Abstract
Purpose
The recombinant Bacillus Calmette–Guérin (BCG) containing the streptococcal inhibitor of the complement gene (rBCG-sic) may be more resistant to antimicrobial peptides and improve internalization; therefore, it can enhance the immunotherapeutic effect of the BCG. Here we determined the optimal dose of rBCG-sic and compared its effectiveness with that of BCG.
Materials and Methods
We fabricated a high-throughput 3D-bioprinted bladder cancer-on-a-chip (BCOC) and used it to evaluate the effectiveness of the rBCG-sic in terms of cell viability, cell migration, and cytokine concentrations. Using an orthotopic mouse model, we evaluated its anticancer effect and toxicity via bioluminescence imaging.
Results
T24 cell viability was decreased after treatment with rBCG-sic 30 multiplicities of infection (MOI) versus the same dosage of mock BCG (42.8%±6.4% vs. 75.7%±6.6%, p<0.05). THP-1 cell migration was positively correlated with rBCG-sic concentration (2.42-fold at 30MOI, p<0.01). The interleukin-6 concentration of rBCG-sic 30MOI was significantly higher than that of mock BCG 30MOI (11.2±1.3 pg/mL vs. 6.7±0.6 pg/mL, p<0.05). In the orthotopic bladder cancer mouse model, lower tumor volume was observed in the rBCG-sic 30MOI group than in the BCG 30MOI group after 10 days of treatment (p<0.05).
Conclusions
We concluded that rBCG-sic is a useful tool for overcoming BCG unresponsiveness in non-muscle invasive bladder cancer. Additionally, high-throughput BCOC with a microfluidic system can successfully reflect the bladder cancer microenvironment.
Keywords: Antimicrobial peptide, Bacillus Calmette–Guérin, Bladder cancer, Genetic recombination
Graphical Abstract
INTRODUCTION
Intravesical immunotherapy using Bacillus Calmette–Guérin (BCG) is a common strategy for the treatment of non-muscle invasive bladder cancer (NMIBC) [1]. Although BCG immunotherapy is the most effective treatment for patients with NMIBC, approximately 30% to 40% of bladder cancer cases are refractory to treatment, while 50% of cases involve recurrence [2,3]. After the intravesical injection of BCG, its low response rate is induced by human innate immunity, such as antimicrobial peptides (AMPs) [4]. The intravesical instillation of BCG causes direct contact between BCG and urothelial cells, followed by BCG internalization. In this process, the innate immune response is activated to prevent BCG internalization. AMPs such as human beta-defensins are related to the innate immune response, which acts against BCG to sterilize the urinary tract [5].
Some bacteria protect themselves against AMPs by secreting variant proteins, such as the streptococcal inhibitor of complement (sic) [6]. Streptococcus pyogenes is an important human pathogen that causes a number of acute suppurative infections [7]. To circumvent the host defensive mechanism and establish an infection, S. pyogenes has developed multiple molecular mechanisms. The sic it produces is a hydrophilic secretory protein that sequesters many AMPs and prevents them from reaching their targets on the cell surface [6]. The sic is almost uniquely expressed by emm1 S. pyogenes and is one of several virulence factors implicated in the propensity for emm1 to cause severe infection. In our previous study, we speculated that BCG unresponsiveness was caused by decreased internalization [8]. We developed a recombinant BCG strain expressing sic (rBCG-sic) and reported its ability to effectively evade BCG-stimulated AMPs and potential to significantly improve immunotherapeutic tools used to treat bladder cancer [9].
A three-dimensional (3D)-bioprinted bladder cancer-on-a-chip (BCOC) is an organ-on-a-chip model that was developed to overcome the limitations of two-dimensional cell culture and reflect cell–cell interactions and the tumor microenvironment in vivo [10]. BCOC is an essential bladder cancer culture model for the development and establishment of an ex vivo cell-based system that can more realistically simulate cellular behavior in vivo [11]. Our BCOC is fabricated using 3D bioprinting technology with a microfluidic system, which can be used to create a bladder cancer-like environment that functions as a drug screening platform [12].
We hypothesized that rBCG-sic may be more resistant to AMPs and improve internalization; therefore, it can enhance the immunotherapeutic effect of BCG. Here, we determined the optimal dose of rBCG-sic and compared the effectiveness of BCG and rBCG-sic in high-throughput 3D-bioprinted BCOC and orthotopic bladder cancer mouse models.
MATERIALS AND METHODS
1. Electroporation of BCG
We followed the plasmid DNA construction method for pMC306-sic as previously described [9]. Mock BCG was electroporated with empty plasmid DNA. After the electroporation, the BCG was resuspended in 7H9 medium containing kanamycin (10 µg/mL) and cultured at 37℃. After 2 days, the bladder cancer cell lines or mice were treated with the rBCG strains.
