Abstract
Background
Although use of gonadotropin-releasing hormone agonist (GnRHa) during chemotherapy is an established strategy to protect ovarian function in premenopausal breast cancer patients, no long-term safety data are available, raising some concerns in women with hormone receptor–positive disease. There are controversial data on its fertility preservation potential.
Methods
The Prevention of Menopause Induced by Chemotherapy: a Study in Early Breast Cancer Patients—Gruppo Italiano Mammella 6 (PROMISE-GIM6) trial is a multicenter, randomized, open-label, phase III superiority trial conducted at 16 Italian centers from October 2003 to January 2008. Eligible patients were randomly assigned to (neo)adjuvant chemotherapy alone (control arm) or combined with the GnRHa triptorelin (GnRHa arm). The primary planned endpoint was incidence of chemotherapy-induced premature ovarian insufficiency. Post hoc endpoints were disease-free survival (DFS), overall survival (OS), and post-treatment pregnancies. Hazard ratios (HRs) and 95% confidence intervals (CIs) were calculated.
Results
Of 281 randomly assigned patients, 80.4% had hormone receptor–positive breast cancer. Median follow-up was 12.4 years (interquartile range = 11.3-13.2 years). No differences in 12-year DFS (65.7% [95% CI = 57.0% to 73.1%] in the GnRHa arm vs 69.2% [95% CI = 60.3% to 76.5%] in the control arm; HR = 1.16, 95% CI = 0.76 to 1.77) or in 12-year OS (81.2% [95% CI = 73.6% to 86.8%] in the GnRHa arm vs 81.3% [95% CI = 73.1% to 87.2%] in the control arm; HR = 1.17, 95% CI = 0.67 to 2.03) were observed. In patients with hormone receptor–positive disease, the hazard ratio was 1.02 (95% CI = 0.63 to 1.63) for DFS and 1.12 (95% CI = 0.59 to 2.11) for OS. In the GnRHa and control arms, 9 and 4 patients had a posttreatment pregnancy, respectively (HR = 2.14, 95% CI = 0.66 to 6.92).
Conclusions
Final analysis of the PROMISE-GIM6 trial provides reassuring results on the safety of GnRHa use during chemotherapy as a strategy to preserve ovarian function in premenopausal patients with early breast cancer, including those with hormone receptor–positive disease.
The majority of breast malignancies arising in premenopausal women are hormone receptor–positive tumors (1). The negative independent prognostic value of young age at diagnosis appears to be specifically related to this breast cancer subtype (2). Therefore, although the integration of genomic tests into the treatment decision-making process is likely to reduce the indication for adjuvant chemotherapy among young patients (3,4), many premenopausal women with hormone receptor–positive early breast cancer remain candidates to receive both chemotherapy and endocrine therapy (5). Among the side effects of chemotherapy in these women, premature ovarian insufficiency (POI) may negatively affect their global health throughout their lives (6,7), leading to both infertility and the short- and long-term consequences of early menopause (8). All guidelines strongly recommend to inform premenopausal patients before treatment initiation about chemotherapy-induced POI risk and to offer the available strategies to counteract this side effect (6,7,9).
Oocyte and embryo cryopreservation are standard options for fertility preservation in young patients wishing future conception (6,7,9). Ovarian suppression obtained with the administration of a gonadotropin-releasing hormone agonist (GnRHa) during chemotherapy is currently recommended as a strategy to preserve ovarian function in premenopausal breast cancer patients but not as a stand-alone fertility preservation technique (6,7,9). Notably, preservation of ovarian function is highly relevant to many premenopausal women irrespective of their age and pregnancy desire (10).
Current guidelines strongly advise to conduct further research efforts in order to collect long-term data from existing trials investigating GnRHa use during chemotherapy (6,7). Specifically, no long-term safety results are available, raising some concerns about concurrent use of GnRHa during chemotherapy in patients with hormone receptor–positive disease (11). Moreover, controversial data are available on its fertility preservation potential, and no evidence exists on the role of this strategy in patients with germline pathogenic variants in the BRCA genes (6).
The randomized phase III Prevention of Menopause Induced by Chemotherapy: a Study in Early Breast Cancer Patients—Gruppo Italiano Mammella 6 (PROMISE-GIM6) study is the largest trial that investigated ovarian suppression with GnRHa use during chemotherapy in premenopausal women with early breast cancer (12,13). The study met its primary endpoint, showing a statistically significant reduction in the occurrence of chemotherapy-induced POI 1 year after the completion of cytotoxic therapy (from 25.9% to 8.9%; odds ratio = 0.28, 95% confidence interval [CI] = 0.14 to 0.59) (12). A subsequent study update at a median follow-up of 7.3 years showed that GnRHa use during chemotherapy was associated with higher 5-year probability of ovarian function recovery (from 64.0% to 72.6%; hazard ratio [HR] = 1.28, 95% CI = 0.98 to 1.68) (13). The present analysis reports the final results of the PROMISE-GIM6 trial at a median follow-up exceeding 12 years.
Methods
Study Design and Patients
Details of the PROMISE-GIM6 study were previously reported (12,13). Briefly, this is a multicenter, randomized, open-label, phase III superiority trial aiming to investigate the benefit of ovarian suppression obtained by administering the GnRHa triptorelin before and during chemotherapy in reducing the risk of treatment-induced POI in premenopausal women with early breast cancer.
The main inclusion criteria were age 18-45 years and premenopausal status at the time of diagnosis of hormone receptor–positive or –negative early breast cancer. Hormone receptor positivity evaluated locally was defined as at least 1% of positive cells for estrogen and/or progesterone receptors.
