Summary
Background & aims
Elective surgery induces skeletal muscle wasting driven by an imbalance between muscle protein synthesis and breakdown. From examination of diverse stable isotope tracer techniques, the dynamic processes driving this imbalance are unclear. This meta-analysis aimed to elucidate the mechanistic driver(s) of postoperative protein catabolism through stable isotope assessment of protein turnover before and after abdominal surgery.
Methods
Meta-analysis was performed of randomized controlled trials and cohort studies in patients undergoing elective abdominal surgery that contained measurements of whole-body or skeletal muscle protein turnover using stable isotope tracer methodologies pre- and postoperatively. Postoperative changes in protein synthesis and breakdown were assessed through subgroup analysis of tracer methodology and perioperative care.
Results
Surgery elicited no overall change in protein synthesis [standardized mean difference (SMD) −0.47, 95% confidence interval (CI): −1.32, 0.39, p = 0.25]. However, subgroup analysis revealed significant suppressions via direct-incorporation methodology [SMD -1.53, 95%CI: −2.89, −0.17, p = 0.03] within skeletal muscle. Changes of this nature were not present among arterio-venous [SMD 0.61, 95%CI: −1.48, 2.70, p = 0.58] or end-product [SMD -0.09, 95%CI: −0.81, 0.64, p = 0.82] whole-body measures. Surgery resulted in no overall change in protein breakdown [SMD 0.63, 95%CI: −0.06, 1.32, p = 0.07]. Yet, separation by tracer methodology illustrated significant increases in urinary end-products (urea/ammonia) [SMD 0.70, 95%CI: 0.38, 1.02, p < 0.001] that were not present among arterio-venous measures [SMD 0.67, 95%CI: −1.05, 2.38, p = 0.45].
Conclusions
Elective abdominal surgery elicits suppressions in skeletal muscle protein synthesis that are not reflected on a whole-body level. Lack of uniform changes across whole-body tracer techniques are likely due to contribution from tissues other than skeletal muscle.
Keywords: Surgery, Postoperative, Muscle protein synthesis, Muscle protein breakdown, Stable isotope studies, Meta-analysis
Abbreviations: AV, Arterio-venous; CI, Confidence interval; DI, Direct-incorporation; EP, End-product; FSR, Fractional synthetic rate; GC-IRMS, Gas chromatography-isotope ratio mass spectrometry; GI, Gastrointestinal; IQR, Interquartile range; RCT, Randomized controlled trial; SD, Standard deviation; SMD, Standardized mean difference
1. Introduction
Skeletal muscle wasting is a key feature of the metabolic response to surgery, known to complicate postoperative recovery and impair clinical outcomes [1]. Although this phenomenon has been observed since early investigations into the metabolic perturbations that occur as a result of trauma and surgery [[2], [3], [4]], the underlying dynamic drivers of these metabolic changes within muscle are yet to be fully defined. Loss of skeletal muscle mass must occur through a chronic imbalance between muscle protein synthesis and muscle protein breakdown, with stable isotope techniques that calculate fractional synthetic rate currently considered a ‘gold standard’ for the measurement of muscle protein synthesis [5]. These techniques have been employed in the perioperative setting [[6], [7], [8]] and have shown distinct synthetic responses when compared with other stable isotope tracer techniques that quantify arterio-venous protein kinetics within the blood [9,10] or tracer kinetics within urinary end-products [11,12]. Comparisons of protein breakdown rates across tracer methodologies are limited by challenges in the assessment of skeletal muscle protein breakdown due to both underlying assumptions in kinetic modelling [13,14] and protocols ill-suited to clinical populations [14,15]. Hence, there is a paucity of information on fractional breakdown rates in the surgical patient, with stable isotope measures of protein breakdown predominantly reflecting whole-body kinetics. Taken together, the dynamic changes driving postoperative muscle wasting are unclear.
Major abdominal surgery has been shown to elicit systemic metabolic dysregulation within skeletal muscle, including alterations in catabolic and inflammatory signaling pathways [16]. In addition, traditional surgical care for these patients has often prescribed prolonged periods of preoperative fasting [17], putting these patients at great risk of postoperative skeletal muscle wasting through energy deficits [18]. Even in light of enhanced recovery programs aimed at reducing the metabolic stress response to surgery [19], in part through recommendations on the avoidance of preoperative fasting and early resumption of oral nutrition postoperatively [1], a recent audit of UK hospitals has illustrated elective surgical procedures - constituted by approximately 70% upper GI, colorectal or general surgery - to routinely involve preoperative fasting of >12 h for food (73% incidence) and clear fluids (21% incidence) [20]. A synthesis of stable isotope studies quantifying protein kinetics in the patient undergoing abdominal surgery may elucidate the changes in protein turnover driving postoperative catabolism, while informing future care strategies aimed at minimizing skeletal muscle wasting to improve patient outcomes and recovery.
The aims of this meta-analysis were to:
-
•
determine postoperative changes in protein kinetics driving skeletal muscle catabolism, through a synthesis of studies utilizing stable isotope research methodologies across a range of elective abdominal surgical procedures and clinical care.
-
•
assess the impact of perioperative care strategies such as nutritional support, neuraxial blockade and minimally invasive (laparoscopic) surgical approaches, and
-
•
evaluate the postoperative time-course of protein turnover responses.
2. Methods
2.1. Search strategy
Electronic searches were performed in PubMed, MEDLINE and Cochrane Library databases to identify suitable articles (i.e. evaluating either whole-body or skeletal muscle protein turnover using stable isotope tracer methodology in adult patients undergoing elective abdominal surgery) published between 01 January 1990 and 08 November 2020. This date restriction was imposed due to the validation of several clinically suitable stable isotope techniques for protein metabolism occurring throughout the 1980s [[21], [22], [23], [24]]; studies which contributed to increased interest into the effects of surgical trauma on protein turnover during the late 1980s [25,26] and to the development of commercially available gas chromatography-isotope ratio mass spectrometers (GC-IRMS) capable of capturing increased signal sensitivity within complex biological matrices [27]. The search terms [“surgery”] AND [“muscle” OR “protein”] AND [“stable isotope” OR “tracer” OR “turnover”] were used to search each database by title and abstract. The bibliographies of all studies which fulfilled the inclusion criteria were manually reviewed to aid in locating additional eligible articles. There were no language restrictions in place during article selection. This meta-analysis was conducted in accordance with the guidance of the PRISMA statement [28] and conforms to AMSTAR-2 guidelines [29].
2.2. Study selection
Articles were screened for suitability by title and abstract on two separate occasions by one reviewer (MJ) and verified by a senior reviewer (MSB). Articles were deemed eligible if they described at least one adult patient cohort undergoing elective abdominal surgery, with pre- and postoperative measures of whole-body or skeletal muscle protein turnover through stable isotope tracer methodologies. Postoperative measures were included if they were performed within two weeks of surgery. Patients receiving a variety of nutritional and analgesic regimens were included due to the inherent heterogeneity of surgical care across different hospital settings and procedures. However, any patient cohort that was specified by study authors to be undergoing non-conventional perioperative care or receiving non-standard drug administration or hormone therapy was excluded. Pre- and postoperative measures of protein turnover had to be performed during the same nutritional state for each patient group, specifically; pre- and postoperative measures had to be both in the postabsorptive or postprandial state to enable accurate comparisons of protein turnover within patients, due to the dynamic regulation of muscle protein turnover with feeding [30]. Patients undergoing emergency, transplant or reconstructive procedures or suffering from burns, preoperative trauma, metabolic disorders, prolonged anti-inflammatory or antibiotic medication, organ dysfunction or failure were excluded. Abdominal surgery was defined as general, urological, or gynecologic, with vascular procedures omitted. Only studies on patients undergoing abdominal surgery were included in this analysis to improve homogeneity in postoperative protein turnover responses, as there is evidence to suggest that the catabolic response to surgery is relative to the magnitude of trauma [11,12,31]. Further, ischemia and reperfusion effects have been shown to impact protein turnover rates within an animal model [32], with great variation in postoperative protein turnover responses in humans previously being demonstrated within a heterogenous abdominal surgical cohort containing vascular procedures [33]. Patients were deemed adults if they were 18 years or older, with all pediatric studies being ineligible. Records containing duplication of study results were omitted, with only the primary publication taken forward for inclusion. Duplication of articles eligible for screening were assessed by title using Python programming language (version 3.6.5), with a subsequent manual check to ensure the full removal of duplicate articles. Duplication of study results was checked manually during full-text screening of eligible articles. For any article where fulfilment of the inclusion criteria was unclear, inclusion was discussed by two reviewers (MJ and MSB) and a final decision was made.
