Abstract
In early-stage HER2-positive breast cancer, biomarkers that guide deescalation and/or escalation of systemic therapy are needed. CelTIL score is a novel, combined biomarker based on stromal tumor-infiltrating lymphocytes and tumor cellularity and is determined in tumor biopsies at week 2 of anti-HER2 therapy only. We evaluated the prognostic value of CelTIL in 196 patients with early-stage HER2-positive disease treated with standard trastuzumab-based chemotherapy in the NeoALTTO phase III trial. Using a prespecified CelTIL cutoff, a better 5-year event-free survival and overall survival was observed between CelTIL-high and CelTIL-low score with a 76.4% (95% confidence interval [CI] = 68.0% to 85.0%) vs 59.7% (95% CI = 50.0% to 72.0%) (hazard ratio = 0.40, 95% CI = 0.17 to 0.94) and 86.4% (95% CI = 80.0% to 94.0%) vs 73.5% (95% CI = 64.0% to 84.0%) (hazard ratio = 0.43, 95% CI = 0.20 to 0.92), respectively. Statistical significance was maintained after adjusting for baseline tumor-infiltrating lymphocytes, hormone receptor status, pretreatment tumor size and nodal status, type of surgery, treatment arm, and pathological complete response. Further studies to support CelTIL as an early readout biomarker to help deescalate or escalate systemic therapy in HER2-positive breast cancer seem warranted.
Use of (neo)adjuvant chemotherapy with at least 1 anti-HER2 agent is recommended for most patients with early-stage HER2-positive breast cancer (1). Several studies have explored strategies to improve HER2 blockade, such as adding 1 year of adjuvant pertuzumab (2), 1 year of neratinib after trastuzumab (3), or trastuzumab-emtansine (T-DM1) in patients who do not achieve a pathological complete response (pCR) (4). Despite such success, most patients are cured with chemotherapy and trastuzumab. Biomarkers to identify patients not requiring these anti-HER2 therapies are needed.
Various biomarkers determined on diagnosis have been explored for their predictive and/or prognostic value in early-stage HER2-positive disease, including T (tumor size) N (nodes) M (metastases) staging, hormone receptor status (5), stromal tumor-infiltrating lymphocytes (TILs) (6,7), PAM50 subtypes (8,9), loss of PTEN (10), PIK3CA mutations (11), and p95HER2 expression (12). However, their clinical utility remains unknown, and further validation is needed. Biological information obtained after neoadjuvant therapy is gaining attention, because it measures individual response to specific therapies and can inform treatment strategies (4). Promising biomarkers being evaluated in residual tumors are PAM50 subtypes (13), TILs (14), and HER2 expression (15). One caveat is the 4- to 6-month wait until completion of neoadjuvant therapy.
We have shown the value of defining predictive and prognostic biomarkers after some weeks of anti-HER2 therapy (16). We developed the CelTIL score, a combined biomarker based on both TILs and tumor cellularity determined by a tumor biopsy after 14 days (± 2 days) of anti-HER2 therapy only in early-stage HER2-positive breast cancer (16). The CelTIL score was determined in the PAMELA (17) and LPT109096 (18) neoadjuvant trials as an early readout of the probability of a pCR at surgery beyond baseline TILs, PAM50 subtypes, and main clinical-pathological characteristics. High CelTIL scores identify tumors that are highly immune infiltrated with reduced tumor cellularity (16).
The value of CelTIL as a biomarker for long-term survival is unknown. Here, we determined the CelTIL score after 2 weeks of anti-HER2 therapy in tumor samples from the neoadjuvant NeoALTTO phase III trial (19) that randomly assigned 455 patients to receive lapatinib, trastuzumab, or trastuzumab-lapatinib for 6 weeks followed by the addition of weekly paclitaxel for 12 weeks and adjuvant fluorouracil, epirubicin, and cyclophosfamide. The lapatinib arm was excluded because of noninclusion of trastuzumab. The trial was approved by the ethics committee and relevant health authorities at each participating institution. All participating patients gave the written informed consent before the study entry.
