Abstract
Because of the coronavirus pandemic, hydroalcoholic gels have become essential products to prevent the spread of COVID-19. This research aims to develop a simple, fast and sustainable microextraction methodology followed by gas chromatography tandem mass spectrometry (GC-MS/MS) to analyze simultaneously 60 personal care products (PCPs) including fragrances allergens, synthetic musks, preservatives and plasticizers in hand sanitizers. Micro-matrix-solid-phase dispersion (μMSPD) and solid-phase microextraction (SPME) were compared with the aim of obtaining high sensitivity and sample throughput. SPME demonstrated higher efficiency being selected as sample treatment. Different dilutions of the sample in ultrapure water were assessed to achieve high sensitivity but, at the same time, to avoid or minimize matrix effect. The most critical parameters affecting SPME (fibre coating, extraction mode and temperature) were optimized by design of experiments (DOE). The method was successfully validated in terms of linearity, precision and accuracy, obtaining recovery values between 80 and 112% for most compounds with relative standard deviation (RSD) values lower than 10%. External calibration using standards prepared in ultrapure water demonstrated suitability due to the absence of matrix effect. Finally, the simple, fast and high throughput method was applied to the analysis of real hydroalcoholic gel samples. Among the 60 target compounds, 39 of them were found, highlighting the high number of fragrance allergens, at concentrations ranging between 0.01 and 217 μg g−1. Most of the samples were not correctly labelled attending cosmetic Regulation (EU) No 1223/2009, and none of them followed the World Health Organization (WHO) recommendation for hand sanitizers formulation.
Keywords: Hydroalcoholic gels, Personal care products, Solid-phase microextraction, Gas chromatography-tandem mass spectrometry, Experimental design, Fragrance allergens
Graphical abstract

1. Introduction
Hydroalcoholic gels have become essential products, being one of the basic tools to prevent and mitigate transmission of COVID-19 [1,2]. The World Health Organization (WHO) published a protocol to homogenise the hydroalcoholic gel formulation and fabrication, assuring their antimicrobial properties. In this context two aqueous formulations were established, containing: (i) ethanol, hydrogen peroxide, and glycerol and (ii) isopropyl alcohol, hydrogen peroxide, and glycerol [3]. This protocol strongly recommended that no ingredients other than those specified above be added and especially fragrances because of the risk of allergic reactions. Attending the classification of these daily consumer products, if the main purpose of the hydroalcoholic gel is cleaning or cleansing the skin they are considered cosmetics by the EU [4].
The world increasing consumer's demand for cosmetics and personal daily care products imply rigorous controls to assure their safety. All cosmetics products marketed on the European Union must comply with the Regulation (EU) No 1223/2009 [4] and this compliance must be analytically verifiable. Fragrances, synthetic musks, preservatives, antioxidants, or plasticizers are among the compounds more frequently found in cosmetic formulations [[5], [6], [7]].
Cosmetics analysis is a challenge task due to the complexity of the samples formed by a high number of chemical substances, from highly lipophilic to moderately polar, exhibiting basic, acidic, or neutral properties, in a wide range of concentrations from trace levels to thousands of μg g−1. For this reason, a previous sample pre-treatment before analytical determination is required. Solid-liquid extraction (SLE) and liquid–liquid extraction (LLE), have been the most employed procedures for cosmetics analysis. However, multiple extraction steps and considerable organic solvent volumes are often required to obtain an optimum extraction yield. Other drawback is that further steps such as centrifugation, concentration, evaporation and reconstitution or solid-phase extraction (SPE) clean-up are required after extraction [6,8,9]. The analytical determination that is usually accomplished by gas chromatography (GC) or liquid chromatography (LC), depending on the chemical nature of the target analytes. The combination with mass spectrometry (MS) or tandem mass spectrometry (MS/MS) detection became the most suitable option, improving analytical selectivity and sensitivity [9,10]. In last years, green analytical chemistry (GAC) principles have been increasingly implemented for cosmetics analysis through the miniaturization of classical extraction procedures, as well as the substitution of hazardous chemicals and solvents by environmentally friendly alternatives, with the main objective of improving the environmental friendliness without compromising method performance [11,12]. In this way, the use of ultrasound-assisted extraction (UAE), pressurized liquid extraction (PLE) or micro-matrix solid-phase dispersion (μMSPD) has been successfully proposed [8,9]. However, most of the methods have been focused on the determination of individual compounds or a small number of compounds belonging to the same family [6,8,[13], [14], [15]] and only few of them, mainly based on μMSPD, include multianalyte determination [16,17].
Solid-phase microextraction (SPME) is a well-established green solvent-free extraction technique with a large number of applications in different fields such as food, forensic, biomedical, and the environment [18]. The combination of SPME-GC-MS results in a valuable analytical tool. Despite this, a low number of applications for cosmetics analysis are reported, being all of them focused on the determination of few compounds from the same families. SPME has been applied to determine allowed ingredients such as preservatives or fragrances, as well as forbidden substances such as nitrosamines or formaldehyde in cosmetics [[19], [20], [21], [22], [23]]. However, to the best of our knowledge, it has never been applied to simultaneously determine multianalytes from different families in cosmetics.
The main goal of this work is the development of a simple, green, miniaturized, high throughput and easy to implement in worldwide laboratories methodology based on SPME-GC-MS/MS to simultaneously determine a high number of compounds including fragrance allergens (23), synthetic musks (11), preservatives (10) and plasticizers (16) in hydroalcoholic gels. The control of these products is essential since they are massively employed several times every day for many people all around the world. The main experimental parameters affecting SPME have been optimized by experiments design to obtain the high extraction efficiency for the 60 target analytes. Finally, the validated SPME-GC-MS/MS methodology was applied to real hand sanitizers samples demonstrating its suitability. This methodology can be easily implemented in any routine laboratory and automated using a SPME autosampler. Target compounds were quantified in the real samples and the compliance with the applicable legislation, as well as WHO recommendations were discussed.
2. Materials and methods
2.1. Chemicals, reagents and materials
The 60 target compounds (23 fragrance allergens, 11 synthetic musks, 10 preservatives and 16 plasticizers), their CAS number, the retention time, the molecular mass and the MS/MS transitions are depicted in Table S1. Methanol, ultrapure water MS grade and ethyl acetate were supplied by Scharlab (Barcelona, Spain) and acetone by Sigma Aldrich Chemie GmbH (Steinheim, Germany). Individual stock solutions were prepared in methanol and further dilutions and mixtures in acetone (spike solutions). All solutions were stored in amber glass vials and protected from light at −20 °C. All solvents and reagents were of analytical grade.
Commercial 65 μm polydimethylsiloxane/divinylbenzene (PDMS/DVB), 50/30 μm divinylbenzene/carboxen/polydimethylsiloxane (DVB/CAR/PDMS) and 85 μm polyacrylate (PA) fibres and manual SPME holders were obtained from Supelco (Bellefonte, PA, USA). Prior the first use, the fibres were conditioned as recommended by the manufacturer, inserting them in the GC injector under helium flow at 250 °C (PDMS/DVB), 270 °C (DVB/CAR/PDMS) and 280 °C (PA) for 30 min.
2.2. Samples
Hydroalcoholic gel (hand sanitizer) samples for personal use were bought in local stores or collected from different buildings (restaurant, bank, pharmacy, university, local markets) in Galicia (Northwest Spain). Samples were kept in 15 mL glass tubes at room temperature until analysis. The analyzed samples are included in Table S2 showing the composition indicated on the label.
2.3. SPME procedure
Under the optimized experimental conditions (see Results and discussion), 10 mg of real hydroalcoholic gel were placed in a 22 mL glass vial and diluted in 10 mL of ultrapure water (1:1000 w/v). Vials were sealed with aluminium caps furnished with PTFE-faced septa and immersed in a water bath maintained at 100 °C. After 5 min of sample equilibration, the SPME fibre (DVB/CAR/PDMS) was exposed for 20 min to the headspace over the sample (HS-SPME). The samples were magnetically stirred, employing a steel nail, during the extraction. Afterwards, the fibre was retracted into the needle of the holder SPME fibre, transferred to the GC injection port, and desorbed for 5 min at 270 °C.
Water standards of ultrapure water were prepared by adding the target compounds to give final concentrations between 0.01 and 5 μg L−1. The standards (10 mL) were analyzed following the SPME procedure previously indicated.
Since one of the studied families of compounds are plasticizers, which are ubiquitously used, plastic material was replaced by metallic and glass material to avoid possible contamination during the experimental procedure and overestimation in the results. Besides, all material was baked at 230 °C before use.
In order to avoid false-positive findings, fibre blanks and procedure blanks employing 10 mL of ultrapure water were carried out every day. In addition, water standards at different concentrations were also daily analyzed to check the instrumental performance.
All the real samples were analyzed mixing 10 mg of sample with 10 mL of water (1:1000, w/v dilution). Due to the presence of the target compounds in a broad range of concentrations, with concentrations above the high level of the calibration curve in some cases, some of the samples were reanalyzed applying a higher dilution factor. In this way, the analyzed samples (1:1000, w/v), were consequently diluted by a factor between 10 and 1000 depending on the concentration of the compounds.
2.4. GC-MS/MS analysis
GC-MS/MS analysis was performed employing a Thermo Scientific Trace 1310 gas chromatograph coupled to a triple quadrupole mass spectrometer (TSQ 8000) with an autosampler IL 1310 from Thermo Scientific (San Jose, CA, USA). Instrumental GC-MS/MS conditions were previously optimized by the authors [24]. Separation was carried out on a Zebron ZB-Semivolatiles (30 m × 0.25 mm i.d. × 0.25 μm film thickness) obtained from Phenomenex (Torrance, CA, USA). Helium (purity 99.999%) was used as carrier gas at a constant flow of 1 mL min−1. The GC oven temperature was programmed from 60 °C (held 1 min), to 100 °C at 8 °C min−1, to 150 °C at 20 °C min−1, to 200 °C at 25 °C min−1 (held 5 min), to 220 °C at 8 °C min−1 and finally to 290 °C at 30 °C min−1 (held 7 min). The total run time was 30 min. The injector temperature was set at 270 °C working in pulsed split/splitless mode (200 kPa, held 1.2 min).
