Abstract
The therapeutic antitumor effect of clarithromycin (CAM) was examined with the 13762NF mammary adenocarcinoma and F-344 rat system. When CAM treatment at a dosage of 2 mg/kg of body weight orally for 21 days was commenced after inoculation of the tumor, no significant decrease in death rate was observed, although the loss in body weight was less than that in the untreated group. When tumor-bearing (TB) rats were treated with CAM in combination with carboplatin or cyclophosphamide, a significant decrease in the death rate was obtained, although neither treatment alone proved to be effective. A beneficial effect was also observed when CAM treatment was combined with surgical treatment. CAM showed no direct cytotoxicity to this tumor in vitro according to the MTT (3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide) assay. Spleen cells obtained from TB rats receiving CAM treatment showed a stronger tumor-neutralizing activity than those from rats which had not received CAM treatment (Winn assay). Enhanced induction of cytotoxic cells to allogeneic tumor was also observed in rats immunized with allogeneic tumor cells together with CAM treatment (51Cr release assay). The 13762NF tumor produces transforming growth factor-β (TGF-β), tumor necrosis factor alpha, and matrix metalloproteinase-9, and treatment of tumor cells with CAM in vitro for 24 h significantly inhibited the expression of the genes coding for these proteins (reverse transcription-PCR). Levels of expression of the TGF-β and interleukin-6 genes of spleen cells obtained from CAM-treated TB rats were both significantly lower than those of spleen cells from CAM-untreated TB rats. This study suggests that CAM has biological response modifier activities resulting in a beneficial therapeutic antitumor effect and might be useful for the treatment of human cancers.
Recently, the biological response modifier (BRM) activities of antimicrobial agents have become a matter of growing concern in the medical field (5, 7, 21). In Japan, Kudoh et al. (15) first reported in 1987 that low-dose long-term treatment with erythromycin (EM) was effective for diffuse panbronchiolitis. Pseudomonas aeruginosa is not sensitive to EM in vitro, so activities other than an antimicrobial one were indicated. Because of this fact, many investigators have taken interest in the BRM activities of macrolides, and so far, a variety of macrolide activities have been reported: e.g., reduction of bronchial hyperresponsiveness in asthmatics (17); inhibition of neutrophil chemotaxis or neutrophil-derived elastolyte-like (9) or NADPH oxidase activity (27); modulation of production various cytokines, such as interleukin-1 (IL-1) (10, 13, 26), IL-2 (13), IL-6 (2), IL-8 (21), and tumor necrosis factor alpha (TNF-α) (13); inhibition of biofilm formation (18); and other miscellaneous activities (1, 11, 19). Besides the activities mentioned above, Mikasa et al. (16) have recently reported that long-term treatment with clarithromycin (CAM) was effective for non-small cell lung cancer in which surgical resection was not indicated. Together with results in humans, Mikasa’s group has also demonstrated antitumor activity of EM in vivo in mouse tumor systems (6), and antiangiogenesis activity of CAM has been found in vitro (22). If macrolide treatment is found to be truly effective for patients with advanced stages of cancer, it would be greatly beneficial in the management of such patients. Thus, to confirm the beneficial effect of CAM reportedly found in patients with lung cancer, we carried out some animal studies with a rat tumor. We report here that CAM has BRM activities which may be of great benefit to patients with cancer.
MATERIALS AND METHODS
Animals.
Male F-344 rats were purchased from the SLC Co. Ltd., Shizuoka, Japan. The experimental rats were 12 to 14 weeks old, weighed 230 to 250 g, and were kept in a clean room. The experimental designs used in this study were approved by the ethical committee of Toyama Medical and Pharmaceutical University.
Tumor.
The 13762NF (subclone MTLn3) mammary adenocarcinoma (20), originating from an F-344 rat, was kindly given by G. L. Nicolson and was maintained in vitro and in vivo. The E4 mammary adenocarcinoma cell line, originating from an SD rat, was obtained from the Japanese Cancer Research Resources Bank (Tokyo, Japan). Cultured cells were maintained in RPMI 1640 medium containing 10% fetal calf serum.
Anticancer drugs and CAM.
Cyclophosphamide (CY) was administered at 60 mg/kg intravenously (i.v.) through the tail vein, and carboplatin (CBDCA) was given at 50 mg/kg of body weight intraperitoneally (i.p.). Clarithromycin (CAM) (Abbot Co., Ltd., North Chicago, Ill.) was dissolved in tap water and administered orally (p.o. [per os]) once a day by means of a gastric tube. The molecular formula of CAM is C38H69NO13, and the molecular weight is 747.96.
Experimental therapy.
