Abstract
Background
We aimed to characterize the outcomes of sleeve resection after neoadjuvant chemoimmunotherapy for the treatment of non-small cell lung cancer (NSCLC), including perioperative and oncologic outcomes, and to identify any impact of operative approach on resultant findings.
Methods
We identified patients with NSCLC who underwent sleeve resection after ≥2 cycles of neoadjuvant chemoimmunotherapy between May 2019 and April 2021 and retrospectively reviewed clinical records. Perioperative data were collected and compared between video-assisted thoracoscopic surgery (VATS) (n=8) and thoracotomy (n=15) groups. Immunohistochemistry (IHC) scores were compared between tumors with and without major pathological response (MPR).
Results
Twenty-three patients met inclusion criteria, with clinical stages as follows: IB, 2 (8.7%); IIIA, 14 (60.9%); and IIIB, 7 (30.4%). Treatment-related adverse events (TRAE) were recorded in 17 patients (73.9%), including anemia and neutropenia, with no patients exhibiting serious TRAE. Radiological evaluation revealed 5 (21.7%) patients with complete response (CR), 14 (60.9%) with partial response (PR), and 4 (17.4%) with stable disease (SD). Complete resection was accomplished for all patients. One VATS procedure was converted to thoracotomy due to extensive pleural adhesions. There were no significant differences in intraoperative blood loss (87.5±51.8 vs. 193.9±145.3 mL), operative time (198.8±79.7 vs. 225.5±55.0 min), number of lymph node examined (16.9±6.6 vs. 18.2±6.5), and hospital stay (5.5±2.8 vs. 9.2±11.2 days) between the VATS and thoracotomy groups (all P>0.05). Postoperative complications occurred in 3 patients, and 1 patient died of bronchopleural fistula (BPF) in the thoracotomy group. Complete pathological response (CPR) and MPR were achieved in seven (30.4%) and 13 (56.5%) patients, respectively. Both preoperative histopathology (P=0.024) and radiological response (P=0.002) were significantly associated with MPR. In postoperative specimens with MPR, the IHC scores of cluster of differentiation (CD)4, CD8, and CD20 were modestly higher, while programmed cell death receptor 1 (PD-1), lymphocyte-activation gene 3 (LAG3) and T cell immunoglobulin and ITIM domain (TIGIT) were lower compared with non-MPR specimens, albeit insignificantly.
Conclusions
Sleeve resection after neoadjuvant chemoimmunotherapy was feasible in patients with locally advanced NSCLC. Perioperative outcomes were comparable between the VATS and thoracotomy groups.
Keywords: Neoadjuvant chemoimmunotherapy, sleeve resection, non-small cell lung cancer (NSCLC)
Introduction
The prognosis of locally advanced non-small cell lung cancer (NSCLC) remains poor, with 5-year survival of approximately 30% over the past few decades (1). Previous investigators have aimed to improve oncologic outcomes in this patient cohort, with multimodality therapies currently considered standard treatment (2,3). However, the value of neoadjuvant chemotherapy is limited, with an increase of only 5% in 5-year survival compared to surgery alone (4,5). Recently, programmed cell death receptor 1 (PD-1) and programmed cell death ligand 1 (PD-L1) checkpoint inhibitors have shown promise in the treatment of locally advanced NSCLC (6,7). Furthermore, adjuvant atezolizumab was shown to improve the disease-free survival in patients with resected NSCLC (8). Therefore, a neoadjuvant immunotherapy approach in this population is attractive.
For lung cancer invading the main bronchus, sleeve resection was recommended to avoid pneumonectomy and reserve more cardiac or pulmonary function, with promising perioperative and survival outcomes (9). However, the safety of sleeve resection after neoadjuvant immunotherapy has not yet been clearly elucidated, with concerns regarding potentially increased surgical risks, such as hemorrhage, persistent air leakage, bronchopleural fistula (BPF), related to both tissue adhesions and fibrosis as well as impact on the obligatory reconstruction of anatomic structures (10,11).
This study aims to characterize perioperative outcomes of sleeve resection after neoadjuvant chemoimmunotherapy, as well as to identify any differences between operative approaches. In addition, we seek to investigate the oncologic outcomes of sleeve resections after induction chemoimmunotherapy and to explore potential predictors of pathological response in the treatment of NSCLC. We present the following article in accordance with the STROBE reporting checklist (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-22-56/rc).
Methods
Study design and patient selection
This study protocol was approved by the Ethics Committee of Shanghai Pulmonary Hospital (No. 19216XW-4), and the study protocols conformed to the provisions of the Declaration of Helsinki (as revised in 2013). For its retrospective nature, written consents from the included patients were not needed. The clinical data of patients who underwent sleeve resection after neoadjuvant chemoimmunotherapy between May 2019 and April 2021 at Shanghai Pulmonary Hospital were retrospectively collected. The primary inclusion criteria in clinical trial were as follows: (I) age 18–75 years; (II) Eastern Cooperative Oncology Group (ECOG) score 0–1; (III) clinical stage II–IIIB [N2]; (IV) epidermal growth factor receptor (EGFR) and anaplastic lymphoma kinase (ALK) wild-type; and (V) anticipation of complete resectability (R0 resection) after neoadjuvant therapy. The exclusion criteria of the study were as follows: (I) patients with concurrent additional malignancies; (II) patients that had undergone any other neoadjuvant treatment for NSCLC, including local radiotherapy, chemotherapy only, and targeted therapy; and (III) patients whose operations included pulmonary arterioplasty and/or bronchoplasty.
Histologic and staging evaluation
Tissue diagnosis and histologic characterization was performed by percutaneous needle biopsy or endobronchial ultrasound-transbronchial needle aspiration (EBUS-TBNA). Computed tomography (CT) scans were performed to evaluate primary tumors. Positron emission tomography (PET), brain magnetic resonance imaging (MRI), and EBUS-TBNA were performed to confirm the clinical stage.