The study was coordinated by the GIM study group. The ethics committees of all 16 Italian participating institutions approved the study, and written informed consent was required from all patients before inclusion.
The trial is registered on Clinicaltrial.gov (identifier: NCT00311636).
Random Assignment
Eligible patients were randomly assigned to receive chemotherapy plus concurrent triptorelin (GnRHa arm) or chemotherapy alone (control arm). Random assignment in a 1:1 allocation ratio was done centrally, with center being the only stratification factor (12). The Clinical Trials Unit of the IRCCS Policlinico San Martino Hospital in Genova (Italy) was responsible for central data collection and study management.
Study Procedures
In patients randomly assigned to the GnRHa arm, intramuscular administration of triptorelin 3.75 mg was started at least 1 week before chemotherapy initiation and was then given every 28 days for the whole duration of cytotoxic therapy. Following chemotherapy completion, adjuvant endocrine therapy for at least 5 years was recommended to patients with hormone receptor–positive disease. In both study arms, patients with hormone–receptor positive disease who resumed their ovarian function were allowed to receive ovarian suppression as part of adjuvant endocrine therapy.
Study Endpoints
Primary endpoint was incidence of chemotherapy-induced POI defined as no resumption of menstrual activity and postmenopausal follicle-stimulating hormone and estradiol levels 1 year after chemotherapy completion. A post hoc extension of the original study design aimed to collect long-term follow-up data (12,13). The current analysis focuses on disease-free survival (DFS) and overall survival (OS). Updated data on post-treatment pregnancies and a descriptive analysis in patients with germline BRCA pathogenic variants were also reported.
Local recurrences, distant metastases, ipsilateral or contralateral breast cancer, second primary malignancy, or death from any cause were considered DFS events; death from any cause was the definition of an OS event. Pregnancy event was defined as any of the following: at term or preterm delivery, miscarriage, and/or induced abortion. Pregnancy desire was not an inclusion criteria, and this information was not collected as part of the trial; patients were asked about post-treatment pregnancies during annual follow-up visits, and those without were asked about the reasons (eg, no desire and/or no attempt). BRCA genetic testing was conducted locally. These data were systematically collected during annual follow-up visits performed in each center according to clinical practice.
Statistical Analysis
The trial was designed to detect a 20% absolute reduction (from 60% to 40%) in the incidence of chemotherapy-induced POI in the experimental arm, with a 2-sided alpha error of 5% and a power of 90% (12). Results on DFS, OS, and post-treatment pregnancies reported in this manuscript are to be considered exploratory considering that long-term outcomes were not preplanned in the trial protocol and the power of the statistical analyses for these endpoints was not prespecified.
All analyses were conducted based on the intention-to-treat population. DFS and OS intervals were computed from the date of random assignment to the date of the first occurrence of a DFS or OS event, respectively. Time to pregnancy was defined as the interval from random assignment to the start of the first pregnancy, irrespective of its outcome. For all endpoints, observation times of patients without the event were censored on the date of their last contact. December 31, 2018, was the cut-off date used to perform an administrative censoring to all time-to-event analyses. Patients were considered to be lost to follow-up if no information on long-term outcomes was available after the cut-off date.
The reverse Kaplan-Meier method was used to calculate median period of follow-up and its interquartile range (IQR). DFS and OS probabilities were computed according to the Kaplan-Meier method. The log-log method was used to calculate the confidence interval of survival time probabilities. Cumulative incidence of pregnancy was estimated accounting for DFS events as competing risk events. The Cox proportional hazards model, or the Fine and Gray model in the presence of competing risks, was used to calculate unadjusted and adjusted hazard ratios with 95% confidence interval as estimates of treatment effect. The multivariable model included the covariates with known prognostic value (ie, tumor size, nodal status, hormone receptor status). Multivariable analyses were conducted after single imputation of missing values of tumor size, nodal status, and hormone receptor status in 7 patients. Single imputation was performed assuming monotone missing patterns and using the logistic regression method. A second multivariable model was used to investigate the adjustment for other covariates (in addition to tumor size, nodal status, hormone receptor status), such as timing of chemotherapy, type of chemotherapy, and time to GnRHa reinstitution after the end of chemotherapy. This latter covariate was included in the model as a time-dependent variable. The Schoenfeld plot was assessed to check for the proportional hazards assumption.
For subgroup analyses of survival outcomes, an interaction test was used to determine the consistency of treatment effect according to hormone receptor status (positive and negative). Statistical significance of all coefficients was tested with the likelihood ratio test. Adjustment for multiple testing was not applied.
All statistical tests were 2-sided; statistical significance was considered with P values of .05 or less. Statistical analyses were conducted by L.B. and E.B. using SAS 9.2 (SAS Institute, Cary, NC, USA).
Results
Patients
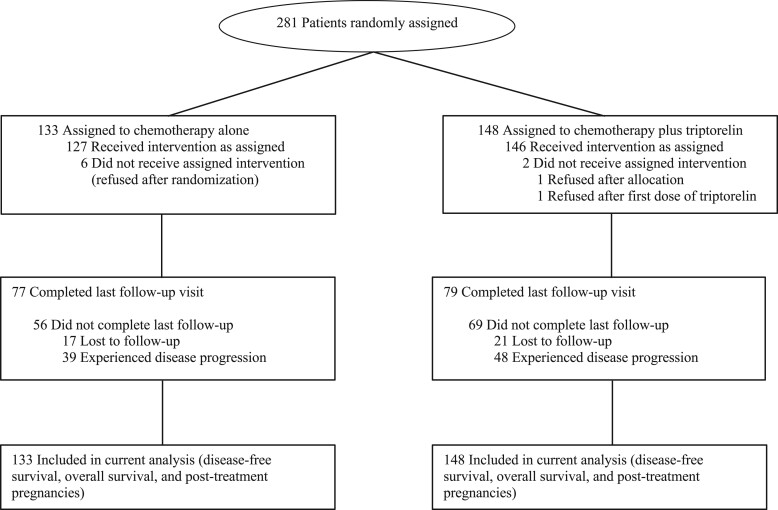
A total of 281 patients were enrolled between October 24, 2003, and January 14, 2008 (Figure 1). At the study cutoff date, 38 patients (13.5%) were lost to follow-up, 21 of 148 (14.2%) in the GnRHa arm and 17 of 133 (12.8%) in the control arm. Median follow-up was 12.4 years (IQR = 11.3-13.2 years).
