Abstract
Objective: This study was designed to investigate the expression of serum autophagy-related protein P62 in patients with severe acute pancreatitis (AP) and its correlation with prognosis. Methods: Eighty patients with AP treated in the First Affiliated Hospital of Gannan Medical University from January 2020 to January 2021 were enrolled as study subjects in this retrospective analysis, and they were placed into the mild AP group (n=52) or the severe AP group (n=28). According to clinical outcomes, these 80 patients were divided into a good prognosis group (GP group, n=51, surviving without serious complications such as organ failure) and a poor prognosis group (PP group, n=29, death or developing organ failure). The differences in C-reactive protein (CRP), P62 and the Acute Physiology and Chronic Health Evaluation II (APACHE-II) were compared upon admission. The changes of CRP, P62 and APACHE-II within 1-7 h after admission were dynamically analyzed in the two groups. Spearman correlation analysis was performed to explore the correlation between P62 and APACHE-II scores, and the receiver operating characteristic (ROC) curve of P62 related to poor AP outcome was plotted. Results: CRP, P62 and APACHE-II in the mild AP group were significantly higher than those in the severe AP group, and these in the PP group were also significantly higher than those in the GP group (P<0.05). Dynamic monitoring showed that within 1-7 h after admission, CRP, P62, and APACHE-II in the severe AP group were significantly higher than those in the mild AP group (P<0.05), and these in the PP group were significantly higher than those in the GP group (P<0.05). Spearman correlation analysis showed that P62 level was significantly positively correlated with both CRP and APACHE-II (r=0.9331, r=0.9500, P<0.0001). ROC curve showed that AUC of P62 was 0.9570 in AP patients with poor prognosis (95% CI=0.8939-1.000, P<0.0001). Conclusion: Serum autophagy-related protein P62 was closely related to the condition and prognosis of AP patients, and P62 could be used as a potential indicator to assess the condition and prognosis of AP patients.
Keywords: Autophagy-related protein, P62, acute pancreatitis, expression, prognosis
Introduction
Acute pancreatitis (AP) is one of the most common clinical acute abdominal syndromes [1]. During the onset of AP, pancreatic enzymes are activated in the pancreas by various factors, which cause pancreatic self-digestion, leading to inflammatory symptoms such as edema, hemorrhage, and even necrosis in the pancreas [2,3]. The manifestations and symptoms of AP include abdominal pain, nausea, vomiting, fever, and elevated blood levels of the pancreatic enzymes [4]. The severity of AP varies from mild to severe. In mild cases, pancreatic edema is the main symptom, which is self-limited and disappears within 3-5 d with a good prognosis. Patients with severe AP may present with hemorrhage and necrosis, often accompanied by systemic inflammatory reactions or local complications (infectious necrosis, pseudocysts, etc.). Patients with peripheral organ involvement may experience multi-organ dysfunction syndrome [5,6]. Although severe AP only accounts for about 10%-25% of all AP cases, it has a high mortality rate of 20%-30% due to the rapid onset and progression [7].
With the deepening of AP research, the pathological activation of trypsinogen and its self-digestive injury to pancreatic tissue are the main pathogenesis of AP [8]. It has been found that abnormal activation of trypsinogen and morphological changes in its zymogen granules are closely associated with autophagic activity, and impaired autophagy is a well-known prominent pathological event in AP [9]. P62 is an autophagic selective substrate that plays a key role in the formation of cytoplasmic protein inclusion bodies, and it is also a marker for many chronic degenerative diseases such as Alzheimer’s disease and Parkinson’s disease. Current studies have shown that P62 protein actively participates in a variety of stress reactions, in which P62 can interact with LC3 located in the autophagosome, and is continuously degraded by the autophagy-lysosome system, promoting mitochondrial damage, peroxisome, and microbial degradation [10]. In recent years, with the progress of treatment technology in China, the overall incidence and mortality rate of AP has declined, and the incidence and mortality rate of severe AP has been reduced to 10%-20% [11]. Early and accurate assessment of the condition plays a critical role in follow-up treatment, improvement of patient prognosis and reduction of mortality. The condition of AP assessment mostly relies on scores of various scales. Although this method is intuitive, it takes a long time and requires good compliance, which makes it difficult to achieve dynamic, rapid, and early assessment of the condition [12]. Therefore, it is important to find biological indicators that can accurately reflect the condition and prognosis of AP. This study intended to investigate the relationship between the expression of serum autophagy-related protein P62 and AP prognosis by enrolling 80 AP patients as the study subjects and grouping them according to the severity of disease and prognosis respectively, to preliminarily demonstrate the role of autophagy in the development of severe pancreatitis, and to find a qualitative index that can be used to evaluate the condition and prognosis of severe AP patients, aiming to provide new ideas and directions for the diagnosis and treatment of AP.
