Abstract
Background:
CD25+ human mast cells (huMCs) have been reported in patients with monoclonal mast cell diseases and in rare association with inflammation. However, the regulation of CD25 expression on huMCs and the possible biologic consequences remain poorly understood.
Objective:
We sought to identify conditions that would upregulate CD25 expression on huMCs and to explore possible functional implications.
Methods:
huMCs were cultured from peripheral blood progenitor cells over 6 to 8 weeks. Expression of CD25 was determined by fluorescence-activated cell sorting and soluble CD25 by ELISA. Signal transducer and activator of transcription 5 (STAT5) phosphorylation induced by IL-2 in huMCs, regulatory T (Treg) cells, or in cocultured huMCs and Treg cells was examined by fluorescence-activated cell sorting.
Results:
Addition of IL-3 to CD34+ progenitors at the initiation of huMC cultures in the presence of stem cell factor and IL-6 upregulated the expression of CD25 in developing huMCs and resulted in shedding of soluble CD25 into the media. Removal of IL-3 after the first week of culture did not affect subsequent expression of CD25. Furthermore, addition of IL-3 14 days after the initiation of the culture did not induce significant CD25 expression. Treatment with anti–IL-3 antibody or the Janus kinase inhibitor tofacitinib blocked IL-3–induced CD25 upregulation. Binding of IL-2 to CD25+ huMCs did not induce STAT5 phosphorylation. However, coincubation of Treg cells with CD25+ huMCs pretreated with IL-2 was sufficient to result in STAT5 phosphorylation in Treg cells.
Conclusions:
IL-3 promotes CD25 expression and shedding by huMCs. Although CD25+ huMCs do not respond to IL-2, they bind IL-2 and may act as a reservoir of IL-2 to then activate lymphocytes.
Keywords: Mast cells, CD25, IL-3, IL-2, STAT5, regulatory T cell
Mast cells (MCs) reside in vascularized tissues and through a number of mechanisms may be activated to both initiate and enhance inflammation.1-3 Human MCs (huMCs) express cluster of differentiation (CD)117 (KIT) and the high-affinity receptor for IgE (FcεRI) on their surface and are often identified by the presence of tryptase and chymase within their granules.4,5 CD25 (IL-2 receptor-α) is minimally expressed, if at all, on normal resting huMCs or on huMCs after exposure to stem cell factor (SCF).6-8 However, CD25 is expressed in most bone marrow mast cells from patients with both indolent and advanced systemic mastocytosis (SM) but with lower to absent levels in some with MC leukemia and well-differentiated SM.9-12 CD25 is absent from MCs from normal/reactive bone marrow, although CD25+ MCs not linked to mastocytosis have been observed in marrows after chemotherapy.13 CD25+ MCs have also been reportedly detected in inflamed tissues such as chronic tonsillitis and lichen planus.14,15 This is in part why CD25 serves as a minor rather than a major diagnostic criteria in the identification of patients with SM. Nevertheless, little is known about the regulation of CD25 expression by huMCs and the possible biologic consequences of such CD25 expression.
To explore CD25 regulation on huMCs, we cultured MC precursors in various cytokines in the presence of SCF, which is obligatory for the culture of huMCs, unlike in the culture of murine MCs where IL-3 alone supports their growth and survival.16,17 As will be shown, among the factors tested, IL-3 was unique in upregulating the expression of CD25 more than 5-fold by huMCs in culture. Furthermore, CD25+ huMCs did not respond to IL-2 but had the ability to bind IL-2 and appeared capable of acting as a reservoir of IL-2 capable of stimulating regulatory T (Treg) cells.
METHODS
Antibodies and reagents
Antibodies for fluorescence-activated cell sorting including BV711-CD25, BV605-CD117, FITC-FcεRI, BV421-CD122, and APC-CD132 were from Biolegend (San Diego, Calif). PE-tryptase was from Santa Cruz (Dallas, Tex). AF647-pSTAT5, PE-pS6RP, and AF488-pERK1/2 were from Cell Signaling Technology (Danvers, Mass). PE-CD123, PE-Cy7-streptavidin, FITC-CD4, and PE-pSTAT3 were from BD Biosciences (San Jose, Calif). FITC-pPI3K, PE-Src, and APC-pPLCr1 were from Abwiz Bio Inc (San Diego, Calif). Tofacitinib was from LC Laboratories (Woburn, Mass).
Human sample collection and processing
Heparinized whole blood (100 mL) and cells from lymphocytapheresis were obtained from healthy adult volunteers after informed consent under protocols approved by the Institutional Review Board of the National Institute of Allergy and Infectious Diseases (protocol nos. 2009-I-0049 and 10-I-0196). PBMCs from lymphocytapheresis were processed as described.18 Bone marrow cells obtained following informed consent (protocol 02-I-0277) were isolated by using Ficoll-Paque Plus (GE Healthcare, Piscataway, NJ) and cryopreserved in a −140°C liquid nitrogen freezer (10-20 × 106 cells/vial) until use.18
huMCculture
Peripheral blood progenitor cells were enriched from PBMCs with EasySep Human Progenitor Cell Enrichment Kit (Stemcell Technologies, Vancouver, BC, Canada) and cultured in StemPro-34 Medium (Invitrogen, Waltham, Mass) supplemented with 100 ng/mL human recombinant SCF and 100 ng/mL human IL-6 (R&D Systems, Minneapolis, Minn). Human IL-3 (PeproTech, Rocky Hill, NJ) was added (IL-3 culture) or not (no IL-3 culture) on day 0 at 30 ng/mL.16 By 7 weeks, with or without IL-3, more than 98% of the cells were MCs, identified as FcεRI/CD117 double-positive, granular cells. The huMC lines LAD219 and HMC1.220 were cultured as described.
