Kim TH, Kishimoto M, Ogusu N, Jeong H, Kobayashi S. Efficacy and safety of brodalumab, an anti-IL17RA monoclonal antibody, in patients with axial spondyloarthritis: 16-week results from a randomised, placebo-controlled, phase 3 trial. Ann Rheum Dis. 2021;80:1014–1021. doi: 10.1136/annrheumdis-2020-219406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mease PJ, Helliwell PS, Hjuler KF, Raymond K, McInnes I. Brodalumab in psoriatic arthritis: results from the randomised phase III AMVISION-1 and AMVISION-2 trials. Ann Rheum Dis. 2021;80:185–193. doi: 10.1136/annrheumdis-2019-216835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gordon KB, Foley P, Krueger JG, Pinter A, Reich K, Vender R, Vanvoorden V, Madden C, White K, Cioffi C, et al. Bimekizumab efficacy and safety in moderate to severe plaque psoriasis (BE READY): a multicentre, double-blind, placebo-controlled, randomised withdrawal phase 3 trial. Lancet. 2021;397:475–486. doi: 10.1016/S0140-6736(21)00126-4. [DOI] [PubMed] [Google Scholar]
- 25.Warren RB, Blauvelt A, Bagel J, Papp KA, Yamauchi P, Armstrong A, Langley RG, Vanvoorden V, De Cuyper D, Cioffi C, et al. Bimekizumab versus adalimumab in plaque psoriasis. N Engl J Med. 2021;385:130–141. doi: 10.1056/NEJMoa2102388. [DOI] [PubMed] [Google Scholar]
- 26.Östör A, Van den Bosch F, Papp K, Asnal C, Blanco R, Aelion J, Alperovich G, Lu W, Wang Z, Soliman AM, et al. Efficacy and safety of risankizumab for active psoriatic arthritis: 24-week results from the randomised, double-blind, phase 3 KEEPsAKE 2 trial. Ann Rheum Dis. 2022;81:351–358. doi: 10.1136/annrheumdis-2021-221048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kristensen LE, Keiserman M, Papp K, McCasland L, White D, Lu W, Wang Z, Soliman AM, Eldred A, Barcomb L, et al. Efficacy and safety of risankizumab for active psoriatic arthritis: 24-week results from the randomised, double-blind, phase 3 KEEPsAKE 1 trial. Ann Rheum Dis. 2022;81:225–231. doi: 10.1136/annrheumdis-2021-221019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Werth VP, Joly P, Mimouni D, Maverakis E, Caux F, Lehane P, Gearhart L, Kapre A, Pordeli P, Chen DM PEMPHIX Study Group. Rituximab versus mycophenolate mofetil in patients with pemphigus vulgaris. N Engl J Med. 2021;384:2295–2305. doi: 10.1056/NEJMoa2028564. [DOI] [PubMed] [Google Scholar]
- 29.Bowman SJ, Fox R, Dörner T, Mariette X, Papas A, Grader-Beck T, Fisher BA, Barcelos F, De Vita S, Schulze-Koops H, et al. Safety and efficacy of subcutaneous ianalumab (VAY736) in patients with primary Sjögren’s syndrome: a randomised, double-blind, placebo-controlled, phase 2b dose-finding trial. Lancet. 2022;399:161–171. doi: 10.1016/S0140-6736(21)02251-0. [DOI] [PubMed] [Google Scholar]
- 30.Mease PJ, Gottlieb AB, van der Heijde D, FitzGerald O, Johnsen A, Nys M, Banerjee S, Gladman DD. Efficacy and safety of abatacept, a T-cell modulator, in a randomised, double-blind, placebo-controlled, phase III study in psoriatic arthritis. Ann Rheum Dis. 2017;76:1550–1558. doi: 10.1136/annrheumdis-2016-210724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ruperto N, Brunner HI, Synoverska O, Ting TV, Mendoza CA, Spindler A, Vyzhga Y, Marzan K, Grebenkina L, Tirosh I, et al. Tofacitinib in juvenile idiopathic arthritis: a double-blind, placebo-controlled, withdrawal phase 3 randomised trial. Lancet. 2021;398:1984–1996. doi: 10.1016/S0140-6736(21)01255-1. [DOI] [PubMed] [Google Scholar]
