Abstract
Objectives
To compare heparin-based anticoagulation and bivalirudin-based anticoagulation within the context of critically ill patients with a severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection.
Design
An observational study.
Setting
At the intensive care unit of a university hospital.
Participants and Interventions
Critically ill patients with a SARS-CoV-2 infection receiving full anticoagulation with heparin or bivalirudin.
Measurements and Main Results
Twenty-three patients received full anticoagulation with bivalirudin and 60 with heparin. Despite patients in the bivalirudin group having higher mortality risk scores (SAPS II 60 ± 16 v 39 ±7, p < 0.001) and a higher need for extracorporeal support compared to the heparin group, hospital mortality was comparable (57% v 45, p = 0.3). No difference in thromboembolic complications was observed, and bleeding events were more frequent in patients treated with bivalirudin (65% v 40%, p = 0.01). Similar results were confirmed in the subgroup analysis of patients undergoing intravenous anticoagulation; in addition to comparable thrombotic complications occurrence and thrombocytopenia rate, however, no difference in the bleeding rate was observed (65% v 35%, p = 0.08).
Conclusions
Although heparin is the most used anticoagulant in the intensive care setting, bivalirudin-based anticoagulation was safe and effective in a cohort of critically ill patients with SARS-CoV-2. Bivalirudin may be given full consideration as an anticoagulation strategy for critically ill patients with SARS-CoV-2, especially in those with thrombocytopenia and on extracorporeal support.
Key Words: anticoagulation, bivalirudin, SARS-CoV-2 infection, critical care, extracorporeal membrane oxygenation
SEVERE ACUTE RESPIRATORY SYNDROME coronavirus 2 (SARS-CoV-2) infection is responsible for a variable spectrum of clinical manifestations—up to severe respiratory and cardiac injury requiring intensive care unit (ICU) treatment.1 , 2 The SARS-CoV-2–induced proinflammatory state and its associated endothelial lung injury have been found to be responsible for a prothrombotic state, involving both large vessels and microcirculation.3, 4, 5 In critically ill patients with a SARS-CoV-2 infection, clinical deterioration toward multiorgan failure appears to be mediated both by respiratory failure and microvascular systemic involvement6; an inherent hypercoagulable state with derangements in laboratory parameters (elevations of D-dimer, fibrin degradation products, prothrombin time, and activated partial thromboplastin time [aPTT]), and both venous and arterial thromboembolic disease are common findings in these patients.7 , 8 For this reason, great attention has been focused on anticoagulation as a prophylactic and therapeutic strategy within the management of SARS-CoV-2 infection and its associated complications.9 , 10 Heparin, traditionally used as a first-line anticoagulation strategy in critically ill patients, presents limitations, including some variability in the anticoagulant effect, bleeding risk, complex titration based on aPTT in the critically ill, and the risk of heparin-induced thrombocytopenia (HIT).11 On the contrary, the use of direct thrombin inhibitors, such as bivalirudin, may represent a valid anticoagulation strategy in critically ill patients, due to the anticoagulation effect on stability, even at a low drug dose, possibly lower bleeding risk, and reduced blood cell activation against platelets, thus avoiding the risk of HIT.12 , 13 Bivalirudin has the ability to bind both the circulating and the clot-bind fibrin and has been shown to reduce the risk of bleeding in critically ill patients on venovenous extracorporeal membrane oxygenation (VV ECMO), compared to heparin.14 , 15 However, reports on the use of bivalirudin in critically ill patients with SARS-CoV-2 infection, beyond extracorporeal support itself, are lacking. The aim of the present observational study was to compare anticoagulation strategies (bivalirudin versus heparin) in critically ill patients admitted to the ICU with respiratory failure due to SARS-CoV-2 infection.
Methods
The study authors performed an observational analytical study involving critically ill patients with SARS-CoV-2 admitted to the ICU at the authors’ institute from February 2020 to May 2020, and treated with anticoagulants in a therapeutic range. The study was in compliance with the Declaration of Helsinki. Data collection was performed in consensus with ethical norms and with the local ethical committee approval.
Demographic, clinical parameters, vital signs, and outcomes data were registered for all patients. Patients' data were electronically stored: all data were anonymized prior to insertion into the database. The primary endpoints of the study were to assess the safety and efficacy of bivalirudin therapy in terms of drug-related adverse events and efficacy in the prevention of thrombotic events compared to heparin. The secondary endpoints were the comparison of bleeding complications rate and hospital survival.
According to the authors’ institutional protocol, patients received heparin as routine anticoagulation while they were administered bivalirudin in the presence of extracorporeal devices (VV ECMO, mechanical circulatory support, etc...), or thrombocytopenia with a suspicion of HIT. Intravenous anticoagulation was performed in unstable patients and in patients with extracorporeal devices or organ failure needing close drug titration. Patients treated with bivalirudin received a starting intravenous continuous infusion of 0.025 mg/kg/h without any bolus, while patients treated with intravenous heparin had a starting dose of 400 U/h. Low starting dosages were administered as routine practice in critically ill patients at the authors’ institution, and in the absence of complications, they were increased rapidly after a few hours due to a minimum 3 times a day monitoring of aPTT, with the target of 60 seconds in patients undergoing full anticoagulation.
