Abstract
Purpose
To perform a retrospective review of the clinicopathological features of patients with conventional and non-conventional renal cell carcinoma (cRCC and ncRCC).
Materials and Methods
A large prospectively maintained uro-oncological registry was accessed to extract clinicopathological data of patients diagnosed with renal tumors who subsequently underwent nephrectomy from 1990–2019. Demographics and operative parameters were extracted. Analyses of overall survival (OS) and cancer-specific survival (CSS) were performed using the Kaplan–Meier method. Cox proportional-hazards analysis was used to identify risk factors which influenced survival.
Results
There were a total of 1,686 consecutive nephrectomies which was retrieved, with 1,286 cRCC and 400 ncRCC. The commonest ncRCC subtypes were papillary (n=198, 11.7%), clear cell papillary (n=50, 3.0%) and chromophobe (n=49, 2.9%) RCC. Kaplan–Meier estimates of OS were higher in cRCC (0.74; 95% confidence interval [CI], 0.71–0.78) than ncRCC (hazard ratio, 1.47; 95% CI, 1.16–1.87). Among individual subtypes, chromophobe RCC had the highest 5-year OS (0.90; 95% CI, 0.79–1.0). Among ncRCC subtypes, acquired cystic RCC demonstrated the highest association with end-stage renal failure and hypertension, with the highest CSS. MiT family translocation RCC had the youngest mean age at presentation (45.6±12.8 y) and excellent CSS. Factors associated with increased OS in the entire cohort included shorter operative time, partial nephrectomy and lower tumor stages.
Conclusions
This study provides a comprehensive contemporary overview of ncRCCs which are yet poorly characterized, in comparison to cRCCs. Data from this study would contribute towards tailored patient counseling and healthcare resource planning.
Keywords: Carcinoma, renal cell; Epidemiology; Nephrectomy; Retrospective studies; Survival analysis
INTRODUCTION
There has been a rising incidence rate of renal cell carcinoma (RCC) globally over the last few decades, attributed to the rising prevalence of risk factors such as hypertension, smoking, and obesity combined with the commonplace use of cross-sectional abdominal imaging [1]. Of all RCC subtypes, clear cell RCC (often referred to as conventional RCC [cRCC]) is the commonest, accounting for approximately 80% of all cases. The International Society of Urological Pathology (ISUP) [2] classification of renal tumors also recognizes rarer, non-conventional RCC (ncRCC) subtypes such as papillary, clear cell papillary, chromophobe, collecting duct, multilocular cystic renal neoplasm of low malignant potential (MCRN-LMP), MiT family translocation and tubulocystic RCC [3].
The histological classification of RCCs is extremely important in the management of the disease, considering the significant prognostic and therapeutic implications of each subtype. There is a multitude of strategies for treating RCC, ranging from active surveillance, minimally invasive ablative therapies, surgical interventions to molecular targeted therapy and novel immunotherapy. Clear preoperative characterization of these renal masses will aid the clinician in tailoring targeted management of these ncRCC to optimize overall survival (OS) and minimize morbidity of overtreatment. In this study, we aim to evaluate and investigate the prevalence, clinicopathological characteristics, surgical management and outcomes of ncRCC using a large, prospectively maintained database of RCC patients over the span of three decades.
MATERIALS AND METHODS
A large prospectively maintained, single-institution, electronic uro-oncological registry was accessed to extract clinicopathological data of patients who were diagnosed with renal tumors and subsequently underwent nephrectomy over a 30-year period from 1990 to 2019. The prospective collection of data in our uro-oncology database extracted for this study was approved by Institutional Review Board (IRB) of the Singapore General Hospital (approval number: 2009/1053/D). However, the conduct of retrospective, anonymized clinical audit study such as this study was exempted from IRB review and approval as per our IRB policy. Data captured included patient demographics, tumor stage (based on American Joint Committee on Cancer TNM Staging, 8th Edition) [4] and size, histological type, and nuclear grade at final pathology. Data was dichotomized into cRCC and ncRCC. Subgroups of ncRCC were: papillary, acquired cystic, clear cell papillary, chromophobe, collecting duct, MCRN-LMP, MiT family translocation, tubulocystic and unclassified.
