Abstract
The antiviral effect of acyclovir elaidate in the female guinea pig model of genital herpes was investigated in a series of experiments. The antiherpesvirus effects of this novel compound, 9-(2′-[trans-9"-octadecenoyloxyl]ethoxymethyl)guanine (code no. P-4010), were studied in both primary and recurrent genital herpes in the female guinea pig, following oral gavage or intraperitoneal injection, with different formulations of the compound, and in comparison with acyclovir (ACV) or penciclovir (PCV). The results indicate that compound P-4010 has a greater capability than either ACV or PCV in reducing the clinical symptoms of primary genital herpes induced following the inoculation of herpes simplex virus type 2 (HSV-2) intravaginally into guinea pigs. In addition, the administration of P-4010 twice daily over a 10-day period by the intraperitoneal route (15 to 40 mg/kg of body weight/day) or by oral gavage (50 to 200 mg/kg/day), commencing 4 h subsequent to intravaginal HSV-2 infection, resulted in a degree of reduction in the incidence and severity of spontaneous, recurrent genital herpes in these animals. The findings are discussed in the light of the value and relevance of the female guinea pig model of genital herpes for the assessment of anti-herpes simplex virus compounds.
The incidence and severity of disease produced by the herpes simplex viruses type 1 and type 2 (HSV-1 and HSV-2, respectively) have been increasing in recent years (6, 19, 33), especially in the immunocompromised host where viral resistance to acyclovir (ACV) represents a particular problem (6). This trend has led not only to a search for completely novel antiherpesvirus compounds but also, because of the success of ACV, to attempts to improve the pharmacokinetics and bioavailability of this proven and highly useful compound through various structural and biochemical modifications. This research has led to the development of a number of ACV-derived, anti-herpes simplex virus drugs such as penciclovir (PCV), famciclovir (4, 32), and valaciclovir (1).
A number of animal models are available for evaluation of the antiviral effects of antiherpesvirus compounds, and among these the guinea pig has been widely employed (2, 3, 5, 12, 14, 21). The female guinea pig model of genital herpes has been used previously for evaluation of ACV (12) and foscarnet (15), in addition to its more recent, extensive use for experimental assessment of vaccines against HSV (10, 17, 26). The model resembles HSV infection of humans, in that both primary and recurrent disease occur (25, 27, 30), although the spontaneous recurrent, genital lesions cease 3 to 5 months after the animals recover from primary disease.
Although both primary and spontaneous, recurrent genital herpes in the guinea pig resemble the disease in humans, differences have been reported between these species in the way deoxyguanosine analogues, such as ACV, and ACV-derivatized compounds are metabolized (8). In guinea pigs, ACV undergoes significant biotransformation, while this is not the case in humans, rodents, or dogs.
The studies reported here are concerned with investigations into the anti-HSV effects of a novel ACV derivative, P-4010, with the female guinea pig model of genital herpes. The effects of the novel compound on both primary and recurrent genital herpes in the guinea pig following administration via the oral or intraperitoneal (i.p.) routes are investigated, with ACV or PCV as comparators in some experiments. The value of the female guinea pig experimental model as a means of assessing novel anti-HSV compounds is discussed.
MATERIALS AND METHODS
Virus strain and virus isolation procedures.
The strain of HSV-2 used throughout this study for intravaginal (i.vag.) infection of guinea pigs was MS (VTCC VR-540). The virus was originally supplied courtesy of L. R. Stanberry (University of Cincinnati College of Medicine, Cincinnati, Ohio), was grown on Vero cell cultures (Flow Laboratories, Irvine, Scotland), and was titrated by standard plaque-forming assay (18, 23).
Vero cell cultures were also used for detection of virus in guinea pig vaginal swabs. Each swab was expressed into 1.0 ml of Eagle’s maintenance medium containing streptomycin and penicillin, and samples were used to infect monolayers in 24-well plates. Each sample was inoculated both undiluted and at serial 10-fold dilutions in triplicate onto Vero cell cultures, and the assay was performed as described earlier (18).
Antiviral compounds.
P-4010, obtained from Norsk Hydro ASA, Porsgrunn, Norway, is a new chemical entity, formed through a combination of ACV (9[2-hydroxyethoxymethyl]guanine) and elaidic acid (trans-9-octadecenoic acid). With a common name of ACV elaidate, P-4010 has a molecular weight of 489.66 and is only slightly soluble in water. The structure of P-4010 is shown in Fig. 1.
FIG. 1.
Structure of P-4010.
P-4010 was supplied in three different forms for use in the study; as a microparticle injection formulation for i.p. inoculation and in a micronized or nonmicronized form to be incorporated into corn oil for delivery by oral gavage. Placebos for use as control preparations matched the base vehicle for each formulation and therefore consisted of either corn oil or the microparticle base vehicle when the i.p. route was employed. Corn oil (C287) was obtained from Sigma Ltd., Poole, Dorset, England. In some experiments, the viscosity of the inoculum was reduced by the incorporation of 5% ethanol. In addition, in most experiments phosphate-buffered saline (pH 7.2; PBS) was also used as a control.
ACV is an analogue of deoxyguanosine in which the deoxyribose is replaced by an acyclic side chain (8) and is a recognized and selective inhibitor of the HSVs (24). For oral or i.p. delivery to guinea pigs, ACV was purchased from the pharmacy at the Royal Hallamshire Hospital, Sheffield, United Kingdom, and prepared for oral administration or i.p. injection based on the manufacturer’s instructions.
PCV has a longer half-life and can achieve higher intracellular concentrations than ACV, although it has lower potency (6, 31). It is a non-water-soluble derivative of ACV, 9-(4-hydroxy-3-hydroxymethylbut-1-yl)guanine (28), and was kindly supplied, as a powder, by Steve Trowbridge, SmithKline Beecham, Worthing, England. For i.p. injection, PCV was prepared as a suspension in PBS, pH 7.2.
Both ACV and PCV were used in the guinea pig experimentation at concentrations equimolar to that of the compound, P-4010, and administered to the animals by using routes, times, and procedures identical to those used for P-4010.
Guinea pigs.
All the female, Dunkin-Hartley guinea pigs used in this study were obtained from David Hall, Darley Oaks Farm, Newchurch, Nr. Burton-on-Trent, England. The animals were all young adults weighing between 360 and 550 g, were caged in groups of two to six, and were fed and watered ad libitum.