- 32.Deodhar A, Sliwinska-Stanczyk P, Xu H, Baraliakos X, Gensler LS, Fleishaker D, Wang L, Wu J, Menon S, Wang C, et al. Tofacitinib for the treatment of ankylosing spondylitis: a phase III, randomised, double-blind, placebo-controlled study. Ann Rheum Dis. 2021;80:1004–1013. doi: 10.1136/annrheumdis-2020-219601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Simpson EL, Forman S, Silverberg JI, Zirwas M, Maverakis E, Han G, Guttman-Yassky E, Marnell D, Bissonnette R, Waibel J, et al. Baricitinib in patients with moderate-to-severe atopic dermatitis: results from a randomized monotherapy phase 3 trial in the United States and Canada (BREEZE-AD5) J Am Acad Dermatol. 2021;85:62–70. doi: 10.1016/j.jaad.2021.02.028. [DOI] [PubMed] [Google Scholar]
- 34.Reich K, Kabashima K, Peris K, Silverberg JI, Eichenfield LF, Bieber T, Kaszuba A, Kolodsick J, Yang FE, Gamalo M, et al. Efficacy and safety of baricitinib combined with topical corticosteroids for treatment of moderate to severe atopic dermatitis: a randomized clinical trial. JAMA Dermatol. 2020;156:1333–1343. doi: 10.1001/jamadermatol.2020.3260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sandborn WJ, Ghosh S, Panes J, Schreiber S, D’Haens G, Tanida S, Siffledeen J, Enejosa J, Zhou W, Othman AA, et al. Efficacy of upadacitinib in a randomized trial of patients with active ulcerative colitis. Gastroenterology. 2020;158:2139–2149.e14. doi: 10.1053/j.gastro.2020.02.030. [DOI] [PubMed] [Google Scholar]
- 36.Guttman-Yassky E, Teixeira HD, Simpson EL, Papp KA, Pangan AL, Blauvelt A, Thaçi D, Chu CY, Hong HC, Katoh N, et al. Once-daily upadacitinib versus placebo in adolescents and adults with moderate-to-severe atopic dermatitis (Measure Up 1 and Measure Up 2): results from two replicate double-blind, randomised controlled phase 3 trials. Lancet. 2021;397:2151–2168. doi: 10.1016/S0140-6736(21)00588-2. [DOI] [PubMed] [Google Scholar]
- 37.Reich K, Teixeira HD, de Bruin-Weller M, Bieber T, Soong W, Kabashima K, Werfel T, Zeng J, Huang X, Hu X, et al. Safety and efficacy of upadacitinib in combination with topical corticosteroids in adolescents and adults with moderate-to-severe atopic dermatitis (AD Up): results from a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2021;397:2169–2181. doi: 10.1016/S0140-6736(21)00589-4. [DOI] [PubMed] [Google Scholar]
- 38.Feagan BG, Danese S, Loftus EV, Jr, Vermeire S, Schreiber S, Ritter T, Fogel R, Mehta R, Nijhawan S, Kempiński R, et al. Filgotinib as induction and maintenance therapy for ulcerative colitis (SELECTION): a phase 2b/3 double-blind, randomised, placebo-controlled trial. Lancet. 2021;397:2372–2384. doi: 10.1016/S0140-6736(21)00666-8. [DOI] [PubMed] [Google Scholar]
- 39.Kruglov AA, Lampropoulou V, Fillatreau S, Nedospasov SA. Pathogenic and protective functions of TNF in neuroinflammation are defined by its expression in T lymphocytes and myeloid cells. J Immunol. 2011;187:5660–5670. doi: 10.4049/jimmunol.1100663. [DOI] [PubMed] [Google Scholar]
- 40.Feldmann M. Translating molecular insights in autoimmunity into effective therapy. Annu Rev Immunol. 2009;27:1–27. doi: 10.1146/annurev-immunol-082708-100732. [DOI] [PubMed] [Google Scholar]
- 41.Keffer J, Probert L, Cazlaris H, Georgopoulos S, Kaslaris E, Kioussis D, Kollias G. Transgenic mice expressing human tumour necrosis factor: a predictive genetic model of arthritis. EMBO J. 1991;10:4025–4031. doi: 10.1002/j.1460-2075.1991.tb04978.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Atretkhany KN, Gogoleva VS, Drutskaya MS, Nedospasov SA. Distinct modes of TNF signaling through its two receptors in health and disease. J Leukoc Biol. 2020;107:893–905. doi: 10.1002/JLB.2MR0120-510R. [DOI] [PubMed] [Google Scholar]