Figure 1.
The PROMISE-GIM6 trial profile.
Median age was 39 years (Table 1). Among the 226 (80.4%) patients with hormone receptor–positive tumors, 69 of 117 (59.0%) and 70 of 109 (64.2%) in the GnRHa and control arms, respectively, received adjuvant GnRHa at the time of ovarian function resumption following chemotherapy completion.
Table 1.
Baseline patient and tumor characteristics
| Characteristics | Control arm (n = 133) | GnRHa arm (n = 148) |
|---|---|---|
| Median age (range), y | 39 (25-45) | 39 (24-45) |
| Tumor size, No. (%) | ||
| pT1 | 75 (56.4) | 90 (60.8) |
| pT2-4 | 54 (40.6) | 56 (37.8) |
| Unknown | 4 (3.0) | 2 (1.4) |
| Axillary nodes, No. (%) | ||
| pN0 | 67 (50.4) | 61 (41.2) |
| pN1-2 | 62 (46.6) | 85 (57.4) |
| Unknown | 4 (3.0) | 2 (1.4) |
| Hormone receptor status, No. (%) | ||
| ER-negative and PR-negative | 22 (16.5) | 29 (19.6) |
| ER-positive, PR-positive, or both | 109 (82.0) | 117 (79.1) |
| Unknown | 2 (1.5) | 2 (1.4) |
| Timing of chemotherapy, No. (%) | ||
| Adjuvant therapy | 117 (88.0) | 133 (89.9) |
| Neoadjuvant therapy | 10 (7.5) | 13 (8.8) |
| Not begun | 6 (4.5) | 2 (1.4) |
| Type of chemotherapy, No. (%) | ||
| Anthracycline-based | 57 (42.9) | 56 (37.8) |
| Anthracycline- and taxane-based | 62 (46.6) | 86 (58.1) |
| CMF-based | 8 (6.0) | 4 (2.7) |
| Cumulative cyclophosphamide dose, median (IQR), mg/m2 | 4008 (3624-5550) | 4080 (3697-5400) |
| Duration of chemotherapy, median (IQR), wk | 16.9 (15.0-21.3) | 17.8 (15.0-21.3) |
| Treatment completed as planned, No. (%) | ||
| Chemotherapy | 121 (91.0) | 143 (96.6) |
| GnRHa during chemotherapy | NA | 142 (95.9) |
| Type of adjuvant endocrine therapy in patients with hormone receptor–positive diseasea, No. (%) | ||
| No treatment | 5 (4.6) | 5 (4.3) |
| GnRHa alone | 3 (2.8) | 6 (5.1) |
| GnRHa + tamoxifen | 65 (59.6) | 61 (52.1) |
| GnRHa + aromatase inhibitor | 2 (1.8) | 2 (1.7) |
| Tamoxifen | 22 (20.2) | 30 (25.6) |
| Tamoxifen followed by aromatase inhibitor | 12 (11.0) | 13 (11.1) |
| Median duration of endocrine therapy (IQR), yb | 5.00 (4.75-5.04) | 5.00 (4.94-5.08) |
| Median duration of adjuvant GnRHa (IQR), yc | 4.10 (2.08-5.04) | 4.08 (2.06-4.92) |
| Median interval between chemotherapy completion and adjuvant GnRHa initiation (IQR), moc | 1.28 (0.46-5.16) | 4.51 (2.96-8.16) |
Calculated on the total number of patients with hormone receptor–positive disease (117 in the GnRHa group and 109 in the control group). CMF = cyclophosphamide, methotrexate, fluorouracil; ER = estrogen receptor; GnRHa = gonadotropin–releasing hormone agonist; IQR = interquartile range; NA = not applicable; PR = progesterone receptor.
Calculated on the total number of patients with hormone receptor–positive disease receiving adjuvant endocrine therapy (112 in the GnRHa group and 104 in the control group).
Calculated on the total number of patients with hormone receptor–positive disease receiving adjuvant GnRHa (69 in the GnRHa group and 70 in the control group).
