Abstract
Moxifloxacin (BAY12-8039) is a new 8-methoxyquinolone shown to be active against Mycobacterium tuberculosis in vitro. We tested moxifloxacin for activity in mice against M. tuberculosis CSU93, a highly virulent, recently isolated clinical strain. The MIC of moxifloxacin for the CSU93 strain was 0.25 μg/ml. The serum moxifloxacin concentration after oral administration in mice peaked within 0.25 h, reaching 7.8 μg/ml with doses of 100 mg/kg of body weight; the maximum concentration and the analysis of the area under the concentration-time curve revealed dose dependency. When mice were infected with a sublethal inoculum of mycobacteria and then treated with moxifloxacin at 100 mg/kg per day for 8 weeks, the log10 CFU counts in the organs of treated mice were significantly lower than those for the control group (0.6 ± 0.2 versus 5.6 ± 0.3 in the lungs and 1.5 ± 0.7 versus 4.9 ± 0.5 in the spleens, respectively; P < 0.001 in both organs). The effectiveness of moxifloxacin monotherapy was comparable to that seen in mice receiving isoniazid alone. Combination therapy with moxifloxacin plus isoniazid was superior to that with moxifloxacin or with isoniazid alone in reducing bacillary counts in the organs studied. Using a sensitive broth-passage subculture method, we demonstrated that 8 weeks of treatment with moxifloxacin (100 mg/kg per day) or with moxifloxacin plus isoniazid (100 mg/kg and 25 mg/kg, respectively, per day) sterilized the lungs in seven of eight and in eight of eight mice, respectively. Among surviving bacilli isolated from animals infected with a high-titer inoculum and treated for 7 weeks with low-dose moxifloxacin (20 mg/kg per day), breakthrough resistance to moxifloxacin was not observed. These results indicate that moxifloxacin is highly effective in reducing M. tuberculosis infection in mice and has activity comparable to that of isoniazid. Combination therapy with moxifloxacin and isoniazid was highly effective, suggesting that moxifloxacin may be useful in multiple-drug regimens for human tuberculosis.
Tuberculosis remains the leading cause of death worldwide from any single infectious agent. The resurgence of tuberculosis in the United States from 1985 to 1992 was accompanied by an increase in the prevalence of resistance to first-line antimycobacterial agents, including isoniazid (INH) and rifampin (5, 6, 19). A survey conducted in New York, N.Y., in 1992 showed that 33% of culture-positive patients had Mycobacterium tuberculosis isolates resistant to one or more antituberculous drugs and 19% had multiple-drug-resistant tuberculosis isolates that were resistant to both INH and rifampin (5). A 35-nation study of the global incidence of drug-resistant tuberculosis found single-drug resistance rates of 36% and multiple-drug resistance rates of 13% among previously treated patients (16). Serious difficulty in controlling infections caused by drug-resistant tuberculosis has further increased demand for potent new drugs to counter M. tuberculosis.
Several fluoroquinolones, such as levofloxacin, ofloxacin, and ciprofloxacin, exhibit MICs of about 1 μg/ml for M. tuberculosis and can attain concentrations in serum that inhibit M. tuberculosis (8, 9, 10, 18, 23). Clinical reports have demonstrated efficacy of ofloxacin and ciprofloxacin against pulmonary tuberculosis, including disease caused by drug-resistant tubercle bacilli, and the use of these agents as tuberculosis chemotherapeutics has increased (10, 11, 18, 24). However, the use of ofloxacin and ciprofloxacin is limited by the development of resistance, which may be related to their inability to achieve concentrations in serum well in excess of the MIC for the organism. In addition, long-acting drugs are important for tuberculosis chemotherapy in view of the importance of directly observed therapy in preventing nonadherence. Hence, the development of long-acting quinolones with improved penetration and antituberculous potency would be valuable for future tuberculosis control efforts.
Moxifloxacin (BAY12-8039) is a new 8-methoxyquinolone with broad-spectrum activity against gram-positive, gram-negative, and anaerobic bacteria. Its serum half-life permits once-daily dosing in humans (1, 2, 4, 7, 20, 21, 26). This drug has more potent in vitro antimicrobial activity than ofloxacin or ciprofloxacin against gram-positive aerobes, including streptococci and staphylococci (4), and also against anaerobes, such as Bacteroides fragilis and Peptostreptococcus and Clostridium spp. (1). Moxifloxacin also has in vitro activity comparable to that of rifampin (MIC of ∼0.5 μg/ml) against three strains of M. tuberculosis that are resistant to one or more of the commonly used antimycobacterial agents and against a fourth, fully susceptible strain (26). In this study, we tested moxifloxacin for activity against M. tuberculosis in a murine tuberculosis model using a virulent clinical isolate (25). We also evaluated its pharmacokinetic properties in mice to determine the effectiveness of a daily dosing regimen in this animal model.