Neoadjuvant therapy strategy
Platinum-based doublet chemotherapy combined with PD-1 inhibitors were prescribed every 21 days for 2–4 treatment cycles depending upon treatment response. The therapeutic regimens of chemotherapy drugs and PD-1 inhibitors are shown in Table 1. According to the protocol, if serious drug-related adverse effects occurred, the use of PD-1 inhibitors were ceased temporarily or permanently. After 2 cycles of neoadjuvant therapy, CT scans or PET-CTs were performed for evaluation of response. If complete resection was not felt to be feasible at that time, additional cycles were considered.
Table 1. Demographic and clinical characteristics of the patients.
| No. | Age | Gender | Smoking | ECOG | Comorbidity | FEV1/FVC | Clinical stage | Diagnosis | Treatment | Cycles | Operation |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 70 | Male | Yes | 1 | NA | 2.3/3.1 L | T3N0M0 IIB | SqCC | TC + pembrolizumab | 2 | VATS RUL SR |
| 2 | 72 | Male | Yes | 0 | Hypertension | 2.8/3.2 L | T2N2M0 IIIA | NSCLC | AC + sintilimab | 2 | LUL SR |
| 3 | 63 | Male | Yes | 0 | Hypertension | 1.8/2.9 L | T4N2M0 IIIB | SqCC | GC + sintilimab | 2 | LUL + DS DSR |
| 4 | 69 | Male | No | 1 | PVC | 2.0/3.4 L | T2N2M0 IIIA | NSCLC | TC + camrelizumab | 2 | LLL SR |
| 5 | 62 | Male | Yes | 1 | NA | 1.8/2.9 L | T4N2M0 IIIB | SqCC | TC + pembrolizumab | 2 | LUL DSR |
| 6 | 62 | Male | No | 1 | Hypertension | 2.3/3.2 L | T3N2M0 IIIB | SqCC | DC + pembrolizumab | 2 | VATS RUL SR |
| 7 | 65 | Male | Yes | 1 | Hypertension | 2.8/3.4 L | T2N2M0 IIIA | AD | AC + pembrolizumab | 3 | VATS RUL SR |
| 8 | 65 | Male | No | 1 | NA | 3.1/4.2 L | T2N2M0 IIIA | SqCC | TC + pembrolizumab | 2 | RUL SR |
| 9 | 68 | Male | Yes | 1 | NA | 1.7/2.4 L | T2N2M0 IIIA | SqCC | TC + sintilimab | 3 | VATS LUL SR |
| 10 | 56 | Male | No | 1 | NA | 3.4/4.3 L | T1N2M0 IIIA | SqCC | TA + pembrolizumab | 3 | VATS LUL SR |
| 11 | 57 | Male | No | 1 | NA | 3.4/4.4 L | T3N1M0 IIIA | SqCC | TC + pembrolizumab | 2 | VATS LUL SR |
| 12 | 71 | Male | No | 1 | NA | 1.8/2.4 L | T4N2M0 IIIB | SqCC | TC + toripalimab | 2 | LUL SR |
| 13 | 74 | Male | Yes | 1 | NA | 1.9/2.6 L | T2N2M0 IIIA | SqCC | TC + camrelizumab | 2 | LUL SR |
| 14 | 57 | Male | Yes | 1 | NA | 2.6/3.8 L | T2N2M0 IIIA | SqCC | TC + pembrolizumab | 2 | RML + LL SR |
| 15 | 68 | Male | Yes | 1 | NA | 2.0/2.9 L | T3N1M0 IIIA | SqCC | GC + camrelizumab | 2 | LLL + LS SR |
| 16 | 69 | Male | Yes | 1 | NA | 2.7/3.3 L | T4N1M0 IIIA | SqCC | TC + pembrolizumab | 3 | LUL DSR |
| 17 | 60 | Male | Yes | 1 | Hypertension | 1.6/2.8 L | T4N1M0 IIIA | SqCC | GC + camrelizumab | 2 | RUL SR |
| 18 | 54 | Male | No | 1 | Hypertension | 2.3/3.0 L | T1N2M0 IIIA | SqCC | TC + pembrolizumab | 3 | RUL SR |
| 19 | 60 | Male | No | 1 | NA | 2.2/3.4 L | T2N1M0 IIB | SqCC | TC + camrelizumab | 3 | VATS RUL SR |
| 20 | 58 | Male | Yes | 1 | CRD | 2.9/3.7 L | T4N2M0 IIIB | AD | AC + camrelizumab | 2 | RUL SR |
| 21 | 45 | Female | No | 1 | NA | 2.2/2.9 L | T3N2M0 IIIB | SqCC | TC + camrelizumab | 3 | VATS RUL + ML SR |
| 22 | 58 | Male | Yes | 1 | Hypertension | 2.6/3.6 L | T2N2M0 IIIA | AD | TC + pembrolizumab + VEGFR | 4 | RUL + DS SR |
| 23 | 70 | Male | Yes | 1 | NA | 2.3/3.1 L | T4N2M0 IIIB | SqCC | TC + camrelizumab | 2 | LLL SR |
ECOG, Eastern Cooperative Oncology Group score; FEV1, forced expiratory volume in one second; FVC, forced vital capacity; NA, not available; PVC, premature ventricular contraction; CRD, coronary heart disease; L, liter; SqCC, squamous cell carcinoma; NSCLC, non-small cell lung cancer; AD, adenocarcinoma; D, docetaxel; C, carboplatin; G, gemcitabine; T, paclitaxel; A, pemetrexed; VEGFR, vascular endothelial growth factor receptor, including bevacizumab and apatinib; VATS, video-assisted thoracoscopic surgery; RUL, right upper lobe; SR, sleeve resection; LUL, left upper lobe; DS, dorsal segment; DSR, double sleeve resection; LLL, left lower lobe; LS, lingular segment; RML, right middle lobe.
