Abstract
Autism spectrum disorders (ASD) are a group of lifelong neurodevelopmental disorders characterized by cognitive deficits and impaired social and communicative development that have been rising in prevalence in recent decades. These disorders may be accompanied by disabling health issues and often lead to a substantial economic burden. The causes and mechanisms of ASD have not yet been fully elucidated, although it has been reported that genetic background, epigenetic modification, and environmental risk factors all contribute to the development of ASD. Environmental factors, which include prenatal circumstances or events, all play a very important role in the early development of autism, yet the exact mechanism remains largely undetermined. In this review, we promote a ‘rethinking’ of autism as a neurodevelopmental disease that originates from early life development. We focus on the impact of the prenatal and maternal risk factors such as maternal diabetes, prenatal chemical exposure, and hormone imbalances during pregnancy on the risk for ASD development in children and offspring, identifying important pathological bases and prevention measures for future decades. Further research focused on understanding the role of the environmental factors in the etiology of ASD will drive forward innovation strategies towards intervention and the prevention of the maternal risk factors for autism.
Keywords: Autism, risk factors, prenatal exposure
Introduction
Before the 21st century, serious mental disorders and illnesses, leprosy, and tuberculosis were some of the major public health issues facing the world. Today, only mental disorders, particularly autism spectrum disorders (ASD), continue to have a large global prevalence and remain incurable. Throughout the past 50 years, autism’s prevalence rate has increased by a factor of over 100, from 1:10000 to 1:59 [1,2]. Despite decades of research, however, there is still no effective cure or prevention strategy for autism. Thus, it is urgent to fully develop basic and clinical applications for autism research that break through traditional thinking to integrate new and innovative conceptualizations of ASD etiology. Past research on autism has generally focused on identifying genetic factors related to autism, but extensive studies in recent years have found that environmental factors are also largely involved. On a relatively heterogeneous genetic basis, the epigenetic network constructed by the interaction of environmental and genetic factors is a key link in determining the risk of ASD development.
Family studies, pedigree analysis, and twin studies have previously suggested that autism has a strong genetic basis, with genetic factors accounting for 30-40% of autism cases [3,4]. However, it is important to recognize that the primary cause is not solely genetic; rather, epigenetics may play a more important role. Many genetic studies have identified common mutations, rare mutations, and copy number variations (CNV) in thousands of children with autism; however, these identified genetic candidate genes often have low prevalence among populations. For example, variants of CACNA1H have only been identified in 6/~461 cases of autism, and CNTN4 mutants have been identified in 7/~2000 cases [5]. On the other hand, environmental factors can directly affect the development of large populations of infants and young children through neurotoxicity and impacts gene expression by modifying epigenetic states, thereby greatly influencing autism’s prevalence. Large-scale studies have found that exposure to harmful environmental factors, including maternal disease and drug abuse, air pollution exposure, family life behaviors, and metal exposure during the intrauterine period and early life stages of development may play a key role in ASD development, in addition to other factors (Figure 1).
Figure 1.
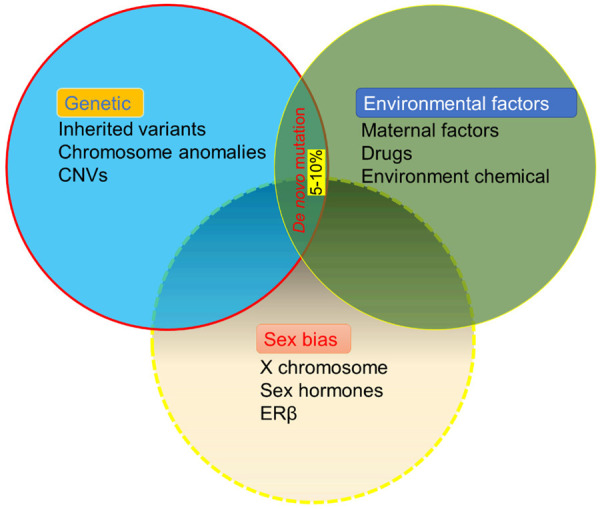
The contributions of genetics, environmental exposure, and sex biases to the etiology of autism spectrum disorders. It is estimated that the genetic and environmental factors contribute equally to ASD development. Sex biases have been observed, with an autism prevalence of 4 males:1 female.