Skin MC isolation
Fresh abdomen or breast skin specimens from healthy donors were obtained from the Cooperative Human Tissue Network of the National Cancer Institute. In brief, skin was minced into fine pieces that were then digested twice for 1 hour at 37°C in an HBSS that contained 1.5 mg/mL collagenase type 2 (Worthington, Lakewood, NJ), 0.7 mg/mL hyaluronidase (Sigma-Aldrich, St Louis, Mo), and 0.15 mg/mL DNase type 1 (Sigma-Aldrich). The dispersed cells from each digestion were passed through a wire mesh. Separated cells were combined and then passed through a 40-μm cell strainer and loaded on top of Percoll Plus (GE Healthcare, Piscataway, NJ). The middle layer cells were collected, washed, and resuspended at 5 × 105 cells/mL in X-Vivo 15 media (Lonza, Walkersville, Md) with 100 ng/mL recombinant human SCF (R&D Systems, Minneapolis, Minn) and cultured for 4 weeks.21
Flow cytometry
For analysis of huMC surface markers, cultured cells were stained in 200 μL of a solution of fluorescent aqua dye (Invitrogen, Waltham, Mass) (1:2000 in PBS) for 20 minutes to detect live/dead cells. Cells were then incubated with 50 μL of an antibody cocktail containing BV711-CD25, BV605-CD117, FITC-FcεRI, BV421-CD122, and APC-CD132 in PBS-3%FBS for 30 minutes at room temperature.16 Cells were acquired on an LSRFortessa flow cytometer (BD, Franklin Lakes, NJ) and analyzed using FlowJo software (TreeStar, Inc, Ashton, Ore).
For intracellular staining of tryptase in huMCs or endogenous IL-3 in cells from human bone marrow, cells were stained with aqua and then fixed with 4% paraformaldehyde. For IL-3 production, LAD2 or HMC1.2 cells were treated with phorbol 12-myristate 13-acetate/ionomycin/brefeldin A for 5 hours, stained with aqua, and then fixed with 4% paraformaldehyde. Cells were permeabilized in 5% milk-saponin solution (PBS-S/milk) for 30 minutes as described,22 stained with antitryptase or anti–IL-3 antibody, and analyzed by flow cytometry.
For detection of phospho-proteins, cells were treated with or without 1 μg/mL IL-2 for 30 minutes at 37°C, and incubated with aqua live/dead cell staining as described above. Cells were then fixed with 4% paraformaldehyde and permeabilized with 95% methanol in PBS on ice for 1 hour and stained with antiphospho antibodies before analysis by flow cytometry. For positive controls, protein phosphorylation in various pathways was induced by treating cells for 20 minutes with the general tyrosine phosphatase inhibitor pervanadate (100 μM), or with a combination of the protein kinase C activator phorbol 12-myristate 13-acetate (PMA) (20 ng/mL) plus the calcium ionophore ionomycin (1 μM).
IL-2 binding assay
LAD2 cells, HMC1.2 cells, or huMCs were harvested and washed once with PBS. Human biotinylated-IL-2 (R&D System, Minneapolis, Minn) was added to the cells in PBS at 1 μg/mL and incubated for 30 minutes at room temperature. Cells were washed in PBS twice and extracellularly bound IL-2 detected by staining with phycoerythrin-streptavidin (BD, San Jose, Calif) for analysis by flow cytometry.
ELISA
For quantitative analysis of IL-3, week 2 huMC culture supernatants (grown without IL-3) were collected and the extracellular IL-3 concentration was determined using an IL-3 ELISA kit (Cusabio Technology LLC, Houston, Tex) following the manufacturer’s instructions. Culture medium without cells was used as control. A soluble CD25 (sCD25) ELISA kit (R&D System, Minneapolis, Minn) was used to measure sCD25 concentration in 2-week, 4-week, and 6-week culture supernatants. The optical density of each well was determined using a Spectramax microplate reader (Molecular Devices, San Jose, Calif) set to 450/570 nm.
Coculture of huMCs and Treg cells
HuMCs were incubated with IL-2 (IL-2-MCs) at 1 μg/mL for 30 minutes at room temperature and then washed 3 times with an excess volume of PBS to remove remaining IL-2 in the supernatant. The supernatant of the final wash served as control to test the effects of residual IL-2 on Treg cells. Resting huMCs or IL-2–exposed huMCs were coincubated either with untreated human Treg cells (Astarte Biologics Inc, Bothell, Wash) at 1:1 or 1:5 ratio or with Treg cells pretreated with 15 mg/mL anti-CD25 antibody for 20 minutes at a 1:1 ratio in serum-free RPMI1640 medium for 1 hour at 37°C and 5% CO2. Cells were then harvested and processed for phospho-flow assay. FITC-CD4, PE-tryptase, and AF647-pSTAT5 antibodies were used for staining.
Statistics
Data are expressed as mean ± SD. P values were determined using unpaired 2-tailed Student t test. P less than .05 (*), P less than .01 (**), or P less than .001 (***) was considered significant.
RESULTS
IL-3 upregulates CD25 expression on huMCs
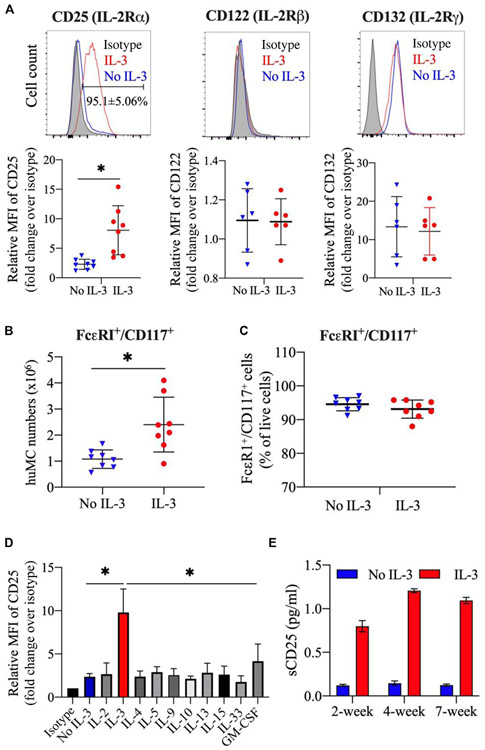
CD25 expression has been detected on huMCs from patients with MC proliferative disorders, and there are reports of CD25+ MCs in association with inflammation.9-12 To determine what cytokines might regulate CD25 expression on huMCs, we first determined the expression of the alpha, beta, and gamma chains of the IL2 receptor (CD25, CD122, and CD132) using a standard culture method where CD34+ progenitor cells enriched from peripheral blood were cultured in the presence of SCF and IL-6 with and without IL-3 (added only the first week of culture). As can be seen in Fig 1, A, under standard culture conditions and when IL-3 was present, 95.1% of huMCs expressed CD25 and CD132, but not CD122. In the absence of IL-3, CD25 expression was markedly reduced, whereas the expression levels of the IL-2 receptor subunits CD132 and CD122 were similar to those in the presence of IL-3. After 7 weeks of culture, huMCs cultured in SCF with or without IL-3 were similarly positive for the IL-3 receptor (CD123) (see Fig E1, A, in this article’s Online Repository at www.jacionline.org), and showed an MC phenotype based on Alcian blue staining (Fig E1, B), tryptase/chymase content (Fig E1, C), and degranulation on IgE aggregation (Fig E1, D). As we have reported,16 addition of IL-3 also increased huMCs in culture (Fig 1, B), with minimal effects on CD117 or FcεRI expression (Fig 1, C) or in the percentage of CD117−/tryptase− cells (<1.4%) (Fig E1, E).
