Abstract
Cardiac stromal cells have been long underestimated in their functions in homeostasis and repair. Recent evidence has changed this perspective in that many more players and facets than just “cardiac fibroblasts” have entered the field. Single cell transcriptomic studies on cardiac interstitial cells have shed light on the phenotypic plasticity of the stroma, whose transcriptional profile is dynamically regulated in homeostatic conditions and in response to external stimuli. Different populations and/or functional states that appear in homeostasis and pathology have been described, particularly increasing the complexity of studying the cardiac response to injury. In this review, we outline current phenotypical and molecular markers, and the approaches developed for identifying and classifying cardiac stromal cells. Significant advances in our understanding of cardiac stromal populations will provide a deeper knowledge on myocardial functional cellular components, as well as a platform for future developments of novel therapeutic strategies to counteract cardiac fibrosis and adverse cardiac remodeling.
Keywords: Cardiac fibroblasts, cardiac stromal cells, fibroblast markers, cardiac fibrosis, heart failure, cardiac remodeling, single-cell sequencing, omics data
Introduction
Stromal cells have been long underestimated in their functions in multiple tissues. A classical view of poorly specialized filler cells has been the reference until recently. Now the scenario is much different, even in proper connective tissues, such as the derma, where multiple populations of fibroblasts have been identified with very different behaviors, particularly concerning tissue repair and fibrosis mechanisms [1,2]. The same change of perspective has affected the heart and its stromal populations, where many more players and facets than just “cardiac fibroblasts” have entered the field.
Several stromal populations have been described in the mammalian heart, with specific homeostatic roles, particularly concerning the synthesis and maintenance of the extracellular matrix (ECM), and the trophic support to other specialized cells, such as endothelial cells or cardiomyocytes. Many membrane and intracellular markers have been associated to each specific stromal phenotype, although with much overlap, and often lacking a unique consensus on the panel to be used to define and/or distinguish a single cell type (Table 1). This complex scenario implicitly suggests the existence of blurred lines separating distinct cell types or subpopulations, and that at least some of those populations may as well be different functional manifestations of a number of cell types much smaller than those described in the literature. Therefore, the field still needs to investigate phenotypes, markers, and functions thoroughly and comparatively.
Table 1.
A summary of cardiac stromal cell markers identified through the years, and sorted as classical or derived from novel omics approaches
| Classical Markers | New Omics Markers | |||
|---|---|---|---|---|
|
|
|
|||
| Cell surface and Intracellular | Extracellular | Transcriptional | Cell surface, Extracellular & Transcriptional | |
| Unactivated Fibroblasts | PDGFRα, DDR2, CD29, CD49e, CD51, CD73, CD90, CD105, Sca1, Vimentin, Filamin A | Collagens, TNC | GATA4, GATA6, Nkx-2.5, Hand2, Tbx18, Tbx20, Tcf-21, WT1 | DCN, ELN, GSN |
| Activated Fibroblasts | ↑CD105, CD90, ↓PDGFRα, ↑Vimentin, ↑DDR2, α-SMA | POSTN, Collagens | ↓Tcf21, ↑Tbx18, ↑Wt1 | Wisp1, Ckap4 |
| Myofibroblasts | ↑CD105, ↓PDGFRα, ↓DDR2, ↑α-SMA | ↑↑Collagens | ↓Tcf21 | MYH11, FAP |
| Matrifibrocytes | CHAD, COMP | |||
| Pericytes | PDGFRα, PDGFRβ, NG2, CD146, CD73, CD90, CD105, CD271, SM-MHC, α-SMA | NCAM2, CD38, CSPG4, ABCC9, KCNJ8 | ||
| Telocytes | CD34, CD117, PDGFRα, PDGFRβ, Vimentin | |||
| Mesenchymal & Progenitor cells | CD51, CD105, CD73, CD90, Sca1, PDGFRα, Filamin A, Vimentin | Collagens | Islet-1, Tbx5, GATA4, Nkx-2.5, MEF2C, Tcf21, NANOG, OCT-4 | CD38, ICAM2, Caecam1, CD36, CD93, CD322, KITL, JAG2, VEGF-C |
The cardiac stromal compartment possesses a fundamental role in physiopathology due to its many roles in ECM remodeling, fibrosis, angiogenic signaling, and crosstalk with the immune compartment. Multiple studies have demonstrated its pivotal role in the pathogenesis and outcome of several diseases, thus suggesting that its targeting may be highly effective for novel therapeutic strategies against cardiac diseases, particularly those involving fibrosis and adverse remodeling. In this review, we are presenting an overview of cardiac stromal cell types and functional states described in the literature (excluding the immune compartment), and the markers used to identify them in different physiological and pathological conditions. Moreover, we are discussing some perspectives on the possible exploitation of stromal cells as mediators or targets of novel therapeutic approaches for the treatment of cardiac diseases.
Cardiac fibroblasts and their classical markers
Fibroblasts have classically been studied and described as a unique cell type with a standard phenotype, independently of tissue origin, whose only function was to synthesize and remodel the ECM. This reductionist view has been challenged in the last decade, particularly by recent single-cell transcriptomic data, demonstrating high phenotypic heterogeneity and plasticity of fibroblasts, both under homeostatic and pathological conditions. In this regard, it is now clear that cardiac fibroblasts (CFs) do not represent a mono-dimensional population within the heart whose only role is to support cardiomyocytes and regulate ECM turnover. Instead, many studies have highlighted the diversity of cells listed as CFs with diverse localization and specialized properties, in both humans and other species. Mature fibroblasts are interspersed in the myocardium, and are able to maintain ECM homeostasis. In humans they are strongly positive for transcription factor 21 (Tcf21) [3], platelet-derived growth factor receptor alpha (PDGFR-α) [4], discoidin domain containing receptor 2 (DDR2) [5,6], and vimentin [7]; conversely, they do not express alpha smooth muscle actin (α-SMA) and Periostin (POSTN). Mature fibroblasts have a low level of proliferation, but after injury they can rapidly proliferate and become activated fibroblasts, characterized by increased expression of Tcf21, PDGFR-α, POSTN, collagens, cell cycle genes, DDR2, and vimentin (as will be more accurately described in a dedicated paragraph below). After activation, a small proportion of these cells differentiate into myofibroblasts, which express α-SMA and produce collagens, while displaying reduced expression of Tcf21 and PDGFR-α.
Below is a brief presentation of the above-mentioned classical markers that are widely used to characterize the dynamic shift of mature fibroblasts into myofibroblasts, as we will explain later. The related intracellular pathways are depicted in Figure 1.
Figure 1.
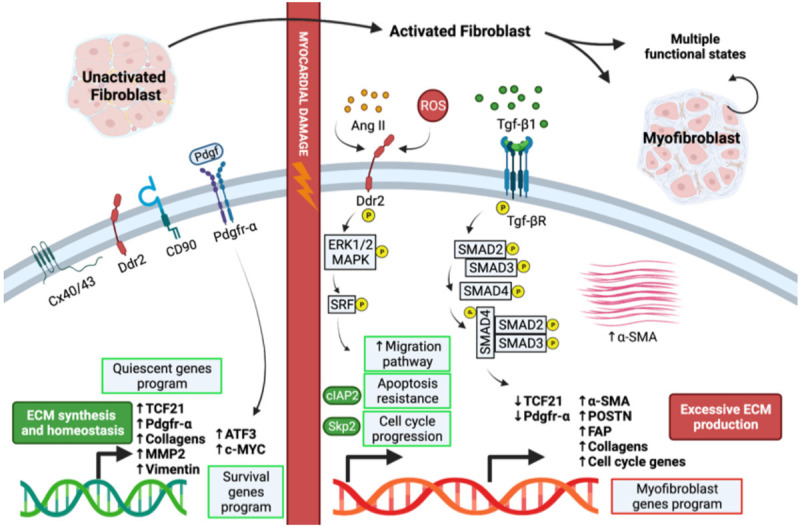
The phenotypic shift of fibroblasts into myofibroblasts. Depiction of signaling pathways and functions involved in the activation of fibroblasts and differentiation into myofibroblasts. Image was created with the Biorender software. Cx40/43: connexin 40/43. MMP2: matrix metallo-proteinase 2. ATF3: activating transcription factor 3. c-MYC: cellular myelocytomatosis oncogene product. Ang II: angiotensin II. ROS: reactive oxygen species. Erk1/2: extracellular signal-regulated kinase 1/2. MAPK: mitogen-activated protein kinase. SMAD: small mother against decapentaplegic. cIAP2: cellular inhibitor of apoptosis 2. SKP2: S-phase kinase associated protein 2. SRF: serum response factor.
● Tcf21 encodes for a transcription factor of the basic helix-loop-helix family, which is mesoderm specific and expressed in the embryonic epicardium. It plays a crucial role in regulating cell differentiation and cell fate specificity through epithelial-to-mesenchymal transition (EMT) during cardiac development, but it is still active in adult resident CFs [3].
● PDGFR-α encodes for a cell surface tyrosine kinase receptor for members of the platelet-derived growth factor family. PDGFR-α signaling directs migration and differentiation of epicardial-derived fibroblasts during heart development [8]. In the adult heart, multiple studies have established that PDGFR-α signaling controls CFs proliferation and activation. Moreover, human CFs require PDGFR-α signaling for survival [4].
● DDR2 encodes for a member of the discoidin domain receptor subclass of the receptor tyrosine kinase protein family. It is expressed on the surface of cells of mesenchymal origin. DDR2 mediates a variety of cell functions, including growth, migration, differentiation, EMT, and is associated with the fibrotic process. The DDR2 receptor is also present in myofibroblasts, therefore it cannot be used to distinguish between cell sub-types unless a combination of markers is used. Moreover, there are controversial results in the literature regarding the abundance of DDR2+ fibroblasts [9].