Experimental protocols.
The results of seven experiments are described in the present study, and these, with details of the compounds used, their dosages, and routes of administration, are listed in Table 1. All experiments included initial i.vag. infection with HSV-2 (strain MS) at concentrations of 105.0 to 105.8 PFU with procedures described in detail elsewhere (17, 30). At times ranging from 4 to 18 h subsequent to HSV-2 i.vag. infection, treatment with compound P-4010, ACV, or PCV and with placebo or PBS as control materials was commenced, and in each experiment these materials were administered twice daily over a 10- or 11-day period. In all but experiment 3, the initial treatment dose was given 4 h subsequent to HSV-2 infection followed by 9 days of treatment, with the final, 20th, dose given on the morning of day 10 postinfection (p.i.). In experiment 3, treatment commenced 18 h after HSV-2 infection and continued over 10 days. In three experiments, experiments 1, 2, and 4, compounds or control materials were given by i.p. injection; in the remaining four experiments, experiments 3, 5, 6, and 7, oral gavage was used. i.p. injection of P-4010, ACV, PCV, or control materials was carried out with an 18-gauge needle and volumes of 500 μl were injected on each occasion. Oral gavage was undertaken with 18-gauge gavage needles (IMS, Dane Mill, Congleton, Cheshire, United Kingdom). Volumes given ranged from 400 to 650 μl, depending on the weight of the animal. Preliminary dose-response experimentation and careful observation throughout the experiments described in this study indicated a degree of toxicity for both ACV and P-4010 given by the oral gavage or i.p. routes in the regimens used. The higher dosages used were up to the tolerance levels displayed by the animals to P-4010. Although all experiments consisted of a primary phase of HSV-2 infection during which test compounds or control materials were administered and the HSV-2-induced disease symptoms were monitored, two experiments, 5 and 7, continued into the recurrent phase of HSV-2 infection in the female guinea pig model (17, 27). Both experiments embraced HSV-2 recurrent-phase monitoring of spontaneous recurrent lesions of all animals and, in experiment 5, a further 10-day treatment protocol by oral gavage. Three of the experiments (1, 2, and 3) were concerned with comparisons between the compound P-4010 and ACV or PCV, while a further three, experiments 4, 5, and 6, comprised dose-response evaluations of compound P-4010. Experiment 7 was primarily an investigation into the effect of P-4010 treatment on the incidence and extent of recurrent HSV-2 disease.
TABLE 1.
Primary genital herpes disease severity in guinea pigs treated with P-4010 or ACV
| Expt no. | No. of animals/ group | Treatment (dose and route)a | Value for parameter of clinical disease
|
Index of disease severityb | |||
|---|---|---|---|---|---|---|---|
| Duration of symptoms (days) | Mean total CLS/ guinea pigc | Mean IES/ guinea pigc | No. of days when CLS ≥ 10 | ||||
| 1 | 10 | P-4010 (40; i.p.) | 8.1 | 109.4*† | 0.30*‡ | 4.4 | 13.5 |
| ACV (20; i.p.) | 8.8 | 185.5 | 0.95 | 7.3 | 21.1 | ||
| Placebo (i.p.) | 9.1 | 223.3 | 1.29 | 7.4 | 24.5 | ||
| 3 | 10 | P-4010 (200; o.g.) | 8.4 | 54.1** | 0.26**† | 1.1 | 6.4 |
| ACV (100; o.g.) | 8.4 | 86.5 | 0.64 | 3.4 | 10.3 | ||
| Placebo (o.g.) | 9.4 | 115.4 | 0.67 | 4.1 | 12.2 | ||
| 4 | 16 | P-4010 (40; i.p.) | 8.5 | 104.3** | 0.65** | 4.6 | 12.3 |
| P-4010 (30; i.p.) | 8.5 | 114.9 | 0.72** | 4.9 | 13.5 | ||
| P-4010 (15; i.p.) | 7.4 | 110.2** | 0.78 | 4.2 | 14.9 | ||
| Placebo (i.p.) | 8.7 | 162.1 | 1.15 | 6.1 | 18.6 | ||
| 6 | 12 | P-4010 (200; o.g.) | 10.0 | 69.3 | 0.26 | 2.2 | 6.9 |
| P-4010 (100; o.g.) | 9.5 | 80.1 | 0.25 | 3.4 | 8.5 | ||
| P-4010 (50; o.g.) | 9.8 | 122.0 | 0.35 | 4.0 | 12.4 | ||
| Placebo (o.g.) | 9.6 | 103.1 | 0.50 | 4.3 | 10.7 | ||
| 7 | 36 | P-4010 (300; o.g.) | 7.1 | 30.1** | 0.16** | 0.9 | 4.2 |
| Placebo (o.g.) | 7.7 | 56.1 | 0.28 | 1.6 | 7.3 | ||
Dose shown as milligrams per kilogram per day; administered i.p. or by oral gavage (o.g.).
Ratio of mean total CLS to duration of symptoms.
* and **, values shown are significantly lower (*, P < 0.005; **, P < 0.05) than those observed for the placebo groups; † and ‡, values shown are significantly lower (†, P < 0.05; ‡, P < 0.005) than those observed for the ACV groups.
In each experiment, except experiment 7, each test or control group consisted of 10 to 16 guinea pigs; however, for experiment 7 there were 72 animals in two groups of 36 each at the commencement of the experiment. Fifty-two surviving animals that had recovered from primary disease with a clear, unscarred vaginal area suitable for recurrency scoring were divided into two groups of 26 each. All animals were weighed daily, commencing 2 or 3 days prior to HSV-2 i.vag. infection. In most experiments, vaginal swabs were collected on day 2, 3, or 4 p.i. with a dry swab, placed in Eagle’s maintenance medium, and stored at −80°C for subsequent analysis by plaque assay.
Monitoring of primary or recurrent HSV-2 disease in guinea pigs.
