Abstract
Radiation resistance represents an imperative obstacle in the treatment of patients with colorectal cancer, which remains difficult to overcome. Here, we explored the anti-proliferative and migration-inhibiting properties of the natural product shikonin on a radiation-resistant human colon carcinoma cell line (SNU-C5RR). Shikonin reduced the viability of these cells in a dose-dependent manner; 38 μM of shikonin was determined as the half-maximal inhibitory concentration. Shikonin induced apoptotic cell death, as demonstrated by increased apoptotic body formation and the number of TUNEL-positive cells. Moreover, shikonin enhanced mitochondrial membrane depolarization and Bax expression and also decreased Bcl-2 expression with translocation of cytochrome c from mitochondria into the cytosol. In addition, shikonin activated mitogen-activated protein kinases, and their specific inhibitors reduced the cytotoxic effects of shikonin. Additionally, shikonin decreased the migration of SNU-C5RR cells via the upregulation of E-cadherin and downregulation of N-cadherin. Taken together, these results suggest that shikonin induces mitochondria-mediated apoptosis and attenuates epithelial-mesenchymal transition in SNU-C5RR cells.
Keywords: Shikonin, Radiation resistance, Human colon cancer, Epithelial to mesenchymal transition, Mitochondria-mediated apoptosis, Mitogen-activated protein kinases
INTRODUCTION
Recent research reports, based on the analysis of morbidity and mortality databases from 39 countries, indicated that the incidence of colorectal cancer continues to increase in young people in countries with a high human development index (Wong et al., 2021). Republic of Korea had the second highest incidence of colorectal cancer in 2018 (Khil et al., 2021). Even after surgical intervention, some microscopic tumor cells may remain, which have the potential to invade the surrounding organs. Radiation therapy is commonly used to eliminate the remaining cells, which is termed adjuvant therapy (Baskar et al., 2012). Unfortunately, many clinical cases are associated with the development of radioresistance, which is difficult to overcome (Jin et al., 2016). Consequently, there is a great need for new chemotherapeutic agents with high anticancer activity to eradicate radioresistant cancer cells.
Recently, the scientific community has started to pay more attention to natural bioactive compounds and their anticancer properties. Shikonin is one of these naturally occurring compounds. Shikonin is extracted from Lithospermum erythrorhizon, Onosma paniculata, and Arnebia euchroma. Shikonin exerts cytotoxic effects by inhibiting protein tyrosine kinase and DNA topoisomerases, which play a crucial role in cancer cell DNA regulation, and decreases the expression of tumor necrosis factor receptor-associated protein 1. In addition, shikonin increases p53 expression and inhibits glycolysis by suppressing the pyruvate kinase M2 (Andújar et al., 2013; Han et al., 2015). Recently, the apoptosis-inducing effect of shikonin was shown in human stomach cancer cells, colon cancer cells, lung cancer cells, promyelocytic leukemia cells, medullary thyroid carcinoma cells, and cervical cancer cells (Lu et al., 2015; He et al., 2016; Jeung et al., 2016; Ko et al., 2016; Trivedi et al., 2016; Wei et al., 2016).
Most colon adenocarcinomas are silent tumors. This means slow growth and no obvious symptoms until the tumor reaches a significant size. Then it becomes invasive and causes metastasis (Ameli and Khalily, 2016). This alteration is related to the epithelial to mesenchymal transition (EMT), the transformation of epithelial cells with adhesive properties that are in close contact with each other into high mobility mesenchymal cells, allowing penetration into surrounding tissues and metastasis (Zhu et al., 2013). The relationship between EMT and metastasis in colon cancer has been proven in many studies (Todosi et al., 2012; Zhu et al., 2013; Matějka et al., 2017). Upregulation of EMT inducers can promote EMT, leading to increased invasiveness and metastasis of colorectal cancer (Vu and Datta, 2017). These inducers can downregulate E-cadherin and upregulate N-cadherin and vimentin through modulating EMT-related signaling pathways, such as WNT/β-catenin and TGF-β, and EMT transcription factors, such as zinc finger E-box binding homeobox 1 (ZEB1) and ZEB2 (Vu and Datta, 2017). Therefore, prevention of EMT is an important step in anticancer therapy (Voon et al., 2017).
Here, we aimed to estimate the apoptosis-inducing and EMT-inhibiting properties of shikonin in radiation-resistant colon cancer cells (SNU-C5RR).
MATERIALS AND METHODS
Reagents
Shikonin (5,8-dihydroxy-2-(1-hydroxy-4-methylpent-3-enyl)naphthalene-1,4-dione, Fig. 1A) was obtained from Cayman Chemical Company (Ann Arbor, MI, USA). 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), Hoechst 33342, trypan blue solution, 5,5-dimethyl-1-pyrroline-N-oxide (DMPO), 2’,7’-dichlorodihydrofluorescein diacetate (DCF-DA), and anti-actin antibody were obtained from Sigma-Aldrich (St. Louis, MO, USA). 5,5’,6,6’-Tetrachloro-1,1’,3,3’-tetraethylbenzimidazolocarbocyanine iodide (JC-1) was obtained from Invitrogen (Thermo Fisher Scientific, Inc., Waltham, MA, USA). Anti-phospho-JNK, anti-JNK, anti-phospho-p38, and anti-caspase-3 antibodies along with SB203580 (p38 inhibitor), and U0126 (ERK inhibitor) were obtained from Cell Signaling Technology, Inc (Danvers, MA, USA). Anti-Bax, anti-Bcl-2, anti-caspase-9, anti-cytochrome c oxidase subunit 4 (COX4), anti-phospho-ERK, anti-ERK, anti-p38, anti-E-cadherin, and anti-N-cadherin antibodies were supplied by Santa Cruz Biotechnology, Inc (Dallas, TX, USA). The JNK inhibitor SP600125 was provided by Selleck Chemicals (Houston, TX, USA). All other chemicals and reagents used were of analytical grade.
