Abstract
Background:
Lung transplantation (LT) is the gold standard for various end-stage chronic lung diseases and could be a salvage therapeutic option in acute respiratory distress syndrome (ARDS). However, LT is uncertain in patients with coronavirus disease 2019 (COVID-19)-related ARDS who failed to recover despite optimal management including extracorporeal membrane oxygenation (ECMO). This study aims to describe the pooled experience of LT for patients with severe COVID-19-related ARDS in Korea.
Methods:
A nationwide multicenter retrospective observational study was performed with consecutive LT for severe COVID-19-related ARDS in South Korea (June 2020–June 2021). Data were collected and compared with other LTs after bridging with ECMO from the Korean Organ Transplantation Registry.
Results:
Eleven patients with COVID-19-related ARDS underwent LT. The median age was 60.0 years [interquartile range (IQR), 57.5–62.5; six males]. All patients were supported with venovenous ECMO at LT listing and received rehabilitation before LT. Patients were transplanted at a median of 49 (IQR, 32–66) days after ECMO cannulation. Primary graft dysfunction within 72 h of LT developed in two (18.2%). One patient expired 4 days after LT due to sepsis and one patient underwent retransplantation for graft failure. After a median follow-up of 322 (IQR, 299–397) days, 10 patients are alive and recovering well. Compared with other LTs after bridging with ECMO (n = 27), post-transplant outcomes were similar between the two groups.
Conclusions:
LT in patients with unresolving COVID-19-related ARDS were effective with reasonable short-term outcome.
Keywords: COVID-19, extracorporeal membrane oxygenations, frailty, lung transplantation, treatment outcome
Background
The coronavirus disease 2019 (COVID-19) caused by severe acute respiratory syndrome coronavirus 2 has spread worldwide. COVID-19 mainly affects the respiratory system with some patients rapidly progressing to acute respiratory distress syndrome (ARDS) where up to 33% may require mechanical ventilation (MV).1–3 Among the patients with COVID-19-related ARDS who require MV, some patients progress to the fibrotic phase of ARDS, which is associated with prolonged ventilator support and increased mortality.4,5 Based on the experience obtained during previous respiratory virus outbreaks,6–9 therefore, extracorporeal membrane oxygenation (ECMO) could be used to bridge such patients to recovery or lung transplantation (LT).10–13
Although LT has been suggested as a salvage therapy for carefully selected patients with ARDS,12–14 there is limited experience on this potentially life-saving procedure for COVID-19-related ARDS. In addition, several concerns including recovery of lung injury, concomitant infections, and uncertainty of long-term outcomes limit the use of LT as a salvage therapy for patients with severe COVID-19-related ARDS. Nonetheless, sporadic cases of LT for patients with COVID-19-related ARDS have been reported. 15 In addition, a case series of patients with COVID-19-related ARDS who were bridged to LT by ECMO at high-volume centers in four Western countries shows that LT could be done successfully with good early post-transplantation outcomes. 16 However, no case series from Asia has been reported.
This study aims to describe the pooled experience of LT for patients with severe COVID-19-related ARDS in Korea. In addition, mortality and short-term outcomes were compared with other LTs after bridging with ECMO from the Korean Organ Transplantation Registry data. 17
Methods
Study design and population
A nationwide multicenter retrospective cohort study was conducted by the Korean LT Study Group. Five of the eight LT centers in Korea performed LT in patients with severe COVID-19-related ARDS and participated in this study. The institutional review boards at each participating hospital approved this study and waived the requirements for informed consent owing to the noninterventional observational nature of the study.
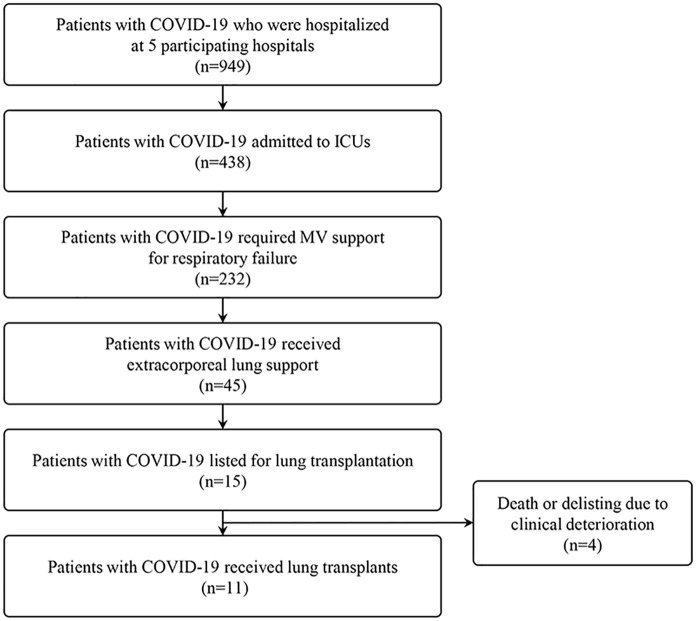
Eleven consecutive LTs for patients with severe COVID-19-related ARDS between June 2020 and June 2021 were enrolled in the study (Figure 1). The patients were followed up until the date of hospital discharge or the date of the latest visit to the outpatient clinics.