Materials and methods
Baseline data
Eighty patients with AP treated in the First Affiliated Hospital of Gannan Medical University from January 2020 to January 2021 were enrolled as the study subjects in this retrospective analysis, including 51 males and 29 females, aged 33-59 years, with a mean age of (46.32±3.65) years.
Inclusion criteria: (1) patients who met the diagnostic criteria for AP in the guidelines for the diagnosis and management of acute pancreatitis (2014) [13]; (2) patients with an onset of ≤24 h; (3) patients with their first episode; (4) this study was approved by the Ethics Committee of First Affiliated Hospital of Gannan Medical University (approval number NCT0125684).
Exclusion criteria: (1) those comorbid with psychiatric disorders; (2) those comorbid with malignancies; (3) those who had received surgery within 1 month prior to the study; (4) those comorbid with systemic inflammatory diseases; (5) pregnant and lactating women; and (6) those with drug or alcohol dependence.
Intervention methods
Fasting venous blood was collected from 80 patients with AP, centrifuged at 3000 r/min for 5 min, and stored at -80°C. After the samples were collected, the serum P62 and C-reactive protein (CRP) levels were measured by enzyme-linked immunosorbent assay (ELISA). The P62 kits (Art. No. 61499) were purchased from Shanghai Enzyme-linked Biotechnology Co., Ltd. (Shanghai, China), and the CRP kits (Art. No. T-039) were purchased from Shanghai Guangrui Biological Technology Co., Ltd. (Shanghai, China). The differences of P62, CRP and the Acute Physiology and Chronic Health Evaluation II (APACHE-II) were compared between mild AP group and severe AP group, as well as good prognosis group (GP group) and poor prognosis group (PP group).
Outcome measurements
The primary indicators of this study included the correlation between serum P62 protein level and the condition and prognosis of patients with severe pancreatitis, and the secondary indicators were serum CRP level and APACHE-II score.
The 80 patients with AP enrolled were divided into a mild AP group (n=52) and a severe AP group (n=28), respectively. Criteria for mild AP: patients had typical clinical symptoms and biochemical changes, but did not develop organ dysfunction or local complications, response to fluid therapy, Ranson score <3. Criteria for severe AP: patients had typical symptoms and biochemical changes as well as local complications or organ failure, Ranson score (the age, blood glucose, AST, LDH, WBC count, blood calcium concentration at admission, and PaO2, alkali deficiency, blood BUN, Hct and body fluid loss at 48 h after admission were assessed) ≥3 [14]. After the blood samples were collected at the time point, the centrifuged serum was used to detect the CRP level by ELISA, and each index was tested three consecutive times, with the average value taken as the final result. The APACHE-II scale was assessed in three aspects [15]: acute physiology score (APS), as well as age and cognitive performance scale (CPS), of which the APS consists of 12 items for the assessment of patient condition, morbidity and mortality in the intensive care unit (ICU), with a theoretical maximum score of 71, with higher scores representing more severe conditions. P62, CRP, and APACHE-II scores at admission were compared, and the differences of P62, CRP, and APACHE-II within 1-7 h after admission were compared between the two groups.
