Abstract
Aim/Background:
Domino liver transplantation (DLT) using liver allografts from patients with metabolic disorders enhances organ utilization. Short- and long-term course and outcome of these patients can impact the decision to offer this procedure to patients, especially those with diseases that can potentially be cured with liver transplant. We reviewed the outcomes of DLT from maple syrup urine disease (MSUD) patients in our large academic pediatric and adult transplant program.
Methods:
All patients receiving DLT were analyzed retrospectively with a minimum of one-year follow-up period for patient and donor characteristics, early and late postoperative complications and patient and graft survival with their MSUD donors in terms of age, weight, MELD/PELD scores, cold ischemia time, postoperative leucine levels, and peak ALT (alanine aminotransferase) levels during the first 48 postoperative hours.
Results:
Between 2006 and May 2019, 21 patients underwent domino liver transplantation with live donor allografts from MSUD patients. Four patients transplanted for different metabolic diseases are focus of a separate report. Seventeen patients with minimum one-year follow-up period are reported herein. The indications were primary sclerosing cholangitis (PSC, n = 4), congenital hepatic fibrosis (CHF, n = 2), alpha-1 antitrypsin deficiency (A-1 ATD, n = 2), progressive familial intrahepatic cholestasis (PFIC, n = 2), cystic fibrosis (n = 1), primary biliary cirrhosis (PBC, n = 1), neonatal hepatitis (n = 1), embryonal sarcoma (n = 1), Caroli disease (n = 1), hepatocellular carcinoma (HCC, n = 1), and chronic rejection after liver transplantations for PSC (n = 1). All patients and grafts survived at median follow-up of 6.4 years (range 1.2–12.9 years). Median domino recipient age was 16.2 years (range 0.6–64.6 years) and median MSUD recipient age was 17.6 years (range 4.8–32.1 years). There were no vascular complications during the early postoperative period, one patient had portal vein thrombosis 3 years after DLT and a meso-Rex bypass was successfully performed. Small for size syndrome (SFSS) occurred in reduced left lobe DLT recipient and was managed successfully with conservative management. Biliary stricture developed in 2 patients and was resolved by stenting. Comparison between DLT and MSUD recipients’ peak postoperative ALT results and PELD/MELD scores lower levels in DLT group (P-value <.05).
Conclusions:
Patient and graft survival in DLT from MSUD donors was excellent short- and long-term follow-up. Metabolic functions have been normal in all recipients on a normal unrestricted protein diet. Ischemia preservation injury based on peak ALT was significantly decreased in DLT recipients. Domino transplantation from pediatric and adult recipients with selected metabolic diseases should be increasingly considered as an excellent option and alternative to deceased donor transplantation, thereby expanding the living donor pool. This, to date, is the largest world experience in DLT utilizing livers from patients with MSUD.
Keywords: live donor liver transplantation, maple syrup urine disease, metabolic liver disease
1 |. INTRODUCTION
There is a perpetual discrepancy between candidates awaiting liver transplantation and the number of available donors. Established options to augment the donor pool include split liver transplant, living donor transplant, extended criteria donors, donation after cardiac death, and hepatocyte transplantation in addition to standard deceased donor transplantation.
Rare genetic metabolic disorders with liver involvement may manifest with liver parenchymal damage and hepatic dysfunction or structurally and functionally normal liver. Domino liver transplantation using allografts with metabolic disorders was first performed and reported by Furtado et al in 1995.1 Patients with familial amyloidotic polyneuropathy (FAP) received allografts from deceased donors and their explanted livers were transplanted to patients with liver cancer. Many centers undertook DLT trials with livers from patients with different metabolic diseases. Unfortunately, primary donor disease occurrence has been reported from several types of domino livers including FAP. Consequently, these organs are transplanted for select indications, mostly in adult recipients, as marginal grafts.2–12 Recent reports with long-term follow-up have demonstrated long-term safety of DLT from patients with MSUD especially in the pediatric recipients of domino livers.13–19
Maple syrup urine disease (MSUD) is an organic acidemia caused by deficiency of branched-chain keto-acid dehydrogenase enzyme complex (BCKDH). Accumulation of branched-chain amino acids (leucine, isoleucine, and valine) leads to episodes of life-threatening ketoacidosis and neurotoxicity. Even with appropriate diet and medical treatment, brain damage, chronic psychological burden in older patients and death may occur. Orthotopic liver transplantation (OLT) for MSUD patients offers enzymatic reprieve with improvement in cognitive functions without neuropsychiatric deterioration on unrestricted diet. BCKDH has activity in whole body, mainly in skeletal muscle (60%), liver (9%−13%), brain and kidney. S These characteristics support MSUD livers as a good source for DLT.20–28 Contrary to series reporting DLT from patients with other metabolic diseases, there has been no report to date, of de novo disease appearance in DLT patients from MSUD donors.13–16
Domino liver transplantation using allografts with metabolic disorders enhances organ utilization but is not well described in children. Documentation of short- and long-term outcomes of these patients is critical to decision regarding safety of this procedure. We reviewed the outcomes of DLT using MSUD livers at our pediatric and adult transplant program. We present the largest series to date of DLT using MSUD livers with the longest follow-up period.
2 |. METHODS
All primary MSUD recipients and their paired donors with end-stage liver disease receiving DLT at Hillman Center for Pediatric Transplantation, Children’s Hospital of Pittsburgh of UPMC and Thomas E. Starzl Transplantation Institute, Division of Transplantation, Department of Surgery at UPMC, Pittsburgh, Pennsylvania, United States were analyzed retrospectively with minimum 1-year follow-up period for patient and donor characteristics, early and late postoperative complications and patient and graft survival. Age, weight, PELD/MELD scores, cold ischemia time, mean postoperative leucine levels, and peak ALT levels during postoperative 48 hours were compared between primary MSUD recipient and domino recipient pairs. Plasma amino acid levels were checked pre-operatively, at first week, first month, first year, and annually thereafter for DLT recipients. Statistical analysis was performed with SPSS, version 25.0 for Windows software (Statistical Package for Social Sciences, SPSS, Inc) for descriptive statistics of the groups and comparison of the groups by independent-samples t test and chi-squared test. P-value <.05 was considered as statistically significant. This study was approved by the institutional review board of the University of Pittsburgh.
2.1 |. Domino liver candidacy
All potential MSUD recipients can be considered as domino donors provided they have normal liver function tests and are not steatotic as evidenced by laboratory tests and, in selected cases, imaging studies. We do not do routine biopsies on MSUD donors. Both MSUD and DLT recipients and/or their guardians are informed about the procedure details and outcomes, and their consents obtained. The domino donors are investigated and prepared according to United Network for Organ Sharing guidelines (https://optn.transplant.hrsa.gov/media/1200/optn_policies.pdf).