TILs and tumor cellularity were centrally determined from formalin-fixed paraffin-embedded hematoxylin and eosin staining of tumor tissues obtained at day 14 (±2 days) of the assigned anti-HER2 therapy. The CelTIL score was centrally evaluated as a continuous variable using a reported formula (CelTIL unscaled score = −0.8 × tumor cellularity [%] + 1.3 × TILs [%]; the score was scaled to reflect a 0-100 range) (16). The primary objective was to evaluate the association of CelTIL (using the predefined 33.59 scaled cutoff score identified in the PAMELA trial) (17) and event-free survival (EFS), defined as the time from randomization to first event (breast cancer relapse after surgery, second primary cancer, or death without recurrence). Secondary objectives were to evaluate the association of CelTIL and overall survival (OS) and pCR defined as ypT0/is ypN0 and assess the prognostic effect of CelTIL per pCR status. For associations with EFS and OS, multivariable Cox proportional hazards regression models using a landmark analysis (from 30-week post randomization) were performed, adjusting for baseline TILs, hormone receptor status, pretreatment tumor size and nodal status, planned type of surgery, pCR status, and assigned treatment arm. The proportionality assumption was tested through evaluation of Schoenfeld residuals. Univariate and multivariable Cox proportional regressions models were used to investigate the association of CelTIL with pCR, and odds ratios and 95% confidence intervals (CIs) were calculated. Baseline TILs were analyzed as a continuous variable (per 10% increase) for association with EFS and OS and as low vs high for associations with pCR (≤5% vs >5%). In all Cox model analyses, the statistical significance level was a 2-sided alpha of .05.
A total 196 of 303 (64.7%) tumor samples were evaluated for CelTIL (108 from the trastuzumab arm and 88 from the trastuzumab-lapatinib arm). Patients’ clinical-pathological characteristics were comparable with the original population in NeoALTTO (Supplementary Table 1, available online). Median age at diagnosis was 49 years, 59.7% of patients had T2 tumors, and 84.7% had clinical N0/N1. Per prespecified CelTIL cutoff, 45.4% of patients had CelTIL-low and 54.6% had CelTIL-high.
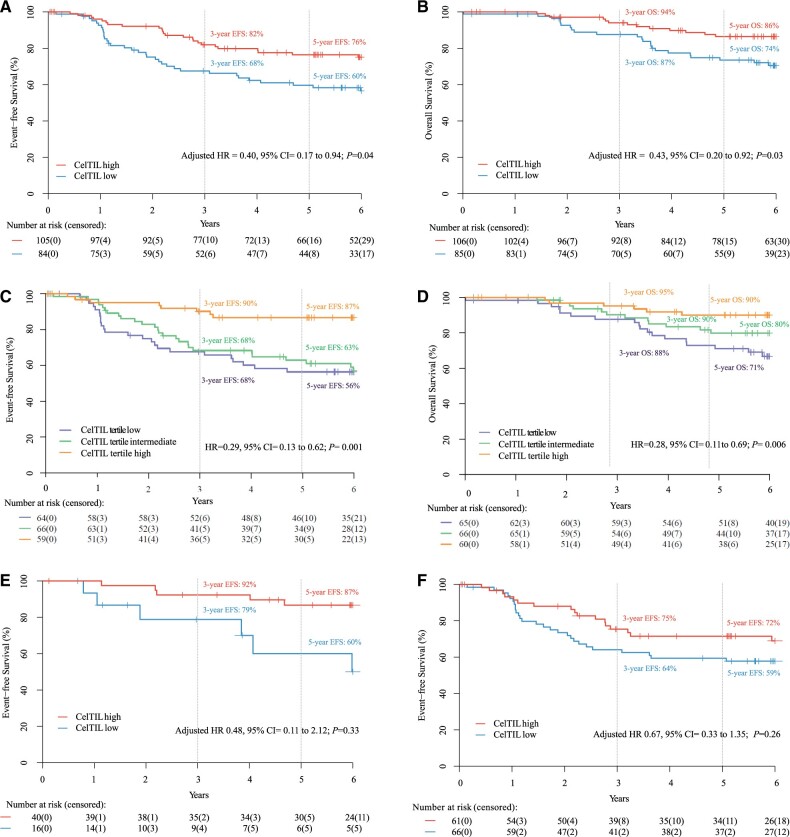
The CelTIL score was independently associated with EFS, OS, and pCR. Five-year EFS was 76.4% (95% CI = 68.0% to 85.0%) and 59.7% (95% CI = 50.0% to 72.0%) in patients with CelTIL-high and CelTIL-low, respectively (adjusted hazard ratio [HR] = 0.40, 95% CI = 0.17 to 0.94, P = .04). Five-year OS rate was 86.4% (95% CI = 80.0% to 94.0%) and 73.5% (95 CI = 64.0% to 84.0%) in patients with CelTIL-high and CelTIL-low, respectively (adjusted HR = 0.43, 95% CI = 0.20 to 0.92, P = .03). When CelTIL was evaluated by tertiles, the upper tertile showed better EFS and OS (EFS univariate HR = 0.29, 95% CI = 0.13 to 0.62, P = .001; OS univariate HR = 0.28, 95% CI = 0.11 to 0.69, P = .006). Patients with CelTIL-high disease had a higher pCR rate vs CelTIL-low group (37% vs 18%, adjusted odds ratio = 2.21, 95% CI = 1.09 to 4.62, P = .03) (Figure 1). All univariate and multivariable survival analyses are shown in Table 1 (Supplementary Table 2, available online). To note, only TILs determined at week 2 (as a continuous variable) were statistically associated with better EFS (Supplementary Table 3, available online).
