Abstract
Malaria is one of the deadliest parasitic diseases in human. Currently, Artemisinin-based combination therapy is considered as the gold standard and most common treatment option. However, the origin and transmission of Plasmodium falciparum from the Greater Mekong Subregion, which has decreased artemisinin (ART) sensitivity, has sparked global concern. The reduced ART sensitivity has been associated with mutations in the Atpase6 and Kelch13 propeller domain of Plasmodium falciparum. A molecular marker is critically needed to monitor the spread of artemisinin resistance. In this article, we reviewed the k13 mutations and potential marker for ART resistance in India. There have been fourteen mutations identified, three of which have been validated by the World Health Organization (WHO) as artemisinin resistance mutations (F446I, R561H/C, and R539T). Among them, the role of F446I and R561H/C in ART resistance is conflicting. R539T and G625R mutation has been identified as an ART- resistance marker in India.
Keywords: Artemisinin, K13 Mutation, Sensitivity, India
Introduction
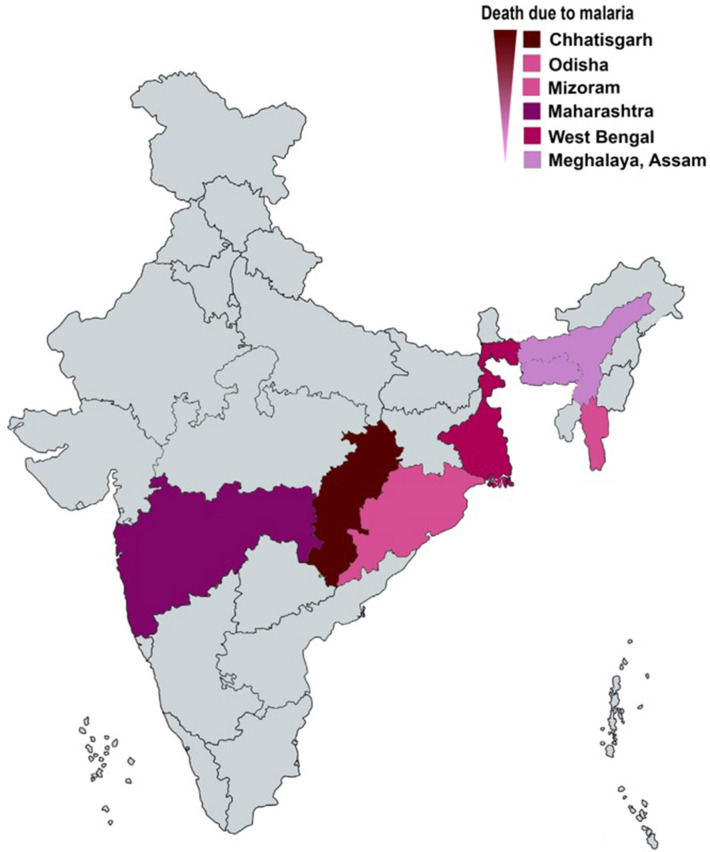
The tremendous efforts on malaria elimination and containments over the recent past decades have reduced malaria-related morbidity and mortality globally (WHO 2019). However still, malaria remains a significant health burden in an endemic region. There were an estimated 228 million cases and 4,05,000 death in 2018 (WHO 2019). The WHO African region recorded 93 per cent of malaria cases in 2018, followed by the WHO South-East Asia region with 3.4 per cent. India accounts for most patients in South-East Asia, with 6.7 million cases (WHO 2019). Plasmodium falciparum, Plasmodium vivax, Plasmodium malariae, Plasmodium ovale curtisi, Plasmodium ovale wallikeri, and Plasmodium knowlesi are the six Plasmodium parasites that causes malaria in humans (Lalremruata et al. 2017; Joste et al. 2018). Plasmodium knowlesi is a zoonotic, crucial human pathogen in Africa and South-East Asia (Oguike et al. 2011; Antinori et al. 2013; Ansari et al. 2016; Lalremruata et al. 2017). The malaria parasite Plasmodium falciparum is predominant to cause malaria in India, along with Plasmodium vivax, and rarely Plasmodium malariae. The map shows India's region with death due to malaria (Fig. 1). Plasmodium ovale curtisi, and Plasmodium ovale wallikeri, two newly described parasites have also been found to cause malaria in India (Chaturvedi et al. 2015).
Fig. 1.
