Abstract
Background
Bone-modifying agent (BMA) therapy is recommended for metastatic castration-resistant prostate cancer but not metastatic castration-sensitive prostate cancer (mCSPC). BMA treatment in mCSPC may therefore constitute overuse.
Methods
In this retrospective cohort study using linked Surveillance, Epidemiology, and End Results–Medicare data, we included patients diagnosed with stage IV prostate adenocarcinoma from 2007 to 2015 who were 66 years of age or older at diagnosis and had received androgen-deprivation or antiandrogen therapy. We excluded patients who had previously received BMAs or had existing osteoporosis, osteopenia, hypercalcemia, or prior bone fracture. The primary outcome was receipt of BMA (zoledronic acid or denosumab) within 180 days of diagnosis (emergence of CRPC within this time frame is unlikely). The secondary outcome was receipt of a BMA within 90 days. Exposures of interest included practice location (physician office vs hospital outpatient) and the specialty (medical oncologist vs urologist) of the treating physician.
Results
Our sample included 2627 patients, of whom 52.9% were treated by medical oncologists and 47.1% by urologists; 77.7% and 22.3% received care in physician office and hospital outpatient locations, respectively. Overall, 23.6% received a BMA within 180 days; 18.4% did within 90 days. BMA therapy was more common among patients treated by oncologists (odds ratio = 8.23, 95% confidence interval = 6.41 to 10.57) and in physician office locations (odds ratio = 1.33, 95% confidence interval = 1.06 to 1.69). Utilization has increased: 17.3% of patients received BMAs from 2007 to 2009 (17.3% zoledronic acid, 0% denosumab) and 28.1% from 2012 to 2015 (8.4% zoledronic acid, 20.3% denosumab).
Conclusions
Among patients with mCSPC who had no evidence of high osteoporotic fracture risk, more than one-quarter have received BMAs in recent years. This overuse may lead to excess costs and toxicity.
Prostate cancer (PCa) is the most prevalent cancer among US men, causing more than 33 000 deaths annually (1). A leading cause of morbidity among patients with advanced PCa is skeletal-related events (SREs), which include pathologic fracture, severe bone pain that requires intervention, hypercalcemia, and spinal cord compression. Of men with PCa and bone metastasis, 30% to 50% will experience 1 or more SREs (2,3). SREs cause clinically significant loss of function and quality of life (4), and they are associated with increased mortality (4,5).
Bone-modifying agents (BMAs), including bisphosphonates and denosumab, are recommended for SRE prevention among patients with metastatic, castration-resistant PCa (mCRPC) with bone involvement (6). Bisphosphonate zoledronic acid and the RANKL inhibitor denosumab are effective in SRE prevention in PCa (7-9). Distinct from SRE prevention, BMAs are also recommended for men at high risk of osteoporotic fracture (6,10), which is especially relevant to patients with PCa because long-term androgen-deprivation therapy accelerates bone mineral density loss.
In contrast to mCRPC, there is evidence against the use of BMAs in metastatic, castration-sensitive prostate cancer (mCSPC). Two phase III randomized trials found no benefit in SRE reduction with the early initiation of zoledronic acid in the mCSPC setting (11,12). Denosumab has demonstrated efficacy only among patients with mCRPC (9) and has not been studied in mCSPC. BMAs can cause severe toxicities. Painful acute-phase reactions are common, and renal injury, hypocalcemia, and osteonecrosis of the jaw are well documented (13-17). BMAs are expensive to the health care system (18,19), as well—especially denosumab; a year of denosumab costs Medicare more than $27 000, compared with $212 for zoledronic acid (20). Given the absence of benefit and the potential for toxicity and excess costs, BMAs are not recommended for patients with PCa who do not have either mCRPC or high fracture risk (6,21).
The real-world use of BMAs among patients with mCSPC has not been previously described. Factors that may have affected BMA use in recent years include the approval of denosumab in 2010; the emergence of clinical data in 2013 that zoledronic acid does not prevent SREs in mCSPC (11,22); and financial incentives under “buy and bill,” in which reimbursement is proportional to drug price (23). These financial incentives favor BMA administration, especially denosumab: denosumab costs approximately $1700 per dose at market entry vs approximately $900 per dose for zoledronic acid (24), a difference that became much greater after the entry of generic zoledronic acid in 2014 (20).
We aimed to characterize BMA use among patients with mCSPC who had no evidence of increased fracture risk, which may represent overuse. We sought to understand temporal trends in the specific drugs used and associated provider factors. Factors of interest included physician specialty and treatment billing location. We hypothesized that medical oncologists’ specialized training would result in greater guideline-concordant BMA use: higher BMA use among patients with increased osteoporotic fracture risk and lower use among patients with mCSPC without that indication. We hypothesized greater use in physician office settings, which has been associated with greater responsiveness to reimbursement incentives (25,26).
Methods
Patient Population
We used linked Surveillance, Epidemiology, and End Results (SEER)–Medicare data. We included fee-for-service Medicare beneficiaries diagnosed with stage IV prostate adenocarcinoma from 2007 to 2015. Patients were eligible if PCa was their first cancer diagnosis or their second cancer diagnosis if their first had occurred 3 years or more prior. Patients were 66 years of age or older at diagnosis and not diagnosed at death. We required continuous enrollment in Medicare Parts A and B and not Medicare Advantage for at least 240 days before the month of diagnosis and 180 days after diagnosis. We required continuous enrollment in Medicare Part D from the month of diagnosis to 180 days after. We included patients with a first claim for a hormone therapy (HT) agent (androgen-deprivation therapy or an antiandrogen) occurring within 60 days before the month of diagnosis (allowing for the possibility that some patients may begin treatment when PCa is suspected but not yet confirmed) to 180 days after. We also excluded patients receiving bisphosphonate therapy before PCa diagnosis or who had a prior diagnosis of hypercalcemia, osteoporosis, osteopenia, or bone fracture (Supplementary Table 1,Supplementary Figure 1, available online).