Surgical resection
The surgical approaches, video-assisted thoracoscopic surgery (VATS) or thoracotomy, were determined at the discretion of surgeon. Surgery was performed 4–6 weeks after the last cycle of neoadjuvant therapy. Surgical procedures included the complete removal of the primary tumor via anatomic resection and systemic lymph node dissection. Double-sleeve resection was performed when the lesions invaded the central vessels. The anastomoses were performed with Prolene running suture and covered by vascular pedicled intercostal muscles, thymus, or pleura. Generally, 3 chest tubes were placed after operation: 2 were placed at the top and the bottom of the pleural cavity for promoting lung recruitment and fluid drainage, respectively. They were removed when patients were discharged without air leak. In addition, another flexible tube was placed at the anastomotic site to prevent the BPF, which was usually removed 1 week after discharge.
Response evaluation
Radiologic and pathologic responses were evaluated by specialists at the Department of Radiology and the Department of Pathology, Shanghai Pulmonary Hospital. The radiologic response was determined according to the Response Evaluation Criteria for Solid Tumors version 1.1 (RECIST 1.1) (12). Major pathological response (MPR) was defined as the presence of 10% or less viable tumor cells in the resected primary tumor, and complete pathological response (CPR) was defined as the tumor without any viable tumor in both the removed primary tumor and dissected lymph nodes (13).
Biomarker analysis
The formalin-fixed and paraffin-embedded specimens were prepared and provided by the Pathology Department of Shanghai Pulmonary Hospital. Slides were stained with PD-1 antibody, PD-L1 antibody, cluster of differentiation (CD)4 antibody, CD8 antibody, CD20 antibody, T cell immunoglobulin mucin 3 (TIM3) antibody, mouse fork head Box Protein P 3 (FXOP3), lymphocyte activation gene 3 (LAG3) antibody, and T cell immunoglobulin and ITIM domain (TIGIT) antibody. For scoring the immunohistochemistry (IHC) images, IHC scores were calculated using the proportion of positive cells of the whole cells (0–100) multiplied by the average intensity of the positive staining (negative staining as 0, weak staining as 1, moderate staining as 2, and strong staining as 3). Thus, for every sample, the IHC score ranged from 1 to 300. The IHC slides were examined and evaluated independently by 3 experienced pathologists, and the mean IHC score was calculated to reduce the potential difference between different observers.
Statistical analyses
Continuous variables were presented as means ± standard deviation and were compared using the Mann-Whitney U test. Categorical variables were presented as frequency and respective percentage and were compared using the Pearson chi-squared test or Fisher exact test, as appropriate. All statistical analyses were performed using R software (version 4.0.3). A 2-sided P value less than 0.05 was considered statistically significant.
Results
Patient characteristics
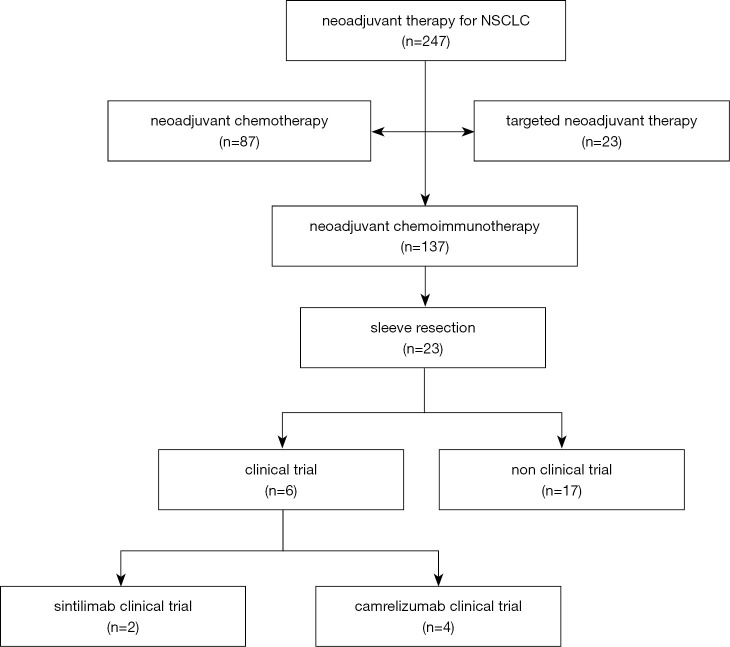
Twenty-three patients met inclusion criteria for this study (Figure 1), of whom 2 (8.7%) patients were from the sintilimab clinical trial (registration number: ChiCTR1900023758), 4 (17.4%) patients were from the camrelizumab clinical trial (registration number: NCT04379739), and 17 (73.9%) patients were treated off-trial.
Figure 1.
Patient selection flow chart. NSCLC, non-small cell lung cancer.
The mean age of included patients was 63.2±7.0 years, and 22 (95.6%) patients were male. Disease distribution in stages IIB, IIIA and IIIB consisted of 2 (8.7%), 14 (60.9%), and 7 (30.4%) patients, respectively. Histopathological diagnosis from pre-treatment biopsies identified 18 (78.3%) patients with squamous cell carcinoma, 3 (13.0%) with adenocarcinoma, and 2 (8.7%) with NSCLC. There were 8 (34.8%) patients who underwent VATS procedures and 15 who underwent thoracotomy (Table 1).
Drug safety
Treatment-related adverse events (TRAEs) were recorded in 17 (73.9%) patients, but no patients exhibited serious TRAEs. TRAEs of grade 1–2 occurred in 17 (73.9%) patients, and TRAEs of grade 3–4 occurred in 2 (8.7%) patients. The most common TRAEs were anemia and neutropenia, in 11 (47.8%) patients and 7 (30.4%) patients, respectively.
