Abstract
Over the last several years, the term PFAS (per- and polyfluoroalkyl substances) has grown to be emblematic of environmental contamination, garnering public, scientific and regulatory concern. PFAS are synthesized by two processes, direct fluorination (e.g., electrochemical) and oligomerization (e.g., fluorotelomerization). More than a megatonne is produced yearly and thousands of PFAS wind up in end-use products. Atmospheric and aqueous fugitive releases during manufacturing, use and disposal have resulted in the global distribution of these compounds. Volatile PFAS facilitate long-range transport, commonly followed by complex transformation schemes to recalcitrant terminal PFAS, which do not degrade under environmental conditions, thereby migrating through the environment and accumulating in biota through multiple pathways. Efforts to remediate PFAS-contaminated matrices still are in their infancy with much current research targeting drinking water.
PRINT PAGE SUMMARY
BACKGROUND:
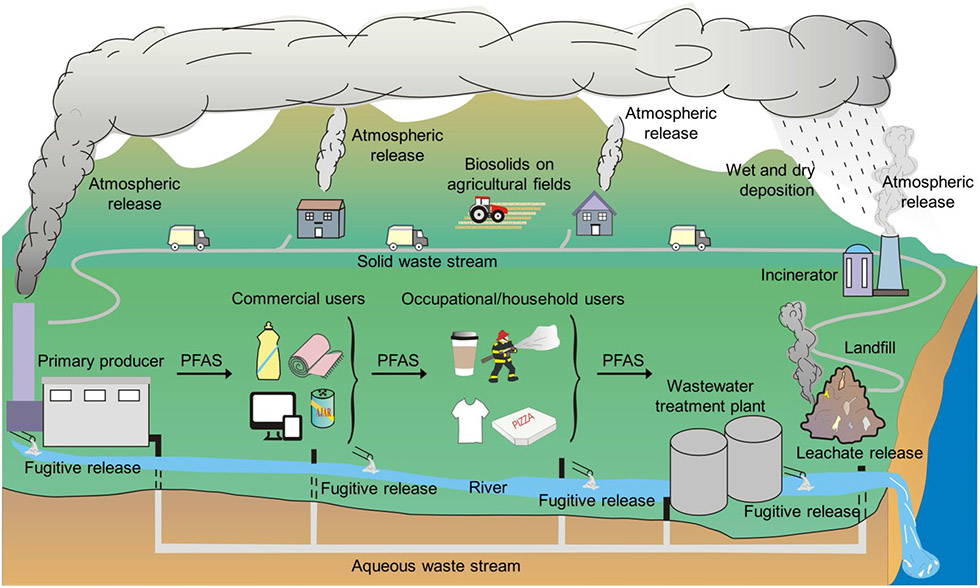
Dubbed “forever chemicals” due to their innate chemical stability, per- and polyfluoroalkyl substances (PFAS), have been found as ubiquitous environmental contaminants, present from the far Arctic reaches of the planet to urban rainwater. Although public awareness of these compounds is still relatively new, PFAS have been manufactured for almost seven decades. In that time, industrial uses of PFAS have extended to more than 200 diverse applications of over 1400 individual PFAS including fast-food containers, anti-staining fabrics, and fire-suppressing foams. These numerous applications are possible and continue to expand because the rapidly broadening development and manufacture of PFAS is creating a physiochemically diverse class of thousands of unique synthetic chemicals, related by their use of highly stable carbon-fluorine bonds. As these products flow through their lifecycle from production to disposal (Print Fig.), PFAS can be released into the environment at each step, potentially taken up by biota, but largely migrating to the oceans and marine sediments in the long term. Bioaccumulation in both aquatic and terrestrial species has been widely observed, and while large-scale monitoring studies have been implemented, the adverse outcomes to ecological and human health, particularly of replacement PFAS, remain largely unknown. Critically, due to the sheer number of PFAS, environmental discovery and characterization studies struggle to keep pace with development and release of next-generation compounds. The rapid expansion of PFAS, combined with their complex environmental interactions, results in a patchwork of data. Whereas the oldest legacy compounds such as perfluorocarboxylic acids (PFCAs) and perfluorosulfonic acids (PFSAs) have known health impacts, more recently developed PFAS are poorly characterized and some novel compounds even lack defined chemical structures, much less known toxicological endpoints.
ADVANCES:
Continued measurement of legacy and next-generation PFAS is critical to assess their behavior in environmental matrices and to improve our understanding of their fate and transport. Studies of well-characterized legacy compounds, such as PFCAs and PFSAs, aid in the elucidation of interactions between PFAS chemistries and realistic environmental heterogeneities (e.g., pH, temperature, mineral assemblages, and co-contaminants). However, the reliability of resulting predictions depends on the degree of similarity between the legacy and novel compounds. Atmospheric transport has been shown to play an important role in global PFAS distribution and, following deposition, mobility within terrestrial settings decreases with increasing molecular weight, whereas bioaccumulation increases. PFAS degradation rates within anaerobic settings and within marine sediments sharply contrast those within aerobic soils, resulting in considerable variation in biotransformation potential and major terminal products between settings such as landfills, oceans, or soils. However, regardless of the degradation pathway, PFAS do not naturally degrade to non-PFAS species, resulting in deposition sites such as landfills serving as time-delayed sources. Thus, PFAS require more drastic, destructive remediation processes for contaminated matrices, including treatment of residuals such as granular activated carbon from drinking-water remediation. Destructive thermal and non-thermal processes for PFAS are being piloted, but there is always a risk of forming yet more PFAS products by incomplete destruction.
OUTLOOK:
Although great strides have been taken in recent decades into understanding the fate, mobility, toxicity, and remediation of PFAS, considerable management concerns still exist across the lifecycle of these persistent chemicals. The study of emerging compounds is complicated by the confidential nature of many PFAS chemistries, manufacturing processes, industrial byproducts, and applications. Furthermore, the diversity and complexity of affected media are difficult to capture in laboratory studies. Unquestionably, it remains a priority for environmental scientists to understand behavior trends of PFAS, and to work collaboratively with global regulatory agencies and industry toward effective environmental-exposure mitigation strategies.
Single-sentence summary:
PFAS are widely distributed and persistent in the environment, potentially causing widespread toxic exposures.
Introduction
The ubiquitous presence of per- and polyfluoroalkyl substances (PFAS) in the environment after decades of manufacturing and consumer use (Fig. 1) has garnered global interest, with an ever-expanding inventory of >1400 individual chemicals in the Toxic Substances Control Act Inventory and >8000 unique known structures (1). PFAS have been incorporated in more than 200 use areas ranging from industrial-mining applications to food production and fire-fighting foams due to the innate chemical and thermal stability of the carbon-fluorine bond and ability to repel oil and water (2). As PFAS flow through commerce from primary manufacturer to commercial user to final disposal, environmental release occurs via both controlled and fugitive waste streams (Print Fig.). The stability of many PFAS degradants fosters their ubiquity in the environment. The growing number of PFAS susceptible to partial degradation (3) further complicates environmental fingerprinting and remediation efforts. Whereas some PFAS transformation pathways have been well-characterized, others degrade through yet unknown pathways, expanding the already immense PFAS inventory by untold numbers. Of the known PFAS, there is a paucity of data adequately describing potential impacts to ecosystems and their provisioning services, and few of these chemicals are well-characterized by ecotoxicity studies, with the widely known perfluorooctanoic acid (PFOA) and perfluorooctane sulfonate (PFOS) alone covering 21% and 39% of the ECOTOX Knowledgebase (4). Furthermore, with their detection in sera across the human population, coupled with epidemiological evidence of the health impacts for legacy PFAS (5, 6), information on associations with human disease for emerging PFAS is needed. With global production volumes of fluoropolymers surpassing 230,000 tonnes/year (2) and estimated cumulative global emissions of perfluoroalkyl acids summing to ≥46,000 tonnes (7), scientists struggle to keep pace with manufacturing, use (Fig. 1) and subsequent release. Here we summarize central concerns in PFAS production, persistence, environmental mobility, exposure, and remediation to inform the international community.
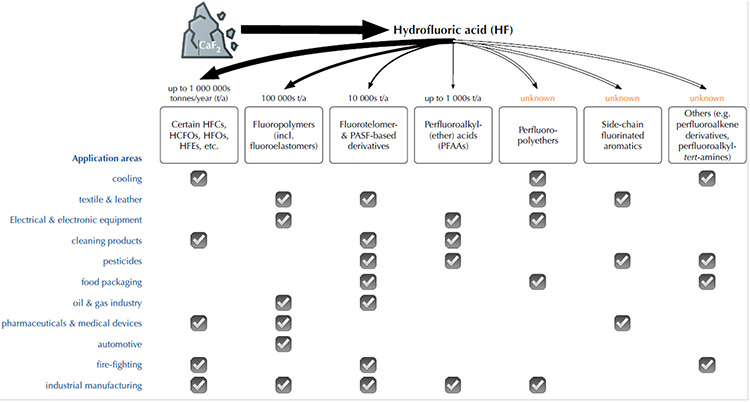
Fig. 1: Summary of PFAS manufacturing, from production to consumer use.
While numerous product fluxes are reasonably documented, considerable lacunae remain. See text for details and citations. HFCs (hydrofluorocarbons); HCFOs (hydrochlorofluoroolefins); HFOs (hydrofluoroolefins); HFEs (hydrofluoroethers); PASF (perfluoroalkanesulfonyl fluoride).
Print Fig: The PFAS lifecycle.
PFAS product flows from primary producer, to commercial user, to consumers, to disposal. Each step is attended by atmospheric and aqueous fugitive releases. Soils constitute a long-term environmental sink, slowly releasing PFAS to the hydrosphere and allowing uptake in biota, but the ultimate reservoir is deep marine sediment.
Major PFAS groups & uses
PFAS are a class of substances within a wide universe of organofluorine compounds (8), as first laid out by Buck et al. in 2011 (9). In 2021, the Organisation for Economic Co-operation and Development released a revised definition of PFAS, "PFAS are fluorinated substances that contain at least one fully fluorinated methyl or methylene carbon atom (without any H/Cl/Br/I atom attached to it)" (10). This revised definition is more inclusive with unambiguous inclusion of PFAS such as side-chain fluorinated aromatics (Fig. 2) (11, 12). In contrast, most historical work within the research community has focused on a small set of perfluoroalkyl(ether) acids and their precursors, with an emphasis on environmental and biological occurrence investigations. Whereas the persistence associated with the perfluorinated-carbon chain is a fundamental underlying concern, PFAS also have a wide range of bioaccumulation and adverse-effect concerns, governed by their varied physiochemical properties.
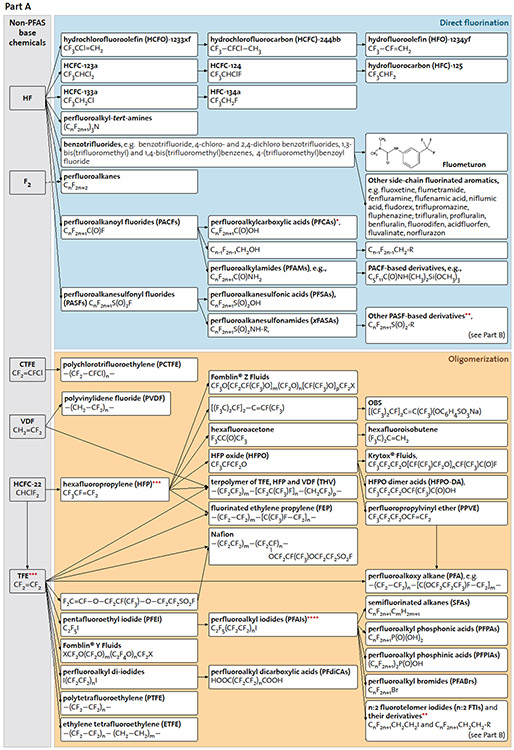
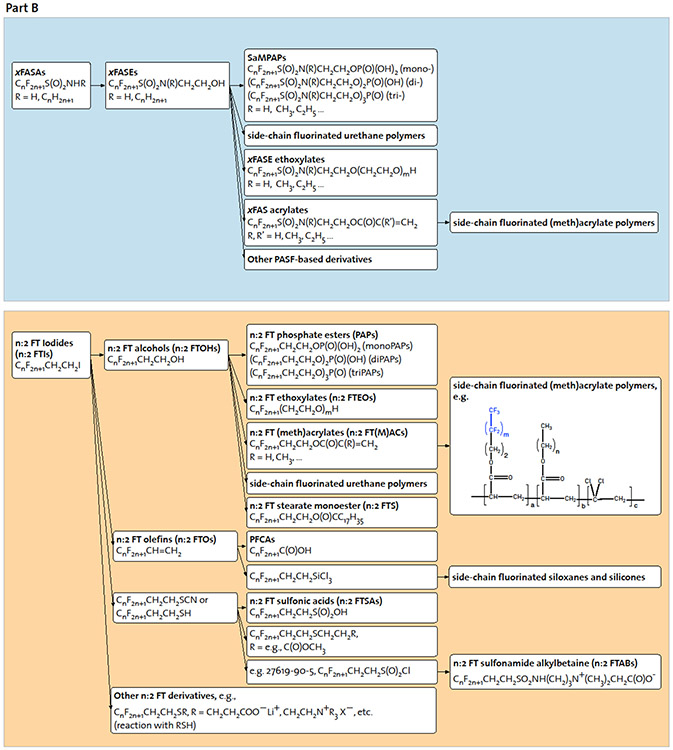
Fig. 2. Intermediate and final manufacturing products.