The map shows India’s region with death due to malaria
(Source: NVBDCP)
Traditionally, anti-malarial drugs are employed to treat uncomplicated malaria as no effective vaccine is available (Wykes 2013; Barik 2015; Draper et al. 2018). Chloroquine was the first synthetic quinine-based anti-malarial compound used to treat uncomplicated malaria and has been a front-line drug for more than three decades. The rise and development of Plasmodium falciparum malaria resistance to the then existing anti-malarial drugs have jeopardized the malaria situation in the endemic region. The past reports of rampant use of counterfeit and substandard drugs (Lon et al.2006) resulted in the loss of chloroquine (CQ) susceptibility in Plasmodium falciparum independently in South America and Southeast Asia (Payne 1987; Chen et al. 2003; Packard 2014). The spread of CQ resistance from these foci was seen in the high infant mortality in South America, Southeast Asia, and Africa (Müller et al. 2019). In 1973, the first evidence of CQ resistance to Plasmodium falciparum was reported in India (Sehgal et al. 1973). Subsequently, a significant rise in the resistance to this drug was reported (Sharma et al. 1996; Vinayak et al. 2003). The Indian agency for prevention and control of disease, the National vector-borne disease control program (NVBDCP), had to drop CQ treatment for Plasmodium falciparum due to rising resistance to the drug. In 1982, sulphadoxine-pyrimethamine (SP) was prescribed as a second-line treatment for falciparum malaria in the CQ resistance region. In the midst of this, a low degree of in vivo SP resistance had previously been established, and an increase in resistant SP allele frequency from low frequencies was observed (Ahmed et al. 2004; Kumar et al. 2015). Thus, in 2005, SP, which was used as a second-line treatment for CQ resistance, was replaced with artesunate plus sulphadoxine-pyrimethamine. As a result, in 2007, artesunate plus sulphadoxine-pyrimethamine (AS + SP) was approved as the first-line treatment for areas with SP resistance. This protocol became the first-line treatment in India in 2010. In 2012, the North-eastern state recorded declining efficacy results for artesunate plus sulphadoxine-pyrimethamine (AS + SP), and treatment failure report was widespread. As a result, the AS + SP protocol was updated with artemether-lumefantrine for uncomplicated malaria cases (Mishra et al. 2014, 2016). Artemisinin (ART) is a fast-acting drug best suited to combine with long-lived partner drugs for better post-treatment prophylactic effects, reducing recurrence probability (Li and Hickman 2015; Tse et al. 2019). Artemether-lumefantrine (A.L.), artesunate-sulphadoxine-pyrimethamine (AS + SP), artesunate-amodiaquine (AS-AQ), artesunate-mefloquine (AS-MQ), and dihydroartemisinin-piperaquine (DHA-PPQ) are the most commonly prescribed regimens for uncomplicated malaria (Sinclair et al. 2009; WHO 2018). Oral artesunate and artemether are self-active compounds converted by blood esterase to the active metabolite dihydroartemisinin (DHA), which is effective against Plasmodium parasites (Woodrow and White 2017; Tse et al. 2019). Artemisinin and its derivatives have a short elimination half-life and kill malaria parasite, thus reducing parasite infliction (Bridgford et al. 2018). However, artemisinin monotherapy does not destroy all parasites, as a subpopulation of ring-stage malaria parasites may escape and enter a state of dormancy, only to re-emerge days to weeks after treatment failure (Codd et al. 2011; Woodrow and White 2017). Since artemisinin has been used in India for decades, it is critical to track the spread of resistance and therapeutic efficacy. This article reviews the current status of Kelch13 mutations, the emergence of artemisinin-resistant malaria, and potential artemisinin resistance marker in India.
The current landscape of Artemisinin resistance in India
India is a gateway to spread resistance to Africa and the rest of the world and shares a porous border with hotbeds for the emergence of drug resistance in Southeast Asia. Thus, it is imperative to have the region's drug resistance profile to manage better and contain the drug resistance menace. The North-eastern Indian bordering terrains are densely covered with hills, forests and receive high rainfall favors conducive climatic conditions for high malaria transmission and parasite load. In 2013–14, an emergence of artemisinin resistance malaria was recorded in India, and known drug failure (Das et al. 2018, 2019; WHO 2018). The first global report of K13 mutation was discovered in western Cambodia, with the most common mutations being C580Y, Y493H, and R539T (Ariey et al. 2014). Since then, more than 300 mutations have been identified around the world (Fairhurst 2015). Table 1 provides the k13 validated associated/candidates for artemisinin resistance in the global malaria-endemic region. Indian bordering areas of China-Myanmar have prevalent F446I, R539T, P574L, N458Y, R561H, and A676D mutations (Ariey et al. 2014; Feng et al. 2015; Wang et al. 2015; Ye et al. 2016). It assumed that the resistant strain Plasmodium falciparum in Myanmar's Sagaing region might spread to neighboring India (Tun et al. 2015). Thus, the threat of the spread of resistance to mainland endemic regions is enormous and requires strict surveillance of resistant parasite strain.
Table 1.