Maternal diabetes induces autism in offspring
Prenatal metabolic disorders are associated with an increased prevalence of ASD and other disorders in offspring. A meta-analysis by Wan et al., for example, identified evidence of a significant association between maternal diabetes and psychiatric disorders in offspring [6]. As diabetes affects up to 15% of pregnant women worldwide [7], the role of maternal diabetes as a factor for autism development in children and offspring should be given much more attention.
Recent epidemiological studies have indicated that maternal diabetes, which includes type 1 diabetes (T1D), type 2 diabetes (T2D), and gestational diabetes mellitus (GDM), diagnosed by the 26th-week mark post-gestation is highly associated with an increased risk of autism in offspring, as shown in Table 1. Maternal pre-existing type 2 diabetes is also significantly associated with the risk of ASD in offspring, while the associated risk is slightly lower than the risk for GDM at 26 weeks [8-10].
Table 1.
The contributions of the environmental factors to ASD development
| Environmental factors | Odds ratio [OR]/Hazard ratios (HR)/Confidence interval [CI] | Increased risk for ASD | Reference |
|---|---|---|---|
| Obesity | OR 1.36, 95% CI 1.08-1.70 | 36% | [16] |
| Maternal diabetes | OR 1.48, 95% CI 1.26-1.75 | 62% | [9] |
| Maternal gestational diabetes mellitus | OR 1.63, 95% CI 1.35-1.97 | 42% | [9] |
| Polycystic ovary syndrome (POS) | OR 1.59, CI 95% 1.34-1.88 | 59% | [17,18] |
| Maternal antidepressant (selective serotonin reuptake inhibitors) | HR 2.17; 95% CI, 1.20-3.93 | N/D | [19] |
| Maternal depression | HR 1.75; 95% CI, 1.03-2.97 | 87% | [20] |
| Maternal hypertension | OR 1.35, 95% CI 1.11-1.64 | 35% | [20] |
| Maternal infection | OR 1.13, 95% CI=1.03-1.23 | 30% | [21] |
| Maternal dichlorodiphenyl dichloroethylene (p,p’-DDE) exposure | OR 2.21, 95% CI 1.32-3.69 | N/D | [22] |
| Prenatal exposure to organophosphate (dialkyl phosphates) | OR 2.0, 95% CI 1.1-3.6 | 60% | [23] |
| In vitro fertilization | OR 1.14, 95% CI 0.94-1.39 | N/D | [24] |
All the analyses were performed using SAS statistical software (SAS Institute Inc., Cary, NC, USA). The distribution of the characteristics of the pregnancy factors was calculated based on the autism and maternal statuses using two-tailed independent sample t-tests of continuous testing and the chi-square tests of the categorical variables. The association between maternal status and ASD was determined using a multivariate logistic regression model to evaluate the odds ratio (OR) and the 95% confidence interval (CI). N/D-Not Defined.
However, the detailed mechanism of the way in which ASD development is mediated by maternal diabetes remains unclear. Our prior research, which focused on using rodent models of diabetes, found that the offspring of diabetic mothers exhibited autistic-like behaviors, such as reduced ultrasonic vocalizations and impaired social interaction. To our knowledge, this is the first time that such a study has been conducted. Furthermore, we found that superoxide dismutase (SOD2) in the amygdala of the offspring of rats were inhibited as a result of oxidative stress-mediated histone methylation (Figure 2). Thus, we suggested that hyperglycemia induces the generation of persistent reactive oxygen species (ROS) and that inhibition of SOD2 is a possible mechanism of maternal diabetic pregnancy-induced autistic behavior [11]. Maternal diabetes is also assumed to impact neurodevelopment in offspring through processes including immune dysfunction, increased oxidative stress and suppressed SOD2 in hematopoietic stem cells (HSCs) [12].
Figure 2.
