Abstract
Rheumatoid arthritis (RA) is a representative autoimmune disease that is primarily characterized by persistent inflammation and progressive destruction of synovial joints. RA has a complex and heterogeneous pathophysiology, involving interactions among various immune and joint stromal cells and a diverse network of cytokines and intracellular signaling pathways. With improved understanding of RA, over the past decades, therapeutic strategies have become considerably advanced and now included targeted molecular therapies, such as tumor necrosis factor inhibitors, IL-6 blockers, B-cell depletion agents, as well as inhibitors of T-cell co-stimulation and Janus kinases. However, a considerable proportion of RA patients experience refractory disease and interrupted treatment owing to the associated risk of developing serious infections and cancers. In contrast, although IL-1β, IL-17A, and p38α play significant roles in RA pathogenesis, several drugs targeting these factors have not been approved because of their low efficacy and severe adverse effects. In this review, we provide an overview of the working mechanism, advantages, and limitations of the currently available targeted drugs for RA. Additionally, we suggest potential mechanistic causes for clinically approved and failed drugs. Thus, this review provides perspectives on approaches for basic and translational studies that hold promise for identifying future next-generation therapeutics for RA.
Keywords: Rheumatoid arthritis, Targeted molecular therapies, Cytokines, Janus kinase
INTRODUCTION
Rheumatoid arthritis (RA) is a common autoimmune disease that causes persistent inflammation, resulting in irreversible joint destruction, which ultimately leads to disability and mortality (1,2). Most evidence from immunological and bio-molecular studies points to an immune-mediated etiology associated with stromal tissue dysregulation, which together propagate chronic inflammation and joint damage in RA. Thus, RA is characterized by the complex process of disordered innate and adaptive immune responses, dysregulated cytokine and signal transduction networks, and disease-progressing semi-autonomous features of joint stromal synovial fibroblasts (3,4).
Over the past three decades, a comprehensive understanding of RA pathogenesis, by virtue of basic and translational research, and the consequent clinical availability of targeted agents, has led to a step-change in RA treatment (5). Currently, two types of clinically successful targeted drugs for RA exist: i) injectable biologic disease-modifying anti-rheumatic drugs (bDMARDs), including TNF-α inhibitors, IL-6 blockers, B-cell depletion agents, and inhibitors of T-cell co-stimulation; (2) oral targeted synthetic DMARDs (tsDMARDs), including small molecules inhibiting the JAK pathway. Current therapies have led to substantial progress toward achieving disease remission and preventing joint deformity in patients with RA. Thus, RA is a notable example of successful immunologic and clinical attempts, based on tissue culture cells, animal models, and human translational studies, which have led to enormous therapeutic advances.
Despite this, at best, these drugs induce significant clinical improvement in less than two-thirds of patients, most of whom will undergo a relapse of the disease after drug withdrawal (1,6). Furthermore, current RA drugs are not curative, nor do they reverse joint destruction. Additionally, current targeted therapies can result in a considerable burden of adverse events, including serious infection and malignancy, which is a consequence of drug cessation (7,8). In contrast, biologic agents blocking IL-1β, IL-17A, and small molecule inhibitors of p38α have exhibited disappointing therapeutic results and severe adverse effects in clinical trials, even though these targets have meaningful roles in RA pathogenesis. Collectively, these findings suggest that further basic and experimental research is required to explore additional therapeutic targets responsible for the complete resolution of inflammation while balancing efficacy and toxicity. An in-depth understanding and key lessons regarding successful and failed targeted agents may provide a novel effective direction for immunobiological research regarding feasible and desirable next-generation therapies for RA.
TNF-α
Identification of TNF-α
TNF-α is a potent chemoattractant and inflammatory cytokine that is primarily produced as a transmembrane protein that gets cleaved by a TNF-converting enzyme, leading to the release of soluble TNF-α (9). TNF-α is predominantly produced by monocyte-derived macrophages; however, it is also produced by other cell types, including endothelial cells, cardiac myocytes, adipose tissue, fibroblasts, and neurons (10,11). Recently, by combining single-cell sequencing and mass cytometry, B cells and T cells have also been identified as major sources of TNF-α in the synovium of patients with RA (12). TNF-α has two receptor isoforms, namely, TNF receptor 1 (TNFR1) and TNFR2. TNFR1 is expressed on most nucleated cells, whereas the expression of TNFR2 is limited to immune cells, such as T cells and myeloid cells, and may be associated with the regulatory function of TNF-α (10).
Role of TNF-α in RA
TNF-α is a key cytokine that has multipotent and central roles in RA pathogenesis by activating joint resident synovial fibroblasts to release various cytokines and chemokines, while also recruiting pro-inflammatory immune cells, including macrophages, monocytes, lymphocytes, and neutrophils (10,11). TNF-α also promotes osteoclast differentiation and activity, while inhibiting osteoblast differentiation and function (13). Furthermore, TNF-α suppresses Foxp3 production by Tregs and promotes the differentiation of Th1 and Th17 cells (14,15).
Indeed, several experimental animal studies have supported the crucial roles of TNF-α in RA. For instance, human TNF-overexpressing transgenic mice spontaneously develop inflammatory arthritis characterized by joint inflammation and bone erosion, similar to the pathologic findings of human RA (16). Moreover, in collagen-induced arthritis (CIA) mice, another representative experimental model of RA, blockade of TNF-α efficiently attenuates joint inflammation and consequent destruction (17).
Targeting of TNF-α in RA
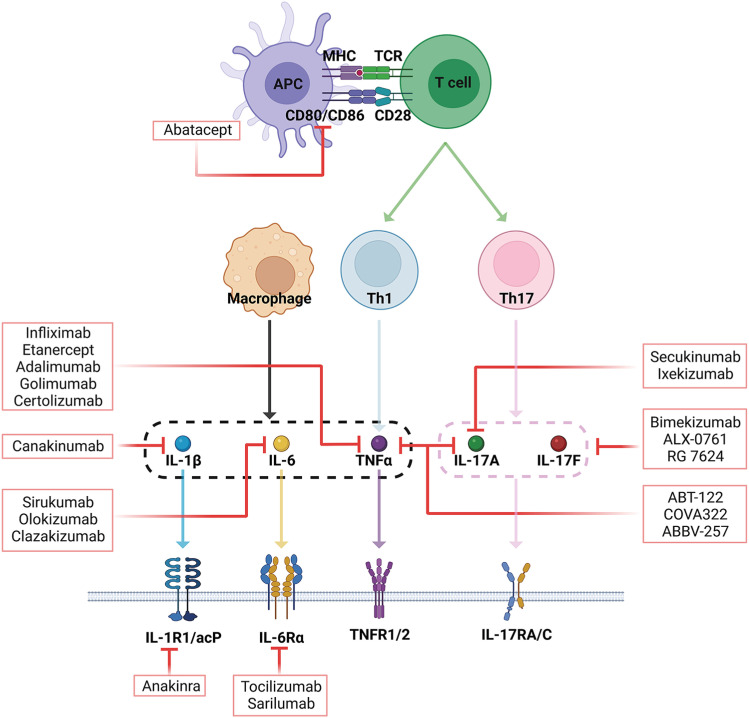
As TNF-α is a potent pro-inflammatory cytokine with a central role in RA pathophysiology, large-scale clinical trials have been conducted to develop several TNF-α inhibitors, including infliximab, which was first approved in 1999 (Fig. 1) (18). Infliximab is a chimeric monoclonal Ab comprising mouse heavy and light chain variable regions (Fab) combined with human heavy and light chain constant Fc regions (19). Infliximab binds to soluble and membrane-attached TNF-α, effectively inhibiting its binding to TNFR. Subsequently, it blocks Ab and complement-dependent responses in cells expressing TNFR (20).
Figure 1. Overview of inhibitors targeting cytokines and their specific receptors in RA treatment. Abatacept blocks CD80 and CD86 in the interface between APC and T cells. Inhibitors of TNF-α, such as infiliximab, etanercept, adalimumab, golimumab, and certolizumab, specifically bind TNF-α. Inhibitors of IL-6, such as tocilizumab and sarilumab, prevent IL-6 from binding to its receptor (IL-6Rα). Sirukumab, olokizumab, and clazakizumab are anti-IL-6 monoclonal Abs that specifically bind IL-6. Secukinumab and ixekizumab are anti-IL-17A monoclonal Abs. ABT-122, COVA-322, and ABBV-257 are dual targeting Abs that block both IL-17A and TNF-α. Bimekizumab, ALX-0761, and RG-7624 target the combination of IL-17A/IL-17F. Anankinra and canakinumab exhibit inhibitory effects by directly binding IL-β and IL-1β receptor 1 (IL-1βR1), respectively. The red lines indicate where the inhibitors block cytokines or their receptors.
Etanercept, another TNF-α inhibitor, contains a human soluble TNFR2 conjugated to the Fc portion of human IgG1. Etanercept binds to soluble TNF-α and TNF-β, not membrane-attached TNF-α, thus interfering with TNF-α binding to its natural receptors on pathologic cells.
Adalimumab is a fully humanized anti-TNF-α monoclonal Ab that binds to both soluble and transmembrane TNF-α. Similarly, golimumab is a fully-humanized IgG1 kappa monoclonal Ab that binds to the soluble and transmembrane bioactive forms of TNF-α (21). Still further, certolizumab is a humanized TNF-α monoclonal Ab Fab fragment linked to polyethylene glycol (22); compared with other TNF-α inhibitors, certolizumab elicits minimal adverse effects. Moreover, considering that it removes the Fc portion required for active transport across the placenta, it is theoretically safer for use during pregnancy (23).