A: Multistep multicomponent syntheses yield a complex PFAS universe.
B: Multistep multicomponent syntheses yield a complex PFAS universe.
Notes:
* PFCAs have also been synthesised using other routes, e.g. from PFAIs or n:2 fluorotelomer iodides. Different synthesis routes may generate PFCAs with different perfluorocarbon chain lengths.
** Additional details on known synthesis routes of PASF-based and n:2 fluorotelomer derivatives can be found in Part (b).
*** For many compounds such as HFP and TFE, there are different synthesis routes with different starting materials, and here shows only one of them.
**** PFAIs may also be synthesized from other initial substances such as (CF3)2CFI.
Sources:
(1) Siegemund G. et al. Fluorine Compounds, Organic, 3rd ed.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2000; Vol. 33.
(2) Banks RE et al. Organofluorine Chemistry: Principles and Commercial Applications. New York: Plenum, 1994.
(3) Buck RC et al. Perfluoroalkyl and polyfluoroalkyl substances in the environment: terminology, classification, and origins, Integr Environ Assess Manag 2011, 7 (4), 513–541.
(4) Wang Z et al. Global emission inventories for C4-C14 perfluoroalkyl carboxylic acid (PFCA) homologues from 1951 to 2030, Part I: production and emissions from quantifiable sources. Environ Int 2014, 70, 62–75.
(5) Zhang W. et al. Manufacture of hydrofluorocarbons and hydrofluoroolefins as the CFCs-alternatives: from fundamental of catalytic reaction to commercialisation. Scientia Sinica Chimica 2017, 47 (11), 1312-1325.
While industrial reviews include general synthetic routes and major applications of some PFAS groups (13), inadequate public information exists for many PFAS internationally, particularly those currently in use, due to confidential-business-information claims and insufficient regulatory structures (14-16). Critical data gaps include PFAS identities, locations/quantities of production and processing, and final uses of products, limiting the capability to identify where environmental and human exposure occur. Here, we summarize synthetic routes, structural traits and uses of major PFAS groups (Figs. 1 & 2) and describe implications and knowledge gaps for future research and action.
The fluorine in PFAS is mined from fluorite (CaF2) mineral deposits, which is digested to form hydrofluoric acid (HF) (Fig. 1). HF and other non-PFAS based chemicals are used in either of two general synthetic techniques to produce starting materials (e.g., perfluoroalkanoyl fluorides in Fig. 2) of individual PFAS groups, namely direct fluorination (i.e., turning non-fluorinated to fluorinated substances; e.g., electrochemical fluorination) and oligomerization (i.e., converting monomers to larger molecules; e.g., fluorotelomerization). Direct fluorination is aggressive and often results in uncontrolled chemical reactions such as carbon-chain shortening and re-arrangement (17-19), leading to a wide range of byproducts including cyclic and branched isomers. Oligomerization is less aggressive and mainly results in homologous series of target compounds (9), as have been observed near fluoropolymer (20) and perfluoropolyether (21) manufacturing and processing sites. Within individual PFAS groups, the functional moieties of starting materials may further react following conventional reaction pathways to yield different PFAS (9); thus, depending on the complexity of synthetic routes, final products may contain a number of unreacted intermediates and degradation products (22, 23). While the summary below focuses on target/intentional PFAS, these unintentional PFAS can constitute an important part of human and environmental exposure, and merit scrutiny.
Major PFAS groups from direct fluorination include those hydrofluorocarbons, hydrofluoroethers, hydrochlorofluoroolefins and hydrofluoroolefins that contain a -CF3 moiety and have an overall global production of over one megatonne/year (24). Including a range of low molecular-weight/low boiling-point compounds that are used as refrigerants, heat-transfer fluids, solvents, and foaming agents (2, 24), these compounds replaced ozone-depleting chlorofluorocarbons and hydrochlorofluorocarbons. Due to their high global-warming potentials, the international community has agreed to phase down and eventually eliminate hydrofluorocarbons (25, 26); an ongoing industrial transition is taking place, including increasing large-scale replacement of hydrofluorocarbons with hydrofluoroethers and hydrofluoroolefins. While having low global-warming potentials, hydrofluoroethers and hydrofluoroolefins can ultimately degrade to highly persistent perfluorocarboxylic acids (PFCAs) such as trifluoroacetate and a steep accumulation of trifluoroacetate in the environment is becoming increasingly evident (27).
Another important PFAS group resulting from direct fluorination is side-chain fluorinated aromatics (11, 12), with unknown, but likely considerable amounts being produced and used annually (e.g., prozac). A common starting point is the synthesis of benzotrifluorides from benzotrichlorides by reaction with HF (8). Addition of the -CF3 moiety can reduce biological degradation, increase biological activity, and assist with membrane transport, making the parent compound longer lasting or more effective; thus, many side-chain fluorinated aromatics are used in pharmaceutical (12) or agricultural (11) applications. They can degrade to PFCAs, such as trifluoroacetate, as well.
Two other major PFAS groups from direct fluorination include perfluoroalkyl-tert-amines (28), and perfluoroalkanoyl/perfluoroalkanesulfonyl fluorides (PACF/PASFs) which are further reacted to produce PFCAs, perfluoroalkanesulfonates (PFSAs), and other derivatives (Fig. 2). Historically, hundreds of PACF/PASF-based derivatives with a wide range of perfluorocarbon-chain lengths were produced, on the order of kilotonnes/year (15, 29), and used for industrial and consumer applications (2). Since the early 2000s, numerous long-chain (fluoroalkyl carbon number ≥6) PACF/PASF-based derivatives have been - and are being - phased out due to widespread concern, whereas shorter-chain PACF/PASF-based derivatives still are being produced and widely used, although in unknown amounts (15, 29). In the environment and biota, PACF/PASF-based derivatives may degrade and partially transform into different perfluoroalkyl acids.
On the oligomerization side, two major PFAS groups are fluoropolymers and perfluoropolyethers. These are high-production polymers possessing fluorinated backbones, with fluoropolymers being produced on the scale of 100s kilotonnes/year and unknown, but likely considerable, amounts for perfluoropolyethers. Despite often possessing simple names such as polytetrafluoroethylene, substances in these two groups can be highly diverse, including both non-functionalized (with -CF3) and functionalized termini, with different structural combinations and molar ratios of monomers (for co-polymers), and from low molecular weight (<1000 Da) to very high (>100000 Da) (30-32); this complexity has not been clearly communicated with a comprehensive overview of different fluoropolymers and perfluoropolyethers on the market. Depending on structure, different fluoropolymers and perfluoropolyethers can be used in a range of industrial and consumer applications (2); in some applications, perfluoropolyethers are used as alternatives to PACF/PASF-based derivatives. Given their variety and complexity, their subsequent bioavailability and degradability are highly variable and complex, which is generally overlooked, understudied and/or unknown.
Three other major PFAS groups formed from oligomerization are fluorotelomers, perfluoroalkyl(ether) carboxylic and sulfonic acids, and perfluoroalkene derivatives. Fluorotelomers share many similarities to PACF/PASF-based derivatives other than perfluoroalkyl(ether) acids, including molecular structures, degradability (9, 23, 29), use applications (2), and manufacturing trends from a wide range of perfluorocarbon chain lengths to predominantly shorter-chains. Fluorotelomers were historically produced on the order of 9 kilotonnes/year (33), with current amounts being unknown. Unknown amounts of perfluoroalkyl(ether) carboxylic and sulfonic acids are being used to replace long-chain PFCAs and PFSAs (34) in industrial applications such as fluoropolymer production and metal plating, respectively. Perfluoroalkene derivatives such as p-perfluorous nonenoxybenzene sulfonate have been produced since the 1980s; large-scale production (on the scale of kilotonnes/year) recently was initiated in China as an alternative to perfluorooctane sulfonate (PFOS) in firefighting and oil production (35). Despite an unsaturated bond, p-perfluorous nonenoxybenzene sulfonate is not readily biodegradable (36).
Environmental stability, degradation schemes and transformation rates
Despite typically high stability as a group, ~20% of PFAS may undergo transformation in the environment (3). These labile compounds are precursors to recalcitrant, terminal transformation products such as PFCAs and PFSAs. For example, frequently detected precursors including perfluorooctane sulfonamides, fluorotelomer alcohols (FTOHs) and fluorotelomer sulfonates, have been found to contribute up to 86% of total PFAS identified in wastewater-treatment-plant sludge (37).
Although PFAS can undergo complete degradation to inorganic components using high-energy remediation technologies, precursor transformations under environmental conditions, including processes such as hydrolysis (38), oxidation (39, 40), reduction, decarboxylation and hydroxylation (41), ultimately yield stable PFAS. Despite low vapor pressure and high water solubilities of many PFAS, some conditions (e.g., within industrial stacks) can promote partitioning to air via particulate sorption, and volatile PFAS such as FTOHs can exist in the gas phase (42), making atmospheric/photochemical transformation possible. In the soil-water environment, microbe-facilitated functional group biotransformation can occur aerobically (43, 44) or anaerobically (45-47), and some microbes that carry out these reactions have been identified (46, 48, 49). Biotransformation of labile PFAS also can be mediated by plant-specific enzymes. For example, microbial transformation of 8:2 FTOH was significantly enhanced with the addition of soybean root exudates in solution (50), and perfluorooctane sulfonamide was transformed in the presence of carrot and lettuce crops, but not in their absence, in amended soils (51). In both studies, enhanced degradation was attributed to the organic carbon content of the soil, as addition of carbon sources can increase microbial degradation rates through co-metabolic processes (52).
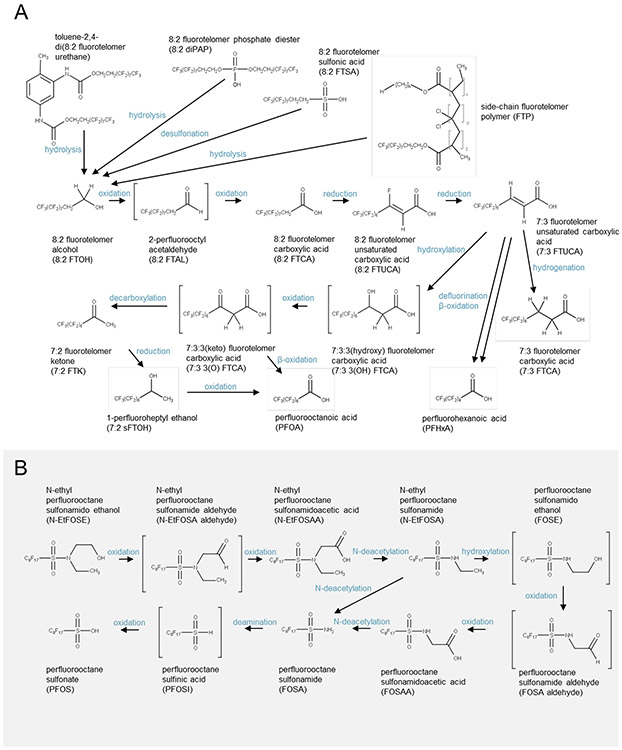
Several PFAS can undergo transformation resulting in the formation of FTOHs through processes such oxidation, reduction (53), desulfonation (54) and hydrolysis (38, 55-58) (Fig 3A). Although some fluorotelomers evidently transform without forming intermediate FTOHs (9, 22, 49, 59), one of the archetypal “legacy PFAS” transformation schemes involves FTOHs which are subject to (bio)transformation through numerous intermediates leading to formation of terminal PFCAs through chain-shortening processes (Fig. 3A). Efficiency of these transformations decreases from aerobic to anoxic to anaerobic (60, 61) conditions, and PFCA yields and rates of formation depend on specific precursor and transformation conditions (9). On average perfluorooctanoic acid (PFOA) yields from 8:2 FTOH were reported to be 25% in aerobic soils compared to <1% in anaerobic sludge (62). This process is initiated with the oxidation of 8:2 FTOH to yield the inferred 8:2 fluorotelomer aldehyde, thence the 8:2 fluorotelomer carboxylic acid, which is reduced through the loss of F to form 7:3 unsaturated fluorotelomer acid, which can form the terminal acid perfluorohexanoic acid (53, 63, 64) (Fig. 3A). A key step in the pathway is hydroxylation in the β position and subsequent oxidation to form the 7:3 3(keto) fluorotelomer carboxylic acid, which then undergoes β-oxidation to form PFOA, as well as α-decarboxylation to form the 7:2 ketone (53, 63, 64). The ketone then is reduced to form the secondary alcohol, 1-perfluoroheptyl ethanol (also known as 7:2(sec) FTOH), which is oxidized to form PFOA (53, 63, 64).
