Abstract
Objective: To investigate clinical effect of probiotics combined with zinc and selenium preparation in the treatment of child rotavirus enteritis. Methods: In this retrospective study, the patients were divided into two groups based on treatment method. The control group (n=42) received probiotic therapy, while the experimental group (n=43) received probiotics combined with zinc and selenium preparation. Clinical efficacy, stool frequency and incidence of adverse reactions after treatment were compared to assess the clinical effect. Results: The clinical effect was improved after intervention (P<0.05), and the total effective rate of two groups was 88.4% (38/43), 50% (21/42), respectively. Time to symptom disappearance was significantly decreased in the experimental group as compared to the control group. The myocardial zymogram indices (CK, CK-MB and AST) were decreased after treatment, and the levels in the experimental group were significantly lower than in the control group (P<0.05). Moreover, we observed that the levels of inflammatory factors (IL-6, IL-8 and hsCRP) in the experimental group were significantly lower than those of the control group after intervention (all P<0.05). Conclusion: Probiotics combined with zinc and selenium preparation can not only significantly improve the clinical effect, but also shorten the course of disease.
Keywords: Probiotics, zinc and selenium preparation, rotavirus enteritis, children
Introduction
Infantile diarrhea mostly occurs in cold weather [1]. Rotavirus is still one of the main pathogens of autumn diarrhea in children [2]. Rotavirus enteritis is an acute infectious digestive tract disease caused by group A rotavirus [3,4]. It occurs primarily in children aged 2-8 years old [5]. Rotavirus infection not only causes symptoms such as diarrhea, vomiting, fever, dehydration, electrolyte disorder and acidosis, but also damages the respiratory system, nervous system, and organs such as heart and liver [6-10]. After entering the human intestine, rotavirus adheres to the small intestinal mucosa and replicates in the intestinal mucosal epithelial cells [11-13]. At present, there is no specific drug treatment. Most drugs have some function in relieving symptoms by maintaining water and supporting treatment [14-17].
Rotavirus infection can lead to the imbalance of intestinal flora in children and cause clinical symptoms such as abdominal pain, diarrhea and vomiting. The key of treatment is to take effective treatment plans and ameliorate symptoms in children [18-20]. At present, montmorillonite powder combined with probiotics is often used in the treatment of rotavirus enteritis, but the effect is poor [21]. Clinical studies showed that the level of trace element zinc in children with rotavirus enteritis is generally decreased [22,23]. Zinc supplementation is suggested to promote recovery in children [24]. However, there is still no evidence on the application of probiotics combined with zinc and selenium preparation in the treatment of children with rotavirus enteritis. Therefore, this retrospective study evaluated the effect of probiotics combined with zinc and selenium preparation.
Data and methods
Study population
This retrospective study was performed in Cangzhou Central Hospital. We enrolled 85 children with rotavirus enteritis from January 2019 to December 2020, and the patients were allocated into two group: the experimental group (n=43 cases) and the control group (n=42 cases) according to the treatment they received. The study was approved by the Ethics Committee of Cangzhou Central Hospital (No.: ECZZX-2019015).
Inclusion and exclusion standard
Inclusion criteria
① Patients with age under 18 years old; ② Patients with confirmed rotavirus enteritis: the diagnostic criteria of acute diarrhea were positive for stool rotavirus antigen, diarrhea >5 times per day; ③ Patients with no previous therapy of probiotics combined with zinc and selenium preparation; ④ Patients with complete clinical data; ⑤ Patients whose parents provided informed consent.
Exclusion criteria
① Patients with a history of gastric surgery; ② Patients with acute enteritis caused by infection of other viruses (such as enteroadenovirus, astrovirus, etc.); ③ Patients with a history of severe heart, liver, kidney and other organ dysfunctions; ④ Patients unwilling to participate in this research; ⑤ Patients with malignant diseases; ⑥ Patients who were undernourished.
Treatment methods
The control group
The children received probiotic therapy: Bifidobacterium triple viable capsule (probiotics) (Shanghai Xinyi Pharmaceutical Co., Ltd.), 0.24 g, TID. After admission, children were given antiviral, rehydration, and symptomatic treatment according to the degree and nature of dehydration, and dietary guidance.