Informed consent is obtained confirming that the donor is willing to donate, is free from inducement and coercion, and has been informed on treatment options that would not involve organ donation. Confidentiality is assured. All prospective domino donors are discussed at the multidisciplinary liver transplant selection committee, and consensus is obtained about their liver transplant candidacy and their suitability to serve as living liver domino donors.
ABO group identical or compatible donor-recipient matching is confirmed, and the patient is registered as a live donor in the United Network for Organ Sharing (UNOS) system. This allows for mandatory UNOS registration and the liver is then allocated to an appropriate recipient based on size, blood type, and urgency.29 If a deceased donor liver was offered, it was accepted for the matched patient, and institutional protocol was initiated to use the domino donor liver for another waitlisted patient who had consented to receive a domino organ. Plasma amino acids on domino recipient and nucleic acid testing on domino donor were ordered on admission.
In the interest of avoiding radiation exposure, our practice is not to image MSUD patients given that they are relatively healthy. Transplantability of the domino liver is based on anatomy and appearance of the liver upon exploration. If, upon exploration, the liver has rounded edges and looks yellow, there is a high chance that it has significant steatosis. In select cases, a liver biopsy is done. Presence of extensive (>30%) steatosis would preclude a domino transplant. We do not do routine liver biopsies on prospective domino donors.
2.2 |. Immunosuppression
Immunosuppression included tacrolimus/prednisolone (Pred) or mycophenolate mofetil (MMF)/tacrolimus/pred. Thymoglobulin was used as induction therapy in selected cases followed by tacrolimus for maintenance immunosuppression. Pre-existing transplant or history of malignancy precluded the use of thymoglobulin.
2.3 |. Operative technique
Three separate teams are involved during DLT. The deceased donor procuring team focusses on safe liver recovery. Upon communication from this team that the liver is acceptable, the MSUD recipient team proceeds with domino donor hepatectomy. To ensure minimal cold ischemia time (CIT) of transplanted organs, MSUD recipient operation starts with the confirmation from the deceased donor procurement team and domino recipient operation starts with the agreement on the quality of the domino graft and suitability of the vasculature at time of removal of the MSUD liver (Figure 1).
FIGURE 1.
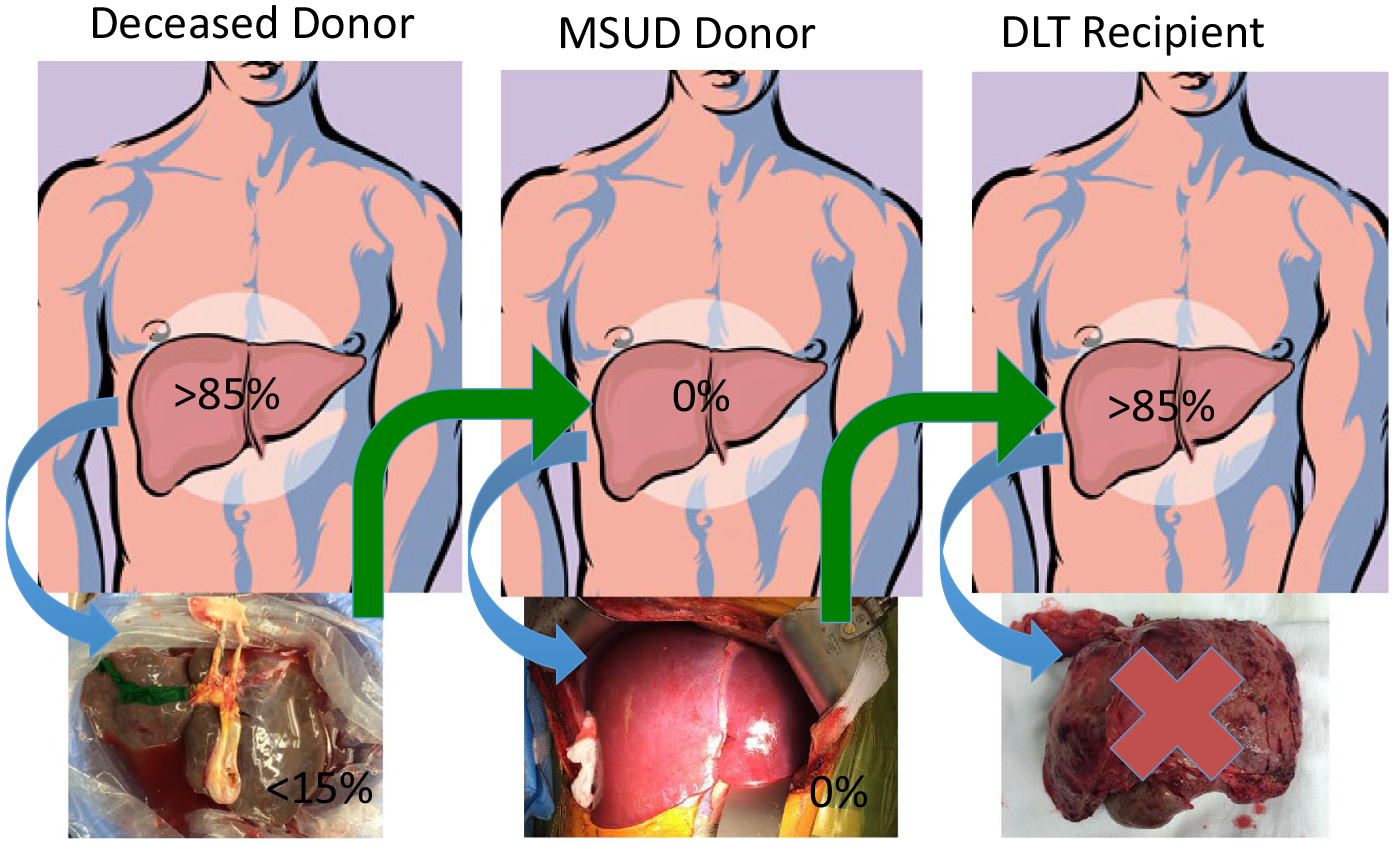
Domino liver transplantation (DLT) involves 3 consecutive but at the same time overlapping operations necessitating 3 separate teams that work on deceased donor, maple syrup urine disease (MSUD) donor and DLT recipient. Explanted liver from deceased donor has approximately 15% normal enzyme activity and is enough for MSUD patient and the explanted enzyme deficient liver from MSUD patient is compensated by approximately 85% normal extrahepatic enzyme activity in domino recipient
2.3.1 |. MSUD donor operation
All primary MSUD recipients have deceased donor allografts procured with standard technique obtaining upper and lower vena cava cuffs. In this series, there were 5 arterial variations in deceased donors, 2 with right hepatic artery (RHA) from superior mesenteric artery (SMA) and left hepatic artery (LHA) from left gastric artery, 2 allografts with LHA branching from left gastric artery and a fourth one with accessory RHA. Arterial reconstructions were done at the back table. All vascular grafts used in both MSUD and DLT recipients were obtained from deceased donors of the pairs. Standard technique was preferred for hepatectomy in MSUD patients (donors) to maximize the venous cuffs when full-size graft is available. Critical steps include:
Isolation of the supra-hepatic and infra-hepatic vena cava for at least 3 cm for proper clamping using the standard technique. Dissection of retro-hepatic space for piggyback technique. Ligation of diaphragmatic veins, opening of diaphragmatic orifice, and dissection of the intra-parenchymal part of hepatic veins ensures effective clamping of the supra-hepatic inferior vena cava and achieves maximum available vein length for anastomosis.