Figure 1.
Study flow.
COVID-19 care and consideration for LT
The patients received treatment for severe COVID-19-related ARDS following the institutional standard of care for each institution. Moreover, the multidisciplinary COVID-19 care team at the respective institutions, which includes infectious disease physicians, pulmonary and critical care physicians, and cardiologists, conducted medical care for patients with severe COVID-19-related ARDS. LT was discussed with the multidisciplinary LT team at least 4 weeks after onset of ARDS for patients with no evidence of lung recovery. Some patients precluded LT evaluation after discussion with the multidisciplinary LT team. Common reasons for preclusion were multiorgan failure, secondary complications (e.g. sepsis or stroke), and general contraindications relevant to LT. Frailty alone was not considered to be exclusive because these patients had been healthy before the onset of COVID-19-related ARDS.
Data collection
Information about LTs, donors, transplantation operations, and postoperative results was retrospectively collected. Data for LTs including pre-COVID-19 demographic information, pre-transplantation status, and donor characteristics were collected. Chest computed tomography (CT) features were classified based on the previous report on the CT finding of COVID-19 pneumonia. 18 Frailty before COVID-19 infection was assessed by the clinical frailty scale. 19 Donor lung score was calculated by Oto et al.’s 20 donor score. Before transplantation, all patients underwent panel reactive antibody (PRA) screening tests for HLA class I and class II antibodies. Calculated panel reactive antibody (cPRA) was measured using the cPRA calculator for anti-HLA antibodies greater than 1000 mean fluorescence intensity (MFI). Transplant surgery data include unilateral or bilateral LT, operation time, ischemic time, and transfusion requirement. Post-transplantation outcome data including primary graft dysfunction (PGD), complication, and mortality were also collected.
The Korean Organ Transplantation Registry data, described in a previous study, was used to compare LTs for severe COVID-19-related ARDS with other LTs after bridging with ECMO. 17
Statistical analysis
Data were summarized using descriptive statistics as median and interquartile ranges (IQR) for continuous variables and as numbers and percentages for categorical variables. To assess the difference between LTs for severe COVID-19-related ARDS and other causes after bridging with ECMO, data were compared using the Mann–Whitney U test and Fisher’s exact test for continuous and categorical variables, respectively. Data were analyzed using R Statistical Software (Version 3.2.5; R Foundation for Statistical Computing, Vienna, Austria).
Results
Patient characteristics
Fifteen patients with severe COVID-19-related ARDS were on the waiting list for LT. However, three patients expired awaiting LT, and one patient was delisted for multiorgan failure (Figure 1). The baseline characteristics of the 11 patients who had LT for severe COVID-19-related ARDS and their clinical characteristics are shown in Tables 1 and 2. Six (54.5%) patients were male, and the median age of all patients was 60.0 (IQR, 57.5–62.5) years. The median clinical frailty scale was 1.0 (IQR, 1.0–2.0). Three (27.3%) patients had comorbidities (e.g. chronic lung disease, diabetes mellitus, or high-dose or long-term steroid use). The patients had a median of 8.0 (IQR, 4.0–10.0) days from diagnosis to intensive care unit (ICU) admission and 8.0 (IQR, 4.5–11.0) days from diagnosis to MV support. COVID-19-specific medical treatments included corticosteroid (n = 9; 81.8%), remdesivir (n = 4; 36.4%), hydroxychloroquine (n = 1; 9.1%), lopinavir/ritonavir (n = 1; 9.1%), and ivermectin (n = 1; 9.1%).
Table 1.
Clinical characteristics of the study cohort.