2.3. Data extraction
Data were extracted by one author (MJ) on two separate occasions and cross-compared to ensure accurate inclusion of article information. These data were then reviewed by a second author (MSB). Where studies contained more than one patient cohort, these cohorts were combined to prevent unit-of-analysis-error in accordance with recommendations from the Cochrane Handbook for Systematic Reviews of Interventions [34]. Data were additionally collected on patient demographics, surgical preparation and underlying conditions necessitating surgical intervention. Where studies did not contain the necessary information, study authors were contacted for retrieval. Where studies did not report the mean and standard deviation of protein turnover measures; median and interquartile ranges were converted to means and standard deviations according to the technique described by Hozo et al. [35]. This technique takes the median as the best estimate of the mean and calculates the SD as follows:
Where relevant, risk of bias for randomized controlled trials (RCTs) was assessed using the Cochrane Collaboration Tool [36]. Publication bias was assessed via funnel plots and tested for via Pustejovsky's and Rodgers' [37] modified test of linear regression for standardized mean difference effect sizes.
2.4. Outcome measures
The primary outcome was to detect changes before and after surgery in whole-body or skeletal muscle protein turnover measured via stable isotope tracer methodology. Secondary outcomes aimed to investigate the influence of tracer methodology, severity of trauma (laparoscopic vs. open procedures), nutritional support and anesthetic regimen on the primary outcome measures. Meta-analysis of these outcomes was achieved through subgroup analyses. The population, intervention, comparator group and outcome (PICO) are summarized in Supplementary Table 1.
2.5. Statistical analyses
Data were prepared in Excel spreadsheet format and imported into R programming language (version 4.1.0, The R Foundation for Statistical Computing, http://www.R-project.org). The ‘meta’ package was used for data analysis. Continuous variables are quoted as standardized mean difference (SMD) with 95% CI and were analyzed using a random-effects, inverse-variance model. The DerSimonian-Laird estimator [38] was used to calculate heterogeneity variance, τ2, with Knapp-Hartung adjustments [39] applied in the calculation of confidence intervals around pooled study effects. Forest plots were generated, with statistical significance determined as p < 0.05 with 2-tailed testing. Study heterogeneity was assessed by I2 statistic [40], with <25% representing low heterogeneity, 25–50% representing moderate heterogeneity and >50% representing high heterogeneity. Meta-regression was performed to investigate time as a continuous variable across postoperative sampling timepoints, to determine whether this impacted the assessment of postoperative protein turnover.
2.6. Protocol registration
The protocol for this meta-analysis was registered on the Prospero database (www.crd.york.ac.uk/prospero), registration number: CRD42021178987.
3. Results
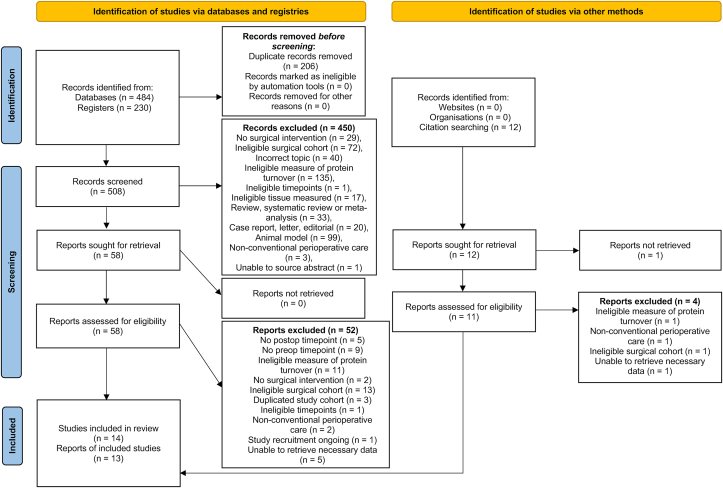
From the 714 studies identified through electronic database searches, 14 studies [[6], [7], [8], [9],11,12,31,[41], [42], [43], [44], [45], [46], [47]] reporting on 190 patients, were included (Fig. 1). Of these, twelve [[6], [7], [8], [9],11,12,[41], [42], [43], [44], [45], [46]] reported measures of protein synthesis (154 patients) and nine [9,11,12,31,[41], [42], [43],46,47] reported measures of protein breakdown (139 patients). From the studies reporting more than one postoperative timepoint [9,11,12,31,42,43], the timepoint closest to surgery was used for analyses, and where differential feeding was involved, its corresponding preoperative baseline value. The full-text from one eligible study [45] was unable to be sourced and attempts to contact the corresponding authors were unsuccessful. However, the abstract contained the necessary information required for inclusion and as such the decision was made between reviewers (MJ and MSB) to include data from this article in the meta-analysis. There were six studies [10,[48], [49], [50], [51], [52]] that fulfilled inclusion criteria but did not contain the necessary information needed for synthesis in the meta-analysis, with the authors being unable to provide the necessary information upon request. These studies were subsequently omitted from the analyses (Supplementary Table 2).
Fig. 1.
PRISMA flow-diagram detailing article identification for meta-analysis.
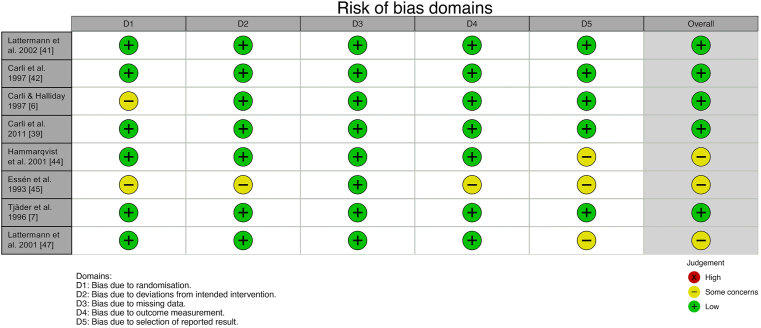
3.1. Risk of bias
Of the 14 studies included in this meta-analysis, eight were RCTs (predominantly investigating parameters related to perioperative catabolism) [6,7,[41], [42], [43], [44], [45],47] and six were cohort studies [8,9,11,12,31,46]. However, none of the RCTs involved randomization of the respective variables of interest within the subgroup analyses performed, with randomized cohorts within these studies thus combined prior to calculation of pooled effect size across studies. Therefore, RCT and cohort studies were not separated throughout this meta-analysis. Additional information on RCT risk of bias can be found in Fig. 2.
Fig. 2.
Risk of bias of the included randomized controlled trials.
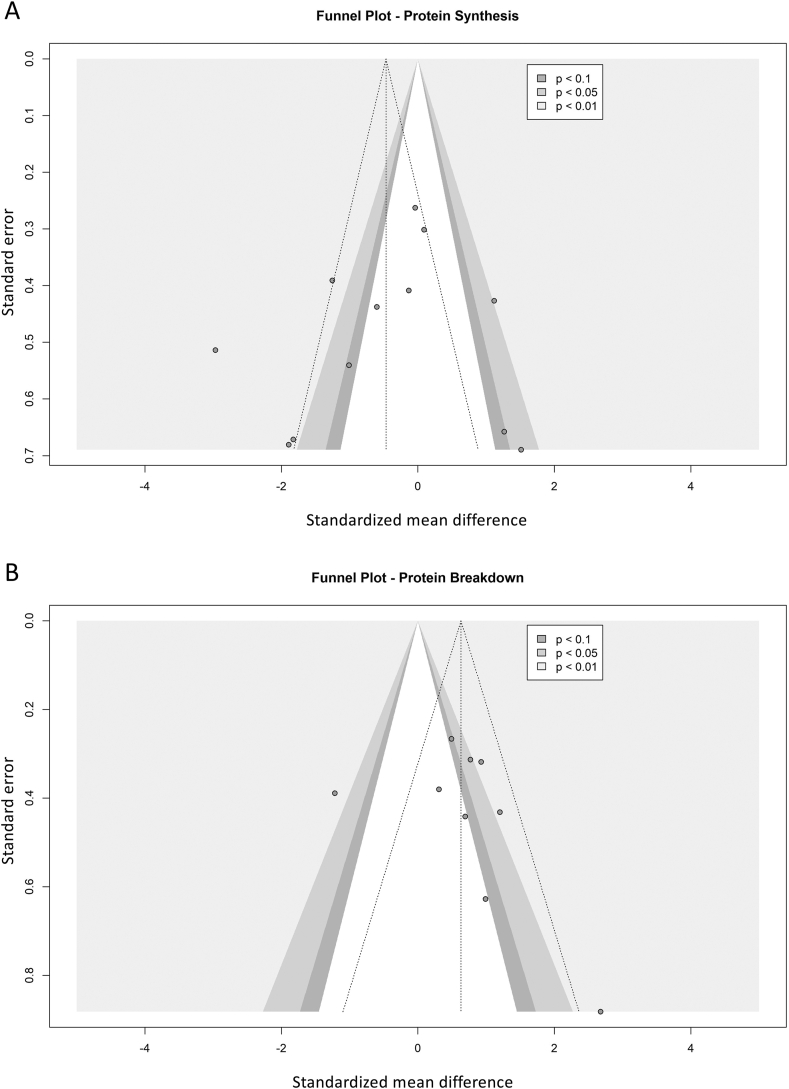
Publication bias was analyzed via funnel plot and Pustejovky's and Rodger's modified test of linear regression [37], for both measures of protein synthesis and protein breakdown across studies (Fig. 3a and b). Neither tests of publication bias for protein synthesis nor protein breakdown were deemed statistically significant (p = 0.97 and p = 0.57 respectively), although interpretation of these results was limited by the low study numbers included. Several studies in each funnel plot were in range of statistical significance, however due to the high heterogeneity expected across studies due to variation in perioperative care and tracer methodology, all studies were subsequently taken forward for further analyses.