In addition to the primary cohort described above, we evaluated BMA use among 2 other mCSPC cohorts. The first consisted of patients who had a prior diagnosis of osteoporosis, osteopenia, hypercalcemia, or bone fracture (elevated fracture risk [EFR] cohort) to assess BMA use among those who may have had an appropriate BMA indication. The second consisted of a subset of the primary cohort that met the additional requirement of having no claims for osteoporosis, osteopenia, any SRE-defining event, or dual-energy x-ray absorptiometry (DEXA) during the follow-up period (lowest fracture risk [LFR] cohort) to address the possibility that some patients might be appropriately diagnosed and treated for high osteoporotic fracture risk after PCa diagnosis has occurred (Supplementary Figure 1, available online).
To assess the intended use (SRE prevention or osteoporotic fracture prevention) of BMA administration, we measured denosumab dosing among patients who received that drug (120 mg per month is administered for SRE prevention vs 60 mg every 6 months for osteoporosis).
The Memorial Sloan Kettering Institutional Review Board found this research exempt under 45 CFR 46.104(d) (4).
Outcome Definition
The primary outcome was any claim for a BMA (zoledronic acid or denosumab) within 180 days of diagnosis. This time point was selected because the emergence, diagnosis, and treatment of mCRPC is unlikely to occur in fewer than 180 days, and patients can therefore be assumed to have mCSPC within this interval. Because a small number of patients may develop mCRPC in less than 180 days, we also assessed receipt of BMAs within 90 days as a secondary outcome. In the EFR cohort specifically, we also included other bisphosphonates (eg, alendronate) in the outcome because these drugs are appropriate for osteoporotic fracture prevention.
Patient Characteristics and Potential Confounders
We identified patient characteristics that may be associated with receipt of BMA therapy. These included age, calendar year of diagnosis, race and ethnicity, history of kidney disease, modified Charlson Comorbidity Index (27), frailty (28), and zip code–level median income (American Community Survey, 2008-2012).
We identified the treatment billing location (hospital outpatient vs physician office), defined by the location of the following: 1) the first billed claim for an HT agent (patients treated with a physician-administered drug) or 2) the first billed BMA claim (patients where BMA claims preceded any HT claims) or 3) the evaluation and management claim of the patient’s first postdiagnosis encounter with a physician specialty type of urology or medical oncology (patients for whom HT was a prescription drug) (25,29,30). The specialty of the treating physician was identified using evaluation and management claims and categorized as either “Urologist only” (≥1 visit with a urologist and none with a medical oncologist during the outcome period) vs “Oncologist involved” (≥1 visit with a medical oncologist during the outcome period).
Statistical Analysis
We calculated descriptive statistics for patient characteristics and outcomes of interest. To assess characteristics associated with BMA use, we performed multivariable logistic regression using the full cohorts. To assess factors associated with the use of denosumab specifically, we performed logistic regression on the subset of each cohort that received BMA therapy (either drug) and was diagnosed during the time period that both drugs were available (2010 onwards).
Estimates with 95% confidence intervals (CIs), excluding the null, were considered statistically significant. All analyses were conducted using SAS statistical software, version 9.4 (SAS Institute Inc, Cary, NC).
Results
The primary cohort comprised 2627 men diagnosed with stage IV PCa from 2007 to 2015 (Supplementary Figure 1, available online). Of these, 1390 (52.9%) had medical oncologists involved in their care, while 1237 (47.1%) saw only urologists (Supplementary Table 2, available online); 586 were treated in a hospital outpatient location and the remaining 2041 in a physician office location. Median age at diagnosis was 75 years, and 77.0% of patients were White. The EFR cohort comprised 1586 patients, and the LFR cohort comprised 2415 patients (Table 1).
Table 1.
Patient characteristicsa
| Characteristic | Primary cohort | EFR cohort | LFR cohort |
|---|---|---|---|
| (n = 2627) | (n = 1586) | (n = 2415) | |
| Age at diagnosis, y | |||
| Median (IQR) | 75 (71-81) | 78 (73-84) | 75 (71-81) |
| Mean (SD) | 76 (7) | 79 (7) | 76 (7) |
| Race, No. (%) | |||
| Black | 314 (12.0) | 142 (9.0) | 297 (12.3) |
| White | 2022 (77.0) | 1291 (81.4) | 1851 (76.7) |
| Other | 291 (11.1) | 153 (9.7) | 267 (11.1) |
| Year of diagnosis, No. (%) | |||
| Before 2010 | 756 (28.8) | 315 (19.9) | 714 (29.6) |
| 2010 or 2011 | 495 (18.8) | 263 (16.6) | 455 (18.8) |
| 2012 or later | 1376 (52.4) | 1008 (63.6) | 1246 (51.6) |
| No. of comorbid conditions, No. (%) | |||
| 0 | 1739 (66.2) | 810 (51.1) | 1597 (66.1) |
| 1 | 471 (17.9) | 332 (20.9) | 431 (17.9) |
| ≥2 | 417 (15.9) | 444 (28.0) | 387 (16.0) |
| Predicted probability of dependence in ADLb, % | |||
| Median (IQR) | 6.6 (4.2-12.1) | 10.2 (5.4-23.9) | 6.6 (4.1-12.2) |
| Mean (SD) | 11.5 (14.2) | 19.5 (22.4) | 11.5 (14.3) |
| Survival time, mo | |||
| Median (IQR) | 20 (11-35) | 18 (10-29) | 21 (11-36) |
| Mean (SD) | 26 (20) | 22 (17) | 27 (21) |
| History of renal disease, No. (%) | |||
| No | 2142 (81.5) | 1207 (76.1) | 1969 (81.5) |
| Yes | 485 (18.5) | 379 (23.9) | 446 (18.5) |
| Zip code median income, No. (%) | |||
| <$50 000 | 1018 (39.7) | 575 (37.2) | 945 (40.1) |
| $50 000-$54 999 | 245 (9.6) | 151 (9.8) | 223 (9.5) |
| $55 000-$59 999 | 200 (7.8) | 130 (8.4) | 180 (7.6) |
| $60 000-$69 999 | 333 (13.0) | 208 (13.5) | 309 (13.1) |
| $70 000 or more | 768 (30.0) | 480 (31.1) | 698 (29.6) |
| Unknown | 63 | 42 | 60 |
The EFR cohort comprised patients with a diagnosis of osteoporosis, osteopenia, or bone fracture before PCa diagnosis. The LFR cohort comprised the subset of the primary cohort that had no claims for osteoporosis, osteopenia, any SRE-defining event, or DEXA during the outcome period. ADL = activity of daily living; DEXA = dual-energy x-ray absorptiometry; EFR = elevated fracture risk; IQR = interquartile range; LFR = lowest fracture risk; PCa = prostate cancer; SD = standard deviation; SRE = skeletal-related event.