Outcomes
The interval between the end of the last neoadjuvant therapy cycle and surgery was 31.5±8.9 days. The mean operative time was 208.1±72.0 min, intraoperative blood loss was 125.2±160.0 mL, postoperative hospitalization stay was 7.9±9.2 days, and chest tube removal time was 9.8±7.0 days. There were no statistical differences in operative time (198.8±79.7 vs. 225.5±55.0 min, P=0.197), estimated blood loss (87.5±51.8 vs. 193.9±145.3 mL, P=0.655), chest tube removal time (7.1±3.4 vs. 11.3±8.0 days, P=0.297), and hospitalization time (5.5±2.8 vs. 9.2±11.2 days, P=0.416) between the VATS and thoracotomy groups. In the VATS group, one patient was converted to open surgery due to extensive pleural adhesion (Table 2).
Table 2. Comparison of perioperative outcomes between the VATS and thoracotomy groups.
| Variables | VATS | Thoracotomy | P |
|---|---|---|---|
| Patients | 8 | 15 | |
| Gender (male), n (%) | 7 (87.5) | 15 (100.0) | 0.348 |
| Age | 60.4±7.9 | 64.7±6.3 | 0.196 |
| ECOG (14,15), n (%) | 8 (100.0) | 13 (86.7) | 0.526 |
| Smoking history, n (%) | 3 (37.5) | 11 (73.3) | 0.179 |
| FEV1 (L) | 2.5±0.6 | 2.3±0.5 | 0.366 |
| Clinical stage (III), n (%) | 7 (87.5) | 14 (93.3) | 1.000 |
| Blood transfusion, n (%) | 0 | 1 (6.7) | |
| Blood loss (mL) | 87.5±51.8 | 193.9±145.3 | 0.655 |
| Operation time (min) | 198.8±79.7 | 225.5±55.0 | 0.197 |
| Drainage volume on days 1–3 (mL) | 995.0±458.0 | 1,030.0±382.1 | 0.746 |
| Drainage time (day) | 7.1±3.4 | 11.3±8.0 | 0.297 |
| Lymph node number | 16.9±6.6 | 18.2±6.5 | 0.674 |
| Lymph node station | 6.4±1.1 | 5.9±1.2 | 0.683 |
| Hospital stay (days) | 5.5±2.8 | 9.2±11.2 | 0.416 |
| Complication, n (%) | |||
| Pulmonary infection | 0 | 1 (6.7) | |
| Acute coronary syndrome | 0 | 1 (6.7) | |
| Bronchopleural fistula | 0 | 1 (6.7) | 0.526 |
| Conversion, n (%) | 1 (12.5) | 0 | |
| Mortality, n (%) | 0 | 1 (6.7) |
VATS, video-assisted thoracoscopic surgery; ECOG, eastern Cooperative Oncology Group; FEV1, forced expiratory volume in one second; L, liter; mL, milliliter; min, minute.
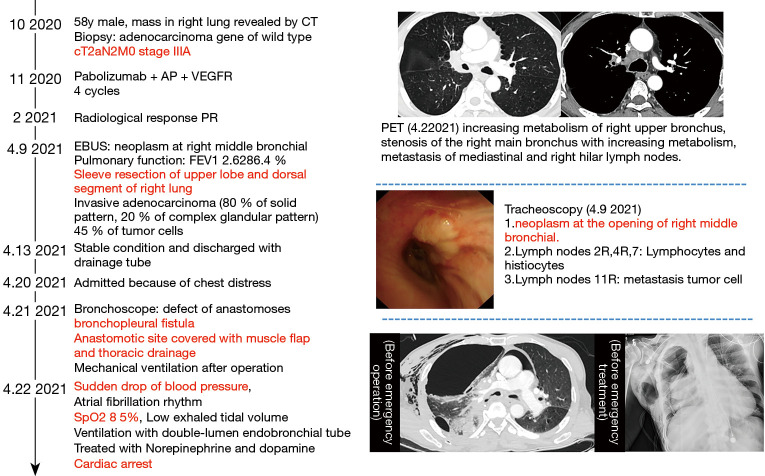
Postoperative complications occurred in 3 (13.0%) patients, including 1 case with BPF on postoperative day 12, 1 acute coronary syndrome, and 1 with pneumonia. All of these patients were in the thoracotomy group. The patient with BPF underwent sleeve resection of the upper lobe and superior segment of the right lung. BPF was diagnosed on day 12 via bronchoscopy, and fistula repair was attempted but failed due to severe inflammation at the site of anastomosis. The patient died of cardiac arrest on postoperative day 13 (Figure 2). With a median follow-up of 15 months (range, 10–30 months), only one patient (Patient 1) had local recurrence at 12 months after surgery. This patient received radiofrequency ablation and was still alive at the time of data cutoff (Feb 13, 2022).
Figure 2.
The treatment process of patients complicated with bronchopleural fistula. CT, computed tomography; AP, pemetrexed and cisplatin; VEGFR, vascular endothelial growth factor receptor; PR, partial response; PET, positron emission tomography; EBUS, endobronchial ultrasound; FEV1, forced expiratory volume in one second.