Disease-Free Survival
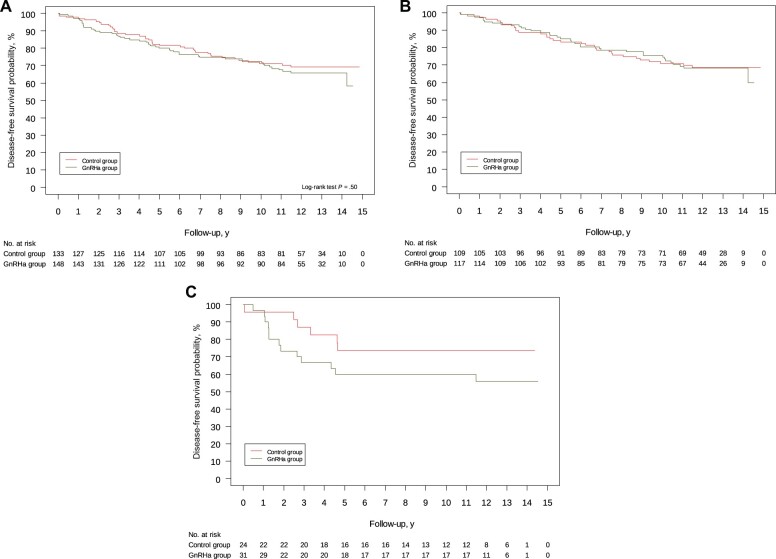
Of 87 DFS events, 48 (32.4%) were observed in the GnRHa arm and 39 (29.3%) in the control arm (Table 2). Twelve-year DFS was 65.7% (95% CI = 57.0% to 73.1%) in the GnRHa arm and 69.2% (95% CI = 60.3% to 76.5%) in the control arm (HR = 1.16, 95% CI = 0.76 to 1.77, P = .50; Figure 2, A). Results from the multivariable Cox proportional hazard models are reported in SupplementaryTables 1 and 2 (available online).
Table 2.
Long-term outcomes according to treatment arma
| Outcomes | Control arm (n = 133) No. (%) |
GnRHa arm (n = 148) No. (%) |
|---|---|---|
| No event | 94 (70.7) | 100 (67.6) |
| Distant recurrence | 19 (14.3) | 21 (14.2) |
| Locoregional recurrence | 9 (6.8) | 10 (6.8) |
| Distant and locoregional recurrence | 0 (0) | 4 (2.7) |
| Contralateral recurrence | 5 (3.8) | 6 (4.1) |
| Second malignancy | 2 (1.5) | 1 (0.7) |
| Death | 4 (3.0) | 6 (4.1) |
GnRHa = gonadotropin–releasing hormone agonist.
Figure 2.
Kaplan-Meier curves for disease-free survival according to treatment arm among (A) all randomly assigned patients, (B) patients with hormone receptor–positive breast cancer, and (C) patients with hormone receptor–negative breast cancer. All statistical tests were 2-sided. GnRHa = gonadotropin-releasing hormone agonist.
Among patients with hormone receptor–positive disease, 12-year DFS was 68.3% (95% CI = 58.4% to 76.3%) in the GnRHa arm and 68.5% (95% CI = 58.6% to 76.6%) in the control arm (HR = 1.02, 95% CI = 0.63 to 1.63; Figure 2, B). Among patients with hormone receptor–negative disease, 12-year DFS was 55.7% (95% CI = 36.0% to 71.5%) in the GnRHa arm and 73.4% (95% CI = 50.1% to 87.1%) in the control group (HR = 1.93, 95% CI = 0.73 to 5.07; Figure 2, C).
Overall Survival
Of 51 OS events, 28 (18.9%) occurred in the GnRHa arm and 23 (17.3%) in the control arm. Twelve-year OS was 81.2% (95% CI = 73.6% to 86.8%) in the GnRHa arm and 81.3% (95% CI = 73.1% to 87.2%) in the control arm (HR = 1.17, 95% CI = 0.67 to 2.03, P = .58; Figure 3, A). Results from the multivariable Cox proportional hazard models are reported in Supplementary Tables 3 and 4 (available online).
Figure 3.
Kaplan-Meier curves for overall survival according to treatment arm among (A) all randomly assigned patients, (B) patients with hormone receptor–positive breast cancer, and (C) patients with hormone receptor–negative breast cancer. All statistical tests were 2-sided. GnRHa = gonadotropin-releasing hormone agonist.
Among patients with hormone receptor–positive disease, 12-year OS was 83.2% (95% CI = 74.7% to 89.1%) in the GnRHa arm and 82.3% (95% CI = 73.2% to 88.5%) in the control arm (HR = 1.12, 95% CI = 0.59 to 2.11; Figure 3, B). Among patients with hormone receptor–negative disease, 12-year OS was 73.0% (95% CI = 53.2% to 85.5%) in the GnRHa arm and 77.6% (95% CI = 54.3% to 90.0%) in the control arm (HR = 1.24, 95% CI = 0.41 to 3.79; Figure 3, C).
Pregnancies
A total of 9 patients in the GnRHa arm and 4 in the control arm had a post-treatment pregnancy (Supplementary Table 5, available online), with a 12-year cumulative incidence estimate of pregnancy of 6.5% (95% CI = 3.5% to 12.3%) and 3.2% (95% CI = 1.2% to 8.3%), respectively (HR = 2.14, 95% CI = 0.66 to 6.92, P = .20; Supplementary Figure 1, available online). The age-adjusted estimate of the hazard ratio was 2.00 (95% CI = 0.63 to 6.40, P = .24). When the analysis was performed by excluding patients who declared no pregnancy attempts (44 patients [29.7%] in the GnRHa arm and 45 patients [33.8%] in the control arm), the hazard ratio was 2.41 (95% CI = 0.64 to 9.03, P = .19).
Of 13 pregnancies, 6 occurred in women with hormone receptor–positive tumors (5 [4.3%] among the 117 patients in the GnRHa arm and 1 [0.9%] among the 109 women in the control arm) and 7 in those with hormone receptor–negative tumors (4 [13.8%] among the 29 patients in the GnRHa arm and 3 [13.6%] among the 22 women in the control arm). The interval from random assignment to first pregnancy ranged between 1.0 and 10.2 years, being 6.2 to 10.2 years and 1.0 to 6.5 years among women with hormone receptor–positive and –negative disease, respectively.