FIG 1.
IL-3 upregulates CD25 expression on huMCs. Enriched CD34+ progenitor cells from human peripheral blood were cultured in SCF and IL-6 with or without IL-3 added at the initiation of cultures. A, CD25 (IL-2Rα), CD122 (IL-2Rβ), and CD132 (IL-2Rγ) expression was analyzed on FcεRI+/CD117+ cells at 7 weeks of culture. Representative FACS histogram (top panel) and summarized data for the expression level of CD25, CD122, and CD132 (geometric mean fluorescent intensity [MFI]) (bottom panel). N = 6 to 8 individual donors. B, Total number of FcεRI+/CD117+ huMCs at 7 weeks of culture. N = 8. C, Summarized data for the frequency of FcεRI+/CD117+ huMCs at 7 weeks. IL-3 was added or not at the initiation of cultures. N = 8. D, Each of the indicated cytokines was added at the culture initiation in addition to SCF and IL-6 and CD25 expression determined at 2 weeks ofculture. CD25 expression levels were normalized to the level of expression in the isotype control. N = 4. E, Bar charts show sCD25 levels in culture supernatants at different time points. N = 3. FACS, Fluorescence-activated cell sorting; IL-2Rα, IL-2 receptor-α; IL-2Rβ, IL-2 receptor-β; IL-2Rγ, IL-2 receptor-γ.
In the next series of experiments, IL-2, IL-3, IL-4, IL-5, IL-9, IL-10, IL-13, IL15, IL-33, or GM-CSF was added once at the initiation of culture containing SCF and IL-6. As shown in Fig 1, D, IL-3 was the only cytokine under these conditions that significantly increased the expression of CD25 by huMCs compared with cultures where none of these cytokines was added. Thus, huMCs from the cultures without IL-3 or huMCs grown with any of the other cytokines exhibited minimal CD25 expression. CD25 is known to be shed from CD25+ cells, and elevated levels of sCD25 are found in sera from patients with mastocytosis.11 As shown in Fig 1, E, with IL-3 added at day 0 of the culture, a significant increase in sCD25 in culture supernatants was detected at 2 weeks of culture and persisted through 7 weeks.
IL-3 upregulates CD25 expression at an early stage in huMC cultures
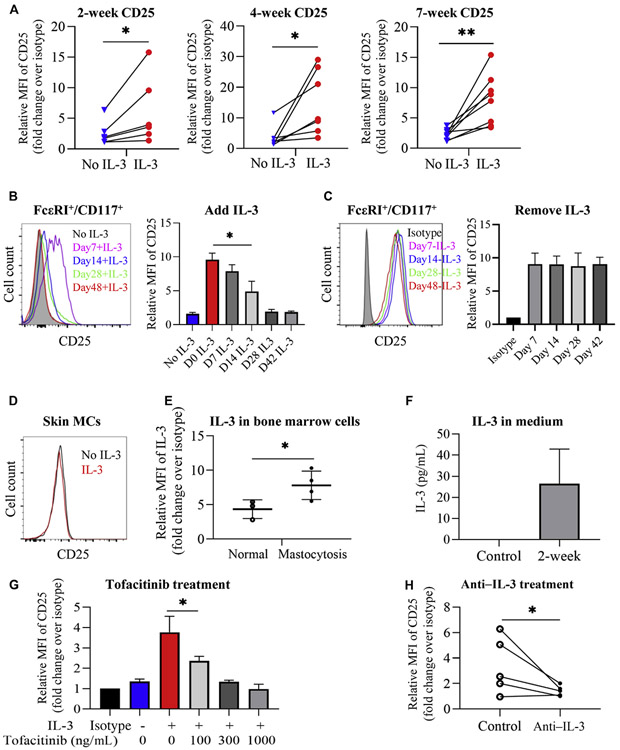
Following documentation that IL-3 upregulates CD25 on huMCs, we next followed the expression of CD25 on cultured cells over 7 weeks when IL-3 was added once at the initiation of cultures (day 0). As can be seen in Fig 2, A, CD25 upregulation was detected as early as 2 weeks and persisted throughout 7 weeks. To determine when IL-3 needed to be present in cultures to upregulate CD25, we next added IL-3 into huMC cultures at day 7, 14, 28, or 42 and analyzed CD25 expression in FcεRI/CD117+ cells at 7 weeks. Compared with cultures where IL-3 was not added, significant expression of CD25 on huMCs was observed when IL-3 was added at 7 days of culture (Fig 2, B). Some expression of CD25 was noted when IL-3 was added to cultures at day 14. However, addition of IL-3 at 28 or 42 days caused minimal to no upregulation of CD25 on huMCs.
FIG 2.
IL-3 upregulates CD25 expression at an early stage in huMC development. FcεRI+/CD117+ huMCs with IL-3 added at initiation of culture were examined at 7 weeks. A, Relative expression of CD25 on FcεRI+/CD117+ cells over 7 weeks. Lines connect paired cultures. N = 6-8. B, Representative FACS histograms showing CD25 expression at 7 weeks (left) and summarized CD25 MFI (right) when IL-3 was added as indicated. N = 3. C, Representative FACS histograms showing CD25 expression in huMCs (left) and average change in CD25 MFI. N = 3 (right). Media was replaced at the indicated time points to remove IL-3. D, Representative FACS histogram (N = 3) showing lack of CD25 expression on skin huMCs cultured for 4 weeks. E, Intracellular IL-3 expression in unstimulated human bone marrow cells from normal individuals or patients with mastocytosis. N = 3-4. F, Extracellular IL-3 concentration in medium of huMCs grown 2 weeks in the absence of IL-3. Control: culture medium. N = 4. G, Effect of tofacitinib, added at initiation of cultures together with IL-3, on CD25 expression. N = 3. H, Changes in CD25 expression induced by treatment with anti–IL-3 antibody (added at initiation of cultures) on huMCs cultured without IL-3. Control: without anti–IL-3. Lines connect paired cultures. N = 5. FACS, Fluorescence-activated cell sorting; MFI, mean fluorescent intensity.