Bleeding episodes were recorded according to GUSTO bleeding criteria.16 Thrombotic episodes were assessed by collecting data concerning ischemic stroke, peripheral ischemia, pulmonary embolism, and any other type of thrombotic event; the parameter "other thrombotic complications" included thrombotic events that were not included in aforementioned cases (such as acute coronary syndrome and venous thrombosis). Thrombocytopenia was defined as mild, moderate, or severe according to platelet count (<100,000 μL, <50,000 μL, <10,000 μL, respectively). Vasoactive agents’ and inotropes’ dosing was standardized with the Vasoactive Inotropic Score (VIS) calculation.17 HIT was presumed if the platelet count was fewer than 100 109/L or decreased more than 50% from the baseline in a patient treated with heparin, thus triggering the performance of an immunologic test (ELISA). If the results were questionable, a heparin-induced platelet aggregation assay also was performed. When HIT was presumed, all sources of heparin were removed.
Data regarding continuous variables with a bell-shaped distribution were presented with mean ± standard deviation. Median and interquartile ranges were reported for not-normally distributed variables. Number and prevalence proportion were recorded for dichotomous variables. The Student t-test and Mann-Whitney U test18 were used to compare variables with normal or not-normal distribution between groups. Dichotomous variables were further analyzed according to the Pearson chi-square calculation to assess statistical significance. A p value < 0.05 was considered statistically significant. The authors performed a comparison between patients receiving full-dose anticoagulation with bivalirudin and heparin. Furthermore, further analysis was performed to compare subgroups of patients undergoing intravenous anticoagulation (either bivalirudin or heparin) to assess data in a more homogeneous population. Another comparison between bivalirudin doses in critically ill patients with SARS-CoV-2 versus critically ill patients who were negative for SARS-CoV-2 was performed with data from a population of 89 critically ill patients without SARS-CoV-2 admitted at the authors’ institution who were treated with bivalirudin by the same team.19
Results
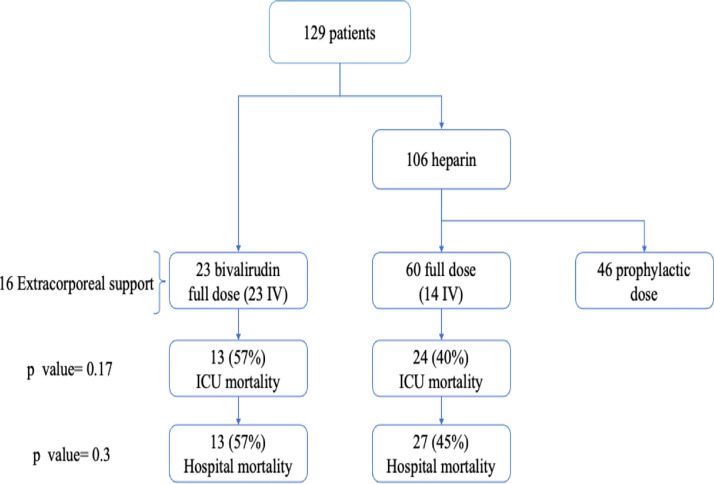
Overall, 129 ICU patients with SARS-CoV-2 infections (110 men [85%] and 19 women [15%]) received anticoagulation in the study period: 23 (17%) received bivalirudin, and 106 patients (82%) were administered heparin, as shown in the flowchart (Fig 1 ). Sixty patients received an anticoagulation strategy with a therapeutic aim (ie, full anticoagulation) (Fig 1), while 46 received a prophylactic regimen and were not included in the study. The dosages of bivalirudin, heparin, and low-molecular-weight heparin administered over days are reported in the supplementary material.
Fig 1.
Study flowchart. The study population receiving full anticoagulation included 23 patients in the bivalirudin arm and 60 patients in the heparin arm. All patients in the bivalirudin group and 14 patients in the heparin group received intravenous anticoagulation; p values refer to the comparison of intensive care unit and hospital mortality. ICU, intensive care unit; IV, intravenous.