Fig 3. Reaction schemes for the (A) 8:2 FTOH (47, 53, 63) and (B) N-EtFOSE (65, 66).
Transformation products proposed by the original investigators are shown with brackets.
In a second major transformation scheme, N-ethyl-perfluorooctanesulfonamido-ethanol is proposed to oxidize to form the aldehyde and subsequently to N-ethyl perfluorooctane sulfonamidoacetic acid (Fig. 3B) (65, 66). N-deacetylation of N-ethyl perfluorooctane sulfonamidoacetic acid then leads to the formation of N-ethylperfluorooctanesulfonamide followed by C-hydroxylation to form perfluorooctane sulfonamido ethanol. Oxidation of perfluorooctane sulfonamido ethanol to perfluorooctane sulfonamido acetic acid is proposed to occur through the perfluorooctanesulfonamide aldehyde. N-deacetylation of perfluorooctane sulfonamido acetic acid to form perfluorooctanesulfonamide is then observed. Perfluorooctanesulfonamide may also form directly from the N-dealkylation of N-ethylperfluorooctanesulfonamide (65, 66). Deamination of perfluorooctanesulfonamide to form perfluorooctane sulfinic acid commonly is followed by oxidation to form the terminal product, PFOS.
PFAS transformation under environmental conditions can be approximated using first-order kinetics (67). Environmental degradation of labile precursors is observed to occur in a ‘tree structure,’ with the formation of numerous intermediates along branching transformation pathways (53, 68). Along each branch, the formation and disappearance of intermediates can be modeled as a sequential decay chain (23), with each step characterized by a pseudo first-order rate constant (67).
In soils and sediment, sorption can slow the observed rate of microbial transformation (69). With long-chain PFAS preferentially adsorbing to soil phases, molecular weight can be used as an approximate indicator of relative stability amongst PFAS sharing common reaction centers (43). To address the effects of reversible sorption, some have proposed use of a double-first-order, in-parallel model (67), wherein rate-limited reversible sorption is included as a first-order process.
In addition to sorption, transformation rate is dependent on a number of other environmental factors including pH, temperature and microbial population (70), and these factors contribute to a wide variation of reported precursor half-lives. For example, biodegradation studies of N-ethyl-perfluorooctanesulfonamido-ethanol in sludge reported a half-life of 0.7-4.2 days, yet the biodegradation in marine sediments was found to proceed at much slower rates (t1/2, 4 °C = 160 days and t1/2, 25 °C = 44 days), which could explain reports of elevated concentrations of N-ethyl-perfluorooctanesulfonamido-ethanol in marine environments (66). Similarly, the anaerobic biotransformations of 6:2 and 8:2 FTOHs slowed substantially (30 and 145 days, respectively) compared to aerobic conditions (<2 and 2-7 days, respectively) (62), which can foster enhanced levels of telomer acids (e.g. 5:3 fluorotelomer carboxylic acid by hydrogenation of the 5:3 fluorotelomer unsaturated carboxylic acid (53)) in landfills (71); as such, PFAS that typically are intermediates in oxidizing settings may exist as terminal products under reducing conditions. For example, variations in PFAS species detected in waste-collection-vehicle leachate compared to landfill leachate suggest alternative biodegradation pathways in long-term anaerobic settings such as landfills (72). Consequently, degradation studies conducted under controlled conditions result in considerable variation in biotransformation potential and possibly different major stable perfluorinated degradation products when extrapolating half-lives and major products from laboratory to environmental conditions.
In addition to accounting for environmental conditions (67), another complicating factor is that contaminants commonly exist as components in complex mixtures. One common precursor source is aqueous-film-forming foam (AFFF), formulations of which contain mixtures of PFAS, and co-contaminants such as non-fluorinated surfactants. High concentrations of organic solvents have been shown to inhibit PFOA degradation under in-situ remedial chemical-oxidation studies, suggesting that interactions of PFAS with other non-PFAS co-contaminants can alter PFAS transformation (40). Additionally, the presence of different PFAS has resulted in changing compositions of microbial communities when comparing cultures spiked with PFOA or PFOS to microbial compositions without PFAS (46). Considering PFAS environmental transformation is mediated primarily by microbes, data suggest that the presence of complex mixtures could indirectly alter biodegradation and that the presence of one PFAS may affect the transformation rate of another, although transformation kinetics of PFAS mixtures has not been reported. Furthermore, these complex mixtures could have downstream implications for PFAS mobility, as co-contaminants in AFFF mixtures impact microbial toxicity, and PFAS solubility, partitioning (73) and remediation (PFAS can be transformed during treatment of organic contaminants (39)).
Taken together, the complexity of real-world environmental conditions acting on primary precursors, intermediates and terminal products can result in divergence from reaction schemes and degradation rates derived under laboratory conditions. These complexities are aggravated by the many experimental challenges associated with larger PFAS, such as fluoropolymers and sidechain fluorinated polymers, the structure, and monomeric compositions of which often are not completely characterized (23, 38, 74). In addition, there remain uncertainties regarding the levels of impurities or synthetic byproducts and lifecycle emissions of these polymers, which may impact degradation rates, further necessitating nontargeted analyses in conjunction with transformation prediction simulators, like EnviPath (75) and the Chemical Transformation Simulator (76), to identify novel PFAS and transformation products in the environment.
Environmental mobility & distribution
Mobility of PFAS in the environment is dictated by properties of the mobile (usually air and water) and immobile phases (e.g., natural organic matter (NOM), mineral assemblages) as well as the PFAS species.
The transformation rates discussed above affect the time available for migration. When transformation rates of short-lived intermediates exceed environmental-transport rates, these intermediates can remain proximate to their precursors, a phenomenon well-established for the environmental distribution of short-lived radionuclides (77) due to secular (radio-decay) equilibrium with long-lived parents (78). And secular equilibrium of short-lived intermediates might contribute to the non-detect status of some inferred compounds (e.g., 2-perfluorooctyl acetaldehyde, Fig. 3). For PFAS with intermediate transformation rates (e.g., FTOHs and fluorotelomer unsaturated carboxylic acids, Fig. 3) relative to environmental transport processes, these compounds can migrate considerable distances before transformation to recalcitrant PFAS, thereby dispersing widely in the environment (79).
Early precursor PFAS include volatile species (FTOHs and sulfonamido ethanols, Fig. 3), the presence of which has been established globally (80-82). Atmospheric residence time governs transport distance (83) and depends on a variety of PFAS properties including volatility, reactivity, molecular weight, and vapor-particulate partitioning (82, 84, 85). Atmospheric lifetimes have been reported for FTOHs of ~20 days (86). Consistent with these atmospheric lifetimes, air samples collected at remote oceanic locations are reported to contain several FTOH and/or perfluorosulfonamido ethanol species in both gas and particulate phases (80). Based on these and related observations, a large portion of PFAS global distribution, including to remote regions, has been attributed to atmospheric transport (79, 87). For example, in a study of soils collected from remote sites globally, all samples contained PFAS, with homologue ratios (e.g., PFOA/perfluorononanoic acid, PFOA/PFNA) consistent with atmospheric transport (79). These soil concentrations have been used to define global-background PFAS ranges in surface soils (means ~10-60 pg/g), such that surface soils rarely contain lower PFAS and higher concentrations suggest local/regional sources (88). Atmospherically transported ionic PFAS also have been shown to disperse widely, perhaps as far afield as >400 kilometers (21, 89, 90), although the form of these species, e.g., free acid, dissolved in droplets or sorbed to particulates, has not been resolved.
In terrestrial settings, PFAS transport usually is via aqueous advection, with migration retarded by sorption on NOM, minerals and at fluid-fluid interfaces (particularly air-water) (91). Most PFAS sorption studies have been conducted with surface soils where NOM, typically present at relatively high concentrations (Fig. 4 (92)), constitutes a major substrate. Exploring surface-soil sorption mechanisms of two PFAS having sulfonate termini revealed an easily extractable fraction, as well as less reversibly sorbed fractions composed of i) perfluoroalkyl groups hydrophobically associating with NOM, ii) sulfonate moieties covalently binding to NOM–OH groups forming ester linkages, and iii) physical entrapment in NOM or minerals (93). Comparing the sorption of cationic, zwitterionic and anionic PFAS showed i) concentration-dependent sorption for cationic and zwitterionic PFAS, ii) pronounced sorption hysteresis for zwitterions, and iii) major electrostatic and NOM sorption for cationic and zwitterionic PFAS (94).
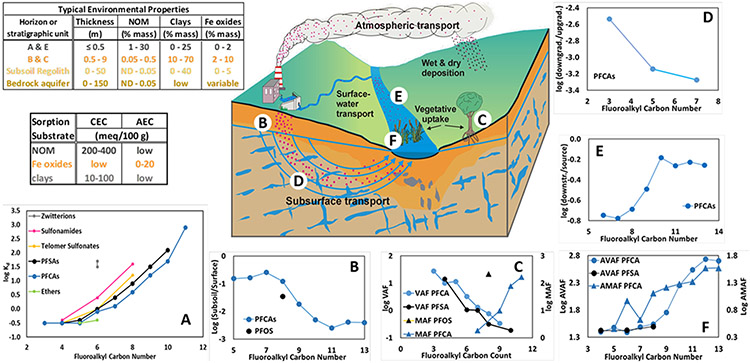
Fig. 4: PFAS partitioning in environmental media (log Kd).
The environmental sorption complex varies grossly with setting, with NOM concentrated in shallow soil horizons, and ferric (oxy)hydroxides commonly dominating in subsurface media (Properties). Log Kd varies as a function of fluoroalkyl number and terminal moiety (A (95); pH=5.2 values depicted). Because of this partitioning behavior, when not complicated by precursor degradation, i) relative mobility amongst PFAS commonly varies with fluoroalkyl carbon number (B (97),D (105),E (106)), ii) terrestrial vegetation accumulation diminishes with increasing fluoroalkyl number, but accumulation in terrestrial detrital feeders increases with fluoroalkyl number (C (101)), and iii) in aquatic settings, vegetative and detrital-feeder accumulation both increase with fluoroalkyl number (F (107)). CEC/AEC (cation-/anion-exchange capacity).
The high NOM concentrations of surface soils typically diminish precipitously in the first several centimeters below ground surface where mineral surfaces come to dominate the vertically more-expansive subsurface realm (Fig. 4) (92). Authigenic minerals typically are abundant in the subsurface and these minerals possess surface charges for electrostatic sorption. Aluminosilicate clays bear permanent negative surface charges, presenting potential sorption sites for cationic and zwitterionic PFAS. Ferric and aluminum (oxy)hydroxides bear pH-dependent, positive surface charges below their zero-point of charge at pH~8, so these minerals can electrostatically sorb anionic PFAS. In the vadose zone, recent studies have shown that the surfactant nature of PFAS also fosters sorption at the air-water interface, retarding PFAS migration (91).
To assess sorption across a wide breadth of PFAS species and complex sorption matrices, experiments have been performed on 29 PFAS in 10 soils (95). The investigators concluded that simple distribution coefficients, Kd (soil/water concentrations), effectively characterized relative distribution amongst PFAS. Recognizing that lower values of log Kd favor partitioning to water, thereby favoring higher environmental mobility, general patterns in these data include (Fig. 4A) i) the distribution coefficient increases logarithmically with fluoroalkyl carbon numbers >5, ii) distribution coefficients converge to similar values amongst PFAS species and chain-lengths having fluorinated carbons ≤5, and iii) for equal fluoroalkyl carbon numbers, sorption generally decreases according to zwitterions > sulfonamides > telomers > PFSAs > PFCAs > ethers. It also was observed that log Kd for anionic PFAS increased with decreasing pH, a pattern consistent with increasing positive electrostatic charge on pH-dependent surfaces of (oxy)hydroxide minerals.
When precursor degradation does not complicate interpretation (96), relative values of log Kd are reflected in PFAS distribution patterns across the spectrum of environmental settings. Fig. 4B depicts geometric mean ratios (subsoil/surface soil) of PFAS for three soil profiles after biosolids application at the ground surface (97); consistent with log Kd values, subsoil accumulation of i) PFCAs exceeds PFSAs for the common fluoroalkyl number =8, ii) shorter chains vary little amongst each other, and iii) shorter chains exceeds that of longer. It is noteworthy that subsoil accumulation for fluoroalkyl number >10 also vary little with chain length, perhaps reflecting facilitated transport of PFAS sorbed to colloids winnowing through the soil column (98).