Trends in presentation, disease management and survival outcomes were analyzed based on histological sub-types and time period. Our cancer registry closely follows international cancer registry standards for hospital-based and population-based cancer registries. This registry is managed by a team of dedicated full-time cancer registry staff who are led by senior urologists and a certified tumor registrar; the registry also carries out yearly validation exercises on data quality.
Comparisons of continuous variables were analyzed using the t-test and chi-squared test. Time to mortality from any cause or cancer-specific mortality was determined by calculating the number of months between the date of diagnosis and date of mortality recorded on the national death registry. Patients who were alive at the point of reference (31 Dec 2020) were censored. Kaplan–Meier estimates were used to evaluate OS and cancer-specific survival (CSS) based on cohorts. Differences in survival curves were compared using the log–rank test, and 5-year survival estimates were generated for different subtypes of RCC. Univariate and multivariate Cox regression models were created to evaluate the proportional hazards of risk factors for OS and CSS, with p<0.05 considered to be statistically significant.
RESULTS
1. Demographic data
A total of 1,686 consecutive nephrectomies with complete dataset were retrieved from the database between 1990 to 2019 and included in the analysis (Table 1). Mean age of the cohort was 58.4±11.6 years, and 66.0% were male. Almost half of our cohort (47.1%) had a Charlson comorbidity index (CCI) of 0–1 points. Mean follow-up time was 38.8±42.5 months. There were a total of 1,286 (76.3%) cRCC and 400 (23.7%) ncRCC. In the ncRCC group, papillary (198 cases, 11.7%), clear cell papillary (50 cases, 3.0%), and chromophobe (49 cases, 2.9%) were the commonest subtypes.
Table 1. Clinicopathological parameters in the overall cohort.
| Characteristic | Value (n=1,686) | ||
|---|---|---|---|
| Sex | |||
| Male | 1,112 (66.0) | ||
| Female | 574 (34.0) | ||
| Age at diagnosis (y) | 58.4±11.6 | ||
| Smoking history | |||
| Non-smoker | 1,103 (65.4) | ||
| Ex-smoker | 281 (16.7) | ||
| Current smoker | 207 (12.3) | ||
| Passive smoker | 3 (0.2) | ||
| BMI (kg/m2) | 25.0±4.38 | ||
| Hypertension | 1,021 (60.6) | ||
| Charlson comorbidity index | |||
| 0–1 | 794 (47.1) | ||
| ≥2 | 850 (50.4) | ||
| End-stage renal failure | 216 (12.8) | ||
| Symptomatic at presentation | 852 (50.5) | ||
| Follow-up time (mo) | 38.8±42.5 | ||
| Histologic subtypes | |||
| Conventional RCC (n=1,286) | |||
| Clear cell | 1,286 (76.3) | ||
| Non-conventional RCC (n=400) | |||
| Papillary | 198 (11.7) | ||
| Acquired cystic disease | 26 (1.5) | ||
| Chromophobe | 49 (2.9) | ||
| Clear cell papillary | 50 (3.0) | ||
| Collecting duct | 2 (0.1) | ||
| MCRN-LMP | 20 (1.2) | ||
| RCC, unclassified | 42 (2.5) | ||
| Translocation | 5 (0.3) | ||
| Tubulocystic | 8 (0.5) | ||
Values are presented as number (%) or mean±standard deviation.
BMI, body mass index; RCC, renal cell carcinoma; MCRN-LMP, multilocular cystic renal neoplasm of low malignant potential.
Table 2 shows demographic characteristics when stratified according to cRCC and ncRCC. When analyzed by group, mean age at diagnosis did not differ significantly in the ncRCC group compared to cRCC (58.1 years vs. 58.5 years, p=0.544). Mean body mass index (BMI) was lower (24.2 kg/m2 vs. 25.2 kg/m2, p<0.001) and end-stage renal failure was more prevalent (27.8% vs. 8.2%, p<0.001) in ncRCC. Follow-up times did not differ significantly.