13762NF tumor cells (2 × 106) were inoculated subcutaneously (s.c.) into the right flank of the F-344 rat on day 0. Tumor size (millimeters) was expressed as (short diameter + long diameter)/2. Animals without a tumor or animals with a regressing tumor on day 50 after tumor inoculation were judged to be cured. In most experiments, all animals judged to be cured survived for more than 90 days.
Tumor-neutralizing assay.
A modified version of the Winn assay (34) was carried out. The mixture of spleen cells (80 × 106) and tumor cells (1 × 106) was inoculated s.c. into syngeneic rats which had been irradiated (2.5 Gy) 24 h before inoculation. The tumor-neutralizing activity of the spleen cells was evaluated by measuring the tumor weight at appropriate points after inoculation.
51Cr release assay.
A mixture of 51Cr-labeled tumor cells (2 × 105) and spleen cells (5 × 106 to 10 × 106) was centrifuged at 500 rpm for 5 min, and then the cells were incubated in a culture medium at 37°C in a 5% CO2 atmosphere for 5 h. The amount of release at the end of the incubation period was measured, and the percentage of cytolysis was calculated by the formula % cytolysis = [(test release − spontaneous release)/(maximum release − spontaneous release)] × 100.
MTT assay.
The MTT (3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide) assay was performed according to the method used in our previous report (23). Briefly, tumor cells (1 × 103 to 4 × 103) were seeded in 96-well flat-bottom plates (Falcon 3072) and cultured in the presence or absence of a drug for 3 days, and 20 μl of MTT (5 mg/ml) was added to each well 4 h before termination of incubation. Following this, the medium in the wells was removed, 200 μl of dimethyl sulfoxide was added, and activity was then measured by a spectrophotometer at 560 nm.
Expression of the TGF-β, IL-6, TNF-α, and MMP-9 genes.
Expression of genes was measured by the reverse transcription-polymerase chain reaction (RT-PCR) method. Total RNA was isolated from the spleen cells or tumor cells by the ISOGEN method (3) and was reverse transcribed with random hexamers by using an RNA-PCR kit (Takara, Tokyo, Japan). cDNA was amplified by the PCR method, and the PCR products were separated on 1.5% agarose gels and stained with ethidium bromide. The primers used for the amplification of the transforming growth factor-β (TGF-β) gene, IL-6 gene, TNF-α gene, or matrix metalloproteinase-9 (MMP-9) gene were as follows: TGF-β gene, 5′-GCCCTGGACACCTATTGC-3′ and 5′-GCTGCACTTGCAGGAGCGCAC-3′; IL-6 gene, 5′-ATGTAGCCGCCCCACACAGA-3′ and 5′-CATCCATCTTTTTCAGCCAT-3′; TNF-α gene, 5′-CAAGGAGGAGTTCCCAA-3′ and 5′-GAATCTGTAGTGCCTCAGGC-3′; and MMP-9 gene, 5′-GGTCCCCCCACTGCTGGCCCTTCTACGGCC-3′ and 5′-GTCCTCAGGGCACTGGAGGATGTCATAGGT-3′. As an internal control, a set of primers for the glyceraldehyde-3-phosphate dehydrogenase (GAPDH) gene (5′-CAAAAGGGTCATCTCTG-3′ and 5′-CCTGCTTCACCACCTTCTTG-3′) were added to each sample. The degree of gene expression was expressed as a ratio of TGF-β (IL-6, TNF-α, or MMP-9) to GAPDH as determined with a densitometer.
Statistical analysis.
Data are shown as means ± standard errors, and statistical significance was evaluated by the Student’s t test or Fisher’s exact probability test. A P value of <0.05 was judged to be significant.
RESULTS
Effect of treatment with CAM alone on the growth of the 13762NF tumor in F-344 rats.
In order to know the effect of CAM treatment alone on the growth of a transplanted 13762NF tumor in F-344 rats, the following protocol was carried out. First, different doses of CAM were administered p.o. once a day for 21 days from day 8 to day 28. As shown in experiment 1 of Table 1, no significant differences in survival rate on day 50 were observed for all doses employed (0.5 to 32 mg/kg). Second, timing of administration was examined at a CAM dosage of 2 mg/kg. As shown in experiment 2 of Table 1, the survival rate on day 50 increased from 0% to 33% when CAM was commenced on day 1 and to 42% when CAM was commenced 5 days before inoculation of the tumor cells. Cured rats rejected reinoculation of the identical tumor (5 × 106 cells s.c.) (data not shown).
TABLE 1.