2. High-throughput BCOC with microfluidic system
1) Fabrication of high-throughput BCOC model
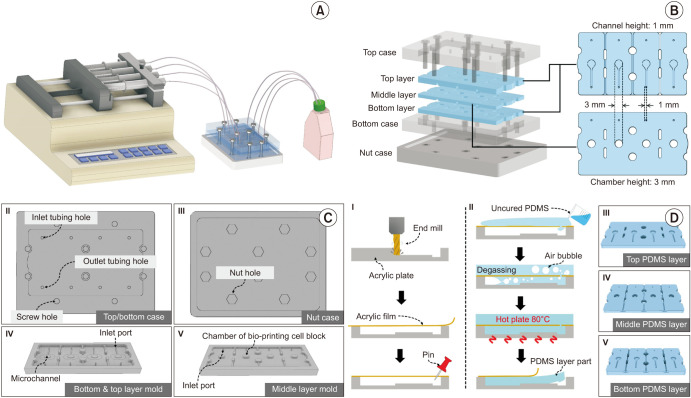
Complementing the previous study [12], we fabricated four BCOC-embedded microfluidic systems to enable efficient bladder cancer screening. Although the compositions of the BCOC models were similar, four stable experiments were performed using a single syringe pump (Fig. 1A). Each BCOC consisted of three microfluidic chip layers (top, middle, and bottom) made of polydimethylsiloxane (PDMS) and an acrylic case that attached and fixed each PDMS layer (Fig. 1B). Acrylic cases were fabricated by cutting the acrylic plate to have a shape that accommodated the PDMS layers (Fig. 1C). In all cases, the PDMS layer molds were fabricated by the engraving of acrylic plates using a milling machine (DAVID 3040; David Motion Technology, Incheon, Korea). The PDMS layer fabrication process is shown in Fig. 1D.
Fig. 1. Fabrication of high-throughput bladder cancer-on-a-chip (BCOC) model with microfluidic system. (A) Schematic illustration of the BCOC system. Four BCOC-embedded systems were operated by a single syringe pump. (B) Components of the BCOC system. The system consists of a top/bottom/nut case and top/middle/bottom polydimethylsiloxane (PDMS) layer. The top and bottom layers have a microchannel with a cross-sectional geometry of 1 mm (width)×1 mm (height), while the middle layer has a cylindrical chamber with a geometry of 3 mm (radius)×3 mm (height). The layers were fabricated from PDMS with the advantage of deformability, allowing for easy connection and leak-proof fluidic connection. The three layers were separated by a polycarbonate track-etched membrane (GVS Filter Technology, Sanford, ME, USA) for stable cell tissue positioning and diffusion-based drug supplied on the middle layer. The three layers containing four microfluidic channels/chambers were assembled by acryl case and tightened with bolts and nuts. The whole system was operated by a single syringe pump (Fusion 200; Chemyx Inc., Stafford, TX, USA) with four syringes. (C) Acrylic cases and PDMS layer molds. The cases and PDMS layer molds were made of acrylic plates. (D) PDMS layer fabrication processes. First of all, a 10-mm-thick acrylic plate was engraved using end milling and the desired shape was obtained. A commercial acrylic film was attached to the engraved acrylic molds to flatten the open side. Next the film was pierced with a pin to allow the PDMS to flow through. Uncured PDMS solution, which is a mixture of a prepolymer and a curing agent at a 10:1 ratio (Sylgard® 184; Dow Inc., Midland, MI, USA), was poured onto the film-attached acrylic mold. The degassing process under vacuum conditions was applied for 20 minutes and the vacuum was released very slowly to allow the PDMS to flow in. After the PDMS was perfectly filled inside the acrylic plate covered with the acrylic membrane, it was cured for 2 hours at 80℃ on a hot plate (MSH-30D; DAIHAN Scientific, Wonju, Korea) to create the PDMS microchannel/microchamber layers. The acrylic case parts were fabricated using computer numerical control milling.
2) Live/dead staining assay
The cell survival rate in the high-throughput BCOC system was assessed at 24 and 72 hours after rBCG-sic treatment. A fluorescent live/dead staining solution (Thermo Fisher Scientific, Waltham, MA, USA) was used according to the manufacturer’s instructions. Cell morphologies were observed using a fluorescence microscope (DMI8; Leica, Wetzlar, Germany). Three independent samples were analyzed.
3) Cell proliferation assay
A Cell Counting Kit-8 (CCK-8; Dojindo Molecular Technologies, Kumamoto, Japan) was used to analyze cell proliferation within the 3D-cell constructs after 24 and 72 hours according to the manufacturer’s instructions. After incubation, 0.2 mL of the solution was transferred to a 96-well plate. The absorbance of each well was measured at 450 nm using a microplate reader (SpectraMax i3x; Molecular Devices, Sunnyvale, CA, USA). Three independent samples were tested in each group.
