Abstract
Clinical presentation of echinococcosis in paediatric population is varied and depends on the site of involvement. The present study was planned to analyse the clinical presentations and therapeutic options for management of echinococcosis in children admitted to a tertiary care hospital. Medical records of children with echinococcosis was reviewed retrospectively over a period of 3 year and 6 months. Demographic data, clinical presentation, management and outcome data were collected and analysed. During the study period, 14 children with hydatid disease were admitted to the hospital. The frequency was higher in male (71.4%) compared to females (28.6%). Liver was found to be the commonest site for hydatid cyst with 78% of all cases had hydatid cyst localised to liver. In half of all cases liver was the only site of involvement. Both liver and lung were involved in 21.4% cases and one patient (7.1%) had pelvic hydatid cyst in addition to liver involvement. Two patients (14.3%) had only pulmonary involvement and one (7.1%) patient had a hydatid cyst in common bile duct. Right upper abdominal pain was the most common presentation (78.5%) with cyst in liver. Cough, breathing difficulty, jaundice was observed in 28.5%, 21.4% and 7.1% of all patients respectively. All children were managed with a combination of surgical and medical therapy. Right upper abdominal pain and chronic cough were the common clinical presentation of hydatid cyst with hepatic and pulmonary involvement respectively. Presence of such chronic symptoms would raise the clinical suspicion of hydatid disease in endemic regions.
Keywords: Hydatid cyst, Laparotomy, Thoracotomy, Albendazole
Introduction
Hydatid disease in human is a zoonotic parasitic infection caused by tapeworm Echinococcus. Few species of echinococcus namely, ehinococcus granulosus, echinococcus multilocularis and echinococcus vogeli cause disease in human. The parasite requires two hosts to complete its life cycle. Dogs, other canines and domestic carnivorous animals are the primary hosts. Sheep, horse and other domestic animals are the secondary hosts (Ozturk et al. 2007; Jairajpuri et al. 2012). Humans are infected by ingesting ova from soil or water contaminated by the feces of dogs (Gulalp et al. 2007; Ozturk et al. 2007; Jairajpuri et al. 2012). After ingestion, the eggs of the parasite hatch in the duodenum, penetrate mucosa and venules and reach the liver through portal vein, where they develop into cysts. The larva can pass through the liver to reach the right side of heart and lungs and may develop into pulmonary cysts. Liver and lung are the commonest organs affected in hydatid diseases (Bhutani and Kajal 2018). Cysts can also be found in other viscera following a hematogenous transmission (Jairajpuri et al. 2012; Bhutani and Kajal 2018).
Hydatid cyst is a chronic, latent disease that often is asymptomatic. It may be found during routine clinical examination and serologic, radiographic, or ultrasonographic screening (Kurt et al. 2003; Dirican et al. 2010). The clinical signs and symptoms of hydatid cysts depend on location, size, relation to adjacent organs, and complications such as cyst rupture. E. granulosus is endemic in South America, Eastern Europe, Russia, the Middle East, and China. Foci of hydatid disease also exist in India where the highest prevalence is reported in Andhra Pradesh and Tamil Nadu than in other parts of the country (Bhutani and Kajal 2018). Rural areas with agricultural background, low socioeconomic status, regional climate, unhygienic animal slaughtering increases the incidence of disease (Jairajpuri et al. 2012; Bhutani and Kajal 2018). The number of surgical cases may actually represent only a fraction of the total infected hosts. In the present study, we describe the spectrum of clinical presentation and management of hydatid disease in children admitted at a tertiary care hospital in West Bengal.
Patients and Methods
This retrospective study was performed in Department of Paediatric surgery at Nilratan Sircar Medical college, West Bengal. Paediatric patients between 3 and 15 years of age, admitted to the hospital between October 2011 to April 2015 with a diagnosis of hydatid disease were included in the study.
The demographic data including age, sex, history of contact with dogs or domestic animals, clinical data, paraclinical data including investigations, imaging and therapeutic data were collected from review of medical records. Clinical data included the site of cyst, clinical presentations and outcome. All patients were managed as per standard guidelines. Evaluations included routine blood, urine, renal function, Liver functions, coagulation profile, ELISA, imaging studies like abdominal ultrasonography, CT scan of abdomen, Chest X ray, CT scan of Thorax. In selected cases Magnetic Resonance Cholangiopancreatography (MRCP), and Urologic evaluation (Micturating cystourethrogram, Magnetic Resonance Urography) was carried out.