The mass spectrometer detector (MSD) was operated in the electron impact (EI) ionization positive mode (+70 eV). The temperatures of the transfer line, and the ion source were set at 290 and 350 °C, respectively. The filament was set at 25 μA and the multiplier voltage was 1950 V. Selected reaction monitoring (SRM) acquisition mode was used, monitoring 2 or 3 transitions per compound (see Table S1). The system was operated by Xcalibur 2.2, and Trace Finder™ 3.2 software.
2.5. Statistical analysis
Basic and descriptive statistical analysis were performed using the software package Statgraphics Centurion XVIII (Manugistics, Rockville, MD; USA).
3. Results and discussion
Sixty compounds were selected considering previous screening studies and our own experience in cosmetic analysis (see Table S1). The studied substances included fragrances allergens, synthetic musks, preservatives and plasticizers. The chromatographic conditions are described in section 2.4. The chromatographic run time was 30 min, offering a high throughput and a good separation of all target compounds.
3.1. Preliminary experiments
3.1.1. Selection of the extraction technique
One of the most critical parameters to isolate the target compounds with high sensitivity, high throughput and efficiency is the selection of the extraction technique. The purpose of this work was to develop a miniaturized and simple procedure in consonance with the green chemistry that allowed the use of a small amount of sample and low waste generation. In this way, micro-matrix solid-phase dispersion (μMSPD) and solid-phase microextraction (SPME) were initially selected. The μMSPD procedure was previously optimized by Celeiro et al. [17] to determine several cosmetics ingredients in personal care and cosmetics products. Briefly, 0.1 g of sample were blended with 0.4 g of Florisil as dispersing agent and 0.4 g of Na2SO4 to remove the moisture of the samples. The resulted mixture was transferred to a glass Pasteur pipette (approximately 150 mm length) containing glass wool and Florisil at the bottom. Then, a small amount of glass wool was placed at the top to compress the mixture and elution was performed by gravity flow using ethyl acetate, collecting 1 mL of extract that was filtered through 0.22 μm PTFE filters and directly analyzed (dilution 1:10 from the sample, w/v) by GC-MS/MS analysis.
Regarding to the SPME procedure, 10 mg of sample were diluted in 10 mL of ultrapure water (dilution 1:1000, w/v) and extracted in the headspace mode at 100 °C using a PDMS/DVB fibre. The conditioning and extraction time were kept constant at 5 and 20 min, respectively, to get maximum analyte response and maximum throughput considering the chromatographic run time (30 min).
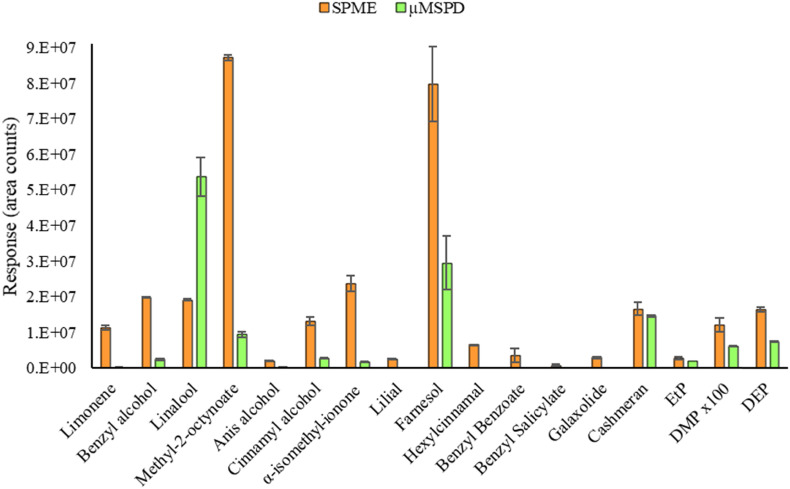
Fig. 1 compares the responses obtained using both techniques for a real non-spiked hydroalcoholic gel (G6 in Table 3 ) containing 17 of the target compounds including ingredients from different cosmetic families. As can be seen, chromatographic responses (area counts) obtained with SPME were much higher (between 2 and 45 times, excluding linalool) than those obtained with μMSPD. Even several compounds such as hexylcinnamal, benzyl benzoate and benzyl salicylate were not detected employing μMSPD. Therefore, SPME allowed higher sensitivity. In addition, it is more environmentally friendly since the use of organic solvents as well as the use of other additional materials (e.g. Florisil and Na2SO4 required for each μMSPD extraction) is avoided.
Fig. 1.
Comparison of the chromatographic responses obtained by SPME and μMSPD in a real non-spiked hydroalcoholic gel sample containing 17 target compounds (responses for DMP were multiplied by 100).
Table 3.
Concentration (μg g−1) of the target compounds in the analyzed real hydroalcoholic gel samples.
| Compounds | G1 | G2 | G3 | G4 | G5 | G6 | G7 | G8 | G9 | G10 |
|---|---|---|---|---|---|---|---|---|---|---|
| Fragrance allergens | ||||||||||
| Pinene | 0.0220 ± 0.0014 | 0.899 ± 0.014 | 0.195 ± 0.013 | 0.048 ± 0.014 | 1.04 ± 0.25 | |||||
| Limonene | 1.29 ± 0.10 | 0.117 ± 0.010 | 3.07 ± 0.73 | 6.80 ± 0.54 | 0.665 ± 0.037 | 12.4 ± 1.2 | 4.4 ± 1.7 | 29.2 ± 9.0 | 0.77 ± 0.10 | |
| Benzyl Alcohol | 2.36 ± 0.23 | 0.1714 ± 0.0027 | 10.23 ± 0.87 | 0.671 ± 0.010 | 11.76 ± 0.78 | 4.5 ± 1.6 | 28.2 ± 7.0 | 0.93 ± 0.10 | ||
| Linalool | 0.182 ± 0.068 | 143.9 ± 5.2 | 1.669 ± 0.011 | 15.5 ± 1.4 | 1.459 ± 0.016 | 1.750 ± 0.084 | 15 ± 4 | 36.23 ± 0.40 | ||
| Methyl-2-octynoate | 0.148 ± 0.053 | 0.279 ± 0.043 | 0.414 ± 0.019 | 2.19 ± 0.57 | 0.650 ± 0.011 | 2.581 ± 0.026 | 1.378 ± 0.010 | 4.1 ± 1.2 | 2.967 ± 0.047 | |
| Citronellol | 2.28 ± 0.61 | 0.1796 ± 0.0011 | 0.122 ± 0.029 | 53.5 ± 2.6 | 7.62 ± 0.96 | 6.85 ± 0.13 | 29.9 ± 0.50 | |||
| Citral | 0.45 ± 0.13 | 2.79 ± 0.43 | 0.210 ± 0.010 | 98.7 ± 2.0 | 36.3 ± 4.1 | 3.85 ± 0.41 | 25.9 ± 7.3 | 2.94 ± 0.11 | ||
| Geraniol | 6.7 ± 1.5 | 3.24 ± 0.37 | 172 ± 12 | 41.6 ± 4.6 | 3.67 ± 0.27 | 217 ± 44 | 26.7 ± 1.2 | |||
| Cinnamaldehyde | 59 ± 14 | 0.2271 ± 0.0012 | 0.173 ± 0.021 | 0.140 ± 0.011 | 5.56 ± 0.50 | |||||
| Anis alcohol | 11 ± 1.0 | |||||||||
| Cinnamyl alcohol | 71 ± 21 | 0.99 ± 0.33 | 159 ± 14 | 2.55 ± 0.12 | ||||||
| Eugenol | 26.0 ± 3.2 | 0.194 ± 0.021 | 5.8 ± 1.7 | 0.634 ± 0.084 | ||||||
| Methyleugenol | 4.73 ± 0.51 | |||||||||
| Isoeugenol | 2.16 ± 0.49 | 2.347 ± 0.050 | 1.172 ± 0.041 | 3.56 ± 0.82 | 1.437 ± 0.086 | |||||
| α-isomethylionone | 2.46 ± 0.59 | 0.027 ± 0.010 | 0.441 ± 0.070 | 0.89 ± 0.10 | 0.0453 ± 0.0041 | 2.24 ± 0.28 | 1.74 ± 0.31 | 9.957 ± 0.048 | ||
| Lilial® | 0.166 ± 0.049 | 0.0109 ± 0.0012 | 0.458 ± 0.020 | 4.910 ± 0.026 | 6.05 ± 0.37 | 7.123 ± 0.016 | ||||
| Amylcinnamyl alcohol | 0.89 ± 0.27 | 11.4 ± 1.0 | 0.539 ± 0.095 | |||||||
| Farnesol | 8.9 ± 2.4 | 0.0613 ± 0.0081 | 2.782 ± 0.023 | 10.85 ± 0.34 | ||||||
| Hexylcinnamal | 0.125 ± 0.010 | 1.15 ± 0.11 | 1.034 ± 0.092 | |||||||
| Benzyl benzoate | 17.8 ± 5.4 | 0.45 ± 0.11 | 0.308 ± 0.016 | 0.75 ± 0.22 | 0.194 ± 0.010 | 0.233 ± 0.039 | 0.685 ± 0.054 | 22.0 ± 5.3 | 9.91 ± 0.51 | |