- 43.Fischer R, Kontermann RE, Pfizenmaier K. Selective targeting of TNF receptors as a novel therapeutic approach. Front Cell Dev Biol. 2020;8:401. doi: 10.3389/fcell.2020.00401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Conrad C, Di Domizio J, Mylonas A, Belkhodja C, Demaria O, Navarini AA, Lapointe AK, French LE, Vernez M, Gilliet M. TNF blockade induces a dysregulated type I interferon response without autoimmunity in paradoxical psoriasis. Nat Commun. 2018;9:25. doi: 10.1038/s41467-017-02466-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Talotta R, Berzi A, Atzeni F, Batticciotto A, Clerici M, Sarzi-Puttini P, Trabattoni D. Paradoxical expansion of Th1 and Th17 lymphocytes in rheumatoid arthritis following infliximab treatment: a possible explanation for a lack of clinical response. J Clin Immunol. 2015;35:550–557. doi: 10.1007/s10875-015-0182-0. [DOI] [PubMed] [Google Scholar]
- 46.Salomon BL. Insights into the biology and therapeutic implications of TNF and regulatory T cells. Nat Rev Rheumatol. 2021;17:487–504. doi: 10.1038/s41584-021-00639-6. [DOI] [PubMed] [Google Scholar]
- 47.Dinarello CA. The IL-1 family of cytokines and receptors in rheumatic diseases. Nat Rev Rheumatol. 2019;15:612–632. doi: 10.1038/s41584-019-0277-8. [DOI] [PubMed] [Google Scholar]
- 48.von Moltke J, Ayres JS, Kofoed EM, Chavarría-Smith J, Vance RE. Recognition of bacteria by inflammasomes. Annu Rev Immunol. 2013;31:73–106. doi: 10.1146/annurev-immunol-032712-095944. [DOI] [PubMed] [Google Scholar]
- 49.Skendros P, Papagoras C, Mitroulis I, Ritis K. Autoinflammation: lessons from the study of familial Mediterranean fever. J Autoimmun. 2019;104:102305. doi: 10.1016/j.jaut.2019.102305. [DOI] [PubMed] [Google Scholar]
- 50.Mantovani A, Dinarello CA, Molgora M, Garlanda C. Interleukin-1 and related cytokines in the regulation of inflammation and immunity. Immunity. 2019;50:778–795. doi: 10.1016/j.immuni.2019.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zahid A, Li B, Kombe AJ, Jin T, Tao J. Pharmacological inhibitors of the NLRP3 inflammasome. Front Immunol. 2019;10:2538. doi: 10.3389/fimmu.2019.02538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Szekanecz Z, McInnes IB, Schett G, Szamosi S, Benkő S, Szűcs G. Autoinflammation and autoimmunity across rheumatic and musculoskeletal diseases. Nat Rev Rheumatol. 2021;17:585–595. doi: 10.1038/s41584-021-00652-9. [DOI] [PubMed] [Google Scholar]
- 53.Grom AA, Horne A, De Benedetti F. Macrophage activation syndrome in the era of biologic therapy. Nat Rev Rheumatol. 2016;12:259–268. doi: 10.1038/nrrheum.2015.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Choy EH, De Benedetti F, Takeuchi T, Hashizume M, John MR, Kishimoto T. Translating IL-6 biology into effective treatments. Nat Rev Rheumatol. 2020;16:335–345. doi: 10.1038/s41584-020-0419-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bataille R, Barlogie B, Lu ZY, Rossi JF, Lavabre-Bertrand T, Beck T, Wijdenes J, Brochier J, Klein B. Biologic effects of anti-interleukin-6 murine monoclonal antibody in advanced multiple myeloma. Blood. 1995;86:685–691. [PubMed] [Google Scholar]
- 56.Gabay C, Emery P, van Vollenhoven R, Dikranian A, Alten R, Pavelka K, Klearman M, Musselman D, Agarwal S, Green J, et al. Tocilizumab monotherapy versus adalimumab monotherapy for treatment of rheumatoid arthritis (ADACTA): a randomised, double-blind, controlled phase 4 trial. Lancet. 2013;381:1541–1550. doi: 10.1016/S0140-6736(13)60250-0. [DOI] [PubMed] [Google Scholar]
- 57.Khanna D, Lin CJ, Furst DE, Goldin J, Kim G, Kuwana M, Allanore Y, Matucci-Cerinic M, Distler O, Shima Y, et al. Tocilizumab in systemic sclerosis: a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Respir Med. 2020;8:963–974. doi: 10.1016/S2213-2600(20)30318-0. [DOI] [PubMed] [Google Scholar]