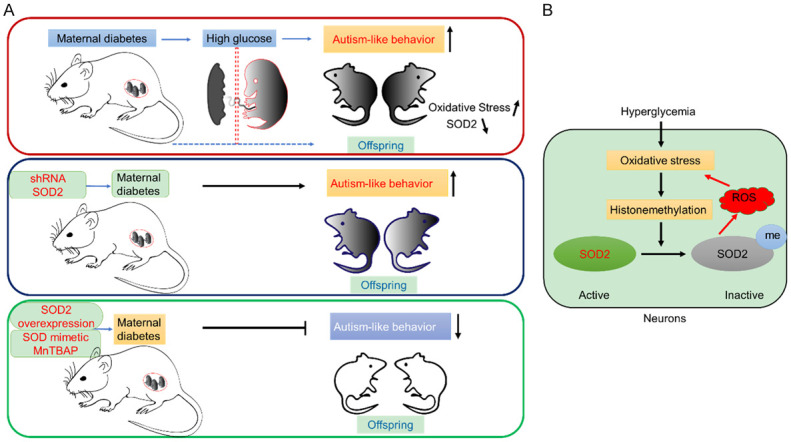
Diabetes during pregnancy induces autism-like behavior in offspring. A. Maternal diabetes-induced ASD-like behaviors in offspring. The overexpression of the SOD2 or SOD mimics can partially reverse autistic behavior in offspring, but inhibiting the expression of SOD2 will induce autistic behavior. B. Hyperglycemia suppresses SOD2 expression through oxidative stress-mediated histone methylation. Histone methylation at H3K9me2 of the SOD2 promoter triggers epigenetic changes, which subsequently lead to SOD2 inactivation. The inhibition of SOD2 activity then aggravates the ROS generation and induces oxidative stress.
Elucidating the role of maternal diabetes in the pathogenesis of ASD in offspring will help people rethink the pathogenesis of autism from the perspective of multi-factorial interaction, thus expanding the understanding of the pathogenesis of ASD and providing a target for ASD diagnosis and drug treatment. As excessive glucose exposure in utero can cause permanent fetal changes, maternal hyperglycemia may have long-term effects on the development and function of the fetal brain. Maternal obesity and diabetes affect the development of the fetus’s brain and nervous system, which may hint at a mechanism for autism development. Previous evidence has shown that the presence of maternal diabetes, in addition to obesity and related metabolic disorders, both before and during pregnancy can damage normal hippocampal development and cause abnormal neurobehavioral development in offspring [13]. Additionally, we have also found that vitamin D deficiency worsens maternal diabetes-induced autism-like phenotypes in offspring through epigenetic modification caused by maternal diabetes [14]. By using this pre-established maternal diabetes-induced autistic mouse model, we have also found that offspring from dams with maternal diabetes show significant oxytocin receptor (OXTR) inhibition in the amygdala region of the brain tissue, while prenatal OXTR knockout mice show worsened maternal diabetes-induced autism symptoms. Further studies have shown that OXTR inhibition is caused by persistent oxidative stress and methylation by hyperglycemia, subsequently causing estrogen receptor β (ERβ) to dissociate from the OXTR promoter [15].
Chemical exposure induces autism
Chemical exposure is one of the most common pregnancy conditions associated with adverse effects on offspring. For example, pregnant women who used marijuana during pregnancy were 1.5 times more likely to give birth to a child with autism, and the risk of autism in offspring has also been found to be associated with maternal pesticide exposure [25-27]. Table 1 lists prenatal exposure to chemicals associated with autism. However, little is known about the underlying mechanisms of these relationships.
The use of animal models can serve to provide insight on autistic offspring behavior and aid in understanding the mechanisms of the effect of xenobiotic exposure during pregnancy on autistic behavior. Maternal exposure to pesticides has been shown to change neuronal cell development and behavior in animal offspring. Epidemiological research has shown that maternal exposure to the herbicide glyphosate increases the incidence of autism in offspring; in one such study, the researchers exposed pregnant mice to glyphosate and found that there was an increase in autistic-like behaviors in offspring, including social interaction deficits and cognitive deficits, in addition to alteration of the composition of gut microbiota. Furthermore, there was significantly increased expression of soluble epoxide hydrolase (sEH) in the brains of offspring after maternal glyphosate exposure, while sEH inhibitors were able to restore the effects of maternal glyphosate exposure on inducing autism-like behaviors in offspring. These findings indicate that increased sEH plays a vital role in ASD-like behaviors in offspring [28]. Thus, the use of rat or mice models to study exposure to environmental factors during pregnancy can help simulate the adverse effects of environmental toxicant exposure that cause human autism.