Comparison of Full Anticoagulation Strategies With Bivalirudin and Heparin
Data regarding the baseline characteristics and anamnestic data of the 60 patients treated with heparin and 23 patients administered bivalirudin with the full anticoagulation target are reported in Table 1 , while the clinical data of patients receiving therapeutic anticoagulation strategy are presented in Table 2 . Higher shock degree and illness severity of the bivalirudin group compared to the heparin counterpart was demonstrated by a higher Simplified Acute Physiology Score II score (60 ± 16 v 39 ± 7, p < 0.001) and the need for extracorporeal support and devices (Table 2). Despite this difference, the need for temporary discontinuation of anticoagulation due to bleeding was not increased (27% in the bivalirudin group, 37% in the heparin group; p = 0.4). In Table 3 , data concerning outcomes and complications of patients receiving full-dose anticoagulation strategy are shown. The efficacy of the 2 anticoagulation strategies in preventing thromboembolic events was comparable. In particular, reported thromboembolic events were ischemic stroke (4% in the bivalirudin group; 8.3% in the heparin group; p = 0.5), ischemic peripheral complications (18% in the bivalirudin group; 10% in the heparin group; p = 0.3), pulmonary embolism (27% in the bivalirudin group; 20% in the heparin group; p = 0.4), and other thrombotic complications (32% in the bivalirudin group; 35% in the heparin group; p = 0.8). The overall bleeding rate was higher among patients in the bivalirudin group (65%) compared to the heparin group (40%) (p = 0.01); this was in line with the higher bleeding risk associated with extracorporeal support devices. In particular, major bleeding episodes were higher in the bivalirudin group compared to the heparin group (26% among the bivalirudin group and 7% among the heparin group, p = 0.01). This finding was associated with a higher need for red blood cells and fresh frozen plasma transfusions in the bivalirudin group 17 (7.5-27) red blood cells units among bivalirudin group versus 1 (0-3) in the heparin group, p = 0.2; 0 (0-8) fresh frozen plasma transfusions in the bivalirudin group and 0 (0-0) in the heparin group, (p = 0.0003) (Table 3). All 6 patients in the bivalirudin group who experienced major bleeding were on VV ECMO due to refractory acute respiratory distress syndrome, and 4 had platelet counts <50,000 μL; 4 patients had intrathoracic bleeding (after a mean of 19 days of support), 1 gastrointestinal bleeding (after 26 days of support), and the last patient had intracranial bleeding (after 11 days of support). However, no difference in the rate of surgical procedures due to bleeding events was recorded (9% among the bivalirudin group and 10% among the heparin group; p = 0.9). Moderate and severe thrombocytopenic episodes were higher among the bivalirudin group (p = 0.02). ECMO duration was notably high: 25 (10-46) days, with 50% of patients having an ECMO run longer than 1 month (maximum length 56 days). No case of heparin-induced thrombocytopenia was recorded.
Table 1.
Baseline Characteristics of Patients Administered Full Anticoagulation
| Parameter | Bivalirudin group (n = 23) | Heparin group (n = 60) | p Value |
|---|---|---|---|
| Age, mean ± SD | 58 ± 12.4 | 62 ± 9 | 0.05 |
| Male, n (%) | 20/23 (87%) | 55/60 (92%) | 0.5 |
| Weight, mean kg ± SD | 89 ± 15 | 86 ±14 | 0.3 |
| Height, mean cm ± SD | 175 ± 8 | 173 ± 7 | 0.5 |
| BMI, mean ± SD | 29.12 ± 5.6 | 28.5 ± 4.0 | 0.5 |
| SAPS II, mean (SD) | 60 ±16 | 39 ±7 | <0.001 |
| Arterial hypertension, n (%) | 5/23 (22%) | 31/60 (52%) | 0.01 |
| Coronary artery disease, n (%) | 0/23 (0%) | 8/60 (13%) | 0.06 |
| Heart rhythm disease, n (%) | 1/23 (4%) | 8/60 (13%) | 0.2 |
| Vascular disease, n (%) | 0/23 (0%) | 10/60 (16%) | 0.03 |
| Overweight BMI <30-≥25, n (%) | 9/23 (40%) | 30/60 (50%) | 0.3 |
| Obesity BMI ≥30, n (%) | 8/23 (35%) | 17/60 (28%) | 0.5 |
| BMI >25, n (%) | 17/23 (74%) | 47/60 (78%) | 0.6 |
| Diabetes, n (%) | 2/23 (8.7%) | 14/60 (23%) | 0.1 |
| COPD, n (%) | 1/23 (4.4%) | 2/60 (3.3%) | 0.8 |
| Asthma, n (%) | 1/23 (4.4%) | 2/60 (3.3%) | 0.8 |
| Respiratory tract diseases, n (%) | 3/23 (10%) | 3/60 (5.0%) | 0.2 |
| Oncohematology, n (%) | 2/23 (8.7%) | 2/60 (3.3%) | 0.3 |
| Hematologic diseases - not oncological, n (%) | 2/23 (8.7%) | 3/60 (5.0%) | 0.5 |
| Oncologic disease, n (%) | 2/23 (8.7%) | 7/60 (12%) | 0.7 |
| Hypothyroidism, n (%) | 3/23 (10%) | 5/60 (8.3%) | 0.5 |
| No comorbidities, n (%) | 1/23 (4.4%) | 2/60 (3.3%) | 0.8 |
| Thrombosis at clinical admission, n (%) | 2/23 (8.7%) | 3/60 (5.0%) | 0.5 |
| Active infectious disease not SARS-CoV-2, n (%) | 1/23 (4.4%) | 2/60 (3.3%) | 0.8 |
Abbreviations: BMI, body mass index; COPD, chronic obstructive pulmonary disease; SAPS II, Simplified Acute Physiology Score II; SARS-CoV-2 infection, severe acute respiratory syndrome coronavirus 2; SD, standard deviation.