● Vimentin is a type III intermediate filament protein that is expressed in multiple cells. It plays a significant role in maintaining cell shape and integrity of the cytoplasm, and in stabilizing cytoskeletal interactions. Vimentin is commonly used as a CFs marker because it labels cells with great sensitivity, however it is not specific. In fact, vascular smooth muscle cells (V-SMCs), endothelial cells, and macrophages also express vimentin [10].
● Alpha-SMA (αSMA) is a member of the highly conserved actin family of proteins, which plays a key role in cell motility, structure, and integrity. It is a classical marker used to distinguish mature fibroblasts from activated fibroblasts and myofibroblasts. However, this actin isoform is also expressed in V-SMCs and pericytes (see also dedicated paragraph). α-SMA is not expressed under homeostatic conditions, but it is up-regulated in response to pro-fibrotic and hypertrophic stimuli in the human heart [11].
● POSTN is a transforming growth factor-beta 1 (TGF-β1)-inducible secreted extracellular protein that plays essential roles in wound healing, ECM deposition, CFs activation and proliferation, and tissue fibrosis. POSTN is highly expressed during cardiac development, but its expression is reduced in un-activated fibroblasts. However, it is up-regulated after injury, such as myocardial infarction; therefore it represents a consensus marker of activated fibroblasts and myofibroblasts [12].
Studies in multiple species, particularly in mice, have shown that, in addition to the above-mentioned classical markers, CFs share the expression of many common fibroblast markers, such as collagens 1α1/1α2, filamin A, and Tenascin C (TNC). In addition, they are characterized by significant heterogeneity of cell surface receptors: virtually all cells are positive for CD29, CD49e, CD51, while a vast majority express CD90 and stem cell antigen 1 (Sca1) [13]. CFs express many other cardiogenic transcription factors, such as Tbx18, Tbx20, GATA4/6, Hand2 and Nkx-2.5 (Table 1; Figure 2), also involved in cardiomyocyte (CM) development and function. Expression programs reveal a heterogeneous landscape of CFs, partially due to their regional specification. Tbx20 is among the highest and most consistently expressed genes in fibroblasts of all cardiac compartments. Furtado et al. have demonstrated that this transcription factor plays a key role in the development and maturation of both myocardial and non-myocardial compartments [13]. The expression of epicardial genes Tcf21 and Wt1 (particularly in ventricular or atrial CFs, respectively) also endorses the epicardial origin of most CFs. Overall, Tcf21, Wt1, and Tbx18 are transcription factors expressed during embryonic development that regulate the fate of epicardial cells and their differentiation towards various lineages, including fibroblasts. In the adult murine heart, instead, Tcf21 is expressed at baseline also by some perivascular and interstitial cells. Cardiac injury, though, increases the expression of Tcf21, first in the epicardial region and then in the myocardial interstitium where fibrosis is induced [8]. Therefore, tissue damage leads to the expression of Tcf21, Wt1, and Tbx18 in different subepicardial mesenchymal populations, recapitulating what happens during embryonic development [14].
Figure 2.
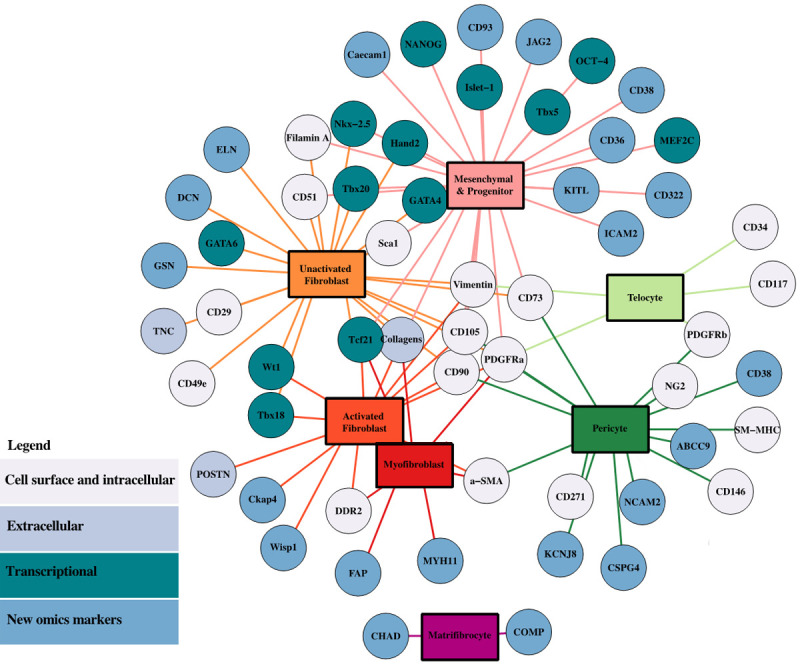
Markers of cardiac stromal cell types. Network of the associations between cardiac stromal cell types and selected markers discussed in the review. The graphic was created by the igraph package of the RStudio software.
Activated fibroblasts and myofibroblasts
The activation of Tcf21+ fibroblasts following cardiac injury is functional to their differentiation into myofibroblasts, typically identified by the expression of α-SMA and their localization within the scar site [15]. In a mouse model of myocardial infarction, activated fibroblasts up-regulate proliferation and migration pathways, as well as cytoskeletal and ECM-modifying genes; then this expression profile is down-regulated few weeks after injury. For some classical markers (e.g., Tcf21, PDGFR-α) the specific time-course of gene expression modulation remains partly debated, with some authors reporting downregulation instead of upregulation [16]. Other genes typical of bone, connective tissue, cartilage, and tendon development or processing, characterize this activated state as well. Induction of the myofibroblast phenotype, instead, has been associated to a multitude of stimuli, and the key effector in this process is the cytokine TGF-β1 (Figure 1). Moreover, myofibroblast activation is a hallmark of several cardiovascular diseases, as these cells are responsible for the excessive deposition of ECM proteins, and are the primary drivers of cardiac fibrosis.
Although α-SMA is a key marker of myofibroblast differentiation (Figure 1), Fu et al. have reported that its expression is extinguished 14 days after myocardial infarction in a mouse model, suggesting that myofibroblasts do not represent a permanent differentiation state [16]. Nevertheless, α-SMA expression persists in these cells. Myofibroblasts initially localize around the damaged area with long cytoskeletal extroflexions. As they downregulate αSMA expression, they reorganize with a linear shape and lose processes. This suggests that α-SMA may regulate the structural organization of myofibroblasts, which initially surround the damaged regions with a network of filaments. During the scar maturation, myofibroblasts lose this phenotype, as collagens gradually become fully supportive of wall integrity. In fact, Fu et al. have shown that blocking collagen maturation leads to persistence of α-SMA expression in these cells [16].
Thus, fibroblasts (Tcf21+), activated fibroblasts (POSTN+), and myofibroblasts (α-SMA+) persist long term within the scar, but myofibroblasts are only a transient differentiated state. Interestingly, Fu et al. have recently identified a further population of cells that becomes detectable after scar formation in humans and mice, and named it “matrifibrocytes” [16] (Figure 2). They appear to differ from myofibroblasts, although closely related to them. In fact, these cells may have the functional role of maintaining the integrity of the mature scar, as they seem to be a more suitable cell type for this environment, expressing unique ECM proteins from dense connective tissues, most likely to confer increased mechanical features to the healing tissue.
Interestingly, Braitsch et al. [8] have demonstrated different expression profiles for the key markers Tcf21, Wt1, and Tbx18 in response to different injuries: for example, ischemia induces all three epicardial progenitor markers associated to epicardial fibrosis; instead, Tcf21 is mostly induced in perivascular fibrosis after pressure overload, but interstitial fibrosis is nonetheless associated to all three markers in different injury models. These transcription factors are not co-expressed with the myofibroblast marker α-SMA, suggesting that they are only activated in differentiated fibroblasts during fibrosis. Moreover, another different profile is associated with chronic heart injury, such as that induced by prolonged angiotensin II exposure, and associated with the upregulation of many fibrosis-related genes in CFs, such as collagen isoforms Col1a1, Col1a2, Col3a1, and Col8a1, fibronectin (Fn1), connective tissue growth factor (CTGF), insulin-like growth factor 1 (IGF-1), protein-lysin 6-oxidase (Lox), and TGF-β1. Moreover, other specific markers, such as α-SMA, DDR2, POSTN, PDGFR-α, S100 calcium binding protein A4 (S100a4), and CD90 are strongly upregulated after injury with a well detectable increase also in the proportion of expressing cells (Table 1; Figure 2).
Cardiac mesenchymal and progenitor cells
The presence of resident cardiac mesenchymal stem cells (recently more cautiously renamed as mesenchymal stromal cells, C-MSCs) has been hypothesized under the quest for resident regenerative cells in adult tissues. Indeed, the C-MSC niche has been described as a reservoir of mesenchymal stem cells and tissue-specific progeny residual from the embryonic development of the heart [17]. Specific criteria have been used through the years for the identification of MSCs, regardless of the tissue of origin. Together with the ability to differentiate towards the three mesodermal lineages (adipocytes, chondrocytes, osteoblasts), and being clonogenic and negative for hematopoietic lineage markers (i.e. CD45, CD34, CD14, CD11b), C-MSCs are largely positive for Endoglin-CD105, the GPI-anchored surface proteins CD73 [18], and CD90 (Table 1; Figure 2). These phenotypic characteristics appear to be similar in all MSCs, although Kang et al. have demonstrated that the percentage of CD90+ cells is reduced by approximately 40% in human C-MSCs compared to bone marrow-derived mesenchymal cells (BM-MSCs). However, it has been shown in animal models that the CD90-negative fraction of C-MSCs possesses stronger cardiovascular trophic functions due to greater production of growth factors (such as HGF, VEGF, and bFGF) compared to CD90+ cells [19], and that the CD90+ fraction is closer to a fibrotic-prone cell type. Another important membrane protein, the integrin alpha ν (CD51), has been reported to mark specifically resident C-MSCs in the heart of postnatal mice [20].