MATERIALS AND METHODS
Antibiotics.
Moxifloxacin (BAY12-8039) was provided by the Bayer Corporation (West Haven, Conn.). Carbenicillin, polymyxin B, trimethoprim, and INH were purchased from Sigma Chemical Co. (St. Louis, Mo.), and amphotericin B was purchased from GIBCO Laboratories (New York, N.Y.).
Bacterial cultivation.
M. tuberculosis CSU93 was cultivated at 37°C in roller bottles in 7H9-ADC broth (Difco Laboratories, Detroit, Mich.) supplemented with 0.05% Tween 80. For animal inoculation, liquid cultures were declumped by brief bath sonication and settling and then diluted in 7H9-ADC broth. Estimated titers measured by hemacytometer counts accorded well with plating dilutions, though the latter were used as the definitive inoculum titers. Colony counts from mouse organs were performed by using Middlebrook 7H10-ADC agar plates made selective by addition of carbenicillin, polymyxin B, trimethoprim, and amphotericin B to final concentrations of 100 μg/ml, 200 U/ml, 20 μg/ml, and 10 μg/ml, respectively. Mycobacterial susceptibility testing was performed by use of the radiometric BACTEC system (Becton Dickinson, Sparks, Md.) according to standard protocols (17). Susceptibility testing on in vivo-grown M. tuberculosis from drug-treated mice was performed by subculturing lung homogenates in selective Middlebrook 7H9 broth at 37°C for 1 week to obtain more than 105 CFU/ml for BACTEC analysis.
Animal model.
Outbred, female Swiss-Webster mice (5 weeks of age; weight range, 18 to 20 g) were purchased from Harlan Sprague Dawley, Inc. (Indianapolis, Ind.), housed in a pathogen-free, biosafety level 3 environment, and allowed to acclimate to their new environment for at least 2 days prior to infection. Food and water were provided ad libitum. Infections were produced by intravenous tail vein inoculation with 0.1 ml of a suspension containing a declumped, diluted, titered M. tuberculosis preparation. Following infection, mice were randomly divided into groups (eight mice per group); in our previous studies this procedure has provided sufficient statistical power. Treatment was initiated 1 week after inoculation (for experiment 1) or on the day following inoculation (for experiment 2). Drugs were administered by esophageal gavage six times weekly; control mice received sterile distilled water by gavage according to the same schedule. Treatment was continued for 4 or 8 weeks (for experiment 1) or for 3 or 7 weeks (for experiment 2), at which points groups of eight mice were euthanized. Lung and spleen colony counts were determined by homogenizing each organ aseptically in 1.0 ml of 7H9 broth in a Ten Broeck glass grinder. At least four serial 10-fold dilutions of the homogenates were plated onto quadrants of selective 7H10 agar plates, and each dilution was plated in duplicate. Colony counts were recorded after incubation at 37°C in a CO2 incubator for 5 weeks. For organs from which no colonies were obtained by the quantitative plate method, the remaining suspension (0.8 ml) was inoculated into bottles containing 5 ml of selective 7H9 liquid medium. After incubation for 5 weeks, the 7H9 medium was subcultivated onto slants of Löwenstein-Jensen medium, which were incubated for 6 weeks. Organs were considered sterilized if no tubercle bacilli grew during any of these steps.
Pharmacokinetics.
Pairs of mice were bled immediately prior to (t = 0) and at five time points following (t = 15, 30, 60, 120, and 240 min) administration of various doses of moxifloxacin by gavage. Serum was prepared and frozen at −70°C until use. Serum moxifloxacin levels were measured by high-performance liquid chromatography. The concentrations were calculated by using drug standards dissolved in human serum.
Statistical analysis.
Student’s t test was used to compare paired data, and a P value of less than 5% denoted statistical significance. No adjustments were made from multiple comparisons.
RESULTS
In vitro activity of moxifloxacin against M. tuberculosis CSU93.
To determine the MIC of moxifloxacin against M. tuberculosis CSU93, we added moxifloxacin at concentrations ranging from 0.06 to 2.0 μg/ml to BACTEC bottles into which 105 CFU of M. tuberculosis CSU93 was inoculated. The BACTEC bottles were then incubated at 37°C for 4 to 7 days. Data were obtained as a growth index, which is a measure of the 14CO2 liberated by M. tuberculosis during the decarboxylation of 14C-labeled palmitate in the medium and is directly proportional to the amount of active growth in the vial. In vitro-grown M. tuberculosis CSU93 was susceptible to moxifloxacin, and the MIC was found to be 0.25 μg/ml by this method.
In vivo activity of moxifloxacin in mice infected with M. tuberculosis.