4) THP-1 cell migration assay
The THP-1 monocytes were differentiated into macrophages by incubation for 24 hours in RPMI 1640 medium with 25 nM phorbol 12-myristate 13-acetate (Sigma, St. Louis, MO, USA). Differentiated THP-1 cells were seeded in the bottom layer of the BCOC at a density of 2×104 cells per chip in culture medium. After that, the polycarbonate track-etched (PCTE; GVS Filter Technology, Sanford, ME, USA) membrane was mounted, the 3D cell constructs were stacked on the second layer, and the BCG was added. Positive THP-1 staining was visualized using an Olympus CKX41 inverted microscope (×100 and ×200; Olympus, Tokyo, Japan). Three equal-sized fields were randomly selected for THP-1 cell counting and the average number was calculated.
5) Measurement of cytokine concentrations
After 6 hours of mock and rBCG-sic treatment, the growth media was collected in a syringe for each fluidic culture solution of BCOC and centrifuged at 3,000 rpm for 10 minutes at 4℃. The levels of tumor necrosis factor alpha (TNF-α) and interleukin-6 (IL-6) in the BCOC culture supernatant were measured using sandwich enzyme-linked immunosorbent assay reagent kits (DY210-05 and DY206-05) purchased from R&D Systems (Minneapolis, MN, USA). The assays were performed according to the manufacturer’s instructions.
3. Orthotopic bladder cancer mouse model
1) Experimental animal
Six-week-old female C3H mice were provided by Orient Bio Co. (Seongnam, Korea). The animals were acclimated for one week under routine laboratory conditions before the start of the experiments. All animals were housed in cages containing five animals and maintained on a daily 12 hours light and dark cycle. The mice were fed a standard balanced diet and water ad libitum.
2) Syngeneic orthotopic mouse bladder cancer model
For intravesical implantation of MBT2-luc cells, the C3H mice were anesthetized with isoflurane. Chemical lesions of the bladder urothelium were created by injecting 100 µL of poly-l-lysine 0.1 mg/mL (molecular weight 70,000–150,000; Sigma) into the bladder of each animal through a 24-gauge catheter (BD Angiocath Plus; Becton-Dickinson and Company, Franklin Lakes, NJ, USA). The presence of tumors in the bladder was confirmed by bioluminescence imaging (BLI) after 1 to 2 weeks. After establishment of the bladder cancer model was confirmed, d-luciferin 150 mg/kg (Gold Bio Technology, St. Louis, MO, USA) was administered intraperitoneally and bioluminescence was detected using a VISQUE In Vivo Smart-LF (Vieworks, Anyang, Korea).
3) In vivo anti-cancer efficacy of rBCG-sic
Mock BCG or rBCG-sic was prepared in 50 µL of phosphate-buffered saline (PBS) and instilled into the bladder lumen via a urinary catheter. The quantitative signal intensities were calculated, and these values are presented as regions of interest. Tumor regression after treatment with BCG or rBCG-sic in the orthotopic mouse model was measured using BLI. Each mouse (5/group) was administered PBS, mock BCG, or rBCG-sic through an intravesical catheter twice a week. Serial BLI values and body weights were measured to monitor bladder cancer progression twice a week for 3 weeks using the VISQUE In Vivo Smart-LF. BLI images were acquired and analyzed using Living Image software Clevue version 3.1.3.2054 (Vieworks).
4. Statistical analysis
The Statistical Package for Social Sciences (SPSS) version 14.0 (SPSS Inc., Chicago, IL, USA) was used to perform the statistical analyses. Student’s t-test was used to compare the means in different groups, and statistical significance was set at p<0.05.
5. Ethics statement
The animal experiments were approved by the Institutional Animal Care and Use Committee of Chung-Ang University (approval number: 2018-00050, date: May 12, 2018; Seoul, Korea) and performed in accordance with the National Institute of Health Guidelines for the Care and Use of Laboratory Animals.