Monitoring of disease has been described in detail elsewhere (10, 17, 20) and was carried out for 12 days following primary HSV-2 infection (in addition to 2 or 3 days prior to infection to establish baseline levels where required) under blind conditions. Briefly, numbers of lesions and levels of edema (proportional increase in vaginal area) were recorded for each individual animal on a daily basis. As primary HSV-2 disease symptoms developed, each animal received a twice-daily abdominal massage by an experienced operator to relieve severe urinary retention, and the urine expressed from each animal during this massage was collected, pooled groupwise, and weighed. Lesions were recorded as the combined lesion score (CLS), that is to say, the total lesions (vesicles, pustules, ulcers, and scabs) were counted. The increase in vaginal area, as calculated from measurements of vaginal size in two directions by a transparent rule, was recorded as the index of edema score (IES). In all but two experiments, experiments 5 and 7, all guinea pigs were sacrificed on day 14 p.i. Involvement of the central nervous system, manifest as temporary hind-leg paralysis lasting 3 to 4 days, was noted in 9.8% of the animals during the primary phase of the disease.
Recurrent HSV-2 disease monitoring of all guinea pigs in experiments 5 and 7 that had recovered from primary HSV-2 disease with no significant vaginal scarring was undertaken on either a daily basis (experiment 7) to day 58 p.i. or on every other day up to day 102 p.i. (for experiment 5). Scoring was for the presence and number of vaginal lesions, providing evidence for spontaneous recurrent HSV vaginal disease under a blinded procedure achieved by cage randomization by an independent worker. Recurrency scoring commenced on day 20 p.i. for experiment 5 and day 29 p.i. for experiment 7.
Statistical analyses were carried out with both a Mann-Whitney nonparametric ranking test not adjusted for multiple comparisons and an unpaired Student t test.
RESULTS
HSV-2-induced primary disease in P-4010-, ACV-, or PCV-treated guinea pigs.
Details of treatment, doses, and routes of administration are summarized in Table 1. The results for the CLS and IES following i.vag. HSV-2 infection of groups of 10 to 16 guinea pigs receiving P-4010, ACV, or PCV treatment or placebo are shown in Fig. 2. The results are from three experiments, two in which P-4010 or ACV was administered by the i.p. route (Fig. 2a to d) and the third, in which the compounds were given by oral gavage (Fig. 2e and f). In each experiment, irrespective of route, the compounds were administered as indicated in Materials and Methods, commencing 4 h subsequent to i.vag. HSV-2 infection and continuing on a twice-daily basis over a 10-day period.
FIG. 2.
Course of primary genital HSV-2 disease in guinea pigs treated with compound P-4010, ACV, or PCV or given placebo or PBS. Shown are CLSs (a, c, and e) or IESs (b, d, and f) in experiments 1 (a and b), 2 (c and d), and 3 (e and f). □, P-4010 (i.p., 40 mg/kg/day [experiments 1 and 2]; oral gavage, 200 mg/kg/day [experiment 3]). ◊, ACV (i.p., 20 mg/kg/day [experiment 1]; oral gavage, 100 mg/kg/day [experiment 3]) or PCV (i.p., 20 mg/kg/day [experiment 2]). ○, Placebo (i.p. [experiments 1 and 2]; oral gavage [experiment 3]). ▵, PBS (i.p. [experiment 2]).
It can be seen in all three experiments that both the CLS and the IES were markedly lower in the animals treated with P-4010 than in those given placebo or PBS over the 12-day period of observation. Thus, in experiment 1 (Fig. 2) both the mean CLS and the mean IES were significantly lower in animals receiving P-4010 than in animals receiving placebo or PBS (P < 0.005). Similar reductions in CLS and IES were observed in experiment 3 (P < 0.05). Differences observed in experiment 2 were not statistically significant. In addition, in all three experiments, the CLS and IES in guinea pigs receiving P-4010 were lower than in those animals treated with ACV or PCV, and significantly lower in experiment 1 (Fig. 2) for both the CLS (P < 0.05) and the IES (P < 0.005), and in experiment 3 for the IES only (P < 0.05). However, no other differences between P-4010 and ACV or PCV with respect to these scores were significant. In all three experiments, the pattern of local, genital HSV-2 disease was essentially identical, with HSV-2-specific lesions and vaginal edema appearing on days 4 or 5 p.i., peak disease levels being observed over days 5 to 8, and the symptoms declining thereafter, although low-level symptoms might persist in some animals up to the end of the observation period and beyond.
HSV-2-induced primary disease in guinea pigs treated with varying concentrations of P-4010.
In three separate experiments, the dose-response characteristics of primary HSV-2 genital disease in groups of 8 to 16 guinea pigs receiving varying concentrations of P-4010 by either the i.p. route (Fig. 3a and b) or oral gavage (Fig. 3c to f) were determined. Animals treated with P-4010 showed markedly, and in some instances significantly, lower CLS and IES than animals receiving placebo or PBS, although a clear-cut dose-response effect was observed only in animals treated with P-4010 by oral gavage (Fig. 3c to f). Thus, in experiments 4 and 5 (Fig. 3), at the highest concentration used animals receiving P-4010 treatment showed significant (P < 0.05 and P < 0.005, respectively) reductions in both mean CLS and mean IES compared to those given placebo or PBS. In experiment 4, the mean IES in animals receiving 30 mg/kg of body weight/day and the mean CLS in animals receiving 15 mg/kg/day were significantly lower (P < 0.05) than those observed in the placebo group. In experiment 5 (Fig. 3), at the lower concentration used both CLS and IES were significantly reduced (P < 0.05) in animals treated with P-4010 compared to animals given placebo. Differences seen in experiment 6 were not significant. As in the initial experiments described (Fig. 2), the courses of HSV-2 genital infection in the guinea pigs in all three experiments were identical, commencing on day 4 or 5 p.i., reaching peaks over days 5 to 9, and decreasing thereafter.
FIG. 3.
Course of primary genital HSV-2 disease in guinea pigs treated with varying doses of P-4010 or given placebo or PBS. Shown are CLSs (a, c, and e) or IESs (b, d, and f) in experiments 4 (a and b), 5 (c and d), and 6 (e and f). □, P-4010 (i.p., 40 mg/kg/day [experiment 4]; oral gavage, 200 mg/kg/day [experiments 5 and 6]). ◊, P-4010 (i.p., 30 mg/kg/day [experiment 4]; oral gavage, 100 mg/kg/day [experiments 5 and 6]). ○, P-4010 (i.p., 15 mg/kg/day [experiment 4]; oral gavage, 50 mg/kg/day [experiment 6]). ⊞, Placebo (i.p. [experiment 4]; oral gavage [experiments 5 and 6]). ▵, PBS (oral gavage [experiment 5]).