Two long-term experimental protocols were evaluated: the first was low-titer infection with high-dose moxifloxacin treatment, and the second was high-titer infection with low-dose moxifloxacin treatment.
(i) Low-titer infection with high-dose moxifloxacin.
In the first experiment, mice were infected with 5.9 × 105 CFU of M. tuberculosis CSU93. One week after inoculation, the infected mice were divided into four groups and treatment was initiated. Groups of 16 mice each were treated with moxifloxacin alone (100 mg/kg of body weight), INH alone (25 mg/kg), a combination of moxifloxacin and INH at the same doses as with the monotherapy, or water (control). Eight mice per group were euthanized at 4 and 8 weeks of treatment.
During the 8-week course of this experiment, the mean body weight of mice in each group increased gradually and no animals, even the control mice that received no treatment, died of tuberculosis. At necropsy, however, nodular lesions measuring 1 to 2 mm in diameter were observed macroscopically in all control mice, whereas in moxifloxacin-treated mice no lung lesions were detected. Histologic examination of lung specimens from control mice revealed severe inflammatory lesions with lymphocytic and monocytic infiltration in regions where numerous acid-fast bacilli were detected by Ziehl-Neelsen staining. In contrast, few acid-fast bacilli and minimal inflammation were detected in specimens from the treatment groups. As shown in Table 1, the mean spleen weights of all treated groups were two- to threefold less than those of the control groups. After 8 weeks of treatment, the mean spleen weights for the groups treated with moxifloxacin monotherapy and moxifloxacin plus INH were significantly lower than those for the mice receiving INH monotherapy or no therapy. Mean spleen weights serve as a sensitive indicator for distinguishing subtle differences in antimycobacterial drug potencies (13). Accordingly, our results suggest a superiority of moxifloxacin over INH after 8 weeks of treatment, whereas INH was more effective in reducing spleen weights after 4 weeks.
TABLE 1.
Spleen weights of mice after treatment with moxifloxacin, INH, or moxifloxacin plus INH following inoculation of M. tuberculosis
| Treatment (daily dose) | Mean spleen weight (mg) of mice after treatment for:a
|
|
|---|---|---|
| 4 weeks | 8 weeks | |
| Control, water | 214 ± 26 | 183 ± 55 |
| INH (25 mg/kg) | 68 ± 14b | 93 ± 10b |
| Moxifloxacin (100 mg/kg) | 84 ± 24b | 71 ± 10bc |
| Moxifloxacin (100 mg/kg) + INH (25 mg/kg) | 71 ± 14b | 70 ± 9bc |
Mice (n = 8 for each treatment at each time) were intravenously inoculated with 5.9 × 105 CFU of M. tuberculosis before treatment. Values shown are the means ± standard deviations.
Significantly lower than the weight in the control group (P < 0.001).
Significantly lower than the weight in the INH group (P < 0.01).
The CFU counts for moxifloxacin-treated mice as well as for the other treated groups showed significant reductions compared to the count for the control group (P < 0.001) at 4 weeks of treatment (Table 2). No significant difference was observed between the CFU counts for mice given monotherapy with moxifloxacin and with INH. However, for reducing bacillary counts the combination of moxifloxacin plus INH was superior to monotherapy with either antimicrobial alone. At 8 weeks after treatment, the log10 CFU counts in the spleens and lungs for the control mice were still high (4.88 ± 0.47 and 5.64 ± 0.31, respectively). In contrast, few colonies appeared on plates of organ homogenates from the groups treated with moxifloxacin, INH, and moxifloxacin plus INH.
TABLE 2.
CFU counts in organ homogenates after treatment with moxifloxacin, INH, or moxifloxacin plus INH following inoculation of M. tuberculosis
| Treatment (daily dose) | CFU counta in homogenates of:
|
|
|---|---|---|
| Lung | Spleen | |
| 4 weeks | ||
| Control, water | 5.64 ± 0.12 | 4.88 ± 0.18 |
| INH (25 mg/kg) | 0.38 ± 0.19b | 1.23 ± 0.18b |
| Moxifloxacin (100 mg/kg) | 0.63 ± 0.22b | 1.51 ± 0.24b |
| Moxifloxacin (100 mg/kg) + INH (25 mg/kg) | 0.17 ± 0.11b | 0.66 ± 0.20bc |
| 8 weeks | ||
| Control, water | 6.45 ± 0.48 | 4.57 ± 0.31 |
| INH (25 mg/kg) | 0.10 ± 0.26b | 0.49 ± 0.47b |
| Moxifloxacin (100 mg/kg) | 0 ± 0bd | 0.74 ± 0.79b |
| Moxifloxacin (100 mg/kg) + INH (25 mg/kg) | 0 ± 0bd | 0 ± 0bd |
Mice (n = 8 for each treatment at each time) were intravenously inoculated with 5.9 × 105 CFU of M. tuberculosis before treatment. The values given are log10 CFU counts (means ± standard deviations).