Studies have also correlated the multipotency and self-renewing features of MSCs with the expression of typical transcription factors of embryonic stem cells, such as Nanog, Oct-4, and Sox-2 [21]. High expression of these latter has been described both in C-MSCs extracted from human aborted fetuses and rat heart tissue [22], while C-MSCs derived from adult cardiac tissue display expression of NANOG, but not OCT-4 and SOX-2 [23].
Positivity to a single marker is not sufficient to define C-MSCs, but characterization of this cell type is still uncertain in the literature. Many recognized markers expressed in C-MSCs are expressed in other cardiac cells, such as cardiac progenitor cells (see paragraphs below), fibroblasts, and pericytes (Figure 2), although these cell types seem to have different functional potential in myocardial homeostasis and repair.
Under the same quest for resident reparative cells, cardiac progenitor cell (CPC) populations within the adult mammalian heart have been described in the last two decades with a high translational interest for regenerative purposes. One of the main surface markers used for the isolation of such a population in mice is Sca1 [24]. Typical features of Sca1+/CD31- CPCs are high clonogenic efficiency [25], a primitive undifferentiated phenotype, long term proliferation, and the ability to differentiate into different cardiac lineages in vitro, such as smooth muscle and endothelial cells [26]. The Sca1+/CD31- population expresses Nanog and the telomerase reverse transcriptase (TERT), two genes associated with pluripotent phenotypes and not expressed by differentiated fibroblasts (Figure 2) [27]. In addition, CPCs express the embryonic heart markers Islet-1 (ISL-1) and TBX5 [28], as well as cardiac-specific transcription factors GATA-4, Nkx-2.5, and MEF2C (Table 1). Conversely, they are negative for markers of mature cardiomyocytes, such as cardiac α-myosin heavy chain (α-MHC) [28]. Therefore, these cells have cardiac-specific features, but do not display markers of neither mature cardiomyocytes, activated fibroblasts or myo-fibroblasts, although similarities may be recognized in the profile of un-activated CFs (Figure 2), or in subpopulations with anti-fibrotic features, as will be further discussed below.
Fate mapping assays have revealed that virtually all Lin- (hematopoietic lineage)/Sca1+ cells derive from Mesp1+ precursors [25], suggesting a mesodermal origin with a possible pro-epicardial contribution [29]. Interestingly, the PDGFR-α+/Tcf21+ fraction also seems to overlap with the so-called side population (SP) dye-efflux phenotype, which is a widely described functional criteria used to identify adult CPCs [30]. Indeed, the Sca1+ population is characterized by a cardiogenic signature and enrichment for stemness-associated markers (e.g. Abcg2, Abcb1b, Klf4). Overall, CPCs identified by the SP phenotype can be defined as PDGFR-α+/Tcf21+ cells, and this expression profile defines more exactly a population enriched for a cardiogenic signature that can be purified by isolation of the PDGFR-α+/CD31- population from Lin-/Sca1+ cells. Consistently, based on single cell analysis, Lin-/Sca1+/Tcf21+/PDGFR-α+/CD31- cells show an enrichment for GATA4/6, Mef2c, Hand2, and Tbx5/20, regardless of their SP status, while the Sca1+/PDGFR-α- pool displays vascular/endothelial features [25].
Another functional criterion used to isolate primitive undifferentiated stromal cells with CPC features is a combination of the explant culture technique (i.e., the classical culture to isolate fibroblasts) with a selection step for spontaneous spheroid growth, which is a widely recognized assay for primitive undifferentiated phenotypes [31-35]. CPCs isolated with this protocol are highly clonogenic, display a mesenchymal signature (CD45-/CD105+) and are largely Sca1+/CD31-, in combination with the expression of several cardiogenic and pluripotent markers (e.g., GATA4, Nkx2.5, Oct4), and with being negative for α-SMA and DDR2. Interestingly, a transcriptomic study on CPCs isolated from the adult human heart has demonstrated very high similarity between human CPCs isolated by different criteria (e.g., Sca1+ versus spheroid selection) [36].
Overall, considering the recurrent overlap of markers, it cannot be excluded that, at least to some extent, C-MSCs, CPCs, un-activated CFs or CFs with an anti-fibrotic signature, may represent different functional states of the same resident stromal pool with a surprising degree of plasticity (Table 1; Figure 2). Several studies have reported that CPC populations can shift in the relative expression of markers associated with the myofibroblast phenotype or cardiac fibrosis, in response to different stimuli [37-42]. These phenotypic shifts seem to exert a biological effect mostly through altered paracrine properties of the cells [43,44] that can indeed propagate anti-fibrotic [45] and anti-inflammatory signals by intercellular communication when maintained in their best reparative phenotype. For example, the relative abundance of cells expressing CD90 (i.e., a marker associated in situ with the activation of CFs and fibrotic processes in the heart [8]) has been negatively correlated with beneficial paracrine profiles, as well as clinical data on reduced functional recovery of the heart after cell transplantation [46,47].
Pericytes
In addition to the stromal phenotypes described so far, other specific non-immune cell types are described in the myocardium. Among them, pericytes are a peculiar cell type which is present in all vascularized organs, anatomically defined as perivascular cells that closely surround endothelial cells in capillaries and microvessels. They are involved in different physiological and pathological functions, including the regulation of blood pressure, tissue healing and scarring, and are well described also in the murine cardiac interstitium [48]. The consistent expression of pericyte markers by human myocardial perivascular cells surrounding microvessels and capillaries has been demonstrated, including neuron-glial antigen 2 (NG2), CD146, α-SMA, smooth muscle myosin heavy chain (SM-MHC), PDGFR-β and PDGFR-α (Table 1) [49]. Different combinations of co-expressed markers (e.g., NG2 and CD146, CD146 and α-SMA, CD146 and SM-MHC) in ventricular pericytes have been described based on the localization around microvessels of different sizes. For example, PDGFR-β can be detected on all myocardial pericytes, while expression of PDGFR-α can be found on nearly all pericytes surrounding microvessels, but not on those surrounding capillaries [50]. Furthermore, studies have reported that perivascular cells share the expression of several mesenchymal markers, such as CD146, CD73, CD90, CD105, CD271, and NG2 (Figure 2) [51].
With the advent of single cell transcriptomics [52], it has been possible to analyze the different patterns activated by pericytes in various conditions. As demonstrated by Litvinukova et al., pericytes also express ABCC9 and KCNJ8, and segregate into several clusters: pericytes resident in ventricles express adhesion molecules (e.g., NCAM2, CD38, and CSPG4) that are involved in microvascular morphogenesis and endothelial cell cross-talk. Conversely, other clusters identified are atria-enriched pericytes, or pericytes with cardiomyocyte features, and so-called stromal pericytes. This latter represents a transitional state between pericytes and endothelial cells [53].
Telocytes
Telocytes represent a stromal population with a typical morphology, including a small cell body and very long and thin moniliform processes, named telopodes. Thanks to these features, they are able to build a 3D physiological network throughout the whole stromal space, in order to communicate with each other and with neighboring cells. In addition, they exert physiological roles such as the release of growth factors, guiding cardiac progenitors during organogenesis. They also play a role in response to pathological states, enhancing angiogenesis, cardiomyocyte renewal, and improving cardiac function [54]. These cells are described in multiple sites in the myocardium, suggesting their significant contribution to cardiac homeostasis and repair mechanisms [55]. Several studies have elucidated their markers profile, that includes CD34, c-kit/CD117, PDGFR-α and β, and Vimentin (Table 1; Figure 2) [56-58]. It was demonstrated that telocytes are implicated in intercellular communication through direct gap junctions or extracellular vesicle release [59]. In particular, the vesicles released can transfer macromolecular signals to adjacent cells to stimulate neovascularization in the infarcted myocardium in mice and rats [60]. Other studies have reported that telocytes play a protective role also by secreting VEGF, expressing angiogenic-associated microRNAs, and establishing direct nano-contacts with newly derived endothelial cells at the border zone of myocardial infarction [59].
An “Omics” perspective on the cardiac stroma
Single cell transcriptomic studies on cardiac interstitial cells have confirmed phenotypic plasticity of the cardiac stroma, whose transcriptional profile is dynamically regulated in homeostatic conditions and in response to external stimuli, with different populations and/or functional states that appear either in homeostasis or disease. In fact, it is known that the heart cellular composition changes during pathological stress, and genome-wide expression analysis specifically occurring within each cell type during cardiac stress is providing unprecedented knowledge on cell dynamics in injury and repair. Dissociated cardiac muscle has been used by Tucker et al. [61] for single-nucleus sequencing (sn-RNA-seq) on nuclei derived from the 4 chambers of the normal human heart of transplant donors, revealing 9 major cell types and 20 subclusters of cell types. Cellular subclasses included 3 fibroblast subsets which constitute 32.4% of observed cells, and display common markers of the fibroblast lineage, such as Decorin (DCN) and Elastin (ELN). The authors identified clusters characterized by upregulation of fibrosis-associated genes, such as NOX4, IGF1, ADAMTS4, VCAN, and AXL. However, as expected in healthy myocardial tissue, they did not identify a subcluster of canonical activated fibroblasts defined by the expression of classical markers of activation (POSTN), myofibroblasts transition (MYH11, fibroblast activation protein-FAP), or transformation into matrifibrocytes (CHAD, COMP) (Figure 2) [61].