Urine retention as a disease indicator in guinea pigs infected with HSV-2 i.vag.
In five of the six experiments described above, the extent of urine retention, as a feature of HSV-2 genital infections in guinea pigs, was assessed by collecting the urine obtained from the routine massage of the animal’s abdomen on a twice-daily basis. The urine thus obtained was pooled for each group and weighed, and the mean total weight of urine per guinea pig expressed from each group, in each experiment, over the observation period was determined (data not shown). For each experiment, with the exception of experiment 6, the amount of urine that could be expressed for each of the groups was greater in control animals, i.e., those treated with either placebo or PBS, than in those animals receiving P-4010, and also generally relatively low in those animals receiving P-4010 as opposed to equimolar amounts of ACV or PCV.
Significant urine retention, as determined from the differences in the weights of urine recovered over the period of primary disease from animals treated with P-4010 compared to groups given placebo or PBS, was noted in experiment 1 (P < 0.05), experiment 3 (P < 0.005), and, with regard to the higher dosage of P-4010 used, in experiment 5 (P < 0.05). No other statistically significant differences were observed. In experiment 6, a marginally greater volume of urine was obtained from animals given P-4010 than from those given placebo.
Severity of HSV-2 primary disease in guinea pigs treated with P-4010, ACV, or PCV or given placebo or PBS.
Table 1 shows the severity of HSV-2 genital infection in treated or control guinea pigs in terms of the duration of local genital disease, total CLS, mean lesion scores per animal, mean increase in edema per animal, and number of animals with relatively high (≥10) lesion scores. As would be expected, the results reflect those shown in Fig. 2 and 3 and indicate that in general groups infected i.vag. with HSV-2 and then treated with relatively high doses of the novel antiherpesvirus compound P-4010 showed a shorter disease time span, lower total mean lesion scores, less edema, and fewer animals presenting with relatively high lesion scores than were observed in those groups receiving lower doses of P-4010 or else treated with ACV or PCV or given placebo or PBS (Table 1). In spite of this, only a very few guinea pigs showed no evidence of local HSV-2 infection. Thus, in experiment 1, in which P-4010, used at a concentration of 40 mg/kg/day, was compared with an equimolar concentration of ACV, both given over a 10-day period by i.p. injection, all animals suffered clinical symptoms. However, the duration of symptoms was 8.1 days for the former and 8.8 days for the latter group, and this compares with 9.1 days for the placebo group. Equivalent values for the mean total CLS per animal over the 12-day period of observation are 109.4, 185.5, and 223.3, while the mean numbers of occasions on which CLSs of ≥10 were recorded were 4.4, 7.3, and 7.4, respectively (Table 1). The mean CLS and IES were significantly lower in the group receiving P-4010 than in the group given placebo. As in experiment 6, similar effects were observed; thus, animals receiving 200, 100, or 50 mg of P-4010 per kg per day showed mean total CLS per animal over the 12-day observation period of 69.3, 80.1, and 122.0, respectively, while the duration of symptoms was 10.0, 9.5, and 9.8 days and the number of occasions on which CLSs of ≥10 were recorded was 2.2, 3.4, and 4.0, respectively (Table 1). All these values can be compared to those obtained for the control group of experiment 6, receiving PBS, which were 103.1 for the mean total CLS per guinea pig, 9.6 days for the duration of symptoms, and 4.3 for number of episodes when the CLS was ≥10 (Table 1). An index of disease severity, obtained by calculating the ratio of mean total CLS to the duration of symptoms, is also presented in Table 1 and shows that, in every experiment, animals receiving P-4010 showed signs of protection against HSV-2 disease symptoms compared to the control materials and that in all experiments this protection was greater than that observed in guinea pigs given ACV. In addition, irrespective of the severity of disease observed in control animals in the different experiments, there was a reduction in the index of disease severity of at least 34% in animals treated with the highest dose of P-4010.
Incidence and level of virus recovery from guinea pig vaginal swabs.
In three of the six experiments described above, experiments 1, 2, and 4, together with one further experiment, experiment 7, aimed at assessing any influence of P-4010 treatment, on both the primary genital HSV-2 infection of guinea pigs and the recurrency phase of infection, vaginal swabs collected on day 2 were tested for the presence and level of HSV-2 by standard plaque assay on Vero cell cultures. The results (Table 2) show that virus was isolated from vaginal swabs from most guinea pigs on day 2, irrespective of the treatment regimen administered, and that these mean titers ranged from 103.06 to 105.06 PFU/ml in the guinea pigs that were shedding detectable virus at this time. As would be expected, the geometric mean titers of virus were lower in vaginal swabs from treated animals (Table 2) when all animals are considered, but in no case did the difference reach a value of 2.0 logs.
TABLE 2.
Incidence and level of virus titers in guinea pig vaginal swabs collected on day 2 p.i.
| Expt no. | Treatment group (dose [mg/kg/day]) | No. of animals/ group | No. positive for virus (%) | Mean virus titer (log10 PFU/ml)
|
|
|---|---|---|---|---|---|
| All animals | Positive animals | ||||
| 1 | P-4010 (40) | 10 | 10 (100.0) | 4.14 | 4.14 |
| ACV (20) | 9 (90.0) | 3.81 | 5.06 | ||
| Placebo | 10 (100.0) | 4.85 | 4.85 | ||
| 2 | P-4010 (40) | 15 | 11 (73.3) | 1.95 | 3.86 |
| PCV (20) | 14 (93.3) | 3.18 | 3.77 | ||
| Placebo | 14 (93.3) | 3.02 | 3.56 | ||
| PBS | 13 (86.7) | 2.29 | 3.13 | ||
| 4 | P-4010 (40) | 16 | 16 (100.0) | 3.25 | 3.25 |
| P-4010 (30) | 13 (81.3) | 2.39 | 3.86 | ||
| P-4010 (15) | 12 (75.0) | 1.92 | 3.57 | ||
| Placebo | 14 (87.5) | 2.65 | 3.61 | ||
| 7 | P-4010 (300) | 36 | 23 (65.7)a | 1.31 | 3.70 |
| Placebo | 31 (86.1) | 2.05 | 3.06 | ||
Swab not available from one animal.