Efficacy of neoadjuvant therapy
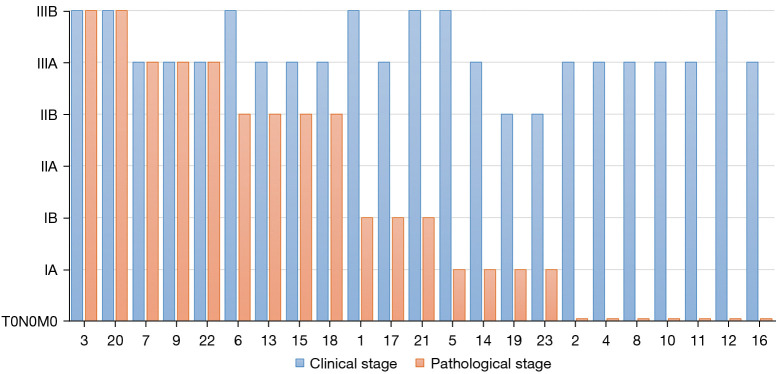
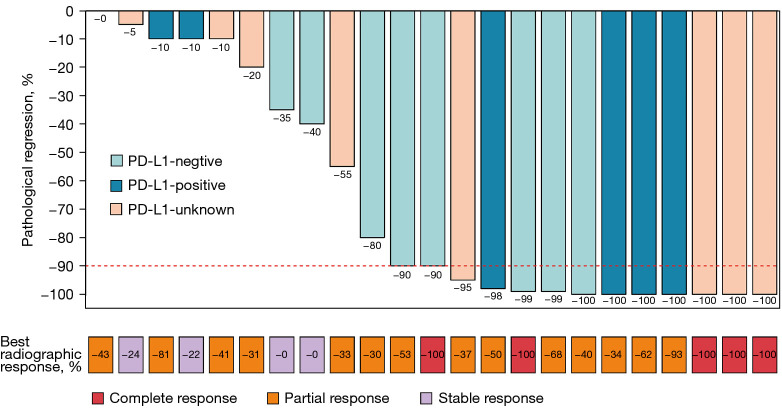
Preoperative radiological evaluation suggested that there were 5 (21.7%) patients with complete response (CR), 14 (60.9%) patients with partial response (PR), and 4 (17.4%) patients with stable disease (SD). There was no progressive disease. A total of 18 (78.3%) patients achieved tumor downstaging. Among them, 16 (69.6%) patients achieved LN downstaging, including N2 downstaging to N1 in 3 (29.2%) patients and N2 downstaging to N0 in 9 (29.2%) patients (Figure 3). Postoperative pathological evaluation of the 23 patients revealed that 13 (56.5%) patients achieved MPR, of which seven (27.0%) patients achieved CPR (Figure 4).
Figure 3.
Stages of patients before and after neoadjuvant therapy. x-axis presented the patient number, and y-axis presented the clinical stage.
Figure 4.
The correlation between radiological response and pathological response. PD-L1, programmed cell death ligand 1.
Clinical factors and biomarkers associated with pathological response
We explored the clinical factors and the IHC score of biomarkers involving antitumor immune response that may be associated with MPR. The patients’ demographic characteristics were not significantly different between the MPR and non-MPR groups. Only preoperative histopathologic type and radiological response were statistically associated with MPR. When analyzing the IHC score of the selected biomarkers, the expression of CD4 (16.5±7.5 vs. 22.3±17.3), CD8 (21.6±16.5 vs. 30.2±30.5), and CD20 (56.1±36.8 vs. 81.5±49.4) were modestly higher in the MPR group, while the expression of PD-1 (14.6±18.7 vs. 2.8±2.9), LAG3 (2.0±6.3 vs. 0), and TIGIT (6.8±7.9 vs. 3.4±3.4) were lower in the MPR group compared to the non-MPR group, although the difference was not significant (Table 3).
Table 3. Comparison of the clinical characteristics and IHC scores between the MPR and non-MPR groups.
| Variables | Non-MPR | MPR | P |
|---|---|---|---|
| Patients | 10 | 13 | |
| Age | 63.2±4.4 | 63.2±8.7 | 0.828 |
| Gender | |||
| Male, n (%) | 10 (100.0) | 12 (92.3) | |
| Female | 0 | 1 (7.7) | 1.000 |
| Smoking history, n (%) | |||
| No | 2 (20.0) | 7 (53.8) | |
| Yes | 8 (80.0) | 6 (46.2) | 0.197 |
| Treatment cycles, n (%) | |||
| 2 | 6 (60.0) | 9 (69.2) | |
| >2 | 4 (40.0) | 4 (30.8) | 0.685 |
| Adverse effect, n (%) | |||
| No | 3 (30.0) | 3 (23.1) | |
| Yes | 7 (70.0) | 10 (76.9) | 1.000 |
| Pretreatment PD-L1 expression, n (%) | |||
| Negative | 3 (30.0) | 5 (38.4) | |
| Positive | 3 (30.0) | 4 (30.8) | |
| NA | 4 (40.0) | 4 (30.8) | 1.000 |
| Pathology, n (%) | |||
| Squamous cell carcinoma | 7 (70.0) | 11 (84.6) | |
| Adenocarcinoma | 3 (30.0) | 0 (7.7) | |
| Non-small cell lung cancer | 0 | 2 (15.4) | 0.024 |
| Clinical stage, n (%) | |||
| II | 2 (20.0) | 0 | |
| III | 8 (80.0) | 13 (100.0) | 0.178 |
| Radiological response, n (%) | |||
| SD | 4 (40.0) | 0 | |
| PR | 6 (60.0) | 8 (61.5) | |
| CR | 0 | 5 (38.5) | 0.002 |
| Postoperative IHC score | |||
| CD4 | 16.5±7.5 | 22.3±17.3 | 0.723 |
| CD8 | 21.6±16.5 | 30.2±30.5 | 0.661 |
| CD20 | 56.1±36.8 | 81.5±49.4 | 0.189 |
| PD-1 | 14.6±18.7 | 2.8±2.9 | 0.080 |
| PD-L1 | 9.5±20.0 | 2.3±4.7 | 1.000 |
| TIM3 | 0.6±1.3 | 3.5±8.5 | 0.925 |
| FXOP3 | 3.5±4.5 | 3.9±8.0 | 0.563 |
| LAG3 | 2.0±6.3 | 0 | 0.146 |
| TIGIT | 6.8±7.9 | 3.4±3.4 | 0.552 |
IHC, immunohistochemistry; MPR, major pathologic response; PD-L1, programmed cell death ligand 1; NA, not available; SD, stable disease; PR, partial response; CR, complete response; CD, cluster of differentiation; PD-1, the programmed cell death receptor 1; TIM3, T cell immunoglobulin mucin 3; FXOP3, mouse fork head Box Protein P 3; LAG3, lymphocyte-activation gene 3; TIGIT, T cell immunoglobulin and ITIM domain.