Fig. 3.
Contour-enhanced funnel plots of protein synthesis (A) and protein breakdown (B) study effects, with significance represented by contour shading at thresholds of p < 0.1, p < 0.05 and p < 0.01.
3.2. Demographics
Indication for surgery was predominantly colorectal cancer [6,11,12,31,41,42] with the remaining indications a mixture of malignant and benign pathologies [[7], [8], [9],[43], [44], [45], [46], [47]]. Two studies [7,45] included patients having open surgery, with the remaining studies not providing this information [6,8,9,11,12,31,[41], [42], [43], [44],46,47]. A mix of anesthetic protocols were employed, with five studies [6,41,43,44,47] selectively providing epidural block as part of the anesthetic regimen (either randomized to patients as part of the study design or based on patient need). Five studies did not provide information on anesthetic protocol [9,11,12,31,46], with anesthetic protocol being unknown for one study [45] due to its inclusion based on abstract only. There was varied perioperative nutrition provided to patients across the study period (Table 1), with eight studies [[7], [8], [9],41,[43], [44], [45], [46]] including patient cohorts that underwent a preoperative fast (of approximately 12 h or more overnight) or bowel preparation. Tracer methodology utilized within studies came under three categories; those that assessed the direct incorporation of stable isotopes into skeletal muscle that measure fractional synthetic rate (FSR), those that assessed whole-body protein kinetics in the blood via arterio-venous (AV) measures and those that assessed the whole-body kinetics of stable isotope labelling in excreted total or specific urinary substrates (EP). There were five studies that measured protein synthesis via FSR [[6], [7], [8],44,45], four [9,[41], [42], [43]] via AV, and three [11,12,46] via EP. Studies that utilized direct-incorporation methodology assessed muscle FSR distant from the site of trauma (quadriceps). Five studies [9,[41], [42], [43],47] measured protein breakdown via AV, and four [11,12,31,46] via EP. Postoperative timepoints for measures of protein turnover were predominantly between 24 and 72 h, with only one study's measures [42] being performed later than this range at 144 h.
Table 1.
Patient demographics of studies included.
| Article | Eligible Patient Cohort | Number of Patients (Total) | Surgical Procedure | Anaesthesia and Analgesia | Perioperative Nutrition | Stable Isotope Tracer | Sampling Timepoints Included (Pre-: Post-operative) |
|---|---|---|---|---|---|---|---|
| Tashiro et al., 1991 [11] | Gastric/colorectal surgery | 11 | Total gastrectomy: 7, hemicolectomy: 3, low anterior resection: 1 | Unknown | TPN exclusively, 1.5 g protein/kg/day and 35 kcal/kg/day | [15 N] Glycine; EP | Pre: Not specified Post: 72 h |
| Lattermann et al., 2002 [41] | General anaesthesia with epidural block/General anaesthesia only | 8/8 (16) | Hemicolectomy/colectomy: 2/5, sigmoid resection: 3/1, anterior resection: 3/1, Ileocolic resection: 0/1 | General anaesthesia with patients randomised to either epidural or IV morphine postoperatively | ∼36 h preoperative fast | L-[1–13C] Leucine; AV | Pre: 0 h Post: 2 h |
| Carli et al., 1997 [42] | Parenteral nutrition control group | 6 | All surgery for non-metastatic adenocarcinoma of the rectosigmoid colon | General anaesthesia with postoperative subcutaneous infusion of papaveretum (3–5 mg/h) for 3–4 days | 0.1 g nitrogen/kg/day and 20 kcal/kg/day. Nonprotein calories were 60% lipid and 40% carbohydrate. Oral intake was started 6 days before surgery under dietetic supervision, and was then changed to parenteral nutrition at 500 ml Vamin 14, 1 L Intralipid 10% and 1 L dextrose 10% 2 days before surgery and continued for 6 days afterward. | L-[1–13C] Leucine; AV | Pre: 0 h Post: 144 h |
| Carli and Halliday 1997 [6] | General anaesthesia with epidural block/general anaesthesia only | 6/6 (12) | Paramedian incision for non-metastatic adenocarcinoma of the rectosigmoid colon | General anaesthesia with patients randomised to either; epidural maintained for 48 h postoperatively supplemented with papaveretum (8–10 mg) given i.m. Every 8 h or continuous subcutaneous infusion of papaveretum set at 3–8 mg/h | 0.1 g nitrogen/kg/day and 20 kcal/kg/day. Nonprotein calories were 60% fat and 40% carbohydrate. Oral intake commenced 6 days before surgery under dietic supervision and changed to parenteral nutrition (500 ml Vamin 14, 1 L Intralipid 10%, 1 L dextrose 10%) 2 days before surgery. Discontinued at midnight day before surgery, recommenced at 4 h postoperatively and maintained for 2 days after surgery. | L-[1–13C] Leucine; FSR | Pre: 0 h Post: 48 h |
| Carli et al., 2011 [43] | Oral Glucose Nutrition/Oral Whey Nutrition | 6/7 (13) | Hemicolectomy/colectomy: 4/4, Sigmoid resection: 0/2, Anterior resection: 2/1 | General anaesthesia with epidural or intraoperative IV analgesia; postoperative epidural for 2 days or PCA with opioids | Preoperative fast of ∼24–36 h. Postoperatively, patients were allowed to drink clear fluids unless contraindicated. Clear fluids consisted of a small portion of apple juice (approximately 110 kcal) and Jell-O® (Kraft Foods, Northfield, Illinois) (approximately 70 kcal). | L-[1–13C] Leucine; AV | Pre: −168 h Post: 48 h |
| Tashiro et al., 1996 [12] | Gastric or colorectal surgery | 22 | Total gastrectomy, hemicolectomy or lower anterior resection, and lymph node dissection. | Unknown | Parenteral nutrition providing 1.5 g amino acid/kg/day and energy intake of 35 kcal/kg/day. No fat was provided as an energy source. PN was started 7 days prior to the operation and maintained across the study duration. Doses of protein and energy were maintained strictly the same throughout the study. | [15 N] Glycine; EP | Pre: Not specified Post: 72 h |
| Hammarqvist et al., 2001 [44] | Glutamine PN group | 8 | Colon resection: 4, rectum resection: 3, retroperitoneal resection: 1 | General anaesthesia. 3 patients were also provided with epidural blockade, although this was not provided continuously throughout the study period. | Postoperative parenteral nutrition containing 0.15 g nitrogen/kg/day including an amino acid solution, supplemented with 0.28 g glutamine/kg/day. Energy provided as glucose and fat, calculated as 1.2-fold of caloric need as determined by Harris-Benedict formula. 75% of parenteral nutrition dose administered in first day after operation (25% across following 2 days). | L-[2H5] Phenylalanine; FSR | Pre: 0 h Post: 72 h |
| Essén et al., 1993 [45] | Saline/Parenteral nutrition | 8/9 (17) | Cholecystectomy | Unable to source full-text article. | Saline or parenteral nutrition for 3 days postoperatively. | L-[1–13C] Leucine; FSR | Pre: Unknown Post: 72 h |
| Tjäder et al., 1996 [7] | Saline | 7 | Cholecystectomy - subcostal incision | General anaesthesia, with diazepam (5 mg) and pancuronium (0.1 mg/kg) for neuromuscular block, with postoperative IV injections of pethidine (synthetic opioid). | Saline perioperatively 3 ml/kg/h, followed by 35 ml/kg/day postoperatively. | L-[2H5] Phenylalanine; FSR | Pre: 0 h Post: 24 h |
| López-Hellín et al., 2004 [46] | Fasted/Parenteral nutrition | 21/8 (29) | Left hemicolectomy: 9; right hemicolectomy: 5; front rectum resection: 4; Miles' resection: 1; gastrectomy: 1; sigmoidectomy: 1 (21). Left hemicolectomy: 3; Miles' resection: 2; front rectum resection: 1; right hemicolectomy: 1; gastrectomy: 1 (8). |
Unknown | Preoperative hypocaloric parenteral nutrition: CHO (28 kJ/kg/day), Amino acids (1 g/kg/day) - followed by either: preoperative fast and postoperative parenteral nutrition of glucose (28 kJ/kg/day) OR TPN (56.1 kJ/kg/day CHO, 56.1 kJ/kg/day Fat, 1.5 g/kg/day Amino acids) administered pre- and post-operatively for 24 h. | [15 N] Glycine; EP | Pre: −72 h Post: 24 h |
| Essén et al., 1992 [8] | Cholecystectomy patient group | 7 | Cholecystectomy - subcostal incision | General anaesthesia, with diazepam (5 mg) and pancuronium-bromide (0.1 mg/kg) for neuromuscular block. | Acute fasted study. | L-[1–13C] Leucine; FSR | Pre: 0 h Post: Immediately after surgery |
| Lattermann et al., 2001 [47] | General anaesthesia/General anaesthesia with epidural block | 7/7 (14) | Elective cystoprostatectomy - Ileal neobladder: 6/6, Ileal conduit: 1/1 | General anaesthesia/General anaesthesia with epidural block. Epidural terminated immediately after surgery – both patient cohorts received IV Piritramide postoperatively. | Parenteral nutrition from 24 h postoperatively until 10 h before postoperative measurement. 2 g/kg/day xylitol and amino acids, equivalent to 0.15 g of N/kg/day. | [15N2] Urea; AV | Pre: −72 h Post: 72 h |
| Tashiro et al., 1996b [31] | Gastric or colorectal surgery | 22 | Total gastrectomy: 11, Hemicolectomy: 4, Low anterior resection: 6, Miles' operation: 1 | Unknown | Parenteral nutrition providing 1.5 g of protein and 40 kcal/kg/day, commenced at least 5 days prior to surgery and maintained throughout study period. | [15 N] Glycine; EP | Pre: Not specified Post: 72 h |
| Carli et al., 1990 [9] | Total abdominal hysterectomy | 6 | Menorrhagia | Unknown | 0.1 g of nitrogen/kg body weight and 1200–1400 calories (5021–5858 kJ)/day was commenced 7 days before surgery by oral intake. The same amount of nitrogen and calories was administered intravenously after surgery starting 4 h from the end of surgery when the cardiorespiratory conditions were stable. The parenteral nutritional support, based on a mixture of glucose, lipid and amino acids (KabiVitrum), was then continued for 4 days after surgery until patients were able to tolerate the pre-operative oral diet again. | L-[1–13C] Leucine: AV | Pre: −48 h Post: 48 h |
FSR: fractional synthetic rate; AV: arterio-venous; EP: end-product.