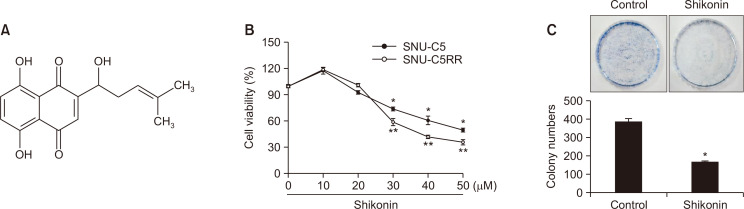
Fig. 1.
Shikonin induces cell death in SNU-C5 and SNU-C5RR cells. (A) The chemical structure of shikonin [5,8-dihydroxy-2-(1-hydroxy-4-methylpent-3-enyl)naphthalene-1,4-dione]. (B) SNU-C5 or SNU-C5RR cells were treated with 10, 20, 30, 40, or 50 μM shikonin for 24 h. Cell viability was measured using the MTT assay. *,**p<0.05 vs the control group in SNU-C5 and SNU-C5RR cells, respectively. (C) For the colony formation assay, SNU-C5RR cells were cultured for 11 days and treated with 38 μM shikonin on day 7. Colonies containing >400 cells were counted. *p<0.05 vs the control group.
Cell culture
Human colorectal adenocarcinoma (SNU-C5) and radiation-resistant (SNU-C5RR) colon cancer cell lines were incubated in a humidified 5% CO2 atmosphere at 37°C in RPMI medium containing 10% heat-inactivated fetal bovine serum (Gibco; Thermo Fisher Scientific, Inc.), penicillin (100 U/mL), and streptomycin (100 μg/mL).
Cell viability assay
SNU-C5 and SNU-C5RR cells were treated with various concentration of shikonin and then incubated for 24 h. In addition, SNU-C5RR cells were treated with shikonin, SB203580, SP600125, U0126, or their combinations, and then incubated for 24 h. To evaluate the EMT in SNU-C5RR cells, cell viability was assessed after 48 h of shikonin treatment. After each treatment, exactly 125 µL of MTT solution (2 mg/mL) was added to each well. After maintaining the cells at 37°C until the formation of formazan crystals, the supernatant was removed by aspiration. Then, we dissolved the formed formazan crystals in dimethyl sulfoxide solution and read the relevant absorbance values at 540 nm with a scanning multi-well spectrophotometer (Shilnikova et al., 2018).
Colony formation assay
SNU-C5RR cells were cultured in 60 mm dishes, and 400 cells per dish were used as the optimum cell density. Cultured cells were incubated, and 38 μM shikonin was added on day 7 of incubation. The cell culture medium was changed every 3 days. On day 11 of incubation, 70% ethanol was applied to fix the cells, which were then stained with 0.4% trypan blue until colonies were detectable. Colony formation was imaged and quantified using ImageJ software (version 1.47; National Institutes of Health, Bethesda, MD, USA).
Nuclear staining with Hoechst 33342
After treating SNU-C5RR cells with 38 µM shikonin or SP600125 along with SB203580, U0126, or a combination for 24 h, Hoechst 33342 staining was performed, followed by incubation at 37°C for 10 min. Then, cells were observed using a fluorescence microscope (Olympus Life Science, Tokyo, Japan).
Terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling (TUNEL) assay
SNU-C5RR cells (1×105 cells/mL) were cultured in chamber slides and maintained for 24 h. 38 μM Shikonin was added, and cells were incubated for 24 h. The slides were then prepared following the manufacturer’s instructions for the DeadEnd Colorimetric TUNEL system (Promega Corporation, Madison, WI, USA). Faramount aqueous mounting medium (Agilent Technologies, Inc., Santa Clara, CA, USA) was used to mount stained cells on microscope slides. The microscopic images were captured via confocal fluorescent microscopy using an appropriate Laser Scanning Microscope 5 PASCAL program (Carl Zeiss AG, Oberkochen, Germany).
Single-cell gel electrophoresis (Comet assay)
Cell pellets were suspended in 1% low-melting agarose (LMA), and the cell-containing suspension was evenly spread on a microscopic slide, which was pre-coated with 1% normal melting agarose. Slides were kept at 4°C to enhance the solidification of agarose and covered with 170 μL of 0.5% LMA. Then, slides were dipped in lysis buffer (2.5 M NaCl, 100 mM Na2EDTA, 1% Triton X-100, 10 mM Tris, and 1% N-lauroyl sarcosinate, pH 10) for 1 h in dark at 4°C. Each slide was subjected to gel electrophoresis, which contained NaOH (300 mM) and Na2EDTA (10 mM, pH 10). Electrophoresis (300 mA, 25 V) was conducted in the dark for 30 min, resulting in DNA unwinding and the expression of alkali-labile damage. After electrophoresis, slides were immersed in neutralizing buffer (0.4 M Tris, pH 7.5) and 70% ethanol. Immediately before detection, slides were stained with 40 μL of ethidium bromide (10 μg/mL) and visualized under a fluorescence microscope (Olympus Life Science), and images were gathered using an image analyzer (Komet 5.5, Kinetic Imaging, Bromborough, UK). Exactly 25 cells per slide were used to quantify the comet tail length and total fluorescence percentage of the tails.