Faurot algorithm (28).
Across the entire study period, 23.6% of patients received BMAs within 180 days of diagnosis, relatively evenly split between denosumab and zoledronic acid (Table 2). Receipt of BMAs was higher in the EFR cohort (41%) and lower in the LFR cohort (17%). The proportion of patients who received BMAs within 90 days was slightly lower at 18.4% (Table 3).
Table 2.
Primary outcomea
| Characteristic | Primary cohort, No. (%) | EFR cohort, No. (%) | LFR cohort, No. (%) |
|---|---|---|---|
| Total No. | 2627 | 1586 | 2415 |
| Any BMA | 619 (23.6) | 643b (40.5) | 407 (16.9) |
| Denosumab | 306 (11.7) | 296 (18.7) | 212 (8.8) |
| Zoledronic acid | 325 (12.4) | 271 (171) | 203 (8.4) |
Receipt of BMAs within 180 days of diagnosis. Because some patients received both denosumab and zoledronic acid, the “Any BMA” total may be less than the sum of the 2 individual drugs. The EFR cohort comprised patients with a diagnosis of osteoporosis, osteopenia, or bone fracture before PCa diagnosis. The LFR cohort comprised the subset of the primary cohort that had no claims for osteoporosis, osteopenia, any SRE, or DEXA during the outcome period. BMA = bone modifying agent; DEXA = dual-energy x-ray absorptiometry; EFR = elevated fracture risk; LFR = lowest fracture risk; PCa = prostate cancer; SRE = skeletal-related event.
For the EFR cohort, “Any BMA” also includes 88 patients who received oral bisphosphonates because orally administered bisphosphonates are appropriate for osteoporotic fracture prevention, and this cohort contains patients with evidence of high osteoporotic fracture risk.
Table 3.
Receipt of bone-modifying agents within 90 days of diagnosis
| Characteristic | Primary cohort, No. (%) | EFR cohort, No. (%) | LFR cohort, No. (%) |
|---|---|---|---|
| Total No. | 2627 | 1586 | 2415 |
| Any BMA | 483 (18.4) | 538a (33.9) | 336 (13.9) |
| Denosumab | 242 (9.2) | 232 (14.6) | 171 (7.1) |
| Zoledronic acid | 246 (9.4) | 225 (14.2) | 167 (6.9) |
For the EFR cohort, “Any BMA” also includes 88 patients who received oral bisphosphonates because orally administered bisphosphonates are appropriate for osteoporotic fracture prevention, and this cohort contains patients with evidence of high osteoporotic fracture risk. BMA = bone-modifying agent; EFR = elevated fracture risk; LFR = lowest fracture risk.
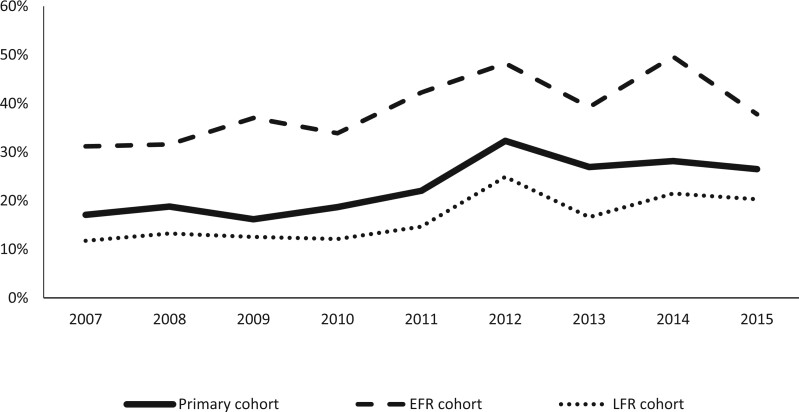
BMA use increased during the study period. Within the primary cohort, the proportion of patients receiving BMAs within 180 days was 17.3% during 2007-2009 and 28.1% during 2012-2015. The use of BMAs increased within the EFR and LFR cohorts, as well (Figure 1).
Figure 1.
Proportion of patients who received any bone-modifying agent within 180 days of diagnosis over time. The elevated fracture risk (EFR) cohort (n = 1586) comprised patients with a diagnosis of osteoporosis, osteopenia, or bone fracture before prostate cancer diagnosis. The lowest fracture risk (LFR) cohort (n = 2415) comprised the subset of the primary cohort that had no claims for osteoporosis, osteopenia, any skeletal-related event, or dual-energy x-ray absorptiometry during the outcome period.
On multivariable regression, the only factors statistically associated with receipt of BMAs were calendar year of diagnosis (2012 or later vs before 2010; odds ratio [OR] = 1.67, 95% CI = 1.31 to 2.13), treatment billing location (physician office vs hospital outpatient; OR = 1.33, 95% CI = 1.06 to 1.69), and physician specialty (oncologist involved vs urologist only; OR = 8.23, 95% CI = 6.41 to 10.57) (Table 4).