| Benzyl salicylate | 0.91 ± 0.25 | 0.044 ± 0.010 | 0.0346 ± 0.0028 | 0.0773 ± 0.0017 | 4.0 ± 1.0 | 5.04 ± 0.88 | ||||
| Benzyl cinnamate | 0.33 ± 0.10 | 0.0201 ± 0.0034 | 0.0192 ± 0.0013 | |||||||
| Synthetic musks | ||||||||||
| Galaxolide | 16.0 ± 4.4 | 0.380 ± 0.060 | 0.129 ± 0.016 | 0.137 ± 0.011 | 32.8 ± 4.1 | 32.4 ± 5.2 | ||||
| Cashmeran | 0.164 ± 0.010 | 0.254 ± 0.055 | 19.1 ± 2.8 | 0.561 ± 0.070 | 0.173 ± 0.020 | 0.131 ± 0.026 | 0.230 ± 0.028 | |||
| Traseolide | 1.82 ± 0.38 | |||||||||
| Ambrettolide | 4.0 ± 1.0 | |||||||||
| Preservatives | ||||||||||
| PhEtOH | 72.49 ± 0.48 | |||||||||
| BHT | 0.298 ± 0.010 | 0.0121 ± 0.0014 | 0.0099 ± 0.0010 | 0.052 ± 0.010 | ||||||
| TCS | 0.32 ± 0.10 | |||||||||
| MeP | 23 ± 3.6 | 2.58 ± 0.27 | 2.54 ± 0.82 | 1.194 ± 0.011 | ||||||
| EtP | 50 ± 11 | 29.6 ± 3.6 | 7.26 ± 0.90 | 1.06 ± 0.10 | 1.81 ± 0.18 | 2.36 ± 0.45 | ||||
| PrP | 3.921 ± 0.024 | 150 ± 13 | ||||||||
| iBuP | 1.23 ± 0.34 | 61.2 ± 1.8 | ||||||||
| Plasticizers | ||||||||||
| DMP | 0.428 ± 0.056 | 0.590 ± 0.010 | 9.6 ± 1.9 | |||||||
| DEP | 1.709 ± 0.075 | 0.2305 ± 0.0034 | 25.48 ± 0.90 | 3.25 ± 0.23 | 0.191 ± 0.025 | 104.2 ± 6.9 | 1.87 ± 0.21 | |||
| DEHP | 0.43 ± 0.13 | 0.128 ± 0.034 | 0.155 ± 0.021 | 0.1145 ± 0.0041 | ||||||
| DMA | 0.221 ± 0.010 | 0.107 ± 0.010 | 0.228 ± 0.040 | 4.07 ± 0.13 | ||||||
| DEA | 1.08 ± 0.32 | |||||||||
3.1.2. Sample dilution factor
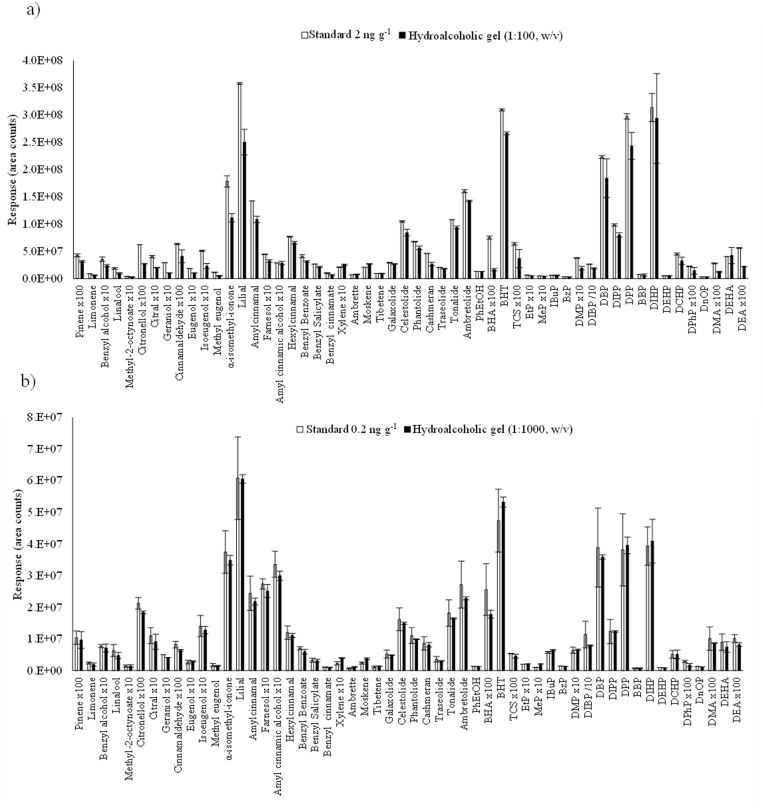
Once SPME was selected as extraction technique, the dilution factor was assessed with the aim of achieving the maximum sensitivity and, at the same time, minimizing or avoiding matrix effect, which could permit quantification by external calibration using standards prepared in ultrapure water. Dilution ratios of 1:100 and 1:1000 (w/v) were evaluated employing 100 or 10 mg, respectively, of a real ‘blank’ sample (it only contained one of the target analytes, DEP) spiked with the target compounds at 0.2 μg g−1 under the same conditions indicated above (section 3.1.1). In Fig. 2 the responses obtained for a) dilution factor of 1:100 (black bars) and its corresponding water standard (2 ng mL−1, white bars) and b) dilution factor of 1:1000 (black bars) and the water standard (0.2 ng mL−1, white bars) are shown.
Fig. 2.
Comparison of the chromatographic responses obtained for ultrapure water standards and spiked hydroalcoholic gel employing different sample dilutions (w/v): a) 1:100 and b) 1:1000 (to easy visualize the results, responses for some compounds were multiplied or divided by a factor).
As could be expected, the dilution factor 1:100 (Fig. 2a) offered higher responses than 1:1000 (Fig. 2b) although in any case the response was 10 times higher (only 2 to 6 times, depending on the compound). Besides, it appeared to be a possible matrix effect for the 1:100 ratio, as the signals were lower than their corresponding in water (see Fig. 2a). In contrast, negligible or no matrix effect was observed for the dilution factor 1:1000 as the responses obtained for the water standard and the cosmetic sample were equivalent (Fig. 2b). Therefore, the dilution ratio of 1:1000 was selected for further experiments, since it could allow the application of external calibration using water standards, making much easier real cosmetic sample quantification using calibration curves prepared with ultrapure water. In addition, it allows working in direct immersion (DI) mode avoiding damages and contaminations in the fibre coating, due to the high dilution of the cosmetic sample.
3.2. Optimization by experimental design
An experimental design approach to simultaneously assess the influence of three parameters affecting the SPME procedure was conducted. Considering preliminary studies, the sample amount (10 mg), dilution factor (1:1000, w/v), and the conditioning (5 min) and extraction time (20 min) were kept fixed. Three, fibre coatings (factor A) were assessed PDMS/DVB, PA and DVB/CAR/PDMS. They were selected based on previous works reported in the literature for the extraction of fragrance allergens and preservatives from cosmetics, and depending on the polarity of the target analytes. In this way, coatings from highly polar (PA) to intermediate polar (PDMS/DVB, DVB/PDMS/CAR) were selected avoiding the use of a highly non-polar coating such as PDMS. The other studied factors in the experimental design were the extraction mode (factor B) at two levels (HS-SPME and DI-SPME), and the extraction temperature (factor C) also at two levels (50 °C and 100 °C). Hence, a multifactorial categorical design (3·22) was carried out evaluating the main factors and their interactions (second order factors).
The analysis of variance (ANOVA) describes the influence of the studied factors on the obtained responses. The F-ratio represents the contribution of each factor and interaction on the variance of the response, and the p-values the statistical significance. Factors or interactions with p-values < 0.05 denote statistically significance at the 95% confidence level. As can be seen in Table 1, the temperature (Factor C) was the most relevant factor being significant for 21 of the 60 target compounds, including 4 fragrance allergens (eugenol, amylcinnamaldehyde, farnesol, benzyl benzoate), 9 synthetic musks (xylene, ambrette, moskene, tibetene, galaxolide, celestolide, phantolide, traseolide, tonalide), 1 preservative (TCS) and 7 plasticizers (DiBP, DBP, DIPP, BBP, DCHP, DPhP, DnOP). The fibre coating (Factor A) was significant for 13 compounds of which 4 were fragrance allergens (linalool, cinnamaldehyde, eugenol, farnesol), 1 synthetic musk (ambrette), 3 preservatives (TCS, MeP, iBuP) and 5 plasticizers (BBP, DCHP, DPhP, DnOP, DMA). Finally, the extraction mode (Factor B) was significant for 14 compounds, including 4 fragrance allergens (eugenol, amylcinnamyl alcohol, benzyl benzoate, benzyl cinnamate), 5 plasticizers (DEP, DBP, BBP, DCHP, DPhP) and 5 preservatives (BHA, BHT, TCS, MeP, iBuP), while it was not statistically significant for any synthetic musk.
Table 1.
ANOVA table and optimal condition for all target compounds. Values in bold denote statistical significance (p-value < 0.05).