Effect of treatment with CAM alone on growth of the 13762NF tumor in F-344 rats
| CAM treatment (mg/kg)a | Days of treatment | Survival rate (% surviving) on day 50b |
|---|---|---|
| Expt 1 | ||
| 32 | 8–28 | 0/9 (0) |
| 8 | 8–28 | 0/9 (0) |
| 2 | 8–28 | 1/9 (11) |
| 0.5 | 8–28 | 2/9 (22) |
| None | 0/10 (0) | |
| Expt 2 | ||
| 2 | −5–∼−1 and +1–∼+16 | 5/12 (42)c |
| 2 | +1–∼+21 | 4/12 (33) |
| 2 | +8–∼+28 | 2/12 (17) |
| None | 0/12 (0) |
Tumor cells (2 × 106) were administered s.c. on day 0. CAM was given p.o. according to the schedule.
Animals without a tumor or animals with a regressing tumor were recorded on day 50 after tumor inoculation.
P < 0.05 versus the untreated group.
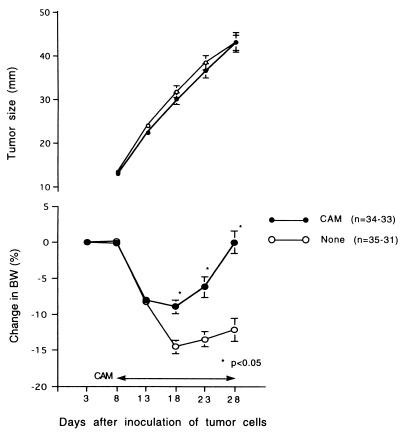
When tumor cells were inoculated s.c. into rats, animals lost weight gradually starting a week later. However, in the group receiving CAM treatment (2 mg/kg p.o. from days 8 to 28), the degree of loss was significantly smaller than that for the untreated group, although there was no significant difference in tumor sizes between the two groups (Fig. 1).
FIG. 1.
Changes in body weight (BW) after tumor inoculation. Tumor cells (2 × 106) were administered s.c. on day 0. CAM (2 mg/kg) was administered p.o. for 21 days from day 8 to day 28. The summarized results of four independent experiments are shown.
Effect of combined treatments with a chemotherapeutic agent and CAM or surgery and CAM on the growth of the 13762NF tumor in rats.
The therapeutic effect of CAM alone was not found to be strong enough, so we examined the combined effect of a chemotherapeutic agent and CAM on the growth of the 13762NF tumor in rats (Table 2). When 60 mg of CY or 50 mg of CBDCA per kg of body weight was combined with CAM, the survival rate significantly increased. We then examined the combined effect of surgical resection and CAM on the death rate. In experiment 1, surgical resection was performed on day 18 and CAM was administered for 21 days from day 23 to day 43. For the surgery-alone group, 3 animals died because of a local recurrence, and 13 of 17 (76%) died because of metastases to the lung (Table 3). Conversely, in the surgery and CAM group, one animal died from surgery, and only 6 of 19 (32%) died because of metastases (P < 0.05). In experiment 2, surgery was carried out at an earlier date (on day 15) to reduce the local recurrence rate, and CAM was administered for 14 days from day 1 to day 14. In the surgery-alone group, 4 animals died because of local recurrence, and 6 of 13 (46%) died because of metastases. Conversely, in the surgery and CAM group, 1 animal died because of surgery, and none of the 16 (0%) died because of metastases (P < 0.05).
TABLE 2.
Combined effect of CY and CAM and CBDCA and CAM on growth of the 13762NF tumor in F-344 rats
| Treatmenta | Day(s) of treatment | Survival rate (% surviving) on day 50b |
|---|---|---|
| Expt 1 | ||
| CY (60 mg/kg) | 8 | 3/11 (27) |
| CAM (2 mg/kg) | 9–29 | 2/12 (17) |
| CY + CAM | 6/12 (50)c | |
| None | 0/12 (0) | |
| Expt 2 | ||
| CBDCA (50 mg/kg) | 8 | 0/12 (100) |
| CAM (2 mg/kg) | 9–29 | 0/12 (100) |
| CBDCA + CAM | 6/12 (50)c | |
| None | 0/12 (100) |
Tumor cells (2 × 106) were administered s.c. on day 0. CY at 60 mg/kg i.v. or CBDCA at 50 mg/kg i.p. was administered on day 8, and CAM at 2 mg/kg p.o. was administered on days 9 to 29.
Animals without tumor or animals with a regressing tumor were recorded on day 50 after tumor inoculation.
P < 0.025 versus the untreated group.
TABLE 3.
Combined effect of surgery and CAM on the death rate due to metastasis
| Treatment (day) | No. of deaths [%] due toa:
|
||
|---|---|---|---|
| Surgery | Local recurrence | Metastasis | |
| Expt 1 (n = 20) | |||
| Surgery (18) | 0 | 3 (24.3) | 13/17 [76] (31.9) |
| Surgery (18) + CAM (23–43) | 1 (10) | 0 | 6/19 [32]b (33.7) |
| Expt 2 (n = 17) | |||
| Surgery (15) | 4 (1.3) | 0 | 6/13 [46] (36.8) |
| CAM (1–14) + surgery (15) | 1 (2) | 0 | 0/16 [0]b (>70) |
Values in parentheses represent mean numbers of survival days from the surgery.
