Abstract
We determined the sequences of the quinolone resistance-determining regions of gyrA, gyrB, and parC genes for 30 clinical strains of Pseudomonas aeruginosa resistant to ciprofloxacin that were previously complemented by wild-type gyrA and gyrB plasmid-borne alleles and studied for their coresistance to imipenem (E. Cambau, E. Perani, C. Dib, C. Petinon, J. Trias, and V. Jarlier, Antimicrob. Agents Chemother. 39:2248–2252, 1995). In the present study, we found mutations in type II topoisomerase genes for all strains. Twenty-eight strains had a missense mutation in gyrA (codon 83 or 87). Ten of them had an additional mutation in parC (codon 80 or 84), including a novel mutation of Ser-80 to Trp, but all were fully complemented by a plasmid-borne wild-type gyrA allele. The remaining two strains harbored the first gyrB mutation described in P. aeruginosa, leading to the substitution of phenylalanine for serine 464. The strains which had two mutations in type II topoisomerase genes (i.e., gyrA and parC) were significantly more resistant to fluoroquinolones than those with a single mutation in gyrA or gyrB (geometric mean MICs of ciprofloxacin, 39.4 versus 10.9 μg/ml, P < 0.01; geometric mean MICs of sparfloxacin, 64.0 versus 22.6, P < 0.01). No mutant with a parC mutation alone was observed, which favors DNA gyrase being the primary target for fluoroquinolones. These results demonstrate that gyrA mutations are the major mechanism of resistance to fluoroquinolones for clinical strains of P. aeruginosa and that additional mutations in parC lead to a higher level of quinolone resistance.
The intrinsic resistance of Pseudomonas aeruginosa to a wide variety of antibiotics represents a major therapeutic challenge. Ciprofloxacin has emerged as one of the most effective quinolones against P. aeruginosa (28). Imipenem has also been widely used for the treatment of P. aeruginosa infections in hospitals. The intensive use of these antibiotics has led to the emergence of resistant strains, some of which are coresistant to quinolones and carbapenems (20, 25, 28). The question of a common mechanism of resistance to quinolones and carbapenems has been raised by several authors (18, 21). The mechanisms of quinolone resistance described in P. aeruginosa are mutations in the DNA gyrase gyrA gene (15, 32, 34) and, recently, in the topoisomerase IV parC gene (19), decreased permeability of the cell wall, and multidrug efflux systems (17). The latter mechanisms can affect both quinolones and a wide variety of antibiotics other than quinolones, including carbapenems (5, 17). However, we have previously demonstrated that in 20 clinical strains of P. aeruginosa, coresistance to ciprofloxacin and imipenem was due to two distinct mechanisms (2). In these strains, imipenem resistance was associated with a lack of a specific outer membrane protein, OprD (4, 24), whereas ciprofloxacin resistance was essentially related to gyrA or gyrB mutations, as suggested by complementation tests with plasmids containing either wild-type gyrA or gyrB genes. However, the wide range of ciprofloxacin MICs (2 to 128 μg/ml) among the strains suggested the association of several mechanisms of resistance in those with the highest level of resistance. Indeed, an additive effect of several mutations affecting gyrA or both gyrA and parC has been described in other gram-negative bacteria (3, 9, 26). To characterize the mutations in type II topoisomerase genes, we analyzed the quinolone resistance-determining regions (QRDR) of the gyrA, gyrB, and parC genes of the 30 clinical ciprofloxacin-resistant P. aeruginosa strains previously studied by complementation tests (2).
MATERIALS AND METHODS
Strains.
Thirty P. aeruginosa strains resistant to ciprofloxacin (MIC, 2 to 128 μg/ml), collected between 1990 and 1992 from clinical specimens in our hospital, were included in the study. Twenty of these strains were resistant to imipenem (MICs, ≥8 μg/ml). MIC determination and transformation with plasmids carrying the wild-type gyrA gene (pPAW207) or gyrB gene (pPBW801) had been previously carried out in these strains and were detailed elsewhere (2). Two wild-type strains were included as controls: strain 65, which was a clinical strain, and the reference strain PAO1, kindly given by E. Collatz.
PCR amplification and DNA sequencing.