Clinical success and limitations of targeting TNF-α in RA
As bDMARDs, the introduction of targeted therapy against TNF-α has markedly improved RA therapy and shifted the primary aim of treatment strategies from symptom control to achieving and maintaining RA remission. Although TNF-α inhibitors have achieved significant clinical success, several limitations have also arisen. First, the use of TNF-α inhibitors is associated with an increased risk of infection, especially tuberculosis (TB). TNF-α plays a vital role in the formation of granulomas, which are critical for protection of the host from TB. Thus, inhibition of TNF-α prevents granuloma formation and promotes the dissemination of TB infection (24). To overcome this issue, physicians can perform a latent TB detection test and administer prophylactic TB medication. Second, TNF-α inhibitor use is also associated with an increased potential risk of malignancy, such as lymphoma and non-melanoma skin cancer. Although a recent meta-analysis has shown that treatment with TNF-α inhibitors does not significantly increase the risk of developing malignancies, TNF-α exhibits selective cytotoxicity and can induce necrosis of tumor cells. Therefore, the risk of malignancy should be considered when prescribing prolonged use of TNF-α inhibitors (25,26). Third, a progressive decrease in TNF-α inhibitor efficiency has been reported. This is believed to be associated with development of anti-drug Abs (ADAs), the prevalence of which ranges from 5% to 20% in RA patients using TNF-α inhibitors (27). To circumvent this issue, TNF-α inhibitors are regularly administered in combination with methotrexate (MTX) to inhibit ADA production (28).
IL-6
Identification of IL-6
IL-6 was first discovered in 1986 as a secreted component that stimulates Ig production and is a multifunctional cytokine with a pivotal role in immunological control, hematopoiesis, and inflammation (29). Most immune cells and stromal cells produce IL-6, including macrophages, monocytes, T cells, B cells, osteoblasts, fibroblasts, and endothelial cells (30). The biological effects of IL-6 are mediated by a fully functional IL-6 receptor (IL-6R), which comprises two subunits: a type I cytokine receptor subunit, IL-6Rα (also called CD126), and a common signal-transducing-receptor subunit, gp130 (also called CD130) (31). Fully functional IL-6R (IL-6Rα/gp130) is primarily expressed in lymphocytes, myelocytes, megakaryocytes, and hepatocytes, whereas gp130 is expressed in a wide range of cells (31,32).
IL-6R exhibits three distinct signaling modes: classical signaling, trans-signaling, and trans-presentation signaling. First, classical signaling is mediated by the membrane-bound IL-6Rα (mIL-6Rα) subunit and gp130. IL-6 binds to mIL-6R, leading to the homo-dimerization of gp130 and activation of an intracellular signal transduction pathway (32). Second, trans-signaling is induced by the soluble form of IL-6Rα (sIL-6Rα). IL-6 binds sIL-6Rα to form the IL-6-s-IL6Rα complex, which binds to, and activates signal transduction in cells expressing only gp130, such as synovial fibroblasts and endothelial cells (33). Third, trans-presentation involves secreted IL-6 binding to mIL-6Rα expressed on dendritic cells (DCs); this complex then interacts with gp130 on CD4 T cells. This type of contact is necessary for Th17 cell priming (34).
Role of IL-6 in RA
IL-6 has multifunctional roles in RA pathogenesis. In an animal experiment, IL-6 deficiency protects against CIA development, whereas neutralizing IL-6 with an Ab improves the CIA clinical score (35). Moreover, IL-6 and sIL-6Rα are expressed in the serum and synovial fluid of RA patients, the levels of which correlate with disease activity and joint damage (36,37). Specifically, recent single-cell transcriptomics revealed that IL-6 is produced by T cells, B cells, and synovial fibroblasts within the RA synovium (12). IL-6 promotes Th17 differentiation, which is critical for inducing joint inflammation and destruction through the JAK/STAT pathway (38). Therefore, IL-6 production exacerbates an imbalance in Th17 cells and Tregs. Furthermore, IL-6 is essential for B-cell differentiation into memory B cells and plasma cells, via affinity maturation and class switching. These memory B cells and plasma cells then produce autoantibodies in the RA synovium (39). In addition, IL-6 upregulates osteoclastogenesis, which causes bone erosion in RA patients, and VEGF expression, which promotes angiogenesis, as well as the recruitment of inflammatory and immune cells into the joints (18,40).
Targeting of IL-6 in RA
IL-6 has been multi-directionally involved in the pathogenesis of RA and has become a target for RA therapeutics. Tocilizumab (TCZ) is a recombinant humanized anti-IL-6Rα monoclonal Ab approved for RA treatment (Fig. 1). TCZ inhibits the binding of IL-6 to mIL-6Rα and sIL-6Rα, thereby preventing the IL-6-mediated inflammatory signaling cascade (41). Thus, TCZ suppresses disease activity and progressive bone erosion in RA patients (42).
Sarilumab, another recombinant humanized anti-IL-6Rα monoclonal Ab, has also been approved by the Food and Drug Administration (FDA) for RA treatment. Sarilumab shows high efficacy in RA patients with moderate-to-severe activity who do not respond to conventional DMARDs (43). Meanwhile, several other biologics, including sirukumab, olokizumab, and clazakizumab, that target IL-6, not IL-6R, are currently undergoing clinical trials as RA therapeutics (Fig. 1) (44).
Clinical success and limitation of targeting IL-6 in RA
Although TCZ was developed after TNF inhibitors, which had already dominated the market, it has exhibited considerable success as a biologic. The tremendous success of IL-6 targeting agents for RA treatment may be due to several factors. First, the efficacy of TCZ supersedes that of adalimumab in RA patients (45). Second, TCZ monotherapy shows comparable clinical efficacy to the combined therapy of TCZ and MTX, whereas anti-TNF-α monotherapy demonstrates lower clinical efficacy than anti-TNF-α combined with MTX. This advantage of TCZ as a monotherapy is highly attractive as one-third of RA patients develop various adverse events to MTX, including nausea, vomiting, skin rash, or aggravation of hepatitis and complicated lung diseases. Third, TCZ has low immunogenicity and thus does not cause significant ADA production (46). The immunogenicity of ADAs can alter the drug’s pharmacokinetics, pharmacodynamics, or other biological activities, thereby impacting the safety and efficacy in RA patients receiving biologics treatment (46). Finally, TCZ inhibits pathological IL-6 signaling, which is associated with C-reactive protein (CRP) production. Thus, TCZ rapidly decreases CRP levels. High CRP levels in RA patients are associated with severe disease activity, whereas a rapid decrease in CRP eventually improves clinical outcomes (47).
Despite the clinical success of IL-6 targeting therapy, several considerations must be made. IL-6-mediated signaling is also required to mount protective immune responses; therefore, blockade of IL-6 increases the risk of infections (48). Moreover, TCZ administration is associated with a significantly increased incidence rate of lower gastrointestinal perforations (GIP), such as diverticulitis, compared to other conventional synthetic DMARDs and bDMARDs, such as TNF-α inhibitors abatacept, and rituximab (48). Therefore, TCZ treatment should be prescribed with caution to RA patients so as to minimize these risks.
CD80/CD86, Co-stimulatory molecule
Identification and role of CD80/CD86 and CTLA-4
Immune synapses that are formed between Ag-presenting cells (APCs) and T cells are required to initiate immune cascades. T cells require three signals for effector functions and differentiation, namely, primary, secondary, and cytokine signaling. The primary signal comprises Ag recognition by TCRs. APCs express MHC class II molecules on their surface, which present Ags to the TCR on T cells. Secondary co-stimulation-mediated signaling then occurs with CD80/CD86, which is upregulated on the surface of APCs and binds to CD28 on T cells. In fact, CD28 signaling acts synergistically with the TCR complex to activate the transcription factors NFAT, NF-κB, and AP-1. Activated T cells differentiate into various subsets, such as Th1, Th2, Th17, Tfh, and Treg, based on the cytokines in their surroundings (49). Meanwhile, CTLA-4 binds CD80/CD86 with a 100-fold higher affinity than CD28 and functions as an inhibitory signal. As such, CTLA4 is a powerful negative regulator of immune responses and is highly expressed on Tregs (50).
Targeting of CD80/CD86 in RA
As the APC-T-cell-B-cell immune cascade is implicated in the pathogenesis of RA, a new class of treatment was developed to inhibit co-stimulation by binding to CD80/CD86. Abatacept is a dimeric fusion protein made up of the CTLA-4 extracellular domain and an IgG Fc region (CTLA4-Ig) (51). As such, abatacept inhibits the co-stimulatory signal required for T-cell activation and the subsequent production of autoantibodies, which are direct drivers of disease activity (Fig. 1) (51). Furthermore, the Fc region of abatacept can bind CD16/CD32, known as FcγRIII and FcγRII, to reduce Fc-mediated processes, complement-dependent cytotoxicity, and Ab-dependent cellular cytotoxicity (50). Indeed, several clinical studies have reported that abatacept exhibits anti-inflammatory effects and radiographic improvement in the MTX-refractory population. Meanwhile, combined abatacept and DMARD therapy also has valuable effects on disease activity in patients with an inadequate response to TNF-α inhibitors (52,53,54).
Clinical success and limitation of targeting CD80/CD86 in RA
The clinical success of abatacept is likely due to two major factors. First, inhibition of the upstream immune synapse, including the APC-T-cell-B-cell axis, by abatacept can minimize compensatory pathway activity by inhibiting various downstream cytokines. In this way, abatacept also reduces autoantibody production (55). Second, abatacept administration is associated with a lower incidence of serious infections in patients with RA, compared to other biologics. The reason is hypothesized that the CD28 and CD80/CD86 interaction, a therapeutic target for abatacept, is higher in activated T cells than in naive T cells. Hence, with the ever increasing number of elderly patients being diagnosed with RA, abatacept is an appropriate treatment option as infection is an important consideration when selecting drugs for elderly patients (56).
Despite these advantages, select considerations must be made when administering abatacept. Some clinicians may be hesitant to choose abatacept for RA patients, as it is slower to reduce disease activity compared to that with other biologics (57). Moreover, abatacept can cross the placenta and therefore should be discontinued at least three months before preparing for pregnancy (58).
IL-17A
Identification of IL-17A
IL-17A was the first member of the IL-17 cytokine family to be identified and defined. This family now includes IL-17A, IL-17B, IL-17C, IL-17D, IL-17E, and IL-17F (59). IL-17A binds to IL-17RA and induces recruitment of IL-17RC to form a heterodimeric receptor complex (60). IL-17A plays a pivotal role in host defense against extracellular bacterial and fungal infections through neutrophil migration at the infection site (61).