Similarly, Litviňuková and colleagues have identified 7 subclusters of CFs combining single cell (sc)-RNA-seq and snRNA-seq data from human healthy transplant donors [52]. In detail, CF compartments share the expression of three markers: DCN, which regulates collagen fibrillogenesis [62], Gelsolin (GSN) [63], and PDGFR-α [4]. However, inside this population the authors found three subclusters displaying different properties: the first has higher expression of genes involved in ECM homeostasis; the second has higher expression of cytokine receptors, such as oncostatin M receptor (OSMR) and interleukin 6 receptor subunit alpha (ILST6); the third is defined by the expression of TGF-β signalling responsive genes (POSTN, TNC, and FAP) thus displaying features of activated CFs (Table 1; Figure 2) [64]. In this context, fibroblast activation could be the result of age-related changes in cardiac physiology, which lead to progressive dominance of fibrotic remodeling circuits. Interestingly, in both studies, fibroblast clusters display chamber specific distribution across the heart, likely related to their diversity in developmental origin and specialized functions. Together, these single cell analyses of healthy human heart provide fascinating information to deepen our understanding of cardiac physiology in homeostatic conditions and normal aging.
According to another single-cell dual-omics approach on mouse cardiac tissue, where transcriptome and epigenome of cardiac non-myocytes were described, CFs can be subdivided into three distinct populations. Each has specific functional states related to cellular response, cytoskeleton organization, and immune response. The three CF sub-types showed a specific distribution, with known markers expressed at comparable levels (e.g., Tcf21, PDGFR-α, Col1a1, and Col3a1). Gene enrichment analysis identified Hsd11b1 and Gfpt2 as the representative marker genes for the differential states described by the authors [65]: state 1 with high levels of Hsd11b1, Inmt, and Cxcl14; state 2, with high levels of Gfpt2, pi16, and Uap1 gene expression; state 3 with the features of fibrocytes (that is a mesenchymal cell type arisen from monocyte precursors). The abundance of each CF population varies after cardiac injury. In fact, early after ischemia, the infarct area is colonized by Hsd11b1+ and Gfpt2+ cells (state 1 and state 2 CFs), with a peak at 7 days post MI for state 3 fibroblasts. Thus, the proportion of cells changes dynamically during time after MI, suggesting a different function for each CF population upon injury [65].
In another study, dissociated mouse hearts were analyzed by single cell transcriptomic, and 12 distinct cell clusters expressing known markers of major cell types were identified. The clusters comprised endothelial cells, fibroblasts, granulocytes, pericytes, SMCs, as well as lymphocytes, dendritic cell (DC)-like cells, and Schwann cells [66]. In this work the authors again confirmed the presence of a cell subpopulation (fibrocytes) [67] expressing intermediate levels of canonical genes corresponding to both fibroblasts (Col1a1, PDGFR-α, Tcf21) and macrophages/leukocytes (Fcgr1, Cd14, Ptprc). Given the overlap of known markers, a strategy was designed to discriminate pericytes, SMCs, Schwann cells, and fibroblasts, by identifying genes with higher expression in one of these cell types. For example, by using staining for the mesenchymal marker ITGA7 and gene expression data, as well as transgenic reporter mouse strains, fibroblasts could be distinguished from mural perivascular cells using mEF-SK4 as a secondary marker, thus proposing a new specific marker to distinguish PDGFR-α+ CFs from mural cells [66].
Transcriptomic studies have also enhanced our understanding of CF functions. Interestingly, the analysis of possible cellular interactions identified fibroblasts as the most trophic cell population with connections to many cell types. For example, the expression of colony stimulating factor 1 (CSF1) and IL34 signal through the CSF1 receptor are essential are essential factors for macrophage growth and survival. Fibroblasts also express growth factors NGF, VEGFA, IGF1, and FGF2, which support neurons of the autonomous nervous system, endothelial cells, and mural cells [68,69]. Thus, CFs appear to establish networks that support not only cardiomyocyte survival and define cardiac ECM, but can also modulate the immune response and support cardiac innervation.
Recent omics studies have allowed us to deepen the understanding of classical markers, as well. Using the PDGFR-α-GFP reporter mouse line, the resident fibroblast population has been divided into two major sub-populations after sc-RNA seq: Sca1high (F-SH) and Sca1low (F-SL), both expressing canonical fibroblast markers such as PDGFR-α, DDR2, and Col1a1 (Table 1). F-SH and F-SL show distinct adhesive and secretory phenotypes, highlighting the likely functional differences between them [70], although both representing populations of quiescent un-activated CFs. In addition, a novel activated fibroblast population, expressing a strong anti-Wingless-related integration site (WNT) transcriptome signature (F-WNT-X) was identified in healthy hearts, as well as after MI [70]. WNT plays complex roles in cardiac biology and disease, impacting immune, vascular, and pro-fibrotic pathways [71,72]. F-WNT-X stromal cells uniquely expressed Wif1, encoding a canonical and non-canonical WNT signaling antagonist [73,74], acting on multiple pathways such as CTGF and VEGF, whose regulation is important for efficient cardiac repair (Figure 1) [75-77]. In addition to WIF1, F-WNT-X cells showed upregulation of other WNT and TGF-β pathway antagonists, overall making these cells paracrine mediators of an anti-WNT/CTGF/TGF-β signaling, essential for anti-fibrotic cardiac repair. Strategies for the enhancement and potentiation of this stromal population after cardiac injury could be of high interest for translational purposes.
In the same study, analysis in injured hearts has revealed a high complexity of signaling, and stromal cells plasticity has shown its importance in driving cardiac tissue response to injury. When mice were subjected to MI, fibroblast populations showed a dynamic change in time and space. At day 3 post-MI both F-SH and F-SL were significantly diminished, apparently converting into an activated state (F-Act) defined as POSTN+Acta2negative-low, with recruitment and activation of cells from areas outside the infarct, then restored by day 7 post-MI [70]. F-Act share some transcriptional features with myofibroblasts, such as activation of collagens and genes associated with wound healing (Figure 1), but they appear more related to resident fibroblasts than to myofibroblasts. These cells showed indeed active proliferation in the first week after MI, consistent with the known proliferation peak observed in CFs [78,79]. Analysis of cells isolated from the infarcted mouse heart revealed the presence of three fibroblast clusters appearing at day 3 post-MI. Cells from two of the three clusters were characterized by the relatively high expression of POSTN, Wisp1, and TNC, classically associated with fibroblast activation [80,81], and were specifically present only after injury [82]. In addition to these known fibrosis markers, the gene cytoskeleton associated protein 4 (Ckap4) was found upregulated specifically in post-MI activated fibroblasts. Ckap4 is a trans-membrane protein and its function in CFs is unknown. Increased Ckap4 expression is specific for the stressed heart and overlaps with vimentin, a marker for CFs in the ischemic heart. Preliminary evidence showed that in activated fibroblast CKAP4 functions to decrease the expression of genes indeed related to activation, making this protein a possible new important modulator of this process [82].
CPC/MSC populations have also been investigated by omics approaches. A single-cell transcriptomic study of cardiac progenitors isolated through cardiospheres has showed that different functional subpopulations exist, which cooperate in heart muscle repair. In this population the expression of the Ly6a gene could evidence 30% of cells being Sca1+ [83]. Cell-cell interactions can be mapped through the analysis of ligands and receptors expression by cell therapy donor and recipient cells, respectively, for a given signaling molecule [66]. Single cell transcriptomic data allowed the construction of a network reflecting the strength of ligand-receptor connections among CPC subpopulations. The number of connections from Sca1+ to Sca1- cells was higher, specifically for angiogenesis related factors, suggesting that Sca1+ CPCs behave as a signaling hub with pro-angiogenic signals. The cardioprotective function of CPCs has been also linked to GATA4 and β-catenin expression [84]. Sca1+ cells show upregulated expression of GATA4 and downregulated expression of β-catenin. Finally, Sca1+ cells show repair activity in infarcted hearts in vivo, a feature not shared with Sca1- cells, which however have a strong proliferative and angiogenic capacity in vitro [83]. The molecular phenotype of Sca1+ resident CPCs was analyzed with a combined transcriptomic and proteomic approach. The data revealed that undifferentiated Sca1+ cells express CD38 and CD105 surface markers, as well as others implicated in cell adhesion, such as Icam2, Ceacam1, CD36, CD93, and CD322 (Table 1; Figure 2). In addition, growth factors like KITL, JAG2, PDGF-β, and VEGFC showed higher expression in Sca1 progenitor cells [85], overall confirming the strong capacity of these cells to provide positive microenvironment cues for cardiac repair.
Targeting cardiac fibroblasts: a translational point of view
The detailed description of stromal cell functions and phenotypes in cardiac disease models might allow the design of targeted therapies against those cell types or functional states responsible to drive remodeling. In this view some therapeutic approaches are being developed to specifically interfere with possible detrimental actions of cardiac stromal cells in the injured cardiac muscle.
