Abstract
Associations of obesity have been established for at least 11 cancer sites in observational studies, though some questions remain as to causality, strength of associations, and timing of associations throughout the life course. In recent years, Mendelian randomization (MR) has provided complementary information to traditional approaches, but the validity requires that the genetic instrumental variables be causally related to cancers only mediated by the exposure. We summarize and evaluate existing evidence from MR studies in comparison with conventional observational studies to provide insights into the complex relationship between obesity and multiple cancers. MR studies further establish the causality of adult obesity with esophageal adenocarcinoma and cancers of the colorectum, endometrium, ovary, kidney, and pancreas, as well as the inverse association of early life obesity with breast cancer. MR studies, which might account for lifelong adiposity, suggest that the associations in observational studies typically based on single measurement may underestimate the magnitude of the association. For lung cancer, MR studies find a positive association with obesity, supporting that the inverse association observed in some conventional observational studies likely reflects reverse causality (loss of lean body mass before diagnosis) and confounding by smoking. However, MR studies have not had sufficient power for gallbladder cancer, gastric cardia cancer, and multiple myeloma. In addition, more MR studies are needed to explore the effect of obesity at different timepoints on postmenopausal breast cancer and aggressive prostate cancer.
Obesity across the globe represents a major threat to public health. The global prevalence of obesity (defined as a body mass index [BMI] ≥30 kg/m2) is estimated to reach 18% in men and surpass 21% in women if post-2000 trends continue (1). In 2017-2018, the prevalence of obesity among adults in the United States has been up to 42.4% (2,3). Consistent with reports from the International Agency for Research on Cancer and the World Cancer Research Fund–American Institute of Cancer Research (WCRF/AICR), associations for 11 cancers including esophageal adenocarcinoma, multiple myeloma, and cancers of the gastric cardia, colon, rectum, biliary tract system, pancreas, breast, endometrium, ovary, and kidney were supported by strong evidence from 204 meta-analyses, which summarized 2179 individual study estimates from 507 unique cohort or case-control studies (4). The reported associations may be causal for some malignancies but are susceptible to potential confounding bias, as obesity co-occurs with various risk factors of cancer, and reverse causality. Despite these concerns, observational studies remain fundamental for evidence-based cancer prevention and control.
In recent years, Mendelian randomization (MR) has received increasing attention with respect to addressing some limitations of conventional observational studies and has been adopted extensively to examine the effects of modifiable exposures on diseases. MR takes advantage of the random assortment of genes from parents to offspring through the process of meiosis and zygote formation (5). Germline genetic variants are generally fixed at conception without being modified by subsequent factors. As such, genetic variants that are robustly associated with levels of modifiable exposures are selected as instrumental variables. The association between genetic variants and diseases, as a proxy of the biological link between exposure and diseases, is generally free of confounding bias and reverse causality that may exist in conventional observational studies. Randomized controlled trials to establish the causality of putative exposure-disease associations are usually not feasible, and MR offers additional evidence for ambiguous or paradoxical associations complementary to conventional studies while remaining in an observational setting.
Despite its strengths, MR has stringent assumptions that may be violated in practice (5). First, the genetic variants used as instrumental variables must be associated with the exposure variable reliably and of sufficient magnitude to ensure enough statistical power. Secondly, the genetic variants must not be associated with any confounders between the modifiable exposure and outcome. Thirdly, the genetic variants must be conditionally independent of the outcome given the exposure (ie, the genetic instrument and the outcome are directly mediated by the exposure). Violations of any of these assumptions may lead to spurious conclusions for causality. Weak genetic instrumental variables would compromise the statistical power, and thus researchers tend to use a larger number of single nucleotide polymorphisms (SNPs) to explain more variability of the exposure (6,7). In addition, it may be challenging to disentangle the life phase–specific effect of obesity.
Neither conventional observational studies nor MR studies can eliminate all biases. Hence, aiming to explicitly determine the relationship between obesity and cancer, we reviewed studies that have adopted an MR framework and assessed their contributions to the existing evidence from large-scale conventional observational studies in combination with the graded evidence from the WCRF/AICR, which is also based on experimental studies.
Breast Cancer
Based on the Breast Cancer Association Consortium and the Genetic Investigation of Anthropometric Traits, Ooi et al. (8) showed that a higher genetically predicted adult BMI was associated with lower overall breast cancer risk (odds ratio [OR] = 0.81, 95% confidence interval [CI] = 0.74 to 0.89 per SD increase). Based on Discovery, Biology and Risk of Inherited Variants in Breast Cancer Project, inverse associations between genetic scores for childhood BMI (OR = 0.71, 95% CI = 0.60 to 0.80 per SD increase), adult BMI (OR = 0.66, 95% CI = 0.57 to 0.77 per SD increase), and breast cancer risk were reported (9). For subtypes of breast cancer, using individual-level data from Breast Cancer Association Consortium, Guo et al. (10) observed that genetically predicted BMI was inversely associated with both premenopausal breast cancer risk (OR = 0.44, 95% CI = 0.31 to 0.62 per 5 kg/m2 increase) and postmenopausal breast cancer (OR = 0.57, 95% CI = 0.46 to 0.71 per 5 kg/m2 increase). In a multivariable MR analysis, Richardson et al. (11) used multiple genetic variants associated with early life body size and adult body size to jointly estimate a direct effect comprising all other pathways from 1 risk factor (eg, early life body size) not mediated by the other factor (adult body size) to the outcome. The authors found a protective direct effect of larger body size (measured as thinner, plumper, and average) at age 10 years on breast cancer risk independent of adult body size (OR = 0.59, 95% CI = 0.50 to 0.71), with less evidence of a direct effect of adult body size (OR = 1.08, 95% CI = 0.93 to 1.27) after adjusting for early life body size (11).
The inverse associations between childhood obesity and breast cancer risk, as well as adult obesity and premenopausal breast cancer risk, in MR studies are consistent with the majority of observational studies (12-16). According to MR effect estimates, the reduction in premenopausal breast cancer risk, as high as 50% per 5 kg/m2 increase in adult BMI, is stronger than those estimated in observational studies. This difference may be attributed to a strong correlation between adult BMI and early life BMI in MR studies because genetic variants were shown to be associated with body size at different timepoints in the life course with varying degrees (11,17). In a large multicenter pooled analysis, the inverse associations of BMI from age 18 through 54 years were robustly present in multiple studies and across strata of birth cohort and risk factors for breast cancer, and the strongest inverse associations were observed for BMI in early adulthood (14). Moreover, in a large cohort study, the inverse association of weight at age 18 years with premenopausal and postmenopausal breast cancer was largely explained by adiposity at age 10 years because the association disappeared after controlling for somatotype at age 10 years (18). Thus, because the etiologic relevant period for the protective effect of BMI is during early life, adult BMI would still show strong associations because of its high correlation with early life BMI. The potential explanation is also corroborated by observational findings that suggested a stronger inverse association of risk (even after menopause) with BMI at younger ages than older ages (14,19).
MR results, which suggest an inverse association between adult body size and postmenopausal breast cancer risk, contrast with observational estimates in favor of a positive association. A longitudinal study examining the dose-response relationship of obesity during the life course among postmenopausal women found that every 10-year increase in adulthood overweight duration was linearly associated with a 5% increase in risk of postmenopausal breast cancer (20). There are several potential explanations for the discrepancy. Predicted adult BMI using genetic variants expressed throughout life is closely related to early life body shape. Longitudinal analysis showed that genetic risk score (GRS) comprising 97 adult BMI-associated variants was positively associated with BMI across all ages from 18 (women) or 21 (men) years to 85 years of age, and the association weakened over old ages (21). As discussed above, the inverse association between later life obesity and postmenopausal breast cancer in MR studies possibly derives from the strong protective effect of early life body size. In the multivariable MR analyses for total breast cancer, the effect estimates for adult body size changed from a strongly inverse association to a suggestively positive association after adjusting for early life BMI (11).
The positive association in observational studies may be driven by adult weight gain (especially in the postmenopausal period) (9). Meta-analyses consistently show that adult weight gain is associated with increased postmenopausal breast cancer risk among no or low hormone-replacement therapy users (22). In contrast, genetic variants serving as instrumental variables in MR studies might be less associated with weight gain in adulthood (10). A study found that a BMI-GRS composed of 31 genome-wide association studies (GWAS)–identified SNPs was associated with increased weight gain from age 20 years to middle adulthood (around age 50 years) but with decreased weight gain during middle adulthood and later adulthood (around age 70 years) (23). In addition, a bodyweight trajectory analysis from age 5 to 60 years reported that the increased risk of postmenopausal breast cancer was associated with trajectories of the lean-moderate increase, lean-marked increase, and heavy-stable increase (24). Finally, long-term weight gain from age 18 years during premenopause and postmenopause was positively associated with postmenopausal breast cancer risk but not premenopausal breast cancer risk (18).