RESULTS
1. Ex vivo immunotherapeutic effects of rBCG-sic in the high-throughput BCOC model
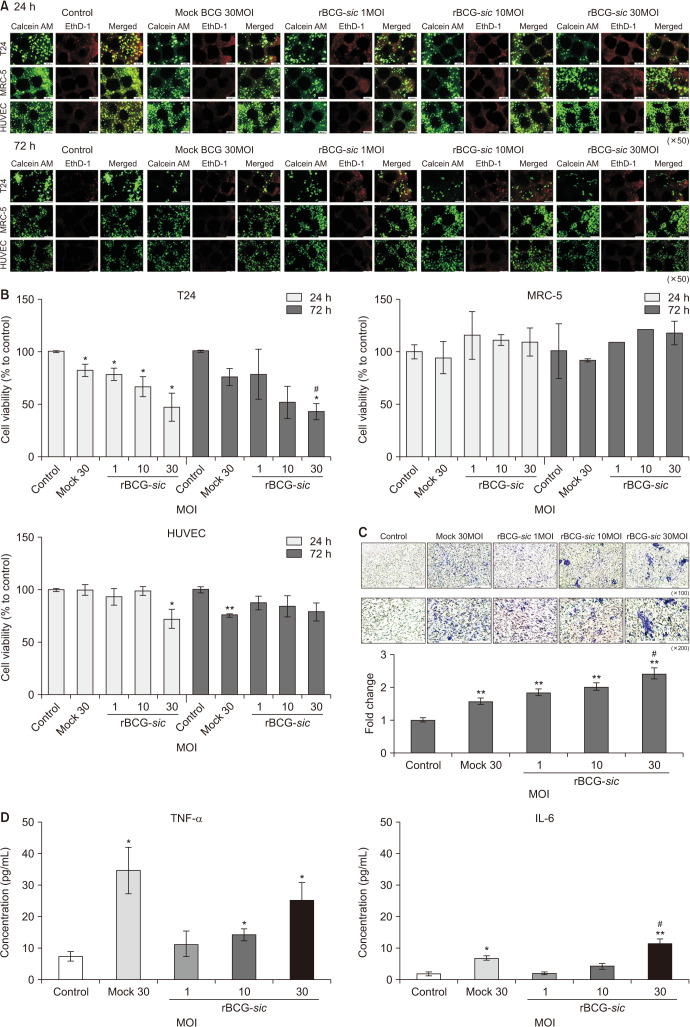
In live and dead staining, the viability of T24 cells showed a reverse correlation with increasing multiplicities of infection (MOI) with rBCG-sic treatment. It was also decreased with mock BCG 30MOI treatment, although the viabilities of the other cell lines (MRC-5 and HUVEC) at 24 and 72 hours did not differ in any of the experimental groups (Fig. 2A). In the CCK-8 assay, the viabilities of T24 cells (mean±SE, % to control group) were significantly decreased at 24 hours after treatment with mock BCG 30MOI and rBCG-sic 1, 10, 30MOI (p<0.05) (Fig. 2B). After 72 hours of treatment, the T24 cell viabilities were significantly reduced in the rBCG-sic 30MOI group (42.8%±6.4%, p<0.05), indicating a more effective reduction than in the mock BCG 30MOI group (75.7%±6.6%, p<0.05) (Fig. 2B). Therefore, we found that rBCG-sic eliminated T24 bladder cancer cells more effectively than mock BCG at 30MOI.
Fig. 2. Ex vivo immunotherapeutic effects of recombinant Bacillus Calmette–Guérin containing the streptococcal inhibitor of the complement gene (rBCG-sic) in high-throughput bladder cancer-on-a-chip models. (A) Live/dead staining assays of T24, MRC-5, and human umbilical vein endothelial cells (HUVECs) on the BCOC models at 24 and 72 hours after mock BCG and rBCG-sic treatment. (B) Cell proliferative assays of T24, MRC-5, and HUVECs on BCOC models after mock BCG and rBCG-sic treatment. (C) Chemotaxic images of monocytic THP-1 cells in the permeable membrane of the BCOC system (upper) and the fold change in THP-1 migration (lower) after mock BCG and rBCG-sic treatment. (D) The concentrations of cytokines (tumor necrosis factor-α [TNF-α] and interleukin-6 [IL-6]) on the BCOC model after mock BCG and rBCG-sic treatment. Data are the mean±SE (n=3–5 per group). *p<0.05 vs. control, **p<0.01 vs. control, #p<0.05 vs. mock-BCG 30 multiplicities of infection (MOI).
We evaluated the chemotaxis of monocytic THP-1 cells in the BCOC model after mock BCG and rBCG-sic treatment, and the chemotaxis of TH1 cells dose-dependently increased with rBCG-sic treatment (Fig. 2C). The fold change in THP-1 cell migration (mean±SE, fold to control) resulted in greater rBCG-sic 1, 10, and 30MOI (p<0.01) and mock BCG 30MOI (1.57±0.01, p<0.01) group values than control group values (Fig. 2C). The rBCG-sic 30MOI treatment showed a higher migration rate than the mock BCG 30MOI treatment (p<0.05).
We hypothesized that the increased internalization of rBCG-sic into the T24 cancer cell line would increase cytokine production and that the cytokines would induce immunological mechanisms. To prove this theory, we measured the concentrations of TNF-α and IL-6 after treatment with mock BCG versus rBCG-sic and found that both were increased in a dose-dependent manner (Fig. 2D). The rBCG-sic 10MOI (14.1±1.7 pg/mL) and 30MOI (25.2±4.5 pg/mL) groups showed greater TNF-α excretions than the control group (7.3±1.1 pg/mL, p<0.05). The IL-6 concentration was higher after rBCG-sic 30MOI treatment than control (11.2±1.3 pg/mL vs. 1.7±0.4 pg/mL, p<0.01) and mock BCG 30MOI (6.7±0.6 pg/mL, p<0.05) treatment (Fig. 2D).