P < 0.05 versus the surgery-alone group.
Studies of mechanisms of the therapeutic effect of CAM treatment. (i) Direct cytotoxicity to tumor cells in vitro.
Whether CAM shows any direct cytotoxicity to 13762NF cells was examined in vitro with the MTT assay. No significant cytotoxicity was observed with 0.5 to 50 μg (0.67 to 67 μM) of CAM per ml. CBDCA (1 to 100 μg/ml [2.67 to 267 μM]) as a positive control showed a significant cytotoxicity to this tumor (data not shown).
(ii) Antitumor cellular immunity.
Antitumor cellular immunity of the hosts was examined by the in vivo tumor-neutralizing assay by using spleen cells. As shown in Table 4, only spleen cells obtained from tumor-bearing (TB) rats which had received CAM treatment showed detectable tumor-neutralizing activity. There were no significant differences between the TB plus CAM and TB groups or between the non-TB plus CAM and non-TB groups.
TABLE 4.
Tumor-neutralizing activity of spleen cells obtained from TB rats treated with CAM
| Treatment of spleen cell donora | Tumor wt (g)b
|
|
|---|---|---|
| Day 8 | Day 12 | |
| TB (day 15) | ||
| CAM (days 1–14) | 0.85 ± 0.08c | 1.97 ± 0.17d |
| None | 1.05 ± 0.17 | 2.82 ± 0.49 |
| Non-TB | ||
| CAM (days 1–14) | 1.11 ± 0.16 | 2.33 ± 0.21 |
| None | 1.38 ± 0.16 | 3.09 ± 0.30 |
| Tumor cell alone | 1.31 ± 0.08 | 2.93 ± 0.33 |
13762NF cells (2 × 106) were administered s.c. on day 0. CAM was administered at 2 mg/kg p.o. on days 1 to 14, with removal of spleens on day 15.
A mixture of tumor cells (1 × 106) and spleen cells (80 × 106) was inoculated s.c. into syngeneic rats, and the tumors were then weighed on days 8 and 12 after the inoculation. Each group contained four to six rats.
P < 0.01 versus the tumor cell-alone group.
P < 0.05 versus the tumor cell-alone group.
(iii) Effect of CAM on expression of the TGF-β, IL-6, TNF-α, and MMP-9 genes.
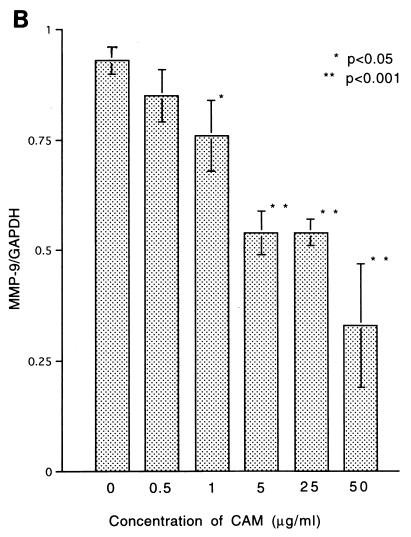
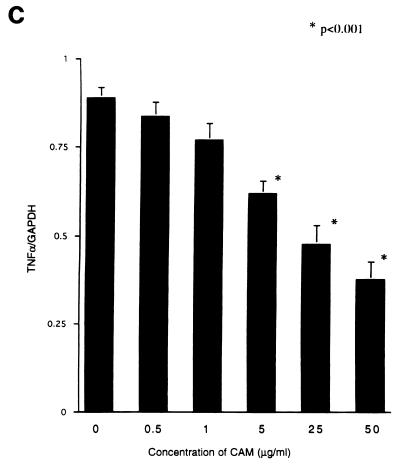
The tumor used in this study has been proved to produce TGF-β and MMP-9 (25), which are well known to have a variety of activities concerning immunosuppression or metastasis (29, 30, 33). To know the effect of CAM on the production of TGF-β, MMP-9, or TNF-α from the tumor cells, we examined the expression of the genes coding for these proteins by the RT-PCR method. Tumor cells incubated with CAM in vitro for 24 h were used for the experiment. Expression of the TGF-β, MMP-9, and TNF-α genes was significantly inhibited by the treatment with CAM at low concentrations (Fig. 2). Inhibition of MMP-9 activity could be confirmed by zymography (8) using 7.5% polyacrylamide gels with 1.5 mg of type IV collagen-derived gelatin per ml embedded in them (data not shown).