Significantly lower than the counts in the control group (P < 0.001).
Significantly lower than the counts in the other treatment groups (P < 0.02).
Zero denotes absence of colonies in any organ homogenates (the lower limit of detection is <0.1).
To evaluate whether 8 weeks of moxifloxacin therapy had a sterilizing effect, we used a sensitive process in which homogenates were amplified in liquid culture for 5 weeks prior to inoculation onto Löwenstein-Jensen slants. Using this method, we found that combination therapy with moxifloxacin plus INH eradicated M. tuberculosis from the lungs and spleens in eight of eight and six of eight mice, respectively. Moxifloxacin alone eradicated the bacteria from the lungs and spleens in seven of eight and three of eight mice, respectively, whereas, in the INH-treated group two of seven and three of seven mice had M. tuberculosis-free cultures of the lungs and spleens, respectively. Hence, moxifloxacin plus INH combination therapy appeared to be better than moxifloxacin or INH alone in sterilizing these organs, although this difference was not statistically significant.
(ii) High-titer infection with low-dose moxifloxacin.
In the second experiment, we challenged mice with a larger inoculum of the M. tuberculosis CSU93 strain and treated them with a lower dose of moxifloxacin. Mice received 1.0 × 107 CFU of M. tuberculosis; on the day following infection they were randomly divided into three groups of 16 mice and started on daily treatment with moxifloxacin (20 mg/kg), moxifloxacin (20 mg/kg) plus INH (25 mg/kg), or water (control). After treatment for 3 weeks, eight mice from each group were euthanized for measurement of CFU counts in the spleens and lungs and for pathological assessment. The remaining mice were euthanized at 7 weeks of treatment.
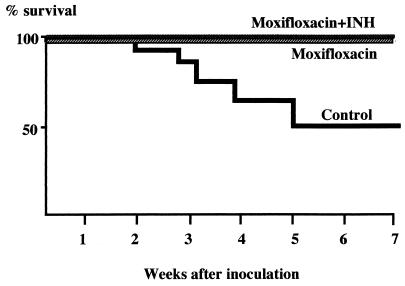
Mice treated with moxifloxacin or with moxifloxacin plus INH gained weight, whereas the body weight curve for the control group declined beginning 3 weeks after treatment. Five mice in the control group died of tuberculosis during the 7-week experiment, while no mice in either group that received drug(s) died (Table 3 and Fig. 1). The lungs from the dead mice were hard and inelastic, and their surface areas had turned white, consistent with overwhelming tuberculosis. Among the survivors examined at 7 weeks, nodular lesions measuring 1 to 3 mm in diameter were observed in all of the untreated mice. Similar lesions were observed in some mice receiving low-dose moxifloxacin but not in those receiving the combination of low-dose moxifloxacin plus INH. As shown in Table 3, at 3 weeks of treatment, the log10 CFU counts in the lungs and spleens for the moxifloxacin-treated mice were significantly lower (P < 0.001) than those for the control group. The combination of moxifloxacin and INH was even more effective in reducing bacillary counts. Whereas this combination reduced the bacillary load in both the lungs and spleens at 7 weeks of treatment compared to that at 3 weeks, we found no difference between the moxifloxacin monotherapy group and the control group in the log10 CFU counts in the lungs and spleens at 7 weeks. As may be seen in Table 3, moxifloxacin monotherapy prevented the expansion of bacillary load between weeks 3 and 7, whereas there was an apparent reduction in counts within the control group. Because only 50% (four of eight mice) of the mice in the control group survived to week 7, it is likely that death of the most heavily infected control mice resulted in a falsely low mean bacillary load in this group at week 7.
TABLE 3.
CFU counts in organ homogenates after treatment with moxifloxacin or moxifloxacin plus INH following inoculation of M. tuberculosis
| Treatment (daily dose) | CFU counta in homogenates of:
|
No. of mice surviving/total no. of mice | |
|---|---|---|---|
| Lung | Spleen | ||
| 3 weeks | |||
| Control, water | 7.01 ± 0.59 | 6.13 ± 0.45 | 14/16 |
| Moxifloxacin (20 mg/kg) | 6.43 ± 0.15b | 4.71 ± 0.30c | 16/16 |
| Moxifloxacin (20 mg/kg) + INH (25 mg/kg) | 2.90 ± 0.36c | 3.26 ± 0.32c | 16/16 |
| 7 weeks | |||
| Control, water | 4.92 ± 1.42 | 4.19 ± 0.76 | 4/7 |
| Moxifloxacin (20 mg/kg) | 6.40 ± 0.23 | 4.27 ± 0.28 | 8/8 |
| Moxifloxacin (20 mg/kg) + INH (25 mg/kg) | 1.67 ± 1.24cd | 1.84 ± 0.87cd | 8/8 |
Mice were intravenously inoculated with 1.0 × 107 CFU of M. tuberculosis before treatment. The values given are log10 CFU counts.