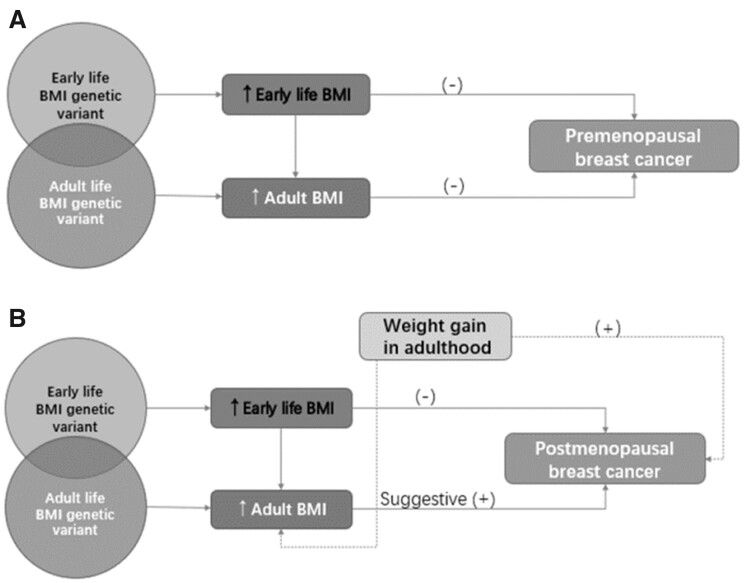
The complex patterns for breast cancer are summarized in Figure 1. Overall, the inverse association between childhood obesity and breast cancer risk (both premenopausal and postmenopausal), as well as the inverse association between adult BMI and premenopausal breast cancer risk, are consistently supported by studies. The differential associations by molecular subtypes are also complicated and inconclusive. The positive association of adult obesity with postmenopausal cancer appears to be limited to estrogen receptor (ER)–positive and progesterone receptor–positive cancer, and the association for hormone receptor-negative cancer was null (25–27). In comparison with the inverse association with ER-positive premenopausal breast cancer, adult obesity is indicated to be associated with a higher risk of ER-negative and triple-negative premenopausal breast cancer (28). Several MR analyses stratified by ER status but not jointly stratified by menopausal status found inverse associations of adult obesity with ER-positive and ER-negative breast cancer (8,29). Likewise, the strong protective effect of early life obesity may also play a role here. Nevertheless, future studies are warranted to tease apart the independent effect of early life obesity and adulthood (particularly postmenopausal) weight gain and examine potential heterogeneous associations by the joint subgroups of menopause status and hormone receptor status.
Figure 1.
Associations between obesity in the life course, weight gain, and risk of breast cancer subtypes. A) Illustration of the association between obesity in the life course, weight gain, and premenopausal breast cancer risk. B) Illustration of the association between obesity in the life course, weight gain, and postmenopausal breast cancer risk. Genetic variants are shown to be associated with body size at different timepoints in the life course. Early life obesity has a direct consistent effect on reducing breast cancer risk (both premenopausal and postmenopausal). Of note, predicted adult body mass index (BMI) using genetic variants expressed throughout life is closely related to early life body shape. As such, in contrast to the positive association in conventional observational studies, adult BMI may show strong, even inverse, associations with postmenopausal breast cancer because of its high correlation with early life BMI in Mendelian randomization studies. In addition, weight gain from age 18 years is an important risk factor of postmenopausal breast cancer according to conventional observational evidence, but genetic variants serving as instrumental variables in Mendelian randomization studies might be less capable to capture weight gain in adulthood. This may also explain the observed discrepancy for postmenopausal breast cancer.
Ovarian Cancer and Endometrial Cancer
Using summary statistics from the Genetic Associations and Mechanisms in Oncology (GAME-ON) consortium (Follow-up of Ovarian Cancer Genetic Association and Interaction Studies [FOCI]), Gao et al. (9) observed a positive association between genetically predicted adult BMI and overall ovarian cancer (OR = 1.35, 95% CI = 1.05 to 1.72 per SD increase). After removing 10 overlapping loci between childhood BMI (before removal, 15 SNPs) and adult BMI (77 SNPs), associations between genetically predicted childhood BMI (OR = 0.58, 95% CI = 0.34 to 1.01 per SD increase), adult BMI (OR = 1.26, 95% CI = 0.93 to 1.72 per SD increase), and ovarian cancer risk were not found. Possibly, the remaining SNPs did not provide adequate statistical power. Dixon et al. (30) found that higher genetically predicted BMI was associated with increased risk of non-high grade serous ovarian cancers (non-HGSC) (OR = 1.29, 95% CI = 1.03 to 1.61 per 5 kg/m2 increase) but not HGSC. For non-HGSC subtypes, the association for invasive low-grade and borderline serous cancers was strongest, and the association for endometrioid and mucinous cancers was weakest (30).
Investigations with measured adult BMI support positive associations with risk of ovarian cancer (4,15,31,32). Interestingly, 2 large prospective cohort studies reported that BMI at age 10 years was inversely associated with ovarian cancer risk, and the association was attenuated after adjusting for BMI change between age 10 and 18 years and BMI change after age 18 years, which were strongly and slightly associated with increased risk of ovarian cancer, respectively (33). In a trajectory analysis, compared with the lean-stable group, no statistically significant differences in ovarian cancer risk were observed in the lean-moderate increase, lean-marked increase, medium-stable, and heavy-stable increase groups. It is possible that early life change in adiposity was more strongly associated with ovarian cancer risk than adulthood change (24). A potential explanation for the similar risk between the lean-stable and heavy-stable group is that the protective effect of the corpulent body in early life is counterbalanced by the deleterious effect of later life obesity.
For endometrial cancer, consistent with observational studies, Painter et al. (34) found a strong positive association with genetically predicted BMI and endometrial cancer risk (OR = 2.06, 95% CI = 1.89 to 2.21 per 5 kg/m2 increase). Concordant with the results of European studies, a statistically significant association with increased BMI was also reported in Japanese women (35). MR studies provide clear evidence to date for a positive relationship between BMI and endometrial cancer, although the MR approach has not investigated the effect of obesity across the life course. Evidence from 2 large prospective cohort studies showed that obesity throughout life from age 5 years to adulthood was positively associated with endometrial cancer risk, with adult obesity an especially strong risk factor (36). Moreover, the longitudinal study examining the cumulative effect of obesity on cancer risk mentioned before found that the risk of endometrial cancer associated with increasing duration and intensity rose exponentially and became statistically significant only after 26 years of being overweight (20).
Colorectal Cancer
Using summary statistics in the GAME-ON consortium (the Colorectal Transdisciplinary Study [CORECT]), Gao et al. (9) found a 39% higher risk of colorectal cancer per SD increment of genetically predicted adult BMI (OR = 1.39, 95% CI =1.06 to 1.82). No associations were found between childhood BMI or waist to hip ratio (WHR) and colorectal cancer risk. Also, after excluding 10 overlapping loci between adult BMI and childhood BMI, neither childhood BMI nor adult BMI was associated with colorectal cancer risk possibly because of low statistical power. In contrast, in MR analysis by Jarvis et al. (37) that did not control for obesity in different periods, the odds ratios for colorectal cancer risk per unit increase in adult BMI, WHR, and childhood obesity were 1.23 (95% CI = 1.02 to 1.49), 1.59 (95% CI = 1.08 to 2.34), and 1.07 (95% CI = 1.03 to 1.13), respectively. In the sex-specific analysis, WHR was associated with colorectal cancer risk only in men while BMI was only associated with risk in women. However, in the latest MR study using more SNPs and larger sample size, higher BMI was associated with 1.23 (95% CI = 1.08 to 1.38 per 4.2 kg/m2 increase) times higher risk of colorectal cancer among men; among women, the positive association was not statistically significant (OR = 1.09, 95% CI = 0.97 to 1.22 per 5.2 kg/m2 increase) (38). Cornish and colleagues’ study (39) showed increased colorectal cancer risk for genetically predicted body fat percentage (OR = 1.14, 95% CI = 1.03 to 1.25), BMI (OR = 1.09, 95% CI = 1.01 to 1.17), and waist circumference (OR = 1.13, 95% CI = 1.02 to 1.26).