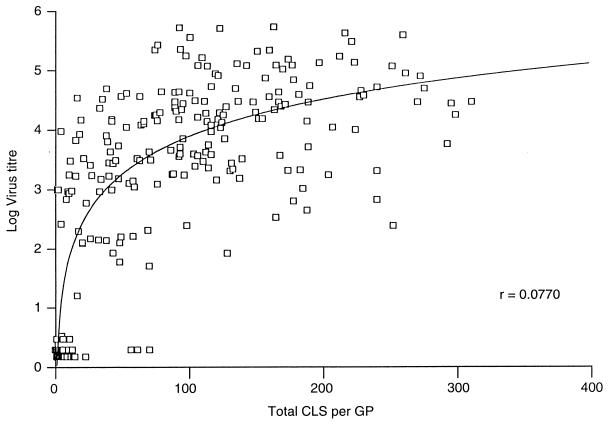
Figure 4 shows the extent of correlation between the amount of HSV-2 recovered from guinea pig vaginal swabs on day 2 p.i. and the mean total CLS over the overall, 12-day observation period for experiments 1, 2, 4, and 7. It can be seen that good correlation (r = 0.77) exists between these two parameters, indicating that the clinical symptoms observed over 12 days are a reflection of the extent of virus replication as determined on day 2 p.i.
FIG. 4.
Correlation of virus titers detected in vaginal swabs collected from HSV-2-infected guinea pigs on day 2 p.i. with the total CLS per guinea pig (GP) over the 12-day scoring period. Each point represents one guinea pig from combined experiments 1, 2, 4, and 7.
Recurrent HSV-2 disease in P-4010-treated guinea pigs.
In two experiments, numbers 5 and 7, following primary HSV-2 infection and treatment by oral gavage during this period with P-4010, placebo, or PBS control, animals recovering from infection were left for 2 weeks (day 28 p.i.) and then scored as described in Materials and Methods for spontaneous, recurrent HSV-2 lesions in the vaginal area. In experiment 5, there were 18 guinea pigs per group, while in experiment 7, there were 26 animals per group. Scoring continued every other day up to day 102 p.i. (with a 14-day break, days 43 to 56 p.i.) for experiment 5 and up to day 58 p.i., without a break and on a daily basis, for experiment 7. In both experiments, guinea pigs received either P-4010 or placebo by oral gavage over a 10-day time span (200 mg/kg/day, twice daily) during the primary illness. In experiment 5 only, P-4010 or placebo was administered on days 62 to 71 p.i. as therapeutic intervention during the recurrency phase.
The results are presented in Fig. 5 and show that, in both experiments, there was a lower mean cumulative recurrency lesion score for animals treated with P-4010 than for those receiving placebo during the primary HSV-2 infection. In experiment 5 (Fig. 5a), this difference was significant (P < 0.05), but significance was not achieved for experiment 7. In experiment 5, therapeutic intervention with P-4010 (or placebo) was found to have no detectable effects on recurrency rate or severity.
FIG. 5.
Mean cumulative recurrent genital lesion scores in guinea pigs undergoing spontaneous HSV-2 recurrent infections over time, subsequent to recovery from primary genital HSV-2 disease. (a) Experiment 5; (b) experiment 7. □, Animals treated with P-4010 during primary genital HSV-2 disease. ◊, Animals receiving placebo or PBS during primary genital HSV-2 disease. ↑, Start and finish day of 10-day oral gavage dosing with P-4010.
Table 3 shows the results of the recurrency scoring for each experiment in terms of the mean total lesion score per guinea pig, the mean number of days on which spontaneous recurrency lesions were observed, the mean number of days for each group on which ≥3 lesions were scored, and the index of recurrent disease severity. It can be seen that, for experiment 5, marked reductions in all those parameters were recorded for the P-4010-treated group, and this difference was significantly lower (P < 0.05) than that for the placebo-treated group with respect to the mean number of days on which spontaneous recurrent lesion scores were equal to or greater than three (Table 3). For experiment 7, similar observations were made. Thus, the total number of lesions scored over the total recurrency scoring period was 912 for the group given the corn oil placebo during primary infection, compared to 543 for the group receiving P-4010, giving mean total lesion scores per guinea pig of 30.4 and 17.5, respectively, and this difference was significant (P < 0.005). In addition, there were significant reductions, in the P-4010-treated group compared to the control group, in the mean number of days on which lesions were observed (P < 0.05) and the mean number of days on which lesion scores of ≥3 were recorded (P < 0.005) over the observation period (Table 3).
TABLE 3.
Incidence and severity of recurrent genital herpes in guinea pigs treated with P-4010 or given placebo during primary HSV-2 infection
| Expt no. | No. of animals in recurrent phase | Treatment during primary infection | Total no. of lesions | Mean total lesion score/guinea pig
|
Mean no. of days on which lesions observed
|
Lesion score of ≥3
|
Index of recurrent disease severitya | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| % of guinea pigs | Mean no. of days
|
||||||||||
| No. | % Redb | No. | % Red | No. | % Red | ||||||
| 5 | 18 | P-4010 | 823 | 48.4 | 42 | 27.2 | 12 | 94 | 5.3c | 48 | 1.78 |
| 18 | Placebo | 1,302 | 69.3 | 31.0 | 100 | 10.2 | 2.24 | ||||
| 7 | 26 | P-4010 | 543 | 17.5c | 30 | 10.9c | 20 | 61 | 1.6c | 64 | 1.61 |
| 26 | Placebo | 912 | 30.4 | 13.6 | 77 | 43 | 2.24 | ||||
Ratio of mean total lesion score per guinea pig to mean number of days on which lesions were observed.
% Red, percent reduction from control (placebo).
Significantly lower than the control (placebo) value.
Finally, the index of recurrent disease severity calculated from the ratio of the mean total lesion score per guinea pig to the mean number of days on which lesions were observed gives values indicating a lower recurrent disease severity for both experiments with guinea pigs treated with P-4010 during primary HSV-2 genital disease than for control animals.