Transport of PFAS into terrestrial plants occurs via a variety of pathways, with the most studied being uptake through roots. As with transport in soils, vegetative accumulation factors (VAF=[PFAS]vegetation/[PFAS]soil) are largely influenced by the propensity of specific PFAS to partition into water as it is transported through plants. These VAFs have revealed plant species- and tissue-specific trends (99-101). However, a recent review of VAFs across numerous species and tissues, reported uniformly declining trends in total VAF with increasing fluoroalkyl number for PFCAs and PFSAs (102) (e.g., Fig. 4C (101)). VAF trends with chain length and amongst terminal moieties, suggest that chemical properties of PFAS also exert a strong influence over plant uptake. Reports of plant uptake of emerging PFAS compounds are limited, but studies examining the concentration of chloroether sulfonic acids (F-53B, a replacement for PFOS in electroplating industry) suggest similar variation with chain length (103).
In contrast to the VAF patterns largely governed by relative PFAS aqueous-sorbed partitioning, soil macroinvertebrates feeding directly on long-chain-rich vegetative detritus and NOM tend to express trends opposite that for VAFs. For example, macroinvertebrate accumulation factors (MAF=[PFAS]macroinvertebrate/[PFAS]soil) reported for earthworms (Eisenia andrei) in biosolid-amended soil possess trends of increasing MAF with fluoroalkyl number (Fig. 4C) (104).
After percolating through the vadose zone, relative PFAS mobility patterns have been reported in groundwater plumes. For example, PFAS concentrations were reported for wells in a groundwater plume flowing from a landfill, to an observation well, then to water-supply well (105). Given travel times exceeding 24 years for flow from the landfill to the water-supply well, several PFCA homologues fell to non-detect levels, but perfluorobutanoic acid, perfluorohexanoic acid and PFOA exhibited a pattern of lower downgradient/upgradient ratios (specifically, downgradient Well 1/upgradient Well OW1f03) with increasing PFCA chain length (Fig. 4D).
In a riverine setting, sediments downstream of a carpet industry have been reported to retain higher ratios of long-chain homologues than short (downstream Site 5/upstream source Site 4; Fig. 4E) (106), consistent with preferential sorption of the longer homologues (perhaps impacted by precursor transformation as well). In turn, this pattern also is expressed at the base aquatic autotrophic level; for example, aquatic vegetative-leaf accumulation (AVAF=[PFAS]vegetation/[PFAS]water; Fig. 4F) was relatively higher for long-chain compounds (107). Mirroring these AVAF trends, aquatic macroinvertebrate accumulation factors (AMAF=[PFAS]macroinvertebrate/[PFAS]sediment; Fig. 4F) for blackworms (Lumbriculus variegatus) increases with fluoroalkyl number as well (107).
Environmental exposure
Widespread global persistence of PFAS has resulted in detectable concentrations of the compounds in the blood of almost the entire human population (6). Human-health effects from exposure to PFAS have been studied extensively, identifying possible carcinogenic, reproductive, endocrine, neurotoxic, dyslipidemia, and immunotoxic effects (6, 108, 109). However, with animal models reflecting similar postulated mechanisms of action, the toxic potential of these compounds to wildlife cannot be dismissed (110). For humans, direct exposure via manufactured products can be managed more expediently than indirect exposure to accumulated sources in aquatic ecosystems. PFAS exposures via food chains are more difficult to resolve, and dietary exposure through drinking water and contaminated food sources (e.g., seafood and other animal products) are among the greatest exposure sources for ecosystems and human populations alike (109, 111). Here we review consequences of PFAS persistence in the environment and resulting bioaccumulation in biota, present ecotoxicological details in the context of environmental distribution and exposure potential, and discuss the ecological effects of PFAS mixtures (112).
Estimation of environmental exposure is hindered by the sheer number of functionally diverse PFAS and is further complicated by their presence as complex mixtures. A fundamental understanding of ecotoxicology requires comprehensive knowledge of all PFAS species to which target organisms have been exposed. Whilst pragmatic limitations have fostered studies reporting summary characterizations such as Total Organic Fluorine and Total Oxidizable Precursor assays as proxies for more informative chemical-specific studies (113-116), more exhaustive approaches providing identification of individual compounds within PFAS mixtures remains the more informative strategy (117, 118). Ideally, such characterizations include details regarding branched- vs. linear-chain homologues, homologue ratios, isomer comparisons, and forensics with high-resolution mass spectrometry. In addition to pinpointing potential point sources, these methods can distinguish between receptor contact with precursor compounds or their terminal products.
An accurate assessment of PFAS risk must consider exposure to precursor compounds, as these compounds transform, and are thus important for characterizing environmental PFAS mixtures (119, 120). PFAS precursors are susceptible to in-vivo metabolic conversion to terminal acids or sulfonamides post-exposure as well as transformation during (or subsequent to) atmospheric or oceanic transport (see previous sections). For example, while PFSAs were the most abundant PFAS in both sediment and water at sites contaminated with AFFF (114), aquatic invertebrates exposed to AFFF displayed elevated concentrations of PFCAs as well as the 6:2 fluorotelomer sulfonate (114, 115). Given the common detection of precursors, environmental-organismal uptake and distribution models should include both parent and degradant PFAS to best describe patterns of exposure and influence on biomagnification, especially considering the rapidly expanding incorporation of novel, shorter-chain PFAS that tend to be detected less frequently in biota (121).
Key to understanding distribution of PFAS in biota are the specific interactions between PFAS and biological molecules. While the bioaccumulation of some persistent organic pollutants often is related to lipid partition coefficients, PFAS are not exclusively associated with lipids (120). Bioaccumulation modeling suggests that both protein interactions and lipid partitioning are important parameters for accurately assessing PFAS (122, 123), although predicting biomacromolecule interactions has proven difficult due to their physiochemical properties. PFAS do not behave like neutral, hydrophobic organic contaminants and instead are hypothesized to involve both phospholipids and proteinaceous tissues due in part to their anionic nature (123). Cooperative binding models have further correlated (and predicted) protein associations, relying on traditional measures of hydrophobicity and its effect on biomacromolecule interactions (124). Consequently, both membrane-water partitioning and protein-water coefficients could be informative bioaccumulation indicators (bioconcentration factor, bioaccumulation factor, trophic magnification factor), and coupled with hepatic- and renal-clearance mechanisms across taxa are all vital in understanding PFAS persistence in organisms. Nevertheless, the specific physiochemical differences, such as chain-length, result in different distribution of PFAS in biological tissues (125).
Ecotoxicological study of PFAS is further complicated by diversity of the PFAS class. Bioaccumulation factors for terrestrial vegetation are greater for PFCAs than PFSAs, with shorter-chain perfluoroalkyl acids bioaccumulating to a greater degree than longer, largely driven by variation in PFAS solubility (126), followed by uptake and translocation into tissues (e.g., Fig. 4C) (100, 101). Conversely, potential perfluoroalkyl-acid bioaccumulation in other fauna is greatest in long-chain compounds (120), with clear trends of bioaccumulation increasing with chain length (Figs. 4C, 4F, 5) (121). Long-chain PFAS concentrations tend to increase with trophic level in aquatic food webs, consistent with biomagnification processes (127). However, transformation of precursors in exposure media and biota can confound interpretation of high concentrations of some PFAS (e.g., PFOS) as biomagnification without explicit identification of trophic magnification (128).
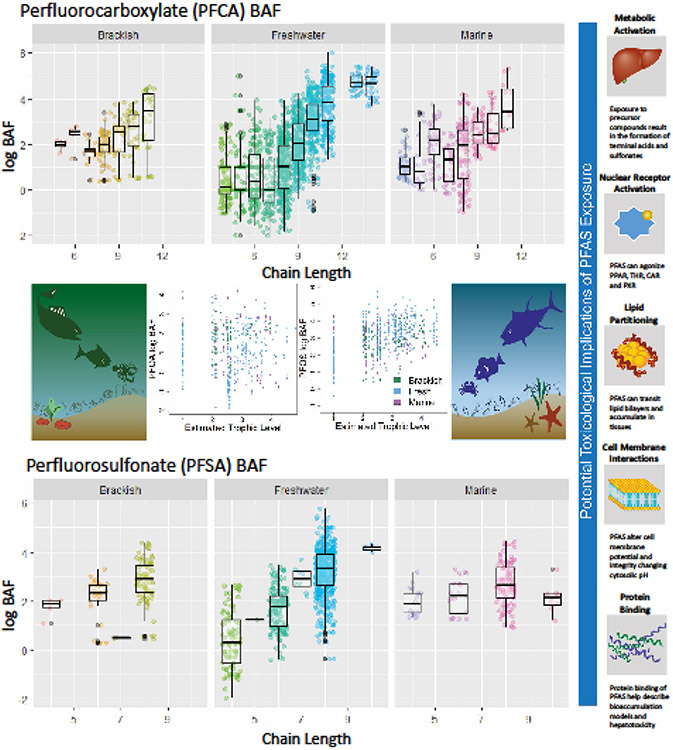
Fig. 5: Trophic transfer and environmental exposures:
Bioaccumulation factors (BAFs) in aquatic food webs are greater for long-chain perfluorocarboxylates (top panel) and perfluorosulfonates (bottom panel) than short-chains. Higher trophic-level organisms demonstrate greater bioaccumulation of PFOS than PFOA (center panel); trophic-level accumulation was estimated for data with a single-prey classification method (FishBase) and standardized bioaccumulation factor by wet weight of organism. Multiple toxicological implications (right panel) reflect the diversity of PFAS physicochemical properties and have been linked to both functional group and fluoroalkyl-carbon-chain length. Data were originally compiled by Burkhard (127).
Biomagnification in predators is related to trophic level, food-chain length, and capacity to metabolize PFAS precursors (125). Seabirds, marine mammals, and terrestrial species show the greatest magnification factors compared with exclusively aquatic food webs in which organisms with gills eliminate perfluoroalkyl acids more efficiently (120). Effects in predators, also frequently seen in humans, seem to be largely cytotoxic, immunological, reproductive, or carcinogenic (125). Exposure models for aquatic food webs at AFFF-contaminated sites found benthic invertebrate consumers to be the avian dietary guild at highest exposure risk (114). At higher trophic levels, PFSAs (e.g., PFOS) bioaccumulate at greater rates than PFCAs (e.g., PFOA) of the same chain length (Fig. 5) (114, 129) and tend to be more toxic (4).
Estuarine, marine, and freshwater environments have demonstrated trophic magnification of long-chain PFAS (Fig. 5) (130, 131). Discrepancies in the relative concentrations of PFAS in fish compared to benthic invertebrates appear largely dependent on the compounds’ functional group and exposure routes, with elevated PFAS concentrations often linked to site-specific sources and/or benthic prey (131-133). Solubilized (i.e., waterborne) rather than dietary exposure was linked to reduced amphipod survival and reproduction (133) while higher trophic-level organisms are exposed primarily through ingestion (109). Counterintuitively, exposure to low concentrations of PFAS can exacerbate bioconcentration, motivating biologically based, physiological models exploring this phenomenon (127). Overall, evidence suggests that the ultimate global reservoirs of PFAS are oceans and marine sediments (134), emphasizing the importance of elucidating consequences of PFAS contamination in these ecosystems (135).
Ecological implications of PFAS exposure to aquatic and terrestrial organisms highlight the need to assess and incorporate new-approach methodologies that prioritize real-world hazard of organismal exposure and subsequent risk. Mechanism-based studies and in-silico approaches are beginning to fill data gaps pinpointing the cellular and molecular pathways resulting in toxicity (136, 137). Elimination half-life has been identified as an endpoint relevant to bioaccumulation and effects (4). In addition to prioritizing chemical selection based on environmental fingerprinting, cross-taxa and sensitive-taxa toxicity testing research should focus on in-silico model development that can determine tissue distribution, molecular perturbations, and trophic-level accumulation. As the scale of assessment expands, so does the need for continued development of adverse-outcome-pathway models to facilitate translation of exposure concentration/dose to organismal-effect endpoints for projection of population-level consequences, including multigenerational effects. For instance, unexposed progeny of fish exposed to PFOA and PFOS had lower survival rates, reduced growth, and thyroid-related effects as revealed by histology (138). Similarly, lipid metabolism (139) and behavioral endpoints (140) were affected in subsequent generations of other species.
Although data are available on potentially common mechanisms of action and toxicity between species (e.g., lipid metabolism, modification of cell-membrane integrity, protein binding and nuclear-receptor activation), the large number of PFAS underscore the need to augment conventional in-vivo testing with in-vitro and in-silico approaches (4). Using these approaches, a number of moderate- and long-chain PFAS have been shown to 1) elicit varying degrees of oxidative stress and modify the antioxidant defense systems of invertebrates, 2) induce neurotoxic and reprotoxic effects across species, and 3) reside in organisms longer than or comparable to any known class of anthropogenic contaminants (120). PFAS toxicity, bioaccumulation and persistence generally are increasingly problematic with increasing chain-length.