Distribution of K13 mutations in the global endemic region
| Country | Mutations | References |
|---|---|---|
| Cambodia |
R539T*, C580Y*, Y493H*, I543T*, N458Y*, R561H*, G449A#, V568G#, P574L#, P553L#, T474I**, A481V**, T508N**, P527T**, G533S**, N537I**, S623C** |
(Ariey et al. 2014; Dwivedi et al. 2016; Kheang et al. 2017) |
| Myanmar | C580Y*, R539T*, N458Y*, R561H*, G449A#, P441L#, P574L#, P553L#, A675V#, F446I# M476I**, A481V**, R515T**, N537I**, D452E**, K479I**, C469F**, R528T**, R575K**, V496F**, P667T**, M476I**, T474I**, M476V**, C469F**, N490T**, Y511H**, G533A**, G538V**, N537I**, E556D**, F673I**, N458I**, E252Q**, A676D** | (Tun et al. 2015;Nyunt et al. 2015; Wang et al. 2017;Nyunt et al. 2017) |
| Thailand | C580Y*, R539T*, Y493H*, R561H*, N458Y*, P441L#, G449A#, P574L#, A481V**, P527H**, E556D**, Y604H**, E605G**, N609S**, N632D**, R575K**, S621F** | (Talundzic et al. 2015; Putaporntip et al. 2016) |
| Nigeria | E433G**, F434I**, F434S**, I684N**, I684T**, E688K** | (Abubakar et al. 2020) |
| Democratic Republic of the Congo | A578S## | (Nzoumbou-Boko et al. 2020) |
| United Republic of Tanzania | R561H*, A578S## | (Bwire et al. 2020) |
| Burkina Faso | C469C**, Y493Y**, G496G**, V589V** | (Somé et al. 2016) |
| Mozambique | V494I** | (Escobar et al. 2015) |
| Niger | M472I**, Y558C**, K563R**, P570L**, P615S** | (Laminou et al. 2018) |
| Uganda | A578S## | (Hawkes et al. 2015) |
| Kenya, Malawi | P553L# | (Taylor et al. 2014) |
| Mali | P553L#, F446I#, A578S## | (Taylor et al. 2014; Kamau et al. 2015; Ouattara et al. 2015) |
| Angola, Equatorial Guinea, Rwanda | R539T*, P574L# | (Nzoumbou-Boko et al. 2020) |
| Congo, Gabon, Ghana, Kenya | A578S## | (Kamau et al. 2015; Nzoumbou-Boko et al. 2020) |
*Validated K13 mutations; # Associated K13 mutations; ## Common mutation but not associated; ** mutation
Recent studies from Indian field isolates show the mutation in the Plasmodium falciparum chromosome kelch13 propeller region. Mishra et al. (2015) conducted a sentinel site study in fifteen Indian states to determine the therapeutic efficacy of ACT (artesunate + sulphadoxine), enrolling 389 subjects in a prospective study. G533A/S, S549Y, R561 H/C, non-synonymous mutations have been found in isolates from Gomati (Tripura), Jalpaiguri (West Bengal), and Changlang (Arunachal Pradesh) (Mishra et al. 2015). Nonetheless, their involvement was not linked to treatment failure. Furthermore, In 254 patients from Mizoram, Tripura, and Arunachal Pradesh, Mishra et al. (2016) discovered non-synonymous mutations F446I and A578S K13propeller region, as well as K189T in the K13 non-propeller region. The therapeutics efficacy studies of ACT in central India reveal the 1.5 per cent incidence of single non-synonymous mutation at codon M579T/H and 37% double mutation at codon M579T/H, N657H (Mishra et al. 2017). Besides that, non-synonymous mutations at A481V, A675V, and D702N were discovered in 134 patients from three northeast states during a routine analysis of the pfk13 propeller domain (Das et al. 2017). Recent research from the Eastern Indian state also discovered a novel mutation, G625R, and a WHO-validated R539T mutation, linked to artemisinin resistance. Table 2 depicts the distribution of Plasmodium falciparum K13 mutations in India. This artemisinin resistance assay was based on criteria of parasite clearance half-life > 5 hrs and parasite survival rate greater than 10% based on ex vivo RSA0-3 h (Das et al. 2018). However, F446I, R539T, R561H/C mutations are validated for artemisinin resistance in Greater Mekong Subregion (WHO 2018). Map showing the reported malaria cases due to Plasmodium falciparum in India and reported k-13 validated mutations (Fig. 2).
Table 2.
Plasmodium falciparum-K13 mutations in India
| States/Districts | Year of study | Mutation observed | Mutation in k13 Propeller region | Assay employed | References |
|---|---|---|---|---|---|
| Arunachal Pradesh (Changlang) |
2014–2015 2009–2013 2014–2015 |
F446I R561H A481V A675V D702N |
F446I R561H A481V A675V D702N |
Molecular marker studies Prospective therapeutics studies Molecular marker studies |
Mishra et al. (2016) Mishra et al. (2015) Das et al. (2017) |
| Mizoram (Lunglei) | 2014–2015 | A578S, K189T | A578S | Molecular marker studies | Mishra et al. (2016) |
| Tripura (Gomati) | 2014–2015 |
K189T G533A |
G533A | Molecular marker studies | Mishra et al. (2016); Mishra et al. (2015) |
| West Bengal |
2013–2014 2009–2013 |
G625R N672S R539T |
R539T G625R N672S S549Y |
Ex vivo Ring-stage survival Assay (RSA 0-3 h) Prospective therapeutics studies |
Das et al. (2018) Mishra et al. (2015) |
| Madhya Pradesh | 2012–2014 |
M579T N657H |
M579T N657H |
Molecular marker studies | Mishra et al. (2017) |
Fig. 2.