Figure 1.
Survival outcomes in NeoALTTO based on CelTIL score. A) Event-free survival (EFS) based on the CelTIL prespecified cutoff score. B) Overall survival (OS). C) EFS based on CelTIL tertile groups. D) OS based on CelTIL tertile groups. E) EFS in patients with a pathological complete response (pCR). F) EFS in patients with a non-pCR. Estimates of EFS and OS were from Kaplan-Meier curves and tests of differences by Cox proportional hazards model. All statistical tests were 2 sided. CelTIL = score based on tumor cellularity and tumor infiltrating lymphocytes at day 14; CI = confidence interval; HR = hazard ratio.
Table 1.
Association of CelTIL score with event-free survival and overall survival in NeoALTTO
| Variable and type of analysis | Event-free survival |
Overall survival |
||
|---|---|---|---|---|
| HR (95% CI) | P a | HR (95% CI) | P a | |
| CelTIL high vs low | ||||
| Univariate | 0.51 (0.30 to 0.85) | .01 | 0.43 (0.22 to 0.84) | .01 |
| Multivariable | 0.40 (0.17 to 0.94) | .04 | 0.43 (0.20 to 0.92) | .03 |
| CelTIL as a continuous variable | ||||
| Univariate | 0.83 (0.73 to 0.95) | .006 | 0.85 (0.72 to 1.01) | .06 |
| Multivariable | 0.78 (0.62 to 1.00) | .05 | 0.85 (0.71 to 1.03) | .09 |
All statistical tests were 2-sided. CelTIL = score based on tumor cellularity and tumor infiltrating lymphocytes at day 14; CI = confidence interval; HR = hazard ratio.
This is the first report, to our knowledge, to show an independent association between an early, optimal on-treatment measurement of TILs and tumor cellularity and long-term-survival outcome in early-stage HER2-positive breast cancer treated with anti-HER2–based therapy. The ability of CelTIL to predict survival benefit to specific drugs is currently unknown. However, with further validation, CelTIL could be used with other clinical-pathological variables as an early survival readout and to select patients in prospective clinical trials for escalation or deescalation of adjuvant regiments. For example, patients achieving pCR and being CelTIL-low may require additional adjuvant anti-HER2 treatments because of their poor prognosis. Additionally, CelTIL could help to identify patients who may do well with standard therapy and thereby not be candidates for adjuvant clinical trials. Determining the CelTIL score has the limitation that an additional biopsy at day 15 should be conducted, which is an invasive procedure. Moreover, the score was initially established for early HER2-positive breast cancer treated with a chemo-free regimen, and its value in other contexts, such as upfront anti-HER2–based chemotherapy, remains unknown. Our data suggest that CelTIL seemed to provide prognostic stratification in patients who did and did not achieve pCR, although this finding is underpowered and did not reach statistical significance (Supplementary Figure 1, available online); further data will be required for confirmation. The limited sample size is a limitation to determine the prognostic effect of 2 or more variables. Finally, CelTIL could be used in window-of-opportunity or preoperative trials to compare biological activity of anti-HER2 drugs and estimate potential survival advantages among strategies. More validations are warranted to draw robust conclusions.