Table 2.
Clinical Data of Patients Administered Full Anticoagulation
| Parameter | Bivalirudin group (n = 23) | Heparin group (n = 60) | p Value |
|---|---|---|---|
| ECMO, n (%) | 15/23 (65%) | 0/60 (0%) | <0.0001 |
| Days on ECMO, median (IQR) | 25 (10 - 46) | – | Not applicable |
| IABP, n (%) | 5/23 (22%) | 1/60 (2%) | 0.002 |
| Impella, n (%) | 1/23 (4%) | 0/60 (0%) | 0.1 |
| CVVH, n (%) | 9/23 (39%) | 13/60 (22%) | 0.1 |
| Cytosorb, n (%) | 16/23 (69%) | 0/60 (0%) | <0.0001 |
| Switch from another anticoagulant, n (%) | 8/23 (35%) | 0/60 (0%) | 0.0001 |
| Need to stop anticoagulation, n (%) | 6/23 (27%) | 22/60 (37%) | 0.4 |
| Orotracheal intubation, n (%) | 23/23 (100%) | 58/60 (97%) | 0.3 |
| Tracheostomy, n (%) | 9/23 (39%) | 20/60 (33%) | 0.6 |
| Inotropic score day 1, median (IQR) | 4 (1-10) | 10 (0-20) | 0.09 |
| Peak inotropic score, median (IQR) | 20 (10-30) | 20 (10-35) | 0.7 |
Abbreviations: CVVH, continuous venovenous hemofiltration; IABP, Intra-aortic balloon pump; ICU, intensive care unit; IQR, interquartile range; VA ECMO, venoarterial extracorporeal membrane oxygenation; VV ECMO; venovenous extracorporeal membrane oxygenation.
Table 3.
Outcomes and Complications of Patients Administered Full Anticoagulation
| Parameter | Bivalirudin (n = 23) | Heparin (n = 60) | p Value |
|---|---|---|---|
| ICU stay days, median (IQR) | 33 (20-48.5) | 14 (11-25) | <0.0001 |
| Hospital stay days, median (IQR) | 42 (28.5-60.5) | 31 (20-49) | 0.1 |
| ICU mortality, n (%) | 13/23 (57%) | 24/60 (40%) | 0.2 |
| Hospital mortality, n (%) | 13/23 (57%) | 27/60 (45%) | 0.3 |
| Need to come back to ICU, n (%) | 0/23 (0%) | 6/60 (10%) | 0.1 |
| Bleeding-associated complications | |||
| Major bleeding, n (%) | 6/23 (26%) | 4/60 (7%) | 0.01 |
| Moderate bleeding, n (%) | 7/23 (30%) | 8/60 (13%) | 0.06 |
| Minor bleeding, (%) | 2/22 (9%) | 12/60 (20%) | 0.2 |
| Surgery due to bleeding, n (%) | 2/22 (9%) | 6/60 (10%) | 0.9 |
| Overall bleeding, (%) | 15/23 (65%) | 24/60 (40%) | 0.03 |
| RBC transfusions, median (IQR) | 17 (7.5-27) | 1 (0-3) | <0.0001 |
| FFP transfusions, median (IQR) | 0 (0-8) | 0 (0-0) | 0.0003 |
| PLT transfusions, median (IQR) | 0 (0-0) | 0 (0-0) | 0.2 |
| Thrombotic-associated complications | |||
| Ischemic stroke, n (%) | 1/23 (4%) | 5/60 (8.3%) | 0.5 |
| Ischemic peripheral complications, n (%) | 4/22 (18%) | 6/60 (10%) | 0.3 |
| Other thrombotic complications, n (%) | 7/22 (32%) | 21/60 (35%) | 0.8 |
| Pulmonary embolism, n (%) | 6/22 (27%) | 12/60 (20%) | 0.4 |
| Surgery due to thrombosis, n (%) | 2/22 (9%) | 2/60 (3.3%) | 0.2 |
| Thrombocytopenia | |||
| Platelets <100,000/mm3, n (%) | 10/20 (50%) | 16/55 (29%) | 0.09 |
| Platelets <50,000/mm3, n (%) | 6/20 (30%) | 2/56 (4%) | <0.001 |
| Platelets <10,000/mm3, n (%) | 2/20 (10%) | 0/56 (0%) | 0.02 |
| Bridge to lung transplant, n (%) | 2/23 (9%) | 0/60 (0%) | 0.02 |
| Pneumothorax, n (%) | 8/21 (38%) | 5/60 (8.3%) | 0.001 |
Abbreviations: FFP, fresh frozen plasma; ICU, intensive care unit; IQR, interquartile range; PLT, platelets; RBC, red blood cells.