- 58.Wallace DJ, Strand V, Merrill JT, Popa S, Spindler AJ, Eimon A, Petri M, Smolen JS, Wajdula J, Christensen J, et al. Efficacy and safety of an interleukin 6 monoclonal antibody for the treatment of systemic lupus erythematosus: a phase II dose-ranging randomised controlled trial. Ann Rheum Dis. 2017;76:534–542. doi: 10.1136/annrheumdis-2016-209668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, Weiner HL, Kuchroo VK. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–238. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 60.Garbers C, Heink S, Korn T, Rose-John S. Interleukin-6: designing specific therapeutics for a complex cytokine. Nat Rev Drug Discov. 2018;17:395–412. doi: 10.1038/nrd.2018.45. [DOI] [PubMed] [Google Scholar]
- 61.Heinrich PC, Behrmann I, Müller-Newen G, Schaper F, Graeve L. Interleukin-6-type cytokine signalling through the gp130/Jak/STAT pathway. Biochem J. 1998;334:297–314. doi: 10.1042/bj3340297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Taniguchi K, Wu LW, Grivennikov SI, de Jong PR, Lian I, Yu FX, Wang K, Ho SB, Boland BS, Chang JT, et al. A gp130-Src-YAP module links inflammation to epithelial regeneration. Nature. 2015;519:57–62. doi: 10.1038/nature14228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yoshida K, Taga T, Saito M, Suematsu S, Kumanogoh A, Tanaka T, Fujiwara H, Hirata M, Yamagami T, Nakahata T, et al. Targeted disruption of gp130, a common signal transducer for the interleukin 6 family of cytokines, leads to myocardial and hematological disorders. Proc Natl Acad Sci U S A. 1996;93:407–411. doi: 10.1073/pnas.93.1.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.O’Shea JJ, Gadina M, Schreiber RD. Cytokine signaling in 2002: new surprises in the Jak/Stat pathway. Cell. 2002;109(Suppl):S121–S131. doi: 10.1016/s0092-8674(02)00701-8. [DOI] [PubMed] [Google Scholar]
- 65.Cua DJ, Tato CM. Innate IL-17-producing cells: the sentinels of the immune system. Nat Rev Immunol. 2010;10:479–489. doi: 10.1038/nri2800. [DOI] [PubMed] [Google Scholar]
- 66.McGeachy MJ, Cua DJ, Gaffen SL. The IL-17 family of cytokines in health and disease. Immunity. 2019;50:892–906. doi: 10.1016/j.immuni.2019.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Veldhoen M. Interleukin 17 is a chief orchestrator of immunity. Nat Immunol. 2017;18:612–621. doi: 10.1038/ni.3742. [DOI] [PubMed] [Google Scholar]
- 68.Akiyama S, Sakuraba A. Distinct roles of interleukin-17 and T helper 17 cells among autoimmune diseases. J Transl Autoimmun. 2021;4:100104. doi: 10.1016/j.jtauto.2021.100104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hirahara K, Poholek A, Vahedi G, Laurence A, Kanno Y, Milner JD, O’Shea JJ. Mechanisms underlying helper T-cell plasticity: implications for immune-mediated disease. J Allergy Clin Immunol. 2013;131:1276–1287. doi: 10.1016/j.jaci.2013.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Langrish CL, Chen Y, Blumenschein WM, Mattson J, Basham B, Sedgwick JD, McClanahan T, Kastelein RA, Cua DJ. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J Exp Med. 2005;201:233–240. doi: 10.1084/jem.20041257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Maitra A, Shen F, Hanel W, Mossman K, Tocker J, Swart D, Gaffen SL. Distinct functional motifs within the IL-17 receptor regulate signal transduction and target gene expression. Proc Natl Acad Sci U S A. 2007;104:7506–7511. doi: 10.1073/pnas.0611589104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Qian Y, Liu C, Hartupee J, Altuntas CZ, Gulen MF, Jane-Wit D, Xiao J, Lu Y, Giltiay N, Liu J, et al. The adaptor Act1 is required for interleukin 17-dependent signaling associated with autoimmune and inflammatory disease. Nat Immunol. 2007;8:247–256. doi: 10.1038/ni1439. [DOI] [PubMed] [Google Scholar]
- 73.Sieper J, Poddubnyy D, Miossec P. The IL-23-IL-17 pathway as a therapeutic target in axial spondyloarthritis. Nat Rev Rheumatol. 2019;15:747–757. doi: 10.1038/s41584-019-0294-7. [DOI] [PubMed] [Google Scholar]