Map showing the reported malaria cases due to Plasmodium falciparum in India
Source: NVBDCP and reported k-13 validated mutations
Single nucleotide polymorphism as a molecular marker for ART Resistance
The F446I and R561 H/C, Single nucleotide polymorphism, has recently been identified from north-eastern India. This polymorphism was previously discovered to be the validated gene marker for ART resistance along with delayed parasite clearance in the bordering area of China and Myanmar (Mishra et al. 2016; WHO, 2018). In India, however, no correlation has been found between this polymorphism and parasite clearance or treatment outcome (Mishra et al. 2016). Since alleles in the k13 propeller domain are heterogeneous, its critical to double-check the ART- resistance marker. The R539T polymorphism is common in Cambodia and has been identified as an ART- resistance marker (Ariey et al. 2014; Straimer et al. 2015). This polymorphism, along with the G625R polymorphism, has been linked to parasite clearance half-life positivity and high survival rates in eastern India, as determined by a ring-stage survival assay (0-3 h), and has been proposed as an ART resistance marker in India.
Surveillance of artemisinin resistance
The surveillance system enables diagnosis and track real-time information on the emergence and spread of anti-malarial drug resistance. This allows prompt steps to mitigate malaria-related morbidity and mortality. Ring-stage survival assay (RSA), either vitro ring-stage survival assay (RSA 0–3 h) or ex vivo RSA, as well as molecular marker studies of validated signatures in the Plasmodium falciparum parasites are currently used to track down artemisinin resistance (Witkowski et al. 2013a; Ariey et al. 2014; WHO 2018; Murmu et al. 2021). The RSA consists of parasite culture (0-3 h) either obtained directly from the infected patient or adapted parasite lines being synchronized by 5% sorbitol solution and then exposed to 700 nM dihydroartemisinin (DHA) for 6 h, maintaining the 37 °C, humid atmosphere,5% O2, 5% CO2, and 90% N2 condition. Then parasites are harvested, DHA is washed out and again cultivated for 66 h. Finally, the viable parasites are determined (Witkowski et al. 2013b) (https://www.wwarn.org/tools-resources/procedures/ring-stage-survival-assays-rsa-evaluate-vitro-and-ex-vivo-susceptibility).However, Genomic DNA is extracted from either Giemsa-stained clinical thick blood films or directly collected finger pricked blood spots from patients in molecular marker studies for validated single nucleotide polymorphism (SNP). The extracted parasite DNA is then PCR amplified, accompanied by PCR products sequencing and SNP analysis.
Concluding remarks
ACT is an effective regimen for severe malaria. Nevertheless, the spread of resistant strain Plasmodium falciparum to India with delay in parasite clearance time and reduced susceptibility is a threat to malaria control and eradication strategy. Artemisinin resistance must be monitored on a large scale by PCHL, RSA, or kelch13 molecular marker studies. The information gleaned from such investigation may aid in the successful management of the Plasmodium falciparum parasites. G625R polymorphism has been verified as a validated marker for ART resistance in eastern India, along with R539T, which was also identified as an ART-resistance marker in Cambodia. Thus, proper mitigation for the containment of parasites is highly essential.
Acknowledgements
The authors want to acknowledge all researchers whose publications were used in our review.
Author contributions
Both authors LKM and TKB conceived the idea, searched the literature, and drafted the final version of the manuscript.