FIG. 2.
Effect of in vitro treatment with CAM on expression of the TGF-β (A), MMP-9 (B), and TNF-α (C) genes of 13762NF tumor cells. An asterisk indicates a statistical difference versus the untreated samples.
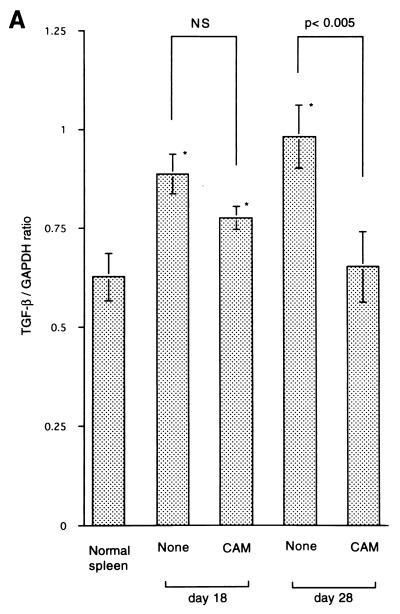
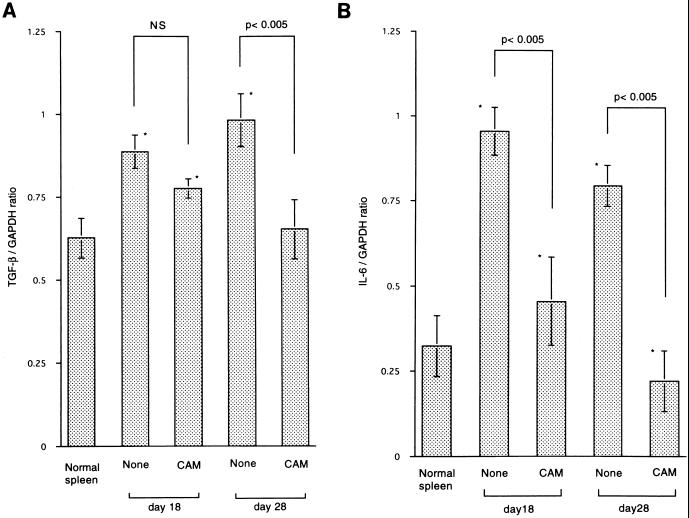
We then examined the expression of the TGF-β gene of spleen cells obtained from TB rats. CAM (2 mg/kg p.o.) treatment was commenced on day 8 after tumor inoculation, and spleens were removed on days 18 (10 days of CAM treatment) and 28 (20 days of CAM treatment). The level of expression of the TGF-β gene on days 18 and 28 was lower in the CAM-treated TB group than that in the CAM-untreated TB group, but a statistical difference was only observed for the 20-day CAM treatment group (Fig. 3A). As shown in Fig. 1, CAM treatment prevented TB rats from losing body weight. Therefore, we also examined the effect of in vivo treatment with CAM on the expression of the IL-6 gene of spleen cells by RT-PCR. As shown in Fig. 3B, the level of expression of the IL-6 gene of spleen cells on days 18 and 28 was significantly lower in the CAM-treated TB group than in the untreated TB group. The levels of IL-6 in serum in TB rats were all below the detection level of the kit (data not shown).
FIG. 3.
Effect of in vivo treatment with CAM on expression of the TGF-β (A) and IL-6 (B) genes of spleen cells. Tumor cells were administered s.c. on day 0. CAM (2 mg/kg) was administered p.o. on days 8 to 17 and on days 8 to 27. Spleens were removed on day 8 or 28 for the assay. An asterisk indicates statistical significance versus the untreated samples. NS, not significant.
(iv) Effect of CAM on the induction of cytotoxic cells to the allogeneic tumor.
The effect of CAM treatment on the induction of cytotoxic cells to allogeneic (SD rat) E4 tumor was examined by the 51Cr release assay. F-344 rats were immunized with viable E4 tumor cells twice on days 0 (3 × 107 cells s.c.) and 9 (3 × 107 cells i.p.), followed by CAM treatment (2 mg/kg p.o.) on days 1 to 14. The assay was performed twice on days 12 and 15 (Table 5). Under this condition, only spleen cells obtained from rats which had been immunized with E4 cells followed by CAM treatment showed a detectable cytotoxicity to E4 tumor cells.
TABLE 5.