For DNA extraction, 2 ml of an 18-h culture in brain heart infusion broth was centrifuged (13,000 × g, 1 min). The pellet was suspended in 500 ml of H2O, and cells were lysed by the freeze-thaw procedure, alternately 1 min at 100°C and 1 min at 0°C, 10 times (29). Two 21-mer primers, PSE1 (5′-GACGGCCTGAAGCCGGTGCAC-3′) and PYO1 (5′-GCCCACGGCGATACCGCTGGA-3′), constructed on the basis of the nucleotide sequence of P. aeruginosa PAO1 gyrA (15), were used to amplify a 417-bp fragment of P. aeruginosa gyrA from positions 115 to 531. Two primers, GYRB1 (5′-GCGCGTGAGATGACCCGCCGT-3′) and GYRB2 (5′-CTGGCGGTAGAAGAAGGTCAG-3′), designed to be homologous to the gyrB gene of Escherichia coli (30) from nucleotides 1162 to 1182 and from nucleotides 1531 to 1551, respectively, were used to amplify a 390-bp fragment of P. aeruginosa gyrB. Two primers, PARC1 (5′-CTGGATGCCGATTCCAAGCAC-3′) and PARC2 (5′-GAAGGACTTGGGATCGTCCGG-3′), constructed on the basis of the partial sequence of P. aeruginosa parC (19), were used to amplify a 186-bp fragment of parC. All amplifications were carried out in a 100-μl volume containing 0.4 μM each nucleotide primer, 0.25 mM each 2′-deoxynucleoside 5′-triphosphate (Pharmacia Biotech, Orsay, France), 10 μl of reaction buffer (Boehringer Mannheim, Meylan, France), 2 μl of a template DNA sample, and 2 U of Taq DNA polymerase (Boehringer Mannheim). The reactions were performed on a DNA thermal cycler (Perkin-Elmer Cetus, Norwalk, Conn.) for 40 cycles, each cycle consisting of 1 min at 94°C for denaturation, 1 min at 65°C (gyrA and gyrB) or 61°C (parC) for annealing, and 1 min at 72°C for polymerization. For each P. aeruginosa strain, the amplified DNA was pooled from two different PCR assays and purified by using the Geneclean kit (Bio 101, La Jolla, Calif.) in accordance with the manufacturer’s recommendations.
Sequencing of the gyrB and parC fragments was done with the same primers as those used for PCR. For the gyrA fragments, we had to design internal primers, PYO2 (5′-ATGAGCGAGCTGGGCAACGAC-3′) and PSE2 (5′-CAGGTCCGCCAGCAGTTCGTG-3′) from positions 154 to 414, to facilitate its sequencing. For gyrA and gyrB, sequence reactions were performed on both DNA strands with the T7 sequencing kit (Pharmacia Biotech) by the sequencing technique of Tabor and Richardson (23) modified as previously described (16). For parC, the purified DNA was sequenced by the Genome Express Society (Paris, France). For each DNA fragment, mutations were confirmed on a new PCR product.
Antibiotic resistance patterns.
The MICs of ciprofloxacin, sparfloxacin, and imipenem were determined in a previous study (2). The log10 MICs were compared by use of the Mann-Whitney U test. Differences between groups were considered to be statistically significant for P values of <0.05.
Nucleotide accession number.
The partial sequence of gyrB from P. aeruginosa PAO1 was submitted to GenBank, EMBL and DDJB under accession no. Y16286.
RESULTS
The mutations found in gyrA, gyrB, and parC QRDR of the 30 clinical ciprofloxacin-resistant P. aeruginosa strains are described in Table 1.
TABLE 1.
Clinical strains of P. aeruginosa: patterns of susceptibility to fluoroquinolones and imipenem, results of complementation tests with wild-type gyrA or gyrB alleles, and DNA mutations found in gyrA, gyrB, or parC QRDR
| Strain | MIC (μg/ml) ofa:
|
Amino acid (codon) encoded by indicated gene at position:
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| CPX
|
SPX
|
IPM
|
gyrA
|
gyrB
|
parC
|
|||||
| NTb | gyrA+c | gyrB+d | NT | NT | 83 | 87 | 464 | 80 | 84 | |
| PAO1 | 0.12 | 0.12 | 0.12 | 0.25 | 1 | Thr (ACC) | Asp (GAC) | Ser (TCC) | Ser (TCG) | Glu (GAG) |
| 65 | 0.25 | 0.25 | 0.25 | 1 | 1 | —e | — | — | — | — |
| 21 | 2 | 0.12 | 2 | 4 | 8 | Ile (ATC) | — | — | — | — |
| 119 | 2 | 0.5 | 4 | 8 | 4 | Ile (ATC) | — | — | — | — |