Table 2. Clinicopathological parameters by group.
| Characteristic | cRCC (n=1,286) | ncRCC (n=400) | p-value | |
|---|---|---|---|---|
| Sex | ||||
| Male | 852 (66.3) | 260 (65.0) | 0.688 | |
| Female | 434 (33.7) | 140 (35.0) | ||
| Age at diagnosis (y) | 58.5±11.5 | 58.1±11.7 | 0.544 | |
| Smoking history | ||||
| Non-smoker | 843 (65.6) | 260 (65.0) | 0.727 | |
| Ex-smoker | 223 (17.3) | 58 (14.5) | ||
| Current smoker | 158 (12.3) | 49 (12.2) | ||
| Passive smoker | 2 (0.2) | 1 (0.2) | ||
| BMI (kg/m2) | 25.2±4.46 | 24.2±4.00 | <0.001 | |
| Hypertension | 754 (58.6) | 267 (66.8) | 0.001 | |
| Charlson comorbidity index | ||||
| 0–1 | 636 (49.5) | 158 (39.5) | 0.002 | |
| ≥2 | 624 (48.5) | 226 (56.5) | ||
| End-stage renal failure | 105 (8.2) | 111 (27.8) | <0.001 | |
| Symptomatic at presentation | 653 (50.8) | 199 (49.8) | 0.814 | |
| Follow-up time (mo) | 40.0±42.1 | 35.3±43.4 | 0.062 | |
Values are presented as number (%) or mean±standard deviation.
cRCC, conventional renal cell carcinoma; ncRCC, non-conventional RCC; BMI, body mass index.
2. Perioperative parameters
Overall, in terms of operative technique, Singapore General Hospital favored a laparoscopic approach (n=934, 55.4%) compared to open (n=533, 31.6%) and robotic (n=190, 11.3%) approaches. The proportion of radical nephrectomies was greater than that of partial nephrectomies (70.9% vs. 28.8%). Mean operative time was 193±73.8 minutes with a mean length of stay of 5.2±4.9 days. Table 3 demonstrates peri-operative parameters when stratified according to histological group. Mean operative time was shorter in the ncRCC group than cRCC group (185 minutes vs. 195 minutes, p=0.010) with similar blood loss (392 mL vs. 422 mL, p=0.536). There was no difference in length of stay between both groups; major Clavien–Dindo complication rate was comparable between both groups (6.2% vs. 5.5%, p=0.809). OS was significantly better in cRCC than ncRCC (81.3% vs. 76.0%, p=0.024).
Table 3. Perioperative parameters by group.
| Variable | cRCC (n=1,286) | ncRCC (n=400) | p-value | |
|---|---|---|---|---|
| Nephrectomy approach | ||||
| Laparoscopic | 710 (55.2) | 224 (56.0) | 0.886 | |
| Open | 411 (32.0) | 122 (30.5) | ||
| Robotic | 146 (11.4) | 44 (11.0) | ||
| Nephrectomy type | ||||
| Radical | 908 (70.6) | 287 (71.8) | 0.482 | |
| Partial | 377 (29.3) | 108 (27.0) | ||
| Operative time (min) | 195±74.9 | 185±69.3 | 0.010 | |
| Blood loss (mL) | 422±603 | 392±680 | 0.536 | |
| Clavien–Dindo score | ||||
| 0–2 | 1,133 (88.1) | 341 (85.2) | 0.809 | |
| ≥3 | 80 (6.2) | 22 (5.5) | ||
| Length of stay (d) | 5.16±5.01 | 5.30±4.69 | 0.627 | |
| Overall survival | 1,046 (81.3) | 304 (76.0) | 0.024 | |
| Cancer-specific survival | 1,131 (87.9) | 338 (84.5) | 0.093 | |
Values are presented as number (%) or mean±standard deviation.
cRCC, conventional renal cell carcinoma; ncRCC, non-conventional RCC.
3. Clinicopathological features
For the whole cohort of patients, stage 1, 2, 3, and 4 tumors comprised 62.3%, 9.1%, 19.2%, and 8.7% of cases respectively (Table 4). Differences in tumor extension into the pelvicalyceal system, perinephric fat, lymphovascular structures, and renal sinus were insignificant between the two groups.