ICU stay was shorter among the heparin group compared to the bivalirudin group, (14 [11-25] days v 33 [20-48.5] days, p < 0.0001). Notably, no statistically significant differences in ICU mortality and hospital mortality were observed: ICU mortality was 57% in the bivalirudin group, and 40% in the heparin group, p = 0.2; hospital mortality was 57% in the bivalirudin group and 45% in the heparin group, p = 0.3.
Comparison of Bivalirudin and Intravenous Heparin Anticoagulation
Demographic characteristics, clinical management, and outcomes of all patients treated with intravenous heparin compared to bivalirudin are reported in Supplementary Tables 1, 2, and 3. Patients in the bivalirudin group had a higher Simplified Acute Physiology Score II (with consequent high predicted mortality risk) compared to the heparin group (60 ± 16 v 44 ± 7, p = 0.01), and a higher need for extracorporeal devices (Supplementary Table 2), thus confirming what was observed in the overall population. Again, the thrombotic complication rate (ischemic stroke, pulmonary embolism, peripheral ischemia, and any other type of thrombotic event) was low and similar between the groups, as shown in Supplementary Table 3. The D-dimers peak value was 5 (3-13) μg/mL in the bivalirudin group and 14 (6-19) μg/mL in the heparin group. In contrast to what was observed in the general population, no statistically significant difference in the overall bleeding rate was observed (65% in the bivalirudin group v 36% in the heparin group, p = 0.08), nor with specific reference to major, moderate, or minor bleeding rate (p = 0.2, p = 0.1, p = 0.3, respectively) (Supplementary Table 3). Similarly, no difference in the thrombocytopenia rate was documented between the bivalirudin and heparin groups in this subgroup analysis. Overall, hospital mortality was comparable between the 2 groups (57% in the bivalirudin group, 57% in the heparin group [p = 0.9]).
Bivalirudin Dosage in SARS-CoV-2 Infection and non–SARS-CoV-2 Infection
The bivalirudin dosage administered to the bivalirudin group was further compared to the bivalirudin dosage administered to the control group of 89 critically ill patients who were negative for SARS-CoV-2 infection. In Supplementary Table 4, a daily dose of bivalirudin of the first 6 days of ICU admission was reported. The median bivalirudin dose administered to patients with a SARS-CoV-2 infection was within a range from 0.04 mg/kg/h-to-0.108 mg/kg/h, higher than the dose administered in the control group (range of 0.014-0.044 mg/kg/h) (p < 0.05).
Discussion
The study authors reported data and results of different anticoagulation strategies in a large population of patients who were critically ill due to SARS-CoV-2 infection. This study demonstrated that intravenous anticoagulation with the direct thrombin inhibitor bivalirudin was a safe and effective strategy even in the most extremely ill patients. Indeed, although being reserved for patients who were highly compromised in the authors’ practice (ie, patients requiring extracorporeal support, patients with severe thrombocytopenia), anticoagulation with bivalirudin allowed adequate antithrombotic effect compared to heparin, even at a low starting dose.
Despite patients treated with bivalirudin being more compromised than the heparin counterpart, as shown by higher mortality risk score and need for extracorporeal support and invasiveness of care, the use of bivalirudin was proved to be associated with an equal overall survival compared to heparin (overall mortality 57% in the bivalirudin group; 42% in the heparin group; p = 0.2). This was further confirmed in the intravenous anticoagulation subgroup (mortality 57% in both groups, p = 0.9).
Patients in the bivalirudin group had a higher incidence of thrombocytopenia; however, this did not translate into a large number of platelet transfusions. Risk factors for thrombocytopenia were several in this population (shock, SARS-CoV-2 infection, therapies, mechanical circulatory support) and, above all, exposure to heparin; they might also act concomitantly. On the contrary, severe bleeding and a need for red blood cells and fresh frozen plasma transfusions were higher in the bivalirudin group compared to the heparin group (Table 3). The higher rate of bleeding and transfusions need, in the authors’ opinion, was linked to the extreme sickness of the patients in the bivalirudin group. Furthermore, in the subgroup analysis on patients treated with an intravenous anticoagulant, which represented the subgroup of most unstable patients, no significant differences in the bleeding rate, thrombocytopenia incidence, and fresh frozen plasma transfusions incidence were observed (Supplementary Tables 1-3).