Careful dissection of hepatic artery, portal vein and bile ducts is performed to preserve tissue viability. Gallbladder is flushed with normal saline after division of common bile duct at the level of cystic duct insertion. Dissection of gastroduodenal artery (GDA) and proximal arterial supply to the level of the splenic artery is performed. The hepatic artery is divided at the GDA branching point. The portal vein is sectioned below the bifurcation at mid-portal level. Two patients had accessory LHA with replaced CHA in one of them. Accessory LHA, if found, was ligated after confirmation of a sizeable LHA and normal RHA.
All inflow is preserved until time of removal of graft, and pre-clamp heparinization is done with 75 μ/kg IV bolus.
Hepatectomy is performed either with standard (n = 15) or piggyback technique (n = 2). Livers procured using piggyback technique were reduced and transplanted as left lateral segments (n = 2). The remaining donor hepatectomies were done in the standard fashion. Deceased donor iliac artery was used in 2 patients as a jump graft between common hepatic artery of the donor and infrarenal aorta of the MSUD recipient.
Back table for domino allograft: The liver is flushed homogenously with Histidine-tryptophan-ketoglutarate solution and stored on ice for transplantation. The lower cava is oversewn for piggyback implantation of the standard allografts. We transplanted 2 reduced left lateral segments to a 23 kg, 8-year-old patient from 107 kg,16-year-old MSUD donor and 7 kg, 0.6-year-old patient from 78 kg,19-year-old MSUD donor. Parenchymal dissection for reduced left lobe was performed at the back table, taking meticulous care to ligate the vessels and bile duct branches.
2.3.2 |. Domino liver transplantation recipient operation
The DLT recipient is prepared in the usual way after visual inspection of anatomy of the MSUD liver during hepatectomy. Critical steps include:
Dissection is carried out on the retro-hepatic vena cava with division of short hepatic veins to the level of right, middle, and left hepatic veins. The hepatic venous cuff is created after sufficient dissection around major hepatic veins and vena cava.
The right and left hepatic arteries are ligated and divided. The portal veins are likewise ligated high in the hilum to give maximum length.
The right, middle, and left hepatic veins are clamped and divided, and a common cuff is created. Transplantation progresses in the standard manner. Extension of the upper vena cava cuff and in another patient lower vena cava cuff with deceased donor iliac vein was necessary in two patients to ensure satisfactory anastomosis30 Two patients with embryonal sarcoma and HCC had tumoral mass close to hepatic veins and standard technique was used with upper and lower cava anastomoses. The native portal vein was ligated in one of the CHF cases due to poor portal flow during dissection, and iliac vein jump graft was created between donor portal vein and superior mesenteric vein (SMV) for optimal flow. Iliac and carotid artery grafts were used in 3 patients between donor PHA and recipient CHA. In reduced left lobe graft, arterial anastomosis was performed between donor PHA (accessory LHA, segment 4 branch, and RHA were ligated) and recipient RHA. In another patient, replaced RHA of allograft was anastomosed to GDA at the back table, and CHA of that donor was anastomosed to RHA of the recipient.
2.4 |. Perioperative care
MSUD patients are given MSUD formula and feeding once notification regarding appropriate donor organ is available. They are made NPO status 6 hours prior to expected time of transplant. They are seen by metabolic genetics team, and blood is sent for leucine, isoleucine and valine levels, and serum osmolality. Urine is tested for ketones. Patients are given iv fluids high in dextrose content (D12.5%) Patients who have elevated leucine levels (>800 μmol/L) or presence of urinary ketones are started on branched-chain amino acid-free TPN. Blood glucose and gases are carefully monitored during liver transplantation. Protein restriction is not necessary during the postoperative period, and patients are given amino acids via TPN as for any non-metabolic liver transplant patient. Full amino acid fractionation studies are sent on postoperative day 1 and at discharge. Patients are advanced to full unrestricted diet. During the follow-up clinic visits patients underwent monthly amino acid profiles in the blood for the next 3 months. They were all on unrestricted diet with no clinical or biochemical features of MSUD. Two patients did not have amino acid levels at the time of publication.
3 |. RESULTS
Between 2006 and May 2019, 21 MSUD patients underwent liver transplantation with deceased allografts and the explanted livers were transplanted to 21 recipients as domino allografts. Four of the DLTs performed on patients with other metabolic diseases were excluded from this study since they are discussed in a separate report.31 These included CN-1, propionic acidemia (n = 2), and carbamoyl phosphate synthetase deficiency. Further analysis was performed on 17 pairs of patients with a minimum of 1-year follow-up period. The non-MSUD recipient indications were PSC (n = 4), CHF (n = 2), A-1 ATD (n = 2), PFIC (n = 2), cystic fibrosis (n = 1), PBC (n = 1), neonatal hepatitis (n = 1), embryonal sarcoma (n = 1), Caroli disease (n = 1), HCC (n = 1), and chronic rejection after liver transplantation for PSC (n = 1).
Median MSUD recipient age was 17.6 years (range 4.8–32.1 years) with 10 female and 7 male patients. All MSUD patients receiving deceased donor liver transplantation were stable on a protein restricted diet. Median DLT recipient age was 16.2 years (range 0.6–64.6 years) with 9 male and 8 female patients. Mean PELD/MELD score at transplantation for MSUD liver (domino) recipient was 24 with a median of 24 and a range of 8–50. Compared to MELD/PELD scores at transplant for MSUD donor this was statistically significant (P = .009, Table 2).
TABLE 2.