| Characteristics | Patients with COVID-19-associated ARDS (N = 11) |
|---|---|
| Gender, male | 6 (54.5) |
| Age, years | 60.0 (57.5–62.5) |
| Body mass index, kg/m2 | 23.7 (20.4–25.3) |
| Blood group | |
| A | 2 (18.2) |
| B | 3 (27.3) |
| O | 1 (9.1) |
| AB | 5 (45.5) |
| Clinical frailty scale | 1.0 (1.0–2.0) |
| Comorbidity | |
| Chronic lung disease | 1 (9.1) |
| Diabetes mellitus | 1 (9.1) |
| High- or long-term corticosteroid use | 1 (9.1) |
| Time from COVID-19 diagnosis to ICU admission, days | 8.0 (4.0–10.0) |
| Time from COVID-19 diagnosis to intubation, days | 8.0 (4.5–11.0) |
| Antiviral medication for COVID-19 | |
| Remdesivir | 4 (36.4) |
| Steroid | 9 (81.8) |
| Others a | 3 (27.3) |
| Time from COVID-19 diagnosis to listing, days | 54.0 (40.0–63.0) |
| At the time of listing | |
| Venovenous ECMO | 11 (100) |
| Time from intubation to ECMO, days | 6.0 (1.0–32.0) |
| Awake ECMO bridging | 5 (45.5) |
| Mechanical ventilation support b | 11 (100.0) |
| Continuous renal replacement therapy | 3 (27.3) |
| Normal left ventricular ejection fraction | 11 (100.0) |
| Evidence of pulmonary bacterial superinfection | 7 (63.6) |
| Evidence of fungal colonization | 1 (9.1) |
| Chest CT findings at the time of listing | |
| Distribution | |
| Peripheral | 1 (9.1) |
| Both central and peripheral | 10 (90.9) |
| Opacity | |
| Ground-glass | 1 (9.1) |
| Ground-glass and consolidation | 9 (81.8) |
| Consolidation | 1 (9.1) |
| Crazy-paving pattern | 8 (72.7) |
| Interlobar septal thickening | 9 (81.8) |
| Fibrous stripe | 8 (72.7) |
| Air bronchogram | 9 (81.8) |
| Number of patients recovered from acute kidney injury | 4 (36.4) |
| Number of patients recovered from sepsis | 9 (81.8) |
| Highest ICU rehabilitation stage on the waiting list | |
| Passive range of motion | 3 (27.3) |
| Active range of motion | 2 (18.2) |
| Sitting on edge of bed | 3 (27.3) |
| Sit to stand | 3 (27.3) |
COVID-19, coronavirus disease 2019; ARDS, acute respiratory distress syndrome; CT, computed tomography; ICU, intensive care unit; ECMO, extracorporeal membrane oxygenation.
Values are presented as median (interquartile range) or number (%).
Others included one hydroxychloroquine, one ivermectin, and one lopinavir/ritonavir.
Ten were tracheostomized patients.
Table 2.
Characteristics of individual transplant recipients.
| Age, years | Center | Comorbidities | Time from MV to LT, days | Tracheostomy | Time on ECMO at time of LT, days | Awake during bridging | Rehabilitation before LT, days | Class I cPRA, % | Class II cPRA, % | Time from LT to ventilator weaning, days | Time in ICU after LT, days | Time in Hospital after LT, days | Follow-up after LT, days | Alive or expired | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Patient 1 | 63 | A | No | 56 | Yes | 56 | Yes | 28 | Negative | Negative | 18 | 32 | 51 | 417 | Alive |
| Patient 2 | 62 | A | No | 75 | Yes | 73 | Yes | 43 | 4% | Negative | 9 | 13 | 165 | 294 | Alive |
| Patient 3 | 60 | A | No | 83 | Yes | 34 | Yes | 12 | Negative | Negative | 2 | 13 | Still admitted | 299 | Alive |
| Patient 4 | 56 | B | No | 84 | Yes | 49 | No | 16 | 91% | 96% | 9 | 15 | 88 | 476 | Alive |
| Patient 5 | 65 | B | Yes | 50 | Yes | 49 | No | 9 | 61% | Negative | 28 | 38 | 146 | 336 | Alive |
| Patient 6 | 62 | B | No | 53 | No | 24 | No | 19 | Negative | Negative | 12 | 19 | 90 | 300 | Alive |
| Patient 7 | 51 | C | No | 112 | Yes | 111 | Yes | 54 | Negative | Negative | 102 | 239 | 239 | 559 | Alive |
| Patient 8 | 63 | C | No | 133 | Yes | 133 | Yes | 94 | 98% | 17% | - | 4 | 4 | 4 | Expired |
| Patient 9 | 59 | D | Yes | 36 | Yes | 30 | No | 32 | Negative | Negative | 5 | 7 | 97 | 224 | Alive |
| Patient 10 | 59 | D | No | 67 | Yes | 59 | No | 43 | Negative | Negative | 7 | 10 | 124 | 338 | Alive |
| Patient 11 | 55 | E | Yes | 64 | Yes | 6 | No | 24 | Negative | Negative | 7 | 13 | 35 | 308 | Alive |
cPRA, calculated panel reactive antibody; ECMO, extracorporeal membrane oxygenation; ICU, intensive care unit; LT, lung transplantation; MV, mechanical ventilation.