Discussion
In the present study, we report the results of sleeve resection after neoadjuvant chemoimmunotherapy in locally advanced NSCLC. Our results showed that TRAEs occurred in 17 (73.9%) patients, and no serious TRAE was recorded from the drugs received. All patients achieved R0 resection. Postoperative complications were observed in 3 patients, with no difference between VATS and thoracotomy. One serious complication occurred, death of BPF, which has led to lessons learned in terms of patient selection and technical caveats. Nonetheless, the objective response rate was 82.6%, and MPR and PCR were achieved in 13 (56.5%) and 7 (27.0%) patients, respectively.
The perioperative outcomes of sleeve resection after neoadjuvant chemoimmunotherapy have remained unclear due to the lack of prior relevant studies. Chen et al.’s study involving 9 patients showed no difference in surgical time and number of lymph nodes between sleeve resection after neoadjuvant chemoimmunotherapy and upfront surgery (16). Our study, representing the largest reported cohort thus far, found that sleeve resection after the neoadjuvant chemoimmunotherapy exhibited similar perioperative outcomes compared with sleeve resection alone (17). In addition, postoperative complications occurred in 3 (13.0%) patients in the thoracotomy group, of which 1 (4.3%) patient died of BPF. Specifically, the patient with BPF received right upper sleeve lobectomy plus S6 segmentectomy, and no bronchial stump coverage was used during operation. It is possible that right upper lobectomy plus S6 segmentectomy can result in a large residual cavity in the right upper thorax. The fluid might be easily accumulated in the anastomotic site, thus leading to an increased risk of BPF. Therefore, adequate drainage and bronchial stump reinforcement were important for patients with a high-risk of BPF. Overall, the introduction of neoadjuvant immunotherapy did not appear to increase the risks of postoperative complications compared to neoadjuvant chemotherapy alone (18). A dominant concern after neoadjuvant immunotherapy was that more anastomosis-related complications might occur, yet the incidence of BPF in this study was comparable to previous literature (19). Therefore, sleeve resection after neoadjuvant immunochemotherapy appeared to show promising perioperative results without increased surgical complications.
In previous studies, most operations after neoadjuvant chemoimmunotherapy were performed via thoracotomy due to concern about the potential risks of intraoperative crisis (14,15), and thus, the use of VATS has been slow to achieve adoption. Bott et al. reported that 7 (54%) patients undergoing a minimally invasive approach required conversion to open surgery, and postoperative morbidity occurred in 10 of 20 (50%) patients (20). Liang et al. also reported a high proportion of conversion after neoadjuvant chemoimmunotherapy due to pleural adhesions (10). In this study, only one patient in the VATS group was converted to thoracotomy due to extensive pleural adhesion, who was in grade 4 (i.e., complete symphysis of the pleural surfaces) in the pleural adhesion grading system documented by Mason et al. (21). VATS did not prolong the operative time or increase the risks of intraoperative blood loss compared with thoracotomy. These results demonstrated the feasibility of VATS in the treatment of locally advanced NSCLC after neoadjuvant chemoimmunotherapy, and patients without extensive pleural adhesion, huge metastasized lymph nodes, and severe invasion to the hilum were found to be the optimal candidates for VATS.
The promising pathological response of neoadjuvant PD-1 inhibitors were hopeful to change the therapeutic regimes of NSCLC. The NADIM clinical trial firstly assessed the effectiveness and safety of neoadjuvant chemoimmunotherapy for NSCLC; they found that 34 (83%) patients had MPR and 26 (63%) patients had CPR (22). Moreover, the clinical trial of neoadjuvant atezolizumab reported that 17 of 30 (57%) patients had an MPR (23). Our results also showed a high MPR (56.5%) after neoadjuvant chemoimmunotherapy. Therefore, neoadjuvant chemoimmunotherapy followed by sleeve resection could potentially improve the therapeutic efficacy and augment the therapeutic strategies offered in the treatment of locally advanced NSCLC.
At present, biomarkers for immunotherapy are investigational. We found that postoperative histopathologic type and radiological response were different between the MPR and non-MPR groups. A similar association between pathologic type and MPR was also observed in another clinical trial, where MPR in squamous cell carcinoma was higher than that in adenocarcinoma (23). This association was possibly due to greater CD8 lymphocyte infiltration in squamous cell carcinoma (24).
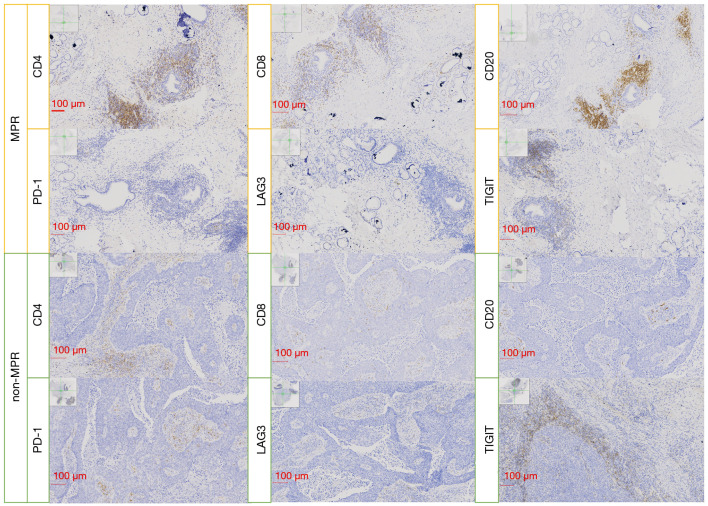
PD-L1 expression was widely established as a significant predictor in immunotherapy, and higher PD-L1 expression was predictive of better radiological response as well as longer overall survival (25). While for neoadjuvant chemoimmunotherapy, PD-L1 expression in the tumor cells was not associated with MPR, which were also indicated in our study (22). Although the IHC scores were not significantly different, the expression of CD4, CD8, and CD20 was modestly higher in the MPR group compared with the non-MPR patients (Figure 5). The combination of chemotherapy and immunotherapy had the potential advantage of generating more abundant and diverse neoantigens to enhance systemic immune response, thus recruiting more lymphocytes into the tumor microenvironment and eliminating the micro-metastasis (26).