The 80 enrolled patients with AP were divided into a GP group (survival, no serious complications such as organ failure, n=51) and a PP group (death or with organ failure, n=29). The differences of P62, CRP, and APACHE-II at admission and within 1-7 h after admission were compared and analyzed between the two groups.
Statistical methods
SPSS 22.0 was the data analysis tool. The measurement data were expressed as mean ± standard deviation (mean ± SD). T-test was performed for data conforming to a normal distribution or with variance consistency; Mann-Whitney U-test was applied for data with variance inconsistency, and Chi-squared test was used for comparison of counting data. For the analysis of the differences between the two groups at multiple time points, analysis of variance (ANOVA) was first implemented to examine whether there were differences between the two groups, and post hoc analysis was used for pairwise comparisons. Spearman correlation analysis was used for correlation analysis. The diagnostic value of P62 and CRP was analyzed by ROC curve. P<0.05 indicated significant difference between the two groups, and the figures were plotted by Graphpad Prism 8.0 [16].
Results
Differences in P62, CRP and APACHE-II regarding the patient’s condition
The 80 patients enrolled were divided into the mild AP group and the severe AP group. The serum P62 and CRP levels as well as APACHE-II scores of patients in the mild AP group were significantly lower than those in the severe AP group (P<0.05) (Figure 1).
Figure 1.
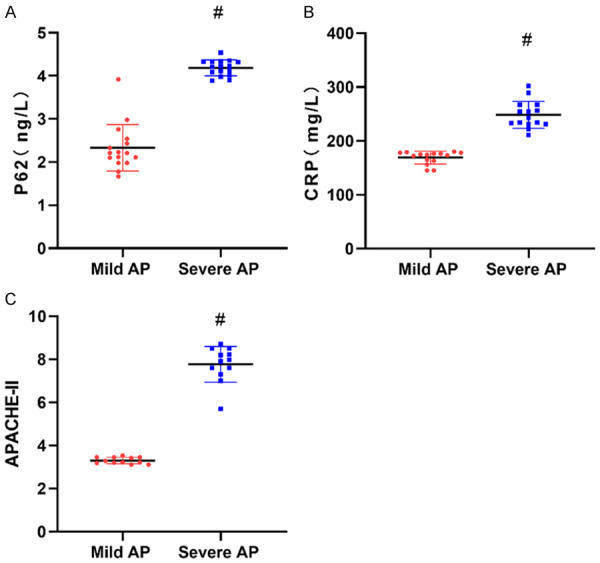
Differences in P62, CRP and APACHE-II among patients with different condition. A: P62 levels; B: CRP levels; C: APACHE-II scores. CRP: C-reactive protein; APACHE-II: Acute Physiology and Chronic Health Evaluation II. #represents P<0.05 compared with the mild AP group. The applied statistical method was t test.
P62, CRP and APACHE-II regarding prognosis
The 80 patients enrolled were divided into either the GP group or the PP group according to the clinical outcomes. The results showed that the serum P62 and CRP levels as well as APACHE-II scores of AP patients in the GP group were significantly lower than those in the PP group (P<0.05) (Figure 2).
Figure 2.
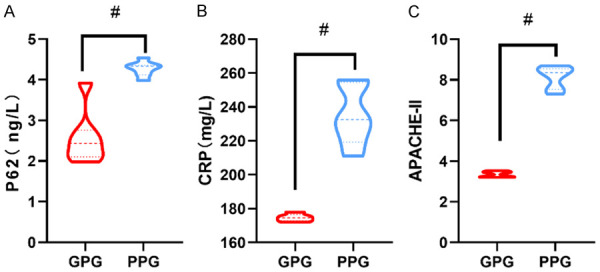
Differences in P62, CRP and APACHE-II among patients with different prognosis. A: P62 levels; B: CRP levels; C: APACHE-II scores. CRP: C-reactive protein; APACHE-II: Acute Physiology and Chronic Health Evaluation II. #represents P<0.05 compared with the good prognosis group. The applied statistical method was t test.