Significantly lower than the counts in the control group (P < 0.01).
Significantly lower than the counts in the control group (P < 0.001).
Significantly lower than the counts in the moxifloxacin group (P < 0.001).
FIG. 1.
Kaplan-Meier survival curve of mice after inoculation with high titer (1.0 × 107 CFU) of M. tuberculosis CSU93. Seven-week Kaplan-Meier survival analysis was conducted for groups of 16 mice infected with high-titer M. tuberculosis (1.0 × 107 CFU) with correction for the sacrifice of 8, 8, and 7 animals in the moxifloxacin, moxifloxacin plus INH, and control groups, respectively, at 3 weeks (see Table 3). There is a statistically significant difference in survival rate between the groups treated with moxifloxacin or moxifloxacin plus INH and the control group (P < 0.05).
Serum moxifloxacin concentrations.
Serum drug concentration-time profiles and pharmacokinetic parametric values of moxifloxacin after a single oral administration in mice are shown in Table 4. The parameters for moxifloxacin derived from this study are shown with previously published values for ofloxacin and sparfloxacin. The values for maximum serum drug concentration (Cmax) of moxifloxacin were similar to those of sparfloxacin and lower than those reported for equivalent doses of ofloxacin. Dose dependency was investigated with Cmax values of moxifloxacin. When the MIC of moxifloxacin for the CSU93 strain determined in this study (0.25 μg/ml) was used, the Cmax/MIC ratios were 8.0, 13.6, and 30.0, respectively, for moxifloxacin at the doses of 25, 50, and 100 mg/kg. As shown in Table 4, the values of the time to Cmax (Tmax) and the terminal elimination half-life (t1/2) of moxifloxacin were similar to those of ofloxacin, although the area under the concentration-time curve (AUC) was not as great. The AUC values of moxifloxacin showed direct proportionality with dose.
TABLE 4.
Pharmacokinetic parametric values of moxifloxacin and reference drugs in mice
Development of M. tuberculosis resistance to moxifloxacin.
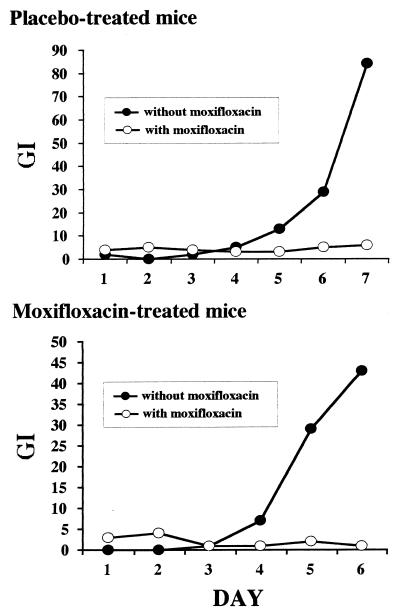
Mouse-passaged bacilli were isolated from the lungs of animals treated with a low dose (20 mg/kg) of moxifloxacin as monotherapy for 7 weeks in experiment 2 (high-titer infection). Homogenates were amplified by liquid subculture for 1 week to obtain a sufficient inoculum of M. tuberculosis. These bacteria were then analyzed for moxifloxacin susceptibility by the BACTEC method. As may be seen in Fig. 2, both moxifloxacin-exposed and untreated samples retained susceptibility to the drug at concentration of 0.25 μg/ml. Since analysis of these samples revealed a mean titer of 2.5 × 106 CFU in the lungs of these mice, this observation suggests that 7 weeks of low-dose (20 mg/kg) moxifloxacin monotherapy does not produce an appreciable subpopulation of quinolone-resistant bacteria.
FIG. 2.
Results of testing susceptibility to moxifloxacin of M. tuberculosis CSU93 harvested from mice exposed to moxifloxacin (20 mg/kg per day) for 49 days. A final concentration of 0.25 μg of moxifloxacin per ml was used in each BACTEC vial for drug susceptibility testing. GI, growth index measured by BACTEC method.
DISCUSSION
Our results clearly indicate that moxifloxacin is active against M. tuberculosis infection in mice. In this study, we used a highly virulent, recently isolated clinical strain of M. tuberculosis (CSU93) which was obtained from the index case of a large tuberculosis outbreak in a small, rural community (25). The in vivo growth rate of this strain in mice has been reported to vastly exceed that of the Erdman strain following aerosol administration. Our intravenously infected mouse model also showed that CSU93 is a virulent strain as demonstrated by the deaths and rising CFU counts, described above, in untreated animals.