A growing body of literature supports the use of bivalirudin in critically ill patients, especially in the case of extracorporeal support since these patients present multiple events of coagulation activation and a precarious balance between bleeding and thrombosis.13 , 20 , 21 A reduction in the bleeding rate of patients on VV ECMO treated with bivalirudin compared to heparin also has been documented specifically in a homogeneous population treated with either one or the other anticoagulant.14 In addition, preliminary evidence also exists on the use of bivalirudin to mitigate the thrombotic risk determined by the peculiar setting of SARS-CoV-2 infection.15 , 22 The authors’ data were in line with existing literature, and the fact that they observed comparable survival between patients treated with bivalirudin and heparin further supported the efficacy and safety of the use of direct thrombin inhibitors in such a complex clinical context. Furthermore, in the setting of SARS-CoV-2 infection, interestingly, a higher risk for antiplatelet factor 4 antibodies development has been claimed, although not unequivocally documented23, 24, 25; this may represent another element to be taken into consideration in the choice of the anticoagulation strategy. At the moment, large prospective randomized trials on the use of bivalirudin in patients with SARS-CoV-2 infection are on the way, and the results will be provided in the future.26
The authors’ data also pointed out that the bivalirudin dose administered to patients affected with SARS-CoV-2 was higher compared to the bivalirudin drug administered to the control group of critically-ill patients negative to SARS-CoV-2 infection. This finding was in line with very recent literature27 and supported the clinical perception that the hypercoagulability status induced by SARS-CoV-2 infection has a relevant clinical effect. Indeed, recommendations supporting higher anticoagulation targets when dealing with ECMO and SARS-CoV-2 infection also have been developed.28 The authors here argue that reaching the appropriate anticoagulation target with higher doses of bivalirudin than what was generally requested played a key role in downregulating thrombotic patterns activation; namely, the authors observed a low thrombotic complication rate, and this also was confirmed in ECMO patients. At the same time, a word of caution is mandatory in the interpretation of coagulation tests, such as aPTT, since they are influenced by the levels of coagulation factors. Namely, levels of factor VIII have been shown to be increased in patients with SARS-CoV-2 infection,29 and this might result in assay artifacts, such as shorter aPTT. In the light of this, the authors might have administered excessively high bivalirudin dosages to their patients, but this did not translate into an increased bleeding risk in the subgroup analysis of patients undergoing intravenous anticoagulation with aPTT monitoring.
Finally, anticoagulation with bivalirudin proved to be as safe and effective as heparin in the authors’ experience; although no specific antidote exists, it has a half-life of 25 minutes.30 Since 20% of it is cleared by the kidneys, dose adjustments were needed in case of renal failure, and a dose increase may be needed in case of renal replacement therapy31 , 32; these may be performed safely following aPTT in the authors’ experience.19
The study also had some limitations: first, the retrospective study design. Moreover, the most compromised patients pertained to the bivalirudin group since the control arm, represented by the heparin group, were not comparable in terms of critical conditions and organ failure. Another confounding factor was that patients in the heparin group were administered different types of drugs (unfractionated heparin versus low-molecular-weight heparin) through different administration routes. However, the authors also performed a subgroup analysis of the most critically ill patients treated with intravenous drugs. Although they might argue that bivalirudin might be a valid alternative to heparin in this clinical context based on their data, the authors are fully aware that the present study was underpowered to assess the effect of anticoagulation regimen on survival; therefore, no conclusion is to be drawn on this topic. Furthermore, this retrospective study was conducted on patients hospitalized from February 2020 to May 2020, when guidelines regarding SARS-CoV-2 infection management were lacking and recommendations were poorly evidence-based. Very recent meta-analyses and multicenter studies are providing new perspectives regarding anticoagulation strategies for critically ill patients with SARS-CoV-2 infection.33 , 34 As a matter of fact, anticoagulant drugs other than heparin represent a new powerful therapeutic tool that may allow patients to overcome adverse reactions from receiving heparin.35 Moreover, direct thrombin inhibitors are now taken into consideration as an anticoagulation strategy to face vaccine-induced immune thrombotic thrombocytopenia, a new prothrombotic condition that cannot be treated with heparin.36 Finally, the authors provided data on the bivalirudin dose needed in contemporary critically ill patients who were negative for SARS-CoV-2 compared with patients with SARS-CoV-2 infection, but a similar analysis could not also be performed in the heparin group.
Conclusions
While heparin is the most extensively used anticoagulant drug, bivalirudin has a good safety and efficacy profile in the most critically ill patients, even in those on extracorporeal support. In addition to the anticoagulant effect, by reducing the thrombin-mediated activation of platelets, bivalirudin also shows antiplatelet effects.30 Furthermore, through the tight cross-talk between coagulation and inflammation, it also might affect inflammatory patterns.37 The pleiotropic clinical effects of heparin administration should be further investigated, also in the perspective of inflammatory downregulation. Prospective trials are needed to assess possible survival implications.
Conflict of Interest
The authors have no conflicts of interest to declare.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1053/j.jvca.2022.03.004.