- 74.Hawkes JE, Yan BY, Chan TC, Krueger JG. Discovery of the IL-23/IL-17 signaling pathway and the treatment of psoriasis. J Immunol. 2018;201:1605–1613. doi: 10.4049/jimmunol.1800013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Genovese MC, Greenwald M, Cho CS, Berman A, Jin L, Cameron GS, Benichou O, Xie L, Braun D, Berclaz PY, et al. A phase II randomized study of subcutaneous ixekizumab, an anti-interleukin-17 monoclonal antibody, in rheumatoid arthritis patients who were naive to biologic agents or had an inadequate response to tumor necrosis factor inhibitors. Arthritis Rheumatol. 2014;66:1693–1704. doi: 10.1002/art.38617. [DOI] [PubMed] [Google Scholar]
- 76.Oppmann B, Lesley R, Blom B, Timans JC, Xu Y, Hunte B, Vega F, Yu N, Wang J, Singh K, et al. Novel p19 protein engages IL-12p40 to form a cytokine, IL-23, with biological activities similar as well as distinct from IL-12. Immunity. 2000;13:715–725. doi: 10.1016/s1074-7613(00)00070-4. [DOI] [PubMed] [Google Scholar]
- 77.Gaffen SL, Jain R, Garg AV, Cua DJ. The IL-23-IL-17 immune axis: from mechanisms to therapeutic testing. Nat Rev Immunol. 2014;14:585–600. doi: 10.1038/nri3707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Floss DM, Schröder J, Franke M, Scheller J. Insights into IL-23 biology: from structure to function. Cytokine Growth Factor Rev. 2015;26:569–578. doi: 10.1016/j.cytogfr.2015.07.005. [DOI] [PubMed] [Google Scholar]
- 79.Puccetti P, Belladonna ML, Grohmann U. Effects of IL-12 and IL-23 on antigen-presenting cells at the interface between innate and adaptive immunity. Crit Rev Immunol. 2002;22:373–390. [PubMed] [Google Scholar]
- 80.Ruiz de Morales JM, Puig L, Daudén E, Cañete JD, Pablos JL, Martín AO, Juanatey CG, Adán A, Montalbán X, Borruel N, et al. Critical role of interleukin (IL)-17 in inflammatory and immune disorders: an updated review of the evidence focusing in controversies. Autoimmun Rev. 2020;19:102429. doi: 10.1016/j.autrev.2019.102429. [DOI] [PubMed] [Google Scholar]
- 81.Thorlacius GE, Wahren-Herlenius M, Rönnblom L. An update on the role of type I interferons in systemic lupus erythematosus and Sjögren’s syndrome. Curr Opin Rheumatol. 2018;30:471–481. doi: 10.1097/BOR.0000000000000524. [DOI] [PubMed] [Google Scholar]
- 82.Okanoue T, Sakamoto S, Itoh Y, Minami M, Yasui K, Sakamoto M, Nishioji K, Katagishi T, Nakagawa Y, Tada H, et al. Side effects of high-dose interferon therapy for chronic hepatitis C. J Hepatol. 1996;25:283–291. doi: 10.1016/s0168-8278(96)80113-9. [DOI] [PubMed] [Google Scholar]
- 83.Chasset F, Arnaud L. Targeting interferons and their pathways in systemic lupus erythematosus. Autoimmun Rev. 2018;17:44–52. doi: 10.1016/j.autrev.2017.11.009. [DOI] [PubMed] [Google Scholar]
- 84.Kalunian KC, Merrill JT, Maciuca R, McBride JM, Townsend MJ, Wei X, Davis JC, Jr, Kennedy WP. A phase II study of the efficacy and safety of rontalizumab (rhuMAb interferon-α) in patients with systemic lupus erythematosus (ROSE) Ann Rheum Dis. 2016;75:196–202. doi: 10.1136/annrheumdis-2014-206090. [DOI] [PubMed] [Google Scholar]
- 85.Khamashta M, Merrill JT, Werth VP, Furie R, Kalunian K, Illei GG, Drappa J, Wang L, Greth W CD1067 study investigators. Sifalimumab, an anti-interferon-α monoclonal antibody, in moderate to severe systemic lupus erythematosus: a randomised, double-blind, placebo-controlled study. Ann Rheum Dis. 2016;75:1909–1916. doi: 10.1136/annrheumdis-2015-208562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Morand EF, Furie R, Tanaka Y, Bruce IN, Askanase AD, Richez C, Bae SC, Brohawn PZ, Pineda L, Berglind A, et al. Trial of anifrolumab in active systemic lupus erythematosus. N Engl J Med. 2020;382:211–221. doi: 10.1056/NEJMoa1912196. [DOI] [PubMed] [Google Scholar]