Because in the culture system used, tissue culture fluid is never totally replaced, we next determined the expression of CD25 on huMCs at 7 weeks of culture when IL-3 was added at day 0 and the tissue culture media completely replaced at day 7, 14, 28, or 42 with SCF and IL-6-containing media without IL-3. As can be seen in Fig 2, C, CD25 expression by huMCs at 7 weeks was unaffected by removal of IL-3 as early as 7 days. Thus, the presence of IL-3 during the first 7 days of culture is sufficient to maintain the expression of CD25 by huMCs through 7 weeks of culture.
We next determined the expression of CD25 by mature huMCs obtained from human skin. As can be seen in Fig 2, D, no expression of CD25 was observed after 4 weeks, even in the presence of IL-3, similar to what was observed in late cultures of huMCs from MC precursors. Of related interest, addition of IL-3 to the human cell lines LAD2 and CD25+ HMC1.2 cells similarly did not upregulate CD25 (see Fig E2 in this article’s Online Repository at www.jacionline.org). These data in total suggest that IL-3 upregulates CD25 expression at an early stage in huMC development but does not affect CD25 expression on mature MCs.
We then examined IL-3 expression in bone marrow cells from healthy volunteers or patients with mastocytosis. Unstimulated bone marrow cells from patients with mastocytosis had an increased percentage of IL-3–positive cells (controls: mean ± SD, 10.6% ± 4.5% [n = 3] vs patients: mean ± SD, 31.4% ± 2.8% [n = 3]), and increased relative intracellular IL-3 expression (Fig 2, E; see Fig E3, A, in this article’s Online Repository at www.jacionline.org). In patients with mastocytosis, some of these IL-3+ cells appeared to be MCs with the highest levels associated with CD25+ MCs (Fig E3, B). We also found that LAD2 and HMC1.2 MC lines in culture produced intracellular IL-3, which was enhanced following the addition of 13-phorbol 12-myristate acetate and ionomycin (see Fig E4 in this article’s Online Repository at www.jacionline.org). To follow up on these observations, we then determined extracellular IL-3 levels in primary MC cultures at 2 weeks where IL-3 was not added and expression of CD25 was minimal (Fig 1, A). We detected low but measurable levels of extracellular IL-3 in the media of these cultures (Fig 2, F). It is possible that these low levels of IL-3 were sufficient to drive the low levels of CD25 expression seen in huMCs cultured in the absence of exogenous IL-3 and which were increased following the addition of recombinant IL-3 (Fig 1, D).
Tofacitinib or anti–IL-3 antibody blocks the effects of IL-3 on CD25 expression
IL-3 activates the Janus kinase (JAK)-signal transducer and activator of transcription 5 (STAT5) signaling pathway to stimulate proliferation and activation of target cells including T lymphocytes and MCs.23,24 To demonstrate the involvement of IL-3 in the induction of CD25 expression by huMCs, we examined the effects of simultaneous treatment of cultures with IL-3 and a JAK inhibitor, tofacitinib. As shown in Fig 2, G, tofacitinib inhibited the effects of IL-3 on CD25 upregulation in a dose-dependent fashion. We also determined whether anti–IL-3 could block the low levels of CD25 expression on huMCs observed in cultures when no exogenous IL-3 was added. As shown in Fig 2, H, IL-3 antibody attenuated background CD25 expression on huMCs. This is consistent with the conclusion that IL-3 drives CD25 expression in huMCs and that the lower levels of CD25 expression observed in huMC cultures in which no IL-3 was added was due to IL-3 production by cells within the cultures.
IL-2 does not induce STAT5 phosphorylation in CD25+ huMCs
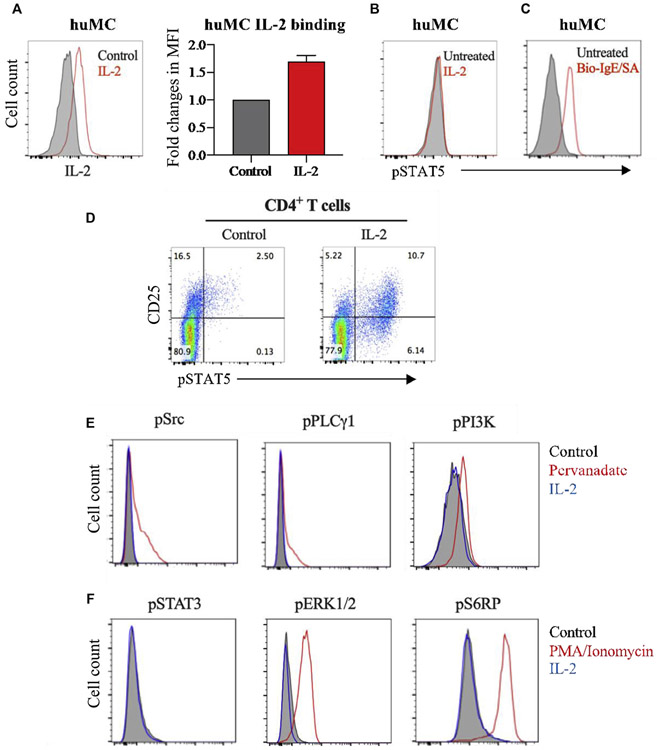
It is known that IL-2 through binding to the IL-2 receptor leads to STAT5 phosphorylation in CD25+ T cells.25 To test whether this is true in CD25+ huMCs, huMCs were exposed to biotinylated-IL-2. We found that IL-2 could bind to CD25+ huMCs (Fig 3, A), but STAT5 phosphorylation was not induced compared with the control (Fig 3, B). However, STAT5 phosphorylation was detected in huMCs following crosslinking of FcεRI (Fig 3, C). As a further control for this assay, IL-2 was verified to induce STAT5 phosphorylation in CD25+ T cells (Fig 3, D). These results are consistent with the conclusion that IL-2 binding to CD25 on huMCs does not activate the JAK-STAT5 pathway within these cells. We then explored other signaling pathways in CD25+ huMCs. On IL-2 treatment, there was no increased phosphorylation of Src, PLCγ1, PI3K, STAT3, ERK1/2, or S6K detectable in huMCs using this approach (Fig 3, E and F).