2. In vivo anti-cancer efficacy of rBCG-sic in the orthotopic bladder cancer mouse model
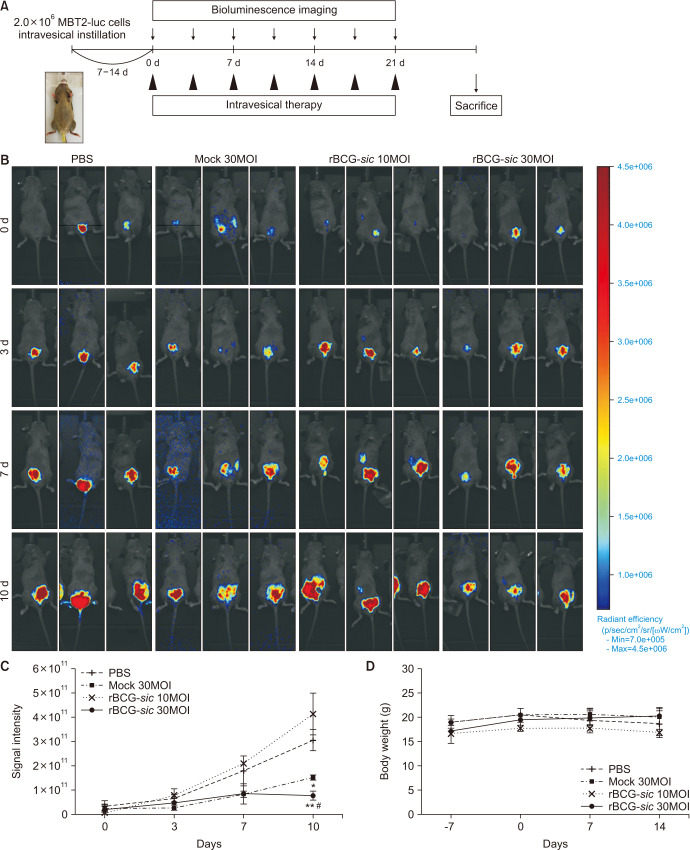
To evaluate the anti-cancer activity of rBCG-sic in vivo, we established an orthotopic bladder cancer mouse model and performed intravesical instillation using mock BCG or rBCG-sic (Fig. 3A). On the seventh day after tumor implantation, all mice that produced BLI were randomly divided into four groups (designated as day 0). There was no significant difference in BLS intensity among the groups at the time of randomization. From the third day, the scale was normalized to eliminate any possible disturbance from the background signal and facilitate the relative comparison of cancer growth among the treatment group. In particular, the rBCG-sic 30MOI group showed weak red signals, representing the effective suppression of tumor growth according to serial intravesical instillation (Fig. 3B).
Fig. 3. In vivo anti-cancer efficacy of recombinant Bacillus Calmette–Guérin containing the streptococcal inhibitor of the complement gene (rBCG-sic) in the orthotopic bladder cancer mouse model. (A) Schematic summary of the intravesical instillation in the orthotopic bladder cancer mouse model. (B) Representative in vivo imaging via bioluminescence signaling in the mouse bladder after intravesical mock BCG and rBCG-sic instillation. (C) Changes in tumor volume via signal intensity in mouse bladder after intravesical serial mock BCG and rBCG-sic instillation. (D) Body weight changes of the mice after intravesical serial mock BCG and rBCG-sic instillation. Data are the mean±SE (n=3–5 per group). *p<0.05 vs. phosphate-buffered saline (PBS), **p<0.01 vs. PBS, #p<0.05 vs. mock BCG 30 multiplicities of infection (MOI).
To compare cancer growth quantitatively, the changes in BLS intensity were measured. All groups showed similar increasing trends of BLS intensity on the seventh day; however, significant differences were observed in the rBCG-sic 30MOI group on the tenth day. The BLI (mean±SE ×109) of rBCG-sic 30MOI group (64.7±22.1 ×109) at the tenth day was significantly lower than that of the control (296.0±31.4 ×109, p<0.01) and mock BCG 30MOI (152.0±8.8 ×109, p<0.05) groups (Fig. 3C).
To determine the adverse side effects of rBCG-sic, we measured the body weights of the mice during the experimental period. We found no significant weight changes in any group, indicating its safety (Fig. 3D). However, the first deaths were observed in the control group on day 10 (2 mice), followed by the mock BCG 30MOI, rBCG-sic 10MOI, and rBCG-sic 30MOI groups on day 14 (2 mice each), and no mice survived until 21 days.