In addition, in terms of metal exposure, a study by Holmes et al. [29] t-tested the levels of lead and mercury in the blood of 203 normal children and found that blood lead levels were positively correlated with autism, and the blood mercury levels were related to an increase in autism-like behavior. In another study, the blood of 34 and 35 children with autism was tested, and it was found that the lead and mercury levels in the children with autism were significantly higher than the corresponding levels in the controls. Studies have shown that zinc and manganese levels in hair are negatively correlated with autism in subjects, and the severity of autism symptoms is positively correlated with the presence of certain heavy metals such as lead and mercury [30-32].
Maternal hormone imbalances are a significant risk factor for ASD
Epidemiological studies have shown that hormone abnormalities in pregnant women are a significant potential risk factor for autism in offspring and that sex hormones may be part of the cause of autism. We conducted a population case epidemiological survey in China and found that prenatal progestin is closely related to the prevalence of ASD. This includes the use of progesterone to prevent abortion, the use of progesterone pills, and maternal uptake progestin-contaminated seafood.
Progesterone has been shown to modulate neurogenic responses and impair the development of cognitive responses by down-regulating the expression of ERβ. It has been reported that the expressions of estrogen receptor β (ERβ) and the estrogen receptor co-factors were significantly suppressed in the brains of autistic patients [33]. Therefore, it is speculated that prenatal exposure to synthetic progesterone can induce autism-like behaviors by inhibiting ERβ in offspring. An in vivo rat model exposed to progesterone showed that prenatal levonorgestrel exposure induced autistic behaviors in offspring and suppressed ERβ expression in the brain. Furthermore, some in vivo research have revealed ERβ knockout mice showed obvious symptoms of anxiety, cognitive deficits and depressive behavior [34]. Since ERβ inhibition has been found to induce autistic behavior, estrogen receptor β agonists and/or overexpression of ERβ may help improve autistic behavior. Further research found that autism-like behaviors induced by prenatal progesterone exposure were rescued through treatment with resveratrol, a drug that activates ERβ [8].
Furthermore, a maternal diagnosis of polycystic ovary syndrome (PCOS) has been found to increase the risk of ASD in the offspring by 59 percent, and prenatal hyperandrogenism has been found to induce autistic-like behavior, such as impaired heterosexual recognition and decreased ultrasonic vocalization frequency, in offspring [35]. We believe that this is because hormones affect the development of neuroendocrine and neuroimmune systems in the early stages of life, leading to the development of ASD. Increasing evidence indicates that the placenta may be particularly important as a mediator of the actions of environmental endocrine hormones on the developing brain, which makes hormone-induced ERβ inhibition in the male brain more sensitive (Figure 3). Understanding how various risk factors affect neurodevelopment may assist in the process of identifying the etiology of ASD more clearly. Our current research suggests that prenatal dihydrotestosterone exposure induces ASD development through hypermethylation on the ERβ promoter, which suppress the expression of ERβ [36]. Previously, we have determined that progestin and norethindrone can increase histone H3 lysine9 dimethylation (H3K9me2) and tri-methylation of H3K27 (H3K27me3) on the ERβ promoter in amygdala neurons, which is responsible for maternal hormone-induced autism-like behavior [37].
Figure 3.