FIG 3.
IL-2 does not induce STAT5 phosphorylation in CD25+ huMCs. Enriched CD34+ progenitor cells from human peripheral blood were cultured in SCF and IL-6 with IL-3 added at culture initiation and examined at 7 weeks. A, huMCs were treated with 1 μg/mL biotinylated-IL-2 (Bio-IL-2) for 30 minutes and stained with PE-streptavidin to detect extracellular IL-2 binding to CD25. Average fold changes in MFI of bound IL-2 over untreated control (N = 3). B and C, Representative FACS histograms (N = 3) showing phosphorylated STAT5 (pSTAT5) intracellular staining in huMCs following IL-2 treatment (Fig 3, B) or biotinylated-IgE crosslinking with streptavidin (Fig 3, C). D, Representative FACS dot plots, showing STAT5 phosphorylation in human CD4+ T cells after IL-2 treatment. N = 3. E and F, Representative FACS histograms showing phosphorylation status of the indicated signaling proteins in huMCs after IL-2 treatment. Pervanadate (Fig 3, E) or PMA/ionomycin (Fig 3, F) was used as positive control. N = 2. FACS, Fluorescence-activated cell sorting; MFI, mean fluorescent intensity; PMA, phorbol 12-myristate 13-acetate.
CD25+ huMCs may serve as a reservoir of IL-2
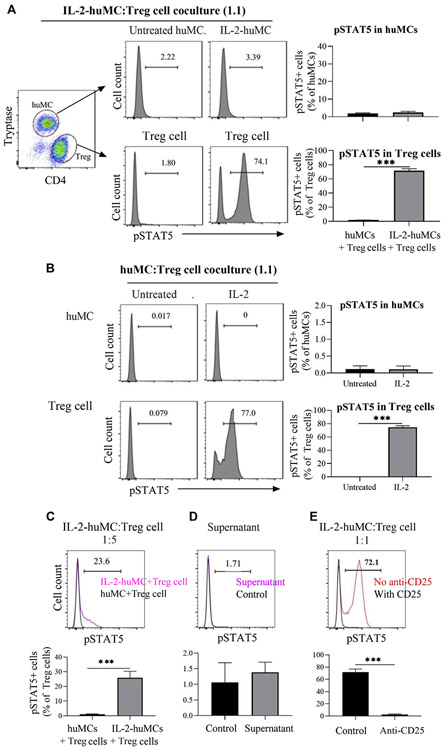
Given that CD25+ huMCs could bind IL-2, we next tested the possibility that CD25+ huMCs might serve as a reservoir of IL-2 and indirectly influence other cell types. Thus, we first preincubated CD25+ huMCs with IL-2 (IL-2-huMCs), and after removing unbound IL-2, we cocultured them with Treg cells at a 1:1 ratio. Coculture of Treg cells with IL-2-huMCs, but not with untreated huMCs, led to STAT5 phosphorylation in the Treg cells (Fig 4, A, lower panels). In contrast, IL-2-huMCs or huMCs in these cocultures did not show evidence of STAT5 activation (Fig 4, A, upper panels). Similar results were obtained when huMC:Treg cocultures were treated with IL-2 directly (Fig 4, B). Reducing the proportion of IL-2-huMCs to Treg cells in the cocultures to 1:5 resulted in diminished STAT5 phosphorylation in Treg cells (Fig 4, C), suggesting that the effect is dependent on the relative ratio of IL-2-huMCs to Treg cells. Treatment of Treg cells with the supernatant from the final wash of IL-2-huMCs did not induce STAT5 phosphorylation in Treg cells, demonstrating that Treg-cell activation was not due to residual amounts of free IL-2 remaining after the washes (Fig 4, D). To determine whether the activation of STAT5 in Treg cells observed in the cocultures with IL-2–pretreated huMCs was mediated through CD25 on Treg cells, Treg cells were pretreated with anti-CD25 before coincubation with IL-2-huMCs. Fig 4, E, shows that STAT5 phosphorylation in Treg cells was blocked by anti-CD25. Supernatant collected from IL-2-huMCs over 4 hours did not trigger STAT5 phosphorylation in Treg cells (see Fig E5 in this article’s Online Repository at www.jacionline.org), suggesting that IL-2 does not slowly dissociate from CD25+ huMCs and that cell proximity or contact may be needed to account for the effects on Treg cells. In total, these results are consistent with the conclusion that CD25+ huMCs bind IL-2, and although binding does not transduce productive signaling in MCs, when placed in coculture with Treg cells, IL-2 bound to CD25+ huMCs provide a source of IL-2 that results in STAT5 phosphorylation in the Treg cells.
FIG 4.
Coculture of CD25+ MCs and Treg cells. A, Representative FACS histograms showing STAT5 phosphorylation on huMCs (upper) and Treg cells (bottom), in cocultures of Treg cells with either untreated huMCs or huMCs pretreated with IL-2 (IL-2-huMCs) for 30 minutes. Bar graphs summarize the percentage of phospho-STAT5+ huMCs (upper) or Treg cells (lower). N = 3. B, HuMCs and Treg cells were mixed at 1:1 ratio and then incubated with or without IL-2 for 1 hour. N = 3. C, HuMCs were pretreated with IL-2 for 30 minutes and washed (IL-2-huMCs). IL-2-huMCs were then coincubated with Treg cells at 1:5 ratio for 1 hour and STAT5 phosphorylation in Treg cells shown as in Fig 4, A. N = 3. D, HuMCs were pretreated with IL-2 for 30 minutes and washed. Treg cells were incubated with 100 μL of supernatant from the final wash for 1 hour. FACS histogram of phospho-STAT5 in Treg cells (upper panel) and summarized percentage of phospho-STAT5+ Treg cells (lower panel). N = 3. E, FACS histogram showing STAT5 phosphorylation in Treg cells (upper panel) and summarized percentage of phospho-STAT5+ Treg cells (lower panel). N = 3. Treg cells were pretreated with anti-CD25 for 20 minutes and then coincubated with IL-2-huMCs at 1:1 ratio for 1 hour. FACS, Fluorescence-activated cell sorting.