Based on conventional observational studies, adult obesity is associated with colorectal cancer risk, with a stronger association among men (4,40,41). Likewise, waist circumference gain during adulthood is associated with higher risk of colorectal cancer in men rather than women, which is consistent with the MR findings (42). Increased body fatness in childhood, adolescence, and early adulthood has been shown to be a risk factor for colorectal cancer carcinogenesis independent of adult obesity especially in women (43). Evidence from MR studies on early life body shape is inadequate, but one study suggested a causal relationship between childhood obesity and colorectal cancer (37). Overall, the observational data support a stronger association with adult obesity and weight gain in men and possibly a stronger association with early life obesity in women. More MR studies are needed to isolate the effect of adult BMI independent of early life effect by sex.
Other Gastrointestinal Cancers
Thrift et al. (44) found that esophageal adenocarcinoma risk increased by 16% (OR = 1.16, 95% CI = 1.01 to 1.33) per 1 kg/m2 increase in BMI. Mao et al. (45) observed that higher genetically predicted BMI was associated with increased stomach cancer risk (OR = 1.07, 95% CI = 1.02 to 1.13 per SD increase in the weighted GRS). Subsite analysis by cardia and noncardia was unavailable. In Carreras-Torres and colleagues’ study (46), the results indicated a robust causal positive association of BMI with pancreatic cancer risk (OR = 1.34, 95% CI = 1.09 to 1.65 per SD increase). Similarly, Lu et al. (47) supported a causal effect of BMI on pancreatic ductal adenocarcinoma (OR = 1.43, 95% CI = 1.20 to 1.71). Langdon et al. (48) also found that BMI (OR = 1.46, 95% CI = 1.20 to 1.78) and hip circumference (OR = 1.42, 95% CI = 1.21 to 1.67 per SD increase) were associated with increased pancreatic cancer risk. For gallbladder cancer, Barahona et al. (49) found that a genetically elevated BMI increased the risk in Chileans (OR = 2.47, 95% CI = 1.10 to 5.54 per 1 kg/m2 increase), but no effect was detected in Europeans (OR = 0.91, 95% CI = 0.22 to 3.78 per 1 kg/m2 increase).
MR studies lend further support to a large body of epidemiological studies showing that obesity has been consistently associated with greater risk of esophageal adenocarcinoma, gallbladder cancer, and pancreatic cancer (4,50,51). Stomach cancers located in the cardia and noncardia anatomical regions may have distinct etiologies. Observational studies have consistently reported a positive association of BMI with gastric cardia cancer, whereas evidence remains inadequate for noncardia cancer (52). Mao et al. (45) performed a secondary MR analysis in the Nanjing/Beijing study and observed an association between genetically predicted BMI and increased noncardia cancer risk. Nevertheless, the sample size of 7004 participants is small, so further confirmatory studies are warranted. Concerning early life obesity, a pooled analysis of 14 cohort studies demonstrated that pancreatic cancer risk was 54% higher for individuals who were overweight in early adulthood compared with those who were not; moreover, a 40% higher risk was observed among individuals who had gained BMI of 10 kg/m2 or more between younger ages and baseline (around age 45 years) compared with individuals whose BMI remained stable (53). However, few MR studies have explored early life obesity and pancreatic cancer.
Lung Cancer
Using summary statistics from GAME-ON consortium (Transdisciplinary Research in Cancer of the Lung [TRICL]), Gao et al. (9) detected a positive association between genetically predicted adult BMI and overall lung cancer (OR = 1.27, 95% CI = 1.09 to 1.49 per SD increase). Carreras-Torres et al. (54) found that an increase in BMI raised the risk for overall lung cancer (OR =1.13, 95% CI = 0.98 to 1.30 per 4.65 kg/m2 increase), which was driven by associations with squamous cell carcinoma (OR = 1.45, 95% CI = 1.16 to 1.62) and small cell carcinoma (OR = 1.81, 95% CI = 1.14 to 2.88). After adding additional samples into the Transdisciplinary Research in Cancer of the Lung and the International Lung Cancer Consortium (TRICL-ILCCO) GWAS, Carreras-Torres et al. (55) still found an increased risk for squamous cell carcinoma (OR = 1.20, 95% CI = 1.01 to 1.43) and small cell lung cancer (OR = 1.52, 95% CI = 1.15 to 2.00) but not adenocarcinoma (OR = 0.93, 95% CI = 0.79 to 1.08). Analyses stratified by smoking status showed a suggestively protective effect of BMI on overall lung cancer in never-smokers (OR = 0.83, 95% CI = 0.60 to 1.16) and a suggestively adverse effect in ever-smokers (OR = 1.12, 95% CI = 0.97 to 1.29) (55).
Zhou et al. (56) performed a univariate MR using BMI-associated SNPs, which indicated an effect of BMI on increased risk of squamous cell carcinoma (OR = 1.36, 95% CI = 1.22 to 1.52) and small cell lung cancer (OR = 1.73, 95% CI = 1.47 to 2.04). Through undertaking multivariable MR to mutually estimate the effects of genetically predicted smoking and BMI, they found a positive direct effect of BMI on small cell lung cancer (OR = 1.28, 95% CI = 1.06 to 1.55) and an inverse direct effect of BMI for lung adenocarcinoma (OR = 0.86, 95% CI = 0.77 to 0.96) but no association with squamous cell carcinoma (OR = 1.02, 95% CI = 0.90 to 1.16). Of note, genetically predicted BMI was positively associated with the number of cigarettes smoked per day (57). Zhou et al.’s bidirectional analysis (56) also showed a much stronger path from BMI to smoking phenotypes rather than from smoking to BMI, indicating a potential causal role of genetically predicted BMI on smoking; hence, it is possible that smoking serves as a mediator between BMI and lung carcinogenesis. In their subgroup analyses to investigate the role of smoking phenotypes, a positive association of BMI among ever-smokers was detected using BMI-all SNPs and BMI and smoking SNPs, which implied a positive total effect (direct effect plus the effect mediated by smoking) of BMI on the risk of lung cancer. On the other hand, a weak inverse association among never-smokers was identified using BMI-only SNPs, in line with meta-analyses (56,58).
The finding that BMI was positively associated with risk of small cell lung cancer in MR studies is in accordance with a pooled analysis (59). However, the conclusions for adenocarcinoma and squamous cell carcinoma are still conflicting (59). In contrast with MR studies, confounding by smoking and reverse causality due to preclinical weight loss presents problems in the interpretation of observational data, which suggests an association between high BMI and a reduced risk of lung cancer (60). In a large, pooled analysis, the inverse association remained among never-smokers even after exclusions of the first 5 years of follow-up, which argues against that the observed inverse association in conventional studies is entirely due to confounding by smoking and reverse causality (59). Another putative explanation is that low BMI may reflect the predominant loss of lean body mass, which may have detrimental effects or be a consequence of preexisting lung diseases. A prospective cohort study demonstrated that low lean body mass rather than fat mass accounted for the observed inverse association between BMI and lung cancer risk, and the highest risk was observed in groups with low BMI and high waist circumference (61).