Figure 5.
The IHC score of biomarkers involved in the immune response in the MPR and non-MPR groups (scale bar, 100 µm). IHC, immunohistochemistry; MPR, major pathological response.
TIM3 was originally identified as a specific marker for T helper cells and CD8 T cells, with evidence for TIM3-dependent inhibitory function (27). Herein, we found that TIM3 expression was higher in MPR, as the tumor microenvironment enriched more T cells with a high pathological response. It has been previously reported that PD-1 inhibitors could mediate endocytosis of PD-1 in T cells (28); similarly, we found the expression of PD-1 was lower in MPR, while the expression of PD-L1 was comparable between two groups. We also found that the expression of LAG3 and TIGIT was higher in the non-MPR group; perhaps, this may be due to the activation of other immunosuppressive pathways in non-MPR patients (29).
Several limitations should be considered in gleaning conclusions from this study. Firstly, this was a retrospective review with potential selection bias. The selection of candidates for neoadjuvant therapy and the use of VATS versus thoracotomy were generally made by the discretion of each surgeon. Furthermore, the included population was relatively small and lacked long-term outcomes, which precluded us from analyzing the predictive characteristics and biomarkers of postoperative complications and MPR, as well as the role of sleeve resection after chemoimmunotherapy on survival.
However, despite these limitations, this represents the largest study to date demonstrating safety and efficacy of the promising role of neoadjuvant chemoimmunotherapy prior to sleeve resection for advanced NSCLC.
Conclusions
In conclusion, we reported the perioperative outcomes of sleeve resection after neoadjuvant chemoimmunotherapy in the treatment of NSCLC. Sleeve resection after neoadjuvant chemoimmunotherapy was feasible and effective in patients with locally advanced NSCLC, with comparable perioperative outcomes between VATS and thoracotomy, and complete resection could be achieved in all patients. Further prospective studies with larger patient sample sizes and long-term follow-up are needed to consolidate these findings.
Supplementary
The article’s supplementary files as
Acknowledgments
The authors appreciate the academic support from the AME Thoracic Surgery Collaborative Group.
Funding: This study was supported by the National Natural Science Foundation of China (Grant No. 82172848), the Shanghai Rising-Star Program (Grant No. 19QA1407400), the Shanghai Municipal Education Commission “Chen Guang” project (19CG19), the Shanghai “Rising Stars of Medical Talent” Youth Development Program Youth Medical Talents-Specialist Program, and Shanghai Pulmonary Hospital Fund (Nos. fkyq1908 and fkzr2105).
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study protocol was approved by the Ethics Committee of Shanghai Pulmonary Hospital (No. 19216XW-4), and the study protocols conformed to the provisions of the Declaration of Helsinki (as revised in 2013). For its retrospective nature, written consents from the included patients were not needed.
Footnotes
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-22-56/rc
Data Sharing Statement: Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-22-56/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-22-56/coif). RAR reports grants from Merck; consulting fees from Astra-Zeneca, Novartis, EMD Serono, and Amgen; payment from Astra-Zeneca, Genentech, and Merck. The other authors have no conflicts of interest to declare.
References
- 1.Chansky K, Detterbeck FC, Nicholson AG, et al. The IASLC Lung Cancer Staging Project: External Validation of the Revision of the TNM Stage Groupings in the Eighth Edition of the TNM Classification of Lung Cancer. J Thorac Oncol 2017;12:1109-21. [DOI] [PubMed] [Google Scholar]
- 2.Migliorino MR, De Petris L, De Santis S, et al. Locally advanced non-small cell lung cancer: role of induction chemotherapy in resectable N2 disease. Ann Oncol 2006;17 Suppl 2:ii28-31. 10.1093/annonc/mdj916 [DOI] [PubMed] [Google Scholar]
- 3.Bogart JA, Aronowitz JN. Localized non-small cell lung cancer: adjuvant radiotherapy in the era of effective systemic therapy. Clin Cancer Res 2005;11:5004s-10s. 10.1158/1078-0432.CCR-05-9010 [DOI] [PubMed] [Google Scholar]
- 4.NSCLC Meta-analysis Collaborative Group . Preoperative chemotherapy for non-small-cell lung cancer: a systematic review and meta-analysis of individual participant data. Lancet 2014;383:1561-71. 10.1016/S0140-6736(13)62159-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pignon JP, Tribodet H, Scagliotti GV, et al. Lung adjuvant cisplatin evaluation: a pooled analysis by the LACE Collaborative Group. J Clin Oncol 2008;26:3552-9. 10.1200/JCO.2007.13.9030 [DOI] [PubMed] [Google Scholar]
- 6.Gong J, Chehrazi-Raffle A, Reddi S, et al. Development of PD-1 and PD-L1 inhibitors as a form of cancer immunotherapy: a comprehensive review of registration trials and future considerations. J Immunother Cancer 2018;6:8. 10.1186/s40425-018-0316-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Forde PM, Chaft JE, Smith KN, et al. Neoadjuvant PD-1 Blockade in Resectable Lung Cancer. N Engl J Med 2018;378:1976-86. 10.1056/NEJMoa1716078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Felip E, Altorki N, Zhou C, et al. Adjuvant atezolizumab after adjuvant chemotherapy in resected stage IB-IIIA non-small-cell lung cancer (IMpower010): a randomised, multicentre, open-label, phase 3 trial. Lancet 2021;398:1344-57. 10.1016/S0140-6736(21)02098-5 [DOI] [PubMed] [Google Scholar]