Declarations
Conflicts of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Abubakar UF, Adam R, Mukhtar MM, Muhammad A, Yahuza AA, Ibrahim SS. Identification of mutations in antimalarial resistance gene kelch13 from Plasmodium falciparum Isolates in Kano. Nigeria Trop Med Infect Dis. 2020;5(2):85. doi: 10.3390/tropicalmed5020085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed A, Bararia D, Vinayak S, Yameen M, Biswas S, Dev V, Kumar A, Ansari MA, Sharma YD. Plasmodium falciparum Isolates in India exhibit a progressive increase in mutations associated with sulfadoxine-pyrimethamine resistance. Antimicrob Agents Chemother. 2004;48(3):879–889. doi: 10.1128/AAC.48.3.879-889.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ansari HR, Templeton TJ, Subudhi AK, Ramaprasad A, Tang J, Lu F, Naeem R, Hashish Y, Oguike MC, Benavente ED, Clark TG, Sutherland CJ, Barnwell JW, Culleton R, Cao J, Pain A. Genome-scale comparison of expanded gene families in Plasmodium ovale wallikeri and Plasmodium ovale curtisi with Plasmodium malariae and with other Plasmodium species. Int J Parasitol. 2016;46(11):685–696. doi: 10.1016/j.ijpara.2016.05.009. [DOI] [PubMed] [Google Scholar]
- Antinori S, Galimberti L, Milazzo L, Corbellino M. Plasmodium knowlesi: the emerging zoonotic malaria parasite. Acta Trop. 2013;125(2):191–201. doi: 10.1016/j.actatropica.2012.10.008. [DOI] [PubMed] [Google Scholar]
- Ariey F, Witkowski B, Amaratunga C, Beghain J, Langlois AC, Khim N, Kim S, Duru V, Bouchier C, Ma L, Lim P, Leang R, Duong S, Sreng S, Suon S, Chuor CM, Bout DM, Ménard S, Rogers WO. A molecular marker of artemisinin-resistant Plasmodium falciparum malaria. Nature. 2014;505:50–55. doi: 10.1038/nature12876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barik T. Antimalarial drug: from its development to deface. Curr Drug Discov Technol. 2015;12(4):225–228. doi: 10.2174/1570163812666150907100019. [DOI] [PubMed] [Google Scholar]
- Bridgford JL, Xie SC, Cobbold SA, Pasaje CFA, Herrmann S, Yang T, Gillett DL, Dick LR, Ralph SA, Dogovski C, Spillman NJ, Tilley L. Artemisinin kills malaria parasites by damaging proteins and inhibiting the proteasome. Nat Commun. 2018;9(1):1–9. doi: 10.1038/s41467-018-06221-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bwire GM, Ngasala B, Mikomangwa WP, Kilonzi M, Kamuhabwa AAR. Detection of mutations associated with artemisinin resistance at k13-propeller gene and a near-complete return of chloroquine susceptible falciparum malaria in Southeast of Tanzania. Sci Rep. 2020;10(1):1–7. doi: 10.1038/s41598-020-60549-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaturvedi N, Bhandari S, Bharti PK, Basak SK, Singh MP, Singh N. Sympatric distribution of Plasmodium ovale curtisi and P. ovale wallikeri in India: implication for the diagnosis of malaria and its control. Trans R Soc Trop Med Hyg. 2015;109(5):352–354. doi: 10.1093/trstmh/trv015. [DOI] [PubMed] [Google Scholar]
- Chen N, Kyle DE, Pasay C, Fowler EV, Baker J, Peters JM, Cheng Q. pfcrt allelic types with two novel amino acid mutations in chloroquine-resistant Plasmodium falciparum Isolates from the Philippines. Antimicrob Agents Chemother. 2003;47(11):3500–3505. doi: 10.1128/AAC.47.11.3500-3505.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Codd A, Teuscher F, Kyle DE, Cheng Q, Gatton ML. Artemisinin-induced parasite dormancy: a plausible mechanism for treatment failure. Malar J. 2011;10(1):56. doi: 10.1186/1475-2875-10-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das M, Kalita M, Chetry S, Dutta P. K13 Kelch Propeller domain and mdr1 sequence polymorphism in field isolates from northeast region. India Hum Parasit Dis. 2017 doi: 10.1177/1179570017706870. [DOI] [Google Scholar]
- Das S, Manna S, Saha B, Hati AK, Roy S. Novel pfkelch13 gene polymorphism associates with artemisinin resistance in Eastern India. Clin Infect Dis. 2019;69(7):1144–1152. doi: 10.1093/cid/ciy1038. [DOI] [PubMed] [Google Scholar]
- Das S, Saha B, Hati AK, Roy S. Evidence of artemisinin-resistant Plasmodium falciparum Malaria in Eastern India. N Engl J Med. 2018;379(20):1962–1964. doi: 10.1056/NEJMc1713777. [DOI] [PubMed] [Google Scholar]