Parameters in domino liver transplantation (DLT) and maple syrup urine disease (MSUD) recipients
| Recipient Demographics and Functional Metrics | MSUD Donor | DLT Recipient | P-value |
|---|---|---|---|
| Mean age at transplantation (y) | 17.5 | 20.4 | .49 |
| Mean weight (kg) | 56.4 | 54.2 | .78 |
| Mean PELD/MELD scores | 37 | 24.1 | .009* |
| Mean donor age (y) | 23.1 | 17.5 | .11 |
| Post-transplant mean cold ischemia time (min) | 348.5 | 288.5 | .77 |
| Post-transplant mean peak ALT in 48 h (IU/L) | 1119.8 | 446.8 | .000* |
| Mean value of post-transplant leucine level (μmol/dL) | 20.3 | 12.9 | .000* |
P-value <.05.
Patient and graft survivals are 100% at current median follow-up of 6.4 years (range 1.2–12.9 years) in both groups. Median hospital stay during transplantation for MSUD patients was 20 days (range 10–39 days) and for DLT patients was 20 days (range 9–37 days).
Four patients had Clavien-Dindo grade III level complication requiring surgical intervention in the immediate postoperative period. These included abdominal paracentesis for ascites in one patient. Small for size syndrome (SFSS) occurred in one of the reduced left lobe DLT recipients. Back-table biopsy of the domino allograft showed only mild (<5%) micro-vesicular steatosis. SFSS was managed successfully by creation of aortic jump graft and splenectomy in the 2nd and 3rd postoperative days (POD), respectively. Partial biliary obstruction occurred in 2 patients on postoperative days 3 and 7, respectively. Revision of hepatico-jejunostomy and endoscopic retrograde cholangiopancreatography (ERCP)/T-tube placement was performed, respectively.
There was no vascular complication in early postoperative period in DLT recipients; one patient who required SMV graft at time of domino transplant had portal vein thrombosis (PVT) 3 years after transplantation and underwent meso-Rex bypass (MRB). The same patient had renal transplantation for calcineurin inhibitor toxicity associated chronic renal insufficiency (CRI) 8 years postliver transplant. Biliary stricture developed in 2 patients during the first year following transplant and was treated by percutaneous trans-hepatic cholangiography (PTC)/stenting-balloon dilatation. One of these patients developed recurrent cholangitis and recurrence of PSC in the third postoperative year. Acute cellular rejection (ACR) was the most common complication in both groups. One patient with alpha 1 antitrypsin deficiency and steroid only induction due to sepsis prior to transplantation had early ACR starting POD 26 and was successfully treated with solumedrol and thymoglobulin. Epstein-Barr Virus (EBV) positive post-transplant lymphoproliferative disorder (PTLD) occurred in this patient and was treated successfully with 4 doses of rituximab. The details of DLT recipients with main characteristics and their follow-up details are outlined in the Table 1.
TABLE 1.
The domino liver transplantation (DLT) recipients with main characteristics and post-transplantation complications
| Patient # | MSUD donor age (y)/weight (kg) | DLT recipient age (y)/weight (kg) | DLT recipient diagnosis/PELD-MELD score | Post-transplant early complications (<3 mo) | Post-transplant late complications |
|---|---|---|---|---|---|
| 1 | 32.1/69.5 | 64.6/83.8 | PSC/11 | Ascites-paracentesis (POM1) | – |
| 2 | 9.2/38.5 | 24.2/45.6 | PFIC/24 | Partial obstruction of hepatico-jejunostomy, revision (POD3), ACR (POD25) | – |
| 3 | 16.6/83.4 | 18.6/59 | CF/11 | – | CRI (POY1), high LFT-no rejection- no infection (POY2) |
| 4 | 21.9/52.5 | 20.3/90 | CHF/13 | Bleeding from biliary anastomosis- revision (POD1), narrowing of biliary anastomosis-ERCP, T-tube placement (POD7) | PVT-MRB, splenectomy (POY3) Renal transplantation (POY8) |
| 5 | 22.3/62.5 | 51.8/90.9 | PBC/18 | – | – |
| 6 | 4.8/19.5 | 6.9/20 | Embryonal sarcoma/30 | – | – |
| 7 | 11.3/46.4 | 23.8/75.7 | CHF/9, PCKD, kidney transplant 2003, PTLD | – | Ventral hernia repair (POY1), CRI (POY2) |
| 8 | 21.9/56 | 15.9/69.3 | PSC/12, UC | ACR (POD7) | ACR (POM9) Biliary stricture-PTC (POY1) cholangitis episodes (POY2) PSC recurrence, infected bilomastent placement (POY3) |
| 9 | 16.5/107.3 | 8.3/23.8 | A-l ATD/50 | SFSS, no HAT-creation of aortic jump graft (POD 2) SFSS, intra-abdominal bleeding- L/T, splenectomy (POD 3) Intra-abdominal bleeding-L/T, ACR (POD 17) |
– |
| 10 | 8.1/22.7 | 16.2/40.7 | PSC/37 Previous liver transplant |
Bile leak-L/T (POD2) Acute cholangitis (POM1.5) | Acute cholangitis (POM3.5) (Normal MRCP) ACR (POM16: 19) |
| 11 | 16.8/55.4 | 24.9/53.3 | PFIC/8 Previous liver transplant |
– | ACR (POM3.5:8.5:15), clindamycin induced esophagitis (POM4) |
| 12 | 19.8/59.9 | 15.5/58.6 | PSC/40 | ACR (POD 28) | ACR (POM 6) |
| 13 | 9.3/29.4 | 8.8/37 | Caroli disease/40 | – | – |
| 14 | 29.9/41.4 | 13.5/58.2 | Neonatal hepatitis/15 | ACR (POD 10, POM1), acute cholangitis (POD18), non-compliance, acute kidney injury, hypertension | Hypertension |
| 15 | 19/78.7 | 0.6/7.3 | A-l AT D/29 | ACR (POD26) | ACR (POM4:5:9:15), severe non-alcoholic fatty liver (POM4), PTLD (POM4), biliary stricture (POM 14) |
| 16 | 17/57 | 16.2/40.8 | HCC/30 | ACR (POD9) | ACR (POM6:14), non-compliance |
| 17 | 21.1/80 | 16.2/68.3 | PSC/34 | ACR (POD14, POM2) | – |
Abbreviations: ACR, acute cellular rejection; L/T, laparotomy; LFT, liver function tests; MRCP, magnetic resonance cholangiopancreatography; MSUD, maple syrup urine disease; POD, postoperative day; POM, postoperative month; PTLD, post-transplant lymphoproliferative disorder; UC, ulcerative colitis.