Statistical analysis was done using SPSS version 21.0 software. Baseline data were expressed using descriptive statistics. Mean and standard deviations were used to express continuous variables. Data related to clinical presentations and outcomes were expressed as percentage of total cases.
Results
During the study period, 14 children with hydatid cyst were admitted to hospital. Age group of the study group ranged from 3 to 15 years with a median age of 5.75 years at presentation. The study group included 10 males (71.4%) and 4 females (28.6%). The average age at presentation was 6.35 ± 2.83 years. Twelve children (85.7%) were from rural areas. A history of contact with dog or domestic animals was present in nine (64.3%) children.
Right upper quadrant abdominal pain (78.5%) and loss of appetite (78.5%) were the most common presenting symptoms in the study cohort. Some degree of fever and fatiguability was present in 9 (64.3%) and 6 (42.8%) of children respectively. Four children (28.6%) had a history of chronic cough and two (14.2%) of them had breathlessness at time of hospitalisation. One child (7.2%) presented with jaundice and pruritus. Right upper quadrant pain (11 out of 11, 100%) was the predominant complaint in children with hepatic cyst and chronic cough (4 out of 5, 80%) was the most common presenting complain in children with lung cyst (Table 1).
Table 1.
Presenting features of the study cohort (n = 14)
| Clinical Symptoms | Number (%) |
|---|---|
| Right upper abdominal Pain | 11 (78.5%) |
| Fever | 9 (64.3%) |
| Fatigue | 6 (42.8%) |
| Loss of apetite | 11 (78.5%) |
| Flank pain | 2 (14.3%) |
| Vomiting | 4 (28.6%) |
| Cough | 4 (28.6%) |
| Breathing difficulty | 2 (14.3%) |
| Jaundice | 1 (7.1%) |
| Pruritus | 1 (7.1%) |
The site of involvement of the cysts was identified using various imaging studies. Plain radiographs, Ultrasonography, Computed tomography were used to localise the site of cysts. Majority of the children had a cyst in the liver. Seven out of 14 (50%) children had a cyst located solely in the liver (Table 2). Three children (21.4%) had both liver and lung cyst. Lung was the only site of involvement in 2 (14.2%) children. The average diameter of cysts was 8.64 ± 1.91 cm (5.9–12.9 cm) and 6.1 ± 0.84 cm (4.9–9.3 cm) in liver and lung respectively (Figs. 1,2). One patient was found to have cyst in right lobe of liver and posterolateral wall of urinary bladder. In the same child, Pelvic ultrasonography revealed an echogenic mass in outer wall of urinary bladder. No significant reflux was detected in micturating cystourethrogram and MR urogram revealed an extrinsic mass of size 5.4 cm × 3.2 cm in the posterolateral wall of urinary bladder compressing the left ureter. In a patient with a hepatic hydatid cyst with features of obstructive jaundice, the diagnosis of type 1 choledochal cyst was confirmed after magnetic resonance cholangiopancreatography (MRCP) and a hydatid cyst of size 2.3 × 1.8 cm within the choledochal cyst was found intraoperatively.
Table 2.
Site of involvement of hydatid cyst in the study cohort (n = 14)
| Site of involvement | Number (%) |
|---|---|
| Liver | 7 (50%) |
| Lung | 2 (14.3%) |
| Both liver and lung | 3 (21.4%) |
| Both liver and urinary bladder | 1 (7.2%) |
| Common bile duct | 1 (7.2%) |
Fig. 1.