| 1311 | 2 | 0.5 | 4 | 8 | 8 | Ile (ATC) | — | — | — | — |
| 44 | 4 | 0.25 | 4 | 8 | 64 | Ile (ATC) | — | — | — | — |
| 47 | 4 | 0.5 | 4 | 8 | 32 | Ile (ATC) | — | — | — | — |
| 128 | 4 | 4 | 0.06 | 16 | 2 | — | — | Phe (TTC) | — | — |
| 1273 | 4 | 0.25 | 4 | 16 | 1 | — | Gly (GGC) | — | — | — |
| 92 | 4 | 0.5 | 8 | 32 | 1 | Ile (ATC) | — | — | — | — |
| 74 | 8 | 0.12 | 4 | 32 | 2 | Ile (ATC) | — | — | — | — |
| 2120 | 8 | 4 | 0.12 | 32 | 1 | — | — | Phe (TTC) | — | — |
| P136 | 8 | 0.12 | 16 | 16 | 16 | Ile (ATC) | — | — | Leu (TTG) | — |
| 2 | 16 | 0.25 | 16 | 32 | 16 | Ile (ATC) | — | — | — | — |
| 57 | 16 | 0.25 | 16 | 32 | 16 | Ile (ATC) | — | — | — | — |
| 78 | 16 | 0.5 | 16 | 32 | 16 | Ile (ATC) | — | — | — | — |
| 134 | 16 | 0.12 | 16 | 32 | 16 | Ile (ATC) | — | — | Leu (TTG) | — |
| D | 32 | 0.25 | 16 | 32 | 32 | Ile (ATC) | — | — | Leu (TTG) | — |
| 6243 | 32 | 0.5 | 32 | 32 | 32 | Ile (ATC) | — | — | — | — |
| C6241 | 32 | 0.25 | 16 | 64 | 16 | Ile (ATC) | — | — | Leu (TTG) | — |
| J15 | 32 | 0.5 | 16 | 32 | 16 | Ile (ATC) | — | — | — | — |
| J388 | 32 | 0.5 | 32 | 32 | 32 | Ile (ATC) | — | — | — | — |
| C5904 | 32 | 0.5 | 16 | 128 | 2 | Ile (ATC) | — | — | Trp (TGG) | — |
| 76 | 32 | 0.25 | 16 | 32 | 16 | Ile (ATC) | — | — | — | — |
| 5299 | 64 | 0.12 | 16 | 64 | 32 | Ile (ATC) | — | — | — | — |
| 8 | 64 | 0.5 | 32 | 64 | 16 | Ile (ATC) | — | — | Leu (TTG) | — |
| 77 | 64 | 0.5 | 64 | 128 | 16 | Ile (ATC) | — | — | Leu (TTG) | — |
| 137 | 64 | 0.25 | 64 | 128 | 8 | Ile (ATC) | — | — | — | Lys (AAG) |
| 1746 | 64 | 0.5 | 32 | 64 | 2 | Ile (ATC) | — | — | Trp (TGG) | — |
| 2863 | 64 | 0.25 | 32 | 128 | 4 | Ile (ATC) | — | — | — | — |
| J431 | 64 | 0.5 | 32 | 32 | 32 | Ile (ATC) | — | — | — | — |
| J1375 | 128 | 0.12 | 128 | 128 | 0.5 | Ile (ATC) | — | — | Leu (TTG) | — |
CPX, ciprofloxacin; SPX, sparfloxacin; IPM, imipenem.
NT, not transformed.
Strain transformed with plasmid pPAW207 carrying the wild-type gyrA gene of E. coli.
Strain transformed with plasmid pPBW801 carrying the wild-type gyrB gene of E. coli.
—, amino acid and codon identical to those of strain PAO1.
gyrA mutations.
The nucleotide sequence of the gyrA fragment obtained from the wild-type ciprofloxacin-sensitive P. aeruginosa strain 65, used as a control, was identical to the sequence of strain PAO1 (15), except for two silent mutations in codons 91 (CGT instead of CGC) and 132 (CAT instead of CAC). Twenty-eight of the 30 ciprofloxacin-resistant P. aeruginosa strains harbored a gyrA mutation. Twenty-seven of them had an ACC-to-ATC mutation in codon 83, leading to the amino acid substitution of an isoleucine for a threonine. The remaining strain had a GAC-to-GGC mutation in codon 87, leading to the substitution of a glycine for an aspartic acid. A polymorphism was found in codon 132, with the nucleotides encoding histidine 132 being CAT for 17 strains, as already mentioned for strain 65, and CAC for the remaining 13 strains, as described for PAO1 (15).
gyrB mutations.
Since the gyrB sequence of P. aeruginosa was not available, we used the gyrB sequence of E. coli (30) to design primers for PCR. Primers were chosen in order to amplify a fragment including the putative QRDR (amino acids 426 to 447 in E. coli) (30). PCR amplification of total DNA from P. aeruginosa with the primers GYRB1 and GYRB2 resulted in several DNA fragments. Only one fragment had the predicted size (390 bp), and it was subsequently purified from the agarose gel and sequenced, leading to the analysis of a 303-bp DNA fragment (Fig. 1). The nucleotide sequence had 86% identity with the corresponding gyrB fragment of Pseudomonas putida and 83% with that of E. coli and Salmonella enterica serovar Typhimurium, which have the same sequence. The deduced amino acid sequence had 98% identity with the corresponding GyrB sequence of P. putida (31) and 92% with that of E. coli (Fig. 2).