Role of IL-17A in RA
The importance of IL-17A in the pathogenesis of RA was determined by identifying the increased production of IL-17A in the synovial tissue of RA patients (62,63). IL-17A exhibits diverse functions in RA, including NF-κB activation and IL-6 production in synovial fibroblasts, augmentation of osteoclastogenesis, as well as enhanced recruitment and activation of neutrophils, macrophages, and B cells (64,65). In the CIA model, administration of IL-17A in joints promotes arthritis with symptoms similar to those observed in RA patients (66). In contrast, inflammatory arthritis is significantly suppressed in IL-17- or IL-17R-deficient mice. Similarly, administration of IL-17A neutralizing Ab reduces the development and progression of arthritis (67,68). Furthermore, the discovery of Th17 cells, an IL-17A-producing specific CD4 T-cell subset, reinforced the pathogenic roles of IL-17 in RA (69).
Limited clinical success of targeting IL-17 in RA
In a phase I clinical trial, secukinumab and ixekizumab, which are fully human monoclonal IL-17A Abs, showed positive clinical responses (Fig. 1) (70,71). However, secukinumab has not successfully improved clinical outcomes in two phase II trials conducted in RA patients (72,73). In fact, secukinumab exhibited lower efficacy than abatacept in a phase III clinical trial (74,75). Thus, despite the promising data from experimental models and phase II clinical trials, the clinical efficacy of IL-17A inhibitors in RA has proven insufficient.
Several potential mechanistic causes may account for the limited clinical success exhibited by targeting IL-17 in RA. First, IL-17A inhibition alone is insufficient to disrupt the inflammatory cascade. IL-17A functions synergistically with other inflammatory cytokines, including TNF-α, IL-1β, and IL-6; thus, neutralization of only IL-17A does not inhibit the pro-inflammatory effects of TNF-α, IL-1β, IL-6 or initiation of chronic inflammatory cascades (76). Nevertheless, IL-17A and IL-17F can synergize with TNF-α; thus, administration of dual neutralizing Abs, such as ABT-122, COVA-322, and ABBV-257 (target the combination of IL-17A and TNF-α) or bimekizumab, ALX-0761, and RG-7624 (target IL-17A/IL-17F), may prove more effective than administration of IL-17A mono-blocking Abs (Fig. 1). Second, IL-17A may have meaningful roles only in the early phase of RA, not in the established phase. We previously observed an increased abundance of Th17 cells during early, or before, disease onset compared to the disease progression phase. Moreover, IL-17A positive Th17 cells convert the IFN-γ positive CD4 T cells in the CIA mice (unpublished data). Accordingly, it may be interesting that IL-17A blockade may serve as an effective preventive strategy for pre-RA patients, before arthritis onset. Third, in RA patients, IL-17A expression is heterogeneous, with not all patients having high IL-17A levels or Th17 cell populations (77). Fourth, IL-17A inhibitors do not only block pathologic Th17 but also regulatory Th17. In patients with RA, IL-10-producing regulatory Th17 cells express IL-17 receptors; thus, IL-17A inhibitors may also affect IL-10-producing non-pathological Th17 cells (78).
IL-1β
Identification of IL-1β
The IL-1 family comprises 11 members, including IL-1α, IL-1β, IL-18, IL-33, IL-37, IL-38, IL-36α, IL-36β, IL-36γ, IL-1 receptor antagonist (IL-1Ra), and IL-36 receptor antagonist (IL-36Ra). Among them, IL-1β is the most frequently studied cytokine in several inflammatory diseases, including RA, gout, systemic-onset juvenile idiopathic arthritis, adult-onset Still’s disease, and osteoarthritis (79). IL-1β exhibits pro-inflammatory activities by binding to IL-1R1, forming a heterotrimeric complex with co-receptor IL-1R3 and triggering recruitment of MYD88 and the subsequent kinase cascade (80).
Role of IL-1β in RA
Several animal studies have found that intra-articular injection, or increased expression of IL-1β, mediates infiltration of leukocytes and subsequent degradation of cartilage, resulting in a severe and aggressive arthritis phenotype, including human RA (81,82,83). Similarly, the presence of IL-1β, and its relevance in arthritis progression, has also been clearly demonstrated in RA patients (12,84). Based on this cumulative evidence, the primary role of IL-1 in RA pathogenesis is believed to involve innate, rather than acquired, immune responses (79). That is, the IL-1β produced by macrophages and monocytes promotes joint destruction by induction of osteoclastogenesis and cartilage degradation (80,85).
Limited clinical success of targeting IL-1β in RA
Anakinra (recombinant IL-1 receptor antagonist) and canakinumab (anti-IL-1β Ab) have been evaluated for their therapeutic efficacy in RA (Fig. 1) and have demonstrated responses that are less potent than those of anti-TNF agents (86,87). In fact, combined therapy of anakinra and methotrexate or etanercept also failed to offer any clinical advantages, yet increased the risk of associated infection (88). Moreover, administration of anakinra also reportedly causes erythema, itchiness, and discomfort at the injection site in some patients. Indeed, adverse injection site reactions were the most notable event resulting in the premature withdrawal of subjects from various clinical trials (89,90). These limited clinical responses and associated adverse events have prompted doubts regarding whether targeting IL-1β in RA is an effective strategy. Specifically, the short half-life (4–6 hours) and inconvenience of daily administration make anakinra a less favored choice for patients and clinicians in RA treatment (91).
p38
Identification of p38
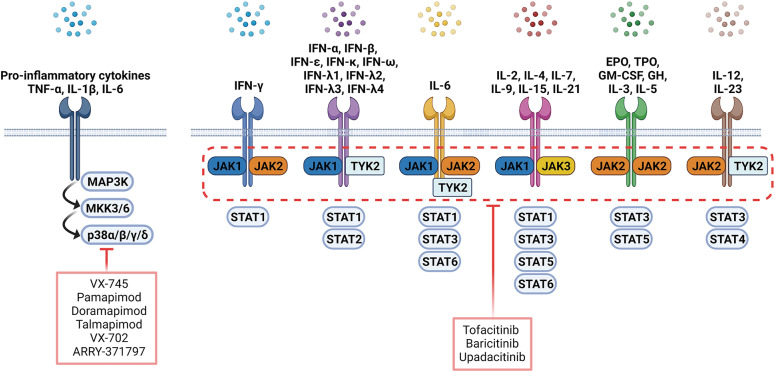
MAPKs consist of 3 major families: ERK, JNK, and p38. ERK and JNK are important for cellular proliferation and differentiation and extracellular matrix regulation (92,93). p38 exists in four isoforms (α, β, γ, and δ) that are widely expressed in not only various immune cells but also stromal cells (94). p38 is activated by various pro-inflammatory cytokines, including IL-1β, IL-6, and TNF-α (95) and is mediated by two upstream kinases, MAPK kinase 3 (MKK3) and MAPK kinase 6 (MKK6). In turn, phosphorylated p38 triggers signaling cascades associated with the expression of various pro-inflammatory molecules (Fig. 2) (96). As such, p38 MAPK has attracted attention as a potential target for autoimmune diseases.
Figure 2. Overview of small molecule inhibitors targeting the p38 MAPK pathway and JAK/STAT pathway in RA treatment. Each cytokine receptor recruits and activates a specific combination in MAPK and JAK/STAT cascades. Tofacitinib is a pan-JAK inhibitor, selective for JAK3 and JAK1 with minor activity for JAK2 and TYK2. Baricitinib is selective for JAK1 and JAK2 and less selective for JAK3 and TYK2.
Role of p38 in RA
The roles of p38 were first recognized in the investigation of LPS-stimulated monocytes, in which p38 was found to be associated with the synthesis of pro-inflammatory cytokines (97). Among the four p38 isoforms, α is thought to be the most important in mediating inflammation, with several studies supporting a pathogenic function of p38α in arthritis. Early in vitro studies demonstrated that the production of IL-1, IL-6, and TNF, key mediators of RA, is regulated by p38α (97,98,99). Meanwhile, deficiency of major upstream kinases of p38α, MKK3 or MKK6, ameliorates arthritis severity and cartilage destruction in K/BxN serum-transferred mice (100,101). Moreover, in CIA mice, deficiency of MAPKAP kinase 2 (MK2), the p38α substrate, also contributes to reduced production of IL-1 and TNF as well as arthritis severity (102). p38α is also, reportedly, associated with collagen- or TNF-α-driven arthritis (103). Meanwhile in RA patients, p38α and γ predominate the synovium in the synovial tissue, in particular within the lining layer and vessels (94). Taken together, these evidences suggest a critical role for p38α and its signaling cascades in RA pathogenesis.
Limited clinical success of targeting of p38 MAPK in RA
After demonstrating the therapeutic effect of p38 inhibitors in animal models of RA (104,105), numerous p38 inhibitors, including VX-745 (106), pamapimod (107,108), doramapimod (BRIB 796) (109), talmapimod (SCIO-469) (110), VX-702 (111), and ARRY-371797 (NCT00729209), were evaluated in clinical trials for RA treatment (Fig. 2). However, no significant improvement was reported disease severity or outcome. These disappointing clinical results have raised questions regarding the potential associated causes of the failure.
First, one of the major limitations of p38 inhibitors is their low efficacy and poor safety profile. Together with their limited efficacy in RA treatment, p38 inhibitors are associated with high rates of hepatotoxicity—talmapimod (110), pamapimod (107), and doramapimod (112), which is the primary cause of patient withdrawal from the clinical trials. Meanwhile, infection, skin disorders, and dizziness are also commonly reported adverse effects of p38 MAPK inhibitors (107,110).
Second, it is unclear whether the α isoform of p38 is the most effective target for RA treatment. Although p38α is the best characterized isoform, and its pathological role has been well demonstrated in vitro (97,98,99) and in vivo (100,101,102,103), recent data suggest an anti-inflammatory effect of p38α. For instance, IL-10 production in macrophages requires p38α regulation (113). Indeed, evidence also exists regarding the importance of other p38 isoforms, namely, β and γ. In particular, it has been suggested that p38β regulates the synthesis of endothelial chemokines and exhibits pro-inflammatory effects (114). Meanwhile, phosphorylated p38γ is also highly expressed in the RA synovium, suggesting a correlation between this isoform and RA pathogenesis (94).