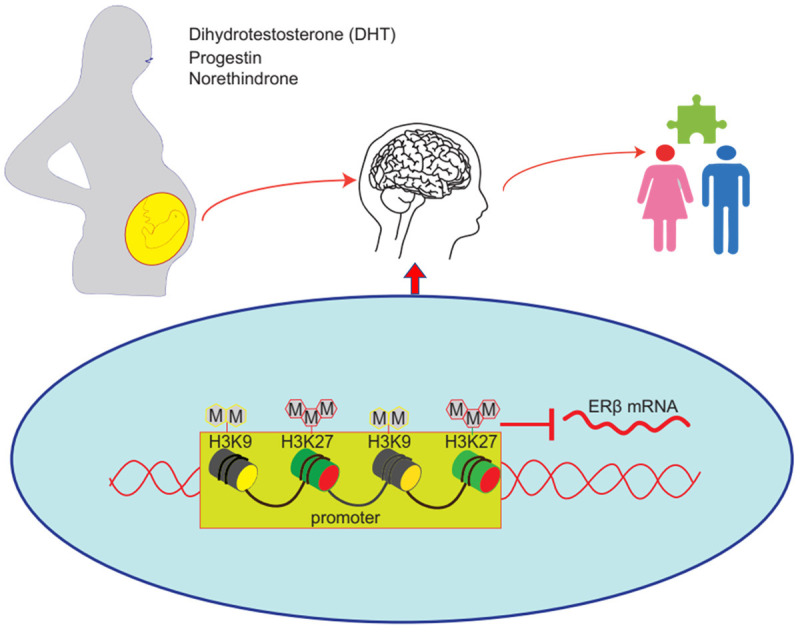
Maternal exposure to hormones during pregnancy is linked to a higher risk of the development of ASD in offspring. Dihydrotestosterone, progestin, and norethindrone exposure can induce significant ERβ promoter methylation and inhibit ERβ expression in offspring.
Sex bias as a significant risk factor for autism
Males and females have significantly different tendencies in the development of neurological diseases. Females are more prone to mood disorders such as anxiety, depression, and bipolar disorder, while males are more likely to suffer from disorders such as Parkinson’s disease (PD), attention deficit hyperactivity disorder (ADHD), and autism.
An interesting phenomenon in autism research is that ASD affects males at a much higher rate than females. The prevalence of autism in males is about four times that of females [38], especially in cases of severe autism, in which the ratio of males to females is nearly 11 to 1. In addition to these differences in prevalence, males and females with autism also tend to display different symptoms. Males with autism tend to show external symptoms, such as aggression and hyperactivity, while females with autism tend to show less social communication in addition to restrictive and repetitive behaviors [39].
Nevertheless, few researchers pay attention to biological sex in the search for factors affecting autism. A better understanding of the molecular mechanisms by which gender differences affect the development of autistic brains will help in designing the best diagnosis and treatment strategies for each gender.
Prof. Donald W. Pfaff proposes a “three-hit theory of autism”-that is, that the interactions among genes, environment, and sex serve to induce ASD development. Based on studies using the Cntnap2 mouse model of autism, the three hits have various synergistic effects on social behavior and social recognition [40].
A possible mechanism for the gender differences in autism prevalence and symptom expression may be understood through the different effects of sex hormones. Many investigations reinforce the notion that estrogen has a certain neuroprotective effect, and a particularly interesting study has found that the expression of estrogen receptor β in children with autism is very low and is not enough to mediate the protective effect of estrogen. Thus, it is suggested that the reduced expression of estrogen receptor beta, in addition to the inactivation of the enzyme aromatase that helps convert testosterone to estrogen, may have a significant impact in autistic individuals and can lead to the higher autism prevalence rates seen in males [33]. Additionally, some interesting studies have shown that the protein and gene expression levels of retinoic acid-related orphan receptor alpha (RORA) and aromatase are down-regulated in the cerebral cortex of males with autism, and it is speculated that deficiencies of RORA and aromatase lead to higher testosterone levels, which may increase the risk for ASD [41]. Thus, testosterone may serve as a male-specific factor that increases susceptibility to autism. In a zebrafish model with mutants of the autism risk gene CNTNAP2, it was found that estrogen receptor agonists can reverse the mutant behavioral phenotype, indicating that sex hormones contribute to genetic backgrounds [42].
Through gender-based de novo enrichment analysis, we can identify candidate gene mutations that affect male and female differences. Recently, Eichler et al. investigated how autism genes differ by sex by combing through the de novo enrichment of 2,133 girls and 4,641 boys diagnosed with autism. They found that only 17 genes were unique to the female patients with autism, and only 18 genes were found exclusively in the males. Another 19 genes were found in both sexes [43]. Thus, if there are any genes on the Y chromosome with de novo enrichment, they may serve to provide information central to uncovering the sex factors that affect the gender bias for autism. In this study, the authors also found that the rare genetic mutations associated with ASD are abundant in female cases at a rate that is much higher than in male cases. A female protective effect (FPE) against autistic behavior was thus identified, and it is predicted that females can have mutations of greater severity or number without the development of ASD and can tolerate more genetic risk variants for autism than males (Figure 4) [44].