Table 4.
Factors associated with receipt of any bone-modifying agent within 180 days of diagnosisa
| Effect | Primary cohort | EFR cohort | LFR cohort |
|---|---|---|---|
| OR (95% CI) | OR (95% CI) | OR (95% CI) | |
| Location | |||
| Hospital outpatient location | [Referent] | [Referent] | [Referent] |
| Physician office location | 1.33 (1.06 to 1.69) | 1.34 (1.04 to 1.73) | 1.21 (0.93 to 1.58) |
| Year of diagnosis | |||
| Before 2010 | [Referent] | [Referent] | [Referent] |
| 2010-2011 | 1.24 (0.91 to 1.70) | 1.10 (0.75 to 1.59) | 1.12 (0.77 to 1.63) |
| 2012 or later | 1.67 (1.31 to 2.13) | 1.23 (0.91 to 1.65) | 1.64 (1.24 to 2.17) |
| Age, y | |||
| <80 | [Referent] | [Referent] | [Referent] |
| ≥80 | 1.04 (0.81 to 1.32) | 1.17 (0.92 to 1.48) | 1.24 (0.95 to 1.63) |
| Race | |||
| Black | 0.90 (0.64 to 1.27) | 0.71 (0.46 to 1.08) | 0.98 (0.66 to 1.44) |
| White | [Referent] | [Referent] | [Referent] |
| Other | 1.04 (0.75 to 1.44) | 1.24 (0.85 to 1.80) | 1.01 (0.70 to 1.48) |
| No. of comorbid conditions | |||
| None | [Referent] | [Referent] | [Referent] |
| 1 | 1.24 (0.95 to 1.60) | 1.05 (0.79 to 1.39) | 1.27 (0.94 to 1.71) |
| ≥2 | 1.01 (0.75 to 1.38) | 1.12 (0.83 to 1.52) | 1.12 (0.79 to 1.58) |
| History of renal failure (vs no history of renal failure) | 1.07 (0.81 to 1.40) | 0.85 (0.64 to 1.13) | 1.07 (0.78 to 1.46) |
| Physician specialty | |||
| Urologist only | [Referent] | [Referent] | [Referent] |
| Oncologist involved | 8.23 (6.41 to 10.57) | 5.53 (4.26 to 7.19) | 7.17 (5.37 to 9.59) |
| Likelihood of dependence in ADLsb | 0.83 (0.36 to 1.93) | 0.64 (0.35 to 1.14) | 0.66 (0.25 to 1.79) |
| Zip code median income | |||
| <$50 000 | [Referent] | [Referent] | [Referent] |
| $50 000-$54 999 | 1.26 (0.88 to 1.79) | 1.18 (0.80 to 1.76) | 1.30 (0.86 to 1.95) |
| $55 000-$59 999 | 0.97 (0.66 to 1.43) | 0.85 (0.55 to 1.31) | 0.90 (0.57 to 1.44) |
| $60 000-$69 999 | 1.12 (0.82 to 1.54) | 1.07 (0.75 to 1.51) | 1.22 (0.85 to 1.75) |
| ≥$70 000 | 1.00 (0.78 to 1.28) | 0.99 (0.76 to 1.30) | 1.00 (0.75 to 1.33) |
Logistic regression models were fit separately within each cohort. The EFR cohort comprised patients with a diagnosis of osteoporosis, osteopenia, or bone fracture before PCa diagnosis. The LFR cohort comprised the subset of the primary cohort that had no claims for osteoporosis, osteopenia, any SRE-defining event, or DEXA during the outcome period. ADL = activity of daily living; CI = confidence interval; DEXA = dual-energy x-ray absorptiometry; EFR = elevated fracture risk; LFR = lowest fracture risk; OR = odds ratio; PCa = prostate cancer; SRE = skeletal-related event.
Faurot algorithm (28).
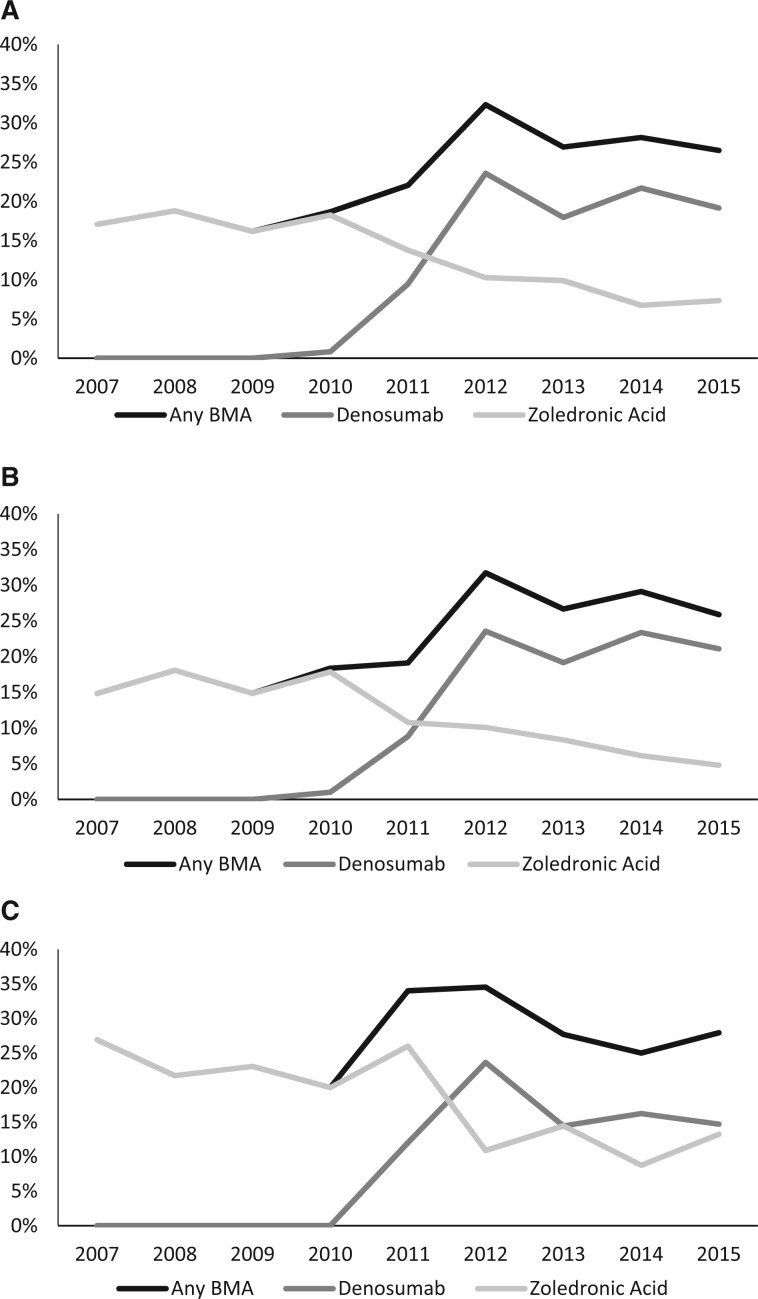
The relative use of the 2 BMA drugs shifted substantially across the study period. Following its approval in 2010, denosumab quickly increased to constitute a majority of BMAs by 2012, while zoledronic acid use declined (Figure 2). Denosumab use has increased from 0% in 2007-2009 to 20.3% of patients during 2012-2015, while zoledronic acid declined from 17.3% to 8.4% of patients.