Pharmacological therapies
Renin-angiotensin-aldosterone system (RAAS) and TGF-β signaling are implicated in the activation of CFs (Figure 1) and the onset of cardiac fibrosis. Inhibition of these pathways using a pharmacological approach is of great interest for the treatment and prevention of cardiac fibrosis. The most studied anti-fibrotic drugs are RAAS inhibitors, which target angiotensin II thus reducing CF proliferation and collagen synthesis [86]. In the past years, several clinical studies have shown that RAAS inhibitors counteract cardiac fibrosis progression [87-89]. Recently, Garvin et al., using single-cell RNA sequencing, have demonstrated that transient ACE (angiotensin-converting enzyme) inhibitor treatment in a spontaneously hypertensive rat model suppresses future fibrogenic capacity and heterogeneity of CF subpopulations [90]. However, inhibition of RAAS only modestly regresses cardiac fibrosis once consolidated, which persists in heart failure patients, indicating a need to develop novel antifibrotic therapies effective also at later time points of disease progression [91]. In this regard, TGF-β1 is another candidate target to treat cardiac fibrosis using a pharmacological approach. Inhibitors of TGF-β signaling have been extensively studied in animal models of fibrosis; however, translation of these findings into treatments for human cardiac diseases has been limited due to the broad range of responses to TGF-β1, and its role in tissue homeostasis. Nonetheless, a few TGF-β inhibitors are currently under clinical evaluation for the treatment of cardiac fibrosis [92].
In situ reprogramming
CFs have also been the target of the so called “direct reprogramming” strategy, which induces a partial dedifferentiation to a state plastic enough to allow the subsequent trans-differentiation into induced cardiomyocytes (iCMs), both in vitro and in situ. This could be an alternative therapeutic strategy to repopulate the myocardial scar with newly formed CMs after injury, thus reducing fibrosis in favor of regeneration. However, the optimal cocktail of transcription factors and/or microRNAs for CF-iCM reprogramming [93,94] has yet to be identified, and new studies are required to accelerate the translation of these technologies to the clinic. Interestingly, the epigenetic profile of induced pluripotent cells generated from CFs appears to be more prone for differentiation towards the cardiac lineage, rather than cell types from heterotopic sources, suggesting that CFs could be a plentiful source of CMs for cell-based therapy or tissue engineering [95].
Specific targeting of CFs
Adverse remodeling might be counteracted by selective ablation of those specific cell populations (or functional states) mediating the fibrotic process (Table 1; Figure 1). Early evidence that this strategy might be useful to reverse fibrosis and ameliorate heart function after injury came from Kaur’s study [96], where POSTN+ activated fibroblasts were genetically ablated in animals engineered to express the diphtheria toxin receptor specifically in activated fibroblasts. In the setting of pressure overload and heart failure injury, the fibrotic burden was decreased without compromising scar stability, and cardiac function was improved by selective removal of pro-fibrotic POSTN+ fibroblasts.
As previously mentioned, FAP expression also distinguishes activated fibroblasts and myofibroblasts from un-activated fibroblasts (Table 1; Figure 1). In fact, FAP is strongly expressed by CFs in response to acute myocardial infarction [97], thus representing a potential target to selectively hit pathological CFs. In this context, Epstein’s group recently presented a very elegant experiment to demonstrate the efficacy of redirected chimeric antigen receptor (CAR)-T-cell immunotherapy to specifically target pathologic cardiac fibrosis. In detail, after CAR binding to FAP, CAR-T cells were able to cause cytotoxic killing of activated pro-fibrotic CFs, decreasing their number in the tissue. This specific ablation resulted in a significant reduction of cardiac fibrosis, and in the restoration of cardiac function in a mouse model of hypertensive cardiac injury and fibrosis [98]. Importantly, extensive analysis revealed no signs of toxicity in this model system, which is in agreement with previous mouse studies in which CAR-T cells against FAP have been used for cancer treatment [99].
Conclusions
The experimental evidence presented in this review on cardiac stromal cells highlights the plasticity and heterogeneity of this compartment, which had long been underestimated (Figure 2). Cardiac stromal cells act as a signaling hub, a support population for cardiomyocytes, and as a potent element of response to injury, which dramatically changes the muscular structure during heart failure progression. The availability of molecular and cellular markers, together with the new description of the stroma dynamics at single cell resolution, allows now a better description of the cellular mechanisms behind cardiac homeostasis and disease, and offers unprecedented information useful for the development of targeted therapies to counteract pathological myocardial remodeling.
Acknowledgements
This work was supported by grant # RG11916B85CDBF76 from Sapienza University to IC, and grant # AR120172B8B543B3 from Sapienza University to VP.
Disclosure of conflict of interest
None.
References
- 1.Thulabandu V, Chen D, Atit RP. Dermal fibroblast in cutaneous development and healing. Wiley Interdiscip Rev Dev Biol. 2018;7:10. doi: 10.1002/wdev.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rinkevich Y, Walmsley GG, Hu MS, Maan ZN, Newman AM, Drukker M, Januszyk M, Krampitz GW, Gurtner GC, Lorenz HP, Weissman IL, Longaker MT. Identification and isolation of a dermal lineage with intrinsic fibrogenic potential. Science. 2015;348:aaa2151. doi: 10.1126/science.aaa2151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Acharya A, Baek ST, Huang G, Eskiocak B, Goetsch S, Sung CY, Banfi S, Sauer MF, Olsen GS, Duffield JS, Olson EN, Tallquist MD. The bHLH transcription factor Tcf21 is required for lineagespecific EMT of cardiac fibroblast progenitors. Dev. 2012;139:2139–2149. doi: 10.1242/dev.079970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ivey MJ, Kuwabara JT, Riggsbee KL, Tallquist MD. Platelet-derived growth factor receptor-α is essential for cardiac fibroblast survival. Am J Physiol Heart Circ Physiol. 2019;317:H330–H344. doi: 10.1152/ajpheart.00054.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Souders CA, Bowers SL, Baudino TA. Cardiac fibroblast: the renaissance cell. Circ Res. 2009;105:1164–1176. doi: 10.1161/CIRCRESAHA.109.209809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Camelliti P, Borg TK, Kohl P. Structural and functional characterisation of cardiac fibroblasts. Cardiovasc Res. 2005;65:40–51. doi: 10.1016/j.cardiores.2004.08.020. [DOI] [PubMed] [Google Scholar]
- 7.Moore-Morris T, Guimarães-Camboa N, Yutzey KE, Pucéat M, Evans SM. Cardiac fibroblasts: from development to heart failure. J Mol Med. 2015;93:823–830. doi: 10.1007/s00109-015-1314-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Braitsch CM, Kanisicak O, van Berlo JH, Molkentin JD, Yutzey KE. Differential expression of embryonic epicardial progenitor markers and localization of cardiac fibrosis in adult ischemic injury and hypertensive heart disease. J Mol Cell Cardiol. 2013;65:108–119. doi: 10.1016/j.yjmcc.2013.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Titus AS, Harikrishnan V, Kailasam S. Coordinated regulation of cell survival and cell cycle pathways by DDR2- dependent SRF transcription factor in cardiac fibroblasts. Am J Physiol Heart Circ Physiol. 2020;318:H1538–H1558. doi: 10.1152/ajpheart.00740.2019. [DOI] [PubMed] [Google Scholar]