DISCUSSION
IL-3 has been used in the culture of huMCs in conjunction with SCF and in the culture of murine MCs as a single cytokine to promote MC growth and differentiation,26-28 although it is not essential for the generation of either human or murine MCs.17 However, IL-3 is usually added at the beginning of huMC cultures because it may contribute to the development and proliferation of hematopoietic progenitor cells.16,26 In the experiments presented in this article, we analyzed the phenotype of huMCs developed from cultures initiated from CD34+ human peripheral progenitor cells in the presence or absence of exogenous IL-3 at the initiation of culture. Consistent with previous reports, IL-3 was not essential for MC development but increased MC numbers. In addition, we observed that IL-3 significantly upregulated the expression of CD25 on huMCs.
CD25 expression on huMCs has been reported in reactive MCs in inflamed tissues14,15 and is expressed in most bone marrow MCs from patients with both indolent and advanced SM but with lower to absent levels in some with MC leukemia and well-differentiated SM.9-12 CD25 is usually absent from MCs from normal/reactive bone marrow.9,13 Thus, CD25 serves as a minor diagnostic criterion in the diagnosis of SM. However, the factors responsible for the expression of CD25 and the function of CD25 on huMCs are largely unknown. Therefore, we investigated whether cytokines such as IL-2, IL-3, IL-4, IL-5, IL-9, IL-10, IL-13, IL-15, IL-33, or GM-CSF might affect CD25 expression by huMCs, and found that IL-3 was the only cytokine that effectively caused CD25 upregulation. In this study, we also found that sCD25 levels, which are elevated in the blood of patients with mastocytosis, were increased in the culture supernatants from human MC cultures with IL-3 added at the culture initiation.
We also found that IL-3 upregulates CD25 expression at an early stage of huMC differentiation but not in mature MCs, because CD25 upregulation was observed only when IL-3 was added in the first week of culture, even if it was removed a week later, and not when added at later times. Similarly, IL-3 did not upregulate CD25 expression in mature MCs isolated from human skin or in the human MC lines. Although we have no direct evidence, this could help explain the observation that huMCs in patients with mastocytosis are often CD25+. This is reinforced by the identification of higher levels of IL-3 in marrow from patients with mastocytosis.
The effects of IL-3 on cell growth and differentiation are associated with the activation of JAK signaling pathway.29 In this study, we found that IL-3–induced CD25 upregulation on huMCs was blocked by tofacitinib. Similarly, neutralizing IL-3 with anti–IL-3 attenuated huMC CD25 expression, further implicating IL-3 on CD25 upregulation. Although we have no direct evidence, the identification of higher levels of IL-3 in the marrow of patients with mastocytosis might help to explain why huMCs in such patients are often CD25+.
IL-2 interacting with CD25 leads to activation of the JAK-STAT5 axis in T lymphocytes.25,30 However, IL-2 addition did not lead to STAT5 phosphorylation in CD25+ huMCs. This result agreed with a similar finding in murine MCs.31 The explanation for this lack of response is believed to relate to the lack of expression of the IL-2 receptor beta chain (Fig 1, A), which, together with the gamma subunit, is critical for IL-2 signaling and high-affinity binding.32,33
Increasing evidence suggest the importance of the crosstalk between MCs and Treg cells in immune responses. Treg cells can modulate inflammatory cytokine production by MCs activated through an IgE-independent mechanism in murine and human systems.34-36 Conversely, MCs can also stimulate or inhibit Treg cells in murine systems37 and induce Treg-cell expansion via varied mediators and surface molecules, such as OX40-OX40L.38-40 As an example, MCs were shown to support IL-2–mediated activation of Treg cells for suppression of chronic inflammation in a mouse model of dermatitis.41 We find that CD25+ huMCs bound to IL-2 can induce STAT5 phosphorylation via CD25 in Treg cells not directly exposed to IL-2. These observations are most consistent with the conclusion that IL-2, although unable to signal in MCs, can bind to CD25+ huMCs, and thus serve as a reservoir of IL-2, which then can cross-stimulate other CD25+ cell types. Reminiscent of this process, it has been proposed that dendritic cell CD25 can capture IL-2 released in the synaptic cleft for presentation in trans to adjacent T cells for activation.33
However, the impact of IL-2 binding by MCs on lymphocyte function in mastocytosis remains speculative. Although CD4+ lymphocytes are increased in patients with mastocytosis, and Treg cells enhance the production of IL-6 by MCs, which is known to be elevated in mastocytosis sera, there are no studies to our knowledge that examine Treg-cell function in mastocytosis nor whether such cells may in some way play a role in the pathogenesis of mastocytosis.38,42,43 Although data are similarly lacking on natural killer–cell function, type 2 innate lymphoid cell (ILC2) populations within those who have mastocytosis are significantly higher and correlate with skin symptoms such as itch.44 Whether the binding of IL-2, potentially with low affinity, within an expanded MC population would have a physiological impact remains to be determined. Perhaps this report will stimulate further research in this area.
CONCLUSIONS
In summary, we did find that IL-3 among the cytokines tested uniquely upregulated CD25 expression on huMCs when presented early in development, but not on mature huMCs. We also found that CD25 is shed by CD25+ huMCs, providing another source of sCD25 found in the blood of patients with MC proliferative disorders and possibly in other general inflammatory conditions. However, perhaps because of the lack of expression of the beta chain of the IL-2 receptor in CD25+ huMCs, IL-2 had no demonstrable direct effects on induction of signaling pathways within such CD25+ huMCs. CD25+ huMCs bind IL-2, and we present evidence that such binding would allow CD25+ huMCs to act perhaps as a temporary reservoir of IL-2.
Supplementary Material
Key messages.
IL-3 upregulates cell membrane CD25 expression on huMCs and CD25+ huMCs shed sCD25.
IL-2 does not induce STAT5 phosphorylation in CD25+ huMCs.
CD25+ huMCs are capable of binding IL-2 and thus may provide a reservoir of IL-2 available for subsequent interaction with and activation of other cell types.