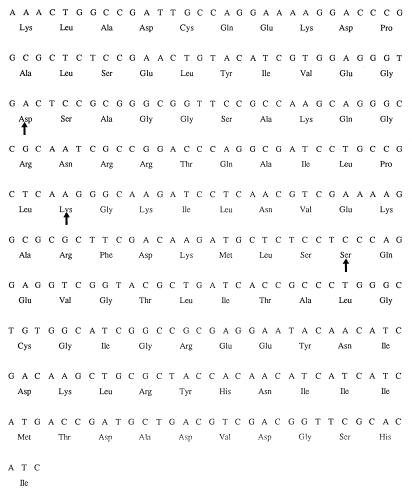
FIG. 1.
Nucleotide sequence of the 303-bp fragment of the gyrB gene of P. aeruginosa PAO1 (corresponding to nucleotides 1216 to 1518 in E. coli gyrB), including the putative QRDR. The arrows indicate codons 426, 447, and 464.
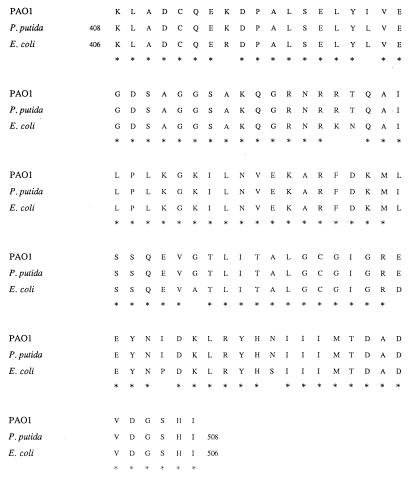
FIG. 2.
Comparison of the amino acid sequences encoded by the 303-bp gyrB fragments of P. aeruginosa PAO1, P. putida, and E. coli. The conserved residues are indicated by asterisks.
The nucleotide sequence of the gyrB fragment from the wild-type P. aeruginosa strain 65 was identical to that of PAO1. Strains 128 and 2120, which have been complemented by a wild-type gyrB gene, did not have mutations in codon 426 or 447, where mutations were described in E. coli (30). However, they had a TCC-to-TTC mutation in codon 464, leading to the amino acid substitution of a phenylalanine for a serine. In all of the other P. aeruginosa strains, we did not find any mutation in the gyrB fragment leading to amino acid changes. Still, compared to the sequence of the gyrB fragment obtained from PAO1, silent mutations were found in codons 406 (AAA to AAG, 7 strains), 407 (CTG to CTT, 1 strain), 436 (CGC to CGT, 1 strain), 454 (GAA to GAG, 6 strains), 456 (GCG to GCA, 4 strains), 472 (ACC to ACT, 12 strains), 479 (GGC to GGT, 6 strains), and 482 (GAA to GAG, 3 strains).
Overall, we found 28 strains with a gyrA mutation and 2 strains with a gyrB mutation. These results were consistent with the results of the complementation tests performed previously (2) except for those for two strains, 128 and J1375, for which there had been a mix of plasmids carrying gyrA or gyrB at the time of complementation. New complementation tests performed with the original clinical strains showed that complementation of strain 128 occurred only with the wild-type gyrB plasmid and that of strain J1375 occurred only with the wild-type gyrA plasmid.
parC mutations.
The nucleotide sequences of the parC fragments from the ciprofloxacin-susceptible strains PAO1 and 65 were identical to that described by Nakano et al. (19). Ten P. aeruginosa strains harbored a mutation in parC. For nine of them, the mutation was located in the codon corresponding to Ser-80 in E. coli parC and led to the amino acid substitution of leucine for serine (seven strains) or of tryptophan for serine (two strains). The remaining strain had a mutation in the codon corresponding to Glu-84 in E. coli, leading to the amino acid substitution of lysine for glutamic acid. All of the strains with parC mutations also had a mutation in the gyrA codon corresponding to Thr-83. In contrast to gyrA and gyrB, no polymorphism was found in the sequences of the parC fragments.
Correlation between antibiotic resistance patterns and topoisomerase mutations.
For all strains, resistance to ciprofloxacin was well correlated with resistance to sparfloxacin (Table 1). Ciprofloxacin and sparfloxacin MICs were significantly higher for strains with a double gyrA-parC mutation than for isolates with a single mutation in gyrA or gyrB (geometric mean MICs of ciprofloxacin, 39.4 versus 10.9 μg/ml, P = 0.008; geometric mean MICs of sparfloxacin, 64.0 versus 22.6 μg/ml, P = 0.003). Ciprofloxacin and sparfloxacin MICs were not statistically different for imipenem-resistant (Imir) and imipenem-susceptible (Imis) strains. However, ciprofloxacin MICs were slightly higher for Imir strains than for Imis ones (geometric mean MICs, 19.0 versus 13.0 μg/ml, P = 0.53), particularly for the strains with a single mutation in gyrA or gyrB (14.4 versus 6.5 μg/ml, P = 0.21), although these differences did not reach statistical significance.