Experimental group
On the basis of treatment in the control group, the children received additional zinc and selenium preparation: (Shandong Xinxibao Co., Ltd), 0.5 g, TID. After admission, children were also given antiviral, rehydration and symptomatic treatment according to the degree and nature of dehydration, and dietary guidance.
Assessment of clinical effect
① The clinical effect can be divided into three levels: markedly effective, effective and ineffective. Markedly effect: the stool form returned to normal after 3 days of treatment, and the stool frequency was less than 3 times every day; Effective: After 3 days of treatment, the moisture in stool decreased significantly, and the stool frequency was 3~4 times every day; Ineffective: There was no improvement in stool form and stool frequency after 3 days of treatment. Total effective rate = significant effective rate + effective rate. ② The cardiac function was assessed by detecting myocardial zymogram indices. ③ 3~5 ml of morning fasting venous blood before and after treatment were collected from patients and centrifuged at 3000 R/min for 10 min to obtain the supernatant. The expressions of interleukin-6 (IL-6), interleukin-8 (IL-8) and high-sensitivity C-reactive protein (hsCRP) were detected by enzyme-linked immunosorbent assay. IL-6, IL-8 and hsCRP kits were provided by Everbright Biotechnology Co., Ltd. The operation was carried out in strict accordance with the operation manual. ④ The time to symptom disappearance and adverse reactions were observed to comprehensively evaluate the effectiveness and safety of probiotics combined with zinc and selenium preparation.
Statistical analysis
Statistical analysis was performed with SPSS 18 (SPSS for Windows software, SPSS Inc., Chicago, IL). Continuous variables were expressed by mean ± standard deviation or median (interquartile interval), and analyzed by independent sample t-test or Mann Whitney U-Rank Sum test; Categorical variables were expressed by absolute value and percentage, and chi square test was used. P<0.05 indicated a significant difference.
Results
Clinical data
Table 1 shows characteristics of the included patients. There were 43 patients in the experimental group with a mean age of (8.3±1.45) years old. There were 42 patients in the control group with a mean age of (7.95±1.24) years old. The two groups were comparable in both demographics and clinical characteristics (all P>0.05).
Table 1.
Comparison of clinical data between groups
| Experimental group (n=43) | Control group (n=42) | t/X2 | P | |
|---|---|---|---|---|
| Age (years old) | 8.3±1.45 | 7.95±1.24 | 2.35 | 0.43 |
| Sex | 7.21 | 0.22 | ||
| Male (n%) | 23 (53.5%) | 27 (64.3%) | ||
| Female (n%) | 20 (46.5%) | 15 (35.7%) | ||
| Average course of disease | 1.52±2.53 | 1.37±2.35 | 2.52 | 0.67 |
| Temperature | 38.35±1.14 | 38.66±0.77 | 3.39 | 0.35 |
| Clinical symptoms | ||||
| Fever | 31 (72.1%) | 34 (81%) | 9.52 | 0.17 |
| Vomit | 13 (30.2%) | 11 (26.2%) | 7.84 | 0.09 |
| Convulsions | 27 (62.8%) | 19 (45.2%) | 6.74 | 0.11 |
| Mild dehydration | 12 (27.9%) | 9 (21.4%) | 4.35 | 0.31 |
| Moderate dehydration | 5 (11.6%) | 6 (14.2%) | 3.34 | 0.27 |
| Daily stool frequency | 3.12 | 0.13 | ||
| >10 times | 18 (41.9%) | 22 (52.4%) | 5.23 | 0.29 |
| 5-10 times | 12 (27.9%) | 9 (21.4%) | 4.35 | 0.31 |
| <5 times | 13 (30.2%) | 11 (26.2%) | 7.84 | 0.09 |
Note: Compared to the control group, a significant difference was P<0.05.
Clinical therapeutic effect
As shown in Table 2, the total effective rate in the experimental group was 88.4% (38/43), which was significantly higher than the 50% in the control group (P<0.05).
Table 2.