Third, the compensatory effect on other kinases caused by blocking downstream p38 must also be considered (115). MKK3, MKK6, and TAK1, which regulate p38 upstream, can be activated by p38 inhibition, leading to the regulation of NF-κB or redirecting signaling cascades (100,116). This suggests that targeting these upstream kinases of p38 may be an attractive alternative for RA treatment.
JAK/STAT PATHWAY
Identification of JAK/STAT
The JAK/STAT pathway comprises intracellular tyrosine kinases (TYKs) that have a pivotal role in the signaling pathways associated with immune responses. When cytokines, growth factors, chemokines, and colony-stimulating factors bind to their cognate receptors, receptors dimerize (Fig. 2) causing cross-phosphorylation by JAK (117). Phosphorylation of receptor-associated tyrosine residues offers docking sites for STAT proteins. Phosphorylated STAT forms a dimer, translocate to the nucleus, and regulates gene expression (117). Humans have 4 different JAKs: JAK1, JAK2, JAK3, TYK2, and 7 STATs, including STAT1, STAT2, STAT3, STAT4, STAT5A, STAT5B, and STAT6 (118).
Role and targeting of JAK/STAT in RA
Cytokines, such as IFN-γ, IL-6, IL-12, IL-17A, and GM-CSF play a crucial role in RA. These cytokines regulate gene expression via the JAK/STAT pathway in immune cells as well as synovial fibroblasts (117). Hence, the JAK/STAT pathway is strongly involved in RA pathophysiology and is a target of RA treatment. The essential cytokines impacted by the JAK/STAT pathway include the interferon family, IL-2, IL-4, IL-6, IL-7, IL 10, IL-12, IL-23, GM-CSF, G-CSF, erythropoietin (EPO), thrombopoietin (TPO), leptin, and growth hormone (Fig. 2). JAKs and STATs combine to generate complex multimers that affect the immune system and hematopoiesis in a variety of biochemical and physiological ways (119). In fact, JAK deficiencies, or genetic gain of functions of other JAKs, can cause several diseases. Specifically, genetic mutations in JAK1 can lead to lymphoid malignancy, while genetic mutations in JAK2 can lead to myeloproliferative disease (120). JAK3 congenital deficiency is strongly associated with severe combined immunodeficiency (SCID), whereas STAT3 congenital deficiency is linked with hyper-IgE syndrome (120).
JAK inhibitors are small molecules and the newest class of tsDMARDs for RA treatment. Tofacitinib was the first targeted synthetic, reversible, and non-selective JAK inhibitor that was FDA approved for the treatment of RA. The structure of tofacitinib and most JAK inhibitors, mimics ATP and competitively binds to the ATP-binding site in the TYK domain (119). Tofacitinib is a pan-JAK inhibitor that has a high degree of selectivity for JAK3 and JAK1; however, it exhibits minimal activity for JAK2 and TYK2 (119). The phase III trial of tofacitinib demonstrated a significantly higher clinical response and limited joint damage progression in patients administered tofacitinib compared to MTX monotherapy (121). Meanwhile, administration of tofacitinib with MTX exhibited similar efficacy to adalimumab with MTX (122).
Baricitinib is more selective for JAK2 and JAK1 compared to JAK3 and TYK2. Baricitinib also successfully passed four phase III clinical trials during the approval process. In the RA-BEGIN trial, baricitinib monotherapy exhibited superior efficacy compared to MTX monotherapy (123). Meanwhile, in the RA-BEAM trial, baricitinib demonstrated superior clinical efficiency and reduced radiographic progression compared to adalimumab (124).
Upadacitinib is a second-generation JAK inhibitor with increased selectivity for JAK1 over JAK2, JAK3, and TYK2 (125). In all five pivotal clinical trials, upadacitinib showed significantly higher rates of remission and low disease activity compared to the placebo, methotrexate, or adalimumab (126).
Clinical success of targeting JAK/STAT in RA
JAK inhibitors are a rapidly-growing therapy option for RA therapy. There are several potential reasons for this success. First, they provide a targeted oral medication with efficacy comparable to that of TNF inhibitors (122,124). In particular, their oral formulations make them a convenient and appealing treatment option for patients with RA. Second, JAK inhibitors block upstream signal transduction, unlike the p38 MAPK inhibitor, which is a small molecule with high associated clinical failure (119). Moreover, it is stable against the activation of compensatory pathways, which can occur when downstream signaling is blocked. Third, JAK inhibitors modulate dysregulated metabolism by inducing oxidative phosphorylation while reducing glycolysis, thus offering opportunities for restoring synovial homeostasis by direct targeting of aggressive RA synovial fibroblasts (127). Finally, the essential cytokines for RA pathophysiology operate through various JAK/STAT pathways; thus, JAK inhibitors simultaneously interfere with signaling pathways across multiple cytokine axes.
However, several adverse events can occur by inhibition of multiple JAK/STAT pathways. The representative risk is hematopoietic toxicity, which is presumed to be caused by JAK2 inhibition.
CONCLUSION
In this review, we identified that selection of the proper therapeutic target is crucial for the clinical success of targeted drugs in RA, such as cytokines with multipotent and pleiotropic activity and kinases in upstream immune synapse or upstream signal transduction pathways (Table 1). Moreover, the success of targeted drugs may be the result of several potential factors, including convenience of treatment, balancing efficacy and safety, and cost. Additionally, as we have suggested regarding JAK inhibitors, direct suppression of aggressive synovial fibroblasts may also contribute to the success of RA drugs. In particular, the immunopathologic contribution of tumor-like synovial fibroblasts to arthritis relapse following discontinuation of drugs appears highly significant in RA; the stromal origin of these cells may render them resistant to current immune-targeted therapies. Thus, we predict that combining an immune-targeted agent with a synovial fibroblast-directed therapy might increase the rate of RA remission without increasing the risk of infection and cancer development. Collectively, the integration of proper targets based on lessons learned from clinically approved or failed drugs, as well as tandem targeting of pathologically relevant immune and stromal cell subsets, is critical to designing effective RA therapies. This approach may facilitate new research directions to achieve curative measures without additional side effects and recurrences for RA patients.
Table 1. Overview of the potential reasons for clinical success or clinical failure of targeted RA therapies.
| Clinical approved | Target | Potential reasons and lessens |
|---|---|---|
| Success | TNF-α | ✓ First approved bDMARD for RA |
| ✓ Multipotent and central role of TNF-α in RA pathophysiology | ||
| IL-6 | ✓ Pleiotropic activity of IL-6 in RA pathogenesis | |
| ✓ Low incidence of anti-drug Ab | ||
| ✓ Rapid reduction of inflammation | ||
| CD80/CD86 | ✓ Blockade of upstream immune-synapse in the inflammatory cascade | |
| ✓ Relatively less susceptible to infection by mainly suppressing activated T cells rather than naive T cells | ||
| JAK/STAT | ✓ Blockade of upstream signal transduction | |
| ✓ Blockade of signaling pathways across multiple cytokine axes simultaneously | ||
| ✓ Oral formulation; convenient to take medicines | ||
| Failure | IL-1β | ✓ Short half-life and need for daily injection |
| ✓ Higher risk of infection compared to efficacy | ||
| IL-17A | ✓ Disease heterogeneity of RA with variable expression of IL-17A | |
| ✓ Meaningful roles of IL-17A only in the early phase of RA, but not in the established phase | ||
| ✓ Blocks not only pathologic Th17 but also regulatory Th17 | ||
| MAPK p38α | ✓ Compensatory activation of upstream kinase pathways upon p38α | |
| ✓ Blocks not only pro-inflammatory p38α but also anti-inflammatory p38α | ||
| ✓ Pro-inflammatory roles of additional isoforms of p38 |
ACKNOWLEDGEMENTS
This study was supported by a grant from National Research Foundation of Korea (NRF-2020R1C1C1007944) and from the Korea Health Technology R&D project through the Korea Health Industry Development Institute (KHIDI) funded by the Korean Ministry of Health and Welfare (grant number: HI14C1277). We would like to thank Editage (www.editage.co.kr) for English language editing.
Abbreviations
- ADA
anti-drug Ab
- APC
Ag-presenting cell
- bDMARD
biologic disease-modifying anti-rheumatic drug
- CIA
collagen-induced arthritis
- CRP
C-reactive protein
- DC
dendritic cell
- EPO
erythropoietin
- FDA
Food and Drug Administration
- GIP
gastrointestinal perforations
- IL-1Ra
IL-1 receptor antagonist
- IL-36Ra
IL-36 receptor antagonist
- IL-6R
IL-6 receptor
- mIL-6Rα
membrane-bound IL-6Rα
- MK2
MAPKAP kinase 2
- MKK3
MAPK kinase 3
- MKK6
MAPK kinase 6
- MTX
methotrexate
- RA
rheumatoid arthritis
- SCID
severe combined immunodeficiency
- sIL-6Rα
soluble form of IL-6Rα
- TB
tuberculosis
- TCZ
tocilizumab
- TNFR1
TNF receptor 1
- TPO
thrombopoietin
- tsDMARD
targeted synthetic disease-modifying anti-rheumatic drug
- TYK
tyrosine kinase
Footnotes
Conflict of Interest: The authors declare no potential conflicts of interest.
- Conceptualization: Kim M, Choe YH, Lee SI.
- Funding acquisition: Kim M, Lee SI.
- Writing - original draft: Kim M, Choe YH, Lee SI.