Hepatectomy in MSUD recipients was performed according to standard technique (n = 15) or piggyback (n = 2). The latter two grafts were reduced for left lobe transplant. All MSUD patients received whole livers except one 9-year-old (29 Kg) from a 14-year-old donor (70 kg). This recipient received a reduced sized graft. The median deceased donor age was 21 years (range 3–39 years). There were no postoperative complications related to portal or biliary system. One patient had intra-operative HAT and had deceased donor iliac artery jump graft to infrarenal aorta for reconstruction. The same patient had biliary stricture at POM3 and stricture resolved in 7 months with ERCP, biliary dilation, and biliary stenting. Four patients had arterial reconstruction. Accessory right and left hepatic arteries of deceased donors were sewn into splenic orifices at the back table in 3 patients, and donor iliac artery graft was used in another patient due to poor flow on the first postoperative day. There was one partial left HAT in a patient with both donor and recipient replaced arterial flow (replaced CHA from SMA and LHA from LGA of donor- total arterial replacement from SMA of recipient) on the fourth postoperative day. Infrarenal aortic interposition graft was created by using 2 carotid arteries of the deceased donor.
The DLT recipients did not have any abnormal amino acid levels during the postoperative period. The MSUD patients had progressive decrease in their branched-chain amino acid levels and none of them experienced metabolic crisis (Figure 2A–C). PELD/MELD scores, postoperative mean leucine levels, and peak ALT levels within first 48 hours in the two patient groups were different with statistical significance (P-value = .000 for all). PELD/MELD scores, post-transplant leucine, peak ALT levels, and CIT were lower in DLT recipient group (Table 2).
FIGURE 2.
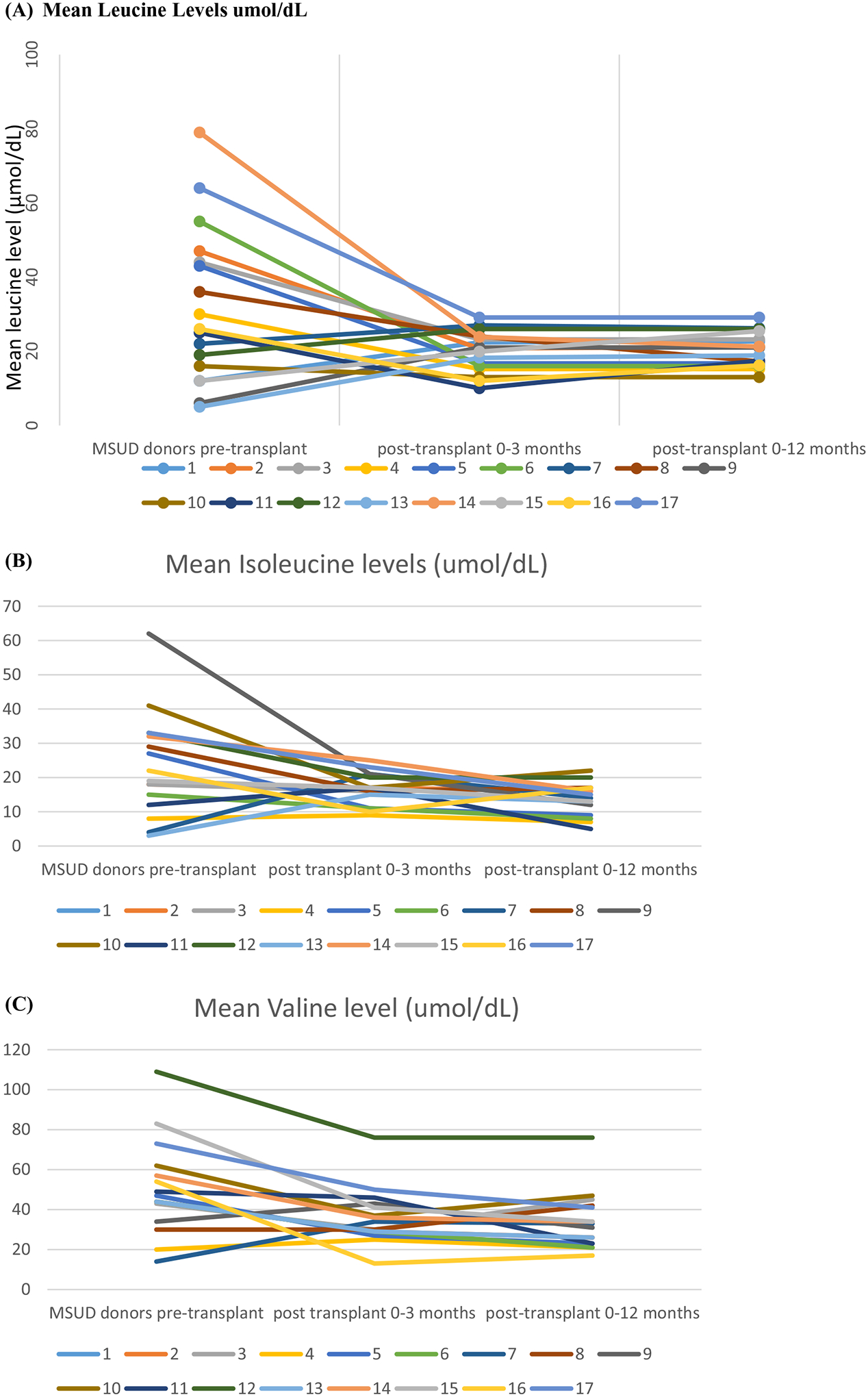
A-C, Pre-transplantation plasma leucine, isoleucine and valine levels of maple syrup urine disease donors decreased to normal or near-normal values during post-liver transplantation period (Normal ranges: leucine 10–20 μmol/dL, isoleucine 3–10 μmol/dL and valine 7–35 μmol/dL).