Mitochondrial membrane potential (Δψm)
SNU-C5RR cells were incubated and treated with 38 µM shikonin, then dyed with JC-1 (10 µg/mL) and maintained at 37°C to enhance the staining for another 15 min. Microscopic slides were prepared using a mounting medium (DAKO, Carpinteria, CA, USA). The mitochondrial polarization and depolarization status were detected via confocal microscopy, which was implemented with the Laser Scanning Microscope 5 PASCAL software. In addition, data obtained via fluorescent microscopy were confirmed using a FACSCalibur flow cytometer (BD Biosciences, Franklin Lakes, NJ, USA).
Western blotting
SNU-C5RR cells were lysed using PRO-PREP™ protein extraction solution (Intron Biotechnology, Inc., Seongnam, Korea). Protein concentrations were quantified using a Bio-Rad protein assay reagent kit (Bio-Rad Laboratories, Inc., Hercules, CA, USA). Proteins were subjected to electrophoresis and transferred to nitrocellulose membranes (Bio-Rad Laboratories, Inc.). Nitrocellulose membranes were blocked in 2% fetal bovine serum (shaking), and incubated with primary antibodies (dilution, 1:1,000) overnight at 4°C. The membranes were further incubated with secondary horseradish peroxidase-conjugated goat anti-rabbit immunoglobulin G (IgG) or goat anti-mouse IgG antibodies (Pierce; Thermo Fisher Scientific, Inc.; dilution, 1:5,000) for 1 h at room temperature. Corresponding protein bands were detected using an increased chemiluminescence western blotting detection kit (GE Healthcare Life Sciences, Little Chalfont, UK) and then exposed to X-ray films.
Cell migration assay
SNU-C5RR cells (1×104 cells/mL) were seeded on 100 mm dishes, incubated for 24 h, and then treated with 3 µM shikonin. Then, scratch lines (diameter, 1.5 mm) were established across the base of the culture dishes. The line areas were measured after 0 and 48 h using a fluorescence microscope (Olympus Life Science). The index of gap width was calculated as follows: (mean gap width, mm)/(mean gap width in control, mm).
Detection of superoxide anion
The xanthine/xanthine oxidase system was used to generate superoxide anions, which interacted with DMPO, and the produced DMPO/∙OOH adducts were measured using an ESR spectrometer. The ESR spectrum was recorded 2.5 min after PBS (pH 7.4) was mixed with 0.02 mL of 3 M DMPO, 5 mM xanthine, 0.25 U xanthine oxidase, and 3 μM shikonin. ESR spectrometer parameters were as follows: Central magnetic field, 336.8 mT; power, 5.00 mW; frequency, 9.4380 GHz; modulation width, 0.2 mT; amplitude, 1000; sweep width, 10 mT; sweep time, 0.5 min; time constant, 0.03 s; temperature, 25°C.
Detection of hydroxyl radicals
Hydroxyl radicals were generated through the Fenton reaction (H2O2+FeSO4) and allowed to interact with DMPO. The resultant DMPO/∙OH adducts were detected using an ESR spectrometer (Chae et al., 2011; Wu et al., 2012). The ESR spectrum was recorded 2.5 min after PBS (pH 7.4) was mixed with 0.02 mL of 0.3 M DMPO, 10 mM FeSO4, 10 mM H2O2, and 3 μM shikonin. ESR spectrometer parameters were as follows: Central magnetic field, 336.8 mT; power, 1.00 mW; frequency, 9.4380 GHz; modulation width, 0.2 mT; amplitude, 600; sweep width, 10 mT; sweep time, 0.5 min; time constant, 0.03 s; temperature, 25°C.
Intracellular reactive oxygen species (ROS) level
SNU-C5RR cells were seeded on a coverslip-containing four-well plate at a density of 1.5×105 cells/well, and then cells were treated with 3 μM shikonin. After 24 h, representative cell groups were treated with DCF-DA (final concentration 20 μM) and incubated for 30 min at 37°C. Stained cells were washed with PBS, and the coverslips were mounted onto microscope slide in a mounting medium (DAKO). Then, the slides were analyzed using a confocal microscope. Relevant images were gathered using the Laser Scanning Microscope 5 PASCAL software (Carl Zeiss, Jena, Germany). In addition, data obtained via fluorescent microscopy were confirmed using a FACS Calibur flow cytometer (BD Biosciences).
Statistical analysis
All experiments were performed in triplicates. Values represent the mean ± standard error of the mean. All data were evaluated via one-way analysis of variance with Tukey’s post-hoc test to analyze the differences. Differences were considered statistically significant at a p value<0.05.
RESULTS
Shikonin decreases cell viability in colon carcinoma cell lines
Shikonin displayed an obvious dose-dependent toxic effect on both parental (SNU-C5) and radiation-resistant (SNU-C5RR) human colon carcinoma cell lines (Fig. 1B). The IC50 was 52 µM for SNU-C5 cells and 38 µM for SNU-C5RR cells. In other words, both parental and radiation-resistant cell lines were strongly sensitive to shikonin. To further confirm this finding, we examined the colony-forming ability of SNU-C5RR cells (Fig. 1C). Shikonin (38 µM) significantly decreased the number of colonies formed compared to that in the control group. Therefore, we used 38 µM as the final concentration for further experiments to test the cytotoxic properties of shikonin in the SNU-C5RR cell line.