Table 4. Clinicopathological characteristics by group.
| Variable | cRCC (n=1,286) | ncRCC (n=400) | p-value | |
|---|---|---|---|---|
| Pathological T | ||||
| T1 | 815 (63.4) | 255 (63.8) | 0.013 | |
| T2 | 126 (9.8) | 54 (13.5) | ||
| T3 | 319 (24.8) | 73 (18.2) | ||
| T4 | 23 (1.8) | 12 (3.0) | ||
| TX | 3 (0.2) | 0 (0.0) | ||
| Pathological N | ||||
| N0 | 975 (75.8) | 286 (71.5) | <0.001 | |
| N1 | 15 (1.2) | 24 (6.0) | ||
| NX | 295 (22.9) | 85 (21.2) | ||
| Pathological M | ||||
| M0 | 1,172 (91.1) | 367 (91.8) | 0.497 | |
| M1 | 106 (8.2) | 27 (6.8) | ||
| MX | 7 (0.5) | 1 (0.2) | ||
| Pathological stage | ||||
| Stage 1 | 801 (62.3) | 249 (62.2) | 0.015 | |
| Stage 2 | 104 (8.1) | 50 (12.5) | ||
| Stage 3 | 261 (20.3) | 62 (15.5) | ||
| Stage 4 | 115 (8.9) | 32 (8.0) | ||
| Pathological size (cm) | 5.36±3.35 | 5.44±4.54 | 0.719 | |
| Collecting duct invasion | 45 (3.5) | 19 (4.8) | 0.275 | |
| Lymphovascular invasion | 170 (13.2) | 41 (10.2) | 0.193 | |
| Renal sinus invasion | 92 (7.2) | 19 (4.8) | 0.137 | |
| Perinephric fat invasion | 212 (16.5) | 61 (15.2) | 0.728 | |
Values are presented as number (%) or mean±standard deviation.
cRCC, conventional renal cell carcinoma; ncRCC, non-conventional RCC.
Supplementary Tables 1, 2, 3 show the detailed breakdown of histological subtype in all RCC subtypes. Males were more likely to be affected with RCC in any form. Chromophobe and MCRN-LMP RCC had favorable pathological stages with corresponding good prognosis and OS; MCRN-LMP had the highest CSS at follow-up of all subtypes at 100%. Acquired cystic RCC demonstrated the highest association with end-stage renal failure (96.2%) and hypertension (92.3%) among all subtypes. MiT family translocation RCC had the youngest mean age at presentation (45.6±12.8 years) in our cohort, with excellent CSS.
4. OSS and CSS
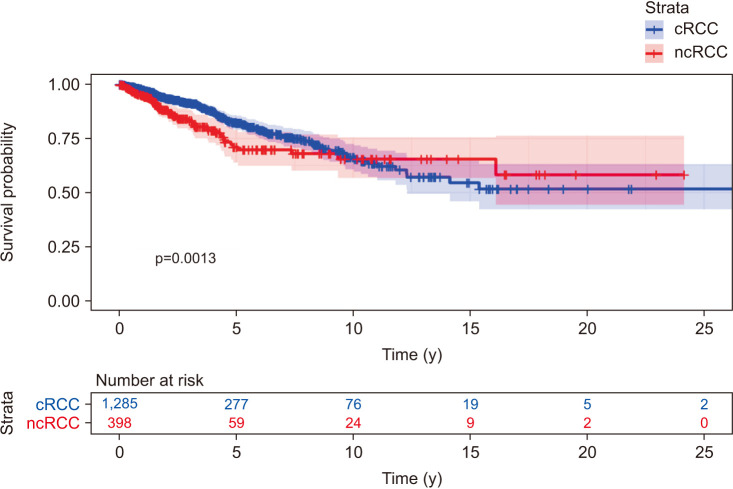
Kaplan–Meier analysis of patient data from this study showed that cRCC had a better prognosis than ncRCC across the duration of the retrospective study (OS hazard ratio [HR], 1.47; 95% confidence interval [CI], 1.16–1.87). Kaplan–Meier estimates of 5-year OS were also significantly higher in cRCC (0.76; 95% CI, 0.72–0.80) compared to ncRCC (0.59; 95% CI, 0.51–0.67); this was corroborated by visual inspection of Kaplan–Meier curves (Fig. 1).