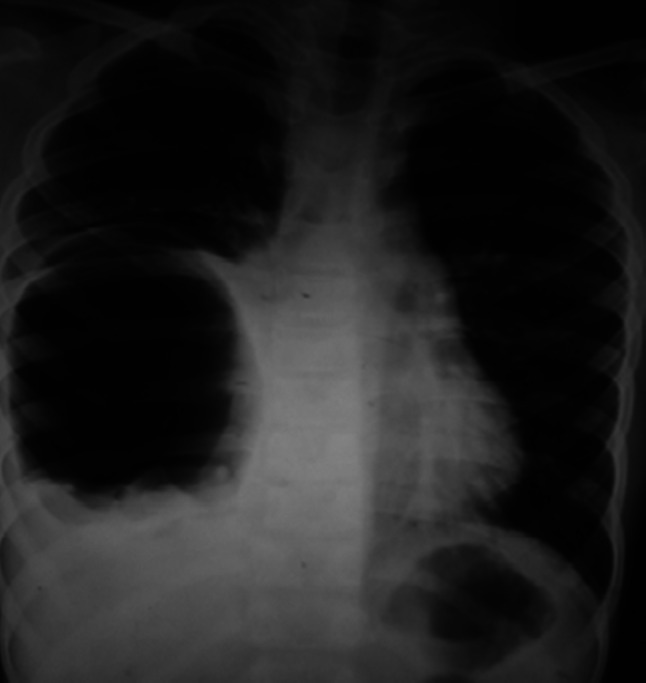
Chest radiograph showing a cavitary lesion in right lower lobe of lung
Fig. 2.

CT scan showing cystic SOL in the liver with septations seen inside the cyst
Surgical excision of cysts was done in all cases. Prior to surgical excision, a course of albendazole (10 mg/kg/day for 3–6 weeks) was given to all cases to sterilise the cysts. Open Laparotomy and thoracotomy were used for surgical excision of cysts involving liver and lung respectively. During surgery special care was taken to avoid any spillage of hydatid fluid, including packing the area with povidone iodine-soaked mops, aspiration of some of the hydatid fluid, instillation of a scolicidal agent (hypertonic saline) into the cyst.
Hepatic cysts were managed with excision of the endocyst containing the daughter cysts. The residual pericystic cavity were partially excised, filled with 3% NS, and closed or obliterated with multiple purse string sutures or omentoplasty. Complete cystectomy could be done in 2 (18.1%) cases. Oral Albendazole was administered for at least 6 weeks during postoperative period to reduce the chances of recurrence.
Lung cysts were managed with excision of endocyst, closure of broncho-pleural fistula and placement of chest drain. For combined liver and lung cysts, initial thoracotomy was done with excision of endocyst, suture ligation of bronchopleural fistulae and insertion of intercostal chest drain. It was followed by a course of albendazole for 6 weeks before excision of liver cysts. In combined liver and pelvic cysts, both cysts were excised in the same setting.
Hydatid cyst within a common bile duct was managed with exploratory laparotomy, excision of the hydatid cyst, excision of choledochal cyst, hepatico-duodenostomy. The hydatid cyst was found intra operatively after incision of the choledochal cyst and subsequently proven by biopsy.
Computed tomography was routinely used in all cases during follow up period to identify any recurrence. Majority of the children remained asymptomatic during follow up period. One child with hepatic cyst had a recurrence, and was subsequently managed by excision and partial cystectomy. The cysts in echinococcosis are often slow growing, which warrants a need for long term follow up to detect any future recurrence.
Discussion
Hydatid disease or echinococcosis is a zoonotic parasitic infection caused by larval form of echinococcus. Echinococcus belongs to cestode (tapeworm) and few of the species causes infection in humans (Jairajpuri et al. 2012). Echinococcus granulosus, echinococcus multilocularis are the predominant species which cause cystic hydatidosis and alveolar hydatid cysts respectively (Jairajpuri et al. 2012; Bhutani and Kajal 2018). Dogs, wild canines and other domestic carnivorous animals are definitive hosts. Sheep, cattle and horses are the intermediate hosts. Humans are the accidentally infected through feco-oral route or after coming in contact with the definitive hosts or after consuming vegetables or water contaminated with eggs of echinococcus. The eggs hatch in intestine, penetrate intestinal mucosa, invade the venules, and through the portal vein reach the liver, where they develop into cysts. The cyst gradually grows in size and can remain asymptomatic for a prolonged period (Gupta et al. 2014; Bhutani and Kajal 2018). The larva may pass through the liver and enter into right side of heart and lungs and may develop into hydatid cysts. Rarely the larva can be transmitted to other viscera through hematogenous route and develop into cysts.