BRCA
Of 43 patients tested for BRCA, 10 harbored germline BRCA pathogenic variants, of whom 5 were in BRCA1 (3 in the GnRHa arm and 2 in the control arm) and 5 in BRCA2 (1 in the GnRHa arm and 4 in the control arm). Supplementary Table 6 (available online) reports baseline characteristics between patients with or without germline BRCA pathogenic variants and those not tested. Among BRCA-mutated patients, incidence of chemotherapy-induced POI was 0% (0 of 4) and 33% (2 of 6) in the GnRHa and control arms, respectively. One post-treatment pregnancy was described in a patient with the BRCA1 pathogenic variant in the control arm.
Discussion
After a median follow-up of 12.4 years, final analysis of the PROMISE-GIM6 study provides reassuring results on the safety of administering GnRHa during chemotherapy as a strategy to preserve ovarian function in premenopausal women with early breast cancer, particularly among women with hormone receptor–positive disease. No statistically significant difference in DFS and OS was observed between treatment arms. Nine patients had a post-treatment pregnancy in the GnRHa arm and 4 in the control arm.
To elucidate the gonadal protective effect of administering GnRHa during chemotherapy, several randomized trials were conducted (14). Among them, the PROMISE-GIM6 study has some unique features (12,13): this is the trial with the largest sample size, the majority of included patients (80%) had hormone receptor–positive disease, and the median follow-up exceeds 12 years.
Despite being a standard approach for ovarian function preservation in premenopausal women with early breast cancer, many physicians offer this strategy only to patients with hormone receptor–negative disease (15). This attitude is a consequence of the safety concerns related to both avoiding chemotherapy-induced amenorrhea and giving antiestrogen therapy during chemotherapy in patients with hormone receptor–positive breast cancer (16). For these reasons, many trials that investigated GnRHa use during chemotherapy, including the POEMS-SWOG S0230 study (17), enrolled only women with hormone receptor–negative breast cancer (14).
Chemotherapy-induced amenorrhea has a positive prognostic value in patients with hormone receptor–positive breast cancer (18-21), and ovarian suppression given as adjuvant endocrine therapy is now recommended in high-risk patients (eg, those previously exposed to chemotherapy) (5,22). To counteract potential concerns in this regard, the PROMISE-GIM6 trials allowed ovarian suppression as adjuvant endocrine therapy in patients with hormone receptor–positive disease that resumed ovarian function after chemotherapy completion. Although this approach was followed by more than 60% of patients, no alarming safety signals arose, with similar results observed in the 2 multivariable models including or not GnRHa reinstitution after the end of chemotherapy. Current evidence supports ovarian function suppression use for 2 to 5 years in high-risk patients (23,24). There is no evidence of any potential beneficial role of prolonging ovarian function suppression beyond 5 years; on the contrary, the possible harmful long-term consequences of early menopause should not be neglected (25).
Preclinical and clinical data suggest a possible antagonism between chemotherapy and tamoxifen (26–28). However, there are no preclinical data to support the same concern with GnRHa use. Moreover, existing clinical data, now supported by our results, are reassuring in this regard (29–32). Considering the risk of relapse beyond 10 years after diagnosis in women with hormone receptor–positive breast cancer (33), final results of the PROMISE-GIM6 trials showing similar DFS and OS are particularly relevant to support the safety of administering GnRHa concurrently with chemotherapy in these patients. On the contrary, a signal of worse outcomes with GnRHa use was observed in women with hormone receptor–negative breast cancer. However, these results should be considered with caution considering the small sample size (n = 51) of this cohort. Reassuring results in these patients are reported in the POEMS-SWOG S0230 trial showing a non-statistically significant improvement in DFS (HR = 0.55, 95% CI = 0.27 to 1.10) and OS (HR = 0.45, 95% CI = 0.19 to 1.04) for the whole trial population of 218 patients with hormone receptor–negative breast cancer (17).
Even though this is a standard strategy for ovarian function preservation in premenopausal women with early breast cancer, the fertility preservation potential of GnRHa use during chemotherapy remains debated (6,7,9). Notably, this strategy has not been studied as a method to preserve fertility: pregnancy desire was not an inclusion criteria in the trials, premenopausal status and not aged younger than 40 years was considered for eligibility, and post-treatment pregnancies were a pre-planned endpoint only in the POEMS-SWOG S0230 study (14). Moreover, most of the trials reported results of their primary endpoint (POI rates) at short-term follow-up (14). Final analysis of the POEMS-SWOG S0230 trial at a median follow-up of 5 years showed a statistically significant higher 5-year cumulative incidence of pregnancy in the GnRHa arm (23.1% vs 12.2%, P = .04) (17). Similar results were observed in our trial but without reaching statistical significance (12-year cumulative incidence of pregnancy 6.5% vs 3.2%). The lower rates reported in our study compared with the POEMS-SWOG S0230 trial can be explained by the fact that the majority of patients in the PROMISE-GIM6 trial had hormone receptor–positive disease. Both the need of 5-10 years of adjuvant endocrine therapy and physicians’ concerns regarding safety of pregnancy in these patients may have discouraged many of them from trying to conceive (15). These data reinforce the current recommendation that GnRHa use during chemotherapy is not an alternative to cryopreservation strategies as a fertility preservation option (6,7,9).