DISCUSSION
In this study, we fabricated a high-throughput BCOC model and investigated the effects of rBCG-sic in BCOC and orthotopic mouse models. We aimed to increase the therapeutic effect of BCG and reduce its side effects and toxicity. BCG toxicity can be overcome by lowering of its dose in every session of intravesical therapy [3]. Ojea et al. [13] recently reported a trial comparing MMC (30 mg) to a one-third (27 mg) and one-sixth dose (13.5 mg) of BCG in 430 patients with bladder cancer. The 27 mg BCG dose had the lowest recurrence rate. Toxicity was similar in both BCG groups but lower in the MMC group. This suggests that the optimal BCG dose was approximately one-third of the full dose. We confirmed that the rBCG-sic 10MOI may have a therapeutic effect similar to that of mock BCG 30MOI in the ex vivo and in vivo experimental models. This indicates that low-dose rBCG-sic has the same effect as standard-dose BCG. Furthermore, rBCG-sic showed a better effect at the same concentration.
In patients in whom intravesical BCG treatment fails, especially high-risk patients, radical therapy such as radical cystectomy, is considered the treatment of choice [14]. Given the BCG shortage and the morbidities of radical cystectomy, there is an urgent need for new therapeutic options for high-risk NMIBC cases [15]. Unfortunately, patients with NMIBC may refuse or be unfit for major surgery. Some alternative immunotherapies, such as the combination of IFN-α, mitomycin C, or bladder wall hyperthermia, have been investigated to enhance therapeutic effects. Intravesical therapy with recombinant BCG technology can be replaced by a new therapeutic strategy between BCG and radical surgery. If these techniques are applied to clinical practice, they may become a new alternative that can overcome BCG unresponsiveness or internalization disorders.
The cancer-on-a-chip model is being actively studied in various oncologic fields [16,17]. The BCOC in this study was characterized by implementing the tumor microenvironment using 3D bio-printing technology and a microfluidic system. We fabricated this high-throughput BCOC model by further developing a previous BCOC. BCG-refractory NMIBC showed a tendency toward frequent recurrence and disease progression to advanced or metastatic stage [18]. Locally advanced or metastatic bladder cancer is primarily treated with platinum-based combination chemotherapy; however, the efficacy of immunotherapy using immune checkpoint inhibitors (ICIs) has been investigated [19]. Although the recent treatment trend of bladder cancer is the combination therapy using ICIs, the problem is that it is not suitable for evaluating the therapeutic efficacy of new combined regimens. The high-throughput BCOC has the advantage of being able to evaluate the therapeutic effect of the ICI combination drug instead of BCG, and it can reduce the error that occurs during the experiment by simultaneously performing the test using four BCOC models.
We also investigated the concentrations of some cytokines and migration of monocytic THP-1 cells. After BCG internalization into urothelial and bladder cancer cells, BCG induces an immune response via cytokine release [20]. This immune cascade induces anti-cancer effects mediated by various immune cells, such as cytotoxic T cells, natural killer cells, neutrophils, and macrophages [21]. The changes in cytokine levels and infiltration of THP-1 cells indicated that the T24 cells were killed by immunologic reactions rather than a direct cytotoxic effect of BCG. The cancer cell-specific immunotherapeutic effect was supported by the constant maintenance of the viability of other cell lines such as MRC-5 and HUVECs. In the orthotopic mouse study, the mice showed no changes in body weight. This suggests that there were no direct toxic effects in the live animals.
Animal cancer models are important for evaluating the in vivo effects of therapeutic drugs. The orthotopic bladder cancer model resembles the human situation resulting from seeding various tumor cells in the bladder mucosa [22]. Whole-body optical imaging allows for repeated real-time in vivo monitoring of tumor growth [23]. BLI, the most sensitive molecular imaging technique to date, does not require expensive or complicated technology. BLI has the potential to become a valuable tool for the early detection of tumor growth such as NMIBC. In our study, the signal intensity of the rBCG-sic 30MOI group was lower than that of the control group. The rBCG-sic 30MOI also confirmed that the proliferation of bladder cancer was inhibited more than in the mock BCG 30MOI group. This can be explained by the fact that recombinant BCG had a significant inhibitory effect on bladder cancer in cell line experiments and animal models. Based on these results, we expect that rBCG-sic will have a strong anti-cancer effect in human clinical trials.