Liver is the most common site for development of cysts in echinococcosis (Gupta et al. 2014; Bhutani and Kajal 2018). Some studies indicate lung to be affected more commonly particularly in pediatric population. A study from Tunisia revealed that hydatid cysts were primarily located in the lung (61.8%) in children and the liver was the second common site (34.85%) (M'rad et al. 2012). In a similar study on children in the northwest of Iran, hydatid cyst in lungs were reported in 67.8% of patients. (Aslanabadi et al. 2013). A recent retrospective study by Gupta et al. have revealed liver to be commonest site of involvement of cysts in Indian children (Gupta et al. 2014). Lung tissue remain more compressible allowing the growth of cyst for a prolonged period before onset of clinical symptoms. A cyst in the liver can also remain asymptomatic for a long time. Pain in the Right hypochondrium is the most common clinical feature of hepatic hydatid cyst. Other clinical features may include fatigue, loss of appetite, fever, vomiting, hepatomegaly, jaundice, portal hypertension (Daali et al. 2001). Complications may occur when cysts rupture and involve nearby viscera. Cysts may rupture spontaneously or following trauma, surgery. Rupture into biliary tree may present with biliary colic and jaundice. Rupture into peritoneal cavity may present with acute abdomen (safioleas et al. 2006). Pulmonary disease may present with cough, breathlessness, fever, chest pain, but can remain asymptomatic and detected accidentally. The cysts may rupture into bronchial tree and present with hydatidoptysis. A rare case of cyst involving urinary bladder was observed in the current study. Primary Hydatid cyst involving urinary tract is rare and reported incidence is around 2–4%. Bladder involvement in hydatid disease has been reported in few case reports in adults (Kirkland et al. 1966; Aggarwal and Bansal 2014). Though few authors have reported renal involvement in hydatid disease, bladder involvement has not been reported in pediatric population. Another rare presentation in the current study was a hydatid cyst inside a choledochal cyst. Few cases in children have been described previously (Sarkar et al. 2013).
Management of hydatid cysts is primarily surgical (Gupta et al. 2014; Bhutani et al. 2018). A course of antihelminthic drug (Albendazole or Mebendazole) may be tried prior to surgery. An older cyst tends to produce more daughter cysts and it remains as an important factor for recurrence of cyst. Medical therapy may reduce the risk of dissemination and may prevent the chances of recurrence. Complete removal of cyst avoiding any spillage of contents (Cystectomy) is the procedure of choice. If the cyst cannot be removed completely, fluid content of the cyst may be aspirated, followed by injection of scolicidal agent into the cyst and re-aspiration of content. The laminated membrane of the cyst should be excised, pouch is irrigated with scolicidal agent and deroofing of the cyst should be carried out. The pericystic cavity should be obliterated by means of purse-string sutures starting from the deepest level (capitonnage) or by omentopexy to prevent secondary infection (Gupta et al. 2014). The operating team should take care to avoid spillage of any contents, as inadvertent implantation of scolices or daughter cysts may result in recurrence (Kjossev et al. 2002).
Percutaneous-aspiration-injection and re-aspiration (PAIR) technique has been a preferred therapy for hydatid liver cysts (Giorgio et al. 2009a, b). Laparoscopic techniques can also be used as an alternative method and recently being popular (Giorgio et al. 2009a, b; Tuxun et al. 2014). Lung conserving surgery is the treatment of choice for pulmonary hydatid cysts. A posterolateral or anterolateral thoracotomy with transpleural approach is commonly performed. Radical procedures are rarely necessary. Median sternotomy is performed in case of bilateral involvement. Recent reports suggest the need for anatomical resection (lobectomy, Pneumonectomy) in less than 10% of all cases (Kavukcu et al. 2006; Bagheri et al. 2011). Removal of the cyst along with cappitonage of the residual cavity can be performed in majority of cases. Some authors have suggested that open surgery, in which the cyst membrane is removed and bronchial openings into the cyst cavity are closed as an appropriate approach for pulmonary hydatid cysts. Recent reports suggest that cappitonage after cystotomy is associated with lesser complications and better post-operative outcome as compared to no cappitonage even in giant cysts (Bilgin et al. 2004; Kosar et al. 2006; Nabi et al. 2010). Lung resection should be restricted even in the complicated cysts, especially for children and young adults, because the affected lung parenchyma in children has a great capacity for healing (Topçu et al. 2000; Kavukcu et al. 2006).