Comparison of clinical therapeutic effect between groups
| Experimental group (n=43) | Control group (n=42) | X2 | P | |
|---|---|---|---|---|
| Significantly effective | 14 (32.6%) | 9 (21.4%) | 7.268 | 0.007 |
| Effective | 24 (55.8%) | 12 (28.6%) | 9.737 | 0.012 |
| Ineffective | 5 (11.6%) | 21 (50%) | 4.061 | 0.003 |
| Total effective rate | 38 (88.4%) | 21 (50%) | 6.378 | 0.002 |
| t | 4.857 | 5.732 | - | - |
| P | 0.13 | 0.21 | - | - |
Note: Compared to the control group, significant difference was P<0.05.
Serum levels of myocardial zymogram indices
As shown in Figure 1, the level of CK (creatine phosphokinase), AST (aspartate aminotransferase) and CK-MB (Muscle kinase isozyme) in the experimental group after intervention were significantly lower than those in the control group (all P<0.05).
Figure 1.
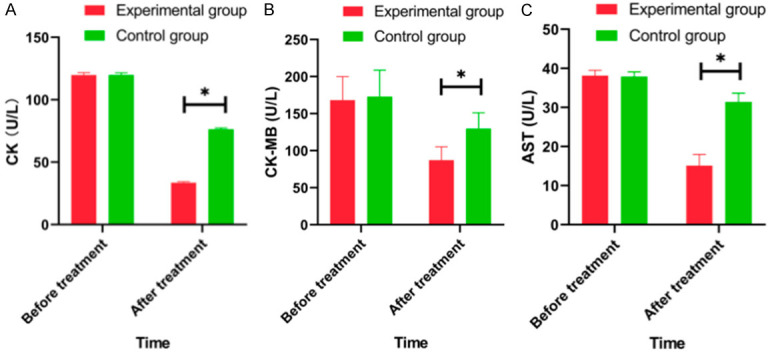
Comparison of serum levels of inflammatory factors between groups before and after intervention. A: CK (creatine kinase); B: CK-MB (creatine kinase-Muscle kinase isozyme); C: AST (aspartate aminotransferase). Note: Compared to experimental group, *P<0.05.
Time to symptom disappearance
As shown in Figure 2, the time to symptom disappearance, including fever, diarrhea, vomiting and dehydration, was significantly shorter in the experimental group than that in the control group (all P<0.05) (Figure 2).
Figure 2.
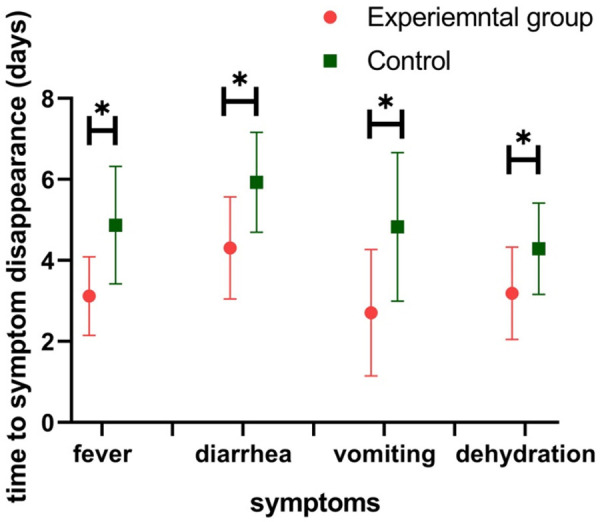
Comparison of time to symptom disappearance between the two groups before and after intervention. Note: Compared with experimental group, *P<0.05.
Adverse reactions
There was statistical significance in the incidence of vomiting and abdominal pain between the groups after intervention (all P<0.05). The incidences of nausea, rash and diarrhea between two groups after intervention were not statistically significant (Table 3).
Table 3.