Fig. 1. Kaplan–Meier overall survival (OS) curves stratified by non-conventional and conventional renal cell carcinoma (ncRCC and cRCC).
Among individual subtypes (Fig. 2), 5-year OS was highest in the chromophobe RCC subtype (0.90; 95% CI, 0.79–1.0). CSS stratified by cRCC versus ncRCC and by subtype are shown in Supplementary Figs. 1, 2. CSS was significantly higher in cRCC than nRCC (HR, 1.47; 95% CI, 1.09–1.98). HRs of OS for various RCC subtypes are also shown in Fig. 3, with the average survival of all patients in the cohort as the baseline comparator. In this analysis, multilocular cystic and chromophobe RCC were found to have the numerically highest HRs but their 95% CIs were not significant compared to the entire cohort, or to clear cell, papillary or clear cell papillary RCC. Collecting duct, translocation and unclassified RCC were found to have statistically significantly lower OS HRs than the rest of the cohort.
Fig. 2. Kaplan–Meier overall survival (OS) curves stratified by subtypes of renal cell carcinoma (RCC). NOS, unclassified.
Fig. 3. Hazard ratios for overall survival (OS) stratified by subtypes of renal cell carcinoma (RCC). NOS, unclassified.
Cox proportional-hazards univariate analysis identified several factors associated with a higher risk of all-cause mortality in all subtypes. For patient demographics, these include male sex, higher age, lower BMI, higher CCI, and presence of pre-nephrectomy end-stage renal failure (Supplementary Table 4). For peri-operative details, robotic surgery, partial nephrectomy, lower operation time, lower post-surgical Clavien–Dindo grade and shorter length of stay were associated with lower mortality. Tumor characteristics indicative of higher mortality included higher T, N, M, and overall stage, as well as tumor extension into the pelvicalyceal system, perinephric fat, lymphovascular structures and renal sinus. In multivariate analysis (Supplementary Table 4), lower BMI, higher age, higher CCI, higher nephrectomy time, higher post-surgical Clavien–Dindo grade, and higher pathological stage were still significantly associated with all-cause mortality, but male sex was no longer significant.
DISCUSSION
RCC accounts for approximately 4% of all new cancer cases globally and 90% to 95% of primary kidney tumors [5]. It is a highly heterogeneous disease from the clinicopathological point of view, as evident in the 2016 World Health Organization (WHO) classification of renal tumors, and exhibits distinct histopathologic features, biologic behavior, and variable response to therapy [3]. A one-size-fits-all approach is suboptimal for management of such a diverse group without considering tumor heterogeneity. To our best knowledge, our report is one of the largest comprehensive retrospective reviews focusing on ncRCC over a span of three decades. The non-conventional histology type represented 12.0% of RCCs in our cohort. Among the non-clear cell RCCs, the most common type is papillary RCC (11.7%), followed by clear cell papillary type (3.0%), chromophobe (2.9%), MCRN-LMP (1.2%), and other rarer subtypes. The trend observed in our cohort is consistent with previously reported proportions of each histologic subtype in other studies.
Surgical resection remains the mainstay of management of localized RCC, regardless of histologic subtypes. Current management of ncRCC is mainly extrapolated from studies and trials conducted for cRCC. It is well known that TNM staging, Eastern Cooperative Oncology Group (ECOG) status, ISUP/WHO grade and tumor necrosis are validated prognostic factors for localized RCC [6], however, the role of histological subtypes being independent prognostic factors remains controversial in the literature [7]. Our analysis suggested patients with cRCC have better 5-year OS compared to ncRCC and among all subtypes of RCC, chromophobe subtype has the best prognosis. Current guidelines of follow-up protocol do not differentiate between different histologic subtypes. However, our data suggests that patients diagnosed with ncRCC may benefit from a dynamic follow-up protocol post-surgical resection.
There is paucity of data regarding diagnostic predictors for ncRCC in the literature. Clinically, most RCC are asymptomatic and incidentally detected on imaging performed for unrelated clinical indications [8]. RCCs exhibit a wide spectrum of morphologic appearances and certain imaging characteristics can be useful in discriminating between the subtypes [9]. Papillary RCC commonly manifests a peripherally located well-circumscribed tumor which may appear hypovascular due to spontaneous tumour necrosis radiologically. Bilaterality and multifocality are also more common in papillary RCC. Chromophobe RCC usually appears as a well circumscribed and homogenous lesion and rarely shows perinephric infiltration or vascular involvement [10]. Detailed pre-operative imaging is important in differentiating between various histologic subtypes, which allows for tailored management even before surgical resection.