In our study, CSU93 was shown to be susceptible to moxifloxacin, and the MIC was 0.25 μg/ml. This agrees well with the results of another study in which the in vitro activity of moxifloxacin against several M. tuberculosis isolates was analyzed and the MIC was found to range from 0.12 to 0.5 μg/ml (26). Based on these results, the MICs for moxifloxacin against M. tuberculosis are below those for ofloxacin and ciprofloxacin (22, 23).
Moxifloxacin given to mice at 100 mg/kg per day exerted a potent therapeutic effect in reducing the bacillary population in the lungs and spleens and in decreasing the spleen weights following infection with 5.9 × 105 CFU per animal. The drug’s activity was comparable to that seen with INH used concurrently in the same animal model. Furthermore, a combination of moxifloxacin and INH was highly effective in reducing M. tuberculosis loads and in sterilizing the lungs, indicating that moxifloxacin may prove useful for combination therapy of active tuberculosis. Moreover, the in vivo bactericidal activity of moxifloxacin alone at 100-mg/kg dosing was sufficiently potent to eradicate M. tuberculosis from the lungs of most mice at 8 weeks, which suggests that moxifloxacin monotherapy may be effective in certain clinical applications, such as for secondary prevention in individuals with latent tuberculosis.
To assess the limits of moxifloxacin’s effectiveness, we conducted a high-titer infection, low-dose therapy experiment with the mouse model. Following high-dose infection with M. tuberculosis (1.0 × 107 CFU per animal), the survival rate for untreated mice was 50%, contrasted with 100% for those receiving moxifloxacin alone (20 mg/kg per day) or moxifloxacin plus INH (20 mg/kg and 25 mg/kg, respectively, per day). Additionally, at 3 weeks of treatment, the CFU counts in the lungs and spleens for the moxifloxacin group were significantly lower than those for the control group, suggesting potent early bactericidal activity for this quinolone. In view of our pharmacokinetic analysis, which showed low serum moxifloxacin levels following 20-mg/kg dosing, the survival benefit observed in mice treated with low-dose therapy indicates significant in vivo potency of this drug.
The quinolones exert concentration-dependent killing, and the Cmax/MIC ratio has been shown to be a pharmacodynamic correlate of efficacy (3, 14). In the neutropenic rat model of Pseudomonas aeruginosa sepsis, the serum peak concentration/MIC ratio was linked to survival, particularly when high ratios (20:1) were obtained (3). These findings suggest that the clinical utility of newer quinolones will depend on their ability to attain high Cmax/MIC ratios (10:1 to 20:1) for clinically important pathogens. When 100 mg of moxifloxacin per kg was administered to mice, the Cmax/MIC ratio (30.0) exceeded the target level described above.
Our mouse pharmacokinetic analysis showed that the t1/2 and the Tmax of moxifloxacin in mice were similar to those of ofloxacin and shorter than those of sparfloxacin. The Cmax of moxifloxacin at 100-mg/kg dosing was considerably lower than that of ofloxacin and as high as that of sparfloxacin (9). The drug half-life for quinolone antimicrobial agents is known to be much shorter in small animals than in humans (15). In fact, the ranges of t1/2 and the Tmax for moxifloxacin in humans were 11.4 to 14.0 and 1.5 to 2.5, respectively, suggesting that daily dosing with moxifloxacin may be appropriate in humans (20, 21). Another concern with the use of quinolones in antituberculosis treatment has been the development of mycobacterial resistance. However, in this study low-dose (20 mg/kg) moxifloxacin monotherapy for 7 weeks did not lead to the development of a detectable subpopulation of quinolone-resistant organisms. It may be that the combination of potent early bactericidal activity and prolonged half-life in serum limits the rapid accumulation of resistance mutations during moxifloxacin therapy. Also, studies of the rate of resistance to moxifloxacin in larger animals will be useful, in view of the relatively small bacterial burden of M. tuberculosis in mice.
In conclusion, moxifloxacin was bactericidal and sterilizing in mice infected with a highly virulent human isolate of M. tuberculosis. A combination of moxifloxacin and INH was also highly effective, suggesting that this new quinolone may be useful in combination therapy for tuberculosis. Further studies to assess the in vivo activity of moxifloxacin against other strains, including multiple-drug-resistant M. tuberculosis, or against other mycobacterial infections, and to evaluate moxifloxacin in combination with standard agents for the treatment of drug-susceptible tuberculosis will be of great value.
ACKNOWLEDGMENTS
This work was supported in part by a grant from the Bayer Corporation.