Comparison of adverse reactions between groups
| Experimental group (n=43) | Control group (n=42) | X2 | P | |
|---|---|---|---|---|
| Nausea | 7 (16.3%) | 10 (23.8%) | 7.153 | 0.643 |
| Vomit | 9 (20.9%) | 16 (38.1%) | 6.378 | 0.038 |
| Rash | 8 (18.6%) | 7 (16.7%) | 3.621 | 0.742 |
| Abdominal pain | 14 (32.6%) | 11 (26.2%) | 4.061 | 0.031 |
| Diarrhea | 5 (11.6%) | 7 (16.7%) | 9.737 | 0.382 |
| Total incidence | 18 (43%) | 21 (51%) | 6.593 | 0.132 |
Note: Compared to the control group, significant difference was P<0.05.
Serum levels of inflammatory factors
The serum levels of inflammatory factors (IL-6, IL-8 and hsCRP) were obviously lower in the two groups after treatment, and those level in the experimental group were significantly lower than those in the control group after intervention (all P<0.05) (Figure 3).
Figure 3.
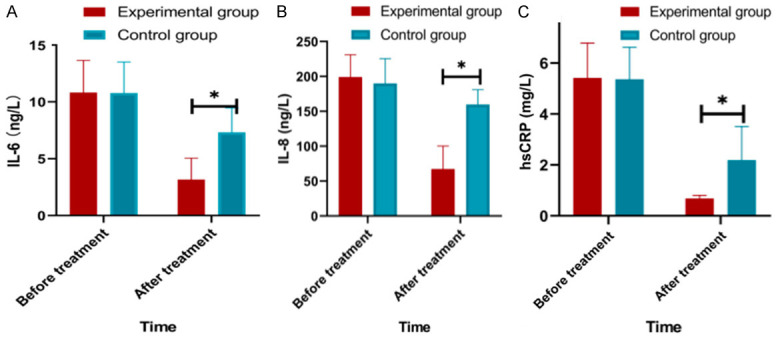
Comparison of serum levels of inflammatory factors between groups before and after intervention. A: Comparison of serum levels of IL-6 between the two groups before and after intervention; B: Comparison of serum levels of IL-8 between the two groups before and after intervention; C: Comparison of serum levels of hsCRP between the two groups before and after intervention; Note: Compared to experimental group, *P<0.05.
Discussion
In our study, we showed that the clinical effect were improved after intervention (P<0.05), and the total effective rate of two group were improved. The time to symptom disappearance (such as fever, vomiting, diarrhea and dehydration) was significantly decreased in the experimental as compared with control group. The myocardial zymogram indices (CK, CK-MB and AST) and inflammatory indices were significantly decreased after treatment.
The main function of microecological agents is to adjust and maintain the microecological balance [25]. The studies on microecological agents such as Peifeikang, Zhengchangsheng and MAMIAI showed that microecological agents are effective in the treatment of acute and chronic infectious diarrhea [26,27] possible through the following mechanisms: (1) direct or indirect regulation of the intestinal beneficial flora. Facultative anaerobic bacteria can grow rapidly in aerobic or anaerobic environment and rapidly consume free oxygen in an ecological environment, and its development in the intestine has the characteristics of colonization, exclusivity, and reproduction. Through the interaction between phosphoteic acid and intestinal mucosal epithelial cells, they form a biologic barrier together with other anaerobic bacteria. Acetic acid, lactic acid, antibiotics, and other substances produced by probiotics can inhibit a variety of pathogenic bacteria and constitute a chemical barrier against pathogenic bacteria infection [29]. (2) Immune function. Kalliomaki et al. showed that some bacteria can down-regulate the expression of pro-inflammatory cytokines and up-regulate the expression of anti-inflammatory cytokines. Fang et al. found that lactobacillus can activate antibody apoptotic protein B and antagonize Pro apoptotic p38MAP kinase [30,31]. (3) Nutritional effect. Probiotics can participate in vitamin metabolism in the intestine and produce Vitamin B complex needed by the human body, such as biotin, folic acid, niacin and pantothenic acid. Anaerobic probiotics can also reduce intestinal pH and redox potential [32]. As shown in our results, the inflammatory factors (IL-6, IL-8 and hsCRP) were obviously lower after receiving the zinc and selenium preparation (P<0.05), which indicated that zinc and selenium preparation works by improving the inflammatory response.