The patients had a median of 54.0 (IQR, 40.0–63.0) days from COVID-19 diagnosis to listing. At lung transplant listing, 10 (90.9%) and eight (72.7%) patients had a tracheostomy and became negative for COVID-19, respectively. However, the three COVID-19 positive patients were also tested negative for COVID-19 while waiting for LT, and eventually all patients were tested negative for COVID-19 before LT. All patients, including vein–pulmonary artery cannulation in one (9.1%), were supported with venovenous ECMO. The median time from MV to ECMO was 6.0 (IQR, 1.0–32.0) days. Of the 11 patients, five (45.5%) were awake while on ECMO. Three (27.3%) were on continuous renal replacement therapy and four (36.4%) recovered from acute kidney injury. Pulmonary (n = 7) and catheter-related (n = 4) superinfections occurred before LT. Nine (81.8%) patients were suffered from sepsis. Nosocomial pathogens were observed in pulmonary and catheter-related infections as follows: methicillin-resistant Staphylococcus aureus, extended-spectrum β-lactamase-producing Klebsiella pneumoniae, Corynebacterium striatum, Burkholderia contaminans, Citrobacter freundii, carbapenem-resistant Acinetobacter baumannii, carbapenem-resistant Enterobacteriaceae, vancomycin-resistant Enterococci, and candida species.
All patients received ICU rehabilitation during the waiting list for LT. The duration of ICU rehabilitation was a median of 28.0 (IQR, 17.5–43.0) days before LT (Table 1).
Intraoperative and donor characteristics
The intraoperative characteristics of the LTs are shown in Table 3. All patients underwent bilateral LT through the clamshell incision. The median time on the waiting list was 26.0 (IQR, 9.0–38.0) days. The median operation time of all patients was 510.0 (IQR, 446.5–541.0) min. Total ischemic time for the right and the left lung was 199.0 (IQR, 142.0–291.5) and 319.0 (IQR, 222.0–350.5) min, respectively. Blood transfusion was required in all patients with a median of 10.0 units of packed red blood cells (IQR, 3.5–12.5) and 2.0 units of fresh frozen plasma (IQR, 1.0–6.5).
Table 3.
Characteristics of lung transplantation.
| Characteristics | Patients with COVID-19-associated ARDS (N = 11) |
|---|---|
| Time on the waiting list, days | 26.0 (9.0–38.0) |
| Intraoperative support | |
| Venoarterial ECMO | 7 (63.6) |
| Venovenous ECMO | 3 (27.3) |
| Cardiopulmonary bypass | 1 (9.1) |
| Operation time, min | 510.0 (446.5–541.0) |
| Total ischemic time, right, min | 199.0 (142.0–291.5) |
| Total ischemic time, left, min | 319.0 (222.0–350.5) |
| Number of intraoperative packed red blood cell | 10.0 (3.5–12.5) |
| Number of intraoperative fresh frozen plasma | 2.0 (1.0–6.5) |
| Donor characteristics | |
| Gender, male | 8 (72.7) |
| Age, years | 51.0 (40.5–58.0) |
| Body mass index, kg/m2 | 23.3 (21.2–25.1) |
| Predicted TLC ratio | 1.0 (1.0–1.1) |
| Smoker | 2 (18.2) |
| Lung donor score | 5.0 (3.0–6.5) |
| PaO2 at FiO2 100% | 504 (379–540) |
| Donor cause of brain death | |
| Brain hemorrhage | 4 (36.4) |
| Suicide | 3 (27.3) |
| Hypoxic brain damage | 4 (36.4) |
COVID-19, coronavirus disease 2019; ARDS, acute respiratory distress syndrome; ECMO, extracorporeal membrane oxygenation; TLC, total lung capacity; PaO2, partial pressure of oxygen in arterial blood; FiO2, fraction of inspired oxygen.
Values are presented as median (interquartile range) or number (in percentage).
The median age of donors was 51.0 (IQR, 40.5–58.0) years and 72.7% were male. The median predicted total lung capacity ratio was 1.0 (IQR, 1.0–1.1). Lung donor score was a median of 5.0 (IQR, 3.0–6.5). All organs were from deceased donors and the most common causes of brain death were brain hemorrhage (36.4%) and hypoxic brain damage (36.4%).
Post-transplantation outcomes
Post-LT course and outcomes are presented in Table 4. PGD developed in two patients (18.2%) within 72 h after LT, and one patient received retransplantation for severe graft failure at 6 days. Ten (90.9%) patients had complications, and the most common complication was infection (n = 7, 63.6%) followed by critical illness polyneuromyopathy (n = 5; 45.5%). All except one patient received rehabilitation after transplantation. Three patients who received renal replacement therapy before LT recovered from renal failure after LT. One patient in the current series expired due to K. pneumoniae bacteremia 4 days after LT, which was not isolated from the donor lung. The median days from LT to ventilator weaning was 9.0 (7.0–16.5) days. From transplantation to ICU discharge, the median time was 13.0 (IQR, 11.5–25.5) days. Of the nine patients discharged from the hospital following LT, seven (77.8%) required re-hospitalization for the management of new infections, wound problems, or airway procedures. After a median follow-up of 322 (IQR, 299–397) days, 10 patients are alive and recovering well.
Table 4.