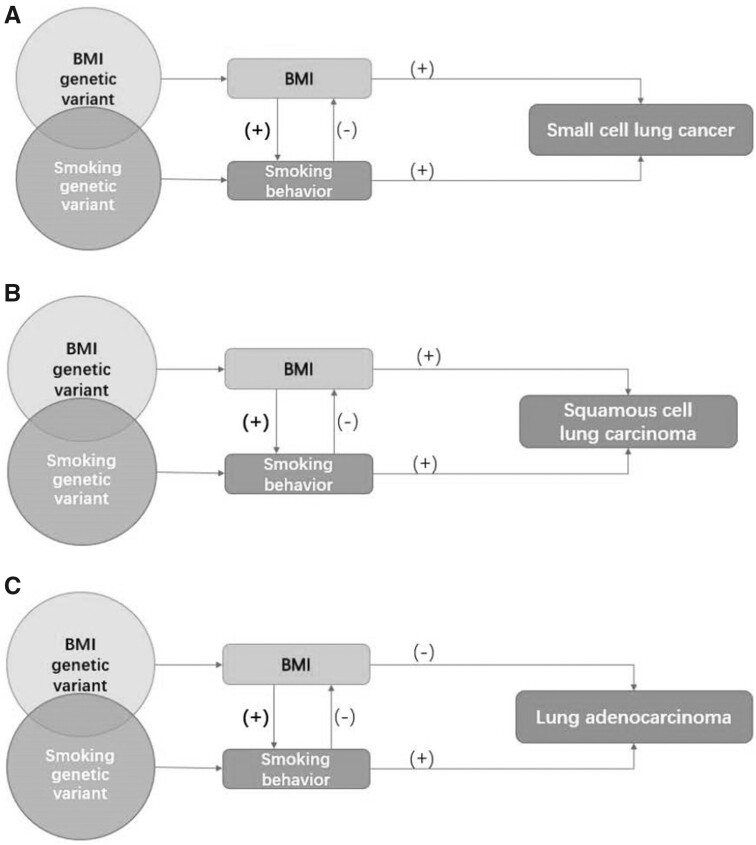
The complex associations between BMI, smoking, and lung cancer are illustrated in Figure 2. Taken together, MR studies adjusting for smoking support a causal relationship between high BMI and increased risk of small cell lung cancer and a causal relationship between low BMI and increased lung cancer risk among nonsmokers. The histology-specific relationship between BMI, smoking, and lung cancer warrants further investigations.
Figure 2.
Associations between obesity, smoking, and risk of lung cancer subtypes. A) Illustration of the association between obesity, smoking, and small cell lung cancer risk. B) Illustration of the association between obesity, smoking, and squamous cell lung carcinoma risk. C) Illustration of the association between obesity, smoking, and lung adenocarcinoma risk. Genetically predicted body mass index (BMI) was positively associated with the number of cigarettes smoked per day; on the contrary, genetically predicted and observed smoking is associated with lower BMI. The bold (+) implies a much stronger path from BMI to smoking phenotypes rather than from smoking to BMI. From this perspective, the presence of smoking can either violate the independence assumption (single variant polymorphisms [SNP]s used are not associated with confounders, such as smoking) or the exclusion restriction assumption (SNPs used affect the outcome only through the effect on BMI) that underpin Mendelian randomization (MR) studies. Using multivariable MR analyses controlling for smoking, the findings that BMI was positively associated with risk of small cell lung cancer but inversely associated with lung adenocarcinoma in MR studies are in accordance with conventional observational studies that carefully address reverse causality and confounding by smoking. For squamous cell carcinoma, a subtype that is most strongly influenced by smoking, MR studies that have advantages in minimizing confounding bias find a positive association, whereas the inverse association observed in conventional observational studies is likely to result in a negative (residual) confounding by smoking.
Kidney Cancer
The longitudinal study among postmenopausal women mentioned before suggested an approximately linear relationship when the duration of being overweight was 0-30 years, but it leveled off after 30 years (20). MR studies provide a causal interpretation for the convincing evidence that being overweight or obese increases kidney cancer risk (3,4,62,63). Johansson and colleagues’ MR analysis (64) indicated that higher BMI (OR = 1.56, 95% CI = 1.44 to 1.70 per SD increase), WHR (OR = 1.63, 95% CI = 1.40 to 1.90 per SD increase), and body fat percentage (OR = 1.66, 95% CI = 1.44 to 1.90 per SD increase) increased the risk of renal cell carcinoma (RCC). However, in Benn and colleagues’ study (65), the causal genetic risk of higher BMI was not associated with kidney cancer, possibly because of limited statistical power. The evidence regarding sex difference from meta-analyses of conventional studies is inconsistent (66,67). In MR studies, little evidence was found for sex heterogeneity in this relation. Besides, emerging evidence suggested that overweight during late adolescence (13-19 years) may be a substantial risk factor for RCC among European men and women (68–70). However, evidence from MR studies examining childhood and adolescent obesity and kidney cancer have been sparse so far. Future MR research focusing on the effect of early life obesity on RCC is necessary to determine the target in efforts for obesity prevention to reduce the burden of kidney cancer.
Prostate Cancer
Existing evidence for BMI and prostate cancer risk remains unclear (71–73). A meta-analysis indicated that obesity may have a dual effect on prostate cancer, possibly a decreased risk for localized prostate cancer and an increased risk for advanced prostate cancer (74). Using summary statistics from GAME-ON consortium (Elucidating Loci Involved in Prostate Cancer Susceptibility [ELLIPSE]), Gao et al. (9) did not observe a statistically significant association between the genetic score for BMI, WHR, or childhood BMI and aggressive prostate cancer, regardless whether they excluded overlapping SNPs between adult and childhood BMI. Davies et al. (75) found suggestive evidence that genetically predicted BMI was associated with a lower prostate cancer risk (OR = 0.98, 95% CI = 0.96 to 1.00 per SD increase in GRS). In addition, no strong evidence was found of a causal effect of either early or later life measures on prostate cancer (early life body size OR = 1.06, 95% CI = 0.81 to 1.40; adult body size OR = 0.87, 95% CI = 0.70 to 1.08) (11). The conflicting evidence implies a complicated role of obesity in prostate cancer. First, mounting evidence shows that obesity is associated with lower prostate-specific antigen levels, which possibly results in a detection bias by masking the presence of prostate cancer (72,76). Alternatively, the lower prostate cancer risk in obese men could be biological rather than entirely due to late detection. For example, the lower prostate-specific antigen level may be a consequence of lower levels of growth-promoting exposures (eg, androgens) in obese men (76). Second, obesity in different phases across the lifespan has been suggested to influence prostate cancer risk differently. Analogous to breast cancer, early life obesity may confer benefits by lowering the risk of both total and advanced prostate cancer, whereas moderate weight gain in initially lean men is likely to be adverse (24,77). In summary, MR researchers should further disentangle the effect of obesity at different timepoints on the different stages of prostate cancer and also take into account potential biases from the screening of the study population.
Multiple Myeloma
Observational epidemiological studies have shown a positive association between obesity and multiple myeloma. A meta-analysis suggested that a 5 kg/m2 increase in BMI was associated with a 12% higher multiple myeloma incidence (78). Nonetheless, according to one MR study, adiposity traits including BMI (OR = 1.17, 95% CI = 0.92 to 1.49), childhood obesity (OR = 0.98, 95% CI = 0.87 to 1.10), BMI-adjusted WHR (OR = 0.82, 95% CI = 0.57 to 1.19), BMI-adjusted hip circumference (OR = 0.77, 95% CI = 0.42 to 1.41), and BMI-adjusted waist circumference (OR = 0.62, 95% CI = 0.37 to 1.02) were not associated with multiple myeloma; yet, the confidence intervals were wide (79). Another MR study reported that BMI (OR = 1.10, 95% CI = 0.99 to 1.22), weight (OR = 1.00, 95% CI = 0.90 to 1.12), whole-body fat mass (OR = 1.00, 95% CI = 0.87 to 1.13), body fat percentage (OR = 1.07, 95% CI = 0.93 to 1.23), trunk fat percentage (OR = 1.11, 95% CI = 0.96 to 1.29), waist circumference (OR = 1.02, 95% CI = 0.87 to 1.18), hip circumference (OR = 1.03, 95% CI = 1.00 to 1.51), and WHR (OR = 0.96, 95% CI = 0.73 to 1.27) were not statistically significantly but suggestively associated with increased risk of multiple myeloma (80). The statistical power given by the study was only 0.57 to detect an effect estimate of 1.10 per SD increase in BMI (80). Given the small number of MR studies, we cannot exclude the possibility that the observed null results were a consequence of low statistical power.
Discussion
We provide an overview of MR approaches that have been adopted to examine the relationship between obesity and cancer and highlight the contributions of MR studies to complement findings from conventional epidemiological studies. The results, summarized in Table 1, show concordance between the MR results and conventional observational results in most circumstances (the rationale for the selection of studies are included in Table 1, and the derivation of effect estimates are provided in Supplementary Methods, available online; the basic information regarding SNPs of those included in MR studies are provided in Table 2). Specifically, the MR approach further establishes the causality of obesity with colorectal cancer, endometrial cancer, ovarian cancer, esophageal adenocarcinoma, kidney cancer, and pancreatic cancer, as well as the inverse relationship of early life obesity with breast cancer. MR studies have not confirmed a positive association for gallbladder cancer, gastric cardia cancer, and multiple myeloma, but this could be due to low power, and larger studies are required. MR also provides insights for a controversial association with lung cancer by addressing reverse causality and residual confounding by smoking.