DISCUSSION
It is clear that, following administration by either oral gavage or i.p. injection, compound P-4010 had the effect of ameliorating the symptoms of clinical disease in the experimental female guinea pig model of genital herpes, compared to control, mock-treated animals; these effects were significant for both primary genital herpes and recurrent disease. Thus, for each of the parameters of primary disease, CLS, IES, urine retention, and disease severity, lower scores were recorded in seven separate experiments for animals receiving treatment with P-4010 over a 10-day period than for animals receiving placebo or PBS. Treatment with P-4010 commencing usually 4 h subsequent to infection, however, had little discernible effect in reducing virus shedding, as determined by virus titer levels in vaginal swabs, in comparison to those detected in mock-treated animals on day 2 p.i. Nevertheless, there was evidence of a strong correlation between the total CLS per guinea pig over the 12-day observation period and the log titer of virus detected in vaginal swabs collected on day 2 from individual guinea pigs. Other workers using the guinea pig model of genital herpes to evaluate the antiherpesvirus effects of ACV have also reported that, although the clinical manifestations of primary genital herpes can be reduced by such treatment, the extent of virus shedding from the genital tract remains unaffected (2, 12, 21).
In the current studies, treatment with P-4010 during primary genital infection was also associated, in two separate experiments, with significant reductions in the incidence and severity of spontaneous recurrent disease, over periods of 1 or 3 months, although therapeutic intervention over this time with P-4010 had no detectable influence on the rates or frequency of recurrent infection. It is of some interest to note that in one further experiment in which only therapeutic, and no prophylactic, treatment of HSV-2-infected animals with P-4010 was carried out, the recurrency scoring patterns of P-4010- and placebo-treated guinea pigs were similar. This implies that treatment of guinea pigs with P-4010 commencing 4 h subsequent to primary infection may be inhibiting the establishment of latent infection, perhaps by limiting the numbers of virions available to invade the innervating nerve tissue. Using a mouse model, other workers have reported that treatment with nucleoside analogues can reduce the establishment of latent infections perhaps through reducing the amount of or slowing the tissue damage that occurs, thereby decreasing the amount of contact between virus and nerve tissue and/or giving more time for both immune and nonimmune defense mechanisms to achieve peak levels of activity (6, 29).
The female guinea pig model of genital herpes has been widely used for the in vivo evaluation of anti-HSV compounds (2, 3, 12, 21) and candidate HSV vaccines (7, 10, 17, 26, 30) and is a model that, unlike the mouse model, is characterized by both primary and spontaneous, recurrent clinical disease (25, 27). In the current studies, besides the clinical parameters of disease lesions and edema associated with the external vaginal surface, the extent of urine retention was measured as a possible correlate of the internal inflammatory response to HSV infection. These measurements showed some discrimination between groups of animals receiving P-4010 or ACV and the control or placebo- or PBS-treated groups, although this was not clear-cut in every experiment; in addition, although there was some correlation of urine retention with other clinical parameters of HSV infection, this, again, was not consistently observed.
A number of compounds related to or derived from ACV have recently been developed with the aim of improving the potency, bioavailability, and pharmacokinetics of ACV; permitting less frequent dosing, increased ACV stability, prolonged drug activity, reduced toxicity, greater activity against ACV-resistant strains, particularly in immunocompromised individuals; and perhaps influencing the establishment of latent infection (6, 16, 22, 28, 29). These compounds include valaciclovir, the l-valyl ester of ACV; ganciclovir, an acyclic nucleoside analogue of guanine; PCV, a derivative of ACV; and famciclovir, a diacetyl prodrug of PCV. The novel compound used in the present studies is 9-(2′-[trans-9"-octadecenoyloxy]ethoxymethyl)guanine (common name, ACV-elaidate) (code no. P-4010), formed through a combination of ACV and elaidic acid (trans-9-octadecenoic acid). In the studies described here, P-4010 was compared with ACV or PCV used at equimolar concentrations, and although statistically significant differences between P-4010 and its comparators, in terms of the extent of clinical genital disease for all parameters, were not achieved in all experiments, compound P-4010 performed markedly better than ACV or PCV in the primary parameters of this model, CLS and IES. Few studies comparing ACV derivative compounds with ACV itself in vivo have been reported, although PCV has been found to have efficacy similar to that of ACV when administered orally to mice, in reducing mortality following HSV-1 intranasal challenge (11), while in an HSV-1 zosteriform mouse model, no differences among ACV, PCV, or its prodrug famciclovir in one or two dosage regimens over a 5-day period were apparent (9).
In the studies described here, P-4010 was essentially well tolerated by the guinea pigs following either i.p. injection or oral gavage, although there was some evidence of failure to gain weight and indeed some weight loss, compared to control animals given placebo or PBS, in some animals given P-4010 by oral gavage.
The anti-HSV activity of P-4010 in the female guinea pig model of genital herpes was manifest under several conditions, including route of administration (oral gavage or i.p. injection) and formulation (as a water-insoluble, nonmicronized powder in corn oil, as a micronized form in corn oil, or as a microparticle formulation for injection), and in both primary and recurrent disease situations in the animals. In all experiments under these sets of conditions, P-4010 was detectably, but not always significantly, more effective in reducing clinical disease than both the control preparations and ACV. However, in two separate experiments with guinea pigs undergoing spontaneous, recurrent clinical disease, therapeutic intervention with P-4010 failed to have any discernible clinical effects.
Although guinea pigs have been used for in vivo evaluation of anti-HSV compounds, there is evidence to suggest that the animal may be less relevant to humans than is the mouse, as the metabolisms of deoxyguanosine analogues such as ACV and ACV-derivatized compounds in guinea pigs and humans are different (8). Significant biotransformation of ACV occurs in guinea pigs, with relatively high percentages of input drug being recovered as 9-carboxymethoxymethylguanine (19 to 29%) or 8-hydroxyacyclovir (2 to 4%) in the urine. In contrast, approximately 90% of input ACV is excreted unchanged in humans. Guinea pigs have high levels of the enzyme aldehyde oxidase (13), which catalyzes the 8-hydroxylation of ACV, and can also efficiently oxidize ACV to 9-carboxymethoxymethylguanine (8). In spite of these differences, other workers (28) have used this model to demonstrate activity of PCV in significantly reducing lesion severity following topical application of this compound to the skin or external genitalia of these animals and used this information as support for activity in humans. In addition, both we and others have shown that the anti-HSV effects of ACV can also be demonstrated in guinea pigs. Furthermore, we have demonstrated (data not shown) that P-4010 has anti-HSV activity in human cell lines in vitro.