Post-lung transplantation course and outcomes.
| Patients with COVID-19-associated ARDS (N = 11) | |
|---|---|
| Induction therapy | 10 (90.9) |
| Postoperative prolonged ECMO | 4 (36.4) |
| PGD at 72 h | |
| PGD 0 | 9 (81.8) |
| PGD 1 | 0 (0.0) |
| PGD 2 | 1 (9.1) |
| PGD 3 | 1 (9.1) |
| Complications | |
| Acute kidney injury required renal replacement therapy | 3 (27.3) |
| Bleeding requiring chest reopening | 2 (18.2) |
| Bleeding managed by medical management | 2 (18.2) |
| Infection | 7 (63.6) |
| Airway complication | 3 (27.3) |
| Critical illness neuropathy | 5 (45.5) |
| Complicated pleural effusion | 3 (27.3) |
| Rehabilitation after transplantation | 10 (90.9) |
| Highest rehabilitation stage | |
| Passive range of motion | 1 (10.0) |
| Active range of motion | 1 (10.0) |
| Sitting on edge of bed | 0 (0.0) |
| Sit to stand | 3 (30.0) |
| Walking in place | 5 (50.0) |
| Length of stay in ICU, days | 88.0 (75.0–98.5) |
| Length of hospital stay, days | 156.0 (137.0–191.3) |
| Time from transplantation to ICU discharge, days | 13.0 (11.5–25.5) |
| Number of patients still in hospital a | 1 (10.0) |
| Overall survival | |
| Alive | 10 (90.9) |
| Expired | 1 (9.1) |
| Follow-up after transplantation, days | 322.0 (299.3–397.3) |
COVID-19, coronavirus disease 2019; ARDS, acute respiratory distress syndrome; ECMO, extracorporeal membrane oxygenation; PGD, primary graft dysfunction; ICU, intensive care unit.
Values are presented as median (interquartile range) or number (in percentage).
Patients in hospital as of December 31, 2021.
The study patients were compared with 27 patients who received LT for other causes after bridging with ECMO (Table 5). The duration from MV to LT (67 versus 18 days; p < 0.001) and from ECMO to LT (49 versus 11 days; p < 0.001) was prolonged in patients with COVID-19-related ARDS. Post-transplant outcomes including the prevalence of PGD, post-transplant acute kidney injury, post-transplant bleeding, and airway complication were similar between the two groups. However, infection was more frequent in patients with COVID-19-related ARDS (63.6% versus 14.8; p = 0.005). Finally, hospital mortality was not significantly different between the two groups (9.1% versus 25.9%; p = 0.395).
Table 5.
Comparison of clinical characteristics and outcomes between COVID-19 patients and other causes patients who received extracorporeal membrane oxygenation as a bridge to lung transplantation.
| Patients with COVID-19-associated ARDS (N = 11) | Control (N = 27) | p | |
|---|---|---|---|
| Gender, male | 6 (54.5) | 21 (77.8) | 0.238 |
| Age, years | 60.0 (57.5–62.5) | 58.0 (53.0–62.0) | 0.287 |
| Body mass index, kg/m2 | 23.7 (20.4–25.3) | 21.4 (18.7–23.4) | 0.122 |
| Comorbidity | |||
| Cardiovascular disease | 0 (0.0) | 1 (3.7) | 1.000 |
| Chronic lung disease | 1 (9.1) | 21 (77.8) a | <0.001 |
| Diabetes mellitus | 1 (9.1) | 3 (11.1) | 1.000 |
| Normal left ventricular ejection fraction | 11 (100.0) | 25 (92.6) | 1.000 |
| Time from intubation to LT, days | 67.0 (54.5–83.5) | 18.0 (7.0–26.5) | <0.001 |
| Time from listing to LT, days | 26.0 (9.0–38.0) | 27.0 (10.5–40.5) | 0.961 |
| Time from ECMO to LT, days | 49.0 (32.0–66.0) | 11.0 (6.0–18.0) | <0.001 |
| Characteristics of LT | |||
| Bilateral lung transplantation | 11 (100.0) | 26 (96.3) | 1.000 |
| Operation time, min | 510 (447–541) | 575 (474–690) | 0.097 |
| Total ischemic time, right, min | 199 (142–292) | 280 (231–363) | 0.053 |
| Total ischemic time, left, min | 319 (222–351) | 331 (250–372) | 0.384 |
| Number of intraoperative packed RBC | 10.0 (3.5–12.5) | 9.0 (6.5–12.0) | 0.821 |
| Number of intraoperative FFP | 2.0 (1.0–6.5) | 3.0 (0.0–6.0) | 0.961 |
| PGD at 72 h | |||
| PGD 0 | 9 (81.8) | 25 (92.6) | 0.435 |
| PGD 1 | 0 (0.0) | 0 (0.0) | |
| PGD 2 | 1 (9.1) | 2 (7.4) | |
| PGD 3 | 1 (9.1) | 0 (0.0) | |
| Complications | |||
| Acute kidney injury required renal replacement therapy | 3 (27.3) | 7 (25.9) | 1.000 |
| Bleeding requiring chest reopening | 2 (18.2) | 6 (22.2) | 1.000 |
| Bleeding requiring medical management | 2 (18.2) | 1 (3.7) | 0.196 |
| Infection | 7 (63.6) | 4 (14.8) | 0.005 |
| Airway complication | 3 (27.3) | 2 (7.4) | 0.134 |
| Postoperative prolonged ECMO | 4 (36.4) | 8 (29.6) | 0.714 |
| Length of stay in ICU, days | 88.0 (75.0–98.5) | 33.0 (23.0–43.5) | <0.001 |
| Hospital survival | 0.395 | ||
| Alive | 10 (90.9) | 20 (74.1) | |
| Expired | 1 (9.1) | 7 (25.9) | |
COVID-19, coronavirus disease 2019; ARDS, acute respiratory distress syndrome; ECMO, extracorporeal membrane oxygenation; FFP, fresh frozen plasma; ICU, intensive care unit; LT, lung transplantation; PGD, primary graft dysfunction; RBC, red blood cell.