Figure 2.
Receipt of any bone-modifying agent (BMA) within 180 days of diagnosis over time. Results for the overall primary cohort (N = 2827) (A), physician office setting (n = 2041) (B), and hospital outpatient setting (n = 586) (C) are shown.
Among the subset of patients who received BMAs, factors associated with receipt of denosumab were treatment within the physician office setting (vs hospital outpatient; OR = 2.68, 95% CI = 1.63 to 4.41), age 80 years or older (vs <80 years; OR = 3.09, 95% CI = 1.71 to 5.57), and treatment by urologist only (vs oncologist involved; OR = 2.81, 95% CI = 1.33 to 5.93) (Supplementary Table 3, available online). Within the primary cohort, 282 of 306 patients who received denosumab (92.2%) received the SRE-prevention dose of 120 mg (not shown).
Discussion
For metastatic PCa, National Comprehensive Cancer Network (NCCN) guidelines recommend BMA therapy for either castration-resistant disease or prevention of osteoporotic fractures in high-risk patients (6). Among patients with mCSPC and without a high risk of osteoporotic fracture, BMA therapy may constitute overuse. We found that 24% of patients in this population received BMA therapy. To our knowledge, this is the first study of BMA use patterns in mCSPC. Although a minority, this is a substantial portion of patients and, because PCa is common, reflects a large number of individuals who may be exposed to unnecessary treatment as well as the associated toxicity and financial cost.
These findings have direct implications for patient outcomes. High-quality clinical trials have found that patients with mCSPC do not benefit from BMA therapy (11,12). As the toxicities of BMAs are well established, it is possible that patients with mCSPC experience net harm from BMA therapy. BMA therapy may also result in avoidable financial consequences. Patients and caregivers may incur additional expenses for transportation and missed work for treatment (31,32), especially for denosumab, which is administered monthly (zoledronic acid can be administered every 3 months). The out-of-pocket cost for denosumab is substantial for Medicare beneficiaries who do not have supplemental insurance and can result in patients needing to seek financial assistance (33).
Our findings also have implications for health care system costs. Medicare reimbursement for zoledronic acid is $53 per infusion vs $2321 per denosumab injection (20). Given this price difference, denosumab is not cost-effective compared with zoledronic acid (19). Unnecessary costs therefore result from both BMA overuse and substitution of denosumab for zoledronic acid when BMA therapy is recommended. The observed increasing utilization of denosumab suggests avoidable spending without commensurate improvement in outcomes. Because PCa is common, the excess financial cost of BMA therapy to the health care system is likely to be substantial; further work is needed to estimate the magnitude of excess costs.
Disease characteristics were important determinants of BMA use. Patients with evidence of high osteoporotic fracture risk (EFR cohort) were more likely to receive BMAs. As BMA therapy would be guideline concordant within this population, higher utilization is appropriate. Although not all patients in the EFR cohort would have needed BMA therapy (eg, those with osteopenia and low Fracture Risk Assessment Tool [FRAX] score), our results suggest that BMAs may be underused for osteoporotic fracture prevention.
We did not find evidence that patient characteristics were associated with BMA use. The lack of association with renal disease is notable; zoledronic acid is more nephrotoxic than denosumab, providing a rationale to use denosumab over zoledronic acid among patients with this comorbidity. The absence of association suggests that a goal of minimizing nephrotoxicity is not an important factor in the increasing use of denosumab. These findings are consistent with observations from multiple myeloma, wherein denosumab use has increased even among patients for whom nephrotoxicity is not a concern (34).
In contrast, provider factors were important determinants of BMA use. Involvement of a medical oncologist was strongly associated with BMA use across all cohorts, which suggests differences among physician specialties with respect to their views of their own therapeutic role, with medical oncologists being more likely to initiate medical therapies such as BMAs. Potential provider-facing interventions aimed at better aligning BMA use with clinical practice guidelines may be more successful if focusing more on medical oncologists than other specialties.
Receiving treatment in the physician office location was associated with receipt of BMA therapy—specifically, denosumab. Higher denosumab use in the physician office could be related to ease of administration, lower availability of infusion chairs, or adherence to clinical pathways that promote its use. It could also reflect greater responsiveness to financial incentives, as the physician office setting has been associated with the delivery of other low-value but highly reimbursed cancer care services (25,26).