References
- 1.McInnes IB, Schett G. The pathogenesis of rheumatoid arthritis. N Engl J Med. 2011;365:2205–2219. doi: 10.1056/NEJMra1004965. [DOI] [PubMed] [Google Scholar]
- 2.Firestein GS. Evolving concepts of rheumatoid arthritis. Nature. 2003;423:356–361. doi: 10.1038/nature01661. [DOI] [PubMed] [Google Scholar]
- 3.Firestein GS, McInnes IB. Immunopathogenesis of rheumatoid arthritis. Immunity. 2017;46:183–196. doi: 10.1016/j.immuni.2017.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nygaard G, Firestein GS. Restoring synovial homeostasis in rheumatoid arthritis by targeting fibroblast-like synoviocytes. Nat Rev Rheumatol. 2020;16:316–333. doi: 10.1038/s41584-020-0413-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Smolen JS, Aletaha D. Rheumatoid arthritis therapy reappraisal: strategies, opportunities and challenges. Nat Rev Rheumatol. 2015;11:276–289. doi: 10.1038/nrrheum.2015.8. [DOI] [PubMed] [Google Scholar]
- 6.de Hair MJ, Jacobs JW, Schoneveld JL, van Laar JM. Difficult-to-treat rheumatoid arthritis: an area of unmet clinical need. Rheumatology (Oxford) 2018;57:1135–1144. doi: 10.1093/rheumatology/kex349. [DOI] [PubMed] [Google Scholar]
- 7.Singh JA, Cameron C, Noorbaloochi S, Cullis T, Tucker M, Christensen R, Ghogomu ET, Coyle D, Clifford T, Tugwell P, et al. Risk of serious infection in biological treatment of patients with rheumatoid arthritis: a systematic review and meta-analysis. Lancet. 2015;386:258–265. doi: 10.1016/S0140-6736(14)61704-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pundole X, Suarez-Almazor ME. Cancer and rheumatoid rrthritis. Rheum Dis Clin North Am. 2020;46:445–462. doi: 10.1016/j.rdc.2020.05.003. [DOI] [PubMed] [Google Scholar]
- 9.Robertshaw HJ, Brennan FM. Release of tumour necrosis factor alpha (TNFalpha) by TNFalpha cleaving enzyme (TACE) in response to septic stimuli in vitro . Br J Anaesth. 2005;94:222–228. doi: 10.1093/bja/aei021. [DOI] [PubMed] [Google Scholar]
- 10.Kalliolias GD, Ivashkiv LB. TNF biology, pathogenic mechanisms and emerging therapeutic strategies. Nat Rev Rheumatol. 2016;12:49–62. doi: 10.1038/nrrheum.2015.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Finsterbusch M, Voisin MB, Beyrau M, Williams TJ, Nourshargh S. Neutrophils recruited by chemoattractants in vivo induce microvascular plasma protein leakage through secretion of TNF. J Exp Med. 2014;211:1307–1314. doi: 10.1084/jem.20132413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang F, Wei K, Slowikowski K, Fonseka CY, Rao DA, Kelly S, Goodman SM, Tabechian D, Hughes LB, Salomon-Escoto K, et al. Defining inflammatory cell states in rheumatoid arthritis joint synovial tissues by integrating single-cell transcriptomics and mass cytometry. Nat Immunol. 2019;20:928–942. doi: 10.1038/s41590-019-0378-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jung YK, Kang YM, Han S. Osteoclasts in the inflammatory arthritis: Implications for pathologic osteolysis. Immune Netw. 2019;19:e2. doi: 10.4110/in.2019.19.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Farrugia M, Baron B. The role of TNF-α in rheumatoid arthritis: a focus on regulatory T cells. J Clin Transl Res. 2016;2:84–90. [PMC free article] [PubMed] [Google Scholar]
- 15.McInnes IB, Buckley CD, Isaacs JD. Cytokines in rheumatoid arthritis - shaping the immunological landscape. Nat Rev Rheumatol. 2016;12:63–68. doi: 10.1038/nrrheum.2015.171. [DOI] [PubMed] [Google Scholar]
- 16.Asquith DL, Miller AM, McInnes IB, Liew FY. Animal models of rheumatoid arthritis. Eur J Immunol. 2009;39:2040–2044. doi: 10.1002/eji.200939578. [DOI] [PubMed] [Google Scholar]
- 17.Williams RO, Feldmann M, Maini RN. Anti-tumor necrosis factor ameliorates joint disease in murine collagen-induced arthritis. Proc Natl Acad Sci U S A. 1992;89:9784–9788. doi: 10.1073/pnas.89.20.9784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guo Q, Wang Y, Xu D, Nossent J, Pavlos NJ, Xu J. Rheumatoid arthritis: pathological mechanisms and modern pharmacologic therapies. Bone Res. 2018;6:15. doi: 10.1038/s41413-018-0016-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Akiho H, Yokoyama A, Abe S, Nakazono Y, Murakami M, Otsuka Y, Fukawa K, Esaki M, Niina Y, Ogino H. Promising biological therapies for ulcerative colitis: a review of the literature. World J Gastrointest Pathophysiol. 2015;6:219–227. doi: 10.4291/wjgp.v6.i4.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Elliott MJ, Maini RN, Feldmann M, Long-Fox A, Charles P, Katsikis P, Brennan FM, Walker J, Bijl H, Ghrayeb J, et al. Treatment of rheumatoid arthritis with chimeric monoclonal antibodies to tumor necrosis factor alpha. Arthritis Rheum. 1993;36:1681–1690. doi: 10.1002/art.1780361206. [DOI] [PubMed] [Google Scholar]
- 21.Braun J, Kay J. The safety of emerging biosimilar drugs for the treatment of rheumatoid arthritis. Expert Opin Drug Saf. 2017;16:289–302. doi: 10.1080/14740338.2017.1273899. [DOI] [PubMed] [Google Scholar]
- 22.Choy E, McKenna F, Vencovsky J, Valente R, Goel N, Vanlunen B, Davies O, Stahl HD, Alten R. Certolizumab pegol plus MTX administered every 4 weeks is effective in patients with RA who are partial responders to MTX. Rheumatology (Oxford) 2012;51:1226–1234. doi: 10.1093/rheumatology/ker519. [DOI] [PubMed] [Google Scholar]
- 23.Goel N, Stephens S. Certolizumab pegol. MAbs. 2010;2:137–147. doi: 10.4161/mabs.2.2.11271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wallis RS. Tumour necrosis factor antagonists: structure, function, and tuberculosis risks. Lancet Infect Dis. 2008;8:601–611. doi: 10.1016/S1473-3099(08)70227-5. [DOI] [PubMed] [Google Scholar]
- 25.Baecklund E, Iliadou A, Askling J, Ekbom A, Backlin C, Granath F, Catrina AI, Rosenquist R, Feltelius N, Sundström C, et al. Association of chronic inflammation, not its treatment, with increased lymphoma risk in rheumatoid arthritis. Arthritis Rheum. 2006;54:692–701. doi: 10.1002/art.21675. [DOI] [PubMed] [Google Scholar]
- 26.Maneiro JR, Souto A, Gomez-Reino JJ. Risks of malignancies related to tofacitinib and biological drugs in rheumatoid arthritis: systematic review, meta-analysis, and network meta-analysis. Semin Arthritis Rheum. 2017;47:149–156. doi: 10.1016/j.semarthrit.2017.02.007. [DOI] [PubMed] [Google Scholar]
- 27.Keystone EC, Schiff MH, Kremer JM, Kafka S, Lovy M, DeVries T, Burge DJ. Once-weekly administration of 50 mg etanercept in patients with active rheumatoid arthritis: results of a multicenter, randomized, double-blind, placebo-controlled trial. Arthritis Rheum. 2004;50:353–363. doi: 10.1002/art.20019. [DOI] [PubMed] [Google Scholar]
- 28.Ma X, Xu S. TNF inhibitor therapy for rheumatoid arthritis. Biomed Rep. 2013;1:177–184. doi: 10.3892/br.2012.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kishimoto T. IL-6: from its discovery to clinical applications. Int Immunol. 2010;22:347–352. doi: 10.1093/intimm/dxq030. [DOI] [PubMed] [Google Scholar]
- 30.Pop VV, Seicean A, Lupan I, Samasca G, Burz CC. IL-6 roles - Molecular pathway and clinical implication in pancreatic cancer - A systemic review. Immunol Lett. 2017;181:45–50. doi: 10.1016/j.imlet.2016.11.010. [DOI] [PubMed] [Google Scholar]
- 31.Rose-John S, Neurath MF. IL-6 trans-signaling: the heat is on. Immunity. 2004;20:2–4. doi: 10.1016/s1074-7613(04)00003-2. [DOI] [PubMed] [Google Scholar]
- 32.Jones SA, Scheller J, Rose-John S. Therapeutic strategies for the clinical blockade of IL-6/gp130 signaling. J Clin Invest. 2011;121:3375–3383. doi: 10.1172/JCI57158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mihara M, Moriya Y, Kishimoto T, Ohsugi Y. Interleukin-6 (IL-6) induces the proliferation of synovial fibroblastic cells in the presence of soluble IL-6 receptor. Br J Rheumatol. 1995;34:321–325. doi: 10.1093/rheumatology/34.4.321. [DOI] [PubMed] [Google Scholar]
- 34.Heink S, Yogev N, Garbers C, Herwerth M, Aly L, Gasperi C, Husterer V, Croxford AL, Möller-Hackbarth K, Bartsch HS, et al. Trans-presentation of IL-6 by dendritic cells is required for the priming of pathogenic TH17 cells. Nat Immunol. 2017;18:74–85. doi: 10.1038/ni.3632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Alonzi T, Fattori E, Lazzaro D, Costa P, Probert L, Kollias G, De Benedetti F, Poli V, Ciliberto G. Interleukin 6 is required for the development of collagen-induced arthritis. J Exp Med. 1998;187:461–468. doi: 10.1084/jem.187.4.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Madhok R, Crilly A, Watson J, Capell HA. Serum interleukin 6 levels in rheumatoid arthritis: correlations with clinical and laboratory indices of disease activity. Ann Rheum Dis. 1993;52:232–234. doi: 10.1136/ard.52.3.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sack U, Kinne RW, Marx T, Heppt P, Bender S, Emmrich F. Interleukin-6 in synovial fluid is closely associated with chronic synovitis in rheumatoid arthritis. Rheumatol Int. 1993;13:45–51. doi: 10.1007/BF00307733. [DOI] [PubMed] [Google Scholar]