Values are presented as median (interquartile range) or number (%).
Twenty-one patients (13 idiopathic pulmonary fibrosis, 5 connective tissue disease–related interstitial lung disease, 2 bronchiolitis obliterans, and 1 emphysema).
Discussion
This multicenter retrospective observational study reported 11 lung transplants for severe COVID-19-related ARDS in Korea. Patients were very fit before being affected with COVID-19-related ARDS. All patients were supported by ECMO at the time of listing, and most patients had recovered from acute kidney injury or sepsis. They received ICU rehabilitation before and after LT. No significant differences in the intraoperative management and immediate outcomes of lung transplants were found for severe COVID-19-related ARDS compared with other lung transplants for other causes after bridging with ECMO. Only one patient was lost in the early postoperative period, resulting in a hospital mortality rate of 9.1%.
Data and experience regarding LT in patients with acute respiratory failure (e.g. ARDS) are still limited. Several case reports and case series currently presented acceptable LT outcomes in carefully selected ARDS patients.12–14 Chang et al. 12 showed a single-center experience of 14 lung transplants for ARDS over 5 years. They reported acceptable outcomes with a 3-year survival rate of 78%. Frick et al. 13 presented post-transplant outcomes of ARDS patients from three high-volume European transplant centers with 13 patients over 22 years, and these patients revealed a 30-day and 1-year survival rate with 92.3% and 71.6%, respectively. Harano et al. 14 analyzed the United Network for Organ Sharing database and presented outcomes of 39 lung-transplanted ARDS patients. They compared postoperative outcomes of ARDS patients with restrictive lung disease patients. The ARDS patients received more ECMO bridging to LT, but survival time and in-hospital mortality rates were not significantly different. Overall, previous studies have shown that LT could be considered in carefully selected ARDS patients. Recent case series of LT for severe COVID-19-related ARDS show similar results with previous studies of ARDS patients.15,16,21 In addition, these studies emphasized a multidisciplinary approach to selecting suitable candidates for LT for severe COVID-19. Moreover, a multidisciplinary approach to selecting suitable COVID-19-related ARDS patients for LT also proceeded. However, the median age of lung transplants was higher than those of other reports.14,15 This may be due to the Korean lung allocation system which is primarily based on the urgency of a transplant. 22 According to the Korean lung allocation system, patients with ECMO on the waiting list had the highest priority for transplantation, regardless of the probability of post-transplant survival. However, the results of this study provide additional information on LT in relatively old patients with COVID-19-related ARDS because the disease is more progressive in older patients.23,24
Previous studies showed that physical function before LT was associated with morbidity and mortality after transplantation.25–27 Similar to other ARDS patients, patients with severe COVID-19-related ARDS were also affected by ICU-acquired weakness. 28 These patients often received deep sedation to prevent patient-ventilator dyssynchrony and ventilator-induced lung injury and also required long ICU stay. Therefore, ICU-acquired weakness may be aggravated during the course of managing COVID-19-related ARDS. All patients in this study received rehabilitation during their ICU stays, and 45.5% were awake while on ECMO. The awake ECMO has several benefits for physical function such as reduced sedatives and active rehabilitation.29–31 In addition, patients who were treated with the awake ECMO as a bridge to transplantation and active rehabilitation would have better outcomes than patients who received MV as a bridge to transplantation. 25 These results suggest that considering the availability of active rehabilitation with awake ECMO may be a key factor for selecting suitable patients for LT. Considering the patient’s physical function and frailty when deciding on LT in COVID-19-related ARDS patients is important based on these experiences with ARDS patients.