4 |. DISCUSSION
The first domino organ transplantation was performed as heart transplantation in 1980s by Yacoub et al to expand the donor pool.32 Removal of heart and lungs in 20 cystic fibrosis patients for combined technique allowed transplantation of CF hearts as domino allografts.28 Furtado et al performed first DLT by using FAP donors for liver cancer patients with short life expectancy.1 Tzakis et al transplanted the liver of a neurogenic intestinal pseudo-obstruction patient who was undergoing a multivisceral organ transplantation for an acute graft failure case due to HAT.33 Since then between 1998 and December 31, 2017 the Domino Liver Transplant Registry (DLTR) reported 1254 cases from 66 centers in 21 countries. The median DLT recipient age was 57.1 years (range 0.3–73.9 years) with the diagnoses of primary hepatic malignancy (n = 519), alcoholic cirrhosis (n = 238), cirrhosis secondary to hepatitis B and C (n = 214), re-transplantation (n = 66), metastatic hepatic malignancy (n = 29), and other miscellaneous diseases (n = 173). Domino livers were mostly from FAP patients. Other domino donors had fibrinogen alpha-chain amyloidosis (FACA), hyper-oxalosis, MSUD, hypercholesterolemia, and hemochromatosis. Major causes of patient losses were tumor recurrence (21%) and septicemia (15%).34 Occurrence of primary disease in both early and late period has been reported in the literature with inadvertent use of allografts from hereditary hemochromatosis and DLT cases from FAP, primary hyperoxaluria, acute intermittent porphyria (AIP), and familial homozygous hypercholesterolemia donors.2–12,35 Disease occurrence is seen in patients in whom liver is the main source of the production of abnormal metabolites in these metabolic disorders. Ethical concerns for justification of performing DLT from these patients can justify giving these livers to patients with terminal diseases like hepatocellular cancer or cholangiocarcinoma. Re-transplantation with a normal graft in case of 5-year primary disease-free survival following a FAP-DLT has been reported.12
However, there has been no report of de novo disease appearance in DLT patients with MSUD livers since liver is only responsible for approximately 9%−13% of BCKDH activity in whole body. Our results have established that MSUD patients can be safe liver donors for candidates with long-term life expectancy rather than being marginal donors for recipients with limited life expectancy or serve as a bridge grafts for a future normal allograft.13–17,36 Pediatric candidates are well suited for this option since DLT serves as an excellent alternative for deceased or live donor allografts, given the difficulty of finding a size-matched organ in this patient population. Table 4 outlines some of the metabolic diseases benefitting from DLT. Current literature supports DLT from donors with MSUD and in select instances methymalonic acidemia as conditions that DLT may be performed without risking development of clinical disease in the recipient.
TABLE 4.
Reports of domino liver transplantation from patients with metabolic disorders
| Authors | Reference | Domino donor diagnosis | Domino recipient diagnosis | Number of domino liver recipients | Domino recipient developed donor disease |
|---|---|---|---|---|---|
| Celik, et al | JIMD Rep. 2019 Jun 19;48(1):83–89 | MSUD | Propionic Acidemia, Crigler-Najjar Syndrome, Carbamoyl phosphate synthetase deficiency | 4 | No |
| Qu, et al | Clin Res Hepatol Gastroenterol. 2019 Mar 7. pii: S2210–7401(19)30039–7 | Hyperhomocysteinemia | Cholangiocarcinoma | 1 | Yes |
| Herden U, et al | Liver Transpl. 2019 Jun;25(6):889–900 | MSUD | Various diagnoses | 15 | No |
| Vollmar, et al | Transpl Int. 2018 Nov;31(11):1207–1215 | Amyloidosis | Various diagnoses | 23 | Yes |
| Karina, et al | Transplantation. 2019 Mar;103(3):536–543 | MSUD | Various diagnoses | 11 | No |
| Golbus JR, et al | J Clin Lipidol. 2017 Sep - Oct;11(5):1284–1288 | Familial hypercholesterolemia | Primary Sclerosing Cholangitis | 1 | Yes |
| Matsunami M, et al | Pediatr Transplant. 2015 May;19(3):E70–4 | MSUD | Protein C deficiency | 1 | No |
| Khanna, et al | JIMD Rep. 2016;25:87–94. Epub 2015 Jul 29 | Methyl malonic acidemia | Primary Sclerosing Cholangitis | 1 | No |
Note: Literature review of domino liver transplantation (DLT) from donors with metabolic diseases. Note that only recipients of domino livers from patients with maple syrup urine disease (MSUD) and methylmalonic acidemia did not develop the disease.
Herein, we report a series with 100% patient and graft survival without any metabolic derangement following DLT using MSUD donors during the short- and long-term follow-up period. We used two reduced grafts and the whole livers were transplanted successfully in other patients. DLT from methyl malonic acidemia donor was first reported by the senior author in 2015.18 Despite having normal liver and renal function, elevated plasma and urine levels of methyl malonate were observed, and long-term results and outcome to see the effect of high unwanted metabolites are pending. The literature and our series showed no abnormal plasma amino acid profile and metabolic decompensation following transplantation confirming sufficient extrahepatic BCKDH activity on normal unrestricted protein intake. The experience with of DLT from MSUD donors is summarized in Table 3.13–16,31
TABLE 3.
Literature review of domino liver transplantation (DLT) using maple syrup urine disease (MSUD) livers
| Patient number | MSUD donor age (y) | DLT recipient age (y) | DLT recipient diagnosis | Follow-up period (mo) | Outcome under protein-free diet | |
|---|---|---|---|---|---|---|
| Khanna, et al14 | 1 | 25 | 51 | HCV, Hepatocellular carcinoma | 7 | Normal leucine No disease symptom Normal graft function |
| Badell, et al37 | 1 | 24 | 52 | Factor-8 deficiency, HIV, HCV | 30 | |
| Feier, et al16 | 1 (received LDLT) |
2 | 2.75 | BA | 13 | |
| Mohan, et al17 | 2 (both received LDLT) |
1.8 3.2 |
2 1.2 |
LCH-sclerosing cholangitis BA | 40–75 | |
| Matsunami, et al13 | 3 (all received LDLT) |
1 3.6 1.2 |
1.9 2.3 2.8 |
Protein C deficiency BA PHC |
8–16 | |
| Herden, et al20 | 14 (8 patients in detail, 1 patient loss with recurrent HCC) | - | 0.3–62.3 | PSC, BA, HCC, unclear cirrhosis, urea cycle disorder, glycogen storage disease type-1 | 2–31 |
There has been no report of difference or disparity in metabolic functions or outcomes of DLT with whole liver and liver segments. We had small for size syndrome in one of our DLT recipients of reduced left lobe but there was no problem related to plasma BCAA levels even during periods of decompensation.13–16
The technical aspects of DLT are reported in a large adult series and in limited pediatric cases with both deceased donor and LDLT confirming feasibility and safety of the procedure without additional risks for donors or recipients. Both standard and piggyback techniques were successfully performed with some modifications during hepatectomy phase of the domino donors ensuring optimum length of supra-hepatic vena cava or hepatic veins. Vascular reconstructions have not caused technical problems especially with deceased donor DLT pairs since there are multiple vascular graft options to use in case of need for both recipients. Bispo et al analyzed early graft function and perioperative bleeding by comparing DLT recipients from FAP and recipients of deceased donors in adult population. They reported younger donor age, shorter CIT, less intra-operative transfusion requirement, less graft dysfunction, and less postoperative bleeding in DLT group. In our study, lower CIT and peak ALT levels were observed with good graft function and minimal ischemia preservation injury in keeping with published reports.13–16,37–39 Biliary reconstruction was performed in two of the 21 recipients of DLT. Although, a small series, this is within acceptable rate of biliary complications seen in liver transplantation. Development of SFSS was promptly identified and addressed with infrarenal aortic graft leading to a good outcome. Mean MELD/PELD at transplantation for domino donors and recipients24 achieved statistical significance (P < .009; Table 2). This is due to the UNOS exception status granted to patients with metabolic diseases and relatively low biological MELD/PELD scores of recipients of domino liver transplants. The latter group are advantaged by early transplantation thus leading to lower waiting list morbidity and mortality.