Clear cell papillary RCC, comprising less than 5% of all RCC, is a histologic subtype that is morphologically, immunohistochemically, and genetically distinct from both clear cell RCC and papillary RCC [11]. Clear cell papillary RCC was previously found in patients with end-stage renal disease, however, subsequent studies have suggested that clear cell papillary RCC also occurs in patients with normal kidneys [12]. There was 3% of our cohort who were diagnosed with this subtype and 34% of them have a history of end-stage renal disease. All 50 patients included in our study had good prognosis with OS of 76% and CSS of 86%, which is consistent with previously reported low malignant potential of this histologic subtype [13]. Clear cell papillary RCC was formally recognized by the ISUP Vancouver classification of renal neoplasia in 2013 and recently added to the WHO Tumours of the Urinary System and Male Genital Organs 2016 edition [3]. Being a relatively newly described and rare histologic subtype, currently there are no recommendations guiding the management of such tumors.
MiT family translocation RCCs are a heterogenous category of renal tumors [14] which all express MiT transcription factors, typically from chromosomal translocation and rarely from gene amplification. This subgroup comprises Xp11 translocation RCC and t(6;11) RCC. Both were first described in and disproportionately involve pediatric patients, though the prevalence in adult patients may be underestimated due to morphological overlap with cRCCs. In our cohort, 5 patients were diagnosed with this subtype at a median age of 45.6 years, with excellent CSS. Both subtypes are indolent neoplasms, with t(6,11) RCCs being generally been more indolent than the Xp11 translocation RCCs [15]. However, they have the potential to recur late (up to 20 or 30 years after diagnosis for Xp11 translocation RCC and up to 8 years for t(6,11) RCC). Being a newly-described RCC subtype, the optimal therapeutic strategy for MiT family translocation RCCs remains to be elucidated due to its rarity.
MCRN-LMP is a rare type of RCC characterized by multilocular cysts with thin fibrous septa, separated from the kidney by a fibrous pseudocapsule. It was first categorized as a separate disease entity based on the WHO 2004 classification of kidney tumors and is known to have a favorable prognosis [16]. Microscopically, it is characterized by a single layer of low grade neoplastic clear cells in the cyst wall. The radiological appearance of this subtype can range from a Bosniak IIF cyst to a Bosniak IV cyst lesion [17], which makes it difficult to differentiate it from other complex cystic renal lesions pre-operatively. In our cohort, 20 patients were diagnosed with MCRN-LMP; most of our patients (90%) presented with stage 1 disease. The CSS and OS were 100% and 95% respectively. Our findings are consistent with previously reported low malignant potential of multilocular cystic RCC [18]. This suggests that patients with this particular subtype might benefit from a nephron sparing approach.
This study reports the clinicopathological outcomes of a large consecutive surgical cohort of patients who underwent nephrectomies over a span of three decades in a single tertiary institution. While the consecutive accrual of patients allows for accurate description of temporal trends, it is still limited by its retrospective analysis nature. Another limitation of our study is the inability to continually adapt and reflect the ever changing histopathological classifications of rare subtypes of RCC prior to official recognition and reclassification by the WHO 2004 classification system and subsequent newer editions. Ideally it would have been optimal to re-examine the specimens to facilitate accurate classification, however in view of the large number and time lapse, it is technically challenging and not feasible due to loss of materials and tissue decay over time.
As our cohort is also derived from a single tertiary centre, the present study outcomes might also not be widely generalizable to other cohorts. Moreover, heterogeneity in terms of clinical and pathological characteristics of different ncRCC subtypes, combined with the rarity of several such subtypes, precluded in-depth survival comparisons between each subtype. Lastly, the authors also acknowledge the limitations of analysis on a predominantly surgical cohort which predominantly consists of patients with clinically organ-confined disease at presentation and may under-represent advanced or de-novo metastatic disease.