Remediation
Treatment and remediation of PFAS-impacted media is especially challenging because the chemistry of PFAS renders them unaffected by most traditional treatment technologies (141). Given the strength of the C-F bond, complete mineralization is difficult, with fluorinated products of incomplete destruction remaining a concern (142, 143). Many existing treatment technologies are only capable of concentrating PFAS (144), and concentrated treatment residuals can result in reintroduction of PFAS into the environment (Fig 6); for example, treatment of drinking water can reduce human exposure at the site of treatment while also acting as a PFAS source where residuals are generated, reinforcing the need for a preventative and holistic approach (145). Therefore, treatment and remediation approaches for contaminated media should be considered in terms of a total-management approach influenced by i) the primary source(s), ii) the impacted media, and iii) the ultimate method of destruction or long-term storage of PFAS.
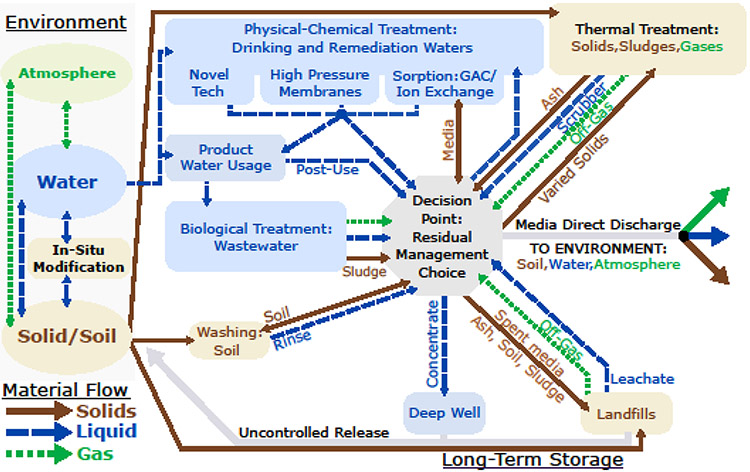
Fig. 6: Site management options for media streams containing PFAS:
Brown, blue, and green indicate solid/semisolid, water/liquid, and air/gas phases. PFAS, including precursors and products of incomplete destruction, cycle through the management options based on treatment and operational choices. Without informed management choices, the persistence of PFAS results in re-releases to the environment. Only complete mineralization, with HF control, offers a permanent solution for breaking the treatment cycle.
PFAS-impacted drinking water often is the primary route of human exposure (146), and treatment techniques for aqueous media are the most well-established—although performance and cost for removal of some short-chain PFAS can be particularly challenging. Management can occur at primary sources (i.e., treatment of industrial wastewater effluent), at secondary concentration source (e.g., drinking-water-treatment plants or landfill leachate), or in diffuse environmental media (e.g., groundwater). Treatment of diffuse media can involve ex-situ “pump-and-treat” approaches to adjoin groundwater to aqueous-treatment technologies. The most established treatments for water are sorption to granular activated carbon (GAC) or ion-exchange stationary phases (141). Powdered sorbents can be used; however, particle-separation technology is needed to physically recover the spent sorbent (e.g., conventional treatment, micro- or ultra-filtration).
Removal performance of sorbents differs among targeted PFAS, concentrations, background water quality, and sorbent properties among other parameters (141, 147, 148). Another concentrative approach is the use of high-pressure membrane systems such as reverse osmosis or nanofiltration. The residual stream for sorbent technologies are the spent media or a regenerate stream for regenerable ion-exchange media, while high-pressure membranes yield an enriched retentate. Both residual streams need to be processed further (Fig 6). GAC typically is reactivated and single-use resins typically are incinerated where little is known regarding PFAS fate in full-scale facilities. Likewise, studies evaluating treatment options for PFAS-laden reverse-osmosis membrane concentrate or ion-exchange regenerant are at their infancy (149). Other, less-used techniques include membrane distillation, electrodialysis-reversal, flotation, electrocoagulation, and evaporation. The niche applications of these technologies are due to performance, cost, and lack of process familiarity.
Environmental media such as soils that can be diffusely contaminated via i) wet/dry deposition, ii) land application of PFAS-enriched materials such as biosolids, wastewater or leachate, and iii) usage of PFAS-containing products such as AFFFs and pesticides, or uncontrolled release via unlined landfills or spills (Print Fig.). Soil contamination is a threat to nearby water sources due to downward and lateral migration of PFAS into receiving water bodies (Fig. 4). In some cases, the large volume of soil that is affected makes ex-situ removal and destruction a considerable logistics problem. Another approach to site management is in-situ modification to enhance mobility of PFAS for pump-and-treat application or to stabilize PFAS migration using GAC or other sorbents (e.g., clays) to limit impacts (150). While this can be an effective short-term site-management technique, it is not a permanent solution, and likely will not retain all PFAS species effectively (148, 150, 151). In-situ treatment of PFAS in soil requires different techniques, such as permeable reactive barriers or addition of powdered activated carbon – of which, none have shown the ability to control PFAS plumes in the long term (150).
The terminal destination of PFAS wastes is of primary concern for the lifecycle management of these compounds. Currently, two commercially viable long-term storage approaches are landfilling affected media or underground injection of contaminated water (145). Such sequestration is a temporary solution. Since most PFAS do not naturally degrade to non-fluorinated chemical species, these long-term sinks are time-delayed sources. For example, landfills are recognized PFAS sources via PFAS-enriched landfill gas and liquid leachates (71). The only permanent solution to PFAS is the destructive remineralization of the underlying fluorine—whether directly acting on contaminated media, or from treatment of residual streams of other treatment techniques, such as spent sorbents or regenerant solutions.
Thermal treatment is a destructive approach that can achieve PFAS mineralization. Incineration by itself has been shown to at least partially destroy even highly fluorinated wastes (143), and advanced thermal oxidation can be used on solid, liquid, and gas samples to convert PFAS to constituent gases with an acid-scrubber cleanup (152). Ideally, this process yields HF, NOx, SOx, and CO2 gases that are handled by traditional air-pollution control technologies. However, thermal treatment requires substantial temperatures (>700 °C) for a sufficient period to convert PFAS into HF and non-fluorinated products, with more highly fluorinated species requiring more time and higher temperature (153, 154). Catalytic oxidation at lower temperatures (e.g., 400 °C) has been demonstrated for some PFAS (155). Thermal processes, however, have not been demonstrated at scale, where inefficiencies can reduce performance. Atmospheric emission of products of incomplete destruction or the air-pollution control technologies associated with thermal-treatment processes, including the regeneration of spent GAC, can become additional PFAS sources. Capture or destruction of these products in the exhaust of thermal processes also is an area of active research, although forefront technologies are like those applied for other media, namely scrubbers, activated-carbon adsorption, and thermal oxidation.
Other destructive treatments for aqueous streams include electrochemical degradation, sonolysis, non-thermal plasma, advanced oxidation (e.g., sulfate radicals) and reduction (solvated electrons), biodegradation (Feammox), zero-valent iron, hydrothermal, and supercritical-water oxidation (149, 156). Although many of these technologies have shown the ability to destroy select PFAS, none have demonstrated long-term performance approaching mineralization at full scale with natural- and industrial-water matrices for a wide assortment of PFAS. Also, the energy costs of many of these technologies limit their sustainability and desirability, and the formation of harmful byproducts (e.g. bromate, perchlorate) remain a concern (144). The lack of widespread testing and limited field usage has led to a reluctance in utilizing these technologies, since additional management of the waste or residual streams will be needed. These unknowns, among others, further demonstrate the need to minimize use of PFAS and find a total waste-management approach where complete destruction of PFAS is assured.
Conclusions
The pool of novel PFAS, for which physical, chemical and toxicological data remain undetermined, is expanding rapidly and now includes untold numbers of compounds having widely varying chemical structures, volatilities and solubilities, as well as uncertain potential exposure consequences. Early studies on structurally similar PFAS suggest that behavioral trends gleaned from legacy PFAS studies can be useful as a basis to predict fate, toxicity, and remediation strategies for emerging compounds. Recently, an internationally authored paper called for PFAS to be managed as a class, based upon widespread use in commerce, shared inclusion of strong C-F bonding, and resulting environmental persistence of common terminal products (157).
Current international reporting practices used to document PFAS synthesis, production volumes, and potential releases vary among countries and are not always tailored to provide the knowledge necessary to adequately track and understand the movement of these compounds in the environment. These efforts typically serve as a critical first step in developing knowledge to be used in future assessment and potential regulation of PFAS. In the United States, expansion of the Toxic Release Inventory will include approximately 172 long-chain PFAS starting in 2021, providing limited, but valuable information in the form of sources, compositions, and quantities released for these compounds. However, under regulatory frameworks around the world, information on many PFAS is protected as confidential-business information and will not be included in this inventory (16), thereby necessitating substantial continued discovery and forensic-identification efforts around the world. Other PFAS, such as those classified as chemical substances of unknown or variable composition, byproducts and biological materials and polymers, may be too complex to fully characterize and can challenge scientific investigation.
While there is an ongoing need to advance responsive PFAS science, particularly regarding investigating environmental sources and sinks, toxicity, and remediation technologies, evidence suggests that preventative upstream actions are critical to facilitate the transition to safer alternatives and minimize the impact of PFAS on human health and the environment. Examples of these upstream actions include the EPA’s Stewardship Program (158), the Amendment to the Polymer Exemption Rule removing side-chain fluorotelomer polymers from the exemption (159), the Significant New Use Rule removing an exemption for a set of PFAS used as coatings (160), the recently announced Comprehensive National Strategy to confront PFAS pollution (161) and a ban on PFAS in food contact paper in Denmark (162). Regardless of the regulatory approach implemented, collaborative efforts between scientists, industrial producers, and policy makers will remain key in finding effective solutions (163).
ACKNOWLEDGMENTS
We thank Tim Collette, Chris Lau, Kara Godineaux, John Johnston, Brian Schumacher, Bill Fisher, Gayle Hagler, Tim Watkins, Bruce Rodan and Susan Burden for helpful comments and suggestions. We thank editors and anonymous referees for comments and helpful suggestions.
Funding:
This research was supported by the EPA Office of Research and Development; North Carolina State University (NCSU), the National Institute of Environmental Health Sciences of the National Institutes of Health (P42ES027706); and ETH Zürich, Institute of Environmental Engineering. It has been subjected to the EPA’s administrative review and approved for publication. Mention of trade names or commercial products does not constitute endorsement or recommendation for use. The views expressed in this article are those of the authors and do not necessarily represent the views or policies of the EPA. J.W.W. is an adjunct faculty member at the University of Georgia, Department of Geology.
Footnotes
Publisher's Disclaimer: This manuscript has been accepted for publication in Science. This version has not undergone final editing. Please refer to the complete version of record at http://www.sciencemag.org/. The manuscript may not be reproduced or used in any manner that does not fall within the fair use provisions of the Copyright Act without the prior, written permission of AAAS.
Competing interests: Z.W. has received compensation from the Organisation of Economic Co-operation and Development (OECD) to develop a synthesis report on side-chain fluorinated polymers. D.K. serves on the North Carolina Secretaries' Science Advisory Board.
REFERENCES
- 1.EPA, PFAS structures in DSSTox. https://comptox.epa.gov/dashboard/chemical_lists/PFASSTRUCTV3 (2020).