Because young age at diagnosis is a criteria to refer breast cancer patients to genetic testing (34), increasing attention should be paid to the oncofertility counseling of women with hereditary cancer syndromes (6). Many studies showed a potential negative effect of germline BRCA pathogenic variants on female reproductive function (35). However, there is no evidence on the efficacy of GnRHa use during chemotherapy in this setting (6). Similar findings to those in the overall trial population favoring GnRHa use were observed in the PROMISE-GIM6 study for women with or without germline BRCA pathogenic variants. Considering the limited number of patients tested for BRCA, this analysis should be regarded as descriptive only. However, we believe these data can raise awareness regarding the importance to counsel young BRCA-mutated patients on the possibility to preserve ovarian function when breast cancer diagnosis is made several years before the recommended age of risk-reducing gynecological surgery.
In terms of study limitations, it should be highlighted that long-term outcomes were not prespecified in the study protocol. However, the steering committee of the PROMISE-GIM6 study decided to collect long-term outcomes with annual systematic follow-up at the time of primary endpoint analysis (12,13). Evaluating differences in long-term safety outcomes between the 2 study arms should have been based on noninferiority testing. We can hypothesize that more than a 20% increase in the hazard ratio estimate could be considered as clinically relevant. Thus, an upper limit of the 95% confidence interval of the observed hazard ratio estimates lower than 1.20 would have confirmed the noninferiority. In our results, the point estimate of the hazard ratio for DFS and OS were 1.16 and 1.17, respectively, but their upper limit of the 95% confidence interval exceeds 1.20 in both the endpoints. The wide 95% confidence interval of the computed hazard ratio estimate is due to the low number of events. Thus, we cannot formally reject the null hypothesis of noninferiority. However, the trial was not powered to investigate differences in DFS or OS, and a higher number of patients would have been needed to demonstrate the noninferiority in these outcomes. Despite that no formal conclusions can be drawn, our results suggest no differences in long-term survival outcomes for patients receiving concomitant GnRHa during chemotherapy compared with controls. In addition, patients’ pregnancy desire and information on genetic testing were not routinely collected in the trial. Therefore, taking into account the above-mentioned limitations these results should be regarded as exploratory and those in BRCA-mutated patients merely descriptive.
Notwithstanding these limitations, these data are highly relevant to improve the oncofertility counseling of premenopausal women with early breast cancer when offering the administration of GnRHa during chemotherapy to preserve ovarian function. Particularly, these findings are important to reassure physicians regarding the safety of this strategy in premenopausal patients with hormone receptor–positive breast cancer.
In conclusion, final analysis of the PROMISE-GIM6 trial provides reassuring results on the safety of GnRHa use during chemotherapy as a strategy to preserve ovarian function in premenopausal women with early breast cancer, including those with hormone receptor–positive disease.
Funding
The study was supported by the IRCCS Ospedale Policlinico San Martino, Genova, Italy, and partly supported by a grant from the Associazione Italiana per la Ricerca sul Cancro (AIRC; grant number MFAG 2020 ID 24698), Italy and the Italian Ministry of Health—5 × 1000 funds 2017 (no grant number), Italy. Triptorelin used in the study was provided by Ipsen, Milan, Italy.
Notes
Role of the funders: The sponsor and funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Disclosures: ML acted as consultant for Roche, AstraZeneca, Eli Lilly, Exact Sciences and Novartis and received speaker honoraria from Roche, Sandoz, Takeda, Pfizer, Eli Lilly, and Novartis outside the submitted work. OG acted as consultant for Novartis, Eisai, MSD, Eli Lilly and received honoraria from Pfizer, Novartis outside the submitted work. GA acted as consultant for Roche, AstraZeneca, Eli Lilly, Pfizer and Novartis and received speaker honoraria from Roche, Sandoz, Takeda, Pfizer, Eli Lilly, and Novartis outside the submitted work. FP acted as consultant for Roche, Merck Sharp and Dohme, received speaker honoraria from Eli Lilly and Novartis, and travel accommodations from Eli Lilly and Takeda outside the submitted work. CB acted as consultant and received speaker honoraria from Novartis, Roche, Eli Lilly and Pfizer, outside the submitted work. LB, AM, EM, AAC, AMM, MG, PC, PF, PP, EB, LDM declare no competing interests.
Author contributions: LDM (principal investigator) had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
ML: Data curation, Formal Analysis, Writing—original draft, Supervision, Validation; LB: Data curation, Formal Analysis Writing—original draft, Methodology, Software, Supervision, Validation; AM: Data curation, Writing—review and editing; EM: Data curation, Writing—review and editing; AAC: Data curation, Writing—review and editing; AMM: Data curation, Writing—review and editing; MG: Data curation, Writing—review and editing; OG: Data curation, Writing—review and editing; GA: Data curation, Writing—review and editing; FP: Data curation, Writing—review and editing; PC: Data curation, Writing—review and editing; CB: Data curation, Writing—review and editing; PF: Data curation, Writing—review and editing; PP: Data curation, Writing—review and editing; EB: Data curation, Formal Analysis, Writing—original draft, Methodology and Software; LDM: Conceptualization, Data curation, Formal Analysis, Writing—original draft, Supervision, Validation.
Acknowledgements: This paper is dedicated to the memory of Dr Marco Venturini, leader of the GIM group. The authors acknowledge all the investigators that participated in the PROMISE-GIM6 trial, Dr Paolo Bruzzi MD who contributed to study design and Mrs Simona Pastorino, Department of Medical Oncology, U.O. Oncologia Medica 2, IRCCS Ospedale Polclinico San Martino, Genova, Italy, for administrative support. Dr Bruzzi and Mrs Pastorino received no compensation for their support to the study.
Prior presentation: This study was presented in the Poster Discussion Session (Breast Cancer—Local/Regional/Adjuvant) at the 2021 American Society of Clinical Oncology (ASCO) Annual Meeting on June 4, 2021.