We are grateful to Vipin Agarwal for measurements of serum levels of moxifloxacin and to Barbara Painter for helpful advice and for reviewing this manuscript. We thank Ping Chen, Nikki Parrish, and Caryn Good for their skillful technical support and valuable advice and Jennifer Doetsch for assistance in the preparation of the manuscript.
ADDENDUM
Similar results documenting the effectiveness of moxifloxacin in a slightly different mouse model of tuberculosis have recently been published by Ji et al. (9a).
REFERENCES
- 1.Aldridge K E, Ashcraft D S. Comparison of the in vitro activities of Bay 12-8039, a new quinolone, and other antimicrobials against clinically important anaerobes. Antimicrob Agents Chemother. 1997;41:709–711. doi: 10.1128/aac.41.3.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boswell F J, Andrews J M, Wise R. Pharmacodynamic properties of BAY 12-8039 on gram-positive and gram-negative organisms as demonstrated by studies of time-kill kinetics and postantibiotic effect. Antimicrob Agents Chemother. 1997;41:1377–1379. doi: 10.1128/aac.41.6.1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Drusano G L, Johnson D E, Rosen M, Standiford H C. Pharmacodynamics of a fluoroquinolone antimicrobial agent in a neutropenic rat model of Pseudomonas sepsis. Antimicrob Agents Chemother. 1993;37:483–490. doi: 10.1128/aac.37.3.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fass R J. In vitro activity of BAY 12-8039, a new 8-methoxyquinolone. Antimicrob Agents Chemother. 1997;41:1818–1824. doi: 10.1128/aac.41.8.1818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Frieden T R, Sterling T, Pablos-Mendez A, Kilburn J O, Cauthen G M, Dooley S W. The emergence of drug-resistant tuberculosis in New York City. N Engl J Med. 1993;328:521–526. doi: 10.1056/NEJM199302253280801. [DOI] [PubMed] [Google Scholar]
- 6.Goble M, Iseman M D, Madsen L A, Waite D, Ackerson L, Horsburgh C R., Jr Treatment of 171 patients with pulmonary tuberculosis resistant to isoniazid and rifampin. N Engl J Med. 1993;328:527–532. doi: 10.1056/NEJM199302253280802. [DOI] [PubMed] [Google Scholar]
- 7.Goldstein E J C, Citron D M, Hudspeth M, Gerardo S H, Merriam C V. In vitro activity of Bay 12-8039, a new 8-methoxyquinolone, compared to the activities of 11 other oral antimicrobial agents against 390 aerobic and anaerobic bacteria isolated from human and animal bite wound skin and soft tissue infections in humans. Antimicrob Agents Chemother. 1997;41:1552–1557. doi: 10.1128/aac.41.7.1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ji B, Lounis N, Truffot-Pernot C, Grosset J. In vitro and in vivo activities of levofloxacin against Mycobacterium tuberculosis. Antimicrob Agents Chemother. 1995;39:1341–1344. doi: 10.1128/aac.39.6.1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ji B, Truffot-Pernot C, Grosset J. In vitro and in vivo activities of sparfloxacin (AT 4140) against Mycobacterium tuberculosis. Tubercle Lung Dis. 1991;72:181–186. doi: 10.1016/0041-3879(91)90004-c. [DOI] [PubMed] [Google Scholar]
- 9a.Ji B, Lounis N, Maslo C, Truffot-Pernot C, Bonnafous P, Grosset J. In vitro and in vivo activities of moxifloxacin and clinafloxacin against Mycobacterium tuberculosis. Antimicrob Agents Chemother. 1998;42:2066–2069. doi: 10.1128/aac.42.8.2066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kennedy N, Berger L, Curram J, Fox R, Gutmann J, Kisyombe G M, Ngowi F I, Ramsay A R C, Saruni A O S, Sam N, Tillotson G, Uiso L O, Yates M, Gillespie S H. Randomized controlled trial of drug regimen that includes ciprofloxacin for the treatment of pulmonary tuberculosis. Clin Infect Dis. 1996;22:827–833. doi: 10.1093/clinids/22.5.827. [DOI] [PubMed] [Google Scholar]
- 11.Kohno S, Koga H, Kaku M, Maesaki S, Hara K. Prospective comparative study of ofloxacin or ethambutol for the treatment of pulmonary tuberculosis. Chest. 1992;102:1815–1818. doi: 10.1378/chest.102.6.1815. [DOI] [PubMed] [Google Scholar]