Conclusion
The present study describes liver as the most common organ affected in children with hydatid disease. Right upper quadrant pain and cough were the commonest clinical features observed with hepatic and pulmonary cysts respectively. The presence of such chronic symptoms would raise the clinical suspicion of hydatid disease particularly in endemic areas. In addition to the usual sites, urinary bladder, biliary tract may be a rare site of involvement of hydatid cysts.
Acknowledgements
We acknowledge the immense help received from the Department of Paediatric surgery, Nilratan Sircar Medical college during acquisition of data during the study period.
Author contributions
A.P.: Conceptualized, designed the study and collected data. B.M.: Collected data and critical input in preparation of draft. D.N.: Analyzed data and prepared the initial draft. A.D.: Supervision and revision of draft. All authors approved the final manuscript.
Funding
None.
Declarations
Conflict of interest
The authors declare that they have no conflict of interest. Consent for Publication: All authors agree for publication.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Aggarwal S, Bansal A. Laparoscopic management of renal hydatid cyst. J Soc Laparoendosc Surgons. 2014;18(2):361–366. doi: 10.4293/108680813X13753907291396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aslanabadi S, Zarrintan S, Abdoli-Oskouei S, Salehpour F, Zarrintan A, Beheshtirouy S, Abdollahi H, Badebarin D. Hydatid cyst in children: a 10-year experience from Iran. Afr J Paediatr Surg. 2013;10(2):140–144. doi: 10.4103/0189-6725.115040. [DOI] [PubMed] [Google Scholar]
- Bagheri R, Haghi SZ, Amini M, Fattahi AS, Noorshafiee S. Pulmonary hydatid cyst: analysis of 1024 cases. Gen Thorac Cardiovasc Surg. 2011;59(2):105–109. doi: 10.1007/s11748-010-0690-z. [DOI] [PubMed] [Google Scholar]
- Bhutani N, Kajal P. Hepatic echinococcosis: a review. Ann Med Surg (lond) 2018;36:99–105. doi: 10.1016/j.amsu.2018.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilgin M, Oguzkaya F, Akçali Y. Is capitonnage unnecessary in the surgery of intact pulmonary hydatic cyst? ANZ J Surg. 2004;74(1–2):40–42. doi: 10.1046/j.1445-1433.2003.02684.x. [DOI] [PubMed] [Google Scholar]
- Daali M, Hssaida R, Zoubir M, Borki K. L'expérience marocaine dans le traitement chirurgical des kystes hydatiques multiple du foie: à propos de 94 cas [Moroccan experience in the surgical treatment of multiple hydatid cysts in the liver] Sante (montrouge, France) 2001;11(3):177–184. [PubMed] [Google Scholar]
- Dirican A, Yilmaz M, Unal B, Tatli F, Piskin T, Kayaalp C. Ruptured hydatid cysts into the peritoneum: a case series. Eur J Trauma Emerg Surg. 2010;36(4):375–379. doi: 10.1007/s00068-009-9056-6. [DOI] [PubMed] [Google Scholar]
- Giorgio A, Di Sarno A, de Stefano G, Farella N, Matteucci P, Scognamiglio U, Giorgio V. Percutaneous treatment of Hydatid Liver Cyst. Recent Pat Antiinfect Drug Discov. 2009;4(1):29–36. doi: 10.2174/157489109787236274. [DOI] [PubMed] [Google Scholar]
- Giorgio A, Di Sarno A, de Stefano G, Liorre G, Farella N, Scognamiglio U, Giorgio V. Sonography and clinical outcome of viable hydatid liver cysts treated with double percutaneous aspiration and ethanol injection as first-line therapy: efficacy and long-term follow-up. AJR Am J Roentgenol. 2009;193(3):W186–W192. doi: 10.2214/AJR.08.1518. [DOI] [PubMed] [Google Scholar]