Differences in P62 and CRP, APACHE-II after admission
Dynamic monitoring showed that serum P62 and CRP levels as well as APACHE-II scores of patients in the severe AP group were significantly higher than those of patients in the mild AP group within 1-7 h after admission (P<0.05). Serum P62 and CRP levels as well as APACHE-II scores were significantly reduced in both groups 7 h after admission (P<0.05) (Figure 3).
Figure 3.
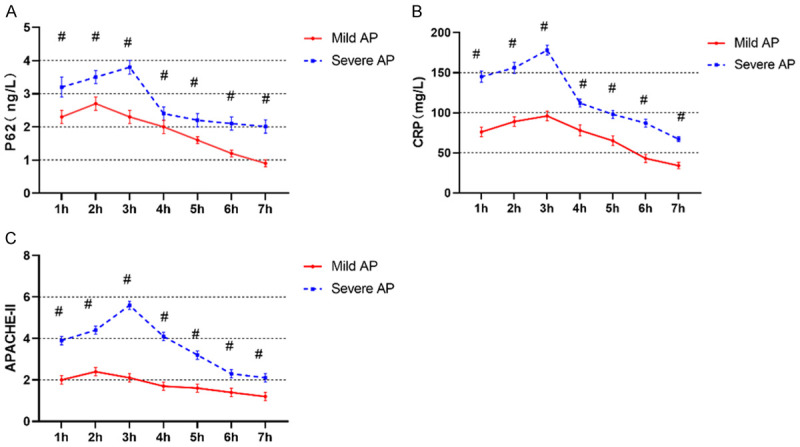
Differences in P62, CRP and APACHE-II among AP patients with different conditions after admission. A: P62 levels; B: CRP levels; C: APACHE-II scores. CRP: C-reactive protein; APACHE-II: Acute Physiology and Chronic Health Evaluation II. #represents P<0.05 compared with the mild AP group. The applied statistical method was t test.
Differences between P62 and CRP, APACHE-II regarding prognosis of AP after admission
Serum P62 and CRP levels as well as APACHE-II scores of AP patients in the PP group were significantly higher than those in the GP group (P<0.05). Serum P62, CRP and APACHE-II were significantly reduced in both groups 7 h after admission (P<0.05) (Figure 4).
Figure 4.
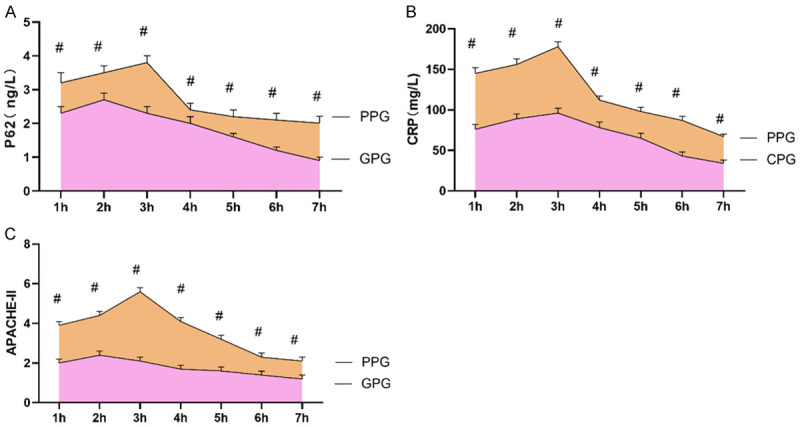
Differences in P62 and CRP, APACHE-II in AP patients with different prognosis after admission. A: P62 levels; B: CRP levels; C: APACHE-II scores. #represents P<0.05 compared with the good prognosis group. The applied statistical method was t test.