Figure 4.
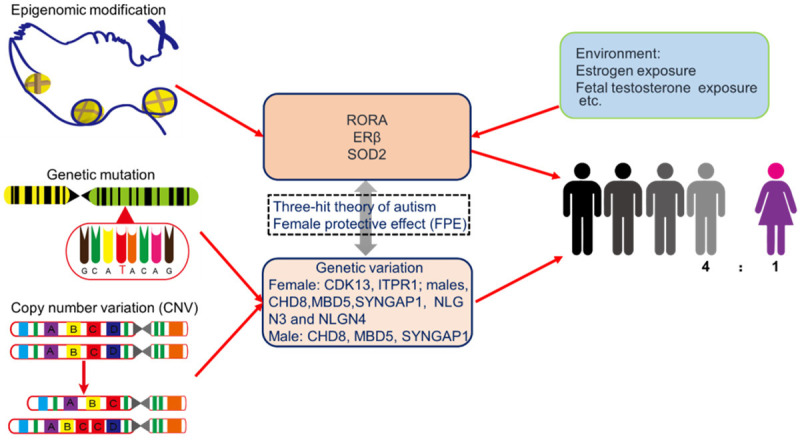
It is speculated that sex hormones and genetic variations play an important role in ASD pathology in both males and females. The three-hit theory of autism: genes, environment, and sex, in addition to the female protective effect (FPE), may be important factors that determine gender differences in autism prevalence and symptom expression.
Conclusion & future prospects
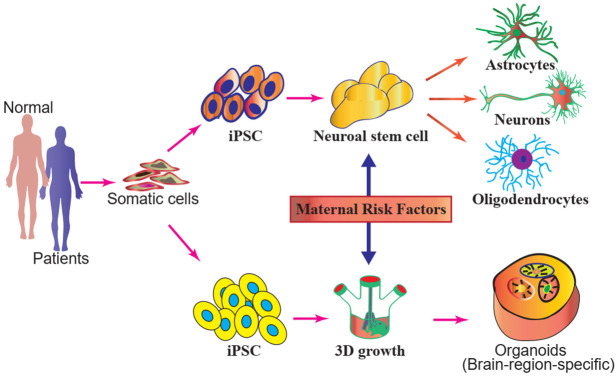
As a neurodevelopmental disorder, ASD is lifelong, irreversible, and incurable, posing large economic burdens in addition to bringing significant impacts on families and societies. At present, the causes of ASD have not yet been fully clarified, although it has been known that genetic and environmental factors are both involved. According to our research and knowledge, the majority of research on autism to date primarily focuses on genetic variations, although many epidemiological studies have established a strong association between prenatal environmental factors and adverse autism outcomes in offspring. Little is known about the environmental factors in the etiology of ASD. The influence of genetics and environment is inseparable when discussing ASD, yet there are relatively few studies on the linkages between genes and environment in affecting ASD. Thus, more convincing studies are needed to support the influence of current simulated environmental exposure on causing epigenetic changes that may lead to ASD development. We believe that environmental factors can govern gene expression by changing epigenetic markers such as DNA methylation and histone acetylation, with histone methylation likely being the most important marker, and affecting the different stages of the biological pathway to brain development, including neuronal differentiation, migration, and the formation of neural tubes, synapses, and myelin, eventually affecting the cognitive and communicative functions of individuals and triggering ASD. Analyzing early development from the perspective of epigenetics enables us to show how environmental factors may influence autism development in children, and approaches such as next-generation DNA sequencing and epigenetic analyses may further reveal the detailed mechanism of the environmental factors affecting gene transcription and epigenetic interactions. Furthermore, the use of induced pluripotent stem cell (iPSC)-derived neuron models and/or brain organoid technology to understand the effects of the environmental factors on early processes during neurogenesis will hold clues to the origins of autism (Figure 5).