- Draper SJ, Sack BK, King CR, Nielsen CM, Rayner JC, Higgins MK, Long CA, Seder RA. Malaria vaccines: recent advances and new horizons. Cell Host Microbe. 2018;24(1):43–56. doi: 10.1016/j.chom.2018.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dwivedi A, Khim N, Reynes C, Ravel P, Ma L, Tichit M, Bourchier C, Kim S, Dourng D, Khean C, Chim P, Siv S, Frutos R, Lek D, Mercereau-Puijalon O, Ariey F, Menard D, Cornillot E. Plasmodium falciparum parasite population structure and gene flow associated to anti-malarial drugs resistance in Cambodia. Malar J. 2016 doi: 10.1186/s12936-016-1370-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escobar C, Pateira S, Lobo E, Lobo L, Teodosio R. Polymorphisms in Plasmodium falciparum K13-Propeller in Angola and mozambique after the introduction of the ACTs. PLoS ONE. 2015;10(3):e0119215. doi: 10.1371/journal.pone.0119215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairhurst RM. Understanding artemisinin-resistant malaria: what a difference a year makes. Curr Opin Infect Dis. 2015;28(5):417–425. doi: 10.1097/QCO.0000000000000199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng J, Zhou D, Lin Y, Xiao H, Yan H, Xia Z. Amplification of pfmdr1, pfcrt, pvmdr1, and K13 propeller polymorphisms associated with Plasmodium falciparum and Plasmodium vivax isolates from the China-Myanmar border. Antimicrob Agents Chemother. 2015;59(5):2554–2559. doi: 10.1128/AAC.04843-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkes M, Conroy AL, Opoka RO, Namasopo S, Zhong K, Liles WC, John CC, Kain KC. Slow Clearance of Plasmodium falciparum in Severe Pediatric Malaria, Uganda, 2011–2013. Emerg Infect Dis. 2015;21(7):1237–1239. doi: 10.3201/eid2107.150213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joste V, Kamaliddin C, Kendjo E, Hubert V, Argy N, Houzé S. Distinction of Plasmodium ovale wallikeri and Plasmodium ovale curtisi using quantitative Polymerase Chain Reaction with High Resolution Melting revelation. Sci Rep. 2018;8(1):1–8. doi: 10.1038/s41598-017-18026-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamau E, Campino S, Amenga-Etego L, Drury E, Ishengoma D, Johnson K, Mumba D, Kekre M, Yavo W, Mead D, Bouyou-Akotet M, Apinjoh T, Golassa L, Randrianarivelojosia M, Andagalu B, Maiga-Ascofare O, Amambua-Ngwa A, Tindana P, Ghansah A. K13-propeller polymorphisms in Plasmodium falciparum parasites from sub-Saharan Africa. J Infect Dis. 2015;211(8):1352–1355. doi: 10.1093/infdis/jiu608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kheang ST, Sovannaroth S, Ek S, Chy S, Chhun P, Mao S, Nguon S, Lek DS, Menard D, Kak N. Prevalence of K13 mutation and Day-3 positive parasitemia in an artemisinin-resistant malaria-endemic area of Cambodia: A cross-sectional study. Malar J. 2017 doi: 10.1186/s12936-017-2024-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A, Moirangthem R, Gahlawat SK, Chandra J, Gupta P, Valecha N, Anvikar A, Singh V. Emergence of sulfadoxine-pyrimethamine resistance in Indian isolates of Plasmodium falciparum in the last two decades. Infect Genet Evol. 2015;36:190–198. doi: 10.1016/j.meegid.2015.08.027. [DOI] [PubMed] [Google Scholar]
- Lalremruata A, Jeyaraj S, Engleitner T, Joanny F, Lang A, Bélard S, Mombo-Ngoma G, Ramharter M, Kremsner PG, Mordmüller B, Held J. Species and genotype diversity of Plasmodium in malaria patients from Gabon analyzed by next-generation sequencing. Malar J. 2017 doi: 10.1186/s12936-017-2044-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laminou I, Lamine M, Arzika I, Mahamadou B, Gora D, Dieye A. Detection of Plasmodium falciparum K13 Propeller A569G mutation after artesunate-amodiaquine treatment failure in Niger. J Adv Biol Biotechnol. 2018;18(2):1–8. doi: 10.9734/jabb/2018/42872. [DOI] [Google Scholar]
- Li Qigui, Hickman M (2015) The Impact of Pharmacokinetic Mismatched Antimalarial Drug Combinations on the Emergence and Spread of Drug-Resistant Parasites. In Ahmed T A (ed) Basic Pharmacokinetic Concepts and Some Clinical Applications, 1st eds. Intech Open, Janeza trdine, pp. 1–32
- Lon CT, Tsuyuoka R, Phanouvong S, Nivanna N, Socheat D, Sokhan C, Blum N, Christophel EM, Smine A. Counterfeit and substandard antimalarial drugs in Cambodia. Trans R Soc Trop Med Hyg. 2006;100(11):1019–1024. doi: 10.1016/j.trstmh.2006.01.003. [DOI] [PubMed] [Google Scholar]