Mean peak ALT levels were significantly lower in DLT recipients compared to MSUD recipients of deceased donor transplantation (P = .000). This is related to the short cold ischemia time seen in live donor (MSUD recipient) transplantation.
Herden et al have recently reported a multicenter experience with DLT from MSUD livers in 15 donor-recipient pairs with good results.20 Our series of 17 patients reported herein and 4 reported elsewhere31 is to date, the largest reported single-center experience of DLT using MSUD livers.
Domino liver transplantation has also been performed between metabolic diseases with two different enzymatic deficiencies involving separate metabolic pathways or if there was production of unwanted metabolites. Matsunami et al and Badell et al13,37 reported successful results and in our experience one PA, two CN-1 and one carbamoyl phosphate synthatase deficiency patient received MSUD livers with metabolically normal post-transplantation follow-up period. Rare cases using APOLT (auxiliary partial orthotopic liver transplantation) technique have been reported as well.38–40 Govil et al have discussed the idea of swap domino auxiliary liver transplantation for liver-based metabolic disorder pairs to perform donor-less transplantation.38 Based on the reports to date, the only metabolic diseases from which a DLT does not result in clinical disease in the recipient are MSUD and methylmalonic acidemia (Table 4).
All of our candidates underwent a formal psychological and social evaluation prior to liver transplantation. Although we did not perform a formal neurocognitive evaluation, patients were seen for regular follow-up visits. None of the domino donors or recipients developed any neuropsychiatric problems during their entire follow-up period.
Successful DLT requires technical expertise, organized team work, multidisciplinary management including detailed metabolic evaluation, care, and follow-up. There are many variations of follow-up protocols and are center-specific. Our experience has demonstrated safety of this procedure over long-term follow-up, even in pediatric recipients. Development of a standardized protocol for follow-up and management of these patients is the key to success.
5 |. CONCLUSION
Patient and graft survivals in DLT from MSUD donors in our series are 100% at current median follow-up of 6.4 years (range 1.2–12.9 years) in both groups. Metabolic function has been normal in all recipients on normal unrestricted protein diet following DLT. Ischemia preservation injury based on peak ALT levels is significantly decreased in DLT recipients.
Domino transplantation from pediatric and adult recipients with selected metabolic diseases should be increasingly considered and adopted by transplant programs worldwide to circumvent organ shortage and as another resource of living donor liver transplantation. The success of DLT is due to surgical expertise, coordinated multidisciplinary teamwork, and a well-organized transplant program. To date, we have not observed any instances of metabolic decompensation in recipients of domino livers from MSUD donors and believe that this strategy can be safely applied even for pediatric recipients who have a longer projected post-transplant life span.
Footnotes
CONFLICT OF INTEREST
None declared.
REFERENCES
- 1.Furtado A, Tomé L, Oliveira FJ, Furtado E, Viana J, Perdigoto R. Sequential liver transplantation. Transplant Proc. 1997;29(1–2):467–468. [DOI] [PubMed] [Google Scholar]
- 2.Ando Y, Ericzon BG, Suhr OB, Tashima K, Ando M. Reuse of a Japanese familial amyloidotic polyneuropathy patient’s liver for a cancer patient: the domino liver transplantation procedure. Intern Med. 1997;36(11):847. [DOI] [PubMed] [Google Scholar]
- 3.Stangou AJ, Heaton ND, Hawkins PN. Transmission of systemic transthyretin amyloidosis by means of domino liver transplantation. N Engl J Med. 2005;352(22):2356. [DOI] [PubMed] [Google Scholar]
- 4.Schielke A, Conti F, Goumard C, Perdigao F, Calmus Y, Scatton O. Liver transplantation using grafts with rare metabolic disorders. Dig Liver Dis. 2015;47(4):261–270. [DOI] [PubMed] [Google Scholar]
- 5.Popescu I, Simionescu M, Tulbure D, et al. Homozygous familial hypercholesterolemia: specific indication for domino liver transplantation. Transplantation. 2003;76(9):1345–1350. [DOI] [PubMed] [Google Scholar]
- 6.Popescu I, Dima SO. Domino liver transplantation: how far can we push the paradigm? Liver Transpl. 2012;18(1):22–28. [DOI] [PubMed] [Google Scholar]
- 7.Liu C, Niu DM, Loong CC, et al. Domino liver graft from a patient with homozygous familial hypercholesterolemia. Pediatr Transplant. 2010;14(3):E30–E33. [DOI] [PubMed] [Google Scholar]
- 8.Franchello A, Paraluppi G, Romagnoli R, et al. Severe course of primary hyperoxaluria and renal failure after domino hepatic transplantation. Am J Transplant. 2005;5(9):2324–2327. [DOI] [PubMed] [Google Scholar]
- 9.Popescu I, Habib N, Dima S, et al. Domino liver transplantation using a graft from a donor with familial hypercholesterolemia: seven-yr follow-up. Clin Transplant. 2009;23(4):565–570. [DOI] [PubMed] [Google Scholar]
- 10.Dowman JK, Gunson BK, Bramhall S, Badminton MN, Newsome PN. Liver transplantation from donors with acute intermittent porphyria. Ann Intern Med. 2011;154(8):571–572. [DOI] [PubMed] [Google Scholar]
- 11.Donckier V, El Nakadi I, Closset J, et al. Domino hepatic transplantation using the liver from a patient with primary hyperoxaluria. Transplantation. 2001;71(9):1346–1348. [DOI] [PubMed] [Google Scholar]
- 12.Azoulay D, Samuel D, Castaing D, et al. Domino liver transplants for metabolic disorders: experience with familial amyloidotic polyneuropathy. J Am Coll Surg. 1999;189(6):584–593. [DOI] [PubMed] [Google Scholar]