- 38.Korn T, Bettelli E, Oukka M, Kuchroo VK. IL-17 and Th17 cells. Annu Rev Immunol. 2009;27:485–517. doi: 10.1146/annurev.immunol.021908.132710. [DOI] [PubMed] [Google Scholar]
- 39.Yoshida Y, Tanaka T. Interleukin 6 and rheumatoid arthritis. BioMed Res Int. 2014;2014:698313. doi: 10.1155/2014/698313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Okamoto K, Takayanagi H. Osteoimmunology. Cold Spring Harb Perspect Med. 2019;9:a031245. doi: 10.1101/cshperspect.a031245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shetty A, Hanson R, Korsten P, Shawagfeh M, Arami S, Volkov S, Vila O, Swedler W, Shunaigat AN, Smadi S, et al. Tocilizumab in the treatment of rheumatoid arthritis and beyond. Drug Des Devel Ther. 2014;8:349–364. doi: 10.2147/DDDT.S41437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nishimoto N, Yoshizaki K, Miyasaka N, Yamamoto K, Kawai S, Takeuchi T, Hashimoto J, Azuma J, Kishimoto T. Treatment of rheumatoid arthritis with humanized anti-interleukin-6 receptor antibody: a multicenter, double-blind, placebo-controlled trial. Arthritis Rheum. 2004;50:1761–1769. doi: 10.1002/art.20303. [DOI] [PubMed] [Google Scholar]
- 43.McCarty D, Robinson A. Efficacy and safety of sarilumab in patients with active rheumatoid arthritis. Ther Adv Musculoskelet Dis. 2018;10:61–67. doi: 10.1177/1759720X17752037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mease PJ, Gottlieb AB, Berman A, Drescher E, Xing J, Wong R, Banerjee S. The efficacy and safety of clazakizumab, an anti-interleukin-6 monoclonal antibody, in a Phase IIb study of adults with active psoriatic arthritis. Arthritis Rheumatol. 2016;68:2163–2173. doi: 10.1002/art.39700. [DOI] [PubMed] [Google Scholar]
- 45.Gabay C, Emery P, van Vollenhoven R, Dikranian A, Alten R, Pavelka K, Klearman M, Musselman D, Agarwal S, Green J, et al. Tocilizumab monotherapy versus adalimumab monotherapy for treatment of rheumatoid arthritis (ADACTA): a randomised, double-blind, controlled phase 4 trial. Lancet. 2013;381:1541–1550. doi: 10.1016/S0140-6736(13)60250-0. [DOI] [PubMed] [Google Scholar]
- 46.Burmester GR, Choy E, Kivitz A, Ogata A, Bao M, Nomura A, Lacey S, Pei J, Reiss W, Pethoe-Schramm A, et al. Low immunogenicity of tocilizumab in patients with rheumatoid arthritis. Ann Rheum Dis. 2017;76:1078–1085. doi: 10.1136/annrheumdis-2016-210297. [DOI] [PubMed] [Google Scholar]
- 47.Kojima T, Yabe Y, Kaneko A, Hirano Y, Ishikawa H, Hayashi M, Miyake H, Takagi H, Kato T, Terabe K, et al. Monitoring C-reactive protein levels to predict favourable clinical outcomes from tocilizumab treatment in patients with rheumatoid arthritis. Mod Rheumatol. 2013;23:977–985. doi: 10.1007/s10165-012-0782-y. [DOI] [PubMed] [Google Scholar]
- 48.Scott LJ. Tocilizumab: a review in rheumatoid arthritis. Drugs. 2017;77:1865–1879. doi: 10.1007/s40265-017-0829-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Abbas A, Lichtman A, Pillai S. Cellular and Molecular Immunology. 9th ed. Amsterdam: Elsevier; 2016. Chapter 9. Activation of T lymphocytes; pp. 209–224. [Google Scholar]
- 50.Sharpe AH, Abbas AK. T-cell costimulation--biology, therapeutic potential, and challenges. N Engl J Med. 2006;355:973–975. doi: 10.1056/NEJMp068087. [DOI] [PubMed] [Google Scholar]
- 51.Malmström V, Trollmo C, Klareskog L. Modulating co-stimulation: a rational strategy in the treatment of rheumatoid arthritis? Arthritis Res Ther. 2005;7(Suppl 2):S15–S20. doi: 10.1186/ar1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kremer JM, Genant HK, Moreland LW, Russell AS, Emery P, Abud-Mendoza C, Szechinski J, Li T, Ge Z, Becker JC, et al. Effects of abatacept in patients with methotrexate-resistant active rheumatoid arthritis: a randomized trial. Ann Intern Med. 2006;144:865–876. doi: 10.7326/0003-4819-144-12-200606200-00003. [DOI] [PubMed] [Google Scholar]
- 53.Genovese MC, Becker JC, Schiff M, Luggen M, Sherrer Y, Kremer J, Birbara C, Box J, Natarajan K, Nuamah I, et al. Abatacept for rheumatoid arthritis refractory to tumor necrosis factor alpha inhibition. N Engl J Med. 2005;353:1114–1123. doi: 10.1056/NEJMoa050524. [DOI] [PubMed] [Google Scholar]
- 54.Schiff M, Pritchard C, Huffstutter JE, Rodriguez-Valverde V, Durez P, Zhou X, Li T, Bahrt K, Kelly S, Le Bars M, et al. The 6-month safety and efficacy of abatacept in patients with rheumatoid arthritis who underwent a washout after anti-tumour necrosis factor therapy or were directly switched to abatacept: the ARRIVE trial. Ann Rheum Dis. 2009;68:1708–1714. doi: 10.1136/ard.2008.099218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Scarsi M, Paolini L, Ricotta D, Pedrini A, Piantoni S, Caimi L, Tincani A, Airò P. Abatacept reduces levels of switched memory B cells, autoantibodies, and immunoglobulins in patients with rheumatoid arthritis. J Rheumatol. 2014;41:666–672. doi: 10.3899/jrheum.130905. [DOI] [PubMed] [Google Scholar]
- 56.Salmon JH, Gottenberg JE, Ravaud P, Cantagrel A, Combe B, Flipo RM, Schaeverbeke T, Houvenagel E, Gaudin P, Loeuille D, et al. Predictive risk factors of serious infections in patients with rheumatoid arthritis treated with abatacept in common practice: results from the Orencia and Rheumatoid Arthritis (ORA) registry. Ann Rheum Dis. 2016;75:1108–1113. doi: 10.1136/annrheumdis-2015-207362. [DOI] [PubMed] [Google Scholar]
- 57.Schiff M, Keiserman M, Codding C, Songcharoen S, Berman A, Nayiager S, Saldate C, Li T, Aranda R, Becker JC, et al. Efficacy and safety of abatacept or infliximab vs placebo in ATTEST: a phase III, multi-centre, randomised, double-blind, placebo-controlled study in patients with rheumatoid arthritis and an inadequate response to methotrexate. Ann Rheum Dis. 2008;67:1096–1103. doi: 10.1136/ard.2007.080002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Martín Mola E, Balsa A, Martínez Taboada V, Sanmartí R, Marenco JL, Navarro Sarabia F, Gómez-Reino J, Alvaro-Gracia JM, Román Ivorra JA, Lojo L, et al. Abatacept use in rheumatoid arthritis: evidence review and recommendations. Reumatol Clin. 2013;9:5–17. doi: 10.1016/j.reuma.2012.05.001. [DOI] [PubMed] [Google Scholar]
- 59.Gu C, Wu L, Li X. IL-17 family: cytokines, receptors and signaling. Cytokine. 2013;64:477–485. doi: 10.1016/j.cyto.2013.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Toy D, Kugler D, Wolfson M, Vanden Bos T, Gurgel J, Derry J, Tocker J, Peschon J. Cutting edge: interleukin 17 signals through a heteromeric receptor complex. J Immunol. 2006;177:36–39. doi: 10.4049/jimmunol.177.1.36. [DOI] [PubMed] [Google Scholar]
- 61.Ishigame H, Kakuta S, Nagai T, Kadoki M, Nambu A, Komiyama Y, Fujikado N, Tanahashi Y, Akitsu A, Kotaki H, et al. Differential roles of interleukin-17A and -17F in host defense against mucoepithelial bacterial infection and allergic responses. Immunity. 2009;30:108–119. doi: 10.1016/j.immuni.2008.11.009. [DOI] [PubMed] [Google Scholar]
- 62.Chabaud M, Durand JM, Buchs N, Fossiez F, Page G, Frappart L, Miossec P. Human interleukin-17: a T cell-derived proinflammatory cytokine produced by the rheumatoid synovium. Arthritis Rheum. 1999;42:963–970. doi: 10.1002/1529-0131(199905)42:5<963::AID-ANR15>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 63.Chabaud M, Garnero P, Dayer JM, Guerne PA, Fossiez F, Miossec P. Contribution of interleukin 17 to synovium matrix destruction in rheumatoid arthritis. Cytokine. 2000;12:1092–1099. doi: 10.1006/cyto.2000.0681. [DOI] [PubMed] [Google Scholar]
- 64.Yao Z, Painter SL, Fanslow WC, Ulrich D, Macduff BM, Spriggs MK, Armitage RJ. Human IL-17: a novel cytokine derived from T cells. J Immunol. 1995;155:5483–5486. [PubMed] [Google Scholar]
- 65.Haugeberg G, Uhlig T, Falch JA, Halse JI, Kvien TK. Bone mineral density and frequency of osteoporosis in female patients with rheumatoid arthritis: results from 394 patients in the Oslo County Rheumatoid Arthritis register. Arthritis Rheum. 2000;43:522–530. doi: 10.1002/1529-0131(200003)43:3<522::AID-ANR7>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 66.Koenders MI, Lubberts E, Oppers-Walgreen B, van den Bersselaar L, Helsen MM, Kolls JK, Joosten LA, van den Berg WB. Induction of cartilage damage by overexpression of T cell interleukin-17A in experimental arthritis in mice deficient in interleukin-1. Arthritis Rheum. 2005;52:975–983. doi: 10.1002/art.20885. [DOI] [PubMed] [Google Scholar]
- 67.Nakae S, Nambu A, Sudo K, Iwakura Y. Suppression of immune induction of collagen-induced arthritis in IL-17-deficient mice. J Immunol. 2003;171:6173–6177. doi: 10.4049/jimmunol.171.11.6173. [DOI] [PubMed] [Google Scholar]