Cytotoxic activity of spleen cells obtained from rats immunized with allogeneic E4 tumor cells followed by CAM
| Treatment of spleen cell donor | % Cytolysis at effector/target cell ratio
|
|||
|---|---|---|---|---|
| Day 12
|
Day 15
|
|||
| 1:50 | 1:25 | 1:50 | 1:25 | |
| Immunization alone | 5.3 ± 0.9 | 3.9 ± 2.1 | 5.1 ± 0.0 | 3.2 ± 1.0 |
| CAM alone | 3.3 ± 1.0 | 1.6 ± 0.7 | 6.9 ± 2.0 | 2.0 ± 1.9 |
| Immunization + CAM | 20.0 ± 1.8b | 15.9 ± 0.6b | 18.0 ± 1.9b | 13.4 ± 1.5b |
| None | 5.4 ± 0.7 | 2.0 ± 1.6 | 6.1 ± 1.6 | 2.0 ± 0.8 |
F-344 rats were immunized with viable E4 tumor cells on day 3 (3 × 107 cells s.c.) and day 9 (3 × 107 i.p.). CAM was administered at 2 mg/kg p.o. on days 1 to 14. Spleens in each group were obtained from three rats. The 51Cr release assay was performed on days 12 and 15.
P < 0.001 versus the immunization-alone group.
DISCUSSION
We have shown in this study that CAM has BRM activities resulting in a beneficial therapeutic effect on a transplanted tumor in rats. A sufficiently good therapeutic effect could not be obtained by CAM treatment alone, but was observed when CAM was combined with chemotherapy or surgery. Concerning the therapeutic antitumor effect of macrolides, Hamada et al. (6) first reported that EM was effective for prolonging the survival time in Ehrlich ascites carcinoma-ddY and P388 leukemia-CDF1 mouse systems. Our results and their results seem to support the finding by Mikasa et al. (16) that long-term treatment with CAM prolonged the survival of patients with advanced non-small cell lung cancer. A particularly interesting finding was that CAM treatment could reduce the death rate due to metastasis (Table 3). CAM treatment was found to be effective when it was administered either before or after surgery. At the time surgical resection of a primary tumor was undertaken, the micrometastases mainly to the lung had already occurred in our system. Control of micrometastasis is very important in the treatment of cancer patients, and a BRM having such an activity has been greatly desired. CAM may become a candidate for BRM to control micrometastasis.
What are the mechanisms for the beneficial therapeutic effect of CAM? CAM showed no cytotoxicity to 13762NF tumor cells in vitro, so a direct cytotoxic effect of CAM seems unlikely. We showed by the Winn assay that the therapeutic antitumor effect of CAM might be partly due to the enhanced antitumor immunity. CAM was found to have an activity which inhibited the tumor cells and spleen cells from producing TGF-β, which has immunosuppressive activity (24, 25). When the 14C-labelled CAM (2 mg/kg) was administered once orally to normal rats, concentrations in various tissues peaked 1 h after administration. The peak concentrations of CAM were as follows: liver, 6.84 ± 1.18 μg/g; lung, 5.28 ± 0.29 μg/g; spleen, 3.11 ± 0.19 μg/g; and blood, 0.18 ± 0.01 μg/ml. We have no data concerning the concentrations of CAM in tumor tissues when it is administered repeatedly. However, concentrations of CAM employed for the in vitro experiments of at least 0.5, 1.0, or 5.0 μg/ml would reflect the in vivo situation. Therefore, we speculate that the enhancement of antitumor immunity may be caused in part by the inhibition of the immunosuppressive factor TGF-β. In Hamadas’ tumor system (6), IL-4 levels in serum were found to be significantly high in TB mice receiving EM, and the antitumor effect was abolished by in vivo treatment with the anti-IL-4 antibody; in their experiment, neither the anti-TNF-α antibody nor the anti-IFN-γ antibody affected it. They speculated that enhanced tumoricidal activity of macrophages induced by EM treatment via the stimulation of IL-4 production might be responsible for the beneficial therapeutic effect of EM. We have not yet examined this possibility in our rat system. Concerning the inhibition of micrometastasis by CAM treatment (Table 3), there are several possible mechanisms. Welch et al. (28) have reported that TGF-β has an activity that stimulates the invasiveness and metastatic potential of tumor cells, and Nakajima et al. (20) showed the close association between the production of MMP-9 and the metastatic potential of tumor cells. The 13762NF tumor we used in this study could produce a large amount of MMP-9, and CAM could inhibit the production of MMP-9 as well as TGF-β from this tumor. Besides the possibilities mentioned above, Sawaki et al. (22) have reported that CAM has an antiangiogenesis activity in vitro via the inhibition of IL-8 production. This activity of CAM is very interesting, but we have not yet studied this possibility.