- 9.Cusumano G, Marra A, Lococo F, et al. Is sleeve lobectomy comparable in terms of short- and long-term results with pneumonectomy after induction therapy? A multicenter analysis. Ann Thorac Surg 2014;98:975-83. 10.1016/j.athoracsur.2014.04.095 [DOI] [PubMed] [Google Scholar]
- 10.Liang H, Yang C, Gonzalez-Rivas D, et al. Sleeve lobectomy after neoadjuvant chemoimmunotherapy/chemotherapy for local advanced non-small cell lung cancer. Transl Lung Cancer Res 2021;10:143-55. 10.21037/tlcr-20-778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rendina EA, Venuta F, De Giacomo T, et al. Safety and efficacy of bronchovascular reconstruction after induction chemotherapy for lung cancer. J Thorac Cardiovasc Surg 1997;114:830-5; discussion 835-7. 10.1016/S0022-5223(97)70088-6 [DOI] [PubMed] [Google Scholar]
- 12.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228-47. 10.1016/j.ejca.2008.10.026 [DOI] [PubMed] [Google Scholar]
- 13.Mouillet G, Monnet E, Milleron B, et al. Pathologic complete response to preoperative chemotherapy predicts cure in early-stage non-small-cell lung cancer: combined analysis of two IFCT randomized trials. J Thorac Oncol 2012;7:841-9. 10.1097/JTO.0b013e31824c7d92 [DOI] [PubMed] [Google Scholar]
- 14.Bott MJ, Cools-Lartigue J, Tan KS, et al. Safety and Feasibility of Lung Resection After Immunotherapy for Metastatic or Unresectable Tumors. Ann Thorac Surg 2018;106:178-83. 10.1016/j.athoracsur.2018.02.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chaft JE, Hellmann MD, Velez MJ, et al. Initial Experience With Lung Cancer Resection After Treatment With T-Cell Checkpoint Inhibitors. Ann Thorac Surg 2017;104:e217-8. 10.1016/j.athoracsur.2017.03.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen Y, Zhang L, Yan B, et al. Feasibility of sleeve lobectomy after neo-adjuvant chemo-immunotherapy in non-small cell lung cancer. Transl Lung Cancer Res 2020;9:761-7. 10.21037/tlcr-20-539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pagès PB, Mordant P, Renaud S, et al. Sleeve lobectomy may provide better outcomes than pneumonectomy for non-small cell lung cancer. A decade in a nationwide study. J Thorac Cardiovasc Surg 2017;153:184-195.e3. 10.1016/j.jtcvs.2016.09.060 [DOI] [PubMed] [Google Scholar]
- 18.Inci I, Benker M, Çitak N, et al. Complex sleeve lobectomy has the same surgical outcome when compared with conventional lobectomy in patients with lung cancer. Eur J Cardiothorac Surg 2020;57:860-6. 10.1093/ejcts/ezz357 [DOI] [PubMed] [Google Scholar]
- 19.Milman S, Kim AW, Warren WH, et al. The incidence of perioperative anastomotic complications after sleeve lobectomy is not increased after neoadjuvant chemoradiotherapy. Ann Thorac Surg 2009;88:945-50; discussion 950-1. 10.1016/j.athoracsur.2009.05.084 [DOI] [PubMed] [Google Scholar]
- 20.Bott MJ, Yang SC, Park BJ, et al. Initial results of pulmonary resection after neoadjuvant nivolumab in patients with resectable non-small cell lung cancer. J Thorac Cardiovasc Surg 2019;158:269-76. 10.1016/j.jtcvs.2018.11.124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mason AC, Miller BH, Krasna MJ, et al. Accuracy of CT for the detection of pleural adhesions: correlation with video-assisted thoracoscopic surgery. Chest 1999;115:423-7. 10.1378/chest.115.2.423 [DOI] [PubMed] [Google Scholar]
- 22.Provencio M, Nadal E, Insa A, et al. Neoadjuvant chemotherapy and nivolumab in resectable non-small-cell lung cancer (NADIM): an open-label, multicentre, single-arm, phase 2 trial. Lancet Oncol 2020;21:1413-22. 10.1016/S1470-2045(20)30453-8 [DOI] [PubMed] [Google Scholar]
- 23.Shu CA, Gainor JF, Awad MM, et al. Neoadjuvant atezolizumab and chemotherapy in patients with resectable non-small-cell lung cancer: an open-label, multicentre, single-arm, phase 2 trial. Lancet Oncol 2020;21:786-95. 10.1016/S1470-2045(20)30140-6 [DOI] [PubMed] [Google Scholar]
- 24.Meng X, Gao Y, Yang L, et al. Immune Microenvironment Differences Between Squamous and Non-squamous Non-small-cell Lung Cancer and Their Influence on the Prognosis. Clin Lung Cancer 2019;20:48-58. 10.1016/j.cllc.2018.09.012 [DOI] [PubMed] [Google Scholar]
- 25.Sezer A, Kilickap S, Gümüş M, et al. Cemiplimab monotherapy for first-line treatment of advanced non-small-cell lung cancer with PD-L1 of at least 50%: a multicentre, open-label, global, phase 3, randomised, controlled trial. Lancet 2021;397:592-604. 10.1016/S0140-6736(21)00228-2 [DOI] [PubMed] [Google Scholar]
- 26.Liu J, Blake SJ, Yong MC, et al. Improved Efficacy of Neoadjuvant Compared to Adjuvant Immunotherapy to Eradicate Metastatic Disease. Cancer Discov 2016;6:1382-99. 10.1158/2159-8290.CD-16-0577 [DOI] [PubMed] [Google Scholar]
- 27.Meyers JH, Sabatos CA, Chakravarti S, et al. The TIM gene family regulates autoimmune and allergic diseases. Trends Mol Med 2005;11:362-9. 10.1016/j.molmed.2005.06.008 [DOI] [PubMed] [Google Scholar]
- 28.Burr ML, Sparbier CE, Chan YC, et al. CMTM6 maintains the expression of PD-L1 and regulates anti-tumour immunity. Nature 2017;549:101-5. 10.1038/nature23643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Anderson AC, Joller N, Kuchroo VK. Lag-3, Tim-3, and TIGIT: Co-inhibitory Receptors with Specialized Functions in Immune Regulation. Immunity 2016;44:989-1004. 10.1016/j.immuni.2016.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The article’s supplementary files as