Funding
This work was supported by PhD4MDgrant of “Departament de Salut” exp SLT008/18/00122 (to NC) and the Breast Cancer Research Foundation (BCRF, grant No. 17–194 grant to RS).
Notes
Role of the funder: The founders had no role in the design of the study; the collection, analysis and interpretation of the data; the writing of the manuscript; and the decision to submit the manuscript to publication.
Disclosures: Aleix Prat has declared personal honoraria from Pfizer, Novartis, Roche, MSD Oncology, Lilly and Daiichi Sankyo, travel, accommodations and expenses paid by Daiichi Sankyo, research funding from Roche and Novartis, consulting/advisory role for NanoString Technologies, Amgen, Roche, Novartis, Pfizer and Bristol-Myers Squibb and patent PCT/EP2016/080056: HER2 AS A PREDICTOR OF RESPONSE TO DUAL HER2 BLOCKADE IN THE ABSENCE OF CYTOTOXIC THERAPY. Evandro de Azambuja has declared honoraria and/or advisory board from Roche/GNE, Novartis, Seattle Genetics and Zodiac; travel grants from Roche/GNE and GSK/Novartis; research grants to his institution from Roche/GNE, AstraZeneca, GSK/Novartis and Servier. Florentine Hilbers declared that her institution received funding from GSK and later Novartis for the conduct of the NeoALTTO trial. Serena Di Cosimo declared honoraria and/or advisory board from Pierre-Fabre and Novartis. Paolo Nuciforo declared that his institution received funding from GSK and later Novartis for the conduct of the NeoALTTO trial. Debora Fumagalli declared that her institution received funding from GSK and later Novartis for the conduct of the NeoALTTO trial. Sherene Loi has declared non-remunerated consultant and research funding to institution of Bristol-Meyers Squibb, Roche Genentech, Puma Biotechnology, Pfizer, Seattle Genetics, Novartis, Merck and AstraZeneca, consulting fees to institution of Aduro Biotech and G1 Therapeutics. Crsitina Saura has declared personal fees as consultant and advisory board of Celgene, Daiichi Sankyo, Genomic health, Novartis, Pierre Fabre, synthon biopharmaceuticals, Merck, Odonate therapeutics, Philips Healthwork, prIME oncology and Sanofi Aventis. The other authors have nothing to declared travel grants.
Author contributions: Study design: N.C., S.L., A.P. and Sh.L. Acquisition of the data: S.L., P.N., R.S., D.F., F.H., Y.W., E.A., I.L., S.C., C.S., J.H., A.P. and Sh.L. Data analysis: S.L and Sh.L. Interpretation of the data: N.C., S.L., A.P. and Sh.L. Writing of the manuscript: N.C., S.L., A.P. and Sh.L. Review of the manuscript: all authors.
Acknowledgments: We thank the patients and their families for participating in the study.
Data Availability
The data underlying this article cannot be shared publicly due to the confidentiality of the patients that were included in the clinical trial. The data will be shared on reasonable request to the corresponding authors.