3.3. Tracer methodology
3.3.1. Protein synthesis
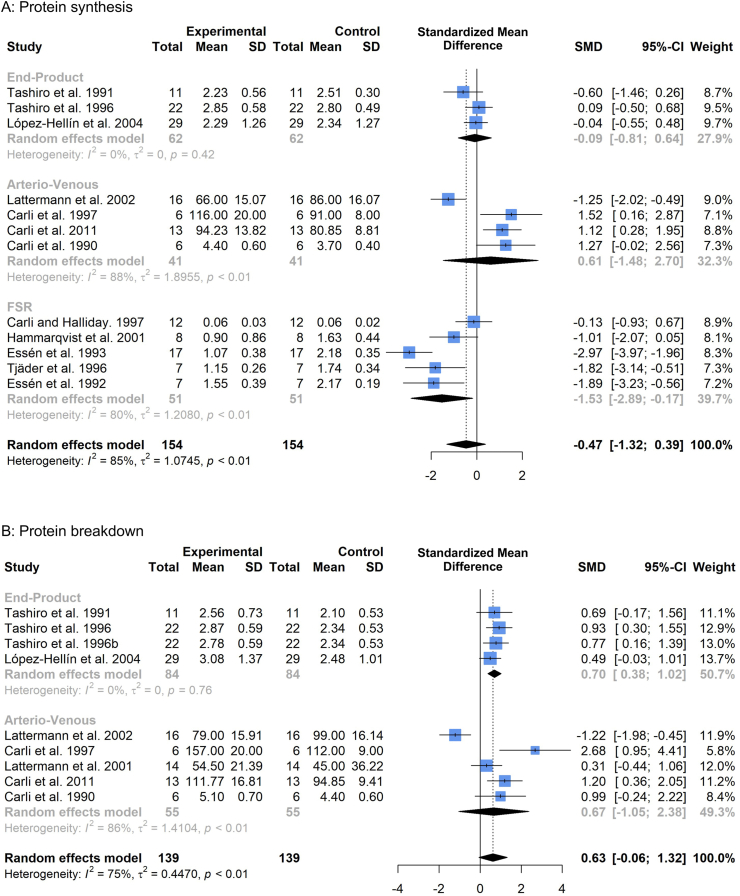
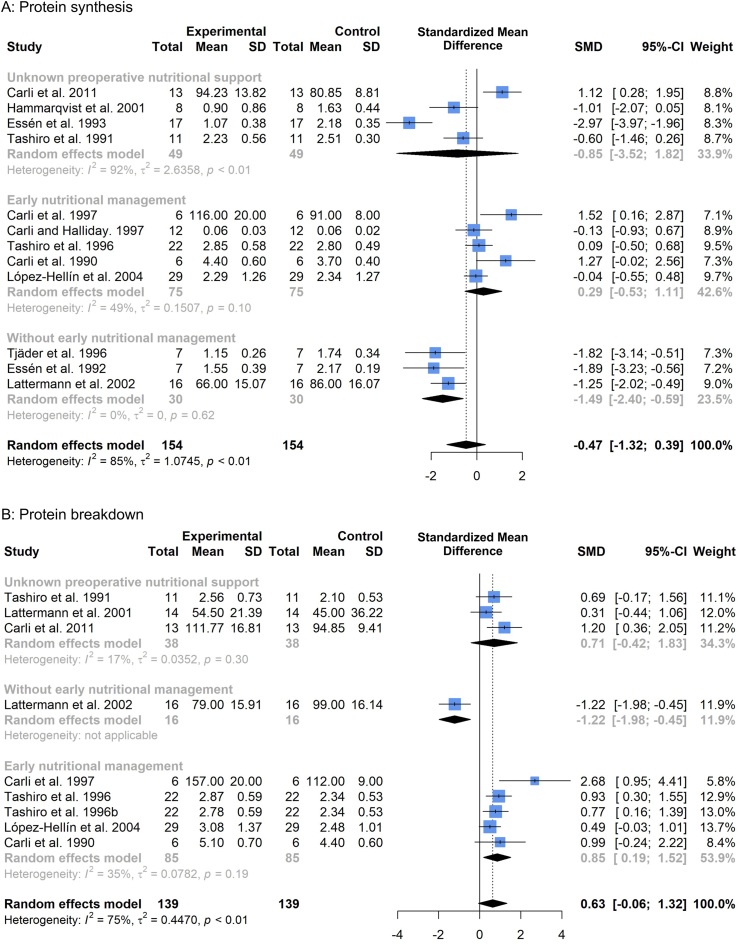
Subgroup analysis of relative changes in protein synthesis (Fig. 4a) pre-post operation illustrated significant suppressions through direct-incorporation methodology (FSR, SMD -1.53, 95%CI: −2.89 to −0.17, p = 0.03). No significant change was observed in whole-body arterio-venous measures (SMD 0.61, 95%CI: −1.48 to 2.70, p = 0.58) or whole-body end-product measures (SMD -0.09, 95%CI: −0.81 to 0.64, p = 0.82). Overall protein synthesis showed a slight trend for suppression, but this did not reach statistical significance (SMD -0.47, 95%CI: −1.32 to 0.39, p = 0.25).
Fig. 4.
Forest plot illustrating relative changes in protein synthesis (A) and protein breakdown (B), before and after surgery, with studies separated into subgroups by stable isotope tracer methodology. A random-effects, inverse-variance model was used to conduct the meta-analysis.
3.3.2. Protein breakdown
Subgroup analysis of relative changes in protein breakdown (Fig. 4b) before and after surgery demonstrated significant increases via whole-body end-product methodology (SMD 0.70, 95%CI: 0.38 to 1.02, p < 0.001). No significant effect was observed via whole-body arterio-venous measures (SMD 0.67, 95%CI: −1.05 to 2.38, p = 0.45). Overall protein breakdown showed a trend for increase, but this did not reach significance (SMD 0.63, 95%CI: −0.06 to 1.32, p = 0.07).
3.4. Preoperative fasting
Nutritional support is a key parameter in the metabolic management of the surgical patient, with recent evidence reinforcing the negative consequences of extended periods of caloric and protein deficits in critically-ill surgical patients [53]. Thus, a key component of many current recommendations on clinical nutrition for the surgical patient advocate the avoidance of prolonged periods of preoperative fasting among elective procedures, particularly within gastrointestinal surgery where bowel preparation has traditionally been common practice [1,19].