Table 1.
A summary of existing evidence for obesity and cancer riska
| Cancer | Mendelian randomization studies |
Conventional observational studies |
WCRF | ||||
|---|---|---|---|---|---|---|---|
| Qualitative | Quantitative OR (95% CI)for 5 kg/m2 in BMI | Referent | Qualitative | Quantitative RR (95% CI)per 5 kg/m2 in BMI | Referent | ||
| Breast | |||||||
| Early life | (-) | 0.69 (0.58 to 0.79) | 9 | (-) | 0.79 (0.63 to 0.98)b | 16 | (-) strong-probable |
| Premenopausal, adult | (-) | 0.44 (0.31 to 0.62) | 10 | (-) | 0.95 (0.94 to 0.97) | 12 | — |
| Postmenopausal, adult | (-) | 0.57 (0.46 to 0.71) | 10 | (+) | 1.13 (1.09 to 1.17) | 4 | (+) strong-convincing |
| Ovary | |||||||
| Early life | (-) suggestive | 0.56 (0.31 to 1.01) | 9 | (-) | 0.84 (0.74 to 0.96) | 4 | — |
| Adult | (+) | 1.29 (1.03 to 1.61) | 30 | (+) | 1.08 (1.04 to 1.12) | 4 | (+) strong-probable |
| Aggressive prostate | |||||||
| Early life | 0 | — | — | 0/- | — | — | — |
| Adult | 0 | — | — | (+) | 1.09(1.02 to 1.16) | 4 | (+) strong-probable |
| Colorectum | |||||||
| Early life | (+) | 1.40 (1.16 to 1.84) | 37 | (+) | 1.18 (1.05 to 1.33)c | 16 | — |
| Men, adult | (+) | 1.27 (1.10 to 1.47) | 38 | (+) |
|
4 | (+) strong-convincing |
| Women, adult | (+) | 1.09 (0.97 to 1.21) | 38 | (+) | Colon: 1.12 (1.05 to 1.17) | 4 | (+) strong-convincing |
| Endometrium, adult | (+) | 2.06 (1.89 to 2.21) | 34 | (+) | 1.54 (1.47 to 1.61) | 4 | (+) strong-convincing |
| Esophageal adenocarcinoma, adult | (+) | 2.10 (1.05 to 4.16) | 44 | (+) | 1.53 (1.41 to 1.67) | 4 | (+) strong-convincing |
| Stomach (cardia), adult | 0 | — | — | (+) | 1.23 (1.07 to 1.40) | 4 | (+) strong-probable |
| Pancreas, adult | (+) | 1.37 (1.10 to 1.72) | 46 | (+) | 1.10 (1.06 to 1.14) | 4 | (+) strong-convincing |
| Gallbladder, adult | (+)suggestive | — | — | (+) | 1.24 (1.13 to 1.35) | 51 | (+) strong-probable |
| Kidney, adult | (+) | 1.60 (1.47 to 1.76) | 64 | (+) | 1.30 (1.23 to 1.36) | 4 | (+) strong-convincing |
| Lung, adult | Limited-no conclusion | ||||||
| Small cell carcinoma | (+) | 1.28 (1.06 to 1.55) | 56 | (+) | 1.09 (1.03 to 1.15) | 59 | — |
| Squamous cell carcinoma | (+) | 1.02 (0.90 to 1.16) | 56 | (-) | 0.94 (0.90 to 0.99) | 59 | — |
| Adenocarcinoma | (-) | 0.86 (0.77 to 0.96) | 56 | (-) | 0.86 (0.84 to 0.89) | 59 | — |
| Multiple myeloma, adult | 0 | — | — | (+) | 1.12 (1.08 to 1.16) | 78 | (+) strong-probable |
(-) inverse; (+) positive; 0 null or limited. BMI = body mass index; CI = confidence interval; OR = odds ratio; RR = relative risk; WCRF = World Cancer Research Fund.
The hazard ratio in the original study was 0.91 (95% CI = 0.83 to 0.99) for 1 z-score increment at age 7 years that approximately corresponds to 1-3 kg/m2.
The hazard ratio in the original study was 1.07 (95% CI = 1.02 to 1.12) for 1 z-score increment at age 7 years that approximately corresponds to 1-3 kg/m2.
Table 2.
Basic information of Mendelian randomization studies presented in Table 1
| Cancer | First author, Year | Data source | No. of SNPs | % of variance explained | 3 basic assumptions assesseda |
||
|---|---|---|---|---|---|---|---|
| Relevance | Independence | Exclusion restriction | |||||
| Breast | |||||||
| Early life | Gao, et al., 2016 (9) | DRIVE | 15 | 2.0 | √ | √b | √ |
| Adult | Guo, et al., 2016 (10) | BCAC, DRIVE | 84 | 1.2 | √ | √ | × |
| Ovary | |||||||
| Early life | Gao, et al., 2016 (9) | FOCI | 15 | 2.0 | √ | √b | √ |
| Adult | Dixon, et al., 2016 (30) | OCAC | 87 | 1.6 | √ | √ | √ |
| Colorectum | |||||||
| Early life | Jarvis, et al., 2016 (37) | CCFR1, CCFR2, COIN, FINLAND, UK1, Scotland1, VQ58 | 9 | Not reported | √ | √c | √ |
| Adult | Bull et al., 2020 (38) | GECCO, CORECT, C-CFR | 312 (185 for women, 152 for men) | 0.3-5.04 | √ | × | √ |
| Endometrium, adult | Painter et al., 2016 (34) | ECAC | 77 | 1.0 | √ (but not reported F-statistics/r-square) | √ | √ |
| Esophageal adenocarcinoma, adult | Thrift et al., 2014 (44) | BEAGSS | 29 | 0.16 kg/m2 | √ | √ | √ |
| Pancreas, adult | Carreras-Torres et al. 2017 (46) | PanScan, PanC4 | 96 | 2.7 | √ | √ | √ |
| Kidney, adult | Johansson et al., 2019 (64) | IARC-1, NCI-1, MDA, UK | 709 | 9.5 | √ | × | √ |
| Lung, adult | Zhou et al., 2020 (56) | TRICL, ILCCO | 842 | 7.3 | √ | √ (smoking) | √ |
Relevance assumption: the genetic variants associate with the risk factor of interest; independence assumption: the genetic variants do not share common causes with the outcome; exclusion restriction: the genetic variants affect the outcome only through their effect on the risk factor of interest. BCAC = Breast Cancer Association Consortium; BEAGSS = Barrett’s and Esophageal Adenocarcinoma Genetic Susceptibility Study; C-CFR = Colon-Cancer Family Registry; CORECT = Colorectal Transdisciplinary Study; DRIVE = Discovery, Biology and Risk of Inherited Variants in Breast Cancer Project; ECAC = Endometrial Cancer Association Consortium; FOCI = Follow-up of Ovarian Cancer Genetic Association and Interaction Studies; GECCO = Genetics and Epidemiology of Colorectal Cancer Consortium; IARC = International Agency for Research on Cancer; ILCCO = International Lung Cancer Consortium; MDA = MD Anderson Cancer Center; NCI = US National Cancer Institute; OCAC = Ovarian Cancer Association Consortium; PanScan = Pancreatic Cancer Cohort Consortium; PanC4 = Pancreatic Cancer Case-Control Consortium; TRICL = Transdisciplinary Research in Cancer of the Lung; UK = United Kingdom cancer research centers.
Only mentioned that there was no confounding by population stratification without showing associations between SNPs and other confounders.
Only discussed the plausibility without showing associations between SNPs and confounders.
MR studies can also redefine the effect estimate of a relationship for an “allocation” to long-term high BMI across the lifespan. Our summary showed that the effect estimates were stronger in nearly all cancers, and the magnitude ranged from 1.14-fold (early life obesity and breast cancer) to 1.37-fold (adult obesity and esophageal adenocarcinoma) stronger. For a cumulative lifetime exposure, MR studies would tend to yield a larger effect estimate than most conventional studies, for which exposures are collected at a single timepoint or over a limited duration. Nevertheless, the concept of “lifetime effect” in MR studies is ambiguously defined, and inadequate attention has been given to time-varying exposures (ie, the relationship between genetic variants and BMI changes over time). A recent meta-analysis of GWAS found that 15 loci influenced BMI differently by age (81). A longitudinal analysis observed that each 10-allele increment in the GRS was associated with different units of BMI change throughout adulthood, and the association varied by baseline BMI (eg, genetically predicted weight gain in early adulthood was more pronounced among individuals who were heavy at approximately 20 years of age) (21). Because the same unit of average genetically conferred BMI increment can derive from diverse trajectories and may not necessarily result in the same effect, the MR estimate would not be a valid estimate of a causal lifetime effect of high BMI (82).