Appendix. Supplementary materials
References
- 1.Barbaro RP, MacLaren G, Boonstra PS, et al. Extracorporeal membrane oxygenation support in COVID-19: An international cohort study of the Extracorporeal Life Support Organization registry (published correction appears in Lancet. 2020 Oct 10;396[10257]:1070) Lancet. 2020;396:1071–1078. doi: 10.1016/S0140-6736(20)32008-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Halacli B, Kaya A, Topeli A. Critically-ill COVID-19 patient. Turk J Med Sci. 2020;50:585–591. doi: 10.3906/sag-2004-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wiersinga WJ, Rhodes A, Cheng AC, et al. Pathophysiology, transmission, diagnosis, and treatment of coronavirus disease 2019 (COVID-19): A review. JAMA. 2020;324:782–793. doi: 10.1001/jama.2020.12839. [DOI] [PubMed] [Google Scholar]
- 4.Levi M, Iba T. COVID-19 coagulopathy: Is it disseminated intravascular coagulation? Intern Emerg Med. 2021;16:309–312. doi: 10.1007/s11739-020-02601-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ciceri F, Beretta L, Scandroglio AM, et al. Microvascular COVID-19 lung vessels obstructive thromboinflammatory syndrome (MicroCLOTS): An atypical acute respiratory distress syndrome working hypothesis. Crit Care Resusc. 2020;22:95–97. doi: 10.51893/2020.2.pov2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Renzi S, Landoni G, Zangrillo A, Ciceri F. MicroCLOTS pathophysiology in COVID 19. Korean J Intern Med. 2020 Sep 9. 10.3904/kjim.2020.336. Online ahead of print [DOI] [PMC free article] [PubMed]
- 7.Klok FA, Kruip MJHA, van der Meer NJM, et al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res. 2020;191:145–147. doi: 10.1016/j.thromres.2020.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tang N, Li D, Wang X, et al. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. 2020;18:844–847. doi: 10.1111/jth.14768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Parisi R, Costanzo S, Di Castelnuovo A, et al. Different anticoagulant regimens, mortality, and bleeding in hospitalized patients with COVID-19: A systematic review and an updated meta-analysis. Semin Thromb Hemost. 2021;47:372–391. doi: 10.1055/s-0041-1726034. [DOI] [PubMed] [Google Scholar]
- 10.Tang N, Bai H, Chen X, et al. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J Thromb Haemost. 2020;18:1094–1099. doi: 10.1111/jth.14817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chong BH. Heparin-induced thrombocytopenia. J Thromb Haemost. 2003;1:1471–1478. doi: 10.1046/j.1538-7836.2003.00270.x. [DOI] [PubMed] [Google Scholar]
- 12.Warkentin TE, Greinacher A, Bivalirudin Koster A. Thromb Haemost. 2008;99:830–839. doi: 10.1160/TH07-10-0644. [DOI] [PubMed] [Google Scholar]
- 13.Mahaffey KW, Lewis BE, Wildermann NM, et al. The anticoagulant therapy with bivalirudin to assist in the performance of percutaneous coronary intervention in patients with heparin-induced thrombocytopenia (ATBAT) study: Main results. J Invasive Cardiol. 2003;15:611–616. [PubMed] [Google Scholar]
- 14.Rivosecchi RM, Arakelians AR, Ryan J, et al. Comparison of anticoagulation strategies in patients requiring venovenous extracorporeal membrane oxygenation: Heparin versus bivalirudin. Crit Care Med. 2021;49:1129–1136. doi: 10.1097/CCM.0000000000004944. [DOI] [PubMed] [Google Scholar]
- 15.Seelhammer TG, Rowse P, Yalamuri S. Bivalirudin for maintenance anticoagulation during venovenous extracorporeal membrane oxygenation for COVID-19. J Cardiothorac Vasc Anesth. 2021;35:1149–1153. doi: 10.1053/j.jvca.2020.06.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.GUSTO investigators An international randomized trial comparing four thrombolytic strategies for acute myocardial infarction. N Engl J Med. 1993;329:673–682. doi: 10.1056/NEJM199309023291001. [DOI] [PubMed] [Google Scholar]
- 17.McIntosh AM, Tong S, Deakyne SJ, et al. Validation of the vasoactive-inotropic score in pediatric sepsis. Pediatr Crit Care Med. 2017;18:750–757. doi: 10.1097/PCC.0000000000001191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mann HB, Whitney DR. On a test of whether one of two random variables is stochastically larger than the other. Ann Math Stat. 1947;18:50–60. [Google Scholar]
- 19.Scandroglio AM, Pieri M, Nardelli P, et al. Impact of CytoSorb on kinetics of vancomycin and bivalirudin in critically ill patients. Artif Organs. 2021;45:1097–1103. doi: 10.1111/aor.13952. [DOI] [PubMed] [Google Scholar]