Interestingly, we observed that children treated with probiotics combined with zinc and selenium preparation could had significantly improved cardiac function, in which the level of CK, CK-MB and AST were decreased after treatment. This result was consistent with other studies [33-35]. Probiotics, such as bifidobacteria and pseudolactobacillus, are natural producers of Vitamin B (B1, B2, B3, B6, B8, B9, B12) [36,37]. They can also enhance the immune system, improve the absorption of vitamins and minerals, and stimulate the production of organic acids and amino acids [38,39]. Probiotics can also produce enzymes such as esterase, lipase and coenzymes A, Q, NAD, and NADP. Some products metabolized by probiotics may also show antibiotic (eosinophils, bacitracin, lactic acid), anticancer and immunosuppressive functions [40-42]. We speculate that those characteristics of probiotics may relieve myocardial damage and ameliorate cardiac function. Moreover, further researches about the pathophysiologic mechanism are needed.
Nevertheless, our study has limitations. One limitation is the small study population, in which we only enrolled children population. The other limitation is the uninvestigated mechanism of action, although the result is promising. Therefore, the results still need further confirmation with larger scale, randomized and control clinical trials.
In summary, probiotics combined with zinc and selenium can effectively alleviate the disease and restore the level of myocardial enzymes in children with rotavirus enteritis. Therefore, probiotics combined with zinc and selenium preparation can significantly improve the clinical symptoms of children with rotavirus colitis.
Acknowledgements
The authors thank all patients who participated in this study.
Disclosure of conflict of interest
None.
References
- 1.Chen Z, Pan WG, Xian WY, Cheng H, Zheng JX, Hu QH, Yu ZJ, Deng QW. Identification of infantile diarrhea caused by breast milk-transmitted staphylococcus aureus infection. Curr Microbiol. 2016;73:498–502. doi: 10.1007/s00284-016-1088-7. [DOI] [PubMed] [Google Scholar]
- 2.Singh S, Singh R, Singh KP, Singh V, Malik YPS, Kamdi B, Singh R, Kashyap G. Immunohistochemical and molecular detection of natural cases of bovine rotavirus and coronavirus infection causing enteritis in dairy calves. Microb Pathog. 2020;138:103814. doi: 10.1016/j.micpath.2019.103814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen F, Knutson TP, Ciarlet M, Sturos M, Marthaler DG. Complete genome characterization of a rotavirus B (RVB) strain identified in Alpine goat kids with enteritis reveals inter-species transmission with RVB bovine strains. J Gen Virol. 2018;99:457–463. doi: 10.1099/jgv.0.001022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang X, Song L, Tan W, Zhao W. Clinical efficacy of oral immunoglobulin Y in infant rotavirus enteritis: systematic review and meta-analysis. Medicine (Baltimore) 2019;98:e16100. doi: 10.1097/MD.0000000000016100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dhama K, Saminathan M, Karthik K, Tiwari R, Shabbir MZ, Kumar N, Malik YS, Singh RK. Avian rotavirus enteritis - an updated review. Vet Q. 2015;35:142–158. doi: 10.1080/01652176.2015.1046014. [DOI] [PubMed] [Google Scholar]
- 6.Hu Y, Gui L, Chang J, Liu J, Xu S, Deng C, Yu F, Ma Z, Wang G, Zhang C. The incidence of infants with rotavirus enteritis combined with lactose intolerance. Pak J Pharm Sci. 2016;29:321–323. [PubMed] [Google Scholar]