| Compound | Fibre coating (A) |
Extraction mode (B) |
Temperature (C) |
AB |
AC |
BC |
Optimum conditions | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| F | P | F | P | F | P | F | P | F | P | F | P | ||
| Fragrance allergens | |||||||||||||
| Pinene | 3.08 | 0.25 | 5.55 | 0.14 | 6.79 | 0.12 | 1.40 | 0.42 | 2.02 | 0.33 | 4.02 | 0.18 | DVB/CAR/PDMS, HS-SPME, 50 °C |
| Limonene | 6.63 | 0.13 | 5.67 | 0.14 | 7.10 | 0.12 | 1.59 | 0.39 | 1.83 | 0.35 | 1.17 | 0.39 | DVB/CAR/PDMS, HS-SPME, 50 °C |
| Benzyl alcohol | 5.82 | 0.15 | 6.33 | 0.13 | 6.35 | 0.13 | 1.65 | 0.38 | 1.86 | 0.35 | 0.48 | 0.56 | DVB/CAR/PDMS, HS-SPME, 50 °C |
| Linalool | 25.1 | 0.04 | 10.1 | 0.09 | 18.0 | 0.05 | 3.76 | 0.21 | 7.39 | 0.12 | 0.62 | 0.51 | DVB/CAR/PDMS, HS-SPME, 50 °C |
| Methyl-2-octynoate | 3.63 | 0.22 | 0.32 | 0.63 | 0.08 | 0.80 | 0.24 | 0.81 | 0.05 | 0.96 | 1.76 | 0.32 | DVB/CAR/PDMS, HS-SPME, 50 °C |
| Citronellol | 3.18 | 0.24 | 0.37 | 0.60 | 0.17 | 0.72 | 0.11 | 0.90 | 0.05 | 0.96 | 2.98 | 0.23 | DVB/CAR/PDMS, HS-SPME, 100 °C |
| Citral | 4.97 | 0.17 | 0.13 | 0.76 | 0.25 | 0.65 | 0.31 | 0.76 | 0.13 | 0.88 | 5.04 | 0.15 | DVB/CAR/PDMS, DI-SPME, 100 °C |
| Geraniol | 6.42 | 0.15 | 0.01 | 0.94 | 0.00 | 0.99 | 0.96 | 0.51 | 0.10 | 0.90 | 4.28 | 0.17 | DVB/CAR/PDMS, HS-SPME, 100 °C |
| Cinnalmaldehyde | 88.1 | 0.01 | 0.03 | 0.88 | 17.1 | 0.05 | 13.2 | 0.07 | 38.8 | 0.03 | 1.51 | 0.34 | DVB/CAR/PDMS, HS-SPME, 100 °C |
| Anis alcohol | 4.93 | 0.17 | 12.7 | 0.07 | 0.16 | 0.73 | 3.41 | 0.23 | 0.75 | 0.57 | 0.05 | 0.84 | DVB/CAR/PDMS, DI-SPME, 100 °C |
| Cinnamyl alcohol | 5.69 | 0.15 | 7.55 | 0.11 | 0.78 | 0.47 | 9.32 | 0.10 | 0.56 | 0.64 | 1.20 | 0.39 | DVB/CAR/PDMS, DI-SPME, 100 °C |
| Eugenol | 571648 | 0.00 | 20182 | 0.00 | 2705 | 0.00 | 22141 | 0.00 | 2662 | 0.00 | 50.4 | 0.02 | PA, HS-SPME, 100 °C |
| Methyleugenol | 11.2 | 0.08 | 0.34 | 0.62 | 8.60 | 0.10 | 3.83 | 0.21 | 0.94 | 0.52 | 16.3 | 0.06 | DVB/CAR/PDMS, DI-SPME, 100 °C |
| Isoeugenol | 7.49 | 0.12 | 0.10 | 0.78 | 7.74 | 0.11 | 2.89 | 0.26 | 0.66 | 0.60 | 13.1 | 0.07 | DVB/CAR/PDMS, DI-SPME, 100 °C |
| α-Isomethyl ionone | 8.72 | 0.10 | 7.55 | 0.11 | 9.13 | 0.09 | 2.20 | 0.31 | 1.52 | 0.40 | 5.13 | 0.15 | DVB/CAR/PDMS, HS-SPME, 100 °C |
| Lilial | 7.11 | 0.12 | 2.31 | 0.27 | 13.1 | 0.07 | 1.36 | 0.42 | 2.33 | 0.30 | 4.74 | 0.16 | DVB/CAR/PDMS, HS-SPME, 100 °C |
| Amylcinnamal | 2.07 | 0.33 | 4.23 | 0.18 | 20.9 | 0.04 | 2.37 | 0.30 | 1.20 | 0.45 | 7.65 | 0.11 | DVB/CAR/PDMS, HS-SPME, 100 °C |
| Amylcinnamyl alcohol | 9.33 | 0.10 | 28.7 | 0.03 | 13.7 | 0.07 | 8.94 | 0.10 | 1.19 | 0.46 | 5.00 | 0.15 | DVB/CAR/PDMS, DI-SPME, 100 °C |
| Farnesol | 47.2 | 0.02 | 18.2 | 0.05 | 21.3 | 0.04 | 12.3 | 0.08 | 2.90 | 0.26 | 17.2 | 0.05 | DVB/CAR/PDMS, DI-SPME, 100 °C |
| Hexylcinnamal | 0.28 | 0.78 | 5.47 | 0.14 | 14.5 | 0.06 | 2.02 | 0.33 | 0.35 | 0.74 | 5.92 | 0.14 | PDMS/DVB, HS-SPME, 100 °C |
| Benzyl benzoate | 6.96 | 0.13 | 19.5 | 0.04 | 21.9 | 0.04 | 1.68 | 0.37 | 2.28 | 0.30 | 17.1 | 0.05 | PDMS/DVB, HS-SPME, 100 °C |
| Benzyl salicylate | 5.16 | 0.16 | 4.30 | 0.17 | 2.43 | 0.26 | 0.90 | 0.52 | 0.20 | 0.83 | 3.52 | 0.20 | PA, HS-SPME, 100 °C |
| Benzyl cinnamate | 11.3 | 0.08 | 91.3 | 0.01 | 8.95 | 0.10 | 5.08 | 0.16 | 0.96 | 0.51 | 0.54 | 0.54 | PA, DI-SPME, 100 °C |
| Synthetic musks | |||||||||||||
| Musk xylene | 13.3 | 0.07 | 1.99 | 0.29 | 57.6 | 0.02 | 3.29 | 0.23 | 4.81 | 0.17 | 16.5 | 0.06 | DVB/CAR/PDMS, HS-SPME, 100 °C |
| Musk ambrette | 48.6 | 0.02 | 13.9 | 0.07 | 104 | 0.01 | 14.0 | 0.07 | 13.8 | 0.07 | 26.5 | 0.04 | DVB/CAR/PDMS, DI-SPME, 100 °C |
| Musk moskene | 9.20 | 0.10 | 7.09 | 0.12 | 58.4 | 0.02 | 3.36 | 0.23 | 3.98 | 0.20 | 22.2 | 0.04 | DVB/CAR/PDMS, HS-SPME, 100 °C |
| Musk tibetene | 6.35 | 0.14 | 2.19 | 0.28 | 80.6 | 0.01 | 4.36 | 0.19 | 2.63 | 0.28 | 23.6 | 0.04 | DVB/CAR/PDMS, HS-SPME, 100 °C |
| Galaxolide | 3.78 | 0.21 | 5.37 | 0.15 | 23.6 | 0.04 | 1.87 | 0.35 | 1.27 | 0.44 | 4.61 | 0.16 | DVB/CAR/PDMS, HS-SPME, 100 °C |
| Celestolide | 5.35 | 0.16 | 10.9 | 0.08 | 18.8 | 0.04 | 2.02 | 0.33 | 1.56 | 0.39 | 6.77 | 0.12 | DVB/CAR/PDMS, HS-SPME, 100 °C |
| Phantolide | 7.40 | 0.12 | 11.0 | 0.08 | 34.2 | 0.03 | 2.16 | 0.32 | 2.66 | 0.27 | 11.8 | 0.08 | DVB/CAR/PDMS, HS-SPME, 100 °C |
| Cashmeran | 8.83 | 0.10 | 3.06 | 0.22 | 8.61 | 0.10 | 1.29 | 0.44 | 1.39 | 0.42 | 6.15 | 0.13 | DVB/CAR/PDMS, HS-SPME, 100 °C |
| Traseolide | 3.01 | 0.25 | 14.1 | 0.06 | 25.6 | 0.04 | 1.81 | 0.36 | 1.13 | 0.47 | 9.14 | 0.09 | DVB/CAR/PDMS, HS-SPME, 100 °C |
| Tonalide | 4.59 | 0.18 | 12.4 | 0.07 | 40.0 | 0.02 | 2.14 | 0.32 | 1.97 | 0.34 | 14.2 | 0.06 | DVB/CAR/PDMS, HS-SPME, 100 °C |
| Ambrettolide | 0.35 | 0.74 | 11.1 | 0.08 | 13.8 | 0.07 | 1.85 | 0.35 | 0.25 | 0.80 | 5.06 | 0.15 | DVB/CAR/PDMS, HS-SPME, 100 °C |
| Preservatives | |||||||||||||
| PhEtOH | 13.6 | 0.07 | 2.56 | 0.25 | 0.25 | 0.67 | 3.86 | 0.21 | 3.43 | 0.23 | 1.55 | 0.34 | DVB/CAR/PDMS, DI-SPME, 100 °C |
| BHA | 6.69 | 0.13 | 19.3 | 0.04 | 0.12 | 0.76 | 3.48 | 0.22 | 0.23 | 0.81 | 19.7 | 0.04 | DVB/CAR/PDMS, DI-SPME, 100 °C |
| BHT | 11.2 | 0.08 | 29.2 | 0.03 | 2.99 | 0.23 | 6.88 | 0.13 | 0.89 | 0.53 | 2.86 | 0.23 | DVB/CAR/PDMS, HS-SPME, 100 °C |
| TCS | 8470 | 0.00 | 138966 | 0.00 | 14456 | 0.00 | 2771 | 0.00 | 880 | 0.00 | 5719 | 0.00 | PA, DI-SPME, 100 °C |
| MeP | 92.9 | 0.01 | 29.6 | 0.03 | 11.5 | 0.07 | 23.8 | 0.04 | 0.12 | 0.89 | 18.1 | 0.05 | PA, HS-SPME, 100 °C |
| EtP | 13.2 | 0.07 | 8.21 | 0.10 | 5.49 | 0.14 | 4.30 | 0.19 | 0.46 | 0.68 | 8.77 | 0.10 | DVB/CAR/PDMS, DI-SPME, 100 °C |