- 10.Zeisberg EM, Kalluri R. Origins of cardiac fibroblasts. Circ Res. 2010;107:1304. doi: 10.1161/CIRCRESAHA.110.231910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shinde AV, Humeres C, Frangogiannis NG. The role of α-smooth muscle actin in fibroblast-mediated matrix contraction and remodeling. Biochim Biophys Acta Mol Basis Dis. 2017;1863:298–309. doi: 10.1016/j.bbadis.2016.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kanisicak O, Khalil H, Ivey MJ, Karch J, Maliken BD, Correll RN, Brody MJ, Lin SCJ, Aronow BJ, Tallquist MD, Molkentin JD. Genetic lineage tracing defines myofibroblast origin and function in the injured heart. Nat Commun. 2016;7:12260. doi: 10.1038/ncomms12260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Furtado MB, Costa MW, Pranoto EA, Salimova E, Pinto AR, Lam NT, Park A, Snider P, Chandran A, Harvey RP, Boyd R, Conway SJ, Pearson J, Kaye DM, Rosenthal NA. Cardiogenic genes expressed in cardiac fibroblasts contribute to heart development and repair. Circ Res. 2014;114:1422–1434. doi: 10.1161/CIRCRESAHA.114.302530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Braitsch CM, Combs MD, Quaggin SE, Yutzey KE. Pod1/Tcf21 is regulated by retinoic acid signaling and inhibits differentiation of epicardium-derived cells into smooth muscle in the developing heart. Dev Biol. 2012;368:345–357. doi: 10.1016/j.ydbio.2012.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Virag JI, Murry CE. Myofibroblast and endothelial cell proliferation during murine myocardial infarct repair. Am J Pathol. 2003;163:2433–2440. doi: 10.1016/S0002-9440(10)63598-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fu X, Khalil H, Kanisicak O, Boyer JG, Vagnozzi RJ, Maliken BD, Sargent MA, Prasad V, Valiente-Alandi I, Blaxall BC, Molkentin JD. Specialized fibroblast differentiated states underlie scar formation in the infarcted mouse heart. J Clin Invest. 2018;128:2127–2143. doi: 10.1172/JCI98215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bastianelli D, Siciliano C, Puca R, Coccia A, Murdoch C, Bordin A, Mangino G, Pompilio G, Calogero A, De Falco E. Influence of Egr-1 in cardiac tissue-derived mesenchymal stem cells in response to glucose variations. Biomed Res Int. 2014;2014:254793. doi: 10.1155/2014/254793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li Q, Qi LJ, Guo ZK, Li H, Zuo HB, Li NN. CD73+ adipose-derived mesenchymal stem cells possess higher potential to differentiate into cardiomyocytes in vitro. J Mol Histol. 2013;44:411–422. doi: 10.1007/s10735-013-9492-9. [DOI] [PubMed] [Google Scholar]
- 19.Shen D, Shen M, Liang H, Tang J, Wang B, Liu C, Wang P, Dong J, Li L, Zhang J, Caranasos TG. Therapeutic benefits of CD90-negative cardiac stromal cells in rats with a 30-day chronic infarct. J Cell Mol Med. 2018;22:1984–1991. doi: 10.1111/jcmm.13517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xie DM, Chen Y, Liao Y, Lin W, Dai G, Lu DH, Zhu S, Yang K, Wu B, Chen Z, Peng C, Jiang MH. Cardiac derived CD51-positive mesenchymal stem cells enhance the cardiac repair through SCF-mediated angiogenesis in mice with myocardial infarction. Front Cell Dev Biol. 2021;9:642533. doi: 10.3389/fcell.2021.642533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Matic I, Antunovic M, Brkic S, Josipovic P, Caput Mihalic K, Karlak I, Ivkovic A, Marijanovic I. Expression of OCT-4 and SOX-2 in bone marrow-derived human mesenchymal stem cells during osteogenic differentiation. Open Access Maced J Med Sci. 2016;4:9–16. doi: 10.3889/oamjms.2016.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Garikipati VNS, Singh SP, Mohanram Y, Gupta AK, Kapoor D, Nityanand S. Isolation and characterization of mesenchymal stem cells from human fetus heart. PLoS One. 2018;13:e0192244. doi: 10.1371/journal.pone.0192244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pierantozzi E, Gava B, Manini I, Roviello F, Marotta G, Chiavarelli M, Sorrentino V. Pluripotency regulators in human mesenchymal stem cells: expression of NANOG but not of OCT-4 and SOX-2. Stem Cells Dev. 2011;20:915–923. doi: 10.1089/scd.2010.0353. [DOI] [PubMed] [Google Scholar]
- 24.Oh H, Bradfute SB, Gallardo TD, Nakamura T, Gaussin V, Mishina Y, Pocius J, Michael LH, Behringer RR, Garry DJ, Entman ML, Schneider MD. Cardiac progenitor cells from adult myocardium: homing, differentiation, and fusion after infarction. Proc Natl Acad Sci U S A. 2003;100:12313–12318. doi: 10.1073/pnas.2132126100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Noseda M, Harada M, McSweeney S, Leja T, Belian E, Stuckey DJ, Abreu Paiva MS, Habib J, Macaulay I, De Smith AJ, Al-Beidh F, Sampson R, Lumbers RT, Rao P, Harding SE, Blakemore AIF, Jacobsen SE, Barahona M, Schneider MD. PDGFRα demarcates the cardiogenic clonogenic Sca1+ stem/progenitor cell in adult murine myocardium. Nat Commun. 2015;6:6930. doi: 10.1038/ncomms7930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang H, Chen H, Feng B, Wang X, He X, Hu R, Yin M, Wang W, Fu W, Xu Z. Isolation and characterization of a Sca-1+/CD31- progenitor cell lineage derived from mouse heart tissue. BMC Biotechnol. 2014;14:75. doi: 10.1186/1472-6750-14-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Takamiya M, Haider KH, Ashraf M. Identification and characterization of a novel multipotent sub-population of sca-1 + cardiac progenitor cells for myocardial regeneration. PLoS One. 2011;6:9. doi: 10.1371/journal.pone.0025265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Laugwitz KL, Moretti A, Lam J, Gruber P, Chen Y, Woodard S, Lin LZ, Cai CL, Lu MM, Reth M, Platoshyn O, Yuan JXJ, Evans S, Chien KB. Postnatal isl1+ cardioblasts enter fully differentiated cardiomyocyte lineages. Nature. 2005;433:647–653. doi: 10.1038/nature03215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhou B, von Gise A, Ma Q, Rivera-Feliciano J, Pu WT. Nkx2-5- and Isl1-expressing cardiac progenitors contribute to proepicardium. Biochem Biophys Res Commun. 2008;375:450–453. doi: 10.1016/j.bbrc.2008.08.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Oyama T, Nagai T, Wada H, Naito AT, Matsuura K, Iwanaga K, Takahashi T, Goto M, Mikami Y, Yasuda N, Akazawa H, Uezumi A, Takeda S, Komuro I. Cardiac side population cells have a potential to migrate and differentiate into cardiomyocytes in vitro and in vivo. J Cell Biol. 2007;176:329–341. doi: 10.1083/jcb.200603014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chimenti I, Gaetani R, Forte E, Angelini F, De Falco E, Zoccai GB, Messina E, Frati G, Giacomello A. Serum and supplement optimization for EU GMP-compliance in cardiospheres cell culture. J Cell Mol Med. 2014;18:624–634. doi: 10.1111/jcmm.12210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chimenti I, Massai D, Morbiducci U, Beltrami AP, Pesce M, Messina E. Stem cell spheroids and ex vivo niche modeling: rationalization and scaling-up. J Cardiovasc Transl Res. 2017;10:150–166. doi: 10.1007/s12265-017-9741-5. [DOI] [PubMed] [Google Scholar]
- 33.Chimenti I, Gaetani R, Barile L, Forte E, Ionta V, Angelini F, Frati G, Messina E, Giacomello A. Isolation and expansion of adult cardiac stem/progenitor cells in the form of cardiospheres from human cardiac biopsies and murine hearts. Methods Mol Biol. 2012;879:327–338. doi: 10.1007/978-1-61779-815-3_19. [DOI] [PubMed] [Google Scholar]
- 34.O’Connor MD, Kardel MD, Eaves CJ. Functional assays for human embryonic stem cell pluripotency. Methods Mol Biol. 2011;690:67–80. doi: 10.1007/978-1-60761-962-8_4. [DOI] [PubMed] [Google Scholar]
- 35.Li TS, Cheng K, Lee ST, Matsushita S, Davis D, Malliaras K, Zhang Y, Matsushita N, Smith RR, Marbán E. Cardiospheres recapitulate a niche-like microenvironment rich in stemness and cell-matrix interactions, rationalizing their enhanced functional potency for myocardial repair. Stem Cells. 2010;28:2088–2098. doi: 10.1002/stem.532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gaetani R, Feyen DA, Doevendans PA, Gremmels H, Forte E, Fledderus JO, Ramjankhan FZ, Messina E, Sussman MA, Giacomello A, Sluijter JP. Different types of cultured human adult cardiac progenitor cells have a high degree of transcriptome similarity. J Cell Mol Med. 2014;18:2147–2151. doi: 10.1111/jcmm.12458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pagano F, Angelini F, Siciliano C, Tasciotti J, Mangino G, De Falco E, Carnevale R, Sciarretta S, Frati G, Chimenti I. Beta2-adrenergic signaling affects the phenotype of human cardiac progenitor cells through EMT modulation. J Cell Mol Med. 2018;127:41–48. doi: 10.1016/j.phrs.2017.01.016. [DOI] [PubMed] [Google Scholar]