Shikonin induces apoptosis in radiation-resistant colon carcinoma cells
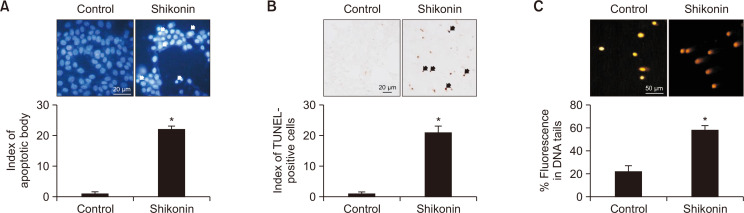
To determine whether the toxic effect of shikonin was related to apoptosis, we detected apoptotic body formation via TUNEL assay as well as the proportion of DNA condensation via Hoechst 33342 nuclear staining. Shikonin-treated cells possessed a higher number of apoptotic bodies and TUNEL-positive cells compared to the control group (Fig. 2A, 2B). Damaged DNA was differentiated from normal DNA due to the influence of the electrophoretic field via comet assay, exhibiting the classic comet tail. Shikonin-treated cells demonstrated elongated nuclear tails and an enhanced DNA index in the tails (Fig. 2C).
Fig. 2.
Shikonin induces apoptosis in SNU-C5RR colon cancer cells. (A) Apoptotic bodies (arrows) were observed via fluorescence microscopy in SNU-C5RR cells stained with Hoechst 33342 and quantified. *p<0.05 vs the control group. (B) Apoptosis in SNU-C5RR cells was examined via the TUNEL assay after 24 h of 38 μM shikonin treatment. TUNEL-positive cells are indicated by arrows. *p<0.05 vs the control group. (C) DNA damage was assessed using an alkaline comet assay. Representative images and the percentage of total DNA fluorescence in the comet tails are shown. *p<0.05 vs the control group.
Shikonin initiates mitochondria-mediated apoptosis
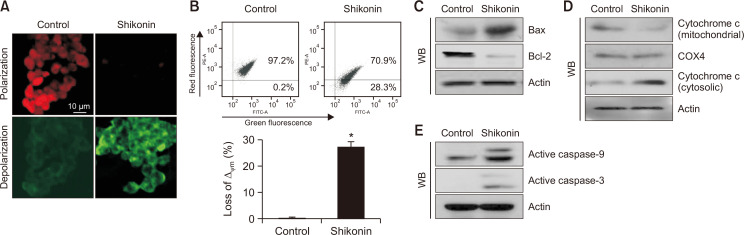
Mitochondria in control cells displayed red fluorescence upon staining with JC-1, which was visualized through Δψm polarization via confocal microscopy. However, shikonin-treated cells displayed a decrease in mitochondrial red fluorescence and elevated green fluorescence, signifying Δψm depolarization (Fig. 3A). The microscopic images were consistent with the flow cytometry analysis, confirming that shikonin destroyed Δψm (Fig. 3B). Furthermore, we investigated the expression of proteins associated with mitochondria-mediated apoptosis. Shikonin enhanced the expression of the pro-apoptotic protein Bax and suppressed the expression of pro-survival/anti-apoptotic protein Bcl-2 (Fig. 3C), leading to the translocation of cytochrome c from mitochondria into the cytosol (Fig. 3D). In addition, shikonin enhanced the expression of active (cleaved) caspase-9 and caspase-3 (Fig. 3E).
Fig. 3.
Shikonin induces mitochondria-mediated apoptosis in SNU-C5RR colon cancer cells. Cells were stained with JC-1, and Δψm was detected via (A) confocal microscopy, and (B) flow cytometry. Cell lysates were subjected to western blotting for the following proteins, *p<0.05 vs control group; (C) Bax, and Bcl-2 protein levels were quantified, and actin was used as the loading control. (D) Mitochondrial cytochrome c, and cytosolic cytochrome c levels were separately measured. COX4 and actin were used as loading controls for the mitochondrial and cytosolic fractions, respectively. (E) Active caspase-9, and active caspase-3 levels were verified via western blotting, and actin was used as the loading control.
Shikonin induces apoptosis via MAPK activation
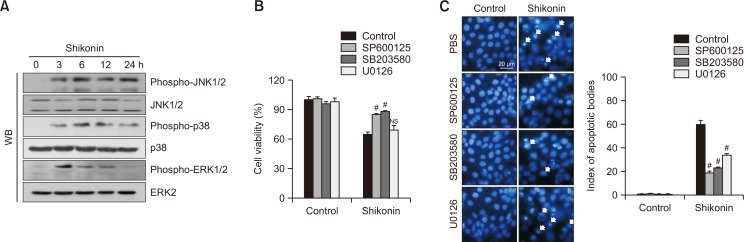
We next examined whether shikonin mediates apoptosis via the MAPK activation cascade. Western blotting data demonstrated that shikonin induced phosphorylation (activation) of JNK, p38, and ERK. Phosphorylation of JNK and p38 reached a maximum at 6 h and remained high after 24 h, whereas phosphorylation of ERK peaked at 3 h and then decreased (Fig. 4A). Thus, ERK signaling demonstrated a faster and shorter reaction to shikonin stimulation than JNK and p38. We then checked whether the inhibitors (JNK, p38, and ERK inhibitors) suppress apoptosis induced by shikonin. Results of the MTT assay and Hoechst 33342 nuclear staining demonstrated the protective effect of SP600125 (JNK inhibitor) and SB203580 (p38 inhibitor) against shikonin-induced cell death (Fig. 4B, 4C). The effect of U0126 (ERK inhibitor) was weaker than the effects of other MAPK inhibitors, which was predicted because cell viability was detected at 24 h, whereas ERK demonstrated maximum phosphorylation at the 3 h time point.
Fig. 4.