Although the results of this study provide important information about the outcome of LT in patients with severe COVID-19-related ARDS, the study has several limitations that should be acknowledged. First, this retrospective study was limited to a small number of patients and was associated with the inherent shortcomings of the study design. Second, the relatively elderly patients bridged with ECMO in this study may reflect the Korean lung allocation system based first on transplant urgency, which is different from the US and the European lung allocation score system based on the expected benefit after LT as well as the disease severity. Therefore, this result has limited generalization with other countries. However, acceptable results also can be obtained even in relatively elderly COVID-19 patients if the physical function before transplantation is good. Finally, COVID-19-related ARDS patients could not be compared with other causes of ARDS because a small number of patients were registered in the KOTRY and a few patients received LT for other causes after bridging with ECMO. Further systematic studies that could directly compare the outcomes of LT between COVID-19-related ARDS and other cases of ARDS are needed.
In conclusion, LT in patients with COVID-19-related ARDS leads to acceptable short-term outcomes. LT could be considered only for patients who are carefully selected with physical function as experienced from previous ARDS patients.
Footnotes
Author contributions: Ryoung-Eun Ko: Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Resources; Writing – original draft; Writing – review & editing.
Dong Kyu Oh: Data curation; Formal analysis; Investigation; Resources; Writing – review & editing.
Sun Mi Choi: Data curation; Formal analysis; Investigation; Resources; Writing – review & editing.
Sunghoon Park: Data curation; Formal analysis; Investigation; Resources; Writing – review & editing.
Ji Eun Park: Data curation; Formal analysis; Investigation; Resources; Writing – review & editing.
Jin Gu Lee: Data curation; Formal analysis; Investigation; Resources; Writing – review & editing.
Young Tae Kim: Conceptualization; Data curation; Formal analysis; Funding acquisition; Investigation; Resources; Writing – review & editing.
Kyeongman Jeon: Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Resources; Supervision; Writing – original draft; Writing – review & editing.
Conflict of interest statement: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by a fund (2014-ER6301-00, 2014-ER6301-01, 2014-ER6301-02, 2017-ER6301-00) by Research of Korea Centers for Disease Control and Prevention Agency. The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
ORCID iDs: Sun Mi Choi  https://orcid.org/0000-0002-0742-6085
https://orcid.org/0000-0002-0742-6085
Kyeongman Jeon  https://orcid.org/0000-0002-4822-1772
https://orcid.org/0000-0002-4822-1772
Contributor Information
Ryoung-Eun Ko, Department of Critical Care Medicine, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, South Korea.
Dong Kyu Oh, Department of Pulmonary and Critical Care Medicine, Asan Medical Center, University of Ulsan College of Medicine, Seoul, South Korea.
Sun Mi Choi, Division of Pulmonary and Critical Care Medicine, Department of Internal Medicine, Seoul National University Hospital, Seoul National University College of Medicine, Seoul, South Korea.
Sunghoon Park, Department of Pulmonary, Allergy and Critical Care Medicine, Hallym University Sacred Heart Hospital, Anyang, South Korea.
Ji Eun Park, Department of Pulmonary and Critical Care Medicine, Ajou University School of Medicine, Suwon, South Korea.
Jin Gu Lee, Department of Thoracic and Cardiovascular Surgery, Severance Hospital, Yonsei University College of Medicine, Seoul, South Korea.
Young Tae Kim, Department of Thoracic and Cardiovascular Surgery, Seoul National University Hospital, Seoul National University College of Medicine, Seoul, South Korea.
Kyeongman Jeon, Division of Pulmonary and Critical Care Medicine, Department of Medicine, Samsung Medical Center, Sungkyunkwan University School of Medicine, 81 Irwon-ro, Gangnam-gu, Seoul 06351, South Korea.