Lastly, an extensive MR framework could help enable us to disentangle the independent (direct) effect of obesity at different timepoints in the life course (11). Observational trajectory analysis emphasized that early life adiposity is associated with lower risk of premenopausal breast cancer, ovarian cancer, and possibly advanced prostate cancer and suggested greater importance of current body mass for endometrial cancer, esophageal adenocarcinoma, and kidney cancer (24). These findings demonstrate a dynamic relationship between obesity and cancer development during different periods of the lifespan. In this case, because genetic variants for anthropometric measures typically overlap with each other, conventional MR studies may fail to find genetic variants that are not associated with obesity in another period and, thus, may be biased especially when the direction of the early and late-life obesity are in opposite directions (eg, breast cancer, ovarian cancer, possibly advanced prostate cancer). Nevertheless, as shown in several studies, multivariable MR studies are useful to study whether obesity at different timepoints influences cancer incidence along the same causal pathway or independently (11,83).
The validity of MR approach depends on 3 core assumptions. In particular, the third assumption can be violated by pleiotropy or colocalization (84). Under pleiotropy, the genetic instrumental variables are causally related to cancer through a pathway that is not mediated by exposure. Under colocalization, the genetic instrumental variables may be in linkage disequilibrium with other variants causally related to cancer through a pathway that does not involve the exposure. Several scenarios violating the MR assumptions have been previously described in detail, including settings with inadequate phenotype definition, time-varying exposures, gene-environment interaction, measurement error, reverse causation, and linkage disequilibrium (85). Some alternative approaches have been suggested to address many, though not all, potential biases (85). Despite these limitations above, if performed appropriately, MR studies can advance and actually have advanced our understanding of obesity in cancer etiology. Importantly, the specific limitations of MR studies and conventional studies may differ, so inferences may be strengthened when the findings are in concordance.
Emerging MR studies currently shift to 2-sample approaches that use GWAS summary statistics to increase statistical power, whereas using individual-level data is superior for mediation and multivariable analyses, as well as validation of MR assumptions (86). Moreover, because the sample sizes of GWAS are increasing and more SNPs associated with obesity are being discovered, the instrumental variables for obesity measures would be stronger, and the statistical power would be further strengthened to resolve the discrepancies between MR and conventional observational studies (eg, multiple myeloma). One limitation of the review is that the SNPs associated with obesity had not been updated at the time when the original MR studies were performed. It is noted that a most recent genome-wide polygenic predictor of obesity comprising of 2.1 million variants, comparing the top decile to the bottom decile, was associated with a 3.5 kg gradient in weight by age 8 years, 12.3 kg by age 18 years, and 13.0 kg among middle-aged adults, which would potentially outperform earlier scores based on fewer variants and provide new insights of quantifying inherited susceptibility to obesity, especially predicting the weight trajectory (87).
The extensions of MR paradigm would provide a broader scope of the causation between obesity and cancer risk. First, for complex effects (eg, BMI and smoking behaviors on lung cancer risk), advanced analyses such as network MR, which uses genetic instruments to investigate mediation in causal pathways, may illuminate the causal relationship (88). Second, integrating high-dimensional omics data may allow us to explore important mediators on causal pathways linking obesity with cancer development (89). Third, when genetic variants tend to be associated with multiple intermediate phenotypes (eg, adult BMI and childhood BMI), multivariable MR studies are useful by disentangling the direct effects of the 2 phenotypes (11). Whether the seemingly opposite effects of early life and later-life obesity on breast cancer and prostate cancer can explain the discrepancies between MR studies and conventional observational studies need to be confirmed. Furthermore, the development of MR methods is warranted to capture nonlinear effects between body size at different points in the lifespan and cancer (90). Future MR studies should prioritize developing a clearer definition of a lifetime effect of obesity and aid in the interpretation of stronger effect size than conventional observational studies (ie, meta-analyses and pooled analyses of cohort studies).
Funding
None.
Notes
Role of the funder: Not applicable.
Disclosures: The authors have declared no conflicts of interest.
Author contributions: ZF: Investigation; Formal analysis; Data visualization; Writing—original draft. ELG: Conceptualization; Methodology; Writing—review and editing; Supervision. MS and DHL: Writing—review and editing.
Disclaimers: The manuscript contents are solely the responsibility of the authors.
Data Availability
No new data were generated or analyzed in support of this research.
Supplementary Material
References
- 1. NCD Risk Factor Collaboration (NCD-RisC). Trends in adult body-mass index in 200 countries from 1975 to 2014: a pooled analysis of 1698 population-based measurement studies with 19.2 million participants. Lancet. 2016;387(10026):1377–1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hales CM, Carroll MD, Fryar CD, Ogden CL.. Prevalence of obesity and severe obesity among adults: United States, 2017-2018. NCHS Data Brief. 2020;(360):1–8. [PubMed] [Google Scholar]
- 3. Lauby-Secretan B, Scoccianti C, Loomis D, et al. Body fatness and cancer–viewpoint of the IARC working group. N Engl J Med. 2016;375(8):794–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kyrgiou M, Kalliala I, Markozannes G, et al. Adiposity and cancer at major anatomical sites: umbrella review of the literature. BMJ. 2017;356:j477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Davey Smith G, Ebrahim S.. Mendelian randomization: Can genetic epidemiology contribute to understanding environmental determinants of disease? Int J Epidemiol . 2003;32(1):1–22. [DOI] [PubMed] [Google Scholar]
- 6. Freeman G, Cowling BJ, Schooling CM.. Power and sample size calculations for Mendelian randomization studies using one genetic instrument. Int J Epidemiol. 2013;42(4):1157–1163. [DOI] [PubMed] [Google Scholar]
- 7. Pierce BL, Ahsan H, Vanderweele TJ.. Power and instrument strength requirements for Mendelian randomization studies using multiple genetic variants. Int J Epidemiol. 2011;40(3):740–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ooi BNS, Loh H, Ho PJ, et al. The genetic interplay between body mass index, breast size and breast cancer risk: a Mendelian randomization analysis. Int J Epidemiol. 2019;48(3):781–794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gao C, Patel CJ, Michailidou K, et al. ; for the Colorectal Transdisciplinary Study (CORECT); Discovery, Biology and Risk of Inherited Variants in Breast Cancer (DRIVE); Elucidating Loci Involved in Prostate Cancer Susceptibility (ELLIPSE); Follow-up of Ovarian Cancer Genetic Association and Interaction Studies (FOCI); and Transdisciplinary Research in Cancer of the Lung (TRICL). Mendelian randomization study of adiposity-related traits and risk of breast, ovarian, prostate, lung and colorectal cancer. Int J Epidemiol. 2016;45(3):896–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Guo Y, Warren AS, Shu XO, et al. Genetically predicted body mass index and breast cancer risk: Mendelian randomization analyses of data from 145,000 women of European descent. PLoS Med. 2016;13(8):e1002105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Richardson TG, Sanderson E, Elsworth B, Tilling K, Davey SG.. Use of genetic variation to separate the effects of early and later life adiposity on disease risk: Mendelian randomisation study. BMJ. 2020;369:m1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Amadou A, Ferrari P, Muwonge R, et al. Overweight, obesity and risk of premenopausal breast cancer according to ethnicity: a systematic review and dose-response meta-analysis. Obes Rev. 2013;14(8):665–678. [DOI] [PubMed] [Google Scholar]
- 13. Cheraghi Z, Poorolajal J, Hashem T, Esmailnasab N, Doosti Irani A.. Effect of body mass index on breast cancer during premenopausal and postmenopausal periods: A meta-analysis. PLoS One. 2012;7(12):e51446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Schoemaker MJ, Nichols HB, Wright LB, et al. ; for the Premenopausal Breast Cancer Collaborative Group. Association of body mass index and age with subsequent breast cancer risk in premenopausal women. JAMA Oncol. 2018;4(11):e181771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Renehan AG, Tyson M, Egger M, Heller RF, Zwahlen M.. Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. Lancet. 2008;371(9612):569–578. [DOI] [PubMed] [Google Scholar]