Supplementary Material
References
- 1. Burstein HJ, Curigliano G, Loibl S, et al. ; Members of the St. Gallen International Consensus Panel on the Primary Therapy of Early Breast Cancer 2019. Estimating the benefits of therapy for early-stage breast cancer: the St. Gallen International Consensus Guidelines for the primary therapy of early breast cancer 2019. Ann Oncol. 2019;30(10):1541–1557. [DOI] [PubMed] [Google Scholar]
- 2. von Minckwitz G, Procter M, de Azambuja E, et al. ; APHINITY Steering Committee and Investigators. Adjuvant pertuzumab and trastuzumab in early HER2-positive breast cancer. N Engl J Med. 2017;377(2):122–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Martin M, Holmes FA, Ejlertsen B, et al. Neratinib after trastuzumab-based adjuvant therapy in HER2-positive breast cancer (ExteNET): 5-year analysis of a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2017;18(12):1688–1700. [DOI] [PubMed] [Google Scholar]
- 4. von Minckwitz G, Huang C-S, Mano MS, et al. Trastuzumab emtansine for residual invasive HER2-positive breast cancer. N Engl J Med. 2019;380(7):617–628. [DOI] [PubMed] [Google Scholar]
- 5. Brandão M, Caparica R, Malorni L, et al. What is the real impact of estrogen receptor status on the prognosis and treatment of HER2-positive early breast cancer? Clin Cancer Res. 2020;26(12):2783–2788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Salgado R, Denkert C, Campbell C, et al. Tumor-infiltrating lymphocytes and associations with pathological complete response and event-free survival in HER2-positive early-stage breast cancer treated with lapatinib and trastuzumab: a secondary analysis of the NeoALTTO trial. JAMA Oncol. 2015;1(4):448–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Salgado R, Denkert C, Demaria S, et al. ; International TILs Working Group 2014. The evaluation of tumor-infiltrating lymphocytes (TILs) in breast cancer: recommendations by an International TILs Working Group 2014. Ann Oncol. 2015;26(2):259–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Prat A, Carey LA, Adamo B, et al. Molecular features and survival outcomes of the intrinsic subtypes within HER2-positive breast cancer. J Natl Cancer Inst. 2014;106(8):dju152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Prat A, Pascual T, De Angelis C, et al. HER2-enriched subtype and ERBB2 expression in HER2-positive breast cancer treated with dual HER2 blockade. J Natl Cancer Inst. 2020;112(1):46–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Carbognin L, Miglietta F, Paris I, et al. Prognostic and predictive implications of PTEN in breast cancer: unfulfilled promises but intriguing perspectives. Cancers. 2019;11(9):1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Loibl S, Majewski I, Guarneri V, et al. PIK3CA mutations are associated with reduced pathological complete response rates in primary HER2-positive breast cancer: pooled analysis of 967 patients from five prospective trials investigating lapatinib and trastuzumab†. Ann Oncol. 2016;27(8):1519–1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Scaltriti M, Rojo F, Ocaña A, et al. Expression of p95HER2, a truncated form of the HER2 receptor, and response to anti-HER2 therapies in breast cancer. J Natl Cancer Inst. 2007;99(8):628–638. [DOI] [PubMed] [Google Scholar]
- 13. Brasó-Maristany F, Griguolo G, Pascual T, et al. Phenotypic changes of HER2-positive breast cancer during and after dual HER2 blockade. Nat Commun. 2020;11(1):385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kurozumi S, Inoue K, Matsumoto H, et al. Prognostic utility of tumor-infiltrating lymphocytes in residual tumor after neoadjuvant chemotherapy with trastuzumab for HER2-positive breast cancer. Sci Rep. 2019;9(1):1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Guarneri V, Dieci MV, Barbieri E, et al. Loss of HER2 positivity and prognosis after neoadjuvant therapy in HER2-positive breast cancer patients. Ann Oncol. 2013;24(12):2990–2994. [DOI] [PubMed] [Google Scholar]
- 16. Nuciforo P, Pascual T, Cortés J, et al. A predictive model of pathologic response based on tumor cellularity and tumor-infiltrating lymphocytes (CelTIL) in HER2-positive breast cancer treated with chemo-free dual HER2 blockade. Ann Oncol. 2018;29(1):170–177. [DOI] [PubMed] [Google Scholar]
- 17. Llombart-Cussac A, Cortés J, Paré L, et al. HER2-enriched subtype as a predictor of pathological complete response following trastuzumab and lapatinib without chemotherapy in early-stage HER2-positive breast cancer (PAMELA): an open-label, single-group, multicentre, phase 2 trial. Lancet Oncol. 2017;18(4):545–554. [DOI] [PubMed] [Google Scholar]
- 18. Holmes FA, Nagarwala YM, Espina VA, et al. Correlation of molecular effects and pathologic complete response to preoperative lapatinib and trastuzumab, separately and combined prior to neoadjuvant breast cancer chemotherapy. JCO. 2011;29(suppl 15):506–506. [Google Scholar]
- 19. de Azambuja E, Holmes AP, Piccart-Gebhart M, et al. Lapatinib with trastuzumab for HER2-positive early breast cancer (NeoALTTO): survival outcomes of a randomised, open-label, multicentre, phase 3 trial and their association with pathological complete response. Lancet Oncol. 2014;15(10):1137–1146. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article cannot be shared publicly due to the confidentiality of the patients that were included in the clinical trial. The data will be shared on reasonable request to the corresponding authors.