It is of interest to note that CAM treatment reduced loss of body weight in TB rats (Fig. 1). TNF-α (4), IL-1, and IL-6 (12) have been reported as factors that induce cachexia. Expression of the TNF-α gene of tumor cells was shown to be inhibited by in vitro treatment with CAM dose dependently (Fig. 2), and expression of the IL-6 gene of spleen cells obtained from CAM-treated TB rats was found to be significantly lower than that of spleen cells from untreated TB rats (Fig. 3B). We suppose that inhibition of the production of cachexia-inducing factors may also contribute to some extent to the therapeutic effect of CAM treatment.
As Mikasa et al. (16) have already reported, CAM treatment was effective for non-small cell lung cancer. However, as they showed, CAM was not effective for small cell lung cancer. There is no agent that is effective for all types of cancer, so we should define for which type of cancer the macrolide is specifically effective. EM and CAM belong to 14-member ring macrolides, but there remains the question of the 15- or 16-member ring macrolides. More study will be required to define the applicability of macrolides as a BRM in cancer treatment.
ACKNOWLEDGMENT
We are grateful to Motowo Nakajima (Novartis Pharma Co. Ltd., Tokyo, Japan) for his kind advice.
REFERENCES
- 1.Anderson R. Erythromycin and roxithromycin potentiate human neutrophil locomotion in vitro by inhibition of leukoattractant-activated superoxide generation and autooxidation. J Infect Dis. 1989;159:966–973. doi: 10.1093/infdis/159.5.966. [DOI] [PubMed] [Google Scholar]
- 2.Bailly S, Pocidalo J-J, Fay M, Gougerot-Pocidalo M-A. Differential modulation of cytokine production by macrolides: interleukin-6 production is increased by spiramycin and erythromycin. Antimicrob Agents Chemother. 1991;35:2016–2019. doi: 10.1128/aac.35.10.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 4.Espat N J, Copeland E M, Moldawer L L. Tumor necrosis factor and cachexia: a current perspective. Surg Oncol. 1994;3:255–262. doi: 10.1016/0960-7404(94)90027-2. [DOI] [PubMed] [Google Scholar]
- 5.Finch R. Immunomodulatory effects of antimicrobial agents. J Antimicrob Chemother. 1980;6:691–699. doi: 10.1093/jac/6.6.691. [DOI] [PubMed] [Google Scholar]
- 6.Hamada K, Kit E, Sawaki M, Mikasa K, Narita N. Antitumor effect of erythromycin in mice. Chemotherapy. 1995;41:59–69. doi: 10.1159/000239325. [DOI] [PubMed] [Google Scholar]
- 7.Hauser W E, Remington J S. Effect of antibiotics on the immune response. Am J Med. 1982;72:683–697. doi: 10.1016/0002-9343(82)90534-4. [DOI] [PubMed] [Google Scholar]
- 8.Heussen C, Dowdle E B. Electrophoretic analysis of plasminogen activators in polyacrylamide gels containing sodium dodecyl sulfate and copolymerized substrates. Anal Biochem. 1980;102:196–202. doi: 10.1016/0003-2697(80)90338-3. [DOI] [PubMed] [Google Scholar]
- 9.Hirakata Y, Kaku M, Mizukane R, Ishida K, Furuya N, Matsumoto T, Tateda K, Yamaguchi K. Potential effects of erythromycin on host defense systems and virulence of Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1992;36:1922–1927. doi: 10.1128/aac.36.9.1922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9a.Ichikawa Y, Ninomiya H, Koga H, Tanaka M, Kinoshita M, Tokunaga N, Yan T, Oizumi K. Erythromycin reduces neutrophils and neutrophil-derived elastolytic-like activity in the lower respiratory tract of bronchiolitis patients. Am Rev Respir Dis. 1992;146:196–203. doi: 10.1164/ajrccm/146.1.196. [DOI] [PubMed] [Google Scholar]
- 10.Katahira J, Haruki K, Shibata Y, Kikuchi K, Hasegawa H, Totsuka K, Shimizu K, Kawada H, Takizawa T. Stimulation of interleukin-1 and tumor necrosis factor production by oral erythromycin. Chemotherapy. 1991;39:320–328. . (In Japanese.) [Google Scholar]
- 11.Keicho N, Kudoh S, Yatsumoto H, Akagawa K S. Erythromycin promotes monocyte to macrophage differentiation. J Antibiot. 1994;47:80–89. doi: 10.7164/antibiotics.47.80. [DOI] [PubMed] [Google Scholar]
- 12.Kishimoto T. The biology of interleukin 6. Blood. 1989;74:1–10. [PubMed] [Google Scholar]