This study has limitations in discussing the therapeutic effect because clinical trials using recombinant BCG have not been conducted and its real-time efficacy has not been proven. We will continue to study whether recombinant BCG could be a new therapeutic alternative. We considered identifying a customized treatment method using patient-derived bladder cancer cells rather than commercial bladder cancer cell lines. In the BCOC study, T24 cell lines constituting BCOC started to die on the first day after treatment with mock BCG or rBCG-sic. We presumed that this was induced by the immediate toxic effect of BCG. After 72 hours, however, the anti-cancer effect on the T24 cells appeared only in the BCOC model treated with rBCG-sic 30MOI. We believe that direct toxicity was a temporary phenomenon, as the immunologic anti-cancer effect of rBCG-sic continued until 72 hours. Additionally, the T24 cell line was only used to evaluate the immunotherapeutic effects of the rBCG-sic. In a previous study, we investigated BCOC experiments with two different bladder cancer cell lines, T24 and 5637 [12]. We had reported better results in the T24 bladder cancer cell line at that time. We will be considered to improve the reliability of research by using various cell lines as much as possible. In the animal study, the mice in this experiment lived for an average of 14 days. We expected the mice to survive for 21 days; however, more than half of them had died by the fourteenth day. We do not believe that rBCG-sic has a direct cytotoxic effect that can kill the host because the groups had similar mortality rates. The early deaths of the mice can be explained by the fact that superficial bladder cancer progresses to advanced bladder cancer after 14 days or BLS of bladder cancer is not sensitive enough to detect early bladder cancer signals. Therefore, it will be necessary to demonstrate the toxicity and safety of rBCG-sic through future large-scale studies.
CONCLUSIONS
Based on the data described here, we conclude that rBCG-sic could be a useful tool for overcoming BCG unresponsiveness and an alternative second-line therapeutic options in patients with NMIBC. Additionally, a high-throughput BCOC model with a microfluidic system can successfully reflect the bladder cancer microenvironment.
Footnotes
CONFLICTS OF INTEREST: The authors have nothing to disclose.
FUNDING: This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology, Republic of Korea (NRF-2021R1A2C1004307 and NRF-2021M3E5E6037643).
- Research conception and design: In Ho Chang, Jung Hoon Kim, Joongwon Choi, and Se Young Choi.
- Data acquisition: Mirinae Kim, Su Jeong Kang, Sung-Hwan Kim, and Young Wook Choi.
- Statistical analysis: Jung Hoon Kim, Young Wook Choi, Su Jeong Kang, and Mirinae Kim.
- Drafting of the manuscript: Jung Hoon Kim.
- Critical revision of the manuscript: In Ho Chang.
- Obtaining funding: In Ho Chang.
- Administrative, technical, or material support: Mirinae Kim, Su Jeong Kang, Sung-Hwan Kim, and Young Wook Choi.
- Supervision: In Ho Chang and Se Young Choi.
- Approval of the final manuscript: In Ho Chang and Jung Hoon Kim.
References
- 1.Patard JJ, Rodriguez A, Leray E, Rioux-Leclercq N, Guillé F, Lobel B. Intravesical Bacillus Calmette-Guerin treatment improves patient survival in T1G3 bladder tumours. Eur Urol. 2002;41:635–641. doi: 10.1016/s0302-2838(02)00173-2. discussion 642. [DOI] [PubMed] [Google Scholar]
- 2.Punnen SP, Chin JL, Jewett MA. Management of bacillus Calmette-Guerin (BCG) refractory superficial bladder cancer: results with intravesical BCG and Interferon combination therapy. Can J Urol. 2003;10:1790–1795. [PubMed] [Google Scholar]
- 3.Witjes JA, Hendricksen K. Intravesical pharmacotherapy for non-muscle-invasive bladder cancer: a critical analysis of currently available drugs, treatment schedules, and long-term results. Eur Urol. 2008;53:45–52. doi: 10.1016/j.eururo.2007.08.015. [DOI] [PubMed] [Google Scholar]
- 4.Reddy KV, Yedery RD, Aranha C. Antimicrobial peptides: premises and promises. Int J Antimicrob Agents. 2004;24:536–547. doi: 10.1016/j.ijantimicag.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 5.Kwon JK, Chi BH, Choi SY, Kim SJ, Lee TJ, Kim K, et al. Murineβ-defensin-2 may regulate the effect of bacillus Calmette-Guérin (BCG) in normal mouse bladder. Urol Oncol. 2015;33:111.e9–111.e16. doi: 10.1016/j.urolonc.2014.10.015. [DOI] [PubMed] [Google Scholar]