- 7.Medici MC, Abelli LA, Martinelli M, Corradi D, Dodi I, Tummolo F, Albonetti V, Martella V, Dettori G, Chezzi C. Clinical and molecular observations of two fatal cases of rotavirus-associated enteritis in children in Italy. J Clin Microbiol. 2011;49:2733–2739. doi: 10.1128/JCM.01358-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Asano KM, Gregori F, Souza SP, Rotava D, Oliveira RN, Villarreal LY, Richtzenhain LJ, Brandão PE. Bovine rotavirus in turkeys with enteritis. Avian Dis. 2011;55:697–700. doi: 10.1637/9765-041911-ResNote.1. [DOI] [PubMed] [Google Scholar]
- 9.Lopez RN, Krishnan U, Ooi CY. Enteritis with pneumatosis intestinalis following rotavirus immunisation in an infant with short bowel syndrome. BMJ Case Rep. 2017;2017:17–22. doi: 10.1136/bcr-2017-219482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cui H, Bai S, Huo Z, Li J, Sun J, An X. A cluster of rotavirus enteritis in pediatric liver recipients. Transpl Infect Dis. 2015;17:477–80. doi: 10.1111/tid.12378. [DOI] [PubMed] [Google Scholar]
- 11.Wang X, Yang Q, Zhou X, Chen T, Dou L, Wang F, Wang W. Shenling Baizhu powder inhibits RV-SA11-induced inflammation and rotavirus enteritis via TLR4/MyD88/NF-κB signaling pathway. Front Pharmacol. 2021;12:642685. doi: 10.3389/fphar.2021.642685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Turner A, Ngwira B, Witte D, Mwapasa M, Dove W, Cunliffe N. Surveillance of rotavirus gastro-enteritis in children in Blantyre, Malawi. Paediatr Int Child Health. 2013;33:42–5. doi: 10.1179/2046905512Y.0000000015. [DOI] [PubMed] [Google Scholar]
- 13.Turner A, Ngwira B, Witte D, Mwapasa M, Dove W, Cunliffe N. Surveillance of rotavirus gastro-enteritis in children in Blantyre, Malawi. Paediatr Int Child Health. 2013;33:42–5. doi: 10.1179/2046905512Y.0000000015. [DOI] [PubMed] [Google Scholar]
- 14.Martinelli D, Fortunato F, Marchetti F, Prato R. Rotavirus vaccine administration patterns in Italy: potential impact on vaccine coverage, compliance and adherence. Hum Vaccin Immunother. 2021;17:1546–1551. doi: 10.1080/21645515.2020.1816109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dong J, Liu G, Gao N, Suo J, Matthijnssens J, Li S, Yuan D, Du Y, Zhang J, Yamashita N, Haga T, Cook FR, Zhu W. A reassortant G3P[12] rotavirus A strain associated with severe enteritis in donkeys (Equus asinus) Equine Vet J. 2022;54:114–120. doi: 10.1111/evj.13425. [DOI] [PubMed] [Google Scholar]
- 16.Martella V, Colombrita D, Lorusso E, Draghin E, Fiorentini S, De Grazia S, Bányai K, Ciarlet M, Caruso A, Buonavoglia C. Detection of a porcine-like rotavirus in a child with enteritis in Italy. J Clin Microbiol. 2008;46:3501–7. doi: 10.1128/JCM.00983-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dong H, Qu S, Chen X, Zhu H, Tai X, Pan J. Changes in the cytokine expression of peripheral Treg and Th17 cells in children with rotavirus enteritis. Exp Ther Med. 2015;10:679–682. doi: 10.3892/etm.2015.2511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jindal N, Chander Y, Patnayak DP, Mor SK, Ziegler AF, Goyal SM. A multiplex RT-PCR for the detection of astrovirus, rotavirus, and reovirus in turkeys. Avian Dis. 2012;56:592–6. doi: 10.1637/9958-100911-ResNote.1. [DOI] [PubMed] [Google Scholar]
- 19.Lee H, Park SY, Clark A, Debellut F, Pecenka C, Kim DS, Kim HM, Kim JH, Cho H, Kim AY, Lee M, Jung SY, Seong BL, Kang HY. Cost-effectiveness analysis of the implementation of a National Immunization Program for rotavirus vaccination in a country with a low rotavirus gastroenteritis-related mortality: a South Korean study. Vaccine. 2019;37:4987–4995. doi: 10.1016/j.vaccine.2019.07.030. [DOI] [PubMed] [Google Scholar]