Acknowledgments
This work was supported by the Division of Intramural Research, National Institute of Allergy and Infectious Diseases, National Institutes of Health.
We thank Dr Melody Carter, Dr Hirsh Komarow, Dr Calman Prussin, and the MCBS nursing staff for the provision of patient materials.
Abbreviations used
- CD
Cluster of differentiation
- FcεRI
High-affinity receptor for IgE
- huMC
Human mast cell
- JAK
Janus kinase
- MC
Mast cell
- SCF
Stem cell factor
- sCD25
Soluble CD25
- SM
Systemic mastocytosis
- STAT5
Signal transducer and activator of transcription 5
- Treg
Regulatory T
Footnotes
Disclosure of potential conflict of interest: The authors declare that they have no relevant conflicts of interest.
REFERENCES
- 1.Kalesnikoff J, Galli SJ. New developments in mast cell biology. Nat Immunol 2008;9:1215–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Olivera A, Beaven MA, Metcalfe DD. Mast cells signal their importance in health and disease. J Allergy Clin Immunol 2018;142:381–93. [DOI] [PubMed] [Google Scholar]
- 3.Falduto GH, Pfeiffer A, Luker A, Metcalfe DD, Olivera A. Emerging mechanisms contributing to mast cell-mediated pathophysiology with therapeutic implications. Pharmacol Ther 2020;220:107718. [DOI] [PubMed] [Google Scholar]
- 4.da Silva EZ, Jamur MC, Oliver C. Mast cell function: a new vision of an old cell. J Histochem Cytochem 2014;62:698–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Elieh Ali Komi D, Wohrl S, Bielory L. Mast cell biology at molecular level: a comprehensive review. Clin Rev Allergy Immunol 2020;58:342–65. [DOI] [PubMed] [Google Scholar]
- 6.Schernthaner GH, Hauswirth AW, Baghestanian M, Agis H, Ghannadan M, Worda C, et al. Detection of differentiation- and activation-linked cell surface antigens on cultured mast cell progenitors. Allergy 2005;60:1248–55. [DOI] [PubMed] [Google Scholar]
- 7.Hauswirth AW, Florian S, Schernthaner GH, Krauth MT, Sonneck K, Sperr WR, et al. Expression of cell surface antigens on mast cells: mast cell phenotyping. Methods Mol Biol 2006;315:77–90. [DOI] [PubMed] [Google Scholar]
- 8.Jordan JH, Schernthaner GH, Fritsche-Polanz R, Sperr WR, Fodinger M, Chott A, et al. Stem cell factor-induced bone marrow mast cell hyperplasia mimicking systemic mastocytosis (SM): histopathologic and morphologic evaluation with special reference to recently established SM-criteria. Leuk Lymphoma 2002;43:575–82. [DOI] [PubMed] [Google Scholar]
- 9.Sotlar K, Horny HP, Simonitsch I, Krokowski M, Aichberger KJ, Mayerhofer M, et al. CD25 indicates the neoplastic phenotype of mast cells: a novel immunohistochemical marker for the diagnosis of systemic mastocytosis (SM) in routinely processed bone marrow biopsy specimens. Am J Surg Pathol 2004;28:1319–25. [DOI] [PubMed] [Google Scholar]
- 10.Akin C, Scott LM, Kocabas CN, Kushnir-Sukhov N, Brittain E, Noel P, et al. Demonstration of an aberrant mast-cell population with clonal markers in a subset of patients with "idiopathic" anaphylaxis. Blood 2007;110:2331–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Akin C, Schwartz LB, Kitoh T, Obayashi H, Worobec AS, Scott LM, et al. Soluble stem cell factor receptor (CD117) and IL-2 receptor alpha chain (CD25) levels in the plasma of patients with mastocytosis: relationships to disease severity and bone marrow pathology. Blood 2000;96:1267–73. [PubMed] [Google Scholar]
- 12.Dasilva-Freire N, Mayado A, Teodosio C, Jara-Acevedo M, Alvarez-Twose I, Matito A, et al. Bone marrow mast cell antibody-targetable cell surface protein expression profiles in systemic mastocytosis. Int J Mol Sci 2019;20:552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cherian S, McCullouch V, Miller V, Dougherty K, Fromm JR, Wood BL. Expression of CD2 and CD25 on mast cell populations can be seen outside the setting of systemic mastocytosis. Cytometry B Clin Cytom 2016;90:387–92. [DOI] [PubMed] [Google Scholar]
- 14.Regauer S, Beham-Schmid C. Benign mast cell hyperplasia and atypical mast cell infiltrates in penile lichen planus in adult men. Histol Histopathol 2014;29:1017–25. [DOI] [PubMed] [Google Scholar]
- 15.Valent P, Akin C, Hartmann K, Nilsson G, Reiter A, Hermine O, et al. Mast cells as a unique hematologic lineage and cell system: from Paul Ehrlich to precision medicine concepts. Theranostics 2020;10:10743–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yin Y, Bai Y, Olivera A, Desai A, Metcalfe DD. An optimized protocol for the generation and functional analysis of human mast cells from CD34(+) enriched cell populations. J Immunol Methods 2017;448:105–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lantz CS, Boesiger J, Song CH, Mach N, Kobayashi T, Mulligan RC, et al. Role for interleukin-3 in mast-cell and basophil development and in immunity to parasites. Nature 1998;392:90–3. [DOI] [PubMed] [Google Scholar]
- 18.Yin Y, Mitson-Salazar A, Wansley DL, Singh SP, Prussin C. Rapamycin preferentially inhibits human IL-5(+) TH2-cell proliferation via an mTORC1/S6 kinase-1-dependent pathway. J Allergy Clin Immunol 2017;139:1701–4.e10. [DOI] [PubMed] [Google Scholar]
- 19.Kirshenbaum AS, Akin C, Wu Y, Rottem M, Goff JP, Beaven MA, et al. Characterization of novel stem cell factor responsive human mast cell lines LAD 1 and 2 established from a patient with mast cell sarcoma/leukemia; activation following aggregation of FcepsilonRI or FcgammaRI. Leuk Res 2003;27:677–82. [DOI] [PubMed] [Google Scholar]