- 87.Lee DS, Rojas OL, Gommerman JL. B cell depletion therapies in autoimmune disease: advances and mechanistic insights. Nat Rev Drug Discov. 2021;20:179–199. doi: 10.1038/s41573-020-00092-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Piccio L, Naismith RT, Trinkaus K, Klein RS, Parks BJ, Lyons JA, Cross AH. Changes in B- and T-lymphocyte and chemokine levels with rituximab treatment in multiple sclerosis. Arch Neurol. 2010;67:707–714. doi: 10.1001/archneurol.2010.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Edwards JC, Szczepanski L, Szechinski J, Filipowicz-Sosnowska A, Emery P, Close DR, Stevens RM, Shaw T. Efficacy of B-cell-targeted therapy with rituximab in patients with rheumatoid arthritis. N Engl J Med. 2004;350:2572–2581. doi: 10.1056/NEJMoa032534. [DOI] [PubMed] [Google Scholar]
- 90.Ivanov A, Beers SA, Walshe CA, Honeychurch J, Alduaij W, Cox KL, Potter KN, Murray S, Chan CH, Klymenko T, et al. Monoclonal antibodies directed to CD20 and HLA-DR can elicit homotypic adhesion followed by lysosome-mediated cell death in human lymphoma and leukemia cells. J Clin Invest. 2009;119:2143–2159. doi: 10.1172/JCI37884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Crickx E, Weill JC, Reynaud CA, Mahévas M. Anti-CD20-mediated B-cell depletion in autoimmune diseases: successes, failures and future perspectives. Kidney Int. 2020;97:885–893. doi: 10.1016/j.kint.2019.12.025. [DOI] [PubMed] [Google Scholar]
- 92.Cree BA, Bennett JL, Kim HJ, Weinshenker BG, Pittock SJ, Wingerchuk DM, Fujihara K, Paul F, Cutter GR, Marignier R, et al. Inebilizumab for the treatment of neuromyelitis optica spectrum disorder (N-MOmentum): a double-blind, randomised placebo-controlled phase 2/3 trial. Lancet. 2019;394:1352–1363. doi: 10.1016/S0140-6736(19)31817-3. [DOI] [PubMed] [Google Scholar]
- 93.Parodis I, Sjöwall C, Jönsen A, Ramsköld D, Zickert A, Frodlund M, Sohrabian A, Arnaud L, Rönnelid J, Malmström V, et al. Smoking and pre-existing organ damage reduce the efficacy of belimumab in systemic lupus erythematosus. Autoimmun Rev. 2017;16:343–351. doi: 10.1016/j.autrev.2017.02.005. [DOI] [PubMed] [Google Scholar]
- 94.Shi F, Xue R, Zhou X, Shen P, Wang S, Yang Y. Telitacicept as a BLyS/APRIL dual inhibitor for autoimmune disease. Immunopharmacol Immunotoxicol. 2021;43:666–673. doi: 10.1080/08923973.2021.1973493. [DOI] [PubMed] [Google Scholar]
- 95.Jeong S, Park SH. Co-stimulatory receptors in cancers and their implications for cancer immunotherapy. Immune Netw. 2020;20:e3. doi: 10.4110/in.2020.20.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Edner NM, Carlesso G, Rush JS, Walker LS. Targeting co-stimulatory molecules in autoimmune disease. Nat Rev Drug Discov. 2020;19:860–883. doi: 10.1038/s41573-020-0081-9. [DOI] [PubMed] [Google Scholar]
- 97.Esensten JH, Helou YA, Chopra G, Weiss A, Bluestone JA. CD28 costimulation: from mechanism to therapy. Immunity. 2016;44:973–988. doi: 10.1016/j.immuni.2016.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Rowshanravan B, Halliday N, Sansom DM. CTLA-4: a moving target in immunotherapy. Blood. 2018;131:58–67. doi: 10.1182/blood-2017-06-741033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Liu M, Yu Y, Hu S. A review on applications of abatacept in systemic rheumatic diseases. Int Immunopharmacol. 2021;96:107612. doi: 10.1016/j.intimp.2021.107612. [DOI] [PubMed] [Google Scholar]
- 100.Larsen CP, Pearson TC, Adams AB, Tso P, Shirasugi N, Strobert E, Anderson D, Cowan S, Price K, Naemura J, et al. Rational development of LEA29Y (belatacept), a high-affinity variant of CTLA4-Ig with potent immunosuppressive properties. Am J Transplant. 2005;5:443–453. doi: 10.1111/j.1600-6143.2005.00749.x. [DOI] [PubMed] [Google Scholar]