- 68.Lubberts E, Koenders MI, Oppers-Walgreen B, van den Bersselaar L, Coenen-de Roo CJ, Joosten LA, van den Berg WB. Treatment with a neutralizing anti-murine interleukin-17 antibody after the onset of collagen-induced arthritis reduces joint inflammation, cartilage destruction, and bone erosion. Arthritis Rheum. 2004;50:650–659. doi: 10.1002/art.20001. [DOI] [PubMed] [Google Scholar]
- 69.Langrish CL, Chen Y, Blumenschein WM, Mattson J, Basham B, Sedgwick JD, McClanahan T, Kastelein RA, Cua DJ. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J Exp Med. 2005;201:233–240. doi: 10.1084/jem.20041257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hueber W, Patel DD, Dryja T, Wright AM, Koroleva I, Bruin G, Antoni C, Draelos Z, Gold MH, et al. Psoriasis Study Group. Effects of AIN457, a fully human antibody to interleukin-17A, on psoriasis, rheumatoid arthritis, and uveitis. Sci Transl Med. 2010;2:52ra72. doi: 10.1126/scitranslmed.3001107. [DOI] [PubMed] [Google Scholar]
- 71.Genovese MC, Van den Bosch F, Roberson SA, Bojin S, Biagini IM, Ryan P, Sloan-Lancaster J. LY2439821, a humanized anti-interleukin-17 monoclonal antibody, in the treatment of patients with rheumatoid arthritis: A phase I randomized, double-blind, placebo-controlled, proof-of-concept study. Arthritis Rheum. 2010;62:929–939. doi: 10.1002/art.27334. [DOI] [PubMed] [Google Scholar]
- 72.Genovese MC, Durez P, Richards HB, Supronik J, Dokoupilova E, Mazurov V, Aelion JA, Lee SH, Codding CE, Kellner H, et al. Efficacy and safety of secukinumab in patients with rheumatoid arthritis: a phase II, dose-finding, double-blind, randomised, placebo controlled study. Ann Rheum Dis. 2013;72:863–869. doi: 10.1136/annrheumdis-2012-201601. [DOI] [PubMed] [Google Scholar]
- 73.Tlustochowicz W, Rahman P, Seriolo B, Krammer G, Porter B, Widmer A, Richards HB. Efficacy and safety of subcutaneous and intravenous loading dose regimens of secukinumab in patients with active rheumatoid arthritis: results from a randomized phase II study. J Rheumatol. 2016;43:495–503. doi: 10.3899/jrheum.150117. [DOI] [PubMed] [Google Scholar]
- 74.Blanco FJ, Möricke R, Dokoupilova E, Codding C, Neal J, Andersson M, Rohrer S, Richards H. Secukinumab in active rheumatoid arthritis: a phase III randomized, double-blind, active comparator- and placebo-controlled study. Arthritis Rheumatol. 2017;69:1144–1153. doi: 10.1002/art.40070. [DOI] [PubMed] [Google Scholar]
- 75.Tahir H, Deodhar A, Genovese M, Takeuchi T, Aelion J, Van den Bosch F, Haemmerle S, Richards HB. Secukinumab in active rheumatoid arthritis after anti-tnfα therapy: A randomized, double-blind placebo-controlled phase 3 study. Rheumatol Ther. 2017;4:475–488. doi: 10.1007/s40744-017-0086-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Benedetti G, Miossec P. Interleukin 17 contributes to the chronicity of inflammatory diseases such as rheumatoid arthritis. Eur J Immunol. 2014;44:339–347. doi: 10.1002/eji.201344184. [DOI] [PubMed] [Google Scholar]
- 77.van Baarsen LG, Lebre MC, van der Coelen D, Aarrass S, Tang MW, Ramwadhdoebe TH, Gerlag DM, Tak PP. Heterogeneous expression pattern of interleukin 17A (IL-17A), IL-17F and their receptors in synovium of rheumatoid arthritis, psoriatic arthritis and osteoarthritis: possible explanation for nonresponse to anti-IL-17 therapy? Arthritis Res Ther. 2014;16:426. doi: 10.1186/s13075-014-0426-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Roberts CA, Durham LE, Fleskens V, Evans HG, Taams LS. TNF blockade maintains an IL-10+ phenotype in human effector CD4+ and CD8+ T cells. Front Immunol. 2017;8:157. doi: 10.3389/fimmu.2017.00157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Dinarello CA. The IL-1 family of cytokines and receptors in rheumatic diseases. Nat Rev Rheumatol. 2019;15:612–632. doi: 10.1038/s41584-019-0277-8. [DOI] [PubMed] [Google Scholar]
- 80.Noack M, Miossec P. Selected cytokine pathways in rheumatoid arthritis. Semin Immunopathol. 2017;39:365–383. doi: 10.1007/s00281-017-0619-z. [DOI] [PubMed] [Google Scholar]
- 81.Henderson B, Pettipher ER. Comparison of the in vivo inflammatory activities after intra-articular injection of natural and recombinant IL-1 alpha and IL-1 beta in the rabbit. Biochem Pharmacol. 1988;37:4171–4176. doi: 10.1016/0006-2952(88)90112-8. [DOI] [PubMed] [Google Scholar]
- 82.Pettipher ER, Higgs GA, Henderson B. Interleukin 1 induces leukocyte infiltration and cartilage proteoglycan degradation in the synovial joint. Proc Natl Acad Sci U S A. 1986;83:8749–8753. doi: 10.1073/pnas.83.22.8749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ghivizzani SC, Lechman ER, Tio C, Mulé KM, Chada S, McCormack JE, Evans CH, Robbins PD. Direct retrovirus-mediated gene transfer to the synovium of the rabbit knee: implications for arthritis gene therapy. Gene Ther. 1997;4:977–982. doi: 10.1038/sj.gt.3300486. [DOI] [PubMed] [Google Scholar]
- 84.Ulfgren AK, Gröndal L, Lindblad S, Khademi M, Johnell O, Klareskog L, Andersson U. Interindividual and intra-articular variation of proinflammatory cytokines in patients with rheumatoid arthritis: potential implications for treatment. Ann Rheum Dis. 2000;59:439–447. doi: 10.1136/ard.59.6.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Farrell SA. Reframing social justice, feminism and abortion. Conscience. 2005;26:42–44. [PubMed] [Google Scholar]
- 86.Mertens M, Singh JA. Anakinra for rheumatoid arthritis: a systematic review. J Rheumatol. 2009;36:1118–1125. doi: 10.3899/jrheum.090074. [DOI] [PubMed] [Google Scholar]
- 87.Gram H. The long and winding road in pharmaceutical development of canakinumab from rare genetic autoinflammatory syndromes to myocardial infarction and cancer. Pharmacol Res. 2020;154:104139. doi: 10.1016/j.phrs.2019.01.023. [DOI] [PubMed] [Google Scholar]
- 88.Genovese MC, Cohen S, Moreland L, Lium D, Robbins S, Newmark R, Bekker P 20000223 Study Group. Combination therapy with etanercept and anakinra in the treatment of patients with rheumatoid arthritis who have been treated unsuccessfully with methotrexate. Arthritis Rheum. 2004;50:1412–1419. doi: 10.1002/art.20221. [DOI] [PubMed] [Google Scholar]
- 89.Bresnihan B, Alvaro-Gracia JM, Cobby M, Doherty M, Domljan Z, Emery P, Nuki G, Pavelka K, Rau R, Rozman B, et al. Treatment of rheumatoid arthritis with recombinant human interleukin-1 receptor antagonist. Arthritis Rheum. 1998;41:2196–2204. doi: 10.1002/1529-0131(199812)41:12<2196::AID-ART15>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 90.Cohen S, Hurd E, Cush J, Schiff M, Weinblatt ME, Moreland LW, Kremer J, Bear MB, Rich WJ, McCabe D. Treatment of rheumatoid arthritis with anakinra, a recombinant human interleukin-1 receptor antagonist, in combination with methotrexate: results of a twenty-four-week, multicenter, randomized, double-blind, placebo-controlled trial. Arthritis Rheum. 2002;46:614–624. doi: 10.1002/art.10141. [DOI] [PubMed] [Google Scholar]
- 91.Goldbach-Mansky R. Blocking interleukin-1 in rheumatic diseases. Ann N Y Acad Sci. 2009;1182:111–123. doi: 10.1111/j.1749-6632.2009.05159.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hahn C, Orr AW, Sanders JM, Jhaveri KA, Schwartz MA. The subendothelial extracellular matrix modulates JNK activation by flow. Circ Res. 2009;104:995–1003. doi: 10.1161/CIRCRESAHA.108.186486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Sun Y, Liu WZ, Liu T, Feng X, Yang N, Zhou HF. Signaling pathway of MAPK/ERK in cell proliferation, differentiation, migration, senescence and apoptosis. J Recept Signal Transduct Res. 2015;35:600–604. doi: 10.3109/10799893.2015.1030412. [DOI] [PubMed] [Google Scholar]
- 94.Korb A, Tohidast-Akrad M, Cetin E, Axmann R, Smolen J, Schett G. Differential tissue expression and activation of p38 MAPK α, β, γ, and δ isoforms in rheumatoid arthritis. Arthritis Rheum. 2006;54:2745–2756. doi: 10.1002/art.22080. [DOI] [PubMed] [Google Scholar]
- 95.Patterson H, Nibbs R, McInnes I, Siebert S. Protein kinase inhibitors in the treatment of inflammatory and autoimmune diseases. Clin Exp Immunol. 2014;176:1–10. doi: 10.1111/cei.12248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Meier FM, McInnes IB. Small-molecule therapeutics in rheumatoid arthritis: scientific rationale, efficacy and safety. Best Pract Res Clin Rheumatol. 2014;28:605–624. doi: 10.1016/j.berh.2014.10.017. [DOI] [PubMed] [Google Scholar]
- 97.Lee JC, Laydon JT, McDonnell PC, Gallagher TF, Kumar S, Green D, McNulty D, Blumenthal MJ, Heys JR, Landvatter SW, et al. A protein kinase involved in the regulation of inflammatory cytokine biosynthesis. Nature. 1994;372:739–746. doi: 10.1038/372739a0. [DOI] [PubMed] [Google Scholar]