DISCUSSION
Mutations in topoisomerases genes are the main mechanism of fluoroquinolone resistance in many bacteria (28). Previous studies have shown that this mechanism is involved in quinolone resistance of P. aeruginosa in clinical practice (2, 15, 19, 34). Herein, we screened for mutations in gyrA, gyrB, and parC in 30 clinical ciprofloxacin-resistant P. aeruginosa strains which were shown to be fully complemented by a plasmid carrying the wild-type gyrA or gyrB allele in a previous work (2).
DNA sequencing of the gyrA QRDR showed a high frequency of point mutations in codon 83 (27 of 30 strains), whereas only one strain had a point mutation in codon 87. These results are consistent with those reported in previous studies on clinical fluoroquinolone-resistant strains of P. aeruginosa in which 13 of 22 strains (19) and 10 of 18 strains (32) harbored a single gyrA mutation in codon 83 and 1 of 22 strains (19) and 1 of 18 (32) harbored only a single mutation in codon 87. We did not find any strain with a double mutation in codons 83 and 87 such as that described by Nakano et al. (19). Strikingly, in our study as well as in others dealing with P. aeruginosa (15, 19, 32) or Campylobacter jejuni (27), the point mutation at position 83 always led to the substitution of an isoleucine for a threonine, despite several other possible amino acid substitutions encoded by a point mutation in an ACC codon (Pro, Ala, Ser, and Asn). This could not be attributed to a clonal relation between the strains of P. aeruginosa studied, since these strains had different antibiotypes and serotypes (4) and were isolated over a long period of time (3 years). In E. coli, mutagenesis studies have shown that the mutation Ser-83 to Ala was responsible for a low level of ciprofloxacin resistance compared to the Ser-to-Leu or Ser-to-Trp mutations described in strains selected in vivo (8, 33). Thus, we assume that the putative mutation Thr-83 to Ala in gyrA would lead to a poor selective advantage in P. aeruginosa in vivo, in contrast to the mutation Thr-83 to Ile. This point could be further investigated in a directed mutagenesis study.
We sequenced a 303-bp gyrB fragment from P. aeruginosa which included the QRDR. This fragment showed a high homology with the corresponding gyrB fragment of Pseudomonas putida (i.e., same genus) but also with that of other members of the family Enterobacteriaceae such as E. coli or S. enterica serovar Typhimurium. Since some regions of the ParE subunit of topoisomerase IV are highly homologous to the GyrB subunit of DNA gyrase in E. coli (13), we compared our sequence to the ParE amino acid sequence of E. coli. Amino acid identity was only 52% with ParE, in contrast to 92% identity with GyrB, suggesting that our DNA fragment corresponded to gyrB and not to parE.
We described the first mutation in the gyrB gene of P. aeruginosa, Ser-464 to Phe, located outside the gyrB QRDR described in E. coli (30) and in Staphylococcus aureus (11). Similar mutations have been described in quinolone-resistant strains of S. enterica serovar Typhimurium: TCC(Ser)-464 to TAC(Tyr) (6, 7) and TCC(Ser)-464 to TTC(Phe) (GenBank accession no. Y08383) (14a). Considering the absence of mutations in codons 426 and 447 and the full complementation of these two strains by a wild-type gyrB allele, we assume that the mutation found in codon 464 is responsible for quinolone resistance in P. aeruginosa. The hydrophobicity and the bulkiness of the R group of phenylalanine in comparison to serine could modify the conformation of the B-subunit domain involved in quinolone interaction. Since there are now four strains belonging to two different genera (S. enterica serovar Typhimurium and P. aeruginosa) resistant to quinolones and harboring a mutation in codon 464 of the gyrB gene, we propose to extend the QRDR in the B subunit of DNA gyrase to the domain of codons 426 to 464.
Analysis of the partial sequences of gyrA and gyrB showed a few silent base substitutions, resulting in a polymorphism of the sequences of the gyrase genes among P. aeruginosa strains. Kureishi et al. mentioned the existence of silent nucleotide changes in P. aeruginosa gyrA but did not provide a detailed description (15). Wobble base changes were reported in C. jejuni (27) and P. putida (31). The polymorphism that we found in P. aeruginosa gyrB is similar, for some codons, to that described in P. putida (codons 406, AAG or AAA; codons 436, CGC or CGT; codons 456, GCA or GCG; codons 472, ACT or ACC). Yamamoto et al. proposed analysis of the gyrB polymorphism as a tool for phylogenetic studies in P. putida, since the base substitution frequency in gyrB in this species turned out to be higher than that in 16S ribosomal DNA (31).