BMA therapy among patients with mCSPC increased during the study period. This increase was temporally correlated with the approval of denosumab in 2010. The dissemination of denosumab into clinical practice may therefore be a contributing factor to the overall increase, offsetting a concurrent decline in zoledronic acid use (Figure 2). Evidence that BMAs do not provide benefit in mCSPC from the Cancer and Leukemia Group B (CALGB) 90202 trial was first presented in February 2013 (22). Therefore, BMA therapy before this date (eg, 2007-2012 within our study period) might not be characterized as overuse unequivocally, although NCCN guidelines recommended against use across the entire study period (21). We did not observe a decline in BMA use following CALGB 90202. In principle, the negative results of CALGB 90202—which studied zoledronic acid only, not denosumab—may have contributed to a relative decline in zoledronic acid vs denosumab that we did observe; however, the “switch” toward denosumab occurred primarily during 2010-2012—before CALGB 90202—suggesting that this trial was unlikely to have been a factor.
Several factors may have contributed to the increase in denosumab use. The drug’s labeled indication includes all patients with metastatic PCa (14), even though guidelines recommend it only for mCRPC, the setting in which it has been evaluated (9). This discrepancy may create confusion among clinicians regarding whether denosumab is recommended for mCSPC, potentially resulting in inadvertent overuse. Additionally, a trial of denosumab found greater SRE reductions vs zoledronic acid in mCRPC; it is possible that providers are extrapolating this finding to mCSPC despite the absence of data for denosumab in this setting. From the practice perspective, subcutaneous administration of denosumab may consume less time and fewer resources than zoledronic acid, which requires an infusion chair for intravenous administration. Finally, denosumab use may be financially motivated, as the billing margin is substantially higher than for zoledronic acid. Such incentives contribute to the delivery of more expensive care across the oncology spectrum (35). Strategies to better align practice with patient value may include coverage policies that favor higher-value drugs (zoledronic acid, in this case) and payment models that decouple provider reimbursement from drug price.
This study has limitations as a consequence of the claims-based design. In addition to SRE prevention, oncology clinical practice guidelines recommend BMA therapy for patients with PCa who have osteoporosis or patients with osteopenia and high fracture risk according to the FRAX algorithm (6,36). If some patients are diagnosed clinically with osteoporosis or osteopenia without a billed claim for it, then they may have remained in our analytic cohort, resulting in an overestimate of BMA overuse (because BMA therapy would be appropriate for such patients), but this is unlikely to have meaningfully influenced our findings for several reasons. In deriving the primary cohort, when we removed patients with evidence of high fracture risk (those with osteoporosis, osteopenia, or prior fracture), 50% of the cohort was excluded; this is comparable to the estimated prevalence of low bone mineral density in the US population (41.8% in men aged 70-79 years, 53.1% in men aged ≥80 years) (37) and the proportion of men eligible for BMA therapy according to the National Osteoporosis Foundation guidelines (29.0% of men aged 70-79 years, 57.1% of men aged ≥80 years) (38). Additionally, we assessed the subset of patients who had no evidence of osteoporosis, osteopenia, an SRE-defining event, or DEXA bone density testing during the outcome period (LFR cohort); although this analysis should be interpreted with a high degree of caution because of the potential for reverse-causality, the relative similarity of BMA use between the LFR and primary cohorts suggests that newly discovered osteoporosis or osteopenia occurring after PCa diagnosis likely accounts for only a small portion of BMA use among patients with mCSPC. Finally, our assessment of dosing strength suggests that the majority of BMA therapy in this population is intended for SRE prevention rather than osteoporotic fracture prevention.
A separate limitation of claims is that castration sensitive vs resistant status is not available, and we aimed to study patients with mCSPC specifically. We addressed this limitation by applying an outcome period close enough to diagnosis that development of CRPC would be unlikely. A small fraction of patients may develop CRPC within this time frame, however, and appropriately receive BMA therapy in response. We assessed this possibility by measuring BMA use within 90 days, in which CRPC would be even more unlikely; the majority of BMA therapy began within 90 days, suggesting that in most cases the decision to administer BMAs was made soon after diagnosis and not in response to the early emergence of CRPC. In contrast, the 180-day outcome period may underestimate the proportion of patients who receive BMAs in the castration-sensitive setting because this approach would not identify patients who initiated BMA therapy later than 180 days but before castration resistance. Finally, the SEER–Medicare data set is limited to older adults residing in SEER regions and may not be generalizable to other populations.
Although BMAs are not recommended for patients with mCSPC, a substantial fraction receive them. The proportion of patients with mCSPC receiving BMAs increased following the 2010 approval of denosumab, while zoledronic acid use declined. Receipt of BMAs is associated with treatment in the physician office and by medical oncologists. Reducing BMA use within this population may reduce health care spending, patient out-of-pocket costs, and drug toxicity without adversely affecting patient outcomes.
Funding
This work was supported by the National Cancer Institute at the National Institutes of Health (grant numbers 1R03CA259863-01 to A.P.M, and P30CA008748).