References
- 1. Richardson S, Hirsch JS, Narasimhan M, et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA 2020; 323: 2052–2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Buckner FS, McCulloch DJ, Atluri V, et al. Clinical features and outcomes of 105 hospitalized patients with COVID-19 in Seattle, Washington. Clin Infect Dis 2020; 71: 2167–2173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wunsch H. Mechanical ventilation in COVID-19: interpreting the current epidemiology. Am J Respir Crit Care Med 2020; 202: 1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lim ZJ, Subramaniam A, Ponnapa Reddy M, et al. Case fatality rates for patients with COVID-19 requiring invasive mechanical ventilation. A meta-analysis. Am J Respir Crit Care Med 2021; 203: 54–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Auld SC, Caridi-Scheible M, Blum JM, et al. ICU and ventilator mortality among critically ill adults with coronavirus disease 2019. Crit Care Med 2020; 48: e799–e804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Davies A, Jones D, Bailey M, et al. Extracorporeal membrane oxygenation for 2009 influenza A(H1N1) acute respiratory distress syndrome. JAMA 2009; 302: 1888–1895. [DOI] [PubMed] [Google Scholar]
- 7. Pham T, Combes A, Rozé H, et al. Extracorporeal membrane oxygenation for pandemic influenza A(H1N1)-induced acute respiratory distress syndrome: a cohort study and propensity-matched analysis. Am J Respir Crit Care Med 2013; 187: 276–285. [DOI] [PubMed] [Google Scholar]
- 8. Alshahrani MS, Sindi A, Alshamsi F, et al. Extracorporeal membrane oxygenation for severe Middle East respiratory syndrome coronavirus. Ann Intensive Care 2018; 8: 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Choi WS, Kang CI, Kim Y, et al. Clinical presentation and outcomes of middle east respiratory syndrome in the Republic of Korea. Infect Chemother 2016; 48: 118–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Barbaro RP, MacLaren G, Boonstra PS, et al. Extracorporeal membrane oxygenation support in COVID-19: an international cohort study of the Extracorporeal Life Support Organization registry. Lancet 2020; 396: 1071–1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ramanathan K, Shekar K, Ling RR, et al. Extracorporeal membrane oxygenation for COVID-19: a systematic review and meta-analysis. Crit Care 2021; 25: 211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chang Y, Lee SO, Shim TS, et al. Lung transplantation as a therapeutic option in acute respiratory distress syndrome. Transplantation 2018; 102: 829–837. [DOI] [PubMed] [Google Scholar]
- 13. Frick AE, Gan CT, Vos R, et al. Lung transplantation for acute respiratory distress syndrome: a multicenter experience. Am J Transplant 2022; 22: 144–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Harano T, Ryan JP, Chan EG, et al. Lung transplantation for the treatment of irreversible acute respiratory distress syndrome. Clin Transplant 2021; 35: e14182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hawkins RB, Mehaffey JH, Charles EJ, et al. Lung transplantation for severe post-coronavirus disease 2019 respiratory failure. Transplantation 2021; 105: 1381–1387. [DOI] [PubMed] [Google Scholar]
- 16. Bharat A, Machuca TN, Querrey M, et al. Early outcomes after lung transplantation for severe COVID-19: a series of the first consecutive cases from four countries. Lancet Respir Med 2021; 9: 487–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ko RE, Lee JG, Kim SY, et al. Extracorporeal membrane oxygenation as a bridge to lung transplantation: analysis of Korean Organ Transplantation Registry (KOTRY) data. Respir Res 2020; 21: 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Fu F, Lou J, Xi D, et al. Chest computed tomography findings of coronavirus disease 2019 (COVID-19) pneumonia. Eur Radiol 2020; 30: 5489–5498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rockwood K, Song X, MacKnight C, et al. A global clinical measure of fitness and frailty in elderly people. CMAJ 2005; 173: 489–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Oto T, Levvey BJ, Whitford H, et al. Feasibility and utility of a lung donor score: correlation with early post-transplant outcomes. Ann Thorac Surg 2007; 83: 257–263. [DOI] [PubMed] [Google Scholar]
- 21. Chen JY, Qiao K, Liu F, et al. Lung transplantation as therapeutic option in acute respiratory distress syndrome for coronavirus disease 2019-related pulmonary fibrosis. Chin Med J 2020; 133: 1390–1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Yu WS, Kim SY, Kim YT, et al. Characteristics of lung allocation and outcomes of lung transplant according to the Korean urgency status. Yonsei Med J 2019; 60: 992–997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Grasselli G, Greco M, Zanella A, et al. Risk factors associated with mortality among patients with COVID-19 in intensive care units in Lombardy, Italy. JAMA Intern Med 2020; 180: 1345–1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet 2020; 395: 1054–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Fuehner T, Kuehn C, Hadem J, et al. Extracorporeal membrane oxygenation in awake patients as bridge to lung transplantation. Am J Respir Crit Care Med 2012; 185: 763–768. [DOI] [PubMed] [Google Scholar]
- 26. Singer JP, Diamond JM, Gries CJ, et al. Frailty phenotypes, disability, and outcomes in adult candidates for lung transplantation. Am J Respir Crit Care Med 2015; 192: 1325–1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Singer JP, Diamond JM, Anderson MR, et al. Frailty phenotypes and mortality after lung transplantation: a prospective cohort study. Am J Transplant 2018; 18: 1995–2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Van Aerde N, Van den Berghe G, Wilmer A, et al. Intensive care unit acquired muscle weakness in COVID-19 patients. Intensive Care Med 2020; 46: 2083–2085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Crotti S, Iotti GA, Lissoni A, et al. Organ allocation waiting time during extracorporeal bridge to lung transplant affects outcomes. Chest 2013; 144: 1018–1025. [DOI] [PubMed] [Google Scholar]
- 30. Biscotti M, Gannon WD, Agerstrand C, et al. Awake extracorporeal membrane oxygenation as bridge to lung transplantation: a 9-year experience. Ann Thorac Surg 2017; 104: 412–419. [DOI] [PubMed] [Google Scholar]
- 31. Na SJ, Jeon K. Extracorporeal membrane oxygenation support in adult patients with acute respiratory distress syndrome. Expert Rev Respir Med 2020; 14: 511–519. [DOI] [PubMed] [Google Scholar]