- Gulalp B, Koseoglu Z, Toprak N, Satar S, Sebe A, Gokel Y, Sakman G, Karcioglu O. Ruptured hydatid cyst following minimal trauma and few signs on presentation. Neth J Med. 2007;65(3):117–118. [PubMed] [Google Scholar]
- Gupta R, Sharma SB, Prabhakar G, Mathur P. Hydatid disease in children: our experience. Formosan J Surg. 2014;47(6):211–220. doi: 10.1016/j.fjs.2014.12.001. [DOI] [Google Scholar]
- Jairajpuri ZS, Jetley S, Hassan MJ, Hussain M. Hydatid disease in childhood: revisited report of an interesting case. J Parasit Dis. 2012;36(2):265–268. doi: 10.1007/s12639-012-0127-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kavukcu S, Kilic D, Tokat AO, Kutlay H, Cangir AK, Enon S, Okten I, Ozdemir N, Gungor A, Akal M, Akay H. Parenchyma-preserving surgery in the management of pulmonary hydatid cysts. J Invest Surg. 2006;19(1):61–68. doi: 10.1080/08941930500444586. [DOI] [PubMed] [Google Scholar]
- Kirkland K. Urological aspects of hydatid disease. Br J Urol. 1966;38(3):241–254. doi: 10.1111/j.1464-410x.1966.tb09707.x. [DOI] [PubMed] [Google Scholar]
- Kjossev K, Losanoff J, Velitchkov N, Belokonski E (2002) Hydatid cyst of the diaphragm: a case report and review of the literature. Internet J Thoracic Cardiovasc Surg 6(1)
- Kosar A, Orki A, Haciibrahimoglu G, Kiral H, Arman B. Effect of capitonnage and cystotomy on outcome of childhood pulmonary hydatid cysts. J Thorac Cardiovasc Surg. 2006;132(3):560–564. doi: 10.1016/j.jtcvs.2006.05.032. [DOI] [PubMed] [Google Scholar]
- Kurt N, Oncel M, Gulmez S, Ozkan Z, Uzun H. Spontaneous and traumatic intra-peritoneal perforations of hepatic hydatid cysts: a case series. J Gastrointest Surg. 2003;7(5):635–641. doi: 10.1016/s1091-255x(02)00434-1. [DOI] [PubMed] [Google Scholar]
- M'rad S, Oudni-M'rad M, Boubaker G, Bouazzi L, Gorcii M, Nouri A, Mezhoud H, Babba H (2012) Étude rétrospective de la distribution et de la fertilité des kystes hydatiques chez l'enfant en Tunisie [Retrospective study of the distribution and the fertility of hydatid cysts in the child in Tunisia]. 60(3): 166–169. 10.1016/j.patbio.2011.03.002 [DOI] [PubMed]
- Nabi MS, Waseem T, Tarif N, Chima KK. Pulmonary hydatid disease: is capitonnage mandatory following cystotomy? Int J Surg. 2010;8(5):373–376. doi: 10.1016/j.ijsu.2010.05.007. [DOI] [PubMed] [Google Scholar]
- Ozturk G, Aydinli B, Yildirgan MI, Basoglu M, Atamanalp SS, Polat KY, Alper F, Guvendi B, Akcay MN, Oren D. Posttraumatic free intraperitoneal rupture of liver cystic echinococcosis: a case series and review of literature. Am J Surg. 2007;194(3):313–316. doi: 10.1016/j.amjsurg.2006.11.014. [DOI] [PubMed] [Google Scholar]
- Safioleas MC, Misiakos EP, Kouvaraki M, Stamatakos MK, Manti CP, Felekouras ES. Hydatid disease of the liver: a continuing surgical problem. Arch Surg. 2006;141(11):1101–1108. doi: 10.1001/archsurg.141.11.1101. [DOI] [PubMed] [Google Scholar]
- Sarkar R, Shukla RM, Maitra S, Bhattacharya M, Mukhopadhyay B. Hydatid cyst within a choledochal cyst. J Indian Assoc Pediatr Surg. 2013;18(4):158–159. doi: 10.4103/0971-9261.121128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Topçu S, Kurul IC, Taştepe I, Bozkurt D, Gülhan E, Cetin G. Surgical treatment of pulmonary hydatid cysts in children. J Thorac Cardiovasc Surg. 2000;120(6):1097–1101. doi: 10.1067/mtc.2000.110181. [DOI] [PubMed] [Google Scholar]
- Tuxun T, Zhang JH, Zhao JM, Tai QW, Abudurexti M, Ma HZ, Wen H. World review of laparoscopic treatment of liver cystic echinococcosis–914 patients. Int J Infect Dis. 2014;24:43–50. doi: 10.1016/j.ijid.2014.01.012. [DOI] [PubMed] [Google Scholar]