- 16. Aarestrup J, Bjerregaard LG, Meyle KD, et al. Birthweight, childhood overweight, height and growth and adult cancer risks: a review of studies using the Copenhagen School Health Records Register. Int J Obes. 2020;44(7):1546–1560. [DOI] [PubMed] [Google Scholar]
- 17. Graff M, Ngwa JS, Workalemahu T, et al. ; for the GIANT Consortium. Genome-wide analysis of BMI in adolescents and young adults reveals additional insight into the effects of genetic loci over the life course. Hum Mol Genet. 2013;22(17):3597–3607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Rosner B, Eliassen AH, Toriola AT, et al. Weight and weight changes in early adulthood and later breast cancer risk. Int J Cancer. 2017;140(9):2003–2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hidayat K, Yang CM, Shi BM.. Body fatness at a young age, body fatness gain and risk of breast cancer: systematic review and meta-analysis of cohort studies. Obes Rev. 2018;19(2):254–268. [DOI] [PubMed] [Google Scholar]
- 20. Arnold M, Jiang L, Stefanick ML, et al. Duration of adulthood overweight, obesity, and cancer risk in the Women’s Health Initiative: a longitudinal study from the United States. PLoS Med. 2016;13(8):e1002081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Song M, Zheng Y, Qi L, et al. Longitudinal analysis of genetic susceptibility and BMI throughout adult life. Diabetes. 2018;67(2):248–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Keum N, Greenwood DC, Lee DH, et al. Adult weight gain and adiposity-related cancers: a dose-response meta-analysis of prospective observational studies. J Natl Cancer Inst. 2015;107(2):djv088. [DOI] [PubMed] [Google Scholar]
- 23. Rukh G, Ahmad S, Ericson U, et al. Inverse relationship between a genetic risk score of 31 BMI loci and weight change before and after reaching middle age. Int J Obes. 2016;40(2):252–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Song M, Willett WC, Hu FB, et al. Trajectory of body shape across the lifespan and cancer risk. Int J Cancer. 2016;138(10):2383–2395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Munsell MF, Sprague BL, Berry DA, Chisholm G, Trentham-Dietz A.. Body mass index and breast cancer risk according to postmenopausal estrogen-progestin use and hormone receptor status. Epidemiol Rev. 2014;36(1):114–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Neuhouser ML, Aragaki AK, Prentice RL, et al. Overweight, obesity, and postmenopausal invasive breast cancer risk: a secondary analysis of the women’s health initiative randomized clinical trials. JAMA Oncol. 2015;1(5):611–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Garcia-Estevez L, Moreno-Bueno G.. Updating the role of obesity and cholesterol in breast cancer. Breast Cancer Res. 2019;21(1):35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Picon-Ruiz M, Morata-Tarifa C, Valle-Goffin JJ, Friedman ER, Slingerland JM.. Obesity and adverse breast cancer risk and outcome: mechanistic insights and strategies for intervention. CA Cancer J Clin. 2017;67(5):378–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Shu X, Wu L, Khankari NK, et al. ; for the Breast Cancer Association Consortium. Associations of obesity and circulating insulin and glucose with breast cancer risk: a Mendelian randomization analysis. Int J Epidemiol. 2019;48(3):795–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Dixon SC, Nagle CM, Thrift AP, et al. ; for the Ovarian Cancer Association Consortium. Adult body mass index and risk of ovarian cancer by subtype: a Mendelian randomization study. Int J Epidemiol. 2016;45(3):884–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Olsen CM, Nagle CM, Whiteman DC, et al. ; for the Ovarian Cancer Association Consortium. Obesity and risk of ovarian cancer subtypes: evidence from the Ovarian Cancer Association Consortium. Endocr Relat Cancer. 2013;20(2):251–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Aune D, Navarro Rosenblatt DA, Chan DS, et al. Anthropometric factors and ovarian cancer risk: a systematic review and nonlinear dose-response meta-analysis of prospective studies. Int J Cancer. 2015;136(8):1888–1898. [DOI] [PubMed] [Google Scholar]
- 33. Huang T, Tworoger SS, Willett WC, Stampfer MJ, Rosner BA.. Associations of early life and adulthood adiposity with risk of epithelial ovarian cancer. Ann Oncol. 2019;30(2):303–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Painter JN, O’Mara TA, Marquart L, et al. Genetic risk score Mendelian randomization shows that obesity measured as body mass index, but not waist:hip ratio, is causal for endometrial cancer. Cancer Epidemiol Biomarkers Prev. 2016;25(11):1503–1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Masuda T, Ogawa K, Kamatani Y, et al. A Mendelian randomization study identified obesity as a causal risk factor of uterine endometrial cancer in Japanese. Cancer Sci. 2020;111(12):4646–4651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Dougan MM, Hankinson SE, Vivo ID, et al. Prospective study of body size throughout the life-course and the incidence of endometrial cancer among premenopausal and postmenopausal women. Int J Cancer. 2015;137(3):625–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Jarvis D, Mitchell JS, Law PJ, et al. Mendelian randomisation analysis strongly implicates adiposity with risk of developing colorectal cancer. Br J Cancer. 2016;115(2):266–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Bull CJ, Bell JA, Murphy N, et al. Adiposity, metabolites, and colorectal cancer risk: Mendelian randomization study. BMC Med. 2020;18(1):396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Cornish AJ, Law PJ, Timofeeva M, et al. Modifiable pathways for colorectal cancer: a Mendelian randomisation analysis. Lancet Gastroenterol Hepatol. 2020;5(1):55–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kim H, Giovannucci EL.. Sex differences in the association of obesity and colorectal cancer risk. Cancer Causes Control. 2017;28(1):1–4. [DOI] [PubMed] [Google Scholar]
- 41. Ma Y, Yang Y, Wang F, et al. Obesity and risk of colorectal cancer: a systematic review of prospective studies. PLoS One. 2013;8(1):e53916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Song M, Hu FB, Spiegelman D, et al. Long-term status and change of body fat distribution, and risk of colorectal cancer: a prospective cohort study. Int J Epidemiol. 2016;45(3):871–883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Zhang X, Wu K, Giovannucci EL, et al. Early life body fatness and risk of colorectal cancer in U.S. women and men: results from two large cohort studies. Cancer Epidemiol Biomarkers Prev. 2015;24(4):690–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Thrift AP, Shaheen NJ, Gammon MD, et al. Obesity and risk of esophageal adenocarcinoma and Barrett’s esophagus: a Mendelian randomization study. J Natl Cancer Inst. 2014;106(11):dju252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Mao Y, Yan C, Lu Q, et al. Genetically predicted high body mass index is associated with increased gastric cancer risk. Eur J Hum Genet. 2017;25(9):1061–1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Carreras-Torres R, Johansson M, Gaborieau V, et al. The role of obesity, type 2 diabetes, and metabolic factors in pancreatic cancer: a Mendelian randomization study. J Natl Cancer Inst. 2017;109(9):djx012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Lu Y, Gentiluomo M, Lorenzo-Bermejo J, et al. Mendelian randomisation study of the effects of known and putative risk factors on pancreatic cancer. J Med Genet. 2020;57(12):820–828. doi:10.1136/jmedgenet-2019-106200. [DOI] [PubMed] [Google Scholar]
- 48. Langdon RJ, Richmond RC, Hemani G, et al. A phenome-wide Mendelian randomization study of pancreatic cancer using summary genetic data. Cancer Epidemiol Biomarkers Prev. 2019;28(12):2070–2078. [DOI] [PubMed] [Google Scholar]
- 49. Barahona Ponce C, Scherer D, Brinster R, et al. Gallstones, body mass index, C-reactive protein and gallbladder cancer–Mendelian randomization analysis of Chilean and European genotype data. Hepatology. 2020;73(5):1783–1796. [DOI] [PubMed] [Google Scholar]
- 50. Murphy N, Jenab M, Gunter MJ.. Adiposity and gastrointestinal cancers: epidemiology, mechanisms and future directions. Nat Rev Gastroenterol Hepatol. 2018;15(11):659–670. [DOI] [PubMed] [Google Scholar]
- 51. Campbell PT, Newton CC, Kitahara CM, et al. Body size indicators and risk of gallbladder cancer: pooled analysis of individual-level data from 19 prospective cohort studies. Cancer Epidemiol Biomarkers Prev. 2017;26(4):597–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.World Cancer Research Fund–American Institute for Cancer Research. Diet, nutrition, physical activity and stomach cancer. Continuous Update Expert Report. 2018. https://www.wcrf.org/wp-content/uploads/2021/02/stomach-cancer-report.pdf. Accessed January 1, 2021.