- 98.Shapiro L, Dinarello CA. Osmotic regulation of cytokine synthesis in vitro . Proc Natl Acad Sci U S A. 1995;92:12230–12234. doi: 10.1073/pnas.92.26.12230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Beyaert R, Cuenda A, Vanden Berghe W, Plaisance S, Lee JC, Haegeman G, Cohen P, Fiers W. The p38/RK mitogen-activated protein kinase pathway regulates interleukin-6 synthesis response to tumor necrosis factor. EMBO J. 1996;15:1914–1923. [PMC free article] [PubMed] [Google Scholar]
- 100.Inoue T, Boyle DL, Corr M, Hammaker D, Davis RJ, Flavell RA, Firestein GS. Mitogen-activated protein kinase kinase 3 is a pivotal pathway regulating p38 activation in inflammatory arthritis. Proc Natl Acad Sci U S A. 2006;103:5484–5489. doi: 10.1073/pnas.0509188103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Yoshizawa T, Hammaker D, Boyle DL, Corr M, Flavell R, Davis R, Schett G, Firestein GS. Role of MAPK kinase 6 in arthritis: distinct mechanism of action in inflammation and cytokine expression. J Immunol. 2009;183:1360–1367. doi: 10.4049/jimmunol.0900483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Hegen M, Gaestel M, Nickerson-Nutter CL, Lin LL, Telliez JB. MAPKAP kinase 2-deficient mice are resistant to collagen-induced arthritis. J Immunol. 2006;177:1913–1917. doi: 10.4049/jimmunol.177.3.1913. [DOI] [PubMed] [Google Scholar]
- 103.O’Keefe SJ, Mudgett JS, Cupo S, Parsons JN, Chartrain NA, Fitzgerald C, Chen SL, Lowitz K, Rasa C, Visco D, et al. Chemical genetics define the roles of p38α and p38β in acute and chronic inflammation. J Biol Chem. 2007;282:34663–34671. doi: 10.1074/jbc.M704236200. [DOI] [PubMed] [Google Scholar]
- 104.Badger AM, Griswold DE, Kapadia R, Blake S, Swift BA, Hoffman SJ, Stroup GB, Webb E, Rieman DJ, Gowen M, et al. Disease-modifying activity of SB 242235, a selective inhibitor of p38 mitogen-activated protein kinase, in rat adjuvant-induced arthritis. Arthritis Rheum. 2000;43:175–183. doi: 10.1002/1529-0131(200001)43:1<175::AID-ANR22>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 105.Nishikawa M, Myoui A, Tomita T, Takahi K, Nampei A, Yoshikawa H. Prevention of the onset and progression of collagen-induced arthritis in rats by the potent p38 mitogen-activated protein kinase inhibitor FR167653. Arthritis Rheum. 2003;48:2670–2681. doi: 10.1002/art.11227. [DOI] [PubMed] [Google Scholar]
- 106.Kumar S, Boehm J, Lee JC. p38 MAP kinases: key signalling molecules as therapeutic targets for inflammatory diseases. Nat Rev Drug Discov. 2003;2:717–726. doi: 10.1038/nrd1177. [DOI] [PubMed] [Google Scholar]
- 107.Cohen SB, Cheng TT, Chindalore V, Damjanov N, Burgos-Vargas R, Delora P, Zimany K, Travers H, Caulfield JP. Evaluation of the efficacy and safety of pamapimod, a p38 MAP kinase inhibitor, in a double-blind, methotrexate-controlled study of patients with active rheumatoid arthritis. Arthritis Rheum. 2009;60:335–344. doi: 10.1002/art.24266. [DOI] [PubMed] [Google Scholar]
- 108.Alten RE, Zerbini C, Jeka S, Irazoque F, Khatib F, Emery P, Bertasso A, Rabbia M, Caulfield JP. Efficacy and safety of pamapimod in patients with active rheumatoid arthritis receiving stable methotrexate therapy. Ann Rheum Dis. 2010;69:364–367. doi: 10.1136/ard.2008.104802. [DOI] [PubMed] [Google Scholar]
- 109.Branger J, van den Blink B, Weijer S, Madwed J, Bos CL, Gupta A, Yong CL, Polmar SH, Olszyna DP, Hack CE, et al. Anti-inflammatory effects of a p38 mitogen-activated protein kinase inhibitor during human endotoxemia. J Immunol. 2002;168:4070–4077. doi: 10.4049/jimmunol.168.8.4070. [DOI] [PubMed] [Google Scholar]
- 110.Genovese MC, Cohen SB, Wofsy D, Weinblatt ME, Firestein GS, Brahn E, Strand V, Baker DG, Tong SE. A 24-week, randomized, double-blind, placebo-controlled, parallel group study of the efficacy of oral SCIO-469, a p38 mitogen-activated protein kinase inhibitor, in patients with active rheumatoid arthritis. J Rheumatol. 2011;38:846–854. doi: 10.3899/jrheum.100602. [DOI] [PubMed] [Google Scholar]
- 111.Damjanov N, Kauffman RS, Spencer-Green GT. Efficacy, pharmacodynamics, and safety of VX-702, a novel p38 MAPK inhibitor, in rheumatoid arthritis: results of two randomized, double-blind, placebo-controlled clinical studies. Arthritis Rheum. 2009;60:1232–1241. doi: 10.1002/art.24485. [DOI] [PubMed] [Google Scholar]
- 112.Genovese MC. Inhibition of p38: has the fat lady sung? Arthritis Rheum. 2009;60:317–320. doi: 10.1002/art.24264. [DOI] [PubMed] [Google Scholar]
- 113.Kim C, Sano Y, Todorova K, Carlson BA, Arpa L, Celada A, Lawrence T, Otsu K, Brissette JL, Arthur JS, et al. The kinase p38 α serves cell type-specific inflammatory functions in skin injury and coordinates pro- and anti-inflammatory gene expression. Nat Immunol. 2008;9:1019–1027. doi: 10.1038/ni.1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Shaik SS, Soltau TD, Chaturvedi G, Totapally B, Hagood JS, Andrews WW, Athar M, Voitenok NN, Killingsworth CR, Patel RP, et al. Low intensity shear stress increases endothelial ELR+ CXC chemokine production via a focal adhesion kinase-p38β MAPK-NF-κB pathway. J Biol Chem. 2009;284:5945–5955. doi: 10.1074/jbc.M807205200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Hammaker D, Firestein GS. “Go upstream, young man”: lessons learned from the p38 saga. Ann Rheum Dis. 2010;69(Suppl 1):i77–i82. doi: 10.1136/ard.2009.119479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Wang C, Deng L, Hong M, Akkaraju GR, Inoue J, Chen ZJ. TAK1 is a ubiquitin-dependent kinase of MKK and IKK. Nature. 2001;412:346–351. doi: 10.1038/35085597. [DOI] [PubMed] [Google Scholar]
- 117.Fragoulis GE, McInnes IB, Siebert S. JAK-inhibitors. New players in the field of immune-mediated diseases, beyond rheumatoid arthritis. Rheumatology (Oxford) 2019;58(Suppl 1):i43–i54. doi: 10.1093/rheumatology/key276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Seif F, Khoshmirsafa M, Aazami H, Mohsenzadegan M, Sedighi G, Bahar M. The role of JAK-STAT signaling pathway and its regulators in the fate of T helper cells. Cell Commun Signal. 2017;15:23. doi: 10.1186/s12964-017-0177-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Harrington R, Al Nokhatha SA, Conway R. JAK inhibitors in rheumatoid arthritis: An evidence-based review on the emerging clinical data. J Inflamm Res. 2020;13:519–531. doi: 10.2147/JIR.S219586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.O’Shea JJ, Holland SM, Staudt LM. JAKs and STATs in immunity, immunodeficiency, and cancer. N Engl J Med. 2013;368:161–170. doi: 10.1056/NEJMra1202117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Lee EB, Fleischmann R, Hall S, Wilkinson B, Bradley JD, Gruben D, Koncz T, Krishnaswami S, Wallenstein GV, Zang C, et al. Tofacitinib versus methotrexate in rheumatoid arthritis. N Engl J Med. 2014;370:2377–2386. doi: 10.1056/NEJMoa1310476. [DOI] [PubMed] [Google Scholar]
- 122.Fleischmann R, Mysler E, Hall S, Kivitz AJ, Moots RJ, Luo Z, DeMasi R, Soma K, Zhang R, Takiya L, et al. Efficacy and safety of tofacitinib monotherapy, tofacitinib with methotrexate, and adalimumab with methotrexate in patients with rheumatoid arthritis (ORAL Strategy): a phase 3b/4, double-blind, head-to-head, randomised controlled trial. Lancet. 2017;390:457–468. doi: 10.1016/S0140-6736(17)31618-5. [DOI] [PubMed] [Google Scholar]
- 123.Fleischmann R, Schiff M, van der Heijde D, Ramos-Remus C, Spindler A, Stanislav M, Zerbini CA, Gurbuz S, Dickson C, de Bono S, et al. Baricitinib, methotrexate, or combination in patients with rheumatoid arthritis and no or limited prior disease-modifying antirheumatic drug treatment. Arthritis Rheumatol. 2017;69:506–517. doi: 10.1002/art.39953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Taylor PC, Keystone EC, van der Heijde D, Weinblatt ME, Del Carmen Morales L, Reyes Gonzaga J, Yakushin S, Ishii T, Emoto K, Beattie S, et al. Baricitinib versus placebo or adalimumab in rheumatoid arthritis. N Engl J Med. 2017;376:652–662. doi: 10.1056/NEJMoa1608345. [DOI] [PubMed] [Google Scholar]
- 125.Parmentier JM, Voss J, Graff C, Schwartz A, Argiriadi M, Friedman M, Camp HS, Padley RJ, George JS, Hyland D, et al. In vitro and in vivo characterization of the JAK1 selectivity of upadacitinib (ABT-494) BMC Rheumatol. 2018;2:23. doi: 10.1186/s41927-018-0031-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Conaghan PG, Mysler E, Tanaka Y, Da Silva-Tillmann B, Shaw T, Liu J, Ferguson R, Enejosa JV, Cohen S, Nash P, et al. Upadacitinib in rheumatoid arthritis: A benefit-risk assessment across a Phase III program. Drug Saf. 2021;44:515–530. doi: 10.1007/s40264-020-01036-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Burja B, Mertelj T, Frank-Bertoncelj M. Hi-JAKi-ng synovial fibroblasts in inflammatory arthritis with JAK inhibitors. Front Med (Lausanne) 2020;7:124. doi: 10.3389/fmed.2020.00124. [DOI] [PMC free article] [PubMed] [Google Scholar]