Figure 5.
iPSC or brain organoids provide a model system to understand pathophysiology and the underlying pathogenetic mechanisms of the maternal risk factors that may induce autism in offspring.
Ultimately, the goal of all research on autism must be to provide prevention and intervention strategies with real-world potentials for application. Future research aimed at understanding the role of the environmental factors in the etiology of ASD will promote innovation that helps achieve this goal.
Acknowledgements
This work was funded by the Science and Technology Innovation Committee of Shenzhen (No. JCYJ20200109150700942, No. JCYJ20180306170922163), the Key Realm R&D Program of Guangdong Province (2019B030335001), the Natural Science Foundation of Guangdong Province (2020A1515011581, 2021A1515010985), the Sanming Project of Medicine (No. SZSM201612079), the Shenzhen Fund for Guangdong Provincial High Level Clinical Key Specialties (No. SZGSP013), and the Shenzhen Key Medical Discipline Construction Fund (No. SZXK042).
Disclosure of conflict of interest
None.
References
- 1.Hyman SL, Levy SE, Myers SM. Identification, evaluation, and management of children with autism spectrum disorder. Pediatrics. 2020;145:e20193447. doi: 10.1542/peds.2019-3447. [DOI] [PubMed] [Google Scholar]
- 2.Baio J, Wiggins L, Christensen DL, Maenner MJ, Daniels J, Warren Z, Kurzius-Spencer M, Zahorodny W, Robinson Rosenberg C, White T, Durkin MS, Imm P, Nikolaou L, Yeargin-Allsopp M, Lee LC, Harrington R, Lopez M, Fitzgerald RT, Hewitt A, Pettygrove S, Constantino JN, Vehorn A, Shenouda J, Hall-Lande J, Van Naarden Braun K, Dowling NF. Prevalence of autism spectrum disorder among children aged 8 years - autism and developmental disabilities monitoring network, 11 sites, United States, 2014. MMWR Surveill Summ. 2018;67:1–23. doi: 10.15585/mmwr.ss6706a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ramaswami G, Geschwind DH. Genetics of autism spectrum disorder. Handb Clin Neurol. 2018;147:321–329. doi: 10.1016/B978-0-444-63233-3.00021-X. [DOI] [PubMed] [Google Scholar]
- 4.Wassink TH, Piven J. The molecular genetics of autism. Curr Psychiatry Rep. 2000;2:170–175. doi: 10.1007/s11920-000-0063-x. [DOI] [PubMed] [Google Scholar]
- 5.Ladd-Acosta C, Hansen KD, Briem E, Fallin MD, Kaufmann WE, Feinberg AP. Common DNA methylation alterations in multiple brain regions in autism. Mol Psychiatry. 2014;19:862–871. doi: 10.1038/mp.2013.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wan H, Zhang C, Li H, Luan S, Liu C. Association of maternal diabetes with autism spectrum disorders in offspring: a systemic review and meta-analysis. Medicine (Baltimore) 2018;97:e9438. doi: 10.1097/MD.0000000000009438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.International Diabetes Federation. IDF policy briefing: diabetes in pregnancy: protecting maternal health. 2013 [Google Scholar]
- 8.Xie W, Ge X, Li L, Yao A, Wang X, Li M, Gong X, Chu Z, Lu Z, Huang X, Jiao Y, Wang Y, Xiao M, Chen H, Xiang W, Yao P. Resveratrol ameliorates prenatal progestin exposure-induced autism-like behavior through ERβ activation. Mol Autism. 2018;9:43. doi: 10.1186/s13229-018-0225-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xiang AH, Wang X, Martinez MP, Walthall JC, Curry ES, Page K, Buchanan TA, Coleman KJ, Getahun D. Association of maternal diabetes with autism in offspring. JAMA. 2015;313:1425–1434. doi: 10.1001/jama.2015.2707. [DOI] [PubMed] [Google Scholar]