| iPrP | 2.12 | 0.32 | 16.7 | 0.06 | 1.17 | 0.39 | 0.96 | 0.51 | 1.61 | 0.38 | 3.06 | 0.22 | DVB/CAR/PDMS, DI-SPME, 50 °C |
| iBuP | 79.0 | 0.01 | 140 | 0.01 | 14.1 | 0.06 | 43.1 | 0.02 | 1.65 | 0.38 | 22.4 | 0.04 | DVB/CAR/PDMS, DI-SPME, 100 °C |
| BzP | 2.44 | 0.29 | 16.7 | 0.05 | 0.42 | 0.58 | 1.39 | 0.42 | 0.15 | 0.87 | 4.59 | 0.17 | PA, DI-SPME, 100 °C |
| Plasticizers | |||||||||||||
| DMP | 0.84 | 0.54 | 1.07 | 0.41 | 1.59 | 0.33 | 0.79 | 0.56 | 0.87 | 0.54 | 1.42 | 0.36 | PDMS/DVB, HS-SPME, 100 °C |
| DEP | 18.8 | 0.05 | 31.8 | 0.03 | 7.72 | 0.11 | 5.03 | 0.17 | 0.17 | 0.85 | 15.7 | 0.06 | DVB/CAR/PDMS, DI-SPME, 100 °C |
| DIBP | 13.8 | 0.07 | 4.71 | 0.16 | 30.0 | 0.03 | 4.84 | 0.17 | 5.82 | 0.15 | 3.43 | 0.21 | DVB/CAR/PDMS, DI-SPME, 100 °C |
| DBP | 3.20 | 0.24 | 20.2 | 0.04 | 20.7 | 0.04 | 3.70 | 0.21 | 0.93 | 0.52 | 1.11 | 0.40 | DVB/CAR/PDMS, DI-SPME, 100 °C |
| DIPP | 0.09 | 0.92 | 0.33 | 0.62 | 21.8 | 0.04 | 1.45 | 0.41 | 0.03 | 0.97 | 3.17 | 0.22 | DVB/CAR/PDMS, DI-SPME, 100 °C |
| DPP | 0.34 | 0.75 | 1.59 | 0.33 | 17.1 | 0.05 | 1.06 | 0.49 | 0.03 | 0.98 | 1.71 | 0.32 | PA, DI-SPME, 100 °C |
| BBP | 101 | 0.01 | 1411 | 0.00 | 209 | 0.00 | 50.7 | 0.02 | 4.44 | 0.14 | 55.6 | 0.02 | PA, DI-SPME, 100 °C |
| DIHP | 2.52 | 0.28 | 0.98 | 0.43 | 11.2 | 0.08 | 0.64 | 0.61 | 1.58 | 0.39 | 3.31 | 0.21 | PA, HS-SPME, 100 °C |
| DEHP | 4.01 | 0.20 | 1.56 | 0.34 | 13.6 | 0.07 | 0.83 | 0.55 | 2.89 | 0.26 | 2.58 | 0.25 | PA, HS-SPME, 100 °C |
| DCHP | 56.1 | 0.02 | 414 | 0.00 | 79.3 | 0.01 | 32.8 | 0.03 | 5.48 | 0.15 | 27.8 | 0.03 | PA, DI-SPME, 100 °C |
| DPhP | 1022 | 0.00 | 9456 | 0.00 | 142 | 0.01 | 1003 | 0.00 | 3.49 | 0.22 | 138 | 0.01 | PA, DI-SPME, 100 °C |
| DNOP | 36.5 | 0.03 | 0.06 | 0.83 | 75.8 | 0.01 | 0.53 | 0.65 | 31.2 | 0.03 | 0.71 | 0.49 | PA, HS-SPME, 100 °C |
| DMA | 57.6 | 0.02 | 2.89 | 0.23 | 11.4 | 0.08 | 0.73 | 0.58 | 3.00 | 0.25 | 2.63 | 0.25 | DVB/CAR/PDMS, HS-SPME, 50 °C |
| DEA | 5.87 | 0.15 | 1.52 | 0.34 | 0.72 | 0.48 | 1.06 | 0.49 | 0.13 | 0.88 | 5.17 | 0.15 | DVB/CAR/PDMS, DI-SPME, 100 °C |
| DEHA | 1.05 | 0.49 | 2.63 | 0.25 | 9.43 | 0.09 | 0.99 | 0.50 | 0.75 | 0.57 | 3.32 | 0.21 | PA, HS-SPME, 100 °C |
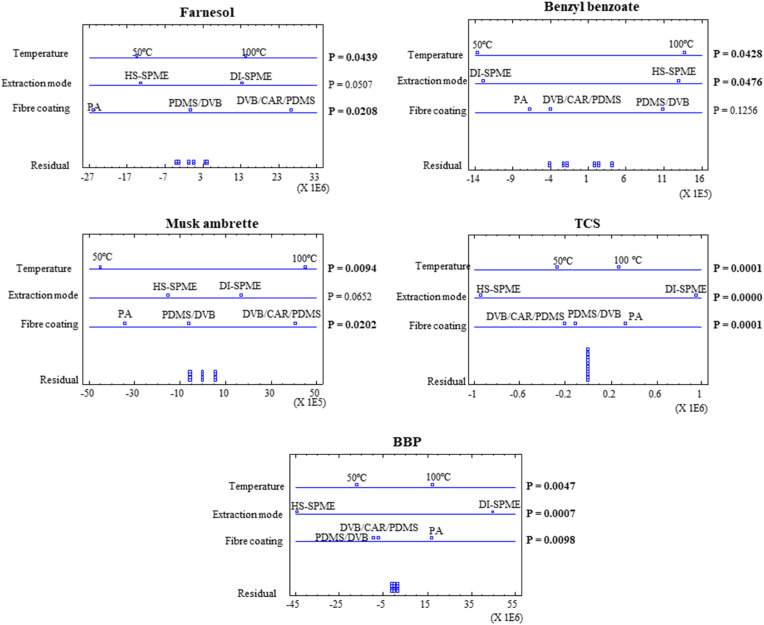
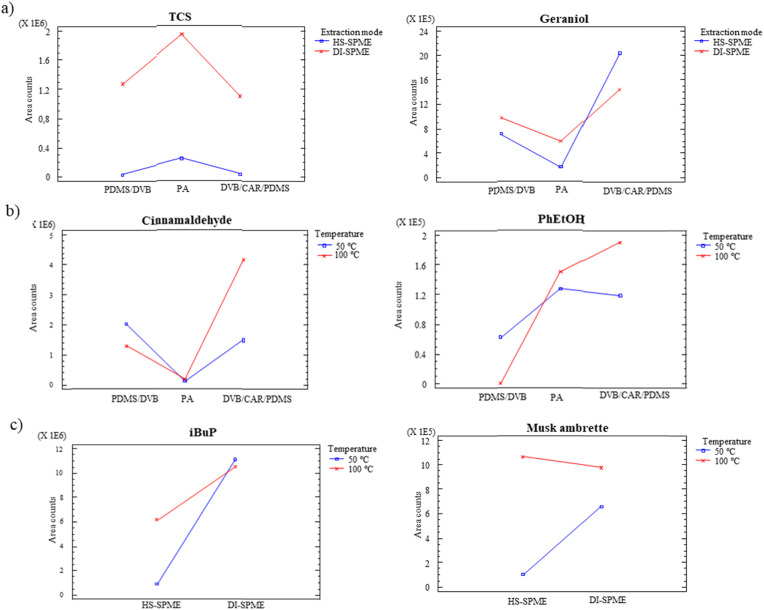
ANOVA results for the main factors are graphically displayed in Fig. 3 for some representative substances, including one compound of each family. These graphs show scaled effects of each factor by comparing the natural variance of the plot points with that of the residuals, displayed at the bottom. Thus, it is easy to identify factors that show differences of greater magnitude than those which could be accounted solely by the experimental error. On the right of these graphs, the most favourable conditions are displayed. The significant effects of the fibre coating (see farnesol, musk ambrette, TCS and BBP in the figure), the extraction mode (benzyl benzoate, TCS and BBP) and the temperature (farnesol, benzyl benzoate, musk ambrette, TCS and BBP) are clearly shown in Fig. 3. In all cases, an extraction temperature of 100 °C was more favourable, while the extraction mode depends on the compound, since some of them preferred direct sampling (see farnesol, TCS and BBP) whereas other compounds preferred the headspace mode (see benzyl benzoate and musk ambrette). Regarding the fibre, PA and DVB/CAR/PDMS allowed higher responses for most compounds (excluding hexylcinnamal, benzyl benzoate and DMP, see Table 1).
Fig. 3.
ANOVA graphs for some representative compounds for each studied family (farnesol, benzyl benzoate, musk ambrette, TCS and BBP).
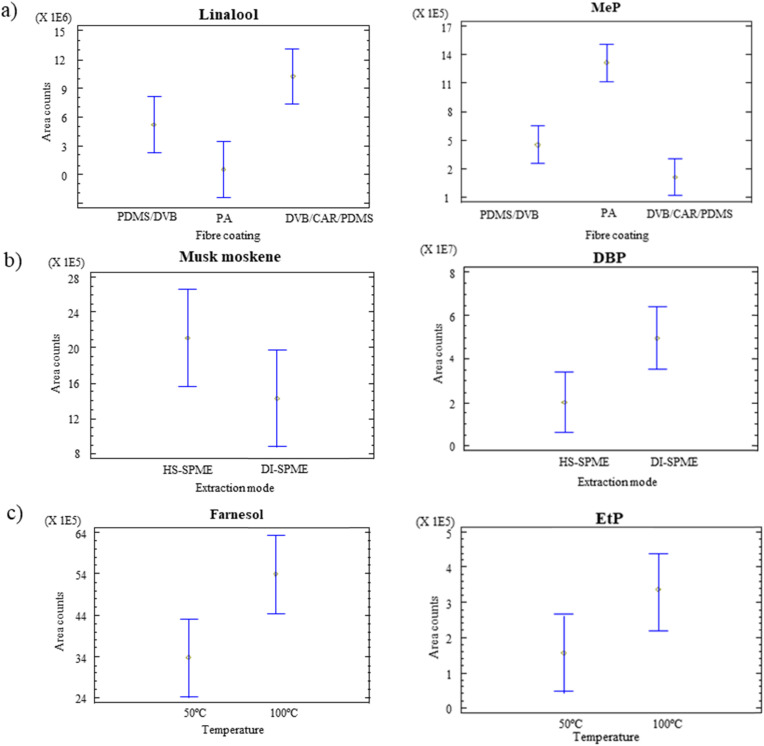
To easily visualize the most favourable extraction conditions, mean plot graphs for some representative compounds are depicted in Fig. 4 . These graphs illustrate the effect of the main factors by showing the mean values as well as the confidence intervals for each level. In general, and especially taking into account statistically significant factors, fragrance allergens and synthetic musks have a common behaviour, as well as preservatives and plasticizers. Fig. 4a shows as example linalool and MeP mean plots for fibre coating. As can be seen, fragrance allergens obtained higher responses employing DVB/CAR/PDMS coating as well as synthetic musks (see as example linalool in Fig. 4a). Divišová et al. [20] also demonstrated the feasibility of employing DVB/CAR/PDMS for the analysis of fragrance allergens in cosmetic products. On the other hand, preservatives and plasticizers achieved, in general, better results using PA fibre for those compounds for which this factor was significant (see as example MeP in Fig. 4a). This result is in consonance with other study found in literature for the extraction of preservatives in cosmetic formulations [21] whereas other authors proposed DVB/CAR/PDMS as more efficient coating [19]. Regarding the extraction mode, the responses were in general improved in HS mode for fragrance allergens and synthetic musks, and in DI for preservatives and plasticizers. On the other hand, 100 °C provided a more efficient extraction for the target substances (see some examples in Fig. 4c), excluding the most volatile analytes (pinene, limonene, benzyl alcohol, linalool and methyl-2-octynoate), for which both temperatures were equivalent.
Fig. 4.
Mean plots obtained for some representative compounds for the different factors: a) fibre coating; b) extraction mode; c) temperature.