- 13.Kita E, Sawaki M, Nishikawa F, Mikasa K, Yagyu Y, Takeuchi S, Yasui K, Narita N, Kashiba S. Enhanced interleukin production after long-term administration of erythromycin stearate. Pharmacology. 1990;41:177–183. doi: 10.1159/000138716. [DOI] [PubMed] [Google Scholar]
- 14.Kobayashi H. Airway biofilm disease: clinical manifestation and therapeutic possibilities using macrolides. J Infect Chemother. 1995;1:1–15. doi: 10.1016/s0002-9343(99)80282-4. [DOI] [PubMed] [Google Scholar]
- 15.Kudoh S, Uetake T, Hagiwara K, Hirayama M, Hus L H, Kimura H, Sugiyama Y. Clinical effect of low-dose long-term erythromycin chemotherapy on diffuse panbronchiolitis. Jpn J Thorac Dis. 1987;25:632–642. . (In Japanese.) [PubMed] [Google Scholar]
- 16.Mikasa K, Sawaki M, Kita E, Hamada K, Teramoto S, Sakamoto M, Maeda K, Konishi M, Narita N. Significant survival benefit to patients with advanced non-small-cell lung cancer from treatment with clarithromycin. Chemotherapy. 1997;43:288–296. doi: 10.1159/000239580. [DOI] [PubMed] [Google Scholar]
- 17.Miyatake H, Taki F, Taniguchi H, Suzuki R, Takagi K, Satake T. Erythromycin reduces the severity of bronchial hyperresponsiveness in asthma. Chest. 1991;99:670–673. doi: 10.1378/chest.99.3.670. [DOI] [PubMed] [Google Scholar]
- 18.Mizushima Y, Kashii T, Kobayashi M. Association between gene alteration and drug sensitivity in human lung carcinoma cell lines. Oncol Rep. 1995;2:277–280. doi: 10.3892/or.2.2.277. [DOI] [PubMed] [Google Scholar]
- 19.Morikawa K, Oseko F, Morikawa S, Iwamoto K. Immunomodulatory effects of three macrolides, midecamycin acetate, josamycin, and clarithromycin, on human T-lymphocyte function in vitro. Antimicrob Agents Chemother. 1994;38:2643–2647. doi: 10.1128/aac.38.11.2643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nakajima M, Welch D R, Wynn D M, Tsuruo T, Nicolson G L. Serum and plasma Mr 92,000 progelatinase levels correlate with spontaneous metastasis of rat 13762NF mammary adenocarcinoma. Cancer Res. 1993;53:5802–5807. [PubMed] [Google Scholar]
- 21.Oda H, Kadota J, Kohno S, Hara K. Erythromycin inhibits neutrophil chemotaxis in bronchoalveoli of diffuse panbronchiolitis. Chest. 1994;106:1116–1123. doi: 10.1378/chest.106.4.1116. [DOI] [PubMed] [Google Scholar]
- 22.Sawaki, M., E. Kita, and N. Narita. 1995. Clarithromycin as a potent anti-angiogenesis agent: possible application for antitumor agent. Can. J. Infect. Dis. 6(Suppl. C):213.
- 23.Sotomayor E A, Teicher B A, Schwartz G N, Holden S A, Menon K, Herman T S, Frei E., III Minocyclin in combination with chemotherapy or radiation therapy in vitro and in vivo. Cancer Chemother Pharmacol. 1992;30:377–384. doi: 10.1007/BF00689966. [DOI] [PubMed] [Google Scholar]
- 24.Sporn M B, Roberts A B, Wakefield L M, Assoian R K. Transforming growth factor-β: biological function and chemical structure. Science. 1986;233:532–534. doi: 10.1126/science.3487831. [DOI] [PubMed] [Google Scholar]
- 25.Sporn M B, Roberts A B, Wakefield L M, de Crombrugghe B. Some recent advances in the chemistry and biology of transforming growth factor-β. J Cell Biol. 1986;105:1039–1045. doi: 10.1083/jcb.105.3.1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Takeshita K, Yamagishi I, Harada M, Otomo S, Nakagawa T, Mizushima Y. Immunological and anti-inflammatory effects of clarithromycin: inhibition of interleukin 1 production of murine peritoneal macrophages. Drugs Exp Clin Res. 1898;15:527–533. [PubMed] [Google Scholar]
- 27.Umeki S. Anti-inflammatory action of erythromycin: its inhibitory effect on neutrophil NADPH oxidase activity. Chest. 1993;104:1191–1193. doi: 10.1378/chest.104.4.1191. [DOI] [PubMed] [Google Scholar]
- 28.Welch D R, Fabra A, Nakajima M. Transforming growth factor β stimulates mammary adenocarcinoma cell invasion and metastatic potential. Proc Natl Acad Sci USA. 1990;87:7678–7682. doi: 10.1073/pnas.87.19.7678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Winn H. Immune mechanisms in homotransplantation. II. Quantitative assay of the immunologic activity of lymphoid cells stimulated by tumor homografts. J Immunol. 1962;86:228–231. [PubMed] [Google Scholar]