Notes
Role of the funder: The funding source had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Disclosures: APM declares receipt of a research abstract award from the Conquer Cancer Foundation, which was partially funded by Merck. PB declares personal fees from Mercer, personal fees and nonfinancial support from United Rheumatology, personal fees from Foundation Medicine, personal fees from Grail, personal fees from Morgan Stanley, personal fees from New York State Rheumatology Society, personal fees and nonfinancial support from Oppenheimer & Co, personal fees from Cello Health, personal fees, nonfinancial support and other from Oncology Analytics, personal fees from Anthem, personal fees from Magellan Health, personal fees and nonfinancial support from Kaiser Permanente Institute for Health Policy, personal fees and nonfinancial support from the Congressional Budget Office, personal fees and nonfinancial support from America’s Health Insurance Plans, grants from Kaiser Permanente, grants from Arnold Ventures, personal fees and nonfinancial support from Geisinger, personal fees from EQRx, personal fees from Meyer Cancer Center of Weill Cornell Medicine, and personal fees from National Pharmaceutical Council. AM declares stock ownership in Johnson & Johnson and Teladoc Health. KP declares stock ownership in Viking Therapeutics; Fluidigm; T2 Biosystems; Adicet Bio, Inc; Chinook Therapeutics; Codexis; Dynavax; AzurRx Biopharma; Curis; Catalyst Biotech; and Vincerx Pharma. MM declares consulting and/or advisory arrangements with Bayer, Endocyte, Advanced Accelerator Applications, ORIC Pharmaceuticals, Johnson & Johnson, Curium Pharma, and Athenex; paid travel for Endocyte and Fujifilm; and research funding from Bayer, Sanofi, Endocyte, Progenics, Corcept Therapeutics, Roche/Genentech, and Janssen. AS has no potential conflicts of interest to disclose.
Author contributions: Dr Mitchell had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Conceptualization: APM, AS, PB, MM. Data curation: APM, AM, KP. Formal analysis: APM, AM, KP. Funding acquisition: APM. Investigation: APM, AM, KP. Methodology: APM, AS, AM, KP. Project administration: APM. Resources: APM, AM, PB. Software: AM. Supervision: APM, PB, MM. Validation: AM. Visualization: APM, AM. Writing—original draft: APM. Writing—review and editing: All authors. Final approval of manuscript: All authors.
Prior presentation: American Society of Clinical Oncology 2021 Genitourinary Cancers Symposium
Data Availability
The data underlying this article were provided by the Centers for Medicare & Medicaid Services (CMS) under license. Data are available from CMS on request and establishment of data use agreement.
Supplementary Material
References
- 1.Cancer stat facts: prostate cancer. National Cancer Institute Surveillance, Epidemiology, and End Results Program Web site. https://seer.cancer.gov/statfacts/html/prost.html. Accessed April 21, 2020.
- 2. Oster G, Lamerato L, Glass AG, et al. Natural history of skeletal-related events in patients with breast, lung, or prostate cancer and metastases to bone: a 15-year study in two large US health systems. Support Care Cancer . 2013;21(12):3279–3286. [DOI] [PubMed] [Google Scholar]
- 3. Baek YH, Jeon HL, Oh IS, Yang H, Park J, Shin JY.. Incidence of skeletal-related events in patients with breast or prostate cancer-induced bone metastasis or multiple myeloma: a 12-year longitudinal nationwide healthcare database study. Cancer Epidemiol. 2019;61:104–110. [DOI] [PubMed] [Google Scholar]
- 4. Broder MS, Gutierrez B, Cherepanov D, Linhares Y.. Burden of skeletal-related events in prostate cancer: unmet need in pain improvement. Support Care Cancer . 2015;23(1):237–247. [DOI] [PubMed] [Google Scholar]
- 5. Howard LE, De Hoedt AM, Aronson WJ, et al. Do skeletal-related events predict overall survival in men with metastatic castration-resistant prostate cancer? Prostate Cancer Prostatic Dis. 2016;19(4):380–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.NCCN Clinical Practice Guidelines in Oncology: prostate cancer, Version 4. National Comprehensive Cancer Network Web site. https://www.nccn.org/professionals/physician_gls/pdf/prostate.pdf. Published August 19, 2019. Accessed January 17, 2020.
- 7. Saad F, Gleason DM, Murray R, et al. ; Zoledronic Acid Prostate Cancer Study Group. Long-term efficacy of zoledronic acid for the prevention of skeletal complications in patients with metastatic hormone-refractory prostate cancer. J Natl Cancer Inst. 2004;96(11):879–882. [DOI] [PubMed] [Google Scholar]
- 8. Lipton A, Small E, Saad F, et al. The new bisphosphonate, Zometa (zoledronic acid), decreases skeletal complications in both osteolytic and osteoblastic lesions: a comparison to pamidronate. Cancer Invest. 2002;20(suppl 2):45–54. [DOI] [PubMed] [Google Scholar]
- 9. Fizazi K, Carducci M, Smith M, et al. Denosumab versus zoledronic acid for treatment of bone metastases in men with castration-resistant prostate cancer: a randomised, double-blind study. Lancet. 2011;377(9768):813–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gillessen S, Attard G, Beer TM, et al. Management of patients with advanced prostate cancer: the report of the Advanced Prostate Cancer Consensus Conference APCCC 2017. Eur Urol. 2018;73(2):178–211. [DOI] [PubMed] [Google Scholar]
- 11. Smith MR, Halabi S, Ryan CJ, et al. Randomized controlled trial of early zoledronic acid in men with castration-sensitive prostate cancer and bone metastases: results of CALGB 90202 (alliance). J Clin Oncol. 2014;32(11):1143–1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. James ND, Sydes MR, Clarke NW, et al. ; STAMPEDE Investigators. Addition of docetaxel, zoledronic acid, or both to first-line long-term hormone therapy in prostate cancer (STAMPEDE): survival results from an adaptive, multiarm, multistage, platform randomised controlled trial. Lancet. 2016;387(10024):1163–1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Saad F, Brown JE, Van Poznak C, et al. Incidence, risk factors, and outcomes of osteonecrosis of the jaw: integrated analysis from three blinded active-controlled phase III trials in cancer patients with bone metastases. Ann Oncol. 2012;23(5):1341–1347. [DOI] [PubMed] [Google Scholar]
- 14.Xgeva [prescribing information]. Thousand Oaks, CA: Amgen Inc; 2010. https://www.accessdata.fda.gov/drugsatfda_docs/label/2010/125320s007lbl.pdf. Accessed April 21, 2020.