- 53. Genkinger JM, Spiegelman D, Anderson KE, et al. A pooled analysis of 14 cohort studies of anthropometric factors and pancreatic cancer risk. Int J Cancer. 2011;129(7):1708–1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Carreras-Torres R, Haycock PC, Relton CL, et al. The causal relevance of body mass index in different histological types of lung cancer: a Mendelian randomization study. Sci Rep. 2016;6:31121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Carreras-Torres R, Johansson M, Haycock PC, et al. Obesity, metabolic factors and risk of different histological types of lung cancer: a Mendelian randomization study. PLoS One. 2017;12(6):e0177875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Zhou W, Liu G, Hung RJ, et al. Causal relationships between body mass index, smoking and lung cancer: univariable and multivariable Mendelian randomization. Int J Cancer. 2021;148(5):1077–1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Carreras-Torres R, Johansson M, Haycock PC, et al. Role of obesity in smoking behaviour: Mendelian randomisation study in UK Biobank. BMJ. 2018;361:k1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Yang Y, Dong J, Sun K, et al. Obesity and incidence of lung cancer: a meta-analysis. Int J Cancer. 2013;132(5):1162–1169. [DOI] [PubMed] [Google Scholar]
- 59. Yu D, Zheng W, Johansson M, et al. Overall and central obesity and risk of lung cancer: a pooled analysis. J Natl Cancer Inst . 2018;110(8):831–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Duan P, Hu C, Quan C, et al. Body mass index and risk of lung cancer: systematic review and dose-response meta-analysis. Sci Rep. 2015;5(1):16938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Jeong SM, Lee DH, Giovannucci EL.. Predicted lean body mass, fat mass and risk of lung cancer: prospective US cohort study. Eur J Epidemiol. 2019;34(12):1151–1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.World Cancer Research Fund–American Institute for Cancer Research. Diet, nutrition, physical activity and kidney cancer. Continuous Update Expert Report. 2018. https://www.wcrf.org/wp-content/uploads/2021/02/kidney-cancer-report.pdf. Accessed January 1, 2021.
- 63. Chow WH, Dong LM, Devesa SS.. Epidemiology and risk factors for kidney cancer. Nat Rev Urol. 2010;7(5):245–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Johansson M, Carreras-Torres R, Scelo G, et al. The influence of obesity-related factors in the etiology of renal cell carcinoma–a Mendelian randomization study. PLoS Med. 2019;16(1):e1002724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Benn M, Tybjærg-Hansen A, Smith GD, Nordestgaard BG.. High body mass index and cancer risk—a Mendelian randomisation study. Eur J Epidemiol. 2016;31(9):879–892. [DOI] [PubMed] [Google Scholar]
- 66. Liu X, Sun Q, Hou H, et al. The association between BMI and kidney cancer risk: an updated dose-response meta-analysis in accordance with PRISMA guideline. Medicine (Baltimore). 2018;97(44):e12860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Wang F, Xu Y.. Body mass index and risk of renal cell cancer: a dose‐response meta‐analysis of published cohort studies. Int J Cancer. 2014;135(7):1673–1686. [DOI] [PubMed] [Google Scholar]
- 68. Leiba A, Kark JD, Afek A, et al. Adolescent obesity and paternal country of origin predict renal cell carcinoma: a cohort study of 1.1 million 16 to 19-year-old males. J Urol. 2013;189(1):25–29. [DOI] [PubMed] [Google Scholar]
- 69. Jensen BW, Meyle KD, Madsen K, Sørensen TIA, Baker JL.. Early life body size in relation to risk of renal cell carcinoma in adulthood: a Danish observational cohort study. Eur J Epidemiol. 2020;35(3):251–258. [DOI] [PubMed] [Google Scholar]
- 70. Landberg A, Fält A, Montgomery S, Sundqvist P, Fall K.. Overweight and obesity during adolescence increases the risk of renal cell carcinoma. Int J Cancer. 2019;145(5):1232–1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.World Cancer Research Fund–American Institute for Cancer Research. Diet, nutrition, physical activity and prostate cancer. Continuous Update Project. 2018. https://www.wcrf.org/wp-content/uploads/2021/02/prostate-cancer-report.pdf. Accessed January 1, 2021.
- 72. Harrison S, Tilling K, Turner EL, et al. Systematic review and meta-analysis of the associations between body mass index, prostate cancer, advanced prostate cancer, and prostate-specific antigen. Cancer Causes Control. 2020;31(5):431–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Freedland SJ, Platz EA.. Obesity and prostate cancer: making sense out of apparently conflicting data. Epidemiol Rev. 2007;29(1):88–97. [DOI] [PubMed] [Google Scholar]
- 74. Discacciati A, Orsini N, Wolk A.. Body mass index and incidence of localized and advanced prostate cancer–a dose-response meta-analysis of prospective studies. Ann Oncol. 2012;23(7):1665–1671. [DOI] [PubMed] [Google Scholar]
- 75. Davies NM, Gaunt TR, Lewis SJ, et al. ; for the PRACTICAL consortium. The effects of height and BMI on prostate cancer incidence and mortality: a Mendelian randomization study in 20,848 cases and 20,214 controls from the. Cancer Causes Control. 2015;26(11):1603–1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Giovannucci E. Adiposity over the life course and prostate cancer: unraveling the complexities. Cancer Causes Control. 2020;31(12):1051–1055. [DOI] [PubMed] [Google Scholar]
- 77. Möller E, Wilson KM, Batista JL, et al. Body size across the life course and prostate cancer in the Health Professionals Follow-up Study. Int J Cancer. 2016;138(4):853–865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Wallin A, Larsson SC.. Body mass index and risk of multiple myeloma: a meta-analysis of prospective studies. Eur J Cancer. 2011;47(11):1606–1615. [DOI] [PubMed] [Google Scholar]
- 79. Went M, Sud A, Law PJ, et al. Assessing the effect of obesity-related traits on multiple myeloma using a Mendelian randomisation approach. Blood Cancer J. 2017;7(6):e573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Went M, Cornish AJ, Law PJ, et al. Search for multiple myeloma risk factors using Mendelian randomization. Blood Adv. 2020;4(10):2172–2179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Winkler TW, Justice AE, Graff M, et al. ; for the CHARGE Consortium. The influence of age and sex on genetic associations with adult body size and shape: a large-scale genome-wide interaction study. PLoS Genet. 2015;11(10):e1005378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Labrecque JA, Swanson SA.. Interpretation and potential biases of Mendelian randomization estimates with time-varying exposures. Am J Epidemiol. 2019;188(1):231–238. [DOI] [PubMed] [Google Scholar]
- 83. Burgess S, Thompson SG.. Multivariable Mendelian randomization: the use of pleiotropic genetic variants to estimate causal effects. Am J Epidemiol. 2015;181(4):251–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Hemani G, Bowden J, Davey Smith G.. Evaluating the potential role of pleiotropy in Mendelian randomization studies. Hum Mol Genet. 2018;27(R2):R195–R208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. VanderWeele TJ, Tchetgen Tchetgen EJ, Cornelis M, Kraft P.. Methodological challenges in mendelian randomization. Epidemiology. 2014;25(3):427–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Glymour MM, Tchetgen Tchetgen EJ, Robins JM.. Credible Mendelian randomization studies: approaches for evaluating the instrumental variable assumptions. Am J Epidemiol. 2012;175(4):332–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Khera AV, Chaffin M, Wade KH, et al. Polygenic prediction of weight and obesity trajectories from birth to adulthood. Cell. 2019;177(3):587–596.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Burgess S, Daniel RM, Butterworth AS, Thompson SG, EPIC-InterAct Consortium. Network Mendelian randomization: using genetic variants as instrumental variables to investigate mediation in causal pathways. Int J Epidemiol. 2015;44(2):484–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Brion M-JA, Benyamin B, Visscher PM, Smith GD.. Beyond the single SNP: emerging developments in Mendelian randomization in the “Omics” Era. Curr Epidemiol Rep. 2014;1(4):228–236. [Google Scholar]
- 90. Staley JR, Burgess S.. Semiparametric methods for estimation of a nonlinear exposure-outcome relationship using instrumental variables with application to Mendelian randomization. Genet Epidemiol. 2017;41(4):341–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No new data were generated or analyzed in support of this research.