- 20.Pieri M, Agracheva N, Bonaveglio E, et al. Bivalirudin versus heparin as an anticoagulant during extracorporeal membrane oxygenation: A case-control study. J Cardiothorac Vasc Anesth. 2013;27:30–34. doi: 10.1053/j.jvca.2012.07.019. [DOI] [PubMed] [Google Scholar]
- 21.Pieri M, Agracheva N, Di Prima AL, et al. Primary anticoagulation with bivalirudin for patients with implantable ventricular assist devices. Artif Organs. 2014;38:342–346. doi: 10.1111/aor.12168. [DOI] [PubMed] [Google Scholar]
- 22.Bissell BD, Gabbard T, Sheridan EA, et al. Evaluation of bivalirudin as the primary anticoagulant in patients receiving extracorporeal membrane oxygenation for SARS-CoV-2-associated acute respiratory failure. Ann Pharmacother. 2022;56:387–392. doi: 10.1177/10600280211036151. [DOI] [PubMed] [Google Scholar]
- 23.Brodard J, Kremer Hovinga JA, et al. COVID-19 patients often show high-titer non-platelet-activating anti-PF4/heparin IgG antibodies. J Thromb Haemost. 2021;19:1294–1298. doi: 10.1111/jth.15262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dragonetti D, Guarini G, Pizzuti M. Detection of anti-heparin-PF4 complex antibodies in COVID-19 patients on heparin therapy. Blood Transfus. 2020;18:328. doi: 10.2450/2020.0164-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lingamaneni P, Gonakoti S, Moturi K, et al. Heparin-induced thrombocytopenia in COVID-19. J Investig Med High Impact Case Rep. 2020;8 doi: 10.1177/2324709620944091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kharma N, Roehrig S, Shible AA, et al. Anticoagulation in critically ill patients on mechanical ventilation suffering from COVID-19 disease, The ANTI-CO trial: A structured summary of a study protocol for a randomised controlled trial. Trials. 2020;21:769. doi: 10.1186/s13063-020-04689-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Trigonis R, Smith N, Porter S, et al. Efficacy of bivalirudin for therapeutic anticoagulation in COVID-19 patients requiring ECMO support. J Cardiothorac Vasc Anesth. 2022;36:414–418. doi: 10.1053/j.jvca.2021.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shekar K, Badulak J, Peek G, et al. Extracorporeal Life Support Organization Coronavirus Disease 2019 Interim Guidelines: A Consensus Document from an International Group of Interdisciplinary Extracorporeal Membrane Oxygenation Providers. ASAIO J. 2020;66:707–721. doi: 10.1097/MAT.0000000000001193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Martín-Rojas RM, Pérez-Rus G, Delgado-Pinos VE, et al. COVID-19 coagulopathy: An in-depth analysis of the coagulation system. Eur J Haematol. 2020;105:741–750. doi: 10.1111/ejh.13501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Di Nisio M, Middeldorp S, Büller HR. Direct thrombin inhibitors. N Engl J Med. 2005;353:1028–1040. doi: 10.1056/NEJMra044440. [DOI] [PubMed] [Google Scholar]
- 31.He T, He J, Wang Z, et al. Modification strategies to improve the membrane hemocompatibility in extracorporeal membrane oxygenator (ECMO) Adv Compos Hybrid Mater. 2021;4:847–864. doi: 10.1007/s42114-021-00244-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Burstein B, Wieruszewski PM, Zhao Y-J, et al. Anticoagulation with direct thrombin inhibitors during extracorporeal membrane oxygenation. World J Crit Care Med. 2019;8:87–98. doi: 10.5492/wjccm.v8.i6.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Investigators INSPIRATION, Sadeghipour P, Talasaz AH, et al. Effect of intermediate-dose vs standard-dose prophylactic anticoagulation on thrombotic events, extracorporeal membrane oxygenation treatment, or mortality among patients with COVID-19 admitted to the intensive care unit: The INSPIRATION randomized clinical trial. JAMA. 2021;325:1620–1630. doi: 10.1001/jama.2021.4152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.REMAP-CAP Investigators; ACTIV-4a Investigators; ATTACC Investigators Therapeutic anticoagulation with heparin in critically ill patients with COVID-19. N Engl J Med. 2021;385:777–789. doi: 10.1056/NEJMoa2103417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Greinacher A, Selleng K, Warkentin TE. Autoimmune heparin-induced thrombocytopenia. J Thromb Haemost. 2017;15:2099–2114. doi: 10.1111/jth.13813. [DOI] [PubMed] [Google Scholar]
- 36.Greinacher A, Thiele T, Warkentin TE, et al. Thrombotic thrombocytopenia after ChAdOx1 nCov-19 vaccination. N Engl J Med. 2021;384:2092–2101. doi: 10.1056/NEJMoa2104840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Foley JH, Conway EM. Cross talk pathways between coagulation and inflammation. Circ Res. 2016;118:1392–1408. doi: 10.1161/CIRCRESAHA.116.306853. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.