Shikonin induces apoptosis via the MAPK signaling pathway in SNU-C5RR colon cancer cells. (A) Cells were harvested after 38 μM shikonin treatment at different time points (0-24 h) and subjected to western blotting using primary antibodies against phospho-JNK1/2, JNK1/2, phospho-p38, p38, phospho-ERK1/2, and ERK2. Following treatment with MAPK inhibitors and/or 38 μM shikonin for 24 h, (B) cell viability was assessed using the MTT assay and (C) apoptotic body formation was observed using Hoechst 33342 nuclear staining. Apoptotic bodies are indicated by arrows. *p<0.05 vs control cells; #p<0.05 vs cells treated with shikonin only; NS, not significant compared to control. SP600125, JNK inhibitor; SB203580, p38 inhibitor; U0126, ERK inhibitor.
Shikonin decreased EMT progression in radiation-resistant colon carcinoma cells
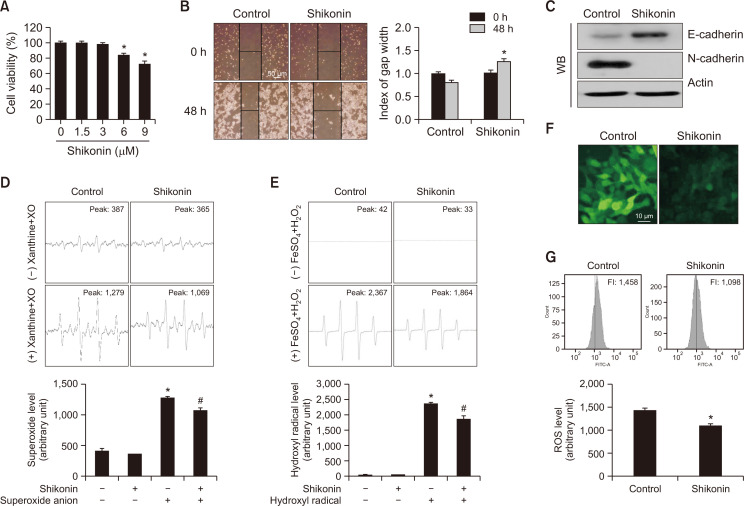
Next, we explored the effect of shikonin on the migration of SNU-C5RR cells to understand its impact on EMT. First, we used a non-toxic concentration of shikonin, which allowed the observation of migrating cells up to 48 h after treatment. MTT data showed that 3 µM of shikonin did not decrease the viability of SNU-C5RR cells 48 h after treatment (Fig. 5A); therefore, we used this concentration in subsequent studies of cellular mobility. We observed wounds established at the base of culture dishes 0 and 48 h after treatment with 3 µM shikonin. While the gap was reduced in the control group, it was expanded in the shikonin-treated group (Fig. 5B). We then determined the expressions of E- and N-cadherin, glycoproteins involved in initiating and stabilizing intercellular contacts. Expression of the epithelial marker E-cadherin and interstitial marker N-cadherin was higher and lower, respectively, after shikonin treatment compared to that in untreated cells (Fig. 5C), confirming that shikonin attenuated EMT and thus the metastatic capacity of SNU-C5RR cells. Increased scavenging of superoxide anions and hydroxyl radicals by 3 µM shikonin was observed in the cell-free system (Fig. 5D, 5E). Furthermore, shikonin reduced cellular ROS production in SNU-C5RR cells, detected using confocal microscopy and flow cytometry after staining with DCF-DA (Fig. 5F, 5G).
Fig. 5.
Shikonin attenuated the epithelial-mesenchymal transition in SNU-C5RR colon cancer cells. (A) SNU-C5RR cells were treated with shikonin at concentrations 0, 1.5, 3, 6, or 9 μM for 48 h, and cell viability was assessed using the MTT assay. *p<0.05 vs control cells. (B) Cells were seeded in 100 mm culture dishes, and following treatment with 3 µM shikonin, a scratch was established along the base of the dish. The wounded area was observed following treatment with or without 3 µM shikonin after 0 and 48 h. The gap was measured, and all data were quantified. Representative images for 48 h incubation are shown. *p<0.05 vs control cells. (C) Cell lysates were electrophoresed and used for western blotting with anti-E-cadherin and anti-N-cadherin antibodies. Actin was used as the loading control. The ability of 3 µM shikonin to scavenge ROS in cell-free conditions was evaluated using (D) the xanthine/xanthine oxidase system for superoxide anion and (E) Fenton reaction (FeSO4+H2O2) system for hydroxyl radical. *p<0.05 vs control, #p<0.05 vs radical only. SNU-C5RR cells were dyed with DCF-DA, and the fluorescence intensity was detected via (F) confocal microscopy, and (G) flow cytometry. *p<0.05 vs control.
DISCUSSION
Our investigation focused on radioresistance and ways to overcome it in colorectal cancer. We found that the herbal compound shikonin significantly suppressed the viability of radiation-resistant human colon carcinoma cells, and we attempted to identify an appropriate mechanism of apoptosis and EMT attenuation initiated by shikonin.
Apoptosis is a type of cell death that leads to several morphological and metabolic alterations, including chromatin condensation, nuclear fragmentation, and activation of special mediators (Wlodkowic et al., 2011). Thereby, the induction of apoptosis is an important strategy for anticancer therapy. Accumulating evidence shows that various apoptotic agents induce single- and double-strand DNA breaks (Lim et al., 2011). Our experiments indicated that the apoptotic body indices and the number of TUNEL-positive cells were elevated in shikonin-treated cells, as well as DNA single- and double-strand breaks, detected via comet assay. These results showed that the cytotoxic effect of shikonin is mediated through the apoptotic mechanism.