- 10.Li M, Fallin MD, Riley A, Landa R, Walker SO, Silverstein M, Caruso D, Pearson C, Kiang S, Dahm JL, Hong X, Wang G, Wang MC, Zuckerman B, Wang X. The association of maternal obesity and diabetes with autism and other developmental disabilities. Pediatrics. 2016;137:e20152206. doi: 10.1542/peds.2015-2206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang X, Lu J, Xie W, Lu X, Liang Y, Li M, Wang Z, Huang X, Tang M, Pfaff DW, Tang YP, Yao P. Maternal diabetes induces autism-like behavior by hyperglycemia-mediated persistent oxidative stress and suppression of superoxide dismutase 2. Proc Natl Acad Sci U S A. 2019;116:23743–23752. doi: 10.1073/pnas.1912625116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lu J, Xiao M, Guo X, Liang Y, Wang M, Xu J, Liu L, Wang Z, Zeng G, Liu K, Li L, Yao P. Maternal diabetes induces immune dysfunction in autistic offspring through oxidative stress in hematopoietic stem cells. Front Psychiatry. 2020;11:576367. doi: 10.3389/fpsyt.2020.576367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Menting MD, van de Beek C, Mintjens S, Wever KE, Korosi A, Ozanne SE, Limpens J, Roseboom TJ, Hooijmans C, Painter RC. The link between maternal obesity and offspring neurobehavior: a systematic review of animal experiments. Neurosci Biobehav Rev. 2019;98:107–121. doi: 10.1016/j.neubiorev.2018.12.023. [DOI] [PubMed] [Google Scholar]
- 14.Liang Y, Yu H, Ke X, Eyles D, Sun R, Wang Z, Huang S, Lin L, McGrath JJ, Lu J, Guo X, Yao P. Vitamin D deficiency worsens maternal diabetes induced neurodevelopmental disorder by potentiating hyperglycemia-mediated epigenetic changes. Ann N Y Acad Sci. 2021;1491:74–88. doi: 10.1111/nyas.14535. [DOI] [PubMed] [Google Scholar]
- 15.Liu J, Liang Y, Jiang X, Xu J, Sun Y, Wang Z, Lin L, Niu Y, Song S, Zhang H, Xue Z, Lu J, Yao P. Maternal diabetes-induced suppression of oxytocin receptor contributes to social deficits in offspring. Front Neurosci. 2021;15:634781. doi: 10.3389/fnins.2021.634781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sanchez CE, Barry C, Sabhlok A, Russell K, Majors A, Kollins SH, Fuemmeler BF. Maternal pre-pregnancy obesity and child neurodevelopmental outcomes: a meta-analysis. Obes Rev. 2018;19:464–484. doi: 10.1111/obr.12643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cherskov A, Pohl A, Allison C, Zhang H, Payne RA, Baron-Cohen S. Polycystic ovary syndrome and autism: a test of the prenatal sex steroid theory. Transl Psychiatry. 2018;8:136. doi: 10.1038/s41398-018-0186-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kosidou K, Dalman C, Widman L, Arver S, Lee BK, Magnusson C, Gardner RM. Maternal polycystic ovary syndrome and the risk of autism spectrum disorders in the offspring: a population-based nationwide study in Sweden. Mol Psychiatry. 2016;21:1441–1448. doi: 10.1038/mp.2015.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brown HK, Ray JG, Wilton AS, Lunsky Y, Gomes T, Vigod SN. Association between serotonergic antidepressant use during pregnancy and autism spectrum disorder in children. JAMA. 2017;317:1544–1552. doi: 10.1001/jama.2017.3415. [DOI] [PubMed] [Google Scholar]
- 20.Maher GM, O’Keeffe GW, Kearney PM, Kenny LC, Dinan TG, Mattsson M, Khashan AS. Association of hypertensive disorders of pregnancy with risk of neurodevelopmental disorders in offspring: a systematic review and meta-analysis. JAMA Psychiatry. 2018;75:809–819. doi: 10.1001/jamapsychiatry.2018.0854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jiang HY, Xu LL, Shao L, Xia RM, Yu ZH, Ling ZX, Yang F, Deng M, Ruan B. Maternal infection during pregnancy and risk of autism spectrum disorders: a systematic review and meta-analysis. Brain Behav Immun. 2016;58:165–172. doi: 10.1016/j.bbi.2016.06.005. [DOI] [PubMed] [Google Scholar]
- 22.Brown AS, Cheslack-Postava K, Rantakokko P, Kiviranta