- Mishra N, Bharti RS, Mallick P, Singh OP, Srivastava B, Rana R, Phookan S, Gupta HP, Ringwald P, Valecha N. Emerging polymorphisms in falciparum Kelch 13 gene in Northeastern region of India. Malar J. 2016 doi: 10.1186/s12936-016-1636-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra N, Kaitholia K, Srivastava B, Shah NK, Narayan JP, Dev V, Phookan S, Anvikar AR, Rana R, Bharti RS, Sonal GS. Declining efficacy of artesunate plus sulphadoxine-pyrimethamine in northeastern India. Malar J. 2014 doi: 10.1186/1475-2875-13-284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra N, Prajapati SK, Kaitholia K, Bharti RS, Srivastava B, Phookan S, Anvikar AR, Dev V, Sonal GS, Dhariwal AC, White NJ, Valecha N. Surveillance of artemisinin resistance in Plasmodium falciparum in India using the kelch13 molecular marker. Antimicrob Agents Chemother. 2015;59(5):2548–2553. doi: 10.1128/AAC.04632-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra S, Bharti PK, Shukla MM, Ali NA, Kashyotia SS, Kumar A, Dhariwal AC, Singh N. Clinical and molecular monitoring of Plasmodium falciparum resistance to antimalarial drug (artesunate+sulphadoxine-pyrimethamine) in two highly malarious districts of Madhya Pradesh, Central India from 2012–2014. Pathog Glob Health. 2017;111(4):186–194. doi: 10.1080/20477724.2017.1331875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller O, Lu GY, Von Seidlein L. Geographic expansion of artemisinin resistance. J Travel Med. 2019 doi: 10.1093/jtm/taz030. [DOI] [PubMed] [Google Scholar]
- Murmu LK, Sahu AA, Barik TK. Diagnosing the drug resistance signature in Plasmodium falciparum: a review from contemporary methods to novel approaches. J Parasit Dis. 2021 doi: 10.1007/s12639-020-01333-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyunt MH, Hlaing T, Oo HW, Tin-Oo LLK, Phway HP, Wang B, Zaw NN, Han SS, Tun T, San KK, Kyaw MP, Han ET. Molecular assessment of artemisinin resistance markers, polymorphisms in the K13 propeller, and a multidrug-resistance gene in the Eastern and Western Border Areas of Myanmar. Clin Infect Dis. 2015;60(8):1208–1215. doi: 10.1093/cid/ciu1160. [DOI] [PubMed] [Google Scholar]
- Nyunt MH, Soe MT, Myint HW, Oo HW, Aye MM, Han SS, Zaw NN, Cho C, Aung PZ, Kyaw KT, Aye TT, San NA, Ortega L. Clinical and molecular surveillance of artemisinin-resistant falciparum malaria in Myanmar (2009–2013) Malar J. 2017 doi: 10.1186/s12936-017-1983-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nzoumbou-Boko R, Panté-Wockama CBG, Ngoagoni C, Petiot N, Legrand E, Vickos U, Gody JC, Manirakiza A, Ndoua C, Lombart JP, Ménard D. Molecular assessment of kelch13 non-synonymous mutations in Plasmodium falciparum isolates from Central African Republic (2017–2019) Malar J. 2020 doi: 10.1186/s12936-020-03264-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oguike MC, Betson M, Burke M, Nolder D, Stothard JR, Kleinschmidt I, Proietti C, Bousema T, Ndounga M, Tanabe K, Ntege E, Culleton R, Sutherland CJ. Plasmodium ovale curtisi and Plasmodium ovale wallikeri circulate simultaneously in African communities. Int J Parasitol. 2011;41(6):677–683. doi: 10.1016/j.ijpara.2011.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouattara A, Kone A, Adams M, Fofana B, Maiga AW, Hampton S, Coulibaly D, Thera MA, Diallo N, Dara A, Sagara I, Gil JP, Bjorkman A, Takala-Harrison S, Doumbo OK, Plowe CV, Djimde AA. Polymorphisms in the K13-propeller gene in artemisinin-susceptible Plasmodium falciparum parasites from Bougoula-Hameau and Bandiagara. Mali Am J Trop Med Hyg. 2015;92(6):1202–1206. doi: 10.4269/ajtmh.14-0605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Packard RM. The origins of antimalarial-drug resistance. N Engl J Med. 2014;371:397–399. doi: 10.1056/NEJMp1403340. [DOI] [PubMed] [Google Scholar]
- Payne D. Spread of chloroquine resistance in Plasmodium falciparum. Parasitol Today. 1987;3(8):241–246. doi: 10.1016/0169-4758(87)90147-5. [DOI] [PubMed] [Google Scholar]
- Putaporntip C, Kuamsab N, Kosuwin R, Tantiwattanasub W, Vejakama P, Sueblinvong T, Seethamchai S, Jongwutiwes S, Hughes AL. Natural selection of K13 mutants of Plasmodium falciparum in response to artemisinin combination therapies in Thailand. Clin Microbiol Infect. 2016;22(3):285.e1–285.e8. doi: 10.1016/j.cmi.2015.10.027. [DOI] [PubMed] [Google Scholar]
- Sehgal PN, Sharma MTD, Sharma SL, et al. Resistance to chloroquine in falciparum malaria in Assam state. India J Commun Dis. 1973;5:175–180. [Google Scholar]
- Sinclair D, Zani B, Donegan S, Olliaro P, Garner P. Artemisinin-based combination therapy for treating uncomplicated malaria. Cochrane Database Syst Rev. 2009 doi: 10.1002/14651858.CD007483.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somé AF, Sorgho H, Zongo I, Bazié T, Nikiéma F, Sawadogo A, Zongo M, Compaoré Y, Ouédraogo J. Polymorphisms in K13, pfcrt, pfmdr1, pfdhfr, and pfdhps in parasites isolated from symptomatic malaria patients in Burkina Faso. Parasite. 2016 doi: 10.1051/parasite/2016069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma YD, Biswas S, Pillai CR, Ansari MA, Adak T, Devi CU. High prevalence of chloroquine resistant Plasmodium falciparum infection in the Rajasthan epidemic. Acta Trop. 1996;62(3):135–141. doi: 10.1016/S0001-706X(96)00031-9. [DOI] [PubMed] [Google Scholar]