- 13.Matsunami M, Fukuda A, Sasaki K, et al. Living donor domino liver transplantation using a maple syrup urine disease donor: a case series of three children - the first report from Japan. Pediatr Transplant. 2016;20(5):633–639. [DOI] [PubMed] [Google Scholar]
- 14.Khanna A, Hart M, Nyhan WL, Hassanein T, Panyard-Davis J, Barshop BA. Domino liver transplantation in maple syrup urine disease. Liver Transpl. 2006;12(5):876–882. [DOI] [PubMed] [Google Scholar]
- 15.Barshop BA, Khanna A. Domino hepatic transplantation in maple syrup urine disease. N Engl J Med. 2005;353(22):2410–2411. [DOI] [PubMed] [Google Scholar]
- 16.Feier FH, Miura IK, Fonseca EA, et al. Successful domino liver transplantation in maple syrup urine disease using a related living donor. Braz J Med Biol Res. 2014;47(6):522–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mohan N, Karkra S, Rastogi A, Vohra V, Soin AS. Living donor liver transplantation in maple syrup urine disease - case series and world’s youngest domino liver donor and recipient. Pediatr Transplant. 2016;20(3):395–400. [DOI] [PubMed] [Google Scholar]
- 18.Khanna A, Gish R, Winter SC, Nyhan WL, Barshop BA. Successful domino liver transplantation from a patient with methylmalonic acidemia. JIMD Rep. 2015;25:87–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stangou AJ, Banner NR, Hendry BM, et al. Hereditary fibrinogen alpha-chain amyloidosis: phenotypic characterization of a systemic disease and the role of liver transplantation. Blood. 2010;115(15):2998–3007. [DOI] [PubMed] [Google Scholar]
- 20.Herden U, Grabhorn E, Santer R, et al. Surgical aspects of liver transplantation and domino liver transplantation in maple syrup urine disease: analysis of 15 donor-recipient pairs. Liver Transpl. 2019;25(6):889–900. [DOI] [PubMed] [Google Scholar]
- 21.Mazariegos GV, Morton DH, Sindhi R, et al. Liver transplantation for classical maple syrup urine disease: long-term follow-up in 37 patients and comparative United Network for Organ Sharing experience. J Pediatr. 2012;160(1):116–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Strauss KA, Wardley B, Robinson D, et al. Classical maple syrup urine disease and brain development: principles of management and formula design. Mol Genet Metab. 2010;99(4):333–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fagiuoli S, Daina E, D’Antiga L, Colledan M, Remuzzi G. Monogenic diseases that can be cured by liver transplantation. J Hepatol. 2013;59(3):595–612. [DOI] [PubMed] [Google Scholar]
- 24.Hoffmann B, Helbling C, Schadewaldt P, Wendel U. Impact of longitudinal plasma leucine levels on the intellectual outcome in patients with classic MSUD. Pediatr Res. 2006;59(1):17–20. [DOI] [PubMed] [Google Scholar]
- 25.Simon E, Schwarz M, Wendel U. Social outcome in adults with maple syrup urine disease (MSUD). J Inherit Metab Dis. 2007;30(2):264. [DOI] [PubMed] [Google Scholar]
- 26.Suryawan A, Hawes JW, Harris RA, Shimomura Y, Jenkins AE, Hutson SM. A molecular model of human branched-chain amino acid metabolism. Am J Clin Nutr. 1998;68(1):72–81. [DOI] [PubMed] [Google Scholar]
- 27.Thompson GN, Bresson JL, Pacy PJ, et al. Protein and leucine metabolism in maple syrup urine disease. Am J Physiol. 1990;258(4 Pt 1):E654–E660. [DOI] [PubMed] [Google Scholar]
- 28.Wendel U, Saudubray JM, Bodner A, Schadewaldt P. Liver transplantation in maple syrup urine disease. Eur J Pediatr. 1999;158(suppl 2):S60–S64. [DOI] [PubMed] [Google Scholar]
- 29.https://optn.transplant.hrsa.gov/media/1200/optn_policies.pdf#nameddest=Policy_09. Accessed 5/28/19.
- 30.Pacheco-Moreira LF, de Oliveira ME, Balbi E, et al. A new technical option for domino liver transplantation. Liver Transplant. 2003;9(6):632–633. [DOI] [PubMed] [Google Scholar]
- 31.Celik N, Squires JE, Soltys K, et al. Domino liver transplantation for select metabolic disorders: expanding the living donor pool. JIMD Rep. 2019;48(1):83–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yacoub MH, Banner NR, Khaghani A, et al. Heart-lung transplantation for cystic fibrosis and subsequent domino heart transplantation. J Heart Transplant. 1990;9(5):459–466; discussion 466–7. [PubMed] [Google Scholar]
- 33.Tzakis AG, Nery JR, Raskin JB, et al. ‘Domino’ liver transplantation combined with multivisceral transplantation. Arch Surg. 1997;132(10):1145–1147. [DOI] [PubMed] [Google Scholar]
- 34.http://www.fapwtr.org/ram_domino.htm Accessed 3/20/19.
- 35.Dwyer JP, Sarwar S, Egan B, Nolan N, Hegarty J. Hepatic iron overload following liver transplantation of a C282y homozygous allograft: a case report and literature review. Liver Int. 2011;31(10):1589–1592. [DOI] [PubMed] [Google Scholar]
- 36.Casas-Melley AT, Thomas PG, Krueger LJ, et al. Domino as a bridge to definitive liver transplantation in a neonate. Pediatr Transplant. 2002;6(3):249–254. [DOI] [PubMed] [Google Scholar]
- 37.Badell IR, Hanish SI, Hughes CB, et al. Domino liver transplantation in maple syrup urine disease: a case report and review of the literature. Transplant Proc. 2013;45(2):806–809. [DOI] [PubMed] [Google Scholar]
- 38.Govil S, Shanmugam NP, Reddy MS, Narasimhan G, Rela M. A metabolic chimera: two defective genotypes make a normal phenotype. Liver Transpl. 2015;21(11):1453–1454. [DOI] [PubMed] [Google Scholar]
- 39.Hashikura Y, Ikegami T, Nakazawa Y, et al. Delayed domino liver transplantation: use of the remnant liver of a recipient of a temporary auxiliary orthotopic liver transplant as a liver graft for another patient. Transplantation. 2004;77(2):324. [DOI] [PubMed] [Google Scholar]
- 40.D’Antiga L, Colledan M. Surgical gene therapy by domino auxiliary liver transplantation. Liver Transpl. 2015;21(11):1338–1339. [DOI] [PubMed] [Google Scholar]