Numerous factors induce apoptosis via mitochondria-related pathways (Xiong et al., 2014). Oxidative stress or similar stress inductors destroy Δψm, resulting in the outflow of cytochrome c from mitochondria into the cytosol. These events are controlled by pro-survival/anti-apoptotic Bcl-2 and the pro-apoptotic protein Bax, leading to apoptosome complex formation. This complex initiates the caspase cascade and induces apoptosis (Yang et al., 2017). Thus, the typical markers of mitochondria-related apoptotic cell death include upregulation of Bax, downregulation of Bcl-2, mitochondrial membrane depolarization, the release of cytochrome c from mitochondria, apoptotic body formation, and activation of the caspase cascade (Wang et al., 2016). Here, we detected the loss of Δψm following shikonin treatment. Moreover, Bcl-2 expression was suppressed, whereas Bax expression increased. In addition, we observed the leakage of cytochrome c from mitochondria into the cytosol and the activation of caspase-9 and caspase-3. These data provide evidence of mitochondria-related apoptosis induced by shikonin.
Many recent investigations have demonstrated that the cytotoxic properties of most compounds with anticancer activities are recognized through the activation of MAPK family members (Sun et al., 2015). Here, we focused on the role of MAPKs in apoptotic cell death induced by shikonin. The expression of activated JNK, p38, and ERK was enhanced with time upon treatment with shikonin, whereas the specific MAPK inhibitors demonstrated a cytoprotective effect against shikonin-induced cell death. Thus, our findings confirm that shikonin induces apoptotic cell death in SNU-C5RR cells via the activation of MAPK cascades.
EMT is closely related to tumor metastasis (Todosi et al., 2012; Zhu et al., 2013; Ameli and Khalily, 2016; Matějka et al., 2017). This process is marked by the downregulation of epithelial markers (E-cadherin and β-catenin) and upregulation of mesenchymal markers (N-cadherin and fibronectin). Changes in N-cadherin expression, as well as the EMT E-cadherin/N-cadherin switch, are known as independent risk factors for cancer growth and metastasis (Di Domenico et al., 2011). These findings have been fully explored in gastric, prostate, and oral carcinoma cell lines (Di Domenico et al., 2011; Feng et al., 2017; Ramamurthy et al., 2018), and there are many reports on EMT in colon cancer (Todosi et al., 2012; Zhu et al., 2013; Matějka et al., 2017). Here, the attenuation of migration capacity of SNU-C5RR cells was observed in the shikonin-treated group, implying that the occurrence of EMT destroys cell polarity, induces cell dedifferentiation, changes connections between cells, and improves the invasive ability. Expression of E-cadherin (an epithelial marker) was increased and expression of N-cadherin (an interstitial marker) was suppressed in the shikonin-treated group, indicating that shikonin inhibits EMT. Excessive ROS production can promote cell invasion and migration, thereby promoting the acquisition of EMT characteristics in cancer cells (Kamiya et al., 2016; Ahn et al., 2017). ROS induce EMT by activating Snail expression and inhibits E-cadherin expression in MCF-7 cells (Lee et al., 2019), as well as mediate EMT and cell invasive potential in colon cancer (Jiao et al., 2016; Kang et al., 2018). ROS also act as a key signaling molecule mediating the fractionated ionizing radiation-induced EMT response through the activation of Akt/Src/Erk pathways (Kim et al., 2020).
Thus, we suggest that the EMT-attenuating property of shikonin may be related to its antioxidant effect. Shikonin significantly decreased ROS levels in SNU-C5RR cells, exerting anti-EMT effects, as increased ROS levels are important for EMT (Wang et al., 2010; Cichon and Radisky, 2014).
Taken together, these results suggest that SNU-C5RR colon cancer cells are sensitive to shikonin and that shikonin activates apoptosis and attenuates EMT. Thus, shikonin can be considered a potential candidate to overcome radiation resistance in colon cancer.
ACKNOWLEDGMENTS
This work was supported by the research grant of Jeju National University in 2021.
Footnotes
CONFLICT OF INTEREST
The authors have no conflicts of interest to declare.
REFERENCES
- Ahn H. M., Yoo J. W., Lee S., Lee H. J., Lee H. S., Lee D. S. Peroxiredoxin 5 promotes the epithelial-mesenchymal transition in colon cancer. Biochem. Biophys. Res. Commun. 2017;487:580–586. doi: 10.1016/j.bbrc.2017.04.094. [DOI] [PubMed] [Google Scholar]
- Ameli M., Khalily F. Analysis of colorectal cancer and polyp for presence herpes simplex virus and cytomegalovirus DNA sequences by polymerase chain reaction. J. Anal. Res. Clin. Med. 2016;4:82–89. doi: 10.15171/jarcm.2016.014. [DOI] [Google Scholar]
- Andújar I., Ríos J. L., Giner R. M., Recio M. C. Pharmacological properties of shikonin - a review of literature since 2002. Planta Med. 2013;79:1685–1697. doi: 10.1055/s-0033-1350934. [DOI] [PubMed] [Google Scholar]