3.4.1. Protein synthesis
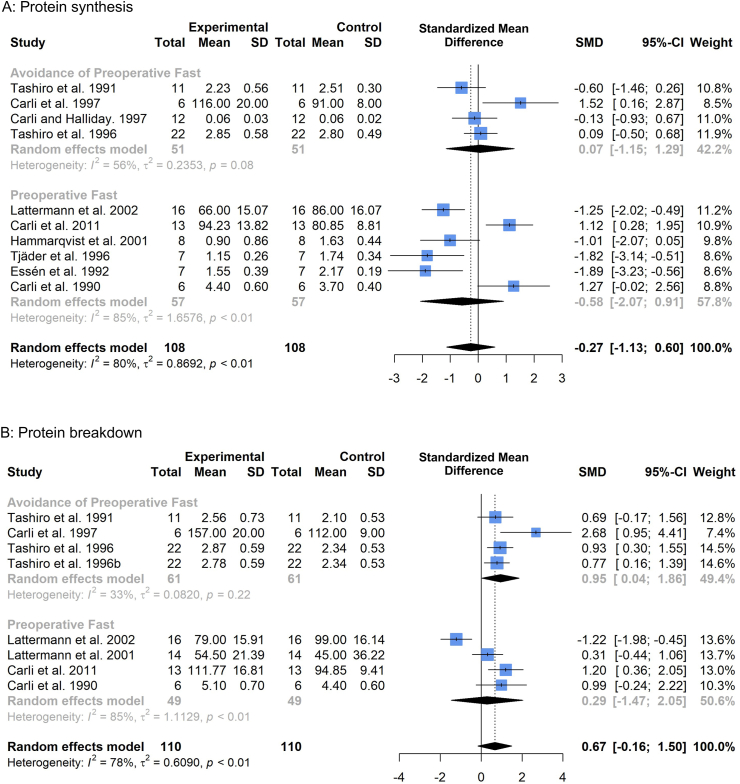
In six studies [[7], [8], [9],41,43,44] measuring protein synthesis within this meta-analysis, patients underwent a preoperative fast as part of conventional perioperative care or bowel preparation. In four studies [6,11,12,42], patients did not undergo a preoperative fast (and were receiving consistent nutritional support prior to operation). One study [46] had to be excluded from subgroup analysis of preoperative fasting due to preoperative study measures of protein synthesis being pooled across two patient cohorts; one of which underwent preoperative fasting and the other avoided preoperative fasting. The study author was unable to provide the necessary information to enable inclusion of these cohorts. Incidence of preoperative fast could not be sourced from a further study [45], due to its inclusion on abstract only, and was consequently excluded from the subgroup analysis. Preoperative fast resulted in no significant changes in protein synthesis (SMD -0.58, 95%CI: −2.07, 0.91, p = 0.45, Fig. 5a), although there was high heterogeneity present among studies (I2: 85%, p < 0.01). Avoidance of preoperative fasting also demonstrated no significant changes (SMD 0.07, 95%CI: −1.15, 1.29, p = 0.92).
Fig. 5.
Forest plot illustrating relative changes in protein synthesis (A) and protein breakdown (B), before and after surgery, with studies separated by whether patients underwent or avoided preoperative fast. A random-effects, inverse-variance model was used to conduct the meta-analysis.
3.4.2. Protein breakdown
In four studies [9,41,43,47] measuring protein breakdown patients underwent a preoperative fast, with four studies [11,12,31,42] containing patient cohorts that avoided preoperative fasting or bowel preparation. As before, one study [46] was excluded due to pooled preoperative baseline measures between fasted and non-fasted patients. Avoidance of preoperative fasting resulted in significant increases in protein breakdown (SMD 0.95, 95%CI: 0.04 to 1.86; p = 0.04, Fig. 5b), with fasted patients demonstrating no significant change (SMD 0.29, 95%CI: −1.47 to 2.05, p = 0.76).
3.5. Preoperative nutritional management
To further examine the role of preoperative nutrition in the metabolic management of the surgical patient, we examined changes in protein turnover following surgery in patients that received controlled nutritional support opposed to those that didn't, as well as for those articles where this information was unknown.
3.5.1. Protein synthesis
Five studies [6,9,12,42,46] provided early nutritional management in the form of controlled dietary intake (Fig. 6a) commenced 3–7 days before surgery. Three studies [7,8,41] did not provide early nutritional management to patients, with this information being unknown for the remaining four studies [11,[43], [44], [45]]. Lack of early nutritional management resulted in significant declines in protein synthesis rates postoperatively (SMD -1.49, 95%CI: −2.40, −0.59, p = 0.001). Postoperative declines in protein synthesis were not present among studies where patients received early nutritional management (SMD 0.29, 95%CI: −0.53, 1.11, p = 0.50). For studies where preoperative nutritional support information was not available, there was a non-significant effect (SMD -0.85, 95%CI: −3.52, 1.82, p = 0.54) and high heterogeneity (I2 = 92%).
Fig. 6.
Forest plot illustrating relative changes in protein synthesis (A) and protein breakdown (B), before and after surgery, with studies separated by whether patients received early nutritional management. A random-effects, inverse-variance model was used to conduct the meta-analysis.
3.5.2. Protein breakdown
Five studies [9,12,31,42,46] provided early nutritional management through controlled dietary intake (Fig. 6b) commenced 3–7 days before surgery. Only one study [41] could be confirmed to have not provided preoperative nutritional management, with information on preoperative nutrition unknown for three studies [11,43,47]. Early nutritional management resulted in elevations in (whole-body) protein breakdown (SMD 0.85, 95%CI: 0.19, 1.52, p = 0.01). The study without preoperative nutritional management [41] demonstrated significant declines in (whole-body) protein breakdown (SMD -1.22, 95%CI: −1.98, −0.45, p = 0.002). Studies where information on preoperative nutritional support was unavailable demonstrated a non-significant effect (SMD 0.71, 95%CI: −0.42, 1.83, p = 0.22).
Further subgroup analyses of nutritional support parameters, such as: nutrient composition, preoperative carbohydrate loading and early postoperative resumption of oral feeding, were not possible with the low study numbers contained within this meta-analysis.
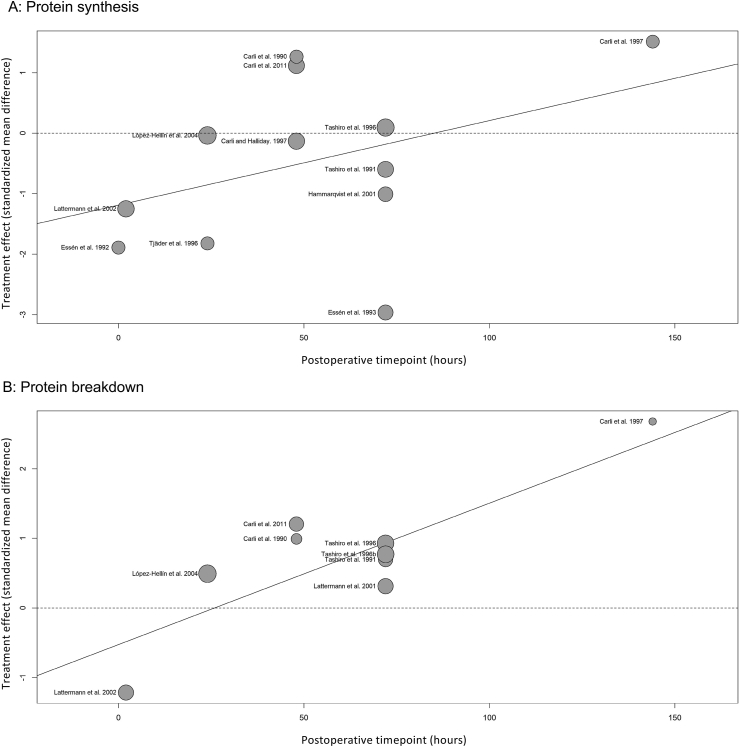
3.6. Time
Meta-regression of postoperative timepoint sampling (representing the proximity of protein turnover measures to surgery) illustrated a trend for early suppressions in protein metabolism with gradual restoration over time towards baseline values. Protein synthesis demonstrated a non-significant trend (p = 0.21, Fig. 7a), while protein breakdown demonstrated a significant trend (p = 0.01, Fig. 7b). However, interpretation of these findings is limited by the small study numbers and with respect to protein breakdown measures, potentially impacted by study homogeneity stemming from three data sets by the same author [11,12,45] being grouped closely together within the meta-regression analysis (Fig. 7b).
Fig. 7.
Bubble plot illustrating meta-regression analysis of postoperative changes in protein synthesis (A) and protein breakdown (B), relative to the timepoint (in hours) of postoperative sampling.
3.7. Anesthesia, epidural blockade and severity of surgical trauma
There was insufficient reporting of open vs. laparoscopic procedures to enable comparisons between the extent of surgical trauma and measures of protein synthesis/breakdown, with specification of these parameters contained within only two studies [7,45]. Anesthetic regimens differed but there were insufficient study numbers to group by minor modalities (specific drug regimens to induce general anesthesia, Table 1). Only three studies [6,41,47] included a patient cohort where all participants received epidural blockade as part of their anesthetic treatment, with a further two studies [43,44] containing patient cohorts receiving mixed anesthetic treatment with and without epidural administration and five studies [9,11,12,31,46] not providing this information. Therefore, no subgroup analyses were performed on these parameters within this meta-analysis.