Data Availability
The data underlying this article will be shared on reasonable request to the corresponding author.
Supplementary Material
References
- 1. Azim HA, Partridge AH.. Biology of breast cancer in young women. Breast Cancer Res. 2014;16(4):427. doi: 10.1186/s13058-014-0427-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Partridge AH, Hughes ME, Warner ET, et al. Subtype-dependent relationship between young age at diagnosis and breast cancer survival. J Clin Oncol. 2016;34(27):3308–3314. doi:10.1200/JClin Oncol.2015.65.8013. [DOI] [PubMed] [Google Scholar]
- 3. Sparano JA, Gray RJ, Ravdin PM, et al. Clinical and genomic risk to guide the use of adjuvant therapy for breast cancer. N Engl J Med. 2019;380(25):2395–2405. doi: 10.1056/NEJMoa1904819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Villarreal-Garza C, Ferrigno AS, De la Garza-Ramos C, Barragan-Carrillo R, Lambertini M, Azim HA.. Clinical utility of genomic signatures in young breast cancer patients: a systematic review. NPJ Breast Cancer. 2020;6:46. doi: 10.1038/s41523-020-00188-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Paluch-Shimon S, Cardoso F, Partridge AH, et al. ; ESO-ESMO 4th International Consensus Guidelines for Breast Cancer in Young Women (BCY4). Ann Oncol. 2020;31(6):674–696. doi: 10.1016/j.annonc.2020.03.284. [DOI] [PubMed] [Google Scholar]
- 6. Lambertini M, Peccatori FA, Demeestere I, et al. ; ESMO Guidelines Committee. Fertility preservation and post-treatment pregnancies in post-pubertal cancer patients: ESMO Clinical Practice Guidelines. Ann Oncol. 2020;31(12):1664–1678. doi: 10.1016/j.annonc.2020.09.006. [DOI] [PubMed] [Google Scholar]
- 7. Anderson RA, Amant F, Braat D, et al. ; ESHRE Guideline Group on Female Fertility Preservation. ESHRE guideline: female fertility preservation. Hum Reprod Open. 2020;2020(4):hoaa052. doi: 10.1093/hropen/hoaa052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Howard-Anderson J, Ganz PA, Bower JE, Stanton AL.. Quality of life, fertility concerns, and behavioral health outcomes in younger breast cancer survivors: a systematic review. J Natl Cancer Inst. 2012;104(5):386–405. doi: 10.1093/jnci/djr541. [DOI] [PubMed] [Google Scholar]
- 9. Oktay K, Harvey BE, Partridge AH, et al. Fertility preservation in patients with cancer: ASCO clinical practice guideline update. J Clin Oncol. 2018;36(19):1994–2001. doi:10.1200/JClin Oncol.2018.78.1914. [DOI] [PubMed] [Google Scholar]
- 10. Blondeaux E, Massarotti C, Fontana V, et al. The PREgnancy and FERtility (PREFER) study investigating the need for ovarian function and/or fertility preservation strategies in premenopausal women with early breast cancer. Front Oncol. 2021;11:690320. doi: 10.3389/fonc.2021.690320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lambertini M, Moore HCF, Leonard RCF, et al. Gonadotropin-releasing hormone agonists during chemotherapy for preservation of ovarian function and fertility in premenopausal patients with early breast cancer: a systematic review and meta-analysis of individual patient-level data. J Clin Oncol. 2018;36(19):1981–1990. doi:10.1200/J Clin Oncol.2018.78.0858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Del Mastro L, Boni L, Michelotti A, et al. Effect of the gonadotropin-releasing hormone analogue triptorelin on the occurrence of chemotherapy-induced early menopause in premenopausal women with breast cancer: a randomized trial. JAMA. 2011;306(3):269–276. doi: 10.1001/jama.2011.991. [DOI] [PubMed] [Google Scholar]
- 13. Lambertini M, Boni L, Michelotti A, et al. ; GIM Study Group. Ovarian suppression with triptorelin during adjuvant breast cancer chemotherapy and long-term ovarian function, pregnancies, and disease-free survival: a randomized clinical trial. JAMA. 2015;314(24):2632–2640. doi: 10.1001/jama.2015.17291. [DOI] [PubMed] [Google Scholar]
- 14. Lambertini M, Horicks F, Del Mastro L, Partridge AH, Demeestere I.. Ovarian protection with gonadotropin-releasing hormone agonists during chemotherapy in cancer patients: from biological evidence to clinical application. Cancer Treat Rev. 2019;72:65–77. doi: 10.1016/j.ctrv.2018.11.006. [DOI] [PubMed] [Google Scholar]
- 15. Lambertini M, Di Maio M, Pagani O, et al. The BCY3/BCC 2017 survey on physicians’ knowledge, attitudes and practice towards fertility and pregnancy-related issues in young breast cancer patients. Breast. 2018;42:41–49. doi: 10.1016/j.breast.2018.08.099. [DOI] [PubMed] [Google Scholar]
- 16. Rugo HS, Rosen MP.. Reducing the long-term effects of chemotherapy in young women with early-stage breast cancer. JAMA. 2011;306(3):312–314. doi: 10.1001/jama.2011.1019. [DOI] [PubMed] [Google Scholar]