DNA sequencing of the parC QRDR showed the higher frequency of point mutations at codon 80 (nine strains), which is homologous to codon 83 in gyrA, than in codon 84 (one strain), which is homologous to codon 87 in gyrA. Nakano et al. recently reported mutations in codon 80 (Ser to Leu) of P. aeruginosa parC for eight clinical strains and in codon 84 (Glu to Lys) for only two strains (19). In the present study, we found a novel substitution of tryptophan for serine encoded by codon 80 for two strains. The mutations Ser to Leu and Ser to Trp which are frequent at position 83 in E. coli GyrA are known to be involved in quinolone resistance (33). Since the A subunit of gyrase and the ParC subunit of topoisomerase IV are highly homologous (13), our results emphasize the key role of the serine at position 83 (or at position corresponding to) in both DNA gyrase and topoisomerase IV for determining quinolone resistance.
Only 10 P. aeruginosa strains had a mutation in parC, whereas all of the strains had a mutation in gyrA or gyrB. Overall, the strains with a double gyrA-parC mutation were three to four times more resistant to ciprofloxacin and sparfloxacin than the strains with a single mutation in gyrA or in gyrB. We thus hypothesize that in P. aeruginosa, mutations in parC occur at a second step in strains already harboring mutations in gyrA or gyrB and lead to a fourfold increase in their fluoroquinolone resistance level. All of the strains with a double gyrA-parC mutation were fully complemented by the wild-type gyrA gene, as already reported for E. coli (9). These findings support the hypothesis that in P. aeruginosa, as in E. coli, DNA gyrase is the primary target for quinolones (9) and that topoisomerase IV is a secondary target.
There was a large overlap in the distribution of fluoroquinolone MICs within both strains with a double gyrA-parC mutation and those with a single mutation in gyrA or gyrB (Table 2). An additional mechanism(s) of resistance could be implicated in some strains, particularly in those with a single mutation and for which the ciprofloxacin MIC was above 8 μg/ml. Mutations in parE can be one of these mechanisms, since E. coli strains with double mutations in gyrA and parE are fully complemented by a plasmid carrying the wild-type gyrA allele (1, 22). Unfortunately, we have not yet succeeded in amplifying the parE gene of P. aeruginosa.
TABLE 2.
Distribution of P. aeruginosa strains by susceptibility to ciprofloxacin and imipenem and number of mutations in topoisomerase genes
| MIC of ciprofloxacin (μg/ml) | No. of strains with:
|
|||
|---|---|---|---|---|
| One mutationa
|
Two mutationsb
|
|||
| Imisc | Imird | Imis | Imir | |
| 2 | 1 | 2 | 0 | 0 |
| 4 | 3 | 2 | 0 | 0 |
| 8 | 2 | 0 | 0 | 1 |
| 16 | 0 | 3 | 0 | 1 |
| 32 | 0 | 4 | 1 | 2 |
| 64 | 1 | 2 | 1 | 3 |
| 128 | 0 | 0 | 1 | 0 |
Strains with a single mutation in gyrA or gyrB.
Strains with one mutation in gyrA and one mutation in parC.
Imis, susceptible to imipenem at MIC range of 0.5 to 4 μg/ml.
Imir, resistant to imipenem at MIC range of 8 to 64 μg/ml.
Twenty of the 30 P. aeruginosa strains studied were coresistant to imipenem and fluoroquinolones (ciprofloxacin and sparfloxacin). We have previously demonstrated that in those strains, imipenem resistance was related to the lack of OprD (2), and we demonstrated in the present work that quinolone resistance was related to mutations in DNA topoisomerase genes. For strains having a single mutation in gyrA or gyrB, not associated with a mutation in parC, the level of resistance to ciprofloxacin, but not that to sparfloxacin, was slightly but not significantly higher in Imir than in Imis strains. Multidrug efflux systems were described in clinical fluoroquinolone-resistant isolates (5, 12, 14), and some of them, such as MexEF-OprN corresponding to the phenotype NfxC, lead to coresistance to quinolones and imipenem (5, 17). The hypothesis that some of the Imir strains used in this study overexpress this efflux system is unlikely, since gyrA-nfxC double mutants are known to be partially complemented with a plasmid carrying a wild-type gyrA gene (5) in contrast to our strains which were all fully complemented as previously shown. For the strains with a double gyrA-parC mutation, the fluoroquinolone MICs were not higher in Imir than in Imis strains. Thus, despite the limited number of strains studied, we assume that the imipenem resistance mechanism has no effect at all on ciprofloxacin resistance when two mutations in topoisomerase genes are present. Taken together, the results of the present work support the hypothesis that the main mechanisms of quinolone resistance in clinical strains of P. aeruginosa are mutations in DNA gyrase or topoisomerase IV genes and that the mechanisms conferring quinolone resistance and those conferring imipenem resistance are independent of each other.
ACKNOWLEDGMENT
This work was supported by a grant from the Institut National de la Santé et de la Recherche Médicale (CRI no. 950601).