- Straimer J, Gnadig NF, Witkowski B, Amaratunga C, Duru V, Ramadani AP, Dacheux M, Khim N, Zhang L, Lam S, Gregory PD, Urnov FD, Mercereau-Puijalon O, Benoit-Vical F, Fairhurst RM, Ménard D, Fidock DA. K13-propeller mutations confer artemisinin resistance in Plasmodium falciparum clinical isolates. Science. 2015;347(6220):428–431. doi: 10.1126/science.1260867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talundzic E, Okoth SA, Congpuong K, Plucinski MM, Morton L, Goldman IF, Kachur PS, Wongsrichanalai C, Satimai W, Barnwell JW, Udhayakumar V. Selection and spread of artemisinin-resistant alleles in thailand prior to the global artemisinin resistance containment campaign. PLoS Pathog. 2015;11(4):e1004862. doi: 10.1371/journal.ppat.1004789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor SM, Parobek CM, De Conti DK, Kayentao K, Coulibaly SO, Greenwood BM, Tagbor H, Williams J, Bojang K, Njie F, Desai M, Kariuki S, Gutman J, Mathanga DP, Mårtensson A, Ngasala B, Conrad MD, Rosenthal PJ, Tshefu AK, et al. Absence of putative artemisinin resistance mutations among Plasmodium falciparum in sub-Saharan Africa: a molecular epidemiologic study. J Infect Dis. 2014;211(5):680–688. doi: 10.1093/infdis/jiu467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tse EG, Korsik M, Todd MH. The past, present, and future of anti-malarial medicines. Malar J. 2019 doi: 10.1186/s12936-019-2724-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tun KM, Imwong M, Lwin KM, Win AA, Hlaing TM, Hlaing T, Lin K, Kyaw MP, Plewes K, Faiz MA, Dhorda M, Cheah PY, Pukrittayakamee S, Ashley EA, Anderson TJC, Nair S, McDew-White M, Flegg JA, Grist EPM, et al. Spread of artemisinin-resistant Plasmodium falciparum in Myanmar: A cross-sectional survey of the K13 molecular marker. Lancet Infect Dis. 2015;15(4):415–421. doi: 10.1016/S1473-3099(15)70032-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinayak S, Biswas S, Dev V, Kumar A, Ansari MA, Sharma YD. Prevalence of the K76T mutation in the pfcrt gene of Plasmodium falciparum among chloroquine responders in India. Acta Trop. 2003;87(2):287–293. doi: 10.1016/S0001-706X(03)00021-4. [DOI] [PubMed] [Google Scholar]
- Wang Z, Wang Y, Cabrera M, Zhang Y, Gupta B, Wu Y, Kemirembe K, Hu Y, Liang X, Brashear A, Shrestha S, Li X, Miao J, Sun X, Yang Z, Cui L. Artemisinin resistance at the China-Myanmar border and association with mutations in the K13 propeller gene. Antimicrob Agents Chemother. 2015;59(11):6952–6959. doi: 10.1128/AAC.01255-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witkowski B, Menard D, Amaratunga C, Fairhurst R (2013a) Ring stage Survival Assays (RSA) to evaluate the in vitro and ex vivo susceptibility of Plasmodium falciparum to artemisinins. Institute Pasteur du Cambodge- National Institutes of Health Procedure RSAv1
- Witkowski B, Amaratunga C, Khim N, Sreng S, Chim P, Kim S, Lim P, Mao S, Fairhurst RM, Menard D. Novel phenotypic assays for the detection of artemisinin-resistant Plasmodium falciparum malaria in Cambodia: in-vitro and ex-vivo drug-response studies. Lancet Infect Dis. 2013;13(12):1043–1049. doi: 10.1016/S1473-3099(13)70252-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodrow CJ, White NJ. The clinical impact of artemisinin resistance in Southeast Asia and the potential for future spread. Microbiol Rev. 2017;41(1):34–48. doi: 10.1093/femsre/fuw037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO (2018) Artemisinin resistance and artemisinin-based combination therapy efficacy. https://www.who.int/malaria/publications/atoz/artemisinin-resistance-august2018/en/. Accessed 12 Oct 2020
- WHO (2019) World Malaria Report 2019. https://www.who.int/publications-detail/world-malaria-report-2019. Accessed 12 Oct 2020
- Wykes MN. Why haven’t we made an efficacious vaccine for malaria? EMBO Rep. 2013;14:661. doi: 10.1038/embor.2013.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye R, Hu D, Zhang Y, Huang Y, Sun X, Wang J, Chen X, Zhou H, Zhang D, Mungthin M, Pan W. Distinctive origin of artemisinin-resistant Plasmodium falciparum on the China-Myanmar border. Sci Rep. 2016 doi: 10.1038/srep20100. [DOI] [PMC free article] [PubMed] [Google Scholar]