Correlation analysis of serum P62 with CRP, APACHE-II
Serum P62 levels in AP patients showed a significant positive correlation with CRP levels (Figure 5A) (r=0.9331, P<0.0001) and APACHE-II scores (Figure 5B) (r=0.9500, P<0.0001). CRP was also positively correlated with APACHE-II scores (r=0.9651, P<0.0001) (Figure 5C).
Figure 5.
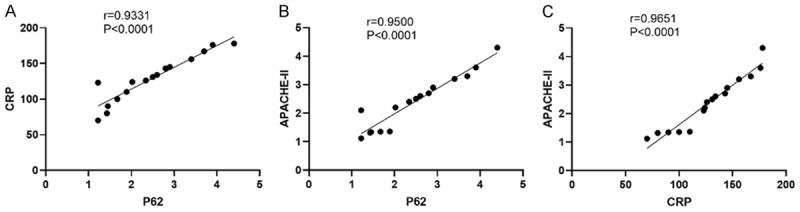
Correlation analysis of serum P62 with CRP and APACHE-II. The serum P62 levels in AP patients showed significant positive correlations with CRP levels (A) (r=0.9331, P<0.0001) and APACHE-II scores (B) (r=0.9500, P<0.0001), and CRP showed the same positive correlation with APACHE-II (r=0.9651, P<0.0001) (C). The applied statistical method was Spearman correlation analysis.
The predictive value of serum P62 for poor prognosis in AP
With 3.0 ng/L as the cut-off value, the receiver operating characteristic (ROC) curve of P62 for predicting poor prognosis in AP patients yielded an AUC of 0.9570 (95% CI=0.8939-1.000, P<0.0001). With 143.19 mg/L as the cut-off value, the ROC curve of CRP for predicting poor prognosis in AP patients yielded an AUC of 0.9311 (95% CI=0.8388-1.000, P<0.0001). With 4.08 as the cut-off value, the ROC curve of APACHE-II scores for predicting poor prognosis in AP patients yielded an AUC of 0.8827 (95% CI=0.7416-1.000, P<0.0001), suggesting that P62 had a superior diagnostic value for poor prognosis in AP patients (Figure 6).
Figure 6.
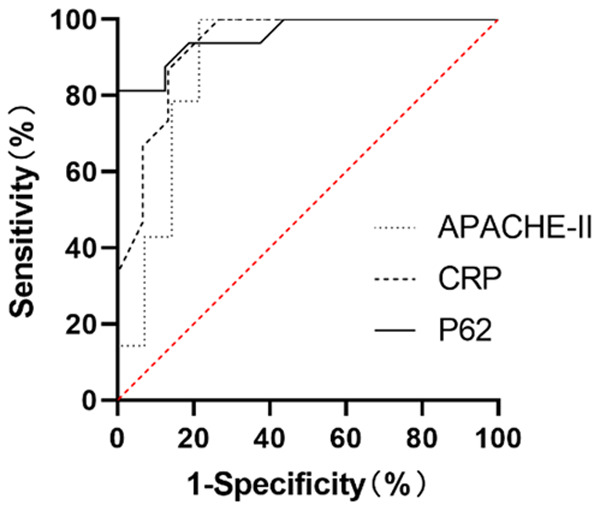
ROC curves of serum P62 AP patients with poor prognosis. Serum AUC of P62: 0.9570 (95% CI=0.8939-1.000, P<0.0001); AUC of CRP: 0.9311 (95% CI=0.8388-1.000, P<0.0001); AUC of APACHE-II: 0.8827 (95% CI=0.7416-1.000, P<0.0001). The applied statistical method was ROC curve analysis.
Discussion
With the changes in people’s dietary habits and lifestyles in recent years, the incidence of AP has been increasing annually [17], reaching about 30/100,000. The pathogenesis of AP remains unclear, and it has been proven that excessive alcohol consumption, gallstones, pancreatic injury, hypercalcemia, and drug allergy can induce the occurrence of AP [18]. Patients with mild AP are generally self-limited and can recover within 1 week after routine treatment, while patients with severe AP require targeted intervention to prevent organ failure or local complications [19]. With recent advances in treatment options, the incidence and mortality of AP are decreasing, but accurate diagnosis and timely intervention are still important means to accelerate the recovery and improve the prognosis of AP patients.