The metabolism of P-4010 in guinea pigs is not known, although as an ACV derivative with a mode of action similar to that of ACV, its superior anti-HSV activity compared to the parent compound in the present study suggests that its bioavailability may be increased over that of ACV in this animal. P-4010 may be considered to act as a prodrug for ACV and appears to be readily converted to ACV in the guinea pig. Determination of the blood plasma levels of ACV in the present study indicates that 1 h following the final i.p. injection of P-4010 at a concentration of 20 mg/kg/day over 10 days, the mean (three animals) level of ACV in plasma detected was 445 ng/ml; at equimolar concentrations under the same dosing schedule, the mean level in blood at 1 h postdosing in three guinea pigs given ACV was 1,195 ng/ml. At 6 h postdosing, mean ACV levels were 112 and 32 ng/ml for animals receiving P-4010 and ACV, respectively. Following oral administration of 200 mg of P-4010 per kg per day or an equimolar concentration of ACV to guinea pigs over a 10-day period, mean levels of ACV in plasma were, respectively, 104 and 1,258 ng/ml at 1 h and 116 and 163 ng/ml at 6 h, postdosing. ACV appears therefore to become rapidly available from P-4010 after dosing, and P-4010 is present at levels that, although initially lower than those present in guinea pigs given ACV, have a greater effect than ACV on HSV disease severity in these animals and may also be converted to ACV over a prolonged time, perhaps maintaining an increased bioavailability compared to that of ACV for a longer period of time. This would be commensurate with its observed superior anti-HSV activity, although blood plasma ACV levels were not tested after 6 h.
The current study emphasizes the value of the female guinea pig experimental model of genital herpes as a tool for assessment of the anti-HSV activity of novel compounds in both primary and recurrent clinical settings and, although further studies are required, suggests that compound P-4010, ACV elaidate, has greater activity than either ACV or PCV in reducing the clinical symptoms of primary, i.vag. HSV-2 infection following administration orally or by i.p. injection, and that this may also influence the incidence and severity of spontaneous, recurrent genital herpesvirus infection in these animals.
REFERENCES
- 1.Beauchamp L M, Orr G F, de Miranda P, Burnette T, Schaeffer H J, Krenitsky T A. Amino acid ester pro-drugs of acyclovir. Antivir Chem Chemother. 1992;3:157–164. [Google Scholar]
- 2.Bernstein D I, Stanberry L R, Harrison C J, Kappes J C, Myers M G. Antibody responses, recurrence patterns and subsequent herpes simplex virus type 2 (HSV-2) re-infection following initial HSV-2 infection of guinea-pigs: effects of acyclovir. J Gen Virol. 1986;67:1601–1612. doi: 10.1099/0022-1317-67-8-1601. [DOI] [PubMed] [Google Scholar]
- 3.Bourne N, Bravo F J, Ashton W T, Meurer L C, Tolman R L, Karkas J D, Stanberry L R. Assessment of a selective inhibitor of herpes simplex virus thymidine kinase (L-653,180) as therapy for experimental recurrent genital herpes. Antimicrob Agents Chemother. 1992;36:2020–2024. doi: 10.1128/aac.36.9.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boyd M R, Bacon T H, Sutton D, Cole M. Antiherpesvirus activity of 9-(4-hydroxy-3-hydroxymethylbut-1-yl)guanine (BRL 39123) in cell culture. Antimicrob Agents Chemother. 1987;31:1238–1242. doi: 10.1128/aac.31.8.1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bravo F J, Stanberry L R, Kier A B, Vogt P E, Kern E R. Evaluation of HPMPC therapy for primary and recurrent genital herpes in mice and guinea-pigs. Antivir Res. 1993;21:59–72. doi: 10.1016/0166-3542(93)90067-s. [DOI] [PubMed] [Google Scholar]
- 6.Cassady K A, Whitley R J. New therapeutic approaches to the alpha-herpesvirus infections. J Antimicrob Chemother. 1997;39:119–128. doi: 10.1093/jac/39.2.119. [DOI] [PubMed] [Google Scholar]
- 7.Clements W L, Armstrong M E, Keys R D, Liu M A. The prophylactic effect of immunisation with DNA encoding herpes simplex virus glycoproteins on HSV-induced disease in guinea-pigs. Vaccine. 1997;15:857–860. doi: 10.1016/s0264-410x(96)00246-0. [DOI] [PubMed] [Google Scholar]
- 8.de Miranda P, Good S S. Species differences in the metabolism and disposition of antiviral nucleoside analogues: 1. Acyclovir Antivir Chem Chemother. 1992;3:1–8. [Google Scholar]
- 9.Ertl P, Snowden W, Lowe D, Miller W, Collins P, Littler E. A comparative study of the in vitro and in vivo antiviral activities of acyclovir and penciclovir. Antivir Chem Chemother. 1995;6:89–97. [Google Scholar]
- 10.Erturk M, Phillpotts R J, Welch M J, Jennings R. Efficacy of HSV-1 ISCOM vaccine in the guinea-pig model of HSV-2 infection. Vaccine. 1991;9:728–734. doi: 10.1016/0264-410x(91)90288-h. [DOI] [PubMed] [Google Scholar]
- 11.Goldthorpe S E, Boyd M R, Field H J. Effects of penciclovir and famciclovir in a murine model of encephalitis induced by intranasal inoculation of herpes simplex virus type 1. Antivir Chem Chemother. 1992;3:37–47. [Google Scholar]
- 12.Kern E R. Acyclovir treatment of experimental genital herpes simplex virus infections. Am J Med. 1982;73:100–107. doi: 10.1016/0002-9343(82)90073-0. [DOI] [PubMed] [Google Scholar]