- 101.Douthwaite J, Moisan J, Privezentzev C, Soskic B, Sabbah S, Cohen S, Collinson A, England E, Huntington C, Kemp B, et al. A CD80-biased CTLA4-Ig fusion protein with superior in vivo efficacy by simultaneous engineering of affinity, selectivity, stability, and FcRn binding. J Immunol. 2017;198:528–537. doi: 10.4049/jimmunol.1600682. [DOI] [PubMed] [Google Scholar]
- 102.Huber M, Kemmner S, Renders L, Heemann U. Should belatacept be the centrepiece of renal transplantation? Nephrol Dial Transplant. 2016;31:1995–2002. doi: 10.1093/ndt/gfw226. [DOI] [PubMed] [Google Scholar]
- 103.Caux C, Massacrier C, Vanbervliet B, Dubois B, Van Kooten C, Durand I, Banchereau J. Activation of human dendritic cells through CD40 cross-linking. J Exp Med. 1994;180:1263–1272. doi: 10.1084/jem.180.4.1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.van Kooten C, Banchereau J. CD40-CD40 ligand. J Leukoc Biol. 2000;67:2–17. doi: 10.1002/jlb.67.1.2. [DOI] [PubMed] [Google Scholar]
- 105.Foy TM, Laman JD, Ledbetter JA, Aruffo A, Claassen E, Noelle RJ. gp39-CD40 interactions are essential for germinal center formation and the development of B cell memory. J Exp Med. 1994;180:157–163. doi: 10.1084/jem.180.1.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Fisher BA, Szanto A, Ng WF, Bombardieri M, Posch MG, Papas AS, Farag AM, Daikeler T, Bannert B, Kyburz D, et al. Assessment of the anti-CD40 antibody iscalimab in patients with primary Sjögren’s syndrome: a multicentre, randomised, double-blind, placebo-controlled, proof-of-concept study. Lancet Rheumatol. 2020;2:e142–e152. doi: 10.1016/S2665-9913(19)30135-3. [DOI] [PubMed] [Google Scholar]
- 107.Villarino AV, Kanno Y, O’Shea JJ. Mechanisms and consequences of Jak-STAT signaling in the immune system. Nat Immunol. 2017;18:374–384. doi: 10.1038/ni.3691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.McLornan DP, Pope JE, Gotlib J, Harrison CN. Current and future status of JAK inhibitors. Lancet. 2021;398:803–816. doi: 10.1016/S0140-6736(21)00438-4. [DOI] [PubMed] [Google Scholar]
- 109.Taylor PC, Keystone EC, van der Heijde D, Weinblatt ME, Del Carmen Morales L, Reyes Gonzaga J, Yakushin S, Ishii T, Emoto K, Beattie S, et al. Baricitinib versus placebo or adalimumab in rheumatoid arthritis. N Engl J Med. 2017;376:652–662. doi: 10.1056/NEJMoa1608345. [DOI] [PubMed] [Google Scholar]
- 110.Papp K, Gordon K, Thaçi D, Morita A, Gooderham M, Foley P, Girgis IG, Kundu S, Banerjee S. Phase 2 trial of selective tyrosine kinase 2 inhibition in psoriasis. N Engl J Med. 2018;379:1313–1321. doi: 10.1056/NEJMoa1806382. [DOI] [PubMed] [Google Scholar]
- 111.Montalban X, Arnold DL, Weber MS, Staikov I, Piasecka-Stryczynska K, Willmer J, Martin EC, Dangond F, Syed S, Wolinsky JS Evobrutinib Phase 2 Study Group. Placebo-controlled trial of an oral BTK inhibitor in multiple sclerosis. N Engl J Med. 2019;380:2406–2417. doi: 10.1056/NEJMoa1901981. [DOI] [PubMed] [Google Scholar]
- 112.Reich DS, Arnold DL, Vermersch P, Bar-Or A, Fox RJ, Matta A, Turner T, Wallström E, Zhang X, Mareš M, et al. Safety and efficacy of tolebrutinib, an oral brain-penetrant BTK inhibitor, in relapsing multiple sclerosis: a phase 2b, randomised, double-blind, placebo-controlled trial. Lancet Neurol. 2021;20:729–738. doi: 10.1016/S1474-4422(21)00237-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.FDA requires warnings about increased risk of serious heart-related events, cancer, blood clots, and death for JAK inhibitors that treat certain chronic inflammatory conditions [Internet] [accessed on 21 January 2022]. Available at https://www.fda.gov/drugs/drug-safety-and-availability/fda-requires-warnings-about-increased-risk-serious-heart-related-events-cancer-blood-clots-and-death.