Regarding interaction effects (see ANOVA in Table 1), extraction mode-temperature (BC) was significant for 10 compounds, the interaction fibre coating-extraction mode (AB) for 7 and fibre coating-temperature (AC) for 4 compounds. The two-factor interaction plots display the least squared means at all combinations of two factors, which allows studying the effect of both factors simultaneously. Fig. 5 shows some illustrative examples. These figure offers an easy visualization of the most favourable conditions. Fig. 5a represents as an example the interaction plot AB for TCS (preservative) and geraniol (fragrance allergen). For the first one the combination of DI mode and PA coating showed the higher responses. On the other hand, geraniol preferred DVB/CAR/PDMS in HS mode. The interaction plot AC represents for fragrance allergens and synthetic musks DVB/CAR/PDMS and 100 °C as optimal conditions (see cinnamaldehyde in Fig. 5b). The plot for PhEtOH, is also included revealing 100 °C as the best extraction temperature for DVB/CAR/PDMS or PA. Regarding the interaction BC, in general, preservatives obtained higher responses in DI mode regardless the temperature (see iBuP in Fig. 5c), while other compounds preferred 100 °C regardless the extraction mode (see musk ambrette in Fig. 5c).
Fig. 5.
Interaction plots for some representative compounds: a) fibre coating-extraction mode; b) fibre coating-temperature; c) extraction mode-temperature.
In brief, the most favourable conditions include a common temperature of 100 °C in all cases but two different behaviours for the other factors: DVB/CAR/PDMS in headspace mode for fragrances allergens and synthetic musks and PA fibre coating in direct mode for preservatives and plasticizers. The optimal conditions for each analyte are summarized in Table 1 (last column).
In order to select the final common conditions to simultaneously extract the 60 target compounds, both optimal settings were compared. As can be seen in Fig. 6 , the fibre coating DVB/CAR/PDMS in the HS mode showed higher efficiency for more compounds, especially for the most volatile analytes (pinene, limonene, DMA…), for which no response was achieved employing the PA fibre in DI mode. Besides, fragrances, which are more volatile and less polar, and the most expected compounds in cosmetics samples, reached up to 70 times higher responses using the DVB/CAR/PDMS fibre in HS mode. By contrast, the DI sampling mode with the PA fibre implied up to 50 times higher responses for 14 of the 60 target compounds (eugenol, benzyl salicylate, benzyl cinnamate, PhEtOH, TCS, MeP, iPrP, DPP, BBP, DIHP, DEHP, DCHP, DPhP and DnOP).
Fig. 6.
Comparison of optimal conditions obtained in the experimental design: PA-DI-SPME-100 °C and DVB/CAR/PDMS–HS–SPME-100 °C (for easy viewing, responses for some compounds were multiplied or divided by a factor).
Therefore, the final experimental conditions imply the use of 10 mg of hydroalcoholic gel diluted in 10 mL of ultrapure water (1:1000, w/v) for the extraction of the 60 target compounds. For the coating, mode and extraction temperature, compromise conditions involve the use of DVB/CAR/PDMS in HS mode at 100 °C, since they were more favourable for most compounds achieving in all cases good or at least a reasonable sensitivity. Furthermore, fibre contamination is minimized since direct contact between the sample and the fibre is avoided.
3.3. SPME-GC-MS/MS performance
Under optimized conditions, the SPME-GC-MS/MS method was validated in terms of linearity, precision and accuracy, following as far as possible the application of the ISO12787 international standard ‘Cosmetics–Analytical methods–Validation criteria for analytical results using chromatographic techniques’. Limits of detection (LODs) and quantification (LOQs) were also calculated. Results are summarized in Table 2.
Table 2.
SPME-GC-MS/MS performance. Linearity, precision, recoveries, LODs and LOQs.
| Compounds | Linearity |
Precision, RSD (%) |
Recovery ± Precision (RSD) (%) |
LODs (μg g −1) | LOQs (μg g −1) | |||
|---|---|---|---|---|---|---|---|---|
| R2 | Linear range (μg L−1)a | Intra-day (n = 3) | Inter-day (n = 5) | 0.2 μg g−1 | 2 μg g−1 | |||
| Fragrance allergens | ||||||||
| Pinene | 0.9906 | 0.01–5 | 3.8 | 2.7 | 86.4 ± 1.4 | 96.1 ± 7.3 | 0.0028 | 0.0092 |
| Limonene | 0.9910 | 0.01–5 | 4.5 | 3.2 | 72.6 ± 3.8 | 78.6 ± 4.8 | 0.0028 | 0.0092 |
| Benzyl Alcohol | 0.9936 | 0.01–5 | 3.2 | 5.3 | 78.2 ± 3.0 | 77.8 ± 3.7 | 0.0030 | 0.0099 |
| Linalool | 0.9922 | 0.01–5 | 0.2 | 1.0 | 84.2 ± 1.0 | 83.6 ± 2.0 | 0.0013 | 0.0043 |
| Methyl-2-octynoate | 0.9909 | 0.1–5 | 1.4 | 10 | 108.2 ± 4.1 | 96.81 ± 0.47 | 0.027 | 0.089 |
| Citronellol | 0.9931 | 0.02–5 | 5.3 | 15 | 110 ± 15 | 89.6 ± 3.6 | 0.0030 | 0.0099 |
| Citral | 0.9908 | 0.02–5 | 3.2 | 5.2 | 112.1 ± 1.0 | 110.4 ± 5.6 | 0.0040 | 0.013 |
| Geraniol | 0.9901 | 0.02–5 | 7.0 | 6.3 | 98.6 ± 6.9 | 108.2 ± 4.6 | 0.0043 | 0.014 |
| Cinnamaldehyde | 0.9951 | 0.1–5 | 14 | 18 | 103 ± 12 | 113 ± 13 | 0.029 | 0.096 |
| Anis alcohol | 0.9918 | 0.1–5 | 3.1 | 12 | 107.2 ± 3.0 | 117.6 ± 3.3 | 0.033 | 0.11 |
| Cinnamyl alcohol | 0.9919 | 0.1–5 | 3.1 | 3.9 | 115.0 ± 1.1 | 100.8 ± 6.9 | 0.030 | 0.099 |
| Eugenol | 0.9951 | 0.02–5 | 2.6 | 2.0 | 116.0 ± 1.1 | 115.7 ± 6.2 | 0.0060 | 0.020 |
| Methyleugenol | 0.9945 | 0.01–5 | 5.3 | 3.9 | 101.97 ± 0.65 | 110.5 ± 5.3 | 0.0012 | 0.0040 |
| Isoeugenol | 0.9924 | 0.01–5 | 3.5 | 6.2 | 97.6 ± 5.9 | 108.6 ± 3.7 | 0.0024 | 0.0079 |
| α-isomethylionone | 0.9934 | 0.01–5 | 9.5 | 6.7 | 97.57 ± 0.22 | 108.2 ± 5.6 | 0.0026 | 0.0086 |
| Lilial® | 0.9911 | 0.01–5 | 7.6 | 10 | 104.85 ± 0.52 | 111.0 ± 4.2 | 0.0027 | 0.0089 |
| Amylcinnamal | 0.9945 | 0.01–5 | 10 | 12 | 113.9 ± 1.8 | 118.4 ± 3.1 | 0.0026 | 0.0085 |
| Amylcinnamyl alcohol | 0.9951 | 0.01–5 | 18 | 13 | 102.5 ± 1.0 | 112.3 ± 1.6 | 0.0014 | 0.0046 |
| Farnesol | 0.9962 | 0.01–5 | 8.4 | 13 | 110.2 ± 6.4 | 113.7 ± 5.2 | 0.0028 | 0.0092 |
| Hexylcinnamal | 0.9950 | 0.01–5 | 15 | 19 | 110.7 ± 3.0 | 111.1 ± 2.3 | 0.0012 | 0.0040 |
| Benzyl benzoate | 0.9929 | 0.01–5 | 9.9 | 18 | 71.0 ± 6.0 | 92.9 ± 4.3 | 0.0028 | 0.0092 |
| Benzyl salicylate | 0.9924 | 0.01–5 | 6.0 | 7.6 | 107.2 ± 2.4 | 86.2 ± 5.7 | 0.0028 | 0.0092 |
| Benzyl cinnamate | 0.9901 | 0.01–5 | 12 | 13 | 86.2 ± 5.5 | 91.2 ± 3.0 | 0.0026 | 0.0086 |
| Synthetic musks | ||||||||
| Musk Xylene | 0.9912 | 0.01–5 | 7.2 | 10 | 107.2 ± 9.0 | 113.1 ± 4.2 | 0.0014 | 0.0046 |
| Musk Ambrette | 0.9902 | 0.01–5 | 1.4 | 8.2 | 113.1 ± 2.0 | 109.6 ± 4.5 | 0.0011 | 0.0036 |
| Musk Moskene | 0.9922 | 0.01–5 | 4.9 | 5.5 | 119.2 ± 3.1 | 115.9 ± 2.6 | 0.0033 | 0.011 |
| Musk Tibetene | 0.9944 | 0.01–5 | 6.9 | 9.6 | 107.5 ± 1.2 | 104.1 ± 1.0 | 0.0014 | 0.0046 |
| Galaxolide | 0.9927 | 0.01–5 | 7.4 | 10 | 95.54 ± 0.86 | 96.4 ± 6.9 | 0.0020 | 0.0066 |
| Celestolide | 0.9983 | 0.01–5 | 11 | 15 | 96.2 ± 1.2 | 100.3 ± 2.4 | 0.0010 | 0.0033 |
| Phantolide | 0.9975 | 0.01–5 | 12 | 16 | 91.38 ± 0.43 | 100.3 ± 2.3 | 0.0032 | 0.010 |
| Cashmeran | 0.9967 | 0.01–5 | 6.3 | 8.6 | 97.9 ± 1.0 | 105.8 ± 2.9 | 0.0027 | 0.0089 |
| Traseolide | 0.9968 | 0.01–5 | 12 | 17 | 91.1 ± 1.1 | 99.5 ± 2.1 | 0.0010 | 0.0033 |
| Tonalide | 0.9964 | 0.01–5 | 19 | 14 | 91.03 ± 0.58 | 99.8 ± 2.2 | 0.0011 | 0.0036 |
| Ambrettolide | 0.9963 | 0.01–5 | 13 | 19 | 92.8 ± 2.8 | 99.6 ± 2.6 | 0.0010 | 0.0033 |
| Preservatives | ||||||||
| PhEtOH | 0.9912 | 0.1–5 | 9.8 | 15 | 101.2 ± 2.2 | 102.9 ± 2.0 | 0.024 | 0.079 |
| BHA | 0.9962 | 0.01–5 | 2.1 | 3.9 | 109.5 ± 1.0 | 102.5 ± 6.0 | 0.0015 | 0.0049 |