- 2.Ĝge J et al. , An overview of the uses of per- and polyfluoroalkyl substances (PFAS). Environmental Science: Processes & Impacts 22, 2345–2373 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jiao X, Shi Q, Gan J, Uptake, accumulation and metabolism of PFASs in plants and health perspectives: A critical review. Critical Reviews in Environmental Science and Technology, 1–32 (2020). [Google Scholar]
- 4.Ankley GT et al. , Assessing the Ecological Risks of Per- and Polyfluoroalkyl Substances: Current State-of-the Science and a Proposed Path Forward. Environmental Toxicology and Chemistry n/a, (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bartell SM, Vieira VM, Critical review on PFOA, kidney cancer, and testicular cancer. Journal of the Air & Waste Management Association 71, 663–679 (2021). [DOI] [PubMed] [Google Scholar]
- 6.Fenton S et al. , Per- and Polyfluoroalkyl Substance Toxicity and Human Health Review: Current State of Knowledge and Strategies for Informing Future Research. Environmental Toxicology and Chemistry 40, 606–630 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Johansson JH et al. , Global transport of perfluoroalkyl acids via sea spray aerosol. Environmental Science: Processes & Impacts 21, 635–649 (2019). [DOI] [PubMed] [Google Scholar]
- 8.Siegemund GSW; Feiring A; Smart B; Behr F; Vogel H; McKusick B, Fluorine Compounds, Organic. (Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim, Germany, ed. 3rd, 2000), vol. 33. [Google Scholar]
- 9.Buck RC et al. , Perfluoroalkyl and Polyfluoroalkyl Substances in the Environment: Terminology, Classification, and Origins. Integrated Environmental Assessment and Management 7, 513–541 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.OECD, "Reconciling Terminology of the Universe of Per- and Polyfluoroalkyl Substances: Recommendations and Practical Guidance," OECD Series on Risk Management No. No. 61 (Paris, France, 2021). [Google Scholar]
- 11.Ogawa Y, Tokunaga E, Kobayashi O, Hirai K, Shibata N, Current Contributions of Organofluorine Compounds to the Agrochemical Industry. iScience 23, 101467 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Inoue M, Sumii Y, Shibata N, Contribution of Organofluorine Compounds to Pharmaceuticals. ACS Omega 5, 10633–10640 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kissa E, Fluorinated Surfactants and Repellents. (Marcel Dekker AG, 2001). [Google Scholar]
- 14.OECD, "Toward a New Comprehensive Global Database of Per- and Polyfluoroalkyl Substances (PFASs): Summary Report on Updating the OECD 2007 List of Per- and Polyfluoroalkyl Substances (PFASs)," Series on Risk Management No. 39 (Organisation for Economic Co-operation and Development, 2018). [Google Scholar]
- 15.Wang Z, Cousins I, Scheringer M, Buck RC, Hungerbuhler K, Global emission inventories for C4–C14 perfluoroalkyl carboxylic acid (PFCA) homologues from 1951 to 2030, part II: The remaining pieces of the puzzle. Environment International 69 166–176 (2014). [DOI] [PubMed] [Google Scholar]
- 16.Gold SC, Wagner WE, Filling gaps in science exposes gaps in chemical regulation. Science 368, 1066–1068 (2020). [DOI] [PubMed] [Google Scholar]
- 17.Jackson DA, Mabury SA, Polyfluorinated Amides as a Historical PFCA Source by Electrochemical Fluorination of Alkyl Sulfonyl Fluorides. Environmental Science & Technology 47, 382–389 (2013). [DOI] [PubMed] [Google Scholar]
- 18.Rand AA, Mabury SA, Perfluorinated Carboxylic Acids in Directly Fluorinated High-Density Polyethylene Material. Environmental Science & Technology 45, 8053–8059 (2011). [DOI] [PubMed] [Google Scholar]
- 19.Gramstad T, Haszeldine RN, 512. Perfluoroalkyl derivatives of sulphur. Part VI. Perfluoroalkanesulphonic acids CF3·[CF2]·SO3H (n= 1—7). Journal of the Chemical Society (Resumed), 2640–2645 (1957). [Google Scholar]
- 20.Newton S et al. , Novel Polyfluorinated Compounds Identified Using High Resolution Mass Spectrometry Downstream of Manufacturing Facilities near Decatur, Alabama. Environmental Science & Technology 51, 1544–1552 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Washington JW et al. , Nontargeted mass-spectral detection of chloroperfluoropolyether carboxylates in New Jersey soils. Science 368, 1103–1107 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Barzen-Hanson KA et al. , Discovery of 40 Classes of Per- and Polyfluoroalkyl Substances in Historical Aqueous Film-Forming Foams (AFFFs) and AFFF-Impacted Groundwater. Environmental Science & Technology 51, 2047–2057 (2017). [DOI] [PubMed] [Google Scholar]
- 23.Washington JW, Jenkins TM, Rankin K, Naile JE, Decades-scale degradation of commercial, side-chain, fluorotelomer-based polymers in soils & water. Environmental Science & Technology 49, 915–923 (2015). [DOI] [PubMed] [Google Scholar]
- 24.Booten C, Nicholson S, Mann M, Abdelaziz O, "Refrigerants: Market Trends and Supply Chain Assessment," Technical Report (Clean Energy Manufacturing Analysis Center, 2020). [Google Scholar]
- 25.EPA., Proposed Rule - Phasedown of Hydrofluorocarbons: Establishing the Allowance Allocation and Trading Program under the AIM Act. https://www.epa.gov/climate-hfcs-reduction/proposed-rule-phasedown-hydrofluorocarbons-establishing-allowance-allocation. (2021).
- 26.U. Nations, CHAPTER XXVII ENVIRONMENT: 2. f Amendment to the Montreal Protocol on Substances that Deplete the Ozone Layer. . https://treaties.un.org/Pages/ViewDetails.aspx?src=TREATY&mtdsg_no=XXVII-2-f&chapter=27&clang=_en (2016).
- 27.Zhai Z et al. , A 17-fold increase of trifluoroacetic acid in landscape waters of Beijing, China during the last decade. Chemosphere 129, 110–117 (2015). [DOI] [PubMed] [Google Scholar]
- 28.Tsai WT, Environmental implications of perfluorotributylamine—a potent greenhouse gas. Mitig Adapt Strateg Glob Change 22, 225–231 (2017). [Google Scholar]
- 29.Wang Z, Cousins IT, Scheringer M, Buck RC, Hungerbuehler K, Global emission inventories for C-4-C-14 perfluoroalkyl carboxylic acid (PFCA) homologues from 1951 to 2030, Part I: production and emissions from quantifiable sources. Environment International 70, 62–75 (2014). [DOI] [PubMed] [Google Scholar]
- 30.Banks RESBE; Tatlow JC, Organofluorine Chemistry: Principles and Commercial Applications. (Plenum, New York, 1994). [Google Scholar]
- 31.Ebnesajjad S, Ed., Introduction to Fluoropolymers: Materials, Tachnology and Applications, (Elsevier, Inc., Amsterdam, The Netherlands, 2013), pp. 418. [Google Scholar]
- 32.Wang Z, Goldenman G, Tugran T, McNeil A, "Per- and polyfluoroalkylether substances: identity, production and use," Nordiske Arbejdspapirer No. 901 (Nordic Council of Ministers, Copenhagen, Denmark, 2020). [Google Scholar]
- 33.EPA, Long-Chain Perfluorinated Chemicals (PFCs) Action Plan. https://www.epa.gov/assessing-and-managing-chemicals-under-tsca/long-chain-perfluorinated-chemicals-pfcs-action-plan, (2009).
- 34.Wang Z, Cousins IT, Scheringer M, Hungerbuhler K, Fluorinated alternatives to long-chain perfluoroalkyl carboxylic acids (PFCAs), perfluoroalkane sulfonic acids (PFSAs) and their potential precursors. Environment International 60, 242–248 (2013). [DOI] [PubMed] [Google Scholar]
- 35.Xu L et al. , Discovery of a Novel Polyfluoroalkyl Benzenesulfonic Acid around Oilfields in Northern China. Environmental Science & Technology 51, 14173–14181 (2017). [DOI] [PubMed] [Google Scholar]
- 36.Bao Y et al. , First assessment on degradability of sodium p-perfluorous nonenoxybenzene sulfonate (OBS), a high volume alternative to perfluorooctane sulfonate in fire-fighting foams and oil production agents in China. RSC Advances 7, 46948–46957 (2017). [Google Scholar]
- 37.Eriksson U, Haglund P, Kärrman A, Contribution of precursor compounds to the release of per- and polyfluoroalkyl substances (PFASs) from waste water treatment plants (WWTPs). Journal of Environmental Sciences 61, 80–90 (2017). [DOI] [PubMed] [Google Scholar]
- 38.Washington JW, Jenkins TM, Abiotic hydrolysis of fluorotelomer polymers as a source of perfluorocarboxylates at the global scale. Environmental Science & Technology 49, 14129–14135 (2015). [DOI] [PubMed] [Google Scholar]
- 39.Dombrowski PM et al. , Technology review and evaluation of different chemical oxidation conditions on treatability of PFAS. Remediation Journal 28, 135–150 (2018). [Google Scholar]
- 40.Bruton TA, Sedlak DL, Treatment of Aqueous Film-Forming Foam by Heat-Activated Persulfate Under Conditions Representative of In Situ Chemical Oxidation. Environmental Science & Technology 51, 13878–13885 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cui J, Gao P, Deng Y, Destruction of Per- and Polyfluoroalkyl Substances (PFAS) with Advanced Reduction Processes (ARPs): A Critical Review. Environmental Science & Technology 54, 3752–3766 (2020). [DOI] [PubMed] [Google Scholar]
- 42.Ahrens L, Harner T, Shoeib M, Lane DA, Murphy JG, Improved Characterization of Gas-Particle Partitioning for Per- and Polyfluoroalkyl Substances in the Atmosphere Using Annular Diffusion Denuder Samplers. Environmental Science & Technology 46, 7199–7206 (2012). [DOI] [PubMed] [Google Scholar]
- 43.Liu C, Liu J, Aerobic biotransformation of polyfluoroalkyl phosphate esters (PAPs) in soil. Environmental Pollution 212, 230–237 (2016). [DOI] [PubMed] [Google Scholar]
- 44.Mejia Avendaño S, Liu J, Production of PFOS from aerobic soil biotransformation of two perfluoroalkyl sulfonamide derivatives. Chemosphere 119, 1084–1090 (2015). [DOI] [PubMed] [Google Scholar]
- 45.Allred BM, Lang JR, Barlaz MA, Field JA, Physical and Biological Release of Poly- and Perfluoroalkyl Substances (PFASs) from Municipal Solid Waste in Anaerobic Model Landfill Reactors. Environmental Science & Technology 49, 7648–7656 (2015). [DOI] [PubMed] [Google Scholar]
- 46.Huang S, Jaffé PR, Defluorination of Perfluorooctanoic Acid (PFOA) and Perfluorooctane Sulfonate (PFOS) by Acidimicrobium sp. Strain A6. Environmental Science & Technology 53, 11410–11419 (2019). [DOI] [PubMed] [Google Scholar]
- 47.Hamid H, Li LY, Grace JR, Review of the fate and transformation of per- and polyfluoroalkyl substances (PFASs) in landfills. Environmental Pollution 235, 74–84 (2018). [DOI] [PubMed] [Google Scholar]
- 48.Yi S et al. , Biotransformation of AFFF Component 6:2 Fluorotelomer Thioether Amido Sulfonate Generates 6:2 Fluorotelomer Thioether Carboxylate under Sulfate-Reducing Conditions. Environmental Science & Technology Letters 5, 283–288 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Harding-Marjanovic KC et al. , Aerobic Biotransformation of Fluorotelomer Thioether Amido Sulfonate (Lodyne) in AFFF-Amended Microcosms. Environmental Science & Technology 49, 7666–7674 (2015). [DOI] [PubMed] [Google Scholar]
- 50.Zhang H et al. , Uptake, Translocation, and Metabolism of 8:2 Fluorotelomer Alcohol in Soybean (Glycine max L. Merrill). Environmental Science & Technology 50, 13309–13317 (2016). [DOI] [PubMed] [Google Scholar]
- 51.Bizkarguenaga E et al. , Uptake of perfluorooctanoic acid, perfluorooctane sulfonate and perfluorooctane sulfonamide by carrot and lettuce from compost amended soil. Science of The Total Environment 571, 444–451 (2016). [DOI] [PubMed] [Google Scholar]
- 52.Lewis M, Kim M-H, Wang N, Chu K-H, Engineering artificial communities for enhanced FTOH degradation. Science of The Total Environment 572, 935–942 (2016). [DOI] [PubMed] [Google Scholar]
- 53.Washington JW, Jenkins TM, Weber EJ, Identification of unsaturated and 2H polyfluorocarboxylate homologous series, and their detection in environmental samples and as polymer degradation products. Environmental Science & Technology 49, 13256–13263 (2015). [DOI] [PubMed] [Google Scholar]
- 54.Shaw DMJ et al. , Degradation and defluorination of 6:2 fluorotelomer sulfonamidoalkyl betaine and 6:2 fluorotelomer sulfonate by Gordonia sp. strain NB4-1Y under sulfur-limiting conditions. The Science of the total environment 647, 690–698 (2019). [DOI] [PubMed] [Google Scholar]
- 55.Bratt U. M. a. L., Hydrolysis of amides. Alkaline and general acid catalyzed alkaline hydrolysis of some substituted acetamides and benzamides. Acta Chemica Scandinavica A. 28, 715–722 (1974). [Google Scholar]
- 56.Lee H, D'Eon J, Mabury SA, Biodegradation of Polyfluoroalkyl Phosphates as a Source of Perfluorinated Acids to the Environment. Environmental Science & Technology 44, 3305–3310 (2010). [DOI] [PubMed] [Google Scholar]