- Baskar R., Lee K. A., Yeo R., Yeoh K. W. Cancer and radiation therapy: current advances and future directions. Int. J. Med. Sci. 2012;9:193. doi: 10.7150/ijms.3635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chae S., Piao M. J., Kang K. A., Zhang R., Kim K. C., Youn U. J., Nam K. W., Lee J. H., Hyun J. W. Inhibition of matrix metalloproteinase-1 induced by oxidative stress in human keratinocytes by mangiferin isolated from Anemarrhena asphodeloides. Biosci. Biotechnol. Biochem. 2011;75:2321–2325. doi: 10.1271/bbb.110465. [DOI] [PubMed] [Google Scholar]
- Cichon M. A., Radisky D. C. ROS-induced epithelial-mesenchymal transition in mammary epithelial cells is mediated by NF-kB-dependent activation of Snail. Oncotarget. 2014;5:2827–2838. doi: 10.18632/oncotarget.1940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Domenico M., Pierantoni G. M., Feola A., Esposito F., Laino L., DE Rosa A., Rullo R., Mazzotta M., Martano M., Sanguedolce F., Perillo L., D'Angelo L., Papagerakis S., Tortorella S., Bufo P., Lo Muzio L., Pannone G., Santoro A. Prognostic significance of N-Cadherin expression in oral squamous cell carcinoma. Anticancer Res. 2011;31:4211–4218. [PubMed] [Google Scholar]
- Feng L. M., Wang X. F., Huang Q. X. Thymoquinone induces cytotoxicity and reprogramming of EMT in gastric cancer cells by targeting PI3K/Akt/mTOR pathway. J. Biosci. 2017;42:547–554. doi: 10.1007/s12038-017-9708-3. [DOI] [PubMed] [Google Scholar]
- Han C. T., Kim M. J., Moon S. H., Jeon Y. R., Hwang J. S., Nam C., Park C. W., Lee S. H., Na J. B., Park C. S., Park H. W., Lee J. M., Jang H. S., Park S. H., Han K. G., Choi Y. W., Lee H. Y., Kang J. K. Acute and 28-day subacute toxicity studies of hexane extracts of the roots of Lithospermum erythrorhizon in Sprague-Dawley rats. Toxicol. Res. 2015;31:403–414. doi: 10.5487/TR.2015.31.4.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He G., He G., Zhou R., Pi Z., Zhu T., Jiang L., Xie Y. Enhancement of cisplatin-induced colon cancer cells apoptosis by shikonin, a natural inducer of ROS in vitro and in vivo. Biochem. Biophys. Res. Commun. 2016;469:1075–1082. doi: 10.1016/j.bbrc.2015.12.100. [DOI] [PubMed] [Google Scholar]
- Jeung Y. J., Kim H. G., Ahn J., Lee H. J., Lee S. B., Won M., Jung C. R., Im J. Y., Kim B. K., Park S. K., Son M. J., Chung K. S. Shikonin induces apoptosis of lung cancer cells via activation of FOXO3a/EGR1/SIRT1 signaling antagonized by p300. Biochim. Biophys. Acta. 2016;1863:2584–2593. doi: 10.1016/j.bbamcr.2016.07.005. [DOI] [PubMed] [Google Scholar]
- Jiao L., Li D. D., Yang C. L., Peng R. Q., Guo Y. Q., Zhang X. S., Zhu X. F. Reactive oxygen species mediate oxaliplatin-induced epithelial-mesenchymal transition and invasive potential in colon cancer. Tumour Biol. 2016;37:8413–8423. doi: 10.1007/s13277-015-4736-9. [DOI] [PubMed] [Google Scholar]
- Jin H., Gao S., Guo H., Ren S., Ji F., Liu Z., Chen X. Re-sensitization of radiation resistant colorectal cancer cells to radiation through inhibition of AMPK pathway. Oncol. Lett. 2016;11:3197–3201. doi: 10.3892/ol.2016.4339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamiya T., Goto A., Kurokawa E., Hara H., Adachi T. Cross talk mechanism among EMT, ROS, and histone acetylation in phorbol ester-treated human breast cancer MCF-7 cells. Oxid. Med. Cell. Longev. 2016;2016:1284372. doi: 10.1155/2016/1284372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang K. A., Ryu Y. S., Piao M. J., Shilnikova K., Kang H. K., Yi J. M., Boulanger M., Paolillo R., Bossis G., Yoon S. Y., Kim S. B., Hyun J. W. DUOX2-mediated production of reactive oxygen species induces epithelial mesenchymal transition in 5-fluorouracil resistant human colon cancer cells. Redox Biol. 2018;17:224–235. doi: 10.1016/j.redox.2018.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khil H., Kim S. M., Hong S., Gil H. M., Cheon E., Lee D. H., Kim Y. A., Keum N. Time trends of colorectal cancer incidence and associated lifestyle factors in South Korea. Sci. Rep. 2021;11:2413. doi: 10.1038/s41598-021-81877-2.91af08c891e34d12a4d8e467a31eb25e [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S., Kim S. H., Lee C. E. SOCS1 represses fractionated ionizing radiation-induced EMT signaling pathways through the counter-regulation of ROS-scavenging and ROS-generating systems. Cell. Physiol. Biochem. 2020;54:1026–1040. doi: 10.33594/000000285.6fe17f5d379645a7a11cb4183d75ef5d [DOI] [PubMed] [Google Scholar]
- Ko H., Kim S. J., Shim S. H., Chang H., Ha C. H. Shikonin induces apoptotic cell death via regulation of p53 and Nrf2 in AGS human stomach carcinoma cells. Biomol. Ther. (Seoul) 2016;24:501–509. doi: 10.4062/biomolther.2016.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S. Y., Ju M. K., Jeon H. M., Lee Y. J., Kim C. H., Park H. G., Han S. I., Kang H. S. Reactive oxygen species induce epithelial-mesenchymal transition, glycolytic switch, and mitochondrial repression through the Dlx-2/Snail signaling pathways in MCF-7 cells. Mol. Med. Rep. 2019;20:2339–2346. doi: 10.3892/mmr.2019.10466. [DOI] [PubMed] [Google Scholar]