4. Discussion
4.1. What our study found
Assessment via stable isotope techniques demonstrated trends for reductions in protein synthesis and elevations in protein breakdown to occur following abdominal surgery, within the context of varied perioperative care. These were characterized by significant suppressions in skeletal muscle protein synthesis that were not reflected within whole-body measures and significant increases in whole-body end-product but not arterio-venous protein breakdown.
The findings of this meta-analysis suggest that suppressions in postoperative protein synthesis were not contributed by preoperative fasting but are more importantly regulated by whether sufficient caloric and protein intake of patients was met in the days leading up to their operation. Avoidance of preoperative fasting resulted in elevated protein breakdown that was not reflected in patients that underwent preoperative fast, with early nutritional management also resulting in elevated protein breakdown postoperatively and lack of early preoperative diet management resulting in suppressed protein breakdown. Care must be taken in the interpretation of these findings, as only whole-body protein breakdown was measured and based on the findings of this meta-analysis, these measures likely do not accurately reflect the protein kinetics of skeletal muscle. However, it overall appears that sufficient preoperative caloric and protein intake facilitates increased rates of protein turnover postoperatively. Meta-regression provides limited support for postoperative suppressions in protein turnover to be most acute during the immediate postoperative period, and to thereafter increase with time. This may suggest early recommencement of nutritional support to be vital in the immediate postoperative period, although examination of this effect was unfortunately not possible within this meta-analysis.
4.2. What is available in the literature
Variation in stable isotope assessment of protein kinetics through techniques measuring distinct metabolic pools has previously been observed in surgical patients undergoing coronary artery bypass grafts [54], who demonstrated significant reductions in muscle protein synthesis (-∼36%) but notable increases in plasma fibrinogen (+∼177%) and albumin (+∼45%) synthesis postoperatively. Discrepancy between these metabolic pools has been suggested to be a result of the different metabolic demands these pools are subject to following surgical trauma [55], wherein amino acids are mobilized from skeletal muscle to necessitate energy and healing demands and liver protein metabolism is accelerated to promote the production of acute phase reactants. Increases in whole-body protein turnover associated with healing-driven hypermetabolism, would be in line with traditional observations correlating early wound healing and elevated urinary nitrogen excretion rates among patients in receipt of good preoperative nutrition [4], where administration of parenteral nutrition during the postoperative period appears to augment hypermetabolism compared to hypocaloric glucose [56], but simultaneously results in improved nitrogen balance [57]. Our findings support these concepts. There is a clear disparity between the postoperative synthetic responses of muscle and whole-body, with muscle alone demonstrating significant reductions postoperatively. Preoperative nutrition aimed at meeting the caloric and protein requirements of patients attenuates reductions in protein synthesis and elevates protein breakdown, with lack of unified magnitude in these responses likely reflective of the inclusion of direct-incorporation methodology within studies measuring protein synthesis. This reaffirms the importance of applying stable isotope techniques specific to the metabolic pool of interest to accurately study protein metabolism.
4.3. Strengths and limitations
Only studies utilizing stable isotope tracer methodologies were included in this meta-analysis, with these believed to provide the most comprehensive insight into protein kinetics within the surgical patient [58]. This meta-analysis is strengthened by a pre-test post-test design that enables the accurate determination of relative changes in protein turnover for each patient cohort through measurement of protein turnover in a controlled nutritional state before and after surgery (either postabsorptive or postprandial stable isotope measures). Many previous insights into perioperative catabolism and the investigation of care strategies aimed at modulating the catabolic response to surgery (as measured through stable isotope techniques) have utilized RCT designs centered on postoperative comparisons between cohorts, with many of these studies measuring postabsorptive protein turnover at baseline but postprandial protein turnover postoperatively [[59], [60], [61], [62], [63], [64], [65]] (Supplementary Table 2). Although this design is suitable in discerning the benefits of care strategies aimed at ameliorating catabolism through between-patient comparisons, they are limited in their ability to discern the mechanistic drivers of these changes during the surgical care period within patients.
However, this exclusion resulted in low study numbers that was unfortunately further contributed by the omission of several eligible articles [10,[48], [49], [50], [51]] (Supplementary Table 2) that did not present the continuous data necessary for inclusion in this meta-analysis. Additionally, data from several included papers had to have their means and standard deviations estimated from median and interquartile range [44,45,47]; although this was performed using an established method [35] that has been employed in numerous published meta-analyses. The use of only continuous data to calculate pooled effect sizes does, however, aid in further strengthening the validity of results in the context of a highly heterogenous data set. With reference to the I2 statistic; for both protein synthesis and protein breakdown, only whole-body [EP] measures under tracer subgrouping had an I2 statistic <25%, with the majority of subgroups having an I2 statistic >50%. With low study numbers, it is difficult to discern whether this reduced heterogeneity may be due to the necessary control of nutritional intake to enable accurate stable isotope measures [66] or whether it is influenced by many of these studies being performed by the same research group potentially utilizing standardized procedures [11,12,45]. Overall, heterogeneity for protein synthesis was 85% and 75% for protein breakdown, potentially lower due to the lack of direct-incorporation measures. This high variation must be taken into consideration when evaluating the findings of this meta-analysis, but with such low study numbers this observation is not unexpected, even within a strictly defined meta-analysis design.
Subgroup analyses investigating the impacts of preoperative nutrition on postoperative changes in protein synthesis and breakdown demonstrated preoperative fasting to result in high heterogeneity among study results (I2 = 85% for both protein synthesis and breakdown). Less heterogeneity was present among studies where preoperative fasting was avoided (I2 = 56% for protein synthesis, I2 = 33% for protein breakdown). For studies measuring protein synthesis, early nutritional management presented moderate heterogeneity (I2 = 49%) and lack of early nutritional management presented low heterogeneity (I2 = 0%), with unknown nutritional management demonstrating expectantly high heterogeneity (I2 = 92%). Interpretation of heterogeneity regarding nutritional management for studies measuring protein breakdown is limited due to the presence of only one study that did not receive early nutritional management. Overall, heterogeneity was low to moderate for these results. Following the high heterogeneity present among tracer methodology subgroup analyses, mixed tracer subgrouping by nutritional parameters resulted in relatively low heterogeneity. These observations may support preoperative nutrition to exert effects on the postoperative response of protein turnover, likely through the administration of regimented dietary intake providing adequate caloric and protein intake among patients for their metabolic demands. Unfortunately, varied pre- and postoperative nutritional regimens and varied postoperative nutritional administration prevented examination of these parameters with the low study numbers contained within this meta-analysis.
5. Conclusions
Elective abdominal surgery elicits suppressions in skeletal muscle protein synthesis remote to the site of trauma that are not reflected on a whole-body level. Lack of uniform changes across whole-body tracer techniques are likely due to contribution from tissues other than skeletal muscle and complicate the discernment of mechanistic processes driving postoperative skeletal muscle wasting. Future work should focus on tissue-specific stable isotope approaches to comprehensively characterize the protein turnover responses of skeletal muscle, within the context of enhanced recovery after surgery care strategies.
Funding
This work was supported by the Medical Research Council [grant number MR/K00414X/1]; Arthritis Research UK [grant number 19891]. Matthew Jaconelli is in receipt of a funded PhD studentship from the Medical Research Council/Versus Arthritis Centre for Musculoskeletal Ageing Research. Matthew S. Brook is a recipient of a Research Fellowship from the European Society for Clinical Nutrition and Metabolism (ESPEN).
Author contributions
Study design – All authors.
Data extraction and analysis – MJ and MSB.
Writing of the manuscript – All authors.
Critical review – PLG, PJA and DNL.
Final approval – All authors.
Conflicts of interest
None of the authors has a conflict of interest to declare.