- 20.Radinger M, Smrz D, Metcalfe DD, Gilfillan AM. Glycogen synthase kinase-3beta is a prosurvival signal for the maintenance of human mast cell homeostasis. J Immunol 2011;187:5587–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McHale C, Mohammed Z, Deppen J, Gomez G. Interleukin-6 potentiates FcepsilonRI-induced PGD2 biosynthesis and induces VEGF from human in situmatured skin mast cells. Biochim Biophys Acta Gen Subj 2018;1862:1069–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yin Y, Mitson-Salazar A, Prussin C. Detection of intracellular cytokines by flow cytometry. Curr Protoc Immunol 2015;110:6.24.1–18. [DOI] [PubMed] [Google Scholar]
- 23.Shelburne CP, McCoy ME, Piekorz R, Sexl VV, Gillespie SR, Bailey DP, et al. Stat5: an essential regulator of mast cell biology. Mol Immunol 2002;38:1187–91. [DOI] [PubMed] [Google Scholar]
- 24.Kohno M, Yamasaki S, Tybulewicz VL, Saito T. Rapid and large amount of autocrine IL-3 production is responsible for mast cell survival by IgE in the absence of antigen. Blood 2005;105:2059–65. [DOI] [PubMed] [Google Scholar]
- 25.Chinen T, Kannan AK, Levine AG, Fan X, Klein U, Zheng Y, et al. An essential role for the IL-2 receptor in Treg cell function. Nat Immunol 2016;17:1322–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shimizu Y, Matsumoto K, Okayama Y, Sakai K, Maeno T, Suga T, et al. Interleukin-3 does not affect the differentiation of mast cells derived from human bone marrow progenitors. Immunol Invest 2008;37:1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bandara G, Metcalfe DD, Kirshenbaum AS. Growth of human mast cells from bone marrow and peripheral blood-derived CD34(+) pluripotent hematopoietic cells. Methods Mol Biol 2015;1220:155–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ohmori K, Luo Y, Jia Y, Nishida J, Wang Z, Bunting KD, et al. IL-3 induces basophil expansion in vivo by directing granulocyte-monocyte progenitors to differentiate into basophil lineage-restricted progenitors in the bone marrow and by increasing the number of basophil/mast cell progenitors in the spleen. J Immunol 2009;182:2835–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shelburne CP, McCoy ME, Piekorz R, Sexl V, Roh KH, Jacobs-Helber SM, et al. Stat5 expression is critical for mast cell development and survival. Blood 2003;102:1290–7. [DOI] [PubMed] [Google Scholar]
- 30.Burchill MA, Yang J, Vogtenhuber C, Blazar BR, Farrar MA. IL-2 receptor beta-dependent STAT5 activation is required for the development of Foxp3+ regulatory T cells. J Immunol 2007;178:280–90. [DOI] [PubMed] [Google Scholar]
- 31.Deho L, Leoni C, Brodie TM, Montagner S, De Simone M, Polletti S, et al. Two functionally distinct subsets of mast cells discriminated by IL-2-independent CD25 activities. J Immunol 2014;193:2196–206. [DOI] [PubMed] [Google Scholar]
- 32.Spolski R, Li P, Leonard WJ. Biology and regulation of IL-2: from molecular mechanisms to human therapy. Nat Rev Immunol 2018;18:648–59. [DOI] [PubMed] [Google Scholar]
- 33.Zelante T, Fric J, Wong AY, Ricciardi-Castagnoli P. Interleukin-2 production by dendritic cells and its immuno-regulatory functions. Front Immunol 2012;3:161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Curotto de Lafaille MA, Lafaille JJ. CD4(+) regulatory T cells in autoimmunity and allergy. Curr Opin Immunol 2002;14:771–8. [DOI] [PubMed] [Google Scholar]
- 35.Elieh-Ali-Komi D, Cao Y. Role of mast cells in the pathogenesis of multiple sclerosis and experimental autoimmune encephalomyelitis. Clin Rev Allergy Immunol 2017;52:436–45. [DOI] [PubMed] [Google Scholar]
- 36.Su W, Fan H, Chen M, Wang J, Brand D, He X, et al. Induced CD4+ forkhead box protein-positive T cells inhibit mast cell function and established contact hypersensitivity through TGF-beta1. J Allergy Clin Immunol 2012;130:444–52.e7. [DOI] [PubMed] [Google Scholar]
- 37.Gri G, Piconese S, Frossi B, Manfroi V, Merluzzi S, Tripodo C, et al. CD4+CD25+ regulatory T cells suppress mast cell degranulation and allergic responses through OX40-OX40L interaction. Immunity 2008;29:771–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ganeshan K, Bryce PJ. Regulatory T cells enhance mast cell production of IL-6 via surface-bound TGF-beta. J Immunol 2012;188:594–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Elieh Ali Komi D, Grauwet K. Role of mast cells in regulation of T cell responses in experimental and clinical settings. Clin Rev Allergy Immunol 2018;54:432–45. [DOI] [PubMed] [Google Scholar]
- 40.Morita H, Arae K, Unno H, Miyauchi K, Toyama S, Nambu A, et al. An interleukin-33-mast cell-interleukin-2 axis suppresses papain-induced allergic inflammation by promoting regulatory T cell numbers. Immunity 2015;43:175–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hershko AY, Suzuki R, Charles N, Alvarez-Errico D, Sargent JL, Laurence A, et al. Mast cell interleukin-2 production contributes to suppression of chronic allergic dermatitis. Immunity 2011;35:562–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nedoszytko B, Lange M, Sokolowska-Wojdylo M, Renke J, Trzonkowski P, Sobjanek M, et al. The role of regulatory T cells and genes involved in their differentiation in pathogenesis of selected inflammatory and neoplastic skin diseases, part II: the Treg role in skin diseases pathogenesis. Postepy Dermatol Alergol 2017;34:405–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kulinski JM, Eisch R, Young ML, Rampertaap S, Stoddard J, Monsale J, et al. Skewed lymphocyte subpopulations and associated phenotypes in patients with mastocytosis. J Allergy Clin Immunol Pract 2020;8:292–301.e2. [DOI] [PubMed] [Google Scholar]
- 44.van der Ploeg EK, Hermans MAW, van der Velden VHJ, Dik WA, van Daele PLA, Stadhouders R. Increased group 2 innate lymphoid cells in peripheral blood of adults with mastocytosis. J Allergy Clin Immunol 2021;147:1490–6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.