- 13.Krenitsky T A, Hall W W, de Miranda P, Beauchamp L M, Schaeffer H J, Whiteman P D. 6-Deoxyacyclovir: a xanthine oxidase-activated pro-drug of acyclovir. Proc Natl Acad Sci USA. 1984;81:3209–3213. doi: 10.1073/pnas.81.10.3209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Landry M L, Lucia H L, Hsuing G D, Provonost A D, Dann P R, August M J, Mayo D R. Effect of acyclovir on genital infections with herpes simplex virus types 1 and 2 in the guinea-pig. Am J Med. 1982;73:143–150. doi: 10.1016/0002-9343(82)90080-8. [DOI] [PubMed] [Google Scholar]
- 15.Mayo D R, Lucia H L, Hsuing G D. Effect of phosphonoformate on symptomatic genital herpes simplex virus type 2 infection of guinea-pigs. Intervirology. 1983;19:26–32. doi: 10.1159/000149333. [DOI] [PubMed] [Google Scholar]
- 16.McGrath B J, Newman C L. Genital herpes simplex infections in patients with the acquired immunodeficiency syndrome. Pharmacotherapy. 1994;14:529–542. [PubMed] [Google Scholar]
- 17.McLean C S, Erturk M, Jennings R, Ni Challanain D, Minson A C, Duncan I, Bournsell M E G, Inglis S C. Protective vaccination against primary and recurrent disease caused by herpes simplex virus (HSV) type 2 using a genetically disabled HSV-1. J Infect Dis. 1994;170:1100–1109. doi: 10.1093/infdis/170.5.1100. [DOI] [PubMed] [Google Scholar]
- 18.McLean C S, Ni Challanain D, Duncan I, Bournsell M E G, Jennings R, Inglis S C. Induction of a protective immune response by mucosal vaccination with a DISC HSV-1 vaccine. Vaccine. 1996;14:987–992. doi: 10.1016/0264-410x(95)00259-4. [DOI] [PubMed] [Google Scholar]
- 19.Nakao M, Hazama M, Mayumi-Aono A, Hinuma S, Fujisawa Y. Immunotherapy of acute and recurrent herpes simplex virus type 2 infection with an adjuvant-free form of recombinant glycoprotein D-interleukin-2 fusion protein. J Infect Dis. 1994;169:787–791. doi: 10.1093/infdis/169.4.787. [DOI] [PubMed] [Google Scholar]
- 20.Phillpotts R J, Welch M J, Ridgeway P H, Walkland A C, Melling J. A test for the relative potency of herpes simplex virus vaccines based upon the female guinea-pig model of HSV-2 genital infection. J Biol Stand. 1988;16:109–118. doi: 10.1016/0092-1157(88)90038-8. [DOI] [PubMed] [Google Scholar]
- 21.Provonost A D, Lucia H L, Dann P R, Hsuing G D. Effect of acyclovir on genital herpes in guinea-pigs. J Infect Dis. 1982;145:904–908. doi: 10.1093/infdis/145.6.904. [DOI] [PubMed] [Google Scholar]
- 22.Purifoy D M, Beauchamp L M, de Miranda P, Ertl P, Lacey S, Roberts G, Rahim S G, Darby G, Krenitsky T A, Powell K L. Review of research leading to new anti-herpesvirus agents in clinical development: valaciclovir hydrochloride (256U, the L-valyl ester of acyclovir) and 882C, a specific agent for varicella zoster virus. J Med Virol Suppl. 1993;1:139–145. doi: 10.1002/jmv.1890410527. [DOI] [PubMed] [Google Scholar]
- 23.Rawls W E, Iwamoto K, Adam E, Melnick J C. Measurement of antibodies to herpesvirus type 1 and 2 in human sera. J Immunol. 1970;104:599–606. [PubMed] [Google Scholar]
- 24.Richards D M, Carmine A A, Brogden R N, Heel R C, Speight T M, Avery G S. Acyclovir: a review of its pharmacodynamic properties and therapeutic efficacy. Drugs. 1983;26:378–438. doi: 10.2165/00003495-198326050-00002. [DOI] [PubMed] [Google Scholar]
- 25.Scriba M. Herpes simplex virus infection in guinea-pigs: an animal model for studying latent and recurrent herpes simplex virus infection. Infect Immun. 1975;12:162–165. doi: 10.1128/iai.12.1.162-165.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stanberry L R, Bernstein D I, Burke R L, Pachl C, Myers M G. Vaccination with recombinant herpes simplex virus glycoproteins: protection against initial and recurrent genital herpes. J Infect Dis. 1978;155:914–920. doi: 10.1093/infdis/155.5.914. [DOI] [PubMed] [Google Scholar]
- 27.Stanberry L R, Kern E R, Richards J T, Abbott T M, Overall J C. Genital herpes in guinea-pigs: pathogenesis of the primary infection and description of recurrent disease. J Infect Dis. 1982;146:397–404. doi: 10.1093/infdis/146.3.397. [DOI] [PubMed] [Google Scholar]
- 28.Sutton, D., and E. R. Kern. 1993. Activity of famciclovir and penciclovir in HSV-infected animals; a review. Antivir. Chem. Chemother. 4(Suppl. 1):37–46.
- 29.Thackray A M, Field H J. Differential effects of famciclovir and valaciclovir on the pathogenesis of herpes simplex virus in a murine infection model including reactivation from latency. J Infect Dis. 1996;173:291–299. doi: 10.1093/infdis/173.2.291. [DOI] [PubMed] [Google Scholar]
- 30.Thornton B, Baskerville A, Bailey N E, Melling J M, Hambleton P. Herpes simplex virus genital infection of the female guinea-pig as a model for evaluation of an experimental vaccine. Vaccine. 1984;2:141–148. doi: 10.1016/0264-410x(84)90006-9. [DOI] [PubMed] [Google Scholar]
- 31.Vere Hodge, R. A., and Y.-C. Cheng. 1993. The mode of action of penciclovir. Antivir. Chem. Chemother. 4(Suppl. 1):13–24.
- 32.Vere Hodge R A, Sutton D, Boyd M R, Harnden M R, Jarvest R L. Selection of an oral prodrug (BRL 42810; famciclovir) for the antiherpesvirus agent (BRL 39123) [9-(4-hydroxy-3-hydromethylbut-1-yl)guanine; penciclovir] Antimicrob Agents Chemother. 1989;33:1765–1773. doi: 10.1128/aac.33.10.1765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wald A, Zeh J, Barnum G, Davis L G, Corey L. Suppression of subclinical shedding of herpes simplex virus type 2 with acyclovir. Ann Intern Med. 1994;124:8–15. doi: 10.7326/0003-4819-124-1_part_1-199601010-00002. [DOI] [PubMed] [Google Scholar]