- 6.Nawrocki KL, Crispell EK, McBride SM. Antimicrobial peptide resistance mechanisms of Gram-positive bacteria. Antibiotics (Basel) 2014;3:461–492. doi: 10.3390/antibiotics3040461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Akesson P, Sjöholm AG, Björck L. Protein SIC, a novel extracellular protein of Streptococcus pyogenes interfering with complement function. J Biol Chem. 1996;271:1081–1088. doi: 10.1074/jbc.271.2.1081. [DOI] [PubMed] [Google Scholar]
- 8.Choi SY, Kim SJ, Chi BH, Kwon JK, Chang IH. Modulating the internalization of bacille Calmette-Guérin by cathelicidin in bladder cancer cells. Urology. 2015;85:964.e7–964.e12. doi: 10.1016/j.urology.2014.12.028. [DOI] [PubMed] [Google Scholar]
- 9.Cho MJ, Kim MJ, Kim K, Choi YW, Lee SJ, Whang YM, et al. The immunotherapeutic effects of recombinant Bacillus Calmette-Guérin resistant to antimicrobial peptides on bladder cancer cells. Biochem Biophys Res Commun. 2019;509:167–174. doi: 10.1016/j.bbrc.2018.12.097. [DOI] [PubMed] [Google Scholar]
- 10.Riedl A, Schlederer M, Pudelko K, Stadler M, Walter S, Unterleuthner D, et al. Comparison of cancer cells in 2D vs 3D culture reveals differences in AKT-mTOR-S6K signaling and drug responses. J Cell Sci. 2017;130:203–218. doi: 10.1242/jcs.188102. [DOI] [PubMed] [Google Scholar]
- 11.Kim MJ, Chi BH, Yoo JJ, Ju YM, Whang YM, Chang IH. Structure establishment of three-dimensional (3D) cell culture printing model for bladder cancer. PLoS One. 2019;14:e0223689. doi: 10.1371/journal.pone.0223689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim JH, Lee S, Kang SJ, Choi YW, Choi SY, Park JY, et al. Establishment of three-dimensional bioprinted bladder cancer-on-a-chip with a microfluidic system using Bacillus Calmette-Guérin. Int J Mol Sci. 2021;22:8887. doi: 10.3390/ijms22168887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ojea A, Nogueira JL, Solsona E, Flores N, Gómez JM, Molina JR, et al. A multicentre, randomised prospective trial comparing three intravesical adjuvant therapies for intermediate-risk superficial bladder cancer: low-dose bacillus Calmette-Guerin (27 mg) versus very low-dose bacillus Calmette-Guerin (13.5 mg) versus mitomycin C. Eur Urol. 2007;52:1398–1406. doi: 10.1016/j.eururo.2007.04.062. [DOI] [PubMed] [Google Scholar]
- 14.Witjes JA. Management of BCG failures in superficial bladder cancer: a review. Eur Urol. 2006;49:790–797. doi: 10.1016/j.eururo.2006.01.017. [DOI] [PubMed] [Google Scholar]
- 15.Chang SS, Boorjian SA, Chou R, Clark PE, Daneshmand S, Konety BR, et al. Diagnosis and treatment of non-muscle invasive bladder cancer: AUA/SUO guideline. J Urol. 2016;196:1021–1029. doi: 10.1016/j.juro.2016.06.049. [DOI] [PubMed] [Google Scholar]
- 16.Thompson CL, Fu S, Knight MM, Thorpe SD. Mechanical stimulation: a crucial element of organ-on-chip models. Front Bioeng Biotechnol. 2020;8:602646. doi: 10.3389/fbioe.2020.602646. Erratum in: Front Bioeng Biotechnol 2021;9:658873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kashaninejad N, Nikmaneshi MR, Moghadas H, Kiyoumarsi Oskouei A, Rismanian M, Barisam M, et al. Organ-tumor-on-a-chip for chemosensitivity assay: a critical review. Micromachines (Basel) 2016;7:130. doi: 10.3390/mi7080130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim HS, Seo HK. Emerging treatments for bacillus Calmette-Guérin-unresponsive non-muscle-invasive bladder cancer. Investig Clin Urol. 2021;62:361–377. doi: 10.4111/icu.20200602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim HS, Seo HK. Immune checkpoint inhibitors for urothelial carcinoma. Investig Clin Urol. 2018;59:285–296. doi: 10.4111/icu.2018.59.5.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fuge O, Vasdev N, Allchorne P, Green JS. Immunotherapy for bladder cancer. Res Rep Urol. 2015;7:65–79. doi: 10.2147/RRU.S63447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thiounn N, Pages F, Mejean A, Descotes JL, Fridman WH, Romet-Lemonne JL. Adoptive immunotherapy for superficial bladder cancer with autologous macrophage activated killer cells. J Urol. 2002;168:2373–2376. doi: 10.1016/S0022-5347(05)64148-1. [DOI] [PubMed] [Google Scholar]
- 22.Kim SJ, Seo HK, Seo HH, Lee SJ, Kwon JK, Lee TJ, et al. Establishment of an orthotopic mouse non-muscle invasive bladder cancer model expressing the mammalian target of rapamycin signaling pathway. J Korean Med Sci. 2014;29:343–350. doi: 10.3346/jkms.2014.29.3.343. Erratum in: J Korean Med Sci 2014;29:617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.van der Horst G, van Asten JJ, Figdor A, van den Hoogen C, Cheung H, Bevers RF, et al. Real-time cancer cell tracking by bioluminescence in a preclinical model of human bladder cancer growth and metastasis. Eur Urol. 2011;60:337–343. doi: 10.1016/j.eururo.2011.05.005. [DOI] [PubMed] [Google Scholar]