- 15. Lipton A, Fizazi K, Stopeck AT, et al. Superiority of denosumab to zoledronic acid for prevention of skeletal-related events: a combined analysis of 3 pivotal, randomised, phase 3 trials. Eur J Cancer. 2012;48(16):3082–3092. [DOI] [PubMed] [Google Scholar]
- 16. Autio KA, Farooki A, Glezerman IG, et al. Severe hypocalcemia associated with denosumab in metastatic castration-resistant prostate cancer: risk factors and precautions for treating physicians. Clin Genitourin Cancer. 2015;13(4):e305–e309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Higuchi T, Soga Y, Muro M, et al. Replacing zoledronic acid with denosumab is a risk factor for developing osteonecrosis of the jaw. Oral Surg Oral Med Oral Pathol Oral Radiol. 2018;125(6):547–551. [DOI] [PubMed] [Google Scholar]
- 18. Reed SD, Radeva JI, Glendenning GA, Saad F, Schulman KA.. Cost-effectiveness of zoledronic acid for the prevention of skeletal complications in patients with prostate cancer. J Urol. 2004;171(4):1537–1542. [DOI] [PubMed] [Google Scholar]
- 19. Snedecor SJ, Carter JA, Kaura S, Botteman MF.. Denosumab versus zoledronic acid for treatment of bone metastases in men with castration-resistant prostate cancer: a cost-effectiveness analysis. J Med Econ. 2013;16(1):19–29. [DOI] [PubMed] [Google Scholar]
- 20.2020 ASP pricing files. Centers for Medicare & Medicaid Services Web site. https://www.cms.gov/medicare/medicare-part-b-drug-average-sales-price/2020-asp-drug-pricing-files. Updated January 1, 2020. Accessed March 3, 2021.
- 21. NCCN Clinical Practice Guidelines in Oncology: prostate cancer. Version 1. 2007. Published January 3, 2007.
- 22. Smith MR, Halabi S, Ryan CJ, et al. Efficacy and safety of zoledronic acid in men with castration-sensitive prostate cancer and bone metastases: results of CALGB 90202 (Alliance) [ASCO abstract 27]. J Clin Oncol. 2013;31(1_suppl):27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Polite BN, Ward JC, Cox JV, et al. Payment for oncolytics in the United States: a history of buy and bill and proposals for reform. J Oncol Pract. 2014;10(6):357–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.2012 ASP drug pricing file. Centers for Medicare & Medicaid Services Web site. https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Part-B-Drugs/McrPartBDrugAvgSalesPrice/2012ASPFiles. Published January 1, 2020. Accessed July 27, 2021.
- 25. Lipitz-Snyderman A, Atoria CL, Schleicher SM, Bach PB, Panageas KS.. Practice patterns for older adult patients with advanced cancer: physician office versus hospital outpatient setting. J Oncol Pract. 2019;15(1):e30–e38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mitchell AP, Kinlaw AC, Peacock-Hinton S, Dusetzina SB, Sanoff HK, Lund JL.. Use of high-cost cancer treatments in academic and nonacademic practice. Oncologist. 2019;25(1):46–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Klabunde CN, Potosky AL, Legler JM, Warren JL.. Development of a comorbidity index using physician claims data. J Clin Epidemiol. 2000;53(12):1258–1267. [DOI] [PubMed] [Google Scholar]
- 28. Faurot KR, Jonsson Funk M, Pate V, et al. Using claims data to predict dependency in activities of daily living as a proxy for frailty. Pharmacoepidemiol Drug Saf. 2015;24(1):59–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bach P, Jain R. Hospital outpatient versus doctor office cost for physician administered cancer drugs. Drug Pricing Lab Web site. https://www.drugpricinglab.org/wp-content/uploads/2017/01/Hospital-outpatient-versus-doctor-office-cost-for-physician-administered-cancer-drugs.pdf. Published January 4, 2017. Accessed October 14, 2021.
- 30. Fitch KV, Pyenson BS. Site of service cost differences for Medicare patients receiving chemotherapy. Milliman Web site. https://www.milliman.com/en/insight/cost-drivers-of-cancer-care-a-retrospective-analysis-of-medicare-and-commercially-insured. Published October 19, 2011. Accessed October 14, 2021.
- 31. Lee A, Shah K, Chino F.. Assessment of parking fees at National Cancer Institute-designated cancer treatment centers. JAMA Oncol. 2020;6(8):1295–1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bradley CJ. Economic burden associated with cancer caregiving. Semin Oncol Nurs. 2019;35(4):333–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Mitchell A, Muluneh B, Patel R, Basch E.. Pharmaceutical assistance programs for cancer patients in the era of orally administered chemotherapeutics. J Oncol Pharm Pract. 2017;24(6):424–432. [DOI] [PubMed] [Google Scholar]
- 34. Gupta A, Wang P, Ali SA, et al. Use of bone-modifying agents among Medicare beneficiaries with multiple myeloma. JAMA Oncol. 2019;6(2):296–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Mitchell AP, Rotter JS, Patel E, et al. Association between reimbursement incentives and physician practice in oncology: a systematic review. JAMA Oncol. 2019;5(6):893–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kanis JA, Oden A, Johansson H, Borgström F, Ström O, McCloskey E.. FRAX and its applications to clinical practice. Bone. 2009;44(5):734–743. [DOI] [PubMed] [Google Scholar]
- 37. Wright NC, Looker AC, Saag KG, et al. The recent prevalence of osteoporosis and low bone mass in the United States based on bone mineral density at the femoral neck or lumbar spine. J Bone Miner Res. 2014;29(11):2520–2526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Dawson-Hughes B, Looker AC, Tosteson ANA, Johansson H, Kanis JA, Melton LJ III.. The potential impact of new National Osteoporosis Foundation guidance on treatment patterns. Osteoporos Int. 2010;21(1):41–52. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article were provided by the Centers for Medicare & Medicaid Services (CMS) under license. Data are available from CMS on request and establishment of data use agreement.