REFERENCES
- 1.Breines D M, Ouabdessalam S, Ng E Y, Tankovic J, Shah S, Soussy C J, Hooper D C. Quinolone resistance locus nfxD of Escherichia coli is a mutant allele of the parE gene encoding a subunit of topoisomerase IV. Antimicrob Agents Chemother. 1997;41:175–179. doi: 10.1128/aac.41.1.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cambau E, Perani E, Dib C, Petinon C, Trias J, Jarlier V. Role of mutations in DNA gyrase genes in ciprofloxacin resistance of Pseudomonas aeruginosa susceptible or resistant to imipenem. Antimicrob Agents Chemother. 1995;39:2248–2252. doi: 10.1128/aac.39.10.2248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Deguchi T, Yasuda M, Kawamura T, Nakano M, Ozeki S, Kanematsu E, Nishino Y, Kawada Y. Improved antimicrobial activity of DU-6859a, a new fluoroquinolone, against quinolone-resistant Klebsiella pneumoniae and Enterobacter cloacae isolates with alterations in GyrA and ParC proteins. Antimicrob Agents Chemother. 1997;41:2544–2546. doi: 10.1128/aac.41.11.2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dib C, Trias J, Jarlier V. Lack of additive effect between mechanisms of resistance to carbapenems and other beta-lactam agents in Pseudomonas aeruginosa. Eur J Clin Microbiol Infect Dis. 1995;14:979–986. doi: 10.1007/BF01691380. [DOI] [PubMed] [Google Scholar]
- 5.Fukuda H, Hosaka M, Iyobe S, Gotoh N, Nishino T, Hirai K. nfxC-type quinolone resistance in a clinical isolate of Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1995;39:790–792. doi: 10.1128/AAC.39.3.790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gensberg K, Jin Y F, Piddock L J V. A novel gyrB mutation in a fluoroquinolone-resistant clinical isolate of Salmonella typhimurium. FEMS Microbiol Lett. 1995;132:57–60. doi: 10.1111/j.1574-6968.1995.tb07810.x. [DOI] [PubMed] [Google Scholar]
- 7.Gensberg K, Jin Y F, Piddock L J V. Corrigendum to “A novel gyrB mutation in a fluoroquinolone-resistant clinical isolate of Salmonella Typhimurium.”. FEMS Microbiol Lett. 1996;137:293. doi: 10.1111/j.1574-6968.1995.tb07810.x. [DOI] [PubMed] [Google Scholar]
- 8.Hallett P, Maxwell A. Novel quinolone resistance mutations of the Escherichia coli DNA gyrase A protein: enzymatic analysis of the mutant proteins. Antimicrob Agents Chemother. 1991;35:335–340. doi: 10.1128/aac.35.2.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Heisig P. Genetic evidence for a role of parC mutations in development of high-level fluoroquinolone resistance in Escherichia coli. Antimicrob Agents Chemother. 1996;40:879–885. doi: 10.1128/aac.40.4.879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huang H, Hancock R E W. Genetic definition of the substrate selectivity of outer membrane porin protein OprD of Pseudomonas aeruginosa. J Bacteriol. 1993;175:7793–7800. doi: 10.1128/jb.175.24.7793-7800.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ito H, Yoshida H, Bogaki-Shonai M, Niga T, Hattori H, Nakamura S. Quinolone resistance mutations in the DNA gyrase gyrA and gyrB genes of Staphylococcus aureus. Antimicrob Agents Chemother. 1994;38:2014–2023. doi: 10.1128/aac.38.9.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jakics E B, Iyobe S, Hirai K, Fukuda H, Hashimoto H. Occurrence of the nfxB type mutation in clinical isolates of Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1992;36:2562–2565. doi: 10.1128/aac.36.11.2562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kato J I, Nishimura Y, Imamura R, Niki H, Hiraga S, Suzuki H. New topoisomerase essential for chromosome segregation in E. coli. Cell. 1990;63:393–404. doi: 10.1016/0092-8674(90)90172-b. [DOI] [PubMed] [Google Scholar]
- 14.Köhler T, Michéa-Hamzehpour M, Plésiat P, Kahr A L, Pechère J C. Differential selection of multidrug efflux systems by quinolones in Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1997;41:2540–2543. doi: 10.1128/aac.41.11.2540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14a.Kratz, B. Unpublished results.