CRP is a commonly used laboratory test item in clinical practice, but its specificity in the evaluation of severe pancreatitis is low. The significance of including CRP in this study is to verify the feasibility of applying CRP in the diagnosis of severe pancreatitis in addition to seeking the specificity detection of P62, and to provide certain data reference for other studies. APACHE-II has been applied for evaluating the severity and prognosis of severe pancreatitis. In this study, the relationship between the expression of autophagy-related protein P62 and prognosis in patients with AP was investigated. Serum P62 and CRP levels as well as APACHE-II scores of patients in the severe AP group were significantly higher than those in the mild AP group. Patients in the AP group with a poor prognosis had significantly higher serum P62 and CRP levels as well as APACHE-II scores than those in the AP group with a good prognosis, suggesting that P62 was associated with the condition and prognosis of AP patients. The effect of autophagy in AP modeled mice was explored [20], and the results showed that the autophagic pathway was significantly restored in mice receiving resolvin D1. The effect of autophagy on murine cells was significantly minimized while serum P62 and LC3-II levels were significantly reduced, suggesting that P62 could be used as an indicator of the severity of autophagy in AP modeled mice. Animal experiments on AP induced by different concentrations of Bafiomycin A1 in mice showed that [21] the serum P62 and LC3 levels of mice in the high-dose Bafiomycin A1-induced group were significantly lower than those in the low-dose Bafiomycin A1-induced group, suggesting that Bafiomycin A1 could inhibit autophagy in the pancreatic tissue of AP mice, exhibiting a better pancreatic protective effect. The correlation between autophagic process and AP condition has been confirmed in studies [22,23], and the mechanism may be related to the ability of AP to activate multiple stress factors, while the autophagic vacuoles or trypsinogen particles in the autophagic vesicles of pancreatic follicular cells are actively released in the early stages of AP, leading to injury to pancreatic cells. As can be seen from the above, the autophagy process is closely related to the development of AP, and the level of P62, an autophagy-related protein, can directly reflect the severity of autophagy, thus P62 can be used as a reference index for diagnosis, treatment and prognosis analysis of AP patients.
The correlation between serum P62 levels and CRP and APACHE-II scores was further explored, which showed a significant positive correlation between serum P62 levels and both CRP and APACHE-II scores. It was reported in a previous study that [24] serum CRP levels were closely related to organ failure in patients with AP, and calculations using CRP=0.24 mg/L as cut-off value showed that the sensitivity, specificity and accuracy of CRP for the development of organ failure in AP were 78.43%, 80.61% and 79.50%, respectively. It has been shown [25] that serum CRP expression is abnormally high in AP patients compared with controls. CRP levels are closely related to the condition of AP patients, and serum CRP is positively correlated with serum procalcitonin and tumor necrosis factor-α. In a study conducted in 101 AP patients [26], it illustrated that APACHE-II score is a key risk factor for death in AP patients. The correlation between P62 and CRP levels as well as APACHE-II scores in this study confirmed the correlation between P62 and the condition, prognosis of AP patients, which is also verified by the ROC curve of autophagy protein P62 for poor prognosis in AP patients.
In conclusion, serum autophagy-related protein P62 is closely associated with the condition and prognosis of AP patients, and P62 can be used as a potential indicator for assessing the condition and prognosis of AP patients. The innovation of this study is that patients with AP were grouped according to their conditions and prognosis, and the differences of P62 protein expression in AP patients with different conditions and prognosis were demonstrated in detail, and finally, the value of P62 protein in determining poor prognosis of AP was explored by ROC curve analysis, providing clinical reference for further studies. The limitations of this study are as follows: (1) In terms of sample size, the number of subjects is small, and the sample source is singular, which may affect the of scientific results to some extent; (2) This study lacks long-term follow-up results of the enrolled patients, which can be used as a focus for further analysis. In the next step, a multicenter randomized study with a larger sample size needs to be conducted.