| BHT | 0.9966 | 0.01–5 | 6.8 | 5.9 | 101.0 ± 3.2 | 108.0 ± 5.3 | 0.0010 | 0.0033 |
| TCS | 0.9909 | 0.01–5 | 2.0 | 2.0 | 116.4 ± 3.9 | 89.8 ± 6.8 | 0.0032 | 0.010 |
| MeP | 0.9912 | 0.02–5 | 6.1 | 5.1 | 96.0 ± 1.0 | 107.3 ± 5.1 | 0.0060 | 0.019 |
| EtP | 0.9965 | 0.1–5 | 1.0 | 20 | 106 ± 16 | 98.6 ± 1.7 | 0.027 | 0.089 |
| iPrP | 0.9912 | 0.05–5 | 3.3 | 8.6 | 81.0 ± 1.0 | 70.8 ± 7.5 | 0.013 | 0.043 |
| PrP | 0.9936 | 0.2–5 | 5.1 | 5.7 | 103 ± 14 | 95.9 ± 1.4 | 0.065 | 0.21 |
| iBuP | 0.9900 | 0.1–5 | 14 | 18 | 116 ± 20 | 76 ± 10 | 0.026 | 0.086 |
| BzP | 0.9903 | 0.02–5 | 6.4 | 7.3 | 94.2 ± 9.2 | 100.0 ± 2.7 | 0.0071 | 0.024 |
| Plasticizers | ||||||||
| DMP | 0.9916 | 0.05–5 | 12 | 16 | 109.3 ± 6.5 | 103 ± 10 | 0.0070 | 0.023 |
| DEP | 0.9971 | 0.1–5 | 0.1 | 1.6 | n.c. | n.c. | 0.035 | 0.12 |
| DIBP | 0.9935 | 0.05–5 | 3.0 | 3.2 | 105.3 ± 8.3 | 100.5 ± 1.0 | 0.0071 | 0.023 |
| DBP | 0.9917 | 0.1–5 | 13 | 19 | 103.7 ± 5.9 | 101.4 ± 2.5 | 0.025 | 0.082 |
| DMEP | 0.9955 | 0.05–5 | 3.1 | 3.1 | 117.0 ± 5.0 | 72.8 ± 3.1 | 0.014 | 0.046 |
| DIPP | 0.9922 | 0.01–5 | 16 | 16 | 93.9 ± 9.5 | 102.0 ± 1.0 | 0.0033 | 0.011 |
| DPP | 0.9935 | 0.01–5 | 16 | 19 | 91.9 ± 9.2 | 72.6 ± 4.2 | 0.0035 | 0.011 |
| BBP | 0.9935 | 0.02–5 | 12 | 17 | 100.8 ± 7.8 | 99.3 ± 6.0 | 0.0041 | 0.014 |
| DIHP | 0.9908 | 0.05–5 | 2.9 | 17 | 117.3 ± 6.7 | 97.3 ± 1.9 | 0.010 | 0.033 |
| DEHP | 0.9916 | 0.1–5 | 8.4 | 9.8 | 117.0 ± 2.4 | 115.1 ± 2.7 | 0.026 | 0.086 |
| DCHP | 0.9951 | 0.05–5 | 5.3 | 4.0 | 93.9 ± 2.1 | 96.9 ± 2.0 | 0.0082 | 0.027 |
| DPhP | 0.9929 | 0.1–5 | 3.0 | 4.9 | 120.0 ± 1.3 | 118.1 ± 9.2 | 0.034 | 0.11 |
| DNOP | 0.9978 | 0.05–5 | 15 | 10 | 107.4 ± 5.4 | 105.8 ± 5.3 | 0.014 | 0.046 |
| DMA | 0.9927 | 0.05–5 | 6.8 | 6.5 | 80.0 ± 7.0 | 83.2 ± 8.3 | 0.014 | 0.046 |
| DEA | 0.9909 | 0.01–5 | 3.3 | 8.0 | 84.6 ± 7.7 | 97.3 ± 5.7 | 0.0010 | 0.0033 |
| DEHA | 0.9925 | 0.1–5 | 16 | 11 | 97.1 ± 6.8 | 101 ± 14 | 0.020 | 0.066 |
Equivalent to μg g−1 in the cosmetic sample. n.c.: not calculated since DEP appeared in the sample.
Since no matrix effects were detected applying the dilution factor 1:1000 (see section 3.1.2), linearity was assessed using spiked ultrapure water at 9 different concentration levels ranging between 0.01 and 5 μg L−1 which correspond to 0.01 and 5 μg g−1 in cosmetic. Each concentration level was analyzed in triplicate. In all cases, the method showed a good linearity, with coefficients of determination (R2) higher than 0.9900. The precision was evaluated within a day (n = 3) and amongst days (n = 5) for all concentration levels. Relative standard deviation (RSD) values for 0.5 μg L−1 are shown in Table 2, and they were lower than 10% for most compounds for both repeatability and intermediate precision.
Accuracy was carried out using a hydroalcoholic gel sample free of the target compounds (except DEP). The sample was fortified at two levels (0.2 μg g−1 and 2 μg g−1). Recoveries were calculated using the calibration curve prepared in ultrapure water. As can be seen in Table 2, recoveries ranged between 80 and 112% with RSD values lower than 10% in most cases. These results demonstrated the absence of matrix effect. Although the number of experimental points is low, the slope for the water calibration curve and the ones obtained including the sample were compared and the results demonstrate a quite good agreement (slope ratio between 0.84 and 1.14 for virtually all 60 target compounds). Therefore, quantification was easily performed by external calibration employing standards prepared in ultrapure water for all studied compound (60), including fragrance allergens, synthetic musks, preservatives and plasticizers.
LODs and LOQs were calculated in real samples as the concentration giving a signal-to-noise ratio of three (S/N = 3) and ten (S/N = 10), respectively. For the plasticizers detected in the procedure blanks (see section 2.3), DEP, DBP, DEHP, DPhP and DEHA, the LODs were estimated as the average concentration of analyte giving a response equal to the blank plus 3 times the standard deviation. The LODs and LOQs for the 60 target compounds are displayed in Table 2 and, as it can be seen, they were at the low ng g−1, showing an excellent sensitivity considering that the samples were diluted by a factor of 1000 and taking into account the levels that can be found in real cosmetic samples.
3.4. Application to real samples
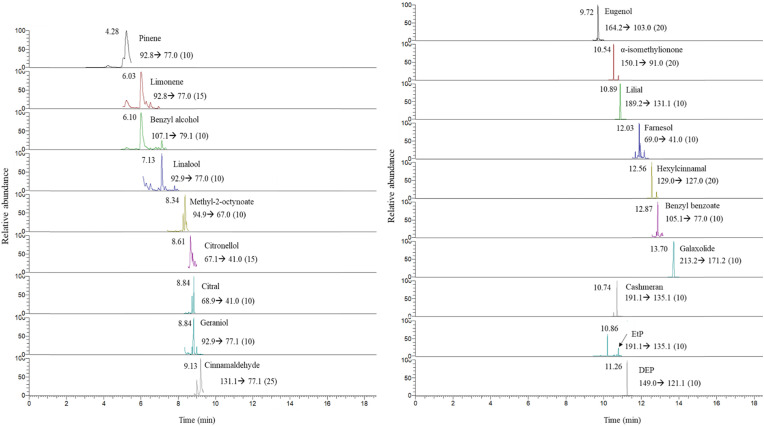
The validated SPME-GC-MS/MS method was applied to the analysis of 10 different hydroalcoholic gel samples in which 39 of the 60 target compounds were found. In this way, none of the analyzed samples complied the WHO recommendations, regarding hand sanitizer formulations in which only the main constituents (see introduction section) should be present. The quantification results for each sample are summarized in Table 3. As an example, a chromatogram of a real sample (G7) in which 19 of the target analytes were found is depicted in Fig. 7 .
Fig. 7.
SRM reconstructed SPME-GC-MS/MS chromatogram of a real hydroalcoholic gel sample (G7) containing 19 compounds from the four studied families (see concentrations in Table 3). The corresponding MS/MS quantification transition and collision energy (eV) is included for each compound.
As can be seen in Table 3, all studied fragrance allergens, excluding amylcinnamal, were detected in the real samples. Sixteen fragrance allergens were found in one sample (G1), 15 in two (G7, G10), 14 in one (G9) and 13 in two (G2 and G5). The other hydroalcoholic gels contained 12, 10, 9 and 6 fragrance allergens. Among the 22 allergens, limonene, methyl-2-octynoate and benzyl benzoate were the most frequently detected, present in 9 of the 10 analyzed hand sanitizers at concentrations up to 144 μg g−1. Other fragrances, such as benzyl alcohol, linalool, citral and α-isomethylionone were found in the 80% of the samples, followed by citronellol and geraniol in the 70% and lilial and benzyl salycilate in the 60% of the analyzed hydroalcoholic gels. The remaining 10 compounds were detected in at least 3 of the samples (30%) excluding anis alcohol and methyleugenol (each in one sample). The compound which reached the highest concentration was geraniol, at concentrations up to 217 μg g−1 in G9 and 172 μg g−1 in G5, followed by cinnamyl alcohol at 159 μg g−1 (G6). In this context, the presence of fragrance allergens above the labelling limit of 0.001% (w/w) (10 μg g−1) in leave-on products established by EC Regulation No. 1223/2009 [4] revealed that all samples (excluding G3 and G8) were under-labelled. Thirteen fragrance allergens found at concentrations higher than 10 μg g−1 in the analyzed hydroalcoholic gels were not specified on the label of the product. As an example, in sample G1 cinnamaldehyde (59 μg g−1), cinnamyl alcohol (71 μg g−1) and benzyl benzoate (18 μg g−1) were not on the label despite exceeding the mentioned limit (see Table S2).
As regards synthetic musks, all samples, except sample G10 which was free of these substances, contained at least 1 or 2 of these cosmetic additives, so the terms ‘parfum’ or ‘aroma’ must be referred on their label. In this way, samples G1, G4 and G9 were under-labelled (see Table S2). Four musks were found in the analyzed hydroalcoholic gels. The one most frequently detected was cashmeran, present in 70% of them. Galaxolide, detected in 60% of the analyzed samples, reached the highest concentrations for the synthetic musks, at concentrations higher than 32 μg g−1 in samples G7 and G9. On the other hand, traseolide and ambrettolide were only found in one sample.