- 15.Kureishi A, Diver J M, Beckthold B, Schollaardt T, Bryan L E. Cloning and nucleotide sequence of Pseudomonas aeruginosa DNA gyrase gyrA gene from strain PAO1 and quinolone-resistant clinical isolates. Antimicrob Agents Chemother. 1994;38:1944–1952. doi: 10.1128/aac.38.9.1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mabilat C, Goussard S, Sougakoff W, Spencer R C, Courvalin P. Direct sequencing of the amplified structural gene and promoter for the extended-broad-spectrum β-lactamase TEM-9 (RHH-1) of Klebsiella pneumoniae. Plasmid. 1990;23:27–34. doi: 10.1016/0147-619x(90)90041-a. [DOI] [PubMed] [Google Scholar]
- 17.Masuda N, Sakagawa E, Ohya S. Outer membrane proteins responsible for multiple drug resistance in Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1995;39:645–649. doi: 10.1128/AAC.39.3.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Michéa-Hamzehpour M, Furet Y X, Pechère J C. Role of protein D2 and lipopolysaccharide in diffusion of quinolones through the outer membrane of Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1991;35:2091–2097. doi: 10.1128/aac.35.10.2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nakano M, Deguchi T, Kawamura T, Yasuda M, Kimura M, Okano Y, Kawada Y. Mutations in the gyrA and parC genes in fluoroquinolone-resistant clinical isolates of Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1997;41:2289–2291. doi: 10.1128/aac.41.10.2289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ogle J W, Reller L B, Vasil M L. Development of resistance in Pseudomonas aeruginosa to imipenem, norfloxacin, and ciprofloxacin during therapy: proof provided by typing with a DNA probe. J Infect Dis. 1988;157:743–748. doi: 10.1093/infdis/157.4.743. [DOI] [PubMed] [Google Scholar]
- 21.Rådberg G, Nilsson L E, Svensson S. Development of quinolone-imipenem cross resistance in Pseudomonas aeruginosa during exposure to ciprofloxacin. Antimicrob Agents Chemother. 1990;34:2142–2147. doi: 10.1128/aac.34.11.2142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Soussy C J, Wolfson J S, Ng E Y, Hooper D C. Limitations of plasmid complementation test for determination of quinolone resistance due to changes in the gyrase A protein and identification of conditional quinolone resistance locus. Antimicrob Agents Chemother. 1993;37:2588–2592. doi: 10.1128/aac.37.12.2588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tabor S, Richardson C C. DNA sequence analysis with a modified bacteriophage T7 DNA polymerase. Proc Natl Acad Sci USA. 1987;84:4767–4771. doi: 10.1073/pnas.84.14.4767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Trias J, Nikaïdo H. Outer membrane protein D2 catalyzes facilitated diffusion of carbapenems and penems through the outer membrane of Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1990;34:52–57. doi: 10.1128/aac.34.1.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Troillet N, Samore M H, Carmeli Y. Imipenem-resistant Pseudomonas aeruginosa: risk factors and antibiotic susceptibility patterns. Clin Infect Dis. 1997;25:1094–1098. doi: 10.1086/516092. [DOI] [PubMed] [Google Scholar]
- 26.Vila J, Ruiz J, Goñi P, Jimenez de Anta T. Quinolone-resistance mutations in the topoisomerase IV parC gene of Acinetobacter baumanii. J Antimicrob Chemother. 1997;39:757–762. doi: 10.1093/jac/39.6.757. [DOI] [PubMed] [Google Scholar]
- 27.Wang Y, Huang W N, Taylor D E. Cloning and nucleotide sequence of the Campylobacter jejuni gyrA gene and characterization of quinolone resistance mutations. Antimicrob Agents Chemother. 1993;37:457–463. doi: 10.1128/aac.37.3.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wolfson J S, Hooper D C. Fluoroquinolone antimicrobial agents. Clin Microbiol Rev. 1989;2:378–424. doi: 10.1128/cmr.2.4.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Woods S A, Cole S T. A rapid method for the detection of potentially viable Mycobacterium leprae in human biopsies: a novel application of PCR. FEMS Microbiol Lett. 1989;65:305–310. doi: 10.1016/0378-1097(89)90235-8. [DOI] [PubMed] [Google Scholar]
- 30.Yamagishi J I, Yoshida H, Yamayoshi M, Nakamura S. Nalidixic acid-resistant mutations of the gyrB gene of Escherichia coli. Mol Gen Genet. 1986;204:367–373. doi: 10.1007/BF00331012. [DOI] [PubMed] [Google Scholar]
- 31.Yamamoto, S., and S. Harayama. PCR amplification and direct sequencing of gyrB genes with universal primers and their application to the detection and taxonomic analysis of Pseudomonas putida strains. Appl. Environ. Microbiol. 61:1104–1109. [DOI] [PMC free article] [PubMed]
- 32.Yonezawa M, Takahata M, Matsubara N, Watanabe Y, Narita H. DNA gyrase gyrA mutations in quinolone-resistant clinical isolates of Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1995;39:1970–1972. doi: 10.1128/aac.39.9.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yoshida H, Bogaki M, Nakamura M, Nakamura S. Quinolone resistance-determining region in the DNA gyrase gyrA gene of Escherichia coli. Antimicrob Agents Chemother. 1990;34:1271–1272. doi: 10.1128/aac.34.6.1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yoshida H, Nakamura M, Bogaki M, Nakamura S. Proportion of DNA gyrase mutants among quinolone-resistant strains of Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1990;34:1273–1275. doi: 10.1128/aac.34.6.1273. [DOI] [PMC free article] [PubMed] [Google Scholar]