Disclosure of conflict of interest
None.
References
- 1.Greenberg JA, Hsu J, Bawazeer M, Marshall J, Friedrich JO, Nathens A, Coburn N, May GR, Pearsall E, McLeod RS. Clinical practice guideline: management of acute pancreatitis. Can J Surg. 2016;59:128–140. doi: 10.1503/cjs.015015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boxhoorn L, Voermans RP, Bouwense SA, Bruno MJ, Verdonk RC, Boermeester MA, van Santvoort HC, Besselink MG. Acute pancreatitis. Lancet. 2020;396:726–734. doi: 10.1016/S0140-6736(20)31310-6. [DOI] [PubMed] [Google Scholar]
- 3.Garg R, Rustagi T. Management of hypertriglyceridemia induced acute pancreatitis. Biomed Res Int. 2018;2018:4721357. doi: 10.1155/2018/4721357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.James TW, Crockett SD. Management of acute pancreatitis in the first 72 hours. Curr Opin Gastroenterol. 2018;34:330–335. doi: 10.1097/MOG.0000000000000456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van Dijk SM, Hallensleben NDL, van Santvoort HC, Fockens P, van Goor H, Bruno MJ, Besselink MG Dutch Pancreatitis Study Group. Acute pancreatitis: recent advances through randomised trials. Gut. 2017;66:2024–2032. doi: 10.1136/gutjnl-2016-313595. [DOI] [PubMed] [Google Scholar]
- 6.Lee PJ, Papachristou GI. New insights into acute pancreatitis. Nat Rev Gastroenterol Hepatol. 2019;16:479–496. doi: 10.1038/s41575-019-0158-2. [DOI] [PubMed] [Google Scholar]
- 7.Mandalia A, Wamsteker EJ, DiMagno MJ. Recent advances in understanding and managing acute pancreatitis. F1000Res. 2018;7 doi: 10.12688/f1000research.14244.1. F1000 Faculty Rev-959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Waller A, Long B, Koyfman A, Gottlieb M. Acute pancreatitis: updates for emergency clinicians. J Emerg Med. 2018;55:769–779. doi: 10.1016/j.jemermed.2018.08.009. [DOI] [PubMed] [Google Scholar]
- 9.Garg PK, Singh VP. Organ failure due to systemic injury in acute pancreatitis. Gastroenterology. 2019;156:2008–2023. doi: 10.1053/j.gastro.2018.12.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Janisch NH, Gardner TB. Advances in management of acute pancreatitis. Gastroenterol Clin North Am. 2016;45:1–8. doi: 10.1016/j.gtc.2015.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Portelli M, Jones CD. Severe acute pancreatitis: pathogenesis, diagnosis and surgical management. Hepatobiliary Pancreat Dis Int. 2017;16:155–159. doi: 10.1016/s1499-3872(16)60163-7. [DOI] [PubMed] [Google Scholar]
- 12.Ismail OZ, Bhayana V. Lipase or amylase for the diagnosis of acute pancreatitis? Clin Biochem. 2017;50:1275–1280. doi: 10.1016/j.clinbiochem.2017.07.003. [DOI] [PubMed] [Google Scholar]
- 13.Majidi S, Golembioski A, Wilson SL, Thompson EC. Acute pancreatitis: etiology, pathology, diagnosis, and treatment. South Med J. 2017;110:727–732. doi: 10.14423/SMJ.0000000000000727. [DOI] [PubMed] [Google Scholar]
- 14.Maheshwari R, Subramanian RM. Severe acute pancreatitis and necrotizing pancreatitis. Crit Care Clin. 2016;32:279–290. doi: 10.1016/j.ccc.2015.12.006. [DOI] [PubMed] [Google Scholar]