Acknowledgements
The authors of this manuscript would like to thank, Dr. Mario Siervo, for his critical feedback on the manuscript prior to final submission.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.clnu.2022.01.023.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Weimann A., Braga M., Carli F., Higashiguchi T., Hübner M., Klek S., et al. ESPEN guideline: clinical nutrition in surgery. Clin Nutr. 2017;36:623–650. doi: 10.1016/j.clnu.2017.02.013. [DOI] [PubMed] [Google Scholar]
- 2.Cuthbertson D.P. Observations on the disturbance of metabolism produced by injury to the limbs. QJM. 1932;1:233–246. [Google Scholar]
- 3.Cuthbertson D.P. The metabolic response to injury and other related explorations in the field of protein metabolism: an autobiographical account. Scot Med J. 1982;27:158–171. doi: 10.1177/003693308202700210. [DOI] [PubMed] [Google Scholar]
- 4.Moore F.D. W. B. Saunders Co; Philadelphia: 1959. Metabolic care of the surgical patient. [Google Scholar]
- 5.Brook M.S., Wilkinson D.J., Phillips B.E., Perez-Schindler J., Philp A., Smith K., et al. Skeletal muscle homeostasis and plasticity in youth and ageing: impact of nutrition and exercise. Acta Physiol. 2016;216:15–41. doi: 10.1111/apha.12532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carli F., Halliday D. Continuous epidural blockade arrests the postoperative decrease in muscle protein fractional synthetic rate in surgical patients. Anesthesiology. 1997;86:1033–1040. doi: 10.1097/00000542-199705000-00005. [DOI] [PubMed] [Google Scholar]
- 7.Tjäder I., Essen P., Thörne A., Garlick P.J., Wernerman J., McNurlan M.A. Muscle protein synthesis rate decreases 24 hours after abdominal surgery irrespective of total parenteral nutrition. JPEN - J Parenter Enter Nutr. 1996;20:135–138. doi: 10.1177/0148607196020002135. [DOI] [PubMed] [Google Scholar]
- 8.Essén P., McNurlan M.A., Wernerman J., Vinnars E., Garlick P.J. Uncomplicated surgery, but not general anesthesia, decreases muscle protein synthesis. Am J Physiol. 1992;262:E253–E260. doi: 10.1152/ajpendo.1992.262.3.E253. [DOI] [PubMed] [Google Scholar]
- 9.Carli F., Webster J., Ramachandra V., Pearson M., Read M., Ford G.C., et al. Aspects of protein metabolism after elective surgery in patients receiving constant nutritional support. Clin Sci. 1990;78:621–628. doi: 10.1042/cs0780621. [DOI] [PubMed] [Google Scholar]
- 10.Carli F., Halliday D. Modulation of protein metabolism in the surgical patient. Effect of 48-hour continuous epidural block with local anesthetics on leucine kinetics. Reg Anesth. 1996;21:430–435. [PubMed] [Google Scholar]
- 11.Tashiro T., Mashima Y., Yamamori H., Horibe K., Nishizawa M., Okui K. Alteration of whole-body protein kinetics according to severity of surgical trauma in patients receiving total parenteral nutrition. JPEN - J Parenter Enter Nutr. 1991;15:169–172. doi: 10.1177/0148607191015002169. [DOI] [PubMed] [Google Scholar]
- 12.Tashiro T., Yamamori H., Takagi K., Morishima Y., Nakajima N. Effect of severity of stress on whole-body protein kinetics in surgical patients receiving parenteral nutrition. Nutrition. 1996;12:763–765. doi: 10.1016/s0899-9007(96)00214-6. [DOI] [PubMed] [Google Scholar]
- 13.Tipton K.D., Hamilton D.L., Gallagher I.J. Assessing the role of muscle protein breakdown in response to nutrition and exercise in humans. Sports Med. 2018;48:53–64. doi: 10.1007/s40279-017-0845-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Holm L., O'Rourke B., Ebenstein D., Toth M.J., Bechshoeft R., Holstein-Rathlou N.-H., et al. Determination of steady-state protein breakdown rate in vivo by the disappearance of protein-bound tracer-labeled amino acids: a method applicable in humans. Am J Physiol Endocrinol Metab. 2013;304:E895–E907. doi: 10.1152/ajpendo.00579.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Holm L., Dideriksen K., Nielsen R.H., Doessing S., Bechshoeft R.L., Højfeldt G., et al. An exploration of the methods to determine the protein-specific synthesis and breakdown rates in vivo in humans. Phys Rep. 2019;7:e14143. doi: 10.14814/phy2.14143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Varadhan K.K., Constantin-Teodosiu D., Constantin D., Greenhaff P.L., Lobo D.N. Inflammation-mediated muscle metabolic dysregulation local and remote to the site of major abdominal surgery. Clin Nutr. 2018;37:2178–2185. doi: 10.1016/j.clnu.2017.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Platell C., Hall J. What is the role of mechanical bowel preparation in patients undergoing colorectal surgery? Dis Colon Rectum. 1998;41:875–882. doi: 10.1007/BF02235369. discussion 882-883. [DOI] [PubMed] [Google Scholar]
- 18.Yuill K.A., Richardson R.A., Davidson H.I.M., Garden O.J., Parks R.W. The administration of an oral carbohydrate-containing fluid prior to major elective upper-gastrointestinal surgery preserves skeletal muscle mass postoperatively--a randomised clinical trial. Clin Nutr. 2005;24:32–37. doi: 10.1016/j.clnu.2004.06.009. [DOI] [PubMed] [Google Scholar]
- 19.Gustafsson U.O., Scott M.J., Hubner M., Nygren J., Demartines N., Francis N., et al. Guidelines for perioperative care in elective colorectal surgery: enhanced Recovery after Surgery (ERAS®) Society recommendations: 2018. World J Surg. 2019;43:659–695. doi: 10.1007/s00268-018-4844-y. [DOI] [PubMed] [Google Scholar]
- 20.El-Sharkawy A.M., Daliya P., Lewis-Lloyd C., Adiamah A., Malcolm F.L., Boyd-Carson H., et al. Fasting and surgery timing (FaST) audit. Clin Nutr. 2021;40:1405–1412. doi: 10.1016/j.clnu.2020.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Matthews D.E., Motil K.J., Rohrbaugh D.K., Burke J.F., Young V.R., Bier D.M. Measurement of leucine metabolism in man from a primed, continuous infusion of L-[1-3C]leucine. Am J Physiol. 1980;238:E473–E479. doi: 10.1152/ajpendo.1980.238.5.E473. [DOI] [PubMed] [Google Scholar]
- 22.Rennie M.J., Edwards R.H., Halliday D., Matthews D.E., Wolman S.L., Millward D.J. Muscle protein synthesis measured by stable isotope techniques in man: the effects of feeding and fasting. Clin Sci (Lond) 1982;63:519–523. doi: 10.1042/cs0630519. [DOI] [PubMed] [Google Scholar]
- 23.Ford G.C., Cheng K.N., Halliday D. Analysis of (1-13C)leucine and (13C)KIC in plasma by capillary gas chromatography/mass spectrometry in protein turnover studies. Biomed Mass Spectrom. 1985;12:432–436. doi: 10.1002/bms.1200120814. [DOI] [PubMed] [Google Scholar]
- 24.Garlick P.J., Wernerman J., McNurlan M.A., Essen P., Lobley G.E., Milne E., et al. Measurement of the rate of protein synthesis in muscle of postabsorptive young men by injection of a “flooding dose” of [1-13C]leucine. Clin Sci. 1989;77:329–336. doi: 10.1042/cs0770329. [DOI] [PubMed] [Google Scholar]
- 25.Harrison R.A., Lewin M.R., Halliday D., Clark C.G. Leucine kinetics in surgical patients. I: a study of the effect of surgical “stress. Br J Surg. 1989;76:505–508. doi: 10.1002/bjs.1800760524. [DOI] [PubMed] [Google Scholar]
- 26.Harrison R.A., Lewin M.R., Halliday D., Clark C.G. Leucine kinetics in surgical patients. II: a study of the effect of malignant disease and tumour burden. Br J Surg. 1989;76:509–511. doi: 10.1002/bjs.1800760525. [DOI] [PubMed] [Google Scholar]
- 27.Wilkinson D.J. Historical and contemporary stable isotope tracer approaches to studying mammalian protein metabolism. Mass Spectrom Rev. 2018;37:57–80. doi: 10.1002/mas.21507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Page M.J., McKenzie J.E., Bossuyt P.M., Boutron I., Hoffmann T.C., Mulrow C.D., et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shea B.J., Reeves B.C., Wells G., Thuku M., Hamel C., Moran J., et al. AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ. 2017;358:j4008. doi: 10.1136/bmj.j4008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Millward D.J., Smith K. The application of stable-isotope tracers to study human musculoskeletal protein turnover: a tale of bag filling and bag enlargement. J Physiol. 2019;597:1235–1249. doi: 10.1113/JP275430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tashiro T., Yamamori H., Takagi K., Morishima Y., Nakajima N. Increased contribution by myofibrillar protein to whole-body protein breakdown according to severity of surgical stress. Nutrition. 1996;12:685–689. doi: 10.1016/s0899-9007(96)00166-9. [DOI] [PubMed] [Google Scholar]