- 57.Royer LA, Lee LS, Russell MH, Nies LF, Turco RF, Microbial transformation of 8:2 fluorotelomer acrylate and methacrylate in aerobic soils. Chemosphere 129, 54–61 (2015). [DOI] [PubMed] [Google Scholar]
- 58.Dasu K, Lee LS, Aerobic biodegradation of toluene-2,4-di(8:2 fluorotelomer urethane) and hexamethylene-1,6-di(8:2 fluorotelomer urethane) monomers in soils. Chemosphere 144, 2482–2488 (2016). [DOI] [PubMed] [Google Scholar]
- 59.Wang N et al. , 6:2 Fluorotelomer sulfonate aerobic biotransformation in activated sludge of waste water treatment plants. Chemosphere 82, 853–858 (2011). [DOI] [PubMed] [Google Scholar]
- 60.Yu X, Takabe Y, Yamamoto K, Matsumura C, Nishimura F, Biodegradation Property of 8:2 Fluorotelomer Alcohol (8:2 FTOH) under Aerobic/Anoxic/Anaerobic Conditions. Journal of Water and Environment Technology 14, 177–190 (2016). [Google Scholar]
- 61.Yu X, Nishimura F, Hidaka T, Effects of microbial activity on perfluorinated carboxylic acids (PFCAs) generation during aerobic biotransformation of fluorotelomer alcohols in activated sludge. The Science of the total environment 610-611, 776–785 (2018). [DOI] [PubMed] [Google Scholar]
- 62.Zhang S et al. , 6:2 and 8:2 Fluorotelomer Alcohol Anaerobic Biotransformation in Digester Sludge from a WWTP under Methanogenic Conditions. Environmental Science & Technology 47, 4227–4235 (2013). [DOI] [PubMed] [Google Scholar]
- 63.Wang N et al. , 8-2 Fluorotelomer alcohol aerobic soil biodegradation: Pathways, metabolites, and metabolite yields. Chemosphere 75, 1089–1096 (2009). [DOI] [PubMed] [Google Scholar]
- 64.Hamid H, Li LY, Grace JR, Aerobic biotransformation of fluorotelomer compounds in landfill leachate-sediment. The Science of the total environment 713, 136547 (2020). [DOI] [PubMed] [Google Scholar]
- 65.Rhoads KR, Janssen EML, Luthy RG, Criddle CS, Aerobic biotransformation and fate of N-ethyl perfluorooctane sulfonamidoethanol (N-EtFOSE) in activated sludge. Environmental Science & Technology 42, 2873–2878 (2008). [DOI] [PubMed] [Google Scholar]
- 66.Benskin JP et al. , Biodegradation of N-Ethyl Perfluorooctane Sulfonamido Ethanol (EtFOSE) and EtFOSE-Based Phosphate Diester (SAmPAP Diester) in Marine Sediments. Environmental Science & Technology 47, 1381–1389 (2013). [DOI] [PubMed] [Google Scholar]
- 67.Sima MW, Jaffé PR, A critical review of modeling Poly- and Perfluoroalkyl Substances (PFAS) in the soil-water environment. The Science of the total environment 757, 143793 (2021). [DOI] [PubMed] [Google Scholar]
- 68.Wang N et al. , Fluorotelomer alcohol biodegradation - Direct evidence that perfluorinated carbon chains breakdown. Environmental Science & Technology 39, 7516–7528 (2005). [DOI] [PubMed] [Google Scholar]
- 69.Mejia-Avendaño S, Vo Duy S, Sauvé S, Liu J, Generation of Perfluoroalkyl Acids from Aerobic Biotransformation of Quaternary Ammonium Polyfluoroalkyl Surfactants. Environmental Science & Technology 50, 9923–9932 (2016). [DOI] [PubMed] [Google Scholar]
- 70.Liu J, Avendano SM, Microbial degradation of polyfluoroalkyl chemicals in the environment: A review. Environment International 61, 98–114 (2013). [DOI] [PubMed] [Google Scholar]
- 71.Lang JR, Allred BM, Field JA, Levis JW, Barlaz MA, National Estimate of Per- and Polyfluoroalkyl Substance (PFAS) Release to U.S. Municipal Landfill Leachate. Environmental Science & Technology 51, 2197–2205 (2017). [DOI] [PubMed] [Google Scholar]
- 72.Liu Y et al. , From Waste Collection Vehicles to Landfills: Indication of Per- and Polyfluoroalkyl Substance (PFAS) Transformation. Environmental Science & Technology Letters 8, 66–72 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Fitzgerald NJM, Temme HR, Simcik MF, Novak PJ, Aqueous film forming foam and associated perfluoroalkyl substances inhibit methane production and Co-contaminant degradation in an anaerobic microbial community. Environmental Science: Processes & Impacts 21, 1915–1925 (2019). [DOI] [PubMed] [Google Scholar]
- 74.Washington JW, Naile JE, Jenkins TM, Lynch DG, Characterizing Fluorotelomer and Polyfluoroalkyl Substances in New and Aged Fluorotelomer-Based Polymers for Degradation Studies with GC/MS and LC/MS/MS. Environmental Science & Technology 48, 5762–5769 (2014). [DOI] [PubMed] [Google Scholar]
- 75.Wackett LP, Robinson SL, The ever-expanding limits of enzyme catalysis and biodegradation: polyaromatic, polychlorinated, polyfluorinated, and polymeric compounds. Biochemical Journal 477, 2875–2891 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tebes-Stevens C, Patel JM, Jones WJ, Weber EJ, Prediction of Hydrolysis Products of Organic Chemicals under Environmental pH Conditions. Environmental Science & Technology 51, 5008–5016 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Rose AW, Hawkes HE, Webb JS, Geochemistry in Mineral Exploration. (Academic Press, New York, NY, ed. second edition, 1979). [Google Scholar]
- 78.Friedlander G, Kennedy JW, Macias ES, Miller JM, Nuclear and Radiochemistry, 3rd Edition. (Wiley Interscience, 1981). [Google Scholar]
- 79.Rankin K, Mabury SA, Jenkins TM, Washington JW, A North American and global survey of perfluoroalkyl substances in surface soils: Distribution patterns and mode of occurrence. Chemosphere 161, 333–341 (2016). [DOI] [PubMed] [Google Scholar]
- 80.Shoeib M, Harner T, Vlahos P, Perfluorinated chemicals in the Arctic atmosphere. Environmental Science & Technology 40, 7577–7583 (2006). [DOI] [PubMed] [Google Scholar]
- 81.Gawor A et al. , Neutral polyfluoroalkyl substances in the global atmosphere. Environ Sci Process Impacts 16, 404–413 (2014). [DOI] [PubMed] [Google Scholar]
- 82.Shoeib M et al. , Survey of polyfluorinated chemicals (PFCs) in the atmosphere over the northeast Atlantic Ocean. Atmospheric Environment 44, 2887–2893 (2010). [Google Scholar]
- 83.Dreyer A, Weinberg I, Temme C, Ebinghaus R, Polyfluorinated Compounds in the Atmosphere of the Atlantic and Southern Oceans: Evidence for a Global Distribution. Environmental Science & Technology 43, 6507–6514 (2009). [DOI] [PubMed] [Google Scholar]
- 84.D'Ambro EL et al. , Characterizing the Air Emissions, Transport, and Deposition of Per- and Polyfluoroalkyl Substances from a Fluoropolymer Manufacturing Facility. Environ Sci Technol 55, 862–870 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Dreyer A, Kirchgeorg T, Weinberg I, Matthias V, Particle-size distribution of airborne poly- and perfluorinated alkyl substances. Chemosphere 129, 142–149 (2015). [DOI] [PubMed] [Google Scholar]
- 86.Ellis DA et al. , Atmospheric lifetime of fluorotelomer alcohols. Environ. Sci. Technol 37, 3816–3820 (2003). [DOI] [PubMed] [Google Scholar]
- 87.MacInnis JJ et al. , Fate and Transport of Perfluoroalkyl Substances from Snowpacks into a Lake in the High Arctic of Canada. Environmental Science & Technology 53, 10753–10762 (2019). [DOI] [PubMed] [Google Scholar]
- 88.Washington JW, Rankin K, Libelo EL, Lynch DG, Cyterski M, Determining global background soil PFAS loads and the fluorotelomer-based polymer degradation rates that can account for these loads. Science of the Total Environment 651, 2444–2449 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.D'Ambro EL et al. , Characterizing the Air Emissions, Transport, and Deposition of Per- and Polyfluoroalkyl Substances from a Fluoropolymer Manufacturing Facility. Environmental Science & Technology 55, 862–870 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Galloway JE et al. , Evidence of Air Dispersion: HFPO–DA and PFOA in Ohio and West Virginia Surface Water and Soil near a Fluoropolymer Production Facility. Environmental Science & Technology 54, 7175–7184 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Brusseau ML, The influence of molecular structure on the adsorption of PFAS to fluid-fluid interfaces: Using QSPR to predict interfacial adsorption coefficients. Water Res. 152, 148–158 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.S. C. S. USDA, Soil Taxonomy: a Basic System of Soil Classification for Making and Interpreting Soil Surveys. Agricultural Handbook No. 436 (US Government Printing Office, Soil Conservation Service, Washington, D.C., 1975). [Google Scholar]
- 93.Zhu X, Song X, Schwarzbauer J, First insights into the formation and long-term dynamic behaviors of nonextractable perfluorooctanesulfonate and its alternative 6:2 chlorinated polyfluorinated ether sulfonate residues in a silty clay soil. Sci. Total Environ 761, 143230 (2021). [DOI] [PubMed] [Google Scholar]
- 94.Xiao F, Jin B, Golovko S, Xing B, Sorption and desorption mechanisms of cationic and zwitterionic per- and polyfluoroalkyl substances in natural soils: thermodynamics and hysteresis. Environ. Sci. Technol 53, 11818–11827 (2019). [DOI] [PubMed] [Google Scholar]
- 95.Nguyen TMH et al. , Influences of Chemical Properties, Soil Properties, and Solution pH on Soil–Water Partitioning Coefficients of Per- and Polyfluoroalkyl Substances (PFASs). Environmental Science & Technology 54, 15883–15892 (2020). [DOI] [PubMed] [Google Scholar]
- 96.McGuire ME et al. , Evidence of remediation-induced alteration of subsurface poly- and perfluoroalkyl substance distribution at a former firefighter training area. Environmental Science & Technology 48, 6644–6652 (2014). [DOI] [PubMed] [Google Scholar]
- 97.Washington JW, Yoo H, Ellington JJ, Jenkins TM, Libelo EL, Concentrations, Distribution, and Persistence of Perfluoroalkylates in Sludge-Applied Soils near Decatur, Alabama, USA. Environmental Science & Technology 44, 8390–8396 (2010). [DOI] [PubMed] [Google Scholar]
- 98.Borthakur A et al. , Release of soil colloids during flow interruption increases the pore-water PFAS concentration in saturated soil. Environmental Pollution 286, (2021). [DOI] [PubMed] [Google Scholar]
- 99.Blaine AC et al. , Perfluoroalkyl Acid Distribution in Various Plant Compartments of Edible Crops Grown in Biosolids-Amended soils. Environmental Science & Technology 48, 7858–7865 (2014). [DOI] [PubMed] [Google Scholar]
- 100.Blaine AC et al. , Perfluoroalkyl acid uptake in lettuce (Lactuca sativa) and strawberry (Fragaria ananassa) irrigated with reclaimed water. Environ Sci Technol 48, 14361–14368 (2014). [DOI] [PubMed] [Google Scholar]
- 101.Blaine AC et al. , Uptake of Perfluoroalkyl Acids into Edible Crops via Land Applied Biosolids: Field and Greenhouse Studies. Environmental Science & Technology 47, 14062–14069 (2013). [DOI] [PubMed] [Google Scholar]
- 102.Lesmeister L et al. , Extending the knowledge about PFAS bioaccumulation factors for agricultural plants – A review. Science of The Total Environment 766, 142640 (2021). [DOI] [PubMed] [Google Scholar]
- 103.Xu C et al. , Occurrence, source apportionment, plant bioaccumulation and human exposure of legacy and emerging per- and polyfluoroalkyl substances in soil and plant leaves near a landfill in China. Science of The Total Environment 776, 145731 (2021). [DOI] [PubMed] [Google Scholar]
- 104.Navarro I et al. , Bioaccumulation of emerging organic compounds (perfluoroalkyl substances and halogenated flame retardants) by earthworm in biosolid amendedsoils. Environmental Research 149, 32–39 (2016). [DOI] [PubMed] [Google Scholar]
- 105.Eschauzier C, Raat KJ, Stuyfzand PJ, De Voogt P, Perfluorinated alkylated acids in groundwater and drinking water: Identification, origin and mobility. Science of the Total Environment 458, 477–485 (2013). [DOI] [PubMed] [Google Scholar]
- 106.Lasier PJ, Washington JW, Hassan SM, Jenkins TM, Perfluorinated chemicals in surface waters and sediments from northwest Georgia, USA, and their bioaccumulation in Lumbriculus variegatus Environmental Toxicology and Chemistry 30, 2194–2201 (2011). [DOI] [PubMed] [Google Scholar]
- 107.Pi N, Ng JZ, Kelly BC, Uptake and elimination kinetics of perfluoroalkyl substances in submerged and free-floating aquatic macrophytes: Results of mesocosm experiments with Echinodorus horemanii and Eichhornia crassipes. Water research 117, 167–174 (2017). [DOI] [PubMed] [Google Scholar]