CONCLUSIONS
In conclusion, the results of this single-institution retrospective study provide a comprehensive epidemiological and clinicopathological picture of ncRCCs. This information will contribute toward tailored patient counseling and healthcare resource planning. Furthermore, it helps to guide follow-up protocols for different subtypes after operative resection, which are currently mainly based on pathologic stage.
Footnotes
CONFLICTS OF INTEREST: The authors have nothing to disclose.
FUNDING: None.
- Research conception and design: John Shyi Peng Yuen and Ee Jean Lim.
- Data acquisition: Ee Jean Lim, Hong Hong Huang, and Jingqiu Li.
- Statistical analysis: Ee Jean Lim, Khi Yung Fong, and Jingqiu Li.
- Data analysis and interpretation: Ee Jean Lim, Khi Yung Fong, Jingqiu Li, and Hong Hong Huang.
- Drafting of the manuscript: Ee Jean Lim, Khi Yung Fong, and Jingqiu Li.
- Critical revision of the manuscript: John Shyi Peng Yuen, Kenneth Chen, Kae Jack Tay, Christopher Wai Sam Cheng, Henry Sun Sien Ho, and Nye Thane Ngo.
- Obtaining funding: No funding, not applicable.
- Administrative, technical, or material support: Hong Hong Huang and John Shyi Peng Yuen.
- Supervision: John Shyi Peng Yuen, Christopher Wai Sam Cheng, and Kae Jack Tay.
- Approval of the final manuscript: John Shyi Peng Yuen, Christopher Wai Sam Cheng, and Nye Thane Ngo.
SUPPLEMENTARY MATERIALS
Supplementary materials can be found via https://doi.org/10.4111/icu.20210373.
Clinical parameters by subtype
Perioperative parameters by subtype
Clinicopathological characteristics by subtype
Cox proportional-hazards analysis of factors affecting mortality in entire cohort
Kaplan–Meier cancer-specific survival (CSS) curves stratified by atypical and conventional RCC.
Kaplan–Meier cancer-specific survival (CSS) curves stratified by subtype. RCC, renal cell carcinoma; NOS, unclassified.
References
- 1.Chow WH, Devesa SS, Warren JL, Fraumeni JF., Jr Rising incidence of renal cell cancer in the United States. JAMA. 1999;281:1628–1631. doi: 10.1001/jama.281.17.1628. [DOI] [PubMed] [Google Scholar]
- 2.Srigley JR, Delahunt B, Eble JN, Egevad L, Epstein JI, Grignon D, et al. The International Society of Urological Pathology (ISUP) Vancouver classification of renal neoplasia. Am J Surg Pathol. 2013;37:1469–1489. doi: 10.1097/PAS.0b013e318299f2d1. [DOI] [PubMed] [Google Scholar]
- 3.Moch H, Cubilla AL, Humphrey PA, Reuter VE, Ulbright TM. The 2016 WHO classification of tumours of the urinary system and male genital organs-part A: renal, penile, and testicular tumours. Eur Urol. 2016;70:93–105. doi: 10.1016/j.eururo.2016.02.029. [DOI] [PubMed] [Google Scholar]
- 4.Amin MB, Edge SB, Greene FL, Byrd DR, Brookland RK, Washington MK, et al. AJCC cancer staging manual [Internet] New York: Springer International Publishing; 2017. [cited 2021 May 12]. Available from: https://www.springer.com/gp/book/9783319406176 . [Google Scholar]
- 5.Tan X, Zhai Y, Chang W, Hou J, He S, Lin L, et al. Global analysis of metastasis-associated gene expression in primary cultures from clinical specimens of clear-cell renal-cell carcinoma. Int J Cancer. 2008;123:1080–1088. doi: 10.1002/ijc.23637. [DOI] [PubMed] [Google Scholar]
- 6.Klatte T, Rossi SH, Stewart GD. Prognostic factors and prognostic models for renal cell carcinoma: a literature review. World J Urol. 2018;36:1943–1952. doi: 10.1007/s00345-018-2309-4. [DOI] [PubMed] [Google Scholar]