- 32.MacLennan P.A., Rennie M.J. Effects of ischaemia, blood loss and reperfusion on rat muscle protein synthesis, metabolite concentrations and polyribosome profiles in vivo. Biochem J. 1989;260:195–200. doi: 10.1042/bj2600195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tjäder I., Essen P., Garlick P.J., McMnurlan M.A., Rooyackers O., Wernerman J. Impact of surgical trauma on human skeletal muscle protein synthesis. Clin Sci (Lond) 2004;107:601–607. doi: 10.1042/CS20040192. [DOI] [PubMed] [Google Scholar]
- 34.Higgins J.P.T., Thomas J., Chandler J., Cumpston M., Li T., Page M.J., et al., editors. Cochrane Handbook for systematic reviews of interventions version 6.2. Cochrane; 2021. www.training.cochrane.org/handbook Available from. [Google Scholar]
- 35.Hozo S.P., Djulbegovic B., Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol. 2005;5:13. doi: 10.1186/1471-2288-5-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Higgins J.P.T., Altman D.G., Gøtzsche P.C., Jüni P., Moher D., Oxman A.D., et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pustejovsky J.E., Rodgers M.A. Testing for funnel plot asymmetry of standardized mean differences. Res Synth Methods. 2019;10:57–71. doi: 10.1002/jrsm.1332. [DOI] [PubMed] [Google Scholar]
- 38.DerSimonian R., Laird N. Meta-analysis in clinical trials. Contr Clin Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 39.Knapp G., Hartung J. Improved tests for a random effects meta-regression with a single covariate. Stat Med. 2003;22:2693–2710. doi: 10.1002/sim.1482. [DOI] [PubMed] [Google Scholar]
- 40.Higgins J.P.T., Thompson S.G. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 41.Lattermann R., Carli F., Wykes L., Schricker T. Epidural blockade modifies perioperative glucose production without affecting protein catabolism. Anesthesiology. 2002;97:374–381. doi: 10.1097/00000542-200208000-00014. [DOI] [PubMed] [Google Scholar]
- 42.Carli F., Webster J.D., Halliday D. Growth hormone modulates amino acid oxidation in the surgical patient: leucine kinetics during the fasted and fed state using moderate nitrogenous and caloric diet and recombinant human growth hormone. Metab Clin Exp. 1997;46:23–28. doi: 10.1016/s0026-0495(97)90162-1. [DOI] [PubMed] [Google Scholar]
- 43.Carli F., Ball J., Wykes L., Kubow S. Oral whey protein decreases protein breakdown and increases protein balance in surgical patients. A stable isotope study. Can J Anesth. 2011;58:S13. [Google Scholar]
- 44.Hammarqvist F., Sandgren A., Andersson K., Essén P., McNurlan M.A., Garlick P.J., et al. Growth hormone together with glutamine-containing total parenteral nutrition maintains muscle glutamine levels and results in a less negative nitrogen balance after surgical trauma. Surgery. 2001;129:576–586. doi: 10.1067/msy.2001.112593. [DOI] [PubMed] [Google Scholar]
- 45.Essén P., McNurlan M.A., Sonnenfeld T., Milne E., Vinnars E., Wernerman J., et al. Muscle protein synthesis after operation: effects of intravenous nutrition. Eur J Surg. 1993;159:195–200. [PubMed] [Google Scholar]
- 46.López-Hellín J., Baena-Fustegueras J.A., Vidal M., Riera S.S., García-Arumí E. Perioperative nutrition prevents the early protein losses in patients submitted to gastrointestinal surgery. Clin Nutr. 2004;23:1001–1008. doi: 10.1016/j.clnu.2003.12.006. [DOI] [PubMed] [Google Scholar]
- 47.Lattermann R., Schricker T., Wachter U., Goertz A., Georgieff M. Intraoperative epidural blockade prevents the increase in protein breakdown after abdominal surgery. Acta Anaesthesiol Scand. 2001;45:1140–1146. doi: 10.1034/j.1399-6576.2001.450915.x. [DOI] [PubMed] [Google Scholar]
- 48.Carli F., Webster J., Pearson M., Forrest J., Venkatesan S., Wenham D., et al. Postoperative protein metabolism: effect of nursing elderly patients for 24 h after abdominal surgery in a thermoneutral environment. Br J Anaesth. 1991;66:292–299. doi: 10.1093/bja/66.3.292. [DOI] [PubMed] [Google Scholar]
- 49.Carli F., Webster J., Pearson M., Pearson J., Bartlett S., Bannister P., et al. Protein metabolism after abdominal surgery: effect of 24-h extradural block with local anaesthetic. Br J Anaesth. 1991;67:729–734. doi: 10.1093/bja/67.6.729. [DOI] [PubMed] [Google Scholar]
- 50.Carli F., Webster J.D., Halliday D. A nitrogen-free hypocaloric diet and recombinant human growth hormone stimulate postoperative protein synthesis: fasted and fed leucine kinetics in the surgical patient. Metabolism. 1997;46:796–800. doi: 10.1016/s0026-0495(97)90125-6. [DOI] [PubMed] [Google Scholar]
- 51.López Hellín J., Baena-Fustegueras J.A., Sabín-Urkía P., Schwartz-Riera S., García-Arumí E. Nutritional modulation of protein metabolism after gastrointestinal surgery. Eur J Clin Nutr. 2008;62:254–262. doi: 10.1038/sj.ejcn.1602732. [DOI] [PubMed] [Google Scholar]
- 52.Carli F., Ramachandra V., Gandy J., Merritt H., Ford G.C., Read M., et al. Effect of general anaesthesia on whole body protein turnover in patients undergoing elective surgery. Br J Anaesth. 1990;65:373–379. doi: 10.1093/bja/65.3.373. [DOI] [PubMed] [Google Scholar]
- 53.Yeh D.D., Fuentes E., Quraishi S.A., Cropano C., Kaafarani H., Lee J., et al. Adequate nutrition may get you home. JPEN - J Parenter Enter Nutr. 2016;40:37–44. doi: 10.1177/0148607115585142. [DOI] [PubMed] [Google Scholar]
- 54.Caso G., Vosswinkel J.A., Garlick P.J., Barry M.K., Bilfinger T.V., McNurlan M.A. Altered protein metabolism following coronary artery bypass graft (CABG) surgery. Clin Sci (Lond) 2008;114:339–346. doi: 10.1042/CS20070278. [DOI] [PubMed] [Google Scholar]
- 55.Gillis C., Carli F. Promoting perioperative metabolic and nutritional care. Anesthesiology. 2015;123:1455–1472. doi: 10.1097/ALN.0000000000000795. [DOI] [PubMed] [Google Scholar]
- 56.Pöyhönen M.J., Takala J.A., Pitkänen O., Kari A., Alhava E., Alakuijala L.A., et al. Urinary excretion of polyamines in patients with surgical and accidental trauma: effect of total parenteral nutrition. Metabolism. 1993;42:44–51. doi: 10.1016/0026-0495(93)90170-s. [DOI] [PubMed] [Google Scholar]
- 57.Svanfeldt M., Thorell A., Nygren J., Ljungqvist O. Postoperative parenteral nutrition while proactively minimizing insulin resistance. Nutrition. 2006;22:457–464. doi: 10.1016/j.nut.2005.06.013. [DOI] [PubMed] [Google Scholar]
- 58.Schricker T., Lattermann R. Perioperative catabolism. Can J Anaesth. 2015;62:182–193. doi: 10.1007/s12630-014-0274-y. [DOI] [PubMed] [Google Scholar]
- 59.Lattermann R., Wykes L., Eberhart L., Carli F., Meterissian S., Schricker T. A randomized controlled trial of the anticatabolic effect of epidural analgesia and hypocaloric glucose. Reg Anesth Pain Med. 2007;32:227–232. doi: 10.1016/j.rapm.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 60.Schricker T., Meterissian S., Lattermann R., Adegoke O.A.J., Marliss E.B., Mazza L., et al. Anticatabolic effects of avoiding preoperative fasting by intravenous hypocaloric nutrition: a randomized clinical trial. Ann Surg. 2008;248:1051–1059. doi: 10.1097/SLA.0b013e31818842d8. [DOI] [PubMed] [Google Scholar]
- 61.Lugli A.K., Schricker T., Wykes L., Lattermann R., Carli F. Glucose and protein kinetics in patients undergoing colorectal surgery: perioperative amino acid versus hypocaloric dextrose infusion. Metab Clin Exp. 2010;59:1649–1655. doi: 10.1016/j.metabol.2010.03.017. [DOI] [PubMed] [Google Scholar]
- 62.Lattermann R., Carli F., Wykes L., Schricker T. Perioperative glucose infusion and the catabolic response to surgery: the effect of epidural block. Anesth Analg. 2003;96:555–562. doi: 10.1097/00000539-200302000-00047. [DOI] [PubMed] [Google Scholar]
- 63.Schricker T., Meterissian S., Wykes L., Eberhart L., Lattermann R., Carli F. Postoperative protein sparing with epidural analgesia and hypocaloric dextrose. Ann Surg. 2004;240:916–921. doi: 10.1097/01.sla.0000143249.93856.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Schricker T., Wykes L., Eberhart L., Carli F., Meterissian S. Randomized clinical trial of the anabolic effect of hypocaloric parenteral nutrition after abdominal surgery. Br J Surg. 2005;92:947–953. doi: 10.1002/bjs.5105. [DOI] [PubMed] [Google Scholar]
- 65.Schricker T., Wykes L., Meterissian S., Hatzakorzian R., Eberhart L., Carvalho G., et al. The anabolic effect of perioperative nutrition depends on the patient's catabolic state before surgery. Ann Surg. 2013;257:155–159. doi: 10.1097/SLA.0b013e31825ffc1f. [DOI] [PubMed] [Google Scholar]
- 66.Fern E.B., Garlick P.J., McNurlan M.A., Waterlow J.C. The excretion of isotope in urea and ammonia for estimating protein turnover in man with [15N]glycine. Clin Sci (Lond) 1981;61:217–228. doi: 10.1042/cs0610217. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.