Considering preservatives, 7 of the 10 studied were found in the samples. Five and four preservatives were found in one sample (G4 and G1, respectively), two in three samples (G5, G8 and G10), while the remaining samples contained only 1. EtP was detected in 6 of the 10 analyzed hydroalcoholic gels, BHT and MeP in 4, and PrP and iBuP in 2. TCS and PhEtOH were found in one sample, the latter present at 72 μg g−1. PrP reached the highest concentration for this family of compounds (150 μg g−1). Regarding the other parabens it is important to highlight that iBuP, found at 1.2 and 61 μg g−1 in samples G1 and G4 respectively, is forbidden in cosmetic products. On the other hand, MeP and EtP do not exceed the legal limit (4 mg g−1) since they ranged between 1.1 and 50 μg g−1.
Among the 16 studied plasticizers, 3 phthalates and 2 adipates were detected in the analyzed hydroalcoholic gels. Phthalates DEP, DEHP and DMP were found in 7, 4 and 3 samples, respectively, reaching concentrations up to 104 μg g−1. It is important to mention that DEHP is prohibited in cosmetics, although its concentration was very low (<0.5 μg g−1). Regarding adipates, DMA was detected in 4 samples at concentrations up to 4 μg g−1 whereas DEA was found in one sample.
4. Conclusions
A methodology based on SPME-GC-MS/MS has been developed for the simultaneous determination of fragrance allergens, synthetic musks, preservatives and plasticizers in hydroalcoholic gels. The most critical parameters affecting SPME were optimized by experimental design, obtaining as optimal conditions the use of DVB/CAR/PDMS fibre coating at 100 °C in the HS mode. Under optimal conditions the proposed method was successfully validated. Since no matrix effect was observed, external calibration using standards prepared in ultrapure water demonstrated suitability obtaining good linearity and quantitative recoveries for the 60 target compounds. Precision was also satisfactory showing RSD values lower than 10% in most cases.
The analysis of 10 real hydroalcoholic gel samples revealed the presence of 39 of the target compounds, including fragrance allergens, synthetic musks, preservatives and plasticizers, showing that WHO recommendations regarding the hand sanitizers formulation were not followed. In addition, the hydroalcoholic gels contained a high number of fragrance allergens at concentrations above 10 μg g−1, indicating that most of these products were under-labelled according to EC Regulation No. 1223/2009. Furthermore, some prohibited compounds (DEHP and iBuP) were found in some cases. This study highlights the need for greater control over the formulations of these frequently diary used cosmetic products to ensure consumer safety without causing undesirable side effects. In brief, the proposed method demonstrated its suitability and high throughput for the analysis of real hydroalcoholic gels being environmentally friendly, fast and simple, and of easy implementation in any routine laboratory.
CRediT authorship contribution statement
Lua Vazquez: Formal analysis, Validation, Data curation, Investigation, Writing – original draft, Writing – review & editing. Maria Celeiro: Methodology, Formal analysis, Investigation, Data curation, Writing – original draft, Writing – review & editing, Visualization, Supervision. Ana Castiñeira-Landeira: Formal analysis, Validation, Data curation, Investigation. Thierry Dagnac: Methodology, Resources, Visualization. Maria Llompart: Conceptualization, Methodology, Writing – review & editing, Visualization, Supervision, Project administration, Funding acquisition.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This work was supported by projects UNST10-1E-491 (Infrastructure Program, Ministry of Science and Innovation, Spain) and ED431 2020/06 (Consolidated Research Groups Program, Xunta de Galicia). The authors belong to the National Network for the Innovation in miniaturized sample preparation techniques, RED2018-102522-T (Ministry of Science, Innovation and Universities, Spain). This study is based upon work from the Sample Preparation Study Group and Network, supported by the Division of Analytical Chemistry of the European Chemical Society, and upon work from the IUPAC project No. 2021-015-2-500 ‘Greenness of official standard sample preparation methods”. All these programmes are co-funded by FEDER (EU).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.aca.2022.339650.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Lotfinejad N., Peters A., Pittet D. Hand hygiene and the novel coronavirus pandemic: the role of healthcare workers. J. Hosp. Infect. 2020;105(4):776. doi: 10.1016/j.jhin.2020.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Berardi A., Perinelli D.R., Merchant H.A., Bisharat L., Basheti I.A., Bonacucina G., Cespi M., Palmieri G.F. Hand sanitisers amid CoViD-19: a critical review of alcohol-based products on the market and formulation approaches to respond to increasing demand. Int. J. Pharm. 2020;584:119431. doi: 10.1016/j.ijpharm.2020.119431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organization (WHO) Guide to local production: WHO-recommended handrub formulations. https://www.who.int/gpsc/5may/Guide_to_Local_Production.pdf available at:
- 4.Regulation (EC) No 1223/2009 of the European parliament and of the council of 30 november 2009 on cosmetic products (text with EEA relevance) EU. Off. J. 2009;342:59–209. [Google Scholar]
- 5.Salvador A., Chisvert A. Analysis of Cosmetic Products. second ed. Elsevier; Netherlands: 2017. [Google Scholar]
- 6.Lores M., Llompart M., Alvarez-Rivera G., Guerra E., Vila M., Celeiro M., Lamas J.P., Garcia-Jares C. Positive lists of cosmetic ingredients: analytical methodology for regulatory and safety controls-A review. Anal. Chim. Acta. 2016;915:1–26. doi: 10.1016/j.aca.2016.02.033. [DOI] [PubMed] [Google Scholar]
- 7.Lores M., Celeiro M., Rubio L., Llompart M., Garcia-Jares C. Extreme cosmetics and borderline products: an analytical-based survey of European regulation compliance. Anal. Bioanal. Chem. 2018;410(27):7085–7102. doi: 10.1007/s00216-018-1312-3. [DOI] [PubMed] [Google Scholar]
- 8.Martin-Pozo L., del Carmen Gomez-Regalado M., Moscoso-Ruiz I., Zafra-Gomez A. Analytical methods for the determination of endocrine disrupting chemicals in cosmetics and personal care products: a review. Talanta. 2021;232:122642. doi: 10.1016/j.talanta.2021.122642. [DOI] [PubMed] [Google Scholar]
- 9.Celeiro M., Garcia-Jares C., Llompart M., Lores M. Recent advances in sample preparation for cosmetics and personal care products analysis. Molecules. 2021;26(16):4900. doi: 10.3390/molecules26164900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhong Z., Li G. Current trends in sample preparation for cosmetic analysis. J. Separ. Sci. 2017;40(1):152–169. doi: 10.1002/jssc.201600367. [DOI] [PubMed] [Google Scholar]
- 11.Anastas P., Eghbali N. Green chemistry: principles and practice. Chem. Soc. Rev. 2010;39(1):301–312. doi: 10.1039/b918763b. [DOI] [PubMed] [Google Scholar]
- 12.Mohamed H.M. Green, environment-friendly, analytical tools give insights in pharmaceuticals and cosmetics analysis. Trac. Trends Anal. Chem. 2015;66:176–192. [Google Scholar]
- 13.Celeiro M., Rubio L., Garcia-Jares C., Lores M. Miniaturized sample preparation methods to simultaneously determine the levels of glycols, glycol ethers and their acetates in cosmetics. Cosmetics. 2021;8(4):102. [Google Scholar]
- 14.Li M., Luo S., Di X., Cui Y. Ultrasound-assisted extraction coupling to high performance liquid chromatography for enantiomerically quantitative analysis of two preservatives in cosmetics and the potentially cytotoxic study. Microchem. J. 2021;172:106937. [Google Scholar]
- 15.Vila M., Facorro R., Lamas J.P., Garcia-Jares C., Dagnac T., Llompart M. Determination of fifteen water and fat-soluble UV filters in cosmetics by pressurized liquid extraction followed by liquid chromatography tandem mass spectrometry. Anal. Methods. 2016;8(37):6787–6794. [Google Scholar]
- 16.Celeiro M., Lamas J.P., Llompart M., Garcia-Jares C. In-vial micro-matrix-solid phase dispersion for the analysis of fragrance allergens, preservatives, plasticizers, and musks in cosmetics. Cosmetics. 2014;1(3):171–201. doi: 10.1016/j.chroma.2014.03.070. [DOI] [PubMed] [Google Scholar]
- 17.Celeiro M., Vazquez L., Lamas J.P., Vila M., Garcia-Jares C., Llompart M. Miniaturized matrix solid-phase dispersion for the analysis of ultraviolet filters and other cosmetic ingredients in personal care products. Separations. 2019;6(2):30. [Google Scholar]
- 18.Llompart M., Celeiro M., García-Jares C., Dagnac T. Environmental applications of solid-phase microextraction. Trac. Trends Anal. Chem. 2019;112:1–12. [Google Scholar]
- 19.Alvarez-Rivera G., Vila M., Lores M., Garcia-Jares C., Llompart M. Development of a multi-preservative method based on solid-phase microextraction-gas chromatography-tandem mass spectrometry for cosmetic analysis. J. Chromatogr. A. 2014;1339:13–25. doi: 10.1016/j.chroma.2014.02.075. [DOI] [PubMed] [Google Scholar]
- 20.Divisová R., Vitova E., DiviÅ¡ P., Zemanova J., Omelkova J. Validation of SPME-GC-FID method for determination of fragrance allergens in selected cosmetic products. Acta Chromatogr. 2015;27(3):509–523. [Google Scholar]
- 21.Tsai T.F., Lee M.R. Determination of antioxidants and preservatives in cosmetics by SPME combined with GC-MS. Chromatographia. 2008;67(5):425–431. [Google Scholar]
- 22.Choi N.R., Kim Y.P., Ji W.H., Hwang G.-S., Ahn Y.G. Identification and quantification of seven volatile n-nitrosamines in cosmetics using gas chromatography/chemical ionization-mass spectrometry coupled with head space-solid phase microextraction. Talanta. 2016;148:69–74. doi: 10.1016/j.talanta.2015.10.045. [DOI] [PubMed] [Google Scholar]
- 23.Rivero R.T., Topiwala V. Quantitative determination of formaldehyde in cosmetics using combined headspace-solid-phase microextraction-gas chromatography. J. Cosmet. Sci. 2004;55(4):343–350. [PubMed] [Google Scholar]
- 24.Celeiro M., Lamas J.P., Vila M., Garcia-Jares C., Homem V., Ratola N., Dagnac T., Llompart M. Determination of multiclass personal care products in continental waters by solid-phase microextraction followed by gas chromatography-tandem mass spectrometry. J. Chromatogr. A. 2019;1607:460398. doi: 10.1016/j.chroma.2019.460398. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.