- Lim S. W., Ting K. N., Bradshaw T. D., Zeenathul N. A., Wiart C., Khoo T. J., Lim K. H., Loh H. S. Acalypha wilkesiana extracts induce apoptosis by causing single strand and double strand DNA breaks. J. Ethnopharmacol. 2011;138:616–623. doi: 10.1016/j.jep.2011.10.005. [DOI] [PubMed] [Google Scholar]
- Lu D., Qian J., Li W., Feng Q., Pan S., Zhang S. β-Hydroxyisovaleryl-shikonin induces human cervical cancer cell apoptosis via PI3K/AKT/mTOR signaling. Oncol. Lett. 2015;10:3434–3442. doi: 10.3892/ol.2015.3769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matějka V. M., Fínek J., Králíčková M. Epithelial-mesenchymal transition in tumor tissue and its role for metastatic spread of cancer. Klin. Onkol. 2017;30:20–27. doi: 10.14735/amko201720. [DOI] [PubMed] [Google Scholar]
- Ramamurthy V. P., Ramalingam S., Gediya L. K., Njar V. The retinamide VNLG-152 inhibits f-AR/AR-V7 and MNK-eIF4E signaling pathways to suppress EMT and castration-resistant prostate cancer xenograft growth. FEBS J. 2018;285:1051–1063. doi: 10.1111/febs.14383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shilnikova K., Piao M. J., Kang K. A., Ryu Y. S., Park J. E., Hyun Y. J., Zhen A. X., Jeong Y. J., Jung U., Kim I. G., Hyun J. W. Shikonin induces mitochondria-mediated apoptosis and attenuates epithelial-mesenchymal transition in cisplatin-resistant human ovarian cancer cells. Oncol. Lett. 2018;15:5417–5424. doi: 10.3892/ol.2018.8065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y., Liu W. Z., Liu T., Feng X., Yang N., Zhou H. F. Signaling pathway of MAPK/ERK in cell proliferation, differentiation, migration, senescence and apoptosis. J. Recept. Signal Transduct. Res. 2015;35:600–604. doi: 10.3109/10799893.2015.1030412. [DOI] [PubMed] [Google Scholar]
- Todosi A. M., Gavrilescu M. M., Aniţei G. M., Filip B., Scripcariu V. Colon cancer at the molecular level--usefulness of epithelial-mesenchymal transition analysis. Rev. Med. Chir. Soc. Med. Nat. Iasi. 2012;116:1106–1111. [PubMed] [Google Scholar]
- Trivedi R., Müller G. A., Rathore M. S., Mishra D. P., Dihazi H. Anti-leukemic activity of shikonin: role of ERP57 in shikonin induced apoptosis in acute myeloid leukemia. Cell. Physiol. Biochem. 2016;39:604–616. doi: 10.1159/000445652. [DOI] [PubMed] [Google Scholar]
- Voon D. C., Huang R. Y., Jackson R. A., Thiery J. P. The EMT spectrum and therapeutic opportunities. Mol. Oncol. 2017;11:878–891. doi: 10.1002/1878-0261.12082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vu T., Datta P. K. Regulation of EMT in colorectal cancer: a culprit in metastasis. Cancers (Basel) 2017;9:171. doi: 10.3390/cancers9120171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q., Zhang L., Yuan X., Ou Y., Zhu X., Cheng Z., Zhang P., Wu X., Meng Y., Zhang L. The relationship between the Bcl-2/Bax proteins and the mitochondria-mediated apoptosis pathway in the differentiation of adipose-derived stromal cells into neurons. PLoS ONE. 2016;11:e0163327. doi: 10.1371/journal.pone.0163327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z., Li Y., Sarkar F. H. Signaling mechanism(s) of reactive oxygen species in epithelial-mesenchymal transition reminiscent of cancer stem cells in tumor progression. Curr. Stem Cell Res. Ther. 2010;5:74–80. doi: 10.2174/157488810790442813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei Y., Li M., Cui S., Wang D., Zhang C. Y., Zen K., Li L. Shikonin inhibits the proliferation of human breast cancer cells by reducing tumor-derived exosomes. Molecules. 2016;21:777. doi: 10.3390/molecules21060777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wlodkowic D., Telford W., Skommer J., Darzynkiewicz Z. Apoptosis and beyond: cytometry in studies of programmed cell death. Methods Cell Biol. 2011;103:55–98. doi: 10.1016/B978-0-12-385493-3.00004-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong M. C. S., Huang J., Lok V., Wang J., Fung F., Ding H., Zheng Z. J. Differences in incidence and mortality trends of colorectal cancer worldwide based on sex, age, and anatomic location. Clin. Gastroenterol. Hepatol. 2021;19:955–966. doi: 10.1016/j.cgh.2020.02.026. [DOI] [PubMed] [Google Scholar]
- Wu H., Sun P., Feng H., Zhou H., Wang R., Liang Y., Lu J., Zhu W., Zhang J., Fang J. Reactive oxygen species in a non-thermal plasma microjet and water system: generation, conversion, and contributions to bacteria inactivation-an analysis by electron spin resonance spectroscopy. Plasma Process. Polym. 2012;9:417–424. doi: 10.1002/ppap.201100065. [DOI] [Google Scholar]
- Xiong S., Mu T., Wang G., Jiang X. Mitochondria-mediated apoptosis in mammals. Protein Cell. 2014;5:737–749. doi: 10.1007/s13238-014-0089-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y., Zong M., Xu W., Zhang Y., Wang B., Yang M., Tao L. Natural pyrethrins induces apoptosis in human hepatocyte cells via Bax-and Bcl-2-mediated mitochondrial pathway. Chem. Biol. Interact. 2017;262:38–45. doi: 10.1016/j.cbi.2016.12.006. [DOI] [PubMed] [Google Scholar]
- Zhu Q. C., Gao R. Y., Wu W., Qin H. L. Epithelial-mesenchymal transition and its role in the pathogenesis of colorectal cancer. Asian Pac. J. Cancer Prev. 2013;14:2689–2698. doi: 10.7314/APJCP.2013.14.5.2689. [DOI] [PubMed] [Google Scholar]