- 15.Restrepo R, Hagerott HE, Kulkarni S, Yasrebi M, Lee EY. Acute pancreatitis in pediatric patients: demographics, etiology, and diagnostic imaging. AJR Am J Roentgenol. 2016;206:632–644. doi: 10.2214/AJR.14.14223. [DOI] [PubMed] [Google Scholar]
- 16.Forsmark CE, Vege SS, Wilcox CM. Acute pancreatitis. N Engl J Med. 2016;375:1972–1981. doi: 10.1056/NEJMra1505202. [DOI] [PubMed] [Google Scholar]
- 17.Habtezion A, Gukovskaya AS, Pandol SJ. Acute pancreatitis: a multifaceted set of organelle and cellular interactions. Gastroenterology. 2019;156:1941–1950. doi: 10.1053/j.gastro.2018.11.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hines OJ, Pandol SJ. Management of severe acute pancreatitis. BMJ. 2019;367:l6227. doi: 10.1136/bmj.l6227. [DOI] [PubMed] [Google Scholar]
- 19.Trikudanathan G, Wolbrink DRJ, van Santvoort HC, Mallery S, Freeman M, Besselink MG. Current concepts in severe acute and necrotizing pancreatitis: an evidence-based approach. Gastroenterology. 2019;156:1994–2007. e1993. doi: 10.1053/j.gastro.2019.01.269. [DOI] [PubMed] [Google Scholar]
- 20.Fonseca Sepúlveda EV, Guerrero-Lozano R. Acute pancreatitis and recurrent acute pancreatitis: an exploration of clinical and etiologic factors and outcomes. J Pediatr (Rio J) 2019;95:713–719. doi: 10.1016/j.jped.2018.06.011. [DOI] [PubMed] [Google Scholar]
- 21.Zhan X, Wan J, Zhang G, Song L, Gui F, Zhang Y, Li Y, Guo J, Dawra RK, Saluja AK, Haddock AN, Zhang L, Bi Y, Ji B. Elevated intracellular trypsin exacerbates acute pancreatitis and chronic pancreatitis in mice. Am J Physiol Gastrointest Liver Physiol. 2019;316:G816–G825. doi: 10.1152/ajpgi.00004.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Murthy P, Singhi AD, Ross MA, Loughran P, Paragomi P, Papachristou GI, Whitcomb DC, Zureikat AH, Lotze MT, Zeh Iii HJ, Boone BA. Enhanced neutrophil extracellular trap formation in acute pancreatitis contributes to disease severity and is reduced by chloroquine. Front Immunol. 2019;10:28. doi: 10.3389/fimmu.2019.00028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Khamaysi I, Hamo-Giladi DB, Abassi Z. Heparanase in acute pancreatitis. Adv Exp Med Biol. 2020;1221:703–719. doi: 10.1007/978-3-030-34521-1_29. [DOI] [PubMed] [Google Scholar]
- 24.Li Z, Yu X, Werner J, Bazhin AV, D’Haese JG. The role of interleukin-18 in pancreatitis and pancreatic cancer. Cytokine Growth Factor Rev. 2019;50:1–12. doi: 10.1016/j.cytogfr.2019.11.001. [DOI] [PubMed] [Google Scholar]
- 25.Sendler M, van den Brandt C, Glaubitz J, Wilden A, Golchert J, Weiss FU, Homuth G, De Freitas Chama LL, Mishra N, Mahajan UM, Bossaller L, Völker U, Bröker BM, Mayerle J, Lerch MM. NLRP3 inflammasome regulates development of systemic inflammatory response and compensatory anti-inflammatory response syndromes in mice with acute pancreatitis. Gastroenterology. 2020;158:253–269. e214. doi: 10.1053/j.gastro.2019.09.040. [DOI] [PubMed] [Google Scholar]
- 26.Cofaru FA, Nica S, FierbinTeanu-Braticevici C. Assessment of severity of acute pancreatitis over time. Rom J Intern Med. 2020;58:47–54. doi: 10.2478/rjim-2020-0003. [DOI] [PubMed] [Google Scholar]