- 7.Kuthi L, Jenei A, Hajdu A, Németh I, Varga Z, Bajory Z, et al. Prognostic factors for renal cell carcinoma subtypes diagnosed according to the 2016 WHO renal tumor classification: a study involving 928 patients. Pathol Oncol Res. 2017;23:689–698. doi: 10.1007/s12253-016-0179-x. [DOI] [PubMed] [Google Scholar]
- 8.Escudier B, Eisen T, Porta C, Patard JJ, Khoo V, Algaba F, et al. Renal cell carcinoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2012;23 Suppl 7:vii65–vii71. doi: 10.1093/annonc/mds227. [DOI] [PubMed] [Google Scholar]
- 9.Kim JK, Kim TK, Ahn HJ, Kim CS, Kim KR, Cho KS. Differentiation of subtypes of renal cell carcinoma on helical CT scans. AJR Am J Roentgenol. 2002;178:1499–1506. doi: 10.2214/ajr.178.6.1781499. [DOI] [PubMed] [Google Scholar]
- 10.Low G, Huang G, Fu W, Moloo Z, Girgis S. Review of renal cell carcinoma and its common subtypes in radiology. World J Radiol. 2016;8:484–500. doi: 10.4329/wjr.v8.i5.484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kuroda N, Ohe C, Kawakami F, Mikami S, Furuya M, Matsuura K, et al. Clear cell papillary renal cell carcinoma: a review. Int J Clin Exp Pathol. 2014;7:7312–7318. [PMC free article] [PubMed] [Google Scholar]
- 12.Aron M, Chang E, Herrera L, Hes O, Hirsch MS, Comperat E, et al. Clear cell-papillary renal cell carcinoma of the kidney not associated with end-stage renal disease: clinicopathologic correlation with expanded immunophenotypic and molecular characterization of a large cohort with emphasis on relationship with renal angiomyoadenomatous tumor. Am J Surg Pathol. 2015;39:873–888. doi: 10.1097/PAS.0000000000000446. [DOI] [PubMed] [Google Scholar]
- 13.Wang K, Zarzour J, Rais-Bahrami S, Gordetsky J. Clear cell papillary renal cell carcinoma: new clinical and imaging characteristics. Urology. 2017;103:136–141. doi: 10.1016/j.urology.2016.12.002. [DOI] [PubMed] [Google Scholar]
- 14.Argani P. MiT family translocation renal cell carcinoma. Semin Diagn Pathol. 2015;32:103–113. doi: 10.1053/j.semdp.2015.02.003. [DOI] [PubMed] [Google Scholar]
- 15.Caliò A, Segala D, Munari E, Brunelli M, Martignoni G. MiT family translocation renal cell carcinoma: from the early descriptions to the current knowledge. Cancers (Basel) 2019;11:1110. doi: 10.3390/cancers11081110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Williamson SR, MacLennan GT, Lopez-Beltran A, Montironi R, Tan PH, Martignoni G, et al. Cystic partially regressed clear cell renal cell carcinoma: a potential mimic of multilocular cystic renal cell carcinoma. Histopathology. 2013;63:767–779. doi: 10.1111/his.12239. [DOI] [PubMed] [Google Scholar]
- 17.Hindman NM, Bosniak MA, Rosenkrantz AB, Lee-Felker S, Melamed J. Multilocular cystic renal cell carcinoma: comparison of imaging and pathologic findings. AJR Am J Roentgenol. 2012;198:W20–W26. doi: 10.2214/AJR.11.6762. [DOI] [PubMed] [Google Scholar]
- 18.Suzigan S, López-Beltrán A, Montironi R, Drut R, Romero A, Hayashi T, et al. Multilocular cystic renal cell carcinoma: a report of 45 cases of a kidney tumor of low malignant potential. Am J Clin Pathol. 2006;125:217–222. doi: 10.1309/AH6F-C77P-YR2V-6YAY. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Clinical parameters by subtype
Perioperative parameters by subtype
Clinicopathological characteristics by subtype
Cox proportional-hazards analysis of factors affecting mortality in entire cohort
Kaplan–Meier cancer-specific survival (CSS) curves stratified by atypical and conventional RCC.
Kaplan–Meier cancer-specific survival (CSS) curves stratified by subtype. RCC, renal cell carcinoma; NOS, unclassified.