- 12.Lalande V, Truffot-Pernot C, Paccaly-Moulin A, Grosset J, Ji B. Powerful bactericidal activity of sparfloxacin (AT-4140) against Mycobacterium tuberculosis in mice. Antimicrob Agents Chemother. 1993;37:407–413. doi: 10.1128/aac.37.3.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lounis N, Ji B, Truffot-Pernot C, Grosset J. Which aminoglycoside or fluoroquinolone is more active against Mycobacterium tuberculosis in mice? Antimicrob Agents Chemother. 1997;41:607–610. doi: 10.1128/aac.41.3.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Meinen J B, McClure J T, Rosin E. Pharmacokinetics of enrofloxacin in clinically normal dogs and mice and drug pharmacodynamics in neutropenic mice with Escherichia coli and staphylococcal infections. Am J Vet Res. 1995;56:1219–1224. [PubMed] [Google Scholar]
- 15.Nakagawa T, Ishigai M, Kato M, Hayakawa N, Kinoshita H, Okutomi T, Ohkubo K, Ozaki A. Pharmacokinetics of the new fluoroquinolone balofloxacin in mice, rats and dogs. Arzneim-Forsch. 1995;45:719–722. [PubMed] [Google Scholar]
- 16.Pablos-Mendez A, Raviglione M, Laszlo A, Binkin N, Rieder H, Bustreo F, Cohn D L, Lambregts-van Weezenbeek C S B, Kim S J, Chaulet P, Nunn P. Global surveillance for antituberculosis-drug resistance, 1994–1997. N Engl J Med. 1998;338:1641–1649. doi: 10.1056/NEJM199806043382301. [DOI] [PubMed] [Google Scholar]
- 17.Siddiqi S. Radiometric (BACTEC) tests for slowly growing mycobacteria. In: Isenberg H D, editor. Clinical microbiology procedures handbook. Vol. 1. Washington, D.C: American Society for Microbiology; 1992. pp. 5.14.1–5.14.25. [Google Scholar]
- 18.Sirgel F A, Botha F J, Parkin D P, Van de Wal B W, Schall R, Donald P R, Mitchison D A. The early bactericidal activity of ciprofloxacin in patients with pulmonary tuberculosis. Am J Respir Crit Care Med. 1997;156:901–905. doi: 10.1164/ajrccm.156.3.9611066. [DOI] [PubMed] [Google Scholar]
- 19.Small P M, Schecter G F, Goodman P C, Sande M A, Chaisson R E, Hopewell P C. Treatment of tuberculosis in patients with advanced human immunodeficiency virus infection. N Engl J Med. 1991;324:289–294. doi: 10.1056/NEJM199101313240503. [DOI] [PubMed] [Google Scholar]
- 20.Stass H, Dalhoff A, Kubitza D, Schühly U. Pharmacokinetics, safety, and tolerability of ascending single doses of moxifloxacin, a new 8-methoxy quinolone, administered to healthy subjects. Antimicrob Agents Chemother. 1998;42:2060–2065. doi: 10.1128/aac.42.8.2060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sullivan J, Woodruff M, Lettieri J, Agarwal V, Krol G, Heller A. Abstracts of the 8th European Congress of Clinical Microbiology and Infectious Diseases 1997. Lausanne, Switzerland: European Society of Clinical Microbiology and Infectious Diseases; 1997. Pharmacokinetics (PK) and tolerability of the new quinolone BAY 12-8039: 10 days’ treatment at 400 mg daily, abstr. P-389; p. 87. [Google Scholar]
- 22.Truffot-Pernot C, Ji B, Grosset J. Activities of pefloxacin and ofloxacin against mycobacteria: in vitro and mouse experiments. Tubercle Lung Dis. 1991;72:57–64. doi: 10.1016/0041-3879(91)90025-n. [DOI] [PubMed] [Google Scholar]
- 23.Tsukamura M. In vitro antituberculosis activity of a new antibacterial substance ofloxacin (DL-8280) Am Rev Respir Dis. 1985;131:348–351. doi: 10.1164/arrd.1985.131.3.348. [DOI] [PubMed] [Google Scholar]
- 24.Tsukamura M, Nakamura E, Yoshii S, Amano H. Therapeutic effect of a new antibacterial substance ofloxacin (DL-8280) on pulmonary tuberculosis. Am Rev Respir Dis. 1985;131:352–356. doi: 10.1164/arrd.1985.131.3.352. [DOI] [PubMed] [Google Scholar]
- 25.Valway S E, Sanchez M P, Shinnick T F, Orme I, Agerton T, Hoy D, Jones J S, Westmoreland H, Onorato I M. An outbreak involving extensive transmission of a virulent strain of Mycobacterium tuberculosis. N Engl J Med. 1998;338:633–639. doi: 10.1056/NEJM199803053381001. [DOI] [PubMed] [Google Scholar]
- 26.Woodcock J M, Andrews J M, Boswell F J, Brenwald N P, Wise R. In vitro activity of BAY 12-8039, a new fluoroquinolone. Antimicrob Agents Chemother. 1997;41:101–106. doi: 10.1128/aac.41.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]