- 20.Anderson JD 4th, Pecenka CJ, Bagamian KH, Rheingans RD. Effects of geographic and economic heterogeneity on the burden of rotavirus diarrhea and the impact and cost-effectiveness of vaccination in Nigeria. PLoS One. 2020;15:e0232941. doi: 10.1371/journal.pone.0232941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rheingans R, Anderson JD 4th, Bagamian KH, Pecenka CJ. Effects of geographic and economic heterogeneity on rotavirus diarrhea burden and vaccination impact and cost-effectiveness in the Lao People’s Democratic Republic. Vaccine. 2018;36:7868–7877. doi: 10.1016/j.vaccine.2018.02.009. [DOI] [PubMed] [Google Scholar]
- 22.Amodio E, Tabacchi G, Cracchiolo M, Sciuto V, Vitale F. Hospitalisation of children aged 0-59 months with rotavirus gastro-enteritis before the introduction of routine vaccination (Sicily 2003-2012) Paediatr Int Child Health. 2015;35:319–23. doi: 10.1080/20469047.2015.1109228. [DOI] [PubMed] [Google Scholar]
- 23.Alfajaro MM, Rho MC, Kim HJ, Park JG, Kim DS, Hosmillo M, Son KY, Lee JH, Park SI, Kang MI, Ryu YB, Park KH, Oh HM, Lee SW, Park SJ, Lee WS, Cho KO. Anti-rotavirus effects by combination therapy of stevioside and Sophora flavescens extract. Res Vet Sci. 2014;96:567–75. doi: 10.1016/j.rvsc.2014.03.011. [DOI] [PubMed] [Google Scholar]
- 24.Corl BA, Odle J, Niu X, Moeser AJ, Gatlin LA, Phillips OT, Blikslager AT, Rhoads JM. Arginine activates intestinal p70(S6k) and protein synthesis in piglet rotavirus enteritis. J Nutr. 2008;138:24–9. doi: 10.1093/jn/138.1.24. [DOI] [PubMed] [Google Scholar]
- 25.Grassi T, De Donno A, Guido M, Gabutti G Collaborative Group for the Surveillance of Rotavirus Infection. The epidemiology and disease burden of rotavirus infection in the Salento peninsula, Italy. Turk J Pediatr. 2008;50:132–6. [PubMed] [Google Scholar]
- 26.Duffy LC, Riepenhoff-Talty M, Byers TE, La Scolea LJ, Zielezny MA, Dryja DM, Ogra PL. Modulation of rotavirus enteritis during breast-feeding. Implications on alterations in the intestinal bacterial flora. Am J Dis Child. 1986;140:1164–8. doi: 10.1001/archpedi.1986.02140250090041. [DOI] [PubMed] [Google Scholar]
- 27.Ballal M, Jyothirlatha , Kotigadde S, Venkatesh A, Shivananda PG. Rotavirus and bacterial enteropathogens causing acute diarrhea. Indian J Pediatr. 1992;59:203–7. doi: 10.1007/BF02759984. [DOI] [PubMed] [Google Scholar]
- 28.Qiu CY, Guo ZX, Zhang GH, Feng YH, Deng YY, Chen XJ, Wu XD, Huang SW. Study on the effectiveness and safety of Xingpi Yanger granule combined with Saccharomyces boulardii for rotavirus enteritis in children: a protocol for systematic review and meta-analysis. Medicine (Baltimore) 2021;100:e25593. doi: 10.1097/MD.0000000000025593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hu Y, Gui L, Chang J, Liu J, Xu S, Deng C, Yu F, Ma Z, Wang G, Zhang C. The incidence of infants with rotavirus enteritis combined with lactose intolerance. Pak J Pharm Sci. 2016;29:321–323. [PubMed] [Google Scholar]
- 30.Fric P. Probiotics in gastroenterology. Z Gastroenterol. 2002;40:197–201. doi: 10.1055/s-2002-22328. [DOI] [PubMed] [Google Scholar]
- 31.Rosenfeldt V, Michaelsen KF, Jakobsen M, Larsen CN, Møller PL, Tvede M, Weyrehter H, Valerius NH, Paerregaard A. Effect of probiotic Lactobacillus strains on acute diarrhea in a cohort of nonhospitalized children attending day-care centers. Pediatr Infect Dis J. 2002;21:417–9. doi: 10.1097/00006454-200205000-00013. [DOI] [PubMed] [Google Scholar]