- 114.June CH, O’Connor RS, Kawalekar OU, Ghassemi S, Milone MC. CAR T cell immunotherapy for human cancer. Science. 2018;359:1361–1365. doi: 10.1126/science.aar6711. [DOI] [PubMed] [Google Scholar]
- 115.Rafiq S, Hackett CS, Brentjens RJ. Engineering strategies to overcome the current roadblocks in CAR T cell therapy. Nat Rev Clin Oncol. 2020;17:147–167. doi: 10.1038/s41571-019-0297-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Mougiakakos D, Krönke G, Völkl S, Kretschmann S, Aigner M, Kharboutli S, Böltz S, Manger B, Mackensen A, Schett G. CD19-targeted CAR T cells in refractory systemic lupus erythematosus. N Engl J Med. 2021;385:567–569. doi: 10.1056/NEJMc2107725. [DOI] [PubMed] [Google Scholar]
- 117.Orvain C, Boulch M, Bousso P, Allanore Y, Avouac J. Is there a place for chimeric antigen receptor-T cells in the treatment of chronic autoimmune rheumatic diseases? Arthritis Rheumatol. 2021;73:1954–1965. doi: 10.1002/art.41812. [DOI] [PubMed] [Google Scholar]
- 118.Brudno JN, Kochenderfer JN. Recent advances in CAR T-cell toxicity: mechanisms, manifestations and management. Blood Rev. 2019;34:45–55. doi: 10.1016/j.blre.2018.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Spolski R, Li P, Leonard WJ. Biology and regulation of IL-2: from molecular mechanisms to human therapy. Nat Rev Immunol. 2018;18:648–659. doi: 10.1038/s41577-018-0046-y. [DOI] [PubMed] [Google Scholar]
- 120.Kolios AG, Tsokos GC, Klatzmann D. Interleukin-2 and regulatory T cells in rheumatic diseases. Nat Rev Rheumatol. 2021;17:749–766. doi: 10.1038/s41584-021-00707-x. [DOI] [PubMed] [Google Scholar]
- 121.Liao W, Lin JX, Leonard WJ. Interleukin-2 at the crossroads of effector responses, tolerance, and immunotherapy. Immunity. 2013;38:13–25. doi: 10.1016/j.immuni.2013.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Churlaud G, Jimenez V, Ruberte J, Amadoudji Zin M, Fourcade G, Gottrand G, Casana E, Lambrecht B, Bellier B, Piaggio E, et al. Sustained stimulation and expansion of Tregs by IL2 control autoimmunity without impairing immune responses to infection, vaccination and cancer. Clin Immunol. 2014;151:114–126. doi: 10.1016/j.clim.2014.02.003. [DOI] [PubMed] [Google Scholar]
- 123.Pinheiro-Rosa N, Torres L, de Almeida Oliveira M, Andrade-Oliveira MF, de Freitas Guimarães MA, Coelho MM, de Lima Alves J, Maioli TU, Faria AMC. Oral tolerance as antigen-specific immunotherapy. Immunother Adv. 2021;1:ltab017. doi: 10.1093/immadv/ltab017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Mowat AM. Anatomical basis of tolerance and immunity to intestinal antigens. Nat Rev Immunol. 2003;3:331–341. doi: 10.1038/nri1057. [DOI] [PubMed] [Google Scholar]
- 125.Faria AM, Weiner HL. Oral tolerance: therapeutic implications for autoimmune diseases. Clin Dev Immunol. 2006;13:143–157. doi: 10.1080/17402520600876804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Ring S, Maas M, Nettelbeck DM, Enk AH, Mahnke K. Targeting of autoantigens to DEC205+ dendritic cells in vivo suppresses experimental allergic encephalomyelitis in mice. J Immunol. 2013;191:2938–2947. doi: 10.4049/jimmunol.1202592. [DOI] [PubMed] [Google Scholar]
- 127.Getts DR, Martin AJ, McCarthy DP, Terry RL, Hunter ZN, Yap WT, Getts MT, Pleiss M, Luo X, King NJ, et al. Microparticles bearing encephalitogenic peptides induce T-cell tolerance and ameliorate experimental autoimmune encephalomyelitis. Nat Biotechnol. 2012;30:1217–1224. doi: 10.1038/nbt.2434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Moorman CD, Sohn SJ, Phee H. Emerging therapeutics for immune tolerance: tolerogenic vaccines, T cell therapy, and IL-2 therapy. Front Immunol. 2021;12:657768. doi: 10.3389/fimmu.2021.657768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Freedman MS, Bar-Or A, Oger J, Traboulsee A, Patry D, Young C, Olsson T, Li D, Hartung HP, Krantz M, et al. A phase III study evaluating the efficacy and safety of MBP8298 in secondary progressive MS. Neurology. 2011;77:1551–1560. doi: 10.1212/WNL.0b013e318233b240. [DOI] [PubMed] [Google Scholar]
- 130.Krienke C, Kolb L, Diken E, Streuber M, Kirchhoff S, Bukur T, Akilli-Öztürk Ö, Kranz LM, Berger H, Petschenka J, et al. A noninflammatory mRNA vaccine for treatment of experimental autoimmune encephalomyelitis. Science. 2021;371:145–153. doi: 10.1126/science.aay3638. [DOI] [PubMed] [Google Scholar]