- 17. Moore HCF, Unger JM, Phillips K-A, et al. Final analysis of the prevention of early menopause study (POEMS)/SWOG Intergroup S0230. J Natl Cancer Inst. 2019;111(2):210–213. doi: 10.1093/jnci/djy185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bianco AR, Del Mastro L, Gallo C, et al. Prognostic role of amenorrhea induced by adjuvant chemotherapy in premenopausal patients with early breast cancer. Br J Cancer. 1991;63(5):799–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Swain SM, Jeong J-H, Geyer CE, et al. Longer therapy, iatrogenic amenorrhea, and survival in early breast cancer. N Engl J Med. 2010;362(22):2053–2065. doi: 10.1056/NEJMoa0909638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zhao J, Liu J, Chen K, et al. What lies behind chemotherapy-induced amenorrhea for breast cancer patients: a meta-analysis. Breast Cancer Res Treat. 2014;145(1):113–128. doi: 10.1007/s10549-014-2914-x. [DOI] [PubMed] [Google Scholar]
- 21. Lambertini M, Campbell C, Bines J, et al. Adjuvant anti-HER2 therapy, treatment-related amenorrhea, and survival in premenopausal HER2-positive early breast cancer patients. J Natl Cancer Inst. 2019;111(1):86–94. doi: 10.1093/jnci/djy094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Burstein HJ, Lacchetti C, Anderson H, et al. Adjuvant endocrine therapy for women with hormone receptor-positive breast cancer: American Society of Clinical Oncology Clinical Practice guideline update on ovarian suppression. J Clin Oncol. 2016;34(14):1689–1701. doi:10.1200/J Clin Oncol.2015.65.9573. [DOI] [PubMed] [Google Scholar]
- 23. Francis PA, Pagani O, Fleming GF, et al. ; SOFT and TEXT Investigators and the International Breast Cancer Study Group. Tailoring adjuvant endocrine therapy for premenopausal breast cancer. N Engl J Med. 2018;379(2):122–137. doi: 10.1056/NEJMoa1803164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kim H-A, Lee JW, Nam SJ, et al. ; Korean Breast Cancer Study Group. Adding ovarian suppression to tamoxifen for premenopausal breast cancer: a randomized phase III trial. J Clin Oncol. 2020;38(5):434–443. doi:10.1200/J Clin Oncol.19.00126. [DOI] [PubMed] [Google Scholar]
- 25. Lambertini M, Blondeaux E, Perrone F, Del Mastro L.. Improving adjuvant endocrine treatment tailoring in premenopausal women with hormone receptor-positive breast cancer. J Clin Oncol. 2020;38(12):1258–1267. doi:10.1200/J Clin Oncol.19.02242. [DOI] [PubMed] [Google Scholar]
- 26. Goldenberg GJ, Froese EK.. Antagonism of the cytocidal activity and uptake of melphalan by tamoxifen in human breast cancer cells in vitro. Biochem Pharmacol. 1985;34(6):763–770. [DOI] [PubMed] [Google Scholar]
- 27. Woods KE, Randolph JK, Gewirtz DA.. Antagonism between tamoxifen and doxorubicin in the MCF-7 human breast tumor cell line. Biochem Pharmacol. 1994;47(8):1449–1452. [DOI] [PubMed] [Google Scholar]
- 28. Albain KS, Barlow WE, Ravdin PM, et al. ; Breast Cancer Intergroup of North America. Adjuvant chemotherapy and timing of tamoxifen in postmenopausal patients with endocrine-responsive, node-positive breast cancer: a phase 3, open-label, randomised controlled trial. Lancet. 2009;374(9707):2055–2063. doi: 10.1016/S0140-6736(09)61523-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.International Breast Cancer Study Group. Late effects of adjuvant oophorectomy and chemotherapy upon premenopausal breast cancer patients. Ann Oncol. 1990;1(1):30–35. [PubMed] [Google Scholar]
- 30. Rivkin SE, Green S, O'Sullivan J, et al. Adjuvant CMFVP versus adjuvant CMFVP plus ovariectomy for premenopausal, node-positive, and estrogen receptor-positive breast cancer patients: a Southwest Oncology Group study. J Clin Oncol. 1996;14(1):46–51. [DOI] [PubMed] [Google Scholar]
- 31. Arriagada R, Lê MG, Spielmann M, et al. Randomized trial of adjuvant ovarian suppression in 926 premenopausal patients with early breast cancer treated with adjuvant chemotherapy. Ann Oncol. 2005;16(3):389–396. doi: 10.1093/annonc/mdi085. [DOI] [PubMed] [Google Scholar]
- 32. Regan MM, Walley BA, Francis PA, et al. Concurrent and sequential initiation of ovarian function suppression with chemotherapy in premenopausal women with endocrine-responsive early breast cancer: an exploratory analysis of TEXT and SOFT. Ann Oncol. 2017;28(9):2225–2232. doi: 10.1093/annonc/mdx285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Pan H, Gray R, Braybrooke J, et al. EBCTCG. 20-year risks of breast-cancer recurrence after stopping endocrine therapy at 5 years. N Engl J Med. 2017;377(19):1836–1846. doi: 10.1056/NEJMoa1701830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Paluch-Shimon S, Cardoso F, Sessa C, et al. ; ESMO Guidelines Committee. Prevention and screening in BRCA mutation carriers and other breast/ovarian hereditary cancer syndromes: ESMO Clinical Practice Guidelines for cancer prevention and screening. Ann Oncol. 2016;27(suppl 5):v103–v110. doi: 10.1093/annonc/mdw327. [DOI] [PubMed] [Google Scholar]
- 35. Vuković P, Peccatori FA, Massarotti C, Miralles MS, Beketić-Orešković L, Lambertini M.. Preimplantation genetic testing for carriers of BRCA1/2 pathogenic variants. Crit Rev Oncol Hematol. 2021;157:103201. doi: 10.1016/j.critrevonc.2020.103201. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author.