- 38.Pagano F, Angelini F, Castaldo C, Picchio V, Messina E, Sciarretta S, Maiello C, Biondi-Zoccai G, Frati G, Di Meglio F, Nurzynska D, Chimenti I. Normal versus pathological cardiac fibroblast-derived extracellular matrix differentially modulates cardiosphere-derived cell paracrine properties and commitment. Stem Cells Int. 2017;2017:7396462. doi: 10.1155/2017/7396462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Belviso I, Angelini F, Di Meglio F, Picchio V, Sacco AM, Nocella C, Romano V, Nurzynska D, Frati G, Maiello C, Messina E, Montagnani S, Pagano F, Castaldo C, Chimenti I. The microenvironment of decellularized extracellular matrix from heart failure myocardium alters the balance between angiogenic and fibrotic signals from stromal primitive cells. Int J Mol Sci. 2020;21:7903. doi: 10.3390/ijms21217903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Angelini F, Pagano F, Bordin A, Picchio V, De Falco E, Chimenti I. Getting old through the blood: circulating molecules in aging and senescence of cardiovascular regenerative cells. Front Cardiovasc Med. 2017;4:62. doi: 10.3389/fcvm.2017.00062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chimenti I, Pagano F, Cavarretta E, Angelini F, Peruzzi M, Barretta A, Greco E, De Falco E, Marullo AG, Sciarretta S, Biondi-Zoccai G, Frati G. B-blockers treatment of cardiac surgery patients enhances isolation and improves phenotype of cardiosphere-derived cells. Sci Rep. 2016;6:36774. doi: 10.1038/srep36774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gago-Lopez N, Awaji O, Zhang Y, Ko C, Nsair A, Liem D, Stempien-Otero A, MaClellan WR. THY-1 receptor expression differentiates cardiosphere-derived cells with divergent cardiogenic differentiation potential. Stem Cell Reports. 2014;2:576–591. doi: 10.1016/j.stemcr.2014.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Siciliano C, Chimenti I, Ibrahim M, Napoletano C, Mangino G, Scafetta G, Zoccai GB, Rendina EA, Calogero A, Frati G, De Falco E. Cardiosphere conditioned media influence the plasticity of human mediastinal adipose tissue-derived mesenchymal stem cells. Cell Transplant. 2015;24:2307–2322. doi: 10.3727/096368914X685771. [DOI] [PubMed] [Google Scholar]
- 44.Pagano F, Picchio V, Angelini F, Iaccarino A, Peruzzi M, Cavarretta E, Biondi-Zoccai G, Sciarretta S, De Falco E, Chimenti I, Frati G. The biological mechanisms of action of cardiac progenitor cell therapy. Curr Cardiol Rep. 2018;20:84. doi: 10.1007/s11886-018-1031-6. [DOI] [PubMed] [Google Scholar]
- 45.De Couto G, Liu W, Tseliou E, Sun B, Makkar N, Kanazawa H, Arditi M, Marbán E. Macrophages mediate cardioprotective cellular postconditioning in acute myocardial infarction. J Clin Invest. 2015;125:3147–3162. doi: 10.1172/JCI81321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cheng K, Ibrahim A, Hensley MT, Shen D, Sun B, Middleton R, Liu W, Smith RR, Marbán E. Relative roles of CD90 and c-Kit to the regenerative efficacy of cardiosphere-derived cells in humans and in a mouse model of myocardial infarction. J Am Heart Assoc. 2014;3:5. doi: 10.1161/JAHA.114.001260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Li TS, Cheng K, Malliaras K, Smith RR, Zhang Y, Sun B, Matsushita N, Blusztajn A, Terrovitis J, Kusuoka H, Marbán L, Marbán E. Direct comparison of different stem cell types and subpopulations reveals superior paracrine potency and myocardial repair efficacy with cardiosphere-derived cells. J Am Coll Cardiol. 2012;59:942–953. doi: 10.1016/j.jacc.2011.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.West CC, Khan NS, Crisan M. Characterization of human pericyte phenotype by immunohistochemistry. Methods Mol Biol. 2021;2235:37–45. doi: 10.1007/978-1-0716-1056-5_4. [DOI] [PubMed] [Google Scholar]
- 49.Armulik A, Genové G, Betsholtz C. Pericytes: developmental, physiological, and pathological perspectives, problems, and promises. Dev Cell. 2011;21:193–215. doi: 10.1016/j.devcel.2011.07.001. [DOI] [PubMed] [Google Scholar]
- 50.Chen WCW, Baily JE, Corselli M, Díaz ME, Sun B, Xiang G, Gray GA, Huard J, Péault B. Human myocardial pericytes: multipotent mesodermal precursors exhibiting cardiac specificity. Stem Cells. 2015;33:557–573. doi: 10.1002/stem.1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lv FJ, Tuan RS, Cheung KM, Leung VY. Concise review: the surface markers and identity of human mesenchymal stem cells. Stem Cells. 2014;32:1408–1419. doi: 10.1002/stem.1681. [DOI] [PubMed] [Google Scholar]
- 52.Litviňuková M, Talavera-López C, Maatz H, Reichart D, Worth CL, Lindberg EL, Kanda M, Polanski K, Heinig M, Lee M, Nadelmann ER, Roberts K, Tuck L, Fasouli ES, DeLaughter DM, McDonough B, Wakimoto H, Gorham JM, Samari S, Mahbubani KT, Saeb-Parsy K, Patone G, Boyle JJ, Zhang H, Zhang H, Viveiros A, Oudit GY, Bayraktar OA, Seidman JG, Seidman CE, Noseda M, Hubner N, Teichmann SA. Cells of the adult human heart. Nature. 2020;588:466–472. doi: 10.1038/s41586-020-2797-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Stallcup WB. The NG2 proteoglycan in pericyte biology. Adv Exp Med Biol. 2018;1109:5–19. doi: 10.1007/978-3-030-02601-1_2. [DOI] [PubMed] [Google Scholar]
- 54.Tao L, Wang H, Wang X, Kong X, Li X. Cardiac telocytes. Curr Stem Cell Res Ther. 2016;11:404–409. doi: 10.2174/1574888x10666150113113420. [DOI] [PubMed] [Google Scholar]
- 55.Marini M, Rosa I, Ibba-Manneschi L, Manetti M. Telocytes in skeletal, cardiac and smooth muscle interstitium: morphological and functional aspects. Histol Histopathol. 2019;33:1151–1165. doi: 10.14670/HH-11-994. [DOI] [PubMed] [Google Scholar]
- 56.Zhou Q, Wei L, Zhong C, Fu S, Bei Y, Huică RI, Wang F, Xiao J. Cardiac telocytes are double positive for CD34/PDGFR-α. J Cell Mol Med. 2015;19:2036–2042. doi: 10.1111/jcmm.12615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rusu MC, Pop F, Hostiuc S, Curca GC, Jianu AM, Paduraru D. Telocytes form networks in normal cardiac tissues. Histol Histopathol. 2012;27:807–816. doi: 10.14670/HH-27.807. [DOI] [PubMed] [Google Scholar]
- 58.Richter M, Kostin S. The failing human heart is characterized by decreased numbers of telocytes as result of apoptosis and altered extracellular matrix composition. J Cell Mol Med. 2015;19:2597–2606. doi: 10.1111/jcmm.12664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Manole CG, Cismaşiu V, Gherghiceanu M, Popescu LM. Experimental acute myocardial infarction: telocytes involvement in neo-angiogenesis. J Cell Mol Med. 2011;15:2284–2296. doi: 10.1111/j.1582-4934.2011.01449.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhao B, Liao Z, Chen S, Yuan Z, Yilin C, Lee KK, Qi X, Shen X, Zheng X, Quinn T, Cai D. Intramyocardial transplantation of cardiac telocytes decreases myocardial infarction and improves post-infarcted cardiac function in rats. J Cell Mol Med. 2014;18:780–789. doi: 10.1111/jcmm.12259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tucker NR, Chaffin M, Fleming SJ, Hall AW, Parsons VA, Bedi KC, Akkad AD, Herndon CN, Arduini A, Papangeli I, Roselli C, Aguet F, Choi SH, Ardlie KG, Babadi M, Margulies KB, Stegmann CM, Ellinor PT. Transcriptional and cellular diversity of the human heart. Circulation. 2020;142:466–482. doi: 10.1161/CIRCULATIONAHA.119.045401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Vu TT, Marquez J, Le LT, Nguyen ATT, Kim HK, Han J. The role of decorin in cardiovascular diseases: more than just a decoration. Free Radic Res. 2018;52:1210–1219. doi: 10.1080/10715762.2018.1516285. [DOI] [PubMed] [Google Scholar]
- 63.Patel VB, Zhabyeyev P, Chen X, Wang F, Paul M, Fan D, McLean BA, Basu R, Zhang P, Shah S, Dawson JF, Pyle WG, Hazra M, Kassiri Z, Hazra S, Vanhaesebroeck B, McCulloch CA, Oudit GY. PI3Kα-regulated gelsolin activity is a critical determinant of cardiac cytoskeletal remodeling and heart disease. Nat Commun. 2018;9:5390. doi: 10.1038/s41467-018-07812-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Soppert J, Kraemer S, Beckers C, Averdunk L, Möllmann J, Denecke B, Goetzenich A, Marx G, Bernhagen J, Stoppe C. Soluble CD74 reroutes MIF/CXCR4/AKT-mediated survival of cardiac myofibroblasts to necroptosis. J Am Heart Assoc. 2018;7:17. doi: 10.1161/JAHA.118.009384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wang L, Yang Y, Ma H, Xie Y, Xu J, Near D, Wang H, Garbutt T, Li Y, Liu J, Qian L. Single-cell dual-omics reveals the transcriptomic and epigenomic diversity of cardiac non-myocytes. Cardiovasc Res. 2021 doi: 10.1093/cvr/cvab134. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Skelly DA, Squiers GT, McLellan MA, Bolisetty MT, Robson P, Rosenthal NA, Pinto AR. Single-cell transcriptional profiling reveals cellular diversity and intercommunication in the mouse heart. Cell Rep. 2018;22:600–610. doi: 10.1016/j.celrep.2017.12.072. [DOI] [PubMed] [Google Scholar]
- 67.Reilkoff RA, Bucala R, Herzog EL. Fibrocytes: emerging effector cells in chronic inflammation. Nat Rev Immunol. 2011;11:427–435. doi: 10.1038/nri2990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bach LA. Endothelial cells and the IGF system. J Mol Endocrinol. 2015;54:1–13. doi: 10.1530/JME-14-0215. [DOI] [PubMed] [Google Scholar]