- 32.Ni RH, Pediatrics DO. Multiple treatments for infantile rotavirus enteritis: a network meta-analysis. World Chinese Journal of Digestology. 2012;33:13–29. [Google Scholar]
- 33.Tunapong W, Apaijai N, Yasom S, Tanajak P, Wanchai K, Chunchai T, Kerdphoo S, Eaimworawuthikul S, Thiennimitr P, Pongchaidecha A, Lungkaphin A, Pratchayasakul W, Chattipakorn SC, Chattipakorn N. Chronic treatment with prebiotics, probiotics and synbiotics attenuated cardiac dysfunction by improving cardiac mitochondrial dysfunction in male obese insulin-resistant rats. Eur J Nutr. 2018;57:2091–2104. doi: 10.1007/s00394-017-1482-3. [DOI] [PubMed] [Google Scholar]
- 34.Silva-Cutini MA, Almeida SA, Nascimento AM, Abreu GR, Bissoli NS, Lenz D, Endringer DC, Brasil GA, Lima EM, Biancardi VC, Andrade TU. Long-term treatment with kefir probiotics ameliorates cardiac function in spontaneously hypertensive rats. J Nutr Biochem. 2019;66:79–85. doi: 10.1016/j.jnutbio.2019.01.006. [DOI] [PubMed] [Google Scholar]
- 35.Kayasaki F, Okagawa T, Konnai S, Kohara J, Sajiki Y, Watari K, Ganbaatar O, Goto S, Nakamura H, Shimakura H, Minato E, Kobayashi A, Kubota M, Terasaki N, Takeda A, Noda H, Honma M, Maekawa N, Murata S, Ohashi K. Direct evidence of the preventive effect of milk replacer-based probiotic feeding in calves against severe diarrhea. Vet Microbiol. 2021;254:108976. doi: 10.1016/j.vetmic.2020.108976. [DOI] [PubMed] [Google Scholar]
- 36.Grassi T, De Donno A, Guido M, Gabutti G Collaborative Group for the Surveillance of Rotavirus Infection. The epidemiology and disease burden of rotavirus infection in the Salento peninsula, Italy. Turk J Pediatr. 2008;50:132–6. [PubMed] [Google Scholar]
- 37.Davidson GP, Gall DG, Petric M, Butler DG, Hamilton JR. Human rotavirus enteritis induced in conventional piglets. Intestinal structure and transport. J Clin Invest. 1977;60:1402–9. doi: 10.1172/JCI108901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Duffy LC, Riepenhoff-Talty M, Byers TE, La Scolea LJ, Zielezny MA, Dryja DM, Ogra PL. Modulation of rotavirus enteritis during breast-feeding. Implications on alterations in the intestinal bacterial flora. Am J Dis Child. 1986;140:1164–8. doi: 10.1001/archpedi.1986.02140250090041. [DOI] [PubMed] [Google Scholar]
- 39.Esposito F, Senese R, Salvatore P, Vallone G. Intrahepatic portal-vein gas associated with rotavirus infection. J Ultrasound. 2011;14:10–3. doi: 10.1016/j.jus.2011.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Legrottaglie R, Rizzi V, Agrimi P. Isolation and identification of avian rotavirus from pheasant chicks with signs of clinical enteritis. Comp Immunol Microbiol Infect Dis. 1997;20:205–10. doi: 10.1016/S0147-9571(97)00005-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kirubakaran C, Davidson GP. Campylobacter as a cause of acute enteritis in children in South Australia. II. Clinical comparison with salmonella, rotavirus and non-specific enteritis. Med J Aust. 1981;2:336–7. doi: 10.5694/j.1326-5377.1981.tb100992.x. [DOI] [PubMed] [Google Scholar]
- 42.Adeyi OA, Costa G, Abu-Elmagd KM, Wu T. Rotavirus infection in adult small intestine allografts: a clinicopathological study of a cohort of 23 patients. Am J Transplant. 2010;10:2683–9. doi: 10.1111/j.1600-6143.2010.03311.x. [DOI] [PubMed] [Google Scholar]