- 108.Bonato M et al. , PFAS Environmental Pollution and Antioxidant Responses: An Overview of the Impact on Human Field. Int. J. Res. Public Health 17, (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Sunderland EM et al. , A review of the pathways of human exposure to poly- and perfluoroalkyl substances (PFASs) and present understanding of health effects. Journal of Exposure Science & Environmental Epidemiology 29, 131–147 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.DeWitt JC, Peden-Adams MM, Keller JM, Germolec DR, Immunotoxicity of perfluorinated compounds: recent developments. Toxicol Pathol 40, 300–311 (2012). [DOI] [PubMed] [Google Scholar]
- 111.E. F. S. Authority, Risk to human health related to the presence of perfluorooctane sulfonic acid and perfluorooctanoic acid in food. EFSA J 16, 1–293 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Goodrum PE, Anderson JK, Luz AL, Ansell GK, Application of a Framework for Grouping and Mixtures Toxicity Assessment of PFAS: A Closer Examination of Dose-Additivity Approaches. Toxicol Sci 179, 262–278 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Dassuncao C et al. , Temporal Shifts in Poly- and Perfluoroalkyl Substances (PFASs) in North Atlantic Pilot Whales Indicate Large Contribution of Atmospheric Precursors. Environmental Science & Technology 51, 4512–4521 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Larson ES, Conder JM, Arblaster JA, Modeling avian exposures to perfluoroalkyl substances in aquatic habitats impacted by historical aqueous film forming foam releases. Chemosphere 201, 335–341 (2018). [DOI] [PubMed] [Google Scholar]
- 115.Koch A et al. , Characterization of an AFFF impacted freshwater environment using total fluorine, extractable organofluorine and suspect per- and polyfluoroalkyl substance screening analysis. Chemosphere 276, 130179 (2021). [DOI] [PubMed] [Google Scholar]
- 116.Miranda DA et al. , Bioaccumulation of Per- and polyfluoroalkyl substances (PFASs) in a tropical estuarine food web. Science of The Total Environment 754, 142146 (2021). [DOI] [PubMed] [Google Scholar]
- 117.Janousek RM, Mayer J, Knepper TP, Is the phase-out of long-chain PFASs measurable as fingerprint in a defined area? Comparison of global PFAS concentrations and a monitoring study performed in Hesse, Germany from 2014 to 2018. TrAC Trends in Analytical Chemistry 120, 115393 (2019). [Google Scholar]
- 118.Benotti MJ et al. , A forensic approach for distinguishing PFAS materials. Environmental Forensics 21, 319–333 (2020). [Google Scholar]
- 119.Glaser D et al. , The impact of precursors on aquatic exposure assessment for PFAS: Insights from bioaccumulation modeling. Integr Environ Assess Manag 17, 705–715 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.De Silva AO et al. , PFAS Exposure Pathways for Humans and Wildlife: A Synthesis of Current Knowledge and Key Gaps in Understanding. Environmental Toxicology and Chemistry 40, 631–657 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Muir D et al. , Levels and trends of poly- and perfluoroalkyl substances in the Arctic environment – An update. Emerging Contaminants 5, 240–271 (2019). [Google Scholar]
- 122.Ng CA, Hungerbühler K, Bioaccumulation of Perfluorinated Alkyl Acids: Observations and Models. Environmental Science & Technology 48, 4637–4648 (2014). [DOI] [PubMed] [Google Scholar]
- 123.Dassuncao C et al. , Shifting Global Exposures to Poly- and Perfluoroalkyl Substances (PFASs) Evident in Longitudinal Birth Cohorts from a Seafood-Consuming Population. Environmental Science & Technology 52, 3738–3747 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Alesio JL, Slitt A, Bothun GD, Critical new insights into the binding of poly- and perfluoroalkyl substances (PFAS) to albumin protein. Chemosphere 287, 131979 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Chen Y et al. , Occurrence, profiles, and ecotoxicity of poly- and perfluoroalkyl substances and their alternatives in global apex predators: A critical review. Journal of Environmental Sciences 109, 219–236 (2021). [DOI] [PubMed] [Google Scholar]
- 126.Yoo H, Washington JW, Jenkins TM, Ellington JJ, Quantitative Determination of Perfluorochemicals and Fluorotelomer Alcohols in Plants from Biosolid-Amended Fields using LC/MS/MS and GC/MS. Environmental Science & Technology 45, 7985–7990 (2011). [DOI] [PubMed] [Google Scholar]
- 127.Burkhard LP, Evaluation of published bioconcentration factor (BCF) and bioaccumulation factor (BAF) data for per- and polyfluoroalkyl substances across aquatic species. Environmental Toxicology and Chemistry n/a, (2021). [DOI] [PubMed] [Google Scholar]
- 128.Borgå K et al. , Trophic magnification factors: considerations of ecology, ecosystems, and study design. Integr Environ Assess Manag 8, 64–84 (2012). [DOI] [PubMed] [Google Scholar]
- 129.Conder JM, Hoke RA, De Wolf W, Russell MH, Buck RC, Are PFCAs bioaccumulative? A critical review and comparison with regulatory lipophilic compounds. Environmental Science & Technology 42, 995–1003 (2008). [DOI] [PubMed] [Google Scholar]
- 130.Loi EIH et al. , Trophic Magnification of Poly- and Perfluorinated Compounds in a Subtropical Food Web. Environmental Science & Technology 45, 5506–5513 (2011). [DOI] [PubMed] [Google Scholar]
- 131.Lescord GL et al. , Perfluorinated and Polyfluorinated Compounds in Lake Food Webs from the Canadian High Arctic. Environmental Science & Technology 49, 2694–2702 (2015). [DOI] [PubMed] [Google Scholar]
- 132.Martin JW, Whittle DM, Muir DCG, Mabury SA, Perfluoroalkyl contaminants in a food web from lake Ontario. Environmental Science & Technology 38, 5379–5385 (2004). [DOI] [PubMed] [Google Scholar]
- 133.Simpson SL et al. , Chronic effects and thresholds for estuarine and marine benthic organism exposure to perfluorooctane sulfonic acid (PFOS)-contaminated sediments: Influence of organic carbon and exposure routes. Science of The Total Environment 776, (2021). [Google Scholar]
- 134.Gonzalez-Gaya B, Casal P, Jurado E, Dachs J, Jimenez B, Vertical transport and sinks of perfluoroalkyl substances in the global open ocean. Environmental Science: Processes & Impacts 21, 1957–1969 (2019). [DOI] [PubMed] [Google Scholar]
- 135.Ahrens L, Bundschuh M, Fate and effects of poly- and perfluoroalkyl substances in the aquatic environment: A review. Environmental Toxicology and Chemistry 33, 1921–1929 (2014). [DOI] [PubMed] [Google Scholar]
- 136.Hoover G, Kar S, Guffey S, Leszczynski J, Sepúlveda MS, In vitro and in silico modeling of perfluoroalkyl substances mixture toxicity in an amphibian fibroblast cell line. Chemosphere 233, 25–33 (2019). [DOI] [PubMed] [Google Scholar]
- 137.McCarthy CJ, Roark SA, Middleton ET, Considerations for toxicity experiments and risk assessments with PFAS mixtures. Integr Environ Assess Manag 17, 697–704 (2021). [DOI] [PubMed] [Google Scholar]
- 138.Ji K et al. , Toxicity of perfluorooctane sulfonic acid and perfluorooctanoic acid on freshwater macroinvertebrates (Daphnia magna and Moina macrocopa) and fish (Oryzias latipes). Environmental Toxicology and Chemistry 27, 2159–2168 (2008). [DOI] [PubMed] [Google Scholar]
- 139.Li Z, Yu Z, Gao P, Yin D, Multigenerational effects of perfluorooctanoic acid on lipid metabolism of Caenorhabditis elegans and its potential mechanism. Science of The Total Environment 703, (2020). [DOI] [PubMed] [Google Scholar]
- 140.Chowdhury MI, Sana T, Panneerselvan L, Dharmarajan R, Megharaj M, Acute toxicity and trans-generational effects of perfluorobutane sulfonate in Caenorhabditis elegans. Environmental Toxicology and Chemistry In press, (2021). [DOI] [PubMed] [Google Scholar]
- 141.Crone BC et al. , Occurrence of Per- and Polyfluoroalkyl Substances (PFAS) in Source Water and Their Treatment in Drinking Water. Critical reviews in environmental science and technology 49, 2359–2396 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Environmental Protection Agency, EPA technical brief on PFAS incineration (2019. https://www.epa.gov/sites/production/files/2019-09/documents/technical_brief_pfas_incineration_ioaa_approved_final_july_2019.pdf).
- 143.Winchell LJ et al. , Per- and polyfluoroalkyl substances thermal destruction at water resource recovery facilities: A state of the science review. Water Environment Research 93, 826–843 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Ross I et al. , A review of emerging technologies for remediation of PFASs. Remediation Journal 28, 101–126 (2018). [Google Scholar]
- 145.Environmental Protection Agency, EPA Interim guidance on PFAS Destruction and Disposal (2020. https://www.regulations.gov/document/EPA-HQ-OLEM-2020-0527-0002).
- 146.Post GB, Gleason JA, Cooper KR, Key scientific issues in developing drinking water guidelines for perfluoroalkyl acids: Contaminants of emerging concern. PLOS Biology 15, (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.EPA, Drinking Water Treatability Database. https://tdb.epa.gov/tdb/home (2021).
- 148.Xiao X, Ulrich BA, Chen B, Higgins CP, Sorption of Poly- and Perfluoroalkyl Substances (PFASs) Relevant to Aqueous Film-Forming Foam (AFFF)-Impacted Groundwater by Biochars and Activated Carbon. Environmental Science & Technology 51, 6342–6351 (2017). [DOI] [PubMed] [Google Scholar]
- 149.Tow EW et al. , Managing and treating per- and polyfluoroalkyl substances (PFAS) in membrane concentrates. AWWA Water Science 3, e1233 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Bolan N et al. , Remediation of poly- and perfluoroalkyl substances (PFAS) contaminated soils – To mobilize or to immobilize or to degrade? Journal of Hazardous Materials 401, 123892 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Barth E, McKernan J, Bless D, Dasu K, Investigation of an immobilization process for PFAS contaminated soils. Journal of Environmental Management 296, 113069 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Chemours, "Thermal oxidizer performance test report: Chemours Company Fayetteville Works," (2020). [Google Scholar]
- 153.Khan MY, So S, da Silva G, Decomposition kinetics of perfluorinated sulfonic acids. Chemosphere 238, 124615 (2020). [DOI] [PubMed] [Google Scholar]
- 154.Rayne S, Forest K, Perfluoroalkyl sulfonic and carboxylic acids: A critical review of physicochemical properties, levels and patterns in waters and wastewaters, and treatment methods. Journal of Environmental Science and Health Part a-Toxic/Hazardous Substances & Environmental Engineering 44, 1145–1199 (2009). [DOI] [PubMed] [Google Scholar]
- 155.Riedel TP et al. , Low temperature thermal treatment of gas-phase fluorotelomer alcohols by calcium oxide. Chemosphere 272, 129859 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Nzeribe BN, Crimi M, Mededovic Thagard S, Holsen TM, Physico-Chemical Processes for the Treatment of Per- And Polyfluoroalkyl Substances (PFAS): A review. Critical Reviews in Environmental Science and Technology 49, 866–915 (2019). [Google Scholar]
- 157.Kwiatkowski CF et al. , Scientific basis for managing PFAS as a chemical class. Environmental Science & Technology Letters 7, 532–543 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.EPA, 2010/2015 PFOA Stewardship Program. OPPT/USEPA, Ed., http://www.epa.gov/assessing-and-managing-chemicals-under-tsca/20102015-pfoa-stewardship-program (http://www.epa.gov/assessing-and-managing-chemicals-under-tsca/20102015-pfoa-stewardship-program, 2010). [Google Scholar]
- 159.EPA, Amendment of Polymer Exemption Rule to Exclude Certain Perfluorinated Polymers. https://www.regulations.gov/document/EPA-HQ-OPPT-2002-0051-0080 (2010).
- 160.EPA, Long-Chain Perfluoroalkyl Carboxylate and Perfluoroalkyl Sulfonate Chemical Substances; Significant New Use Rule. https://www.federalregister.gov/documents/2020/07/27/2020-13738/long-chain-perfluoroalkyl-carboxylate-and-perfluoroalkyl-sulfonate-chemical-substances-significant (2020).
- 161.EPA, Comprehensive National Strategy to Confront PFAS Pollution. https://www.epa.gov/newsreleases/epa-administrator-regan-announces-comprehensive-national-strategy-confront-pfas (2021).
- 162.DMEF, Danish Ministry of Environment and Food. Executive Order on Food Contact Materials and Penal Code for Violation of Related EU Acts. https://www.sgs.com/en/news/2020/05/safeguards-07320-denmark-bans-pfas-chemicals-in-food-contact-paper-and-board (2019).
- 163.Wang Z et al. , We need a global science-policy body on chemicals and waste. Science 371, 774–776 (2021). [DOI] [PubMed] [Google Scholar]