- 69.Glebova NO, Ginty DD. Heterogeneous requirement of NGF for sympathetic target innervation in vivo. J Neurosci. 2004;24:743–751. doi: 10.1523/JNEUROSCI.4523-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Farbehi N, Patrick R, Dorison A, Xaymardan M, Janbandhu V, Wystub-Lis K, Ho JW, Nordon RE, Harvey RP. Single-cell expression profiling reveals dynamic flux of cardiac stromal, vascular and immune cells in health and injury. Elife. 2019;8:e43882. doi: 10.7554/eLife.43882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Foulquier S, Daskalopoulos EP, Lluri G, Hermans KCM, Deb A, Blankesteijn WM. WNT signaling in cardiac and vascular disease. Pharmacol Rev. 2018;70:68–141. doi: 10.1124/pr.117.013896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Palevski D, Levin-Kotler LP, Kain D, Naftali-Shani N, Landa N, Ben-Mordechai T, Konfino T, Holbova R, Molotski N, Rosin-Arbesfeld R, Lang RA, Leor J. Loss of macrophage Wnt secretion improves remodeling and function after myocardial infarction in mice. J Am Heart Assoc. 2017;6:1. doi: 10.1161/JAHA.116.004387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bányai L, Kerekes K, Patthy L. Characterization of a Wnt-binding site of the WIF-domain of Wnt inhibitory factor-1. FEBS Lett. 2012;586:3122–3126. doi: 10.1016/j.febslet.2012.07.072. [DOI] [PubMed] [Google Scholar]
- 74.Meyer IS, Jungmann A, Dieterich C, Zhang M, Lasitschka F, Werkmeister S, Haas J, Müller OJ, Boutros M, Nahrendorf M, Katus HA, Hardt SE, Leuschner F. The cardiac microenvironment uses non-canonical WNT signaling to activate monocytes after myocardial infarction. EMBO Mol Med. 2017;9:1279–1293. doi: 10.15252/emmm.201707565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ko YB, Kim BR, Yoon K, Choi EK, Seo SH, Lee Y, Lee MA, Yang JB, Park MS, Rho SB. WIF1 can effectively co-regulate pro-apoptotic activity through the combination with DKK1. Cell Signal. 2014;26:2562–2572. doi: 10.1016/j.cellsig.2014.07.026. [DOI] [PubMed] [Google Scholar]
- 76.Hu J, Dong A, Fernandez-Ruiz V, Shan J, Kawa M, Martínez-Ansó E, Prieto J, Qian C. Blockade of Wnt signaling inhibits angiogenesis and tumor growth in hepatocellular carcinoma. Cancer Res. 2009;69:6951–6959. doi: 10.1158/0008-5472.CAN-09-0541. [DOI] [PubMed] [Google Scholar]
- 77.Lu D, Dong W, Zhang X, Quan X, Bao D, Lu Y, Zhang L. WIF1 causes dysfunction of heart in transgenic mice. Transgenic Res. 2013;22:1179–1189. doi: 10.1007/s11248-013-9738-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ivey MJ, Kuwabara JT, Pai JT, Moore RE, Sun Z, Tallquist MD. Resident fibroblast expansion during cardiac growth and remodeling. J Mol Cell Cardiol. 2018;114:161–174. doi: 10.1016/j.yjmcc.2017.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Fu X, Khalil H, Kanisicak O, Boyer JG, Vagnozzi RJ, Maliken BD, Sargent MA, Prasad V, Valiente-Alandi I, Blaxall BC, Molkentin JD. Specialized fibroblast differentiated states underlie scar formation in the infarcted mouse heart. J Clin Invest. 2018;128:2127–2143. doi: 10.1172/JCI98215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Li L, Fan D, Wang C, Wang JY, Cui XB, Wu D, Zhou Y, Wu LL. Angiotensin II increases periostin expression via Ras/p38 MAPK/CREB and ERK1/2/TGF-β1 pathways in cardiac fibroblasts. Cardiovasc Res. 2011;91:80–89. doi: 10.1093/cvr/cvr067. [DOI] [PubMed] [Google Scholar]
- 81.Maqbool A, Spary EJ, Manfield IW, Ruhmann M, Zuliani-Alvarez L, Gamboa-Esteves FO, Porter KE, Drinkhill MJ, Midwood KS, Turner NA. Tenascin C upregulates interleukin-6 expression in human cardiac myofibroblasts via toll-like receptor 4. World J Cardiol. 2016;8:340. doi: 10.4330/wjc.v8.i5.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Gladka MM, Molenaar B, De Ruiter H, Van Der Elst S, Tsui H, Versteeg D, Lacraz GPA, Huibers MMH, Van Oudenaarden A, Van Rooij E. Single-cell sequencing of the healthy and diseased heart reveals cytoskeleton-associated protein 4 as a new modulator of fibroblasts activation. Circulation. 2018;138:166–180. doi: 10.1161/CIRCULATIONAHA.117.030742. [DOI] [PubMed] [Google Scholar]
- 83.Gao L, Zhang H, Cui J, Pei L, Huang S, Mao Y, Liu Z, Wei K, Zhu H. Single-cell transcriptomics of cardiac progenitors reveals functional subpopulations and their cooperative crosstalk in cardiac repair. Protein Cell. 2021;12:152–157. doi: 10.1007/s13238-020-00788-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ibrahim AGE, Li C, Rogers R, Fournier M, Li L, Vaturi SD, Antes T, Sanchez L, Akhmerov A, Moseley JJ, Tobin B, Rodriguez-Borlado L, Smith RR, Marbán L, Marbán E. Augmenting canonical Wnt signalling in therapeutically inert cells converts them into therapeutically potent exosome factories. Nat Biomed Eng. 2019;3:695–705. doi: 10.1038/s41551-019-0448-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Samal R, Ameling S, Wenzel K, Dhople V, Völker U, Felix SB, Könemann S, Hammer E. OMICS-based exploration of the molecular phenotype of resident cardiac progenitor cells from adult murine heart. J Proteomics. 2012;75:5304–5315. doi: 10.1016/j.jprot.2012.06.010. [DOI] [PubMed] [Google Scholar]
- 86.Kong P, Christia P, Frangogiannis NG. The pathogenesis of cardiac fibrosis. Cell Mol Life Sci. 2014;71:549–574. doi: 10.1007/s00018-013-1349-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Shimada YJ, Passeri JJ, Baggish AL, O’Callaghan C, Lowry PA, Yannekis G, Abbara S, Ghoshhajra BB, Rothman RD, Ho CY, Januzzi JL, Seidman CE, Fifer MA. Effects of losartan on left ventricular hypertrophy and fibrosis in patients with nonobstructive hypertrophic cardiomyopathy. JACC Heart Fail. 2013;1:480. doi: 10.1016/j.jchf.2013.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Deswal A, Richardson P, Bozkurt B, Mann DL. Results of the Randomized Aldosterone Antagonism in heart failure with Preserved Ejection Fraction trial (RAAM-PEF) J Card Fail. 2011;17:634–642. doi: 10.1016/j.cardfail.2011.04.007. [DOI] [PubMed] [Google Scholar]
- 89.Brilla CG, Funck RC, Rupp H. Lisinopril-mediated regression of myocardial fibrosis in patients with hypertensive heart disease. Circulation. 2000;102:1388–1393. doi: 10.1161/01.cir.102.12.1388. [DOI] [PubMed] [Google Scholar]
- 90.Garvin AM, De Both MD, Talboom JS, Lindsey ML, Huentelman MJ, Hale TM. Transient ACE (Angiotensin-Converting Enzyme) inhibition suppresses future fibrogenic capacity and heterogeneity of cardiac fibroblast subpopulations. Hypertension. 2021;77:904–918. doi: 10.1161/HYPERTENSIONAHA.120.16352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Fang L, Murphy AJ, Dart AM. A clinical perspective of anti-fibrotic therapies for cardiovascular disease. Front Pharmacol. 2017;8:186. doi: 10.3389/fphar.2017.00186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Parichatikanond W, Luangmonkong T, Mangmool S, Kurose H. Therapeutic targets for the treatment of cardiac fibrosis and cancer: focusing on TGF-β signaling. Front Cardiovasc Med. 2020;7:34. doi: 10.3389/fcvm.2020.00034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Tani H, Sadahiro T, Ieda M. Direct cardiac reprogramming: a novel approach for heart regeneration. Int J Mol Sci. 2018;19:2629. doi: 10.3390/ijms19092629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Pagano F, Calicchio A, Picchio V, Ballarino M. The noncoding side of cardiac differentiation and regeneration. Curr Stem Cell Res Ther. 2020;15:723–738. doi: 10.2174/1574888X15666200123120249. [DOI] [PubMed] [Google Scholar]
- 95.Chen W, Bian W, Zhou Y, Zhang J. Cardiac fibroblasts and myocardial regeneration. Front Bioeng Biotechnol. 2021;9:227. doi: 10.3389/fbioe.2021.599928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kaur H, Takefuji M, Ngai CY, Carvalho J, Bayer J, Wietelmann A, Poetsch A, Hoelper S, Conway SJ, Möllmann H, Looso M, Troidl C, Offermanns S, Wettschureck N. Targeted ablation of periostin-expressing activated fibroblasts prevents adverse cardiac remodeling in mice. Circ Res. 2016;118:1906–1917. doi: 10.1161/CIRCRESAHA.116.308643. [DOI] [PubMed] [Google Scholar]
- 97.Tillmanns J, Hoffmann D, Habbaba Y, Schmitto JD, Sedding D, Fraccarollo D, Galuppo P, Bauersachs J. Fibroblast activation protein alpha expression identifies activated fibroblasts after myocardial infarction. J Mol Cell Cardiol. 2015;87:194–203. doi: 10.1016/j.yjmcc.2015.08.016. [DOI] [PubMed] [Google Scholar]
- 98.Aghajanian H, Kimura T, Rurik JG, Hancock AS, Leibowitz MS, Li L, Scholler J, Monslow J, Lo A, Han W, Wang T, Bedi K, Morley MP, Linares Saldana RA, Bolar NA, McDaid K, Assenmacher CA, Smith CL, Wirth D, June CH, Margulies KB, Jain R, Puré E, Albelda SM, Epstein JA. Targeting cardiac fibrosis with engineered T cells. Nature. 2019;573:430–433. doi: 10.1038/s41586-019-1546-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hiltbrunner S, Britschgi C, Schuberth P, Bankel L, Nguyen-Kim TDL, Gulati P, Weder W, Opitz I, Lauk O, Caviezel C, Bachmann H, Tabor A, Schröder P, Knuth A, Münz C, Stahel R, Boyman O, Renner C, Petrausch U, Curioni-Fontecedro A. Local delivery of CAR T cells targeting fibroblast activation protein is safe in patients with pleural mesothelioma: first report of FAPME, a phase I clinical trial. Ann Oncol. 2021;32:120–121. doi: 10.1016/j.annonc.2020.10.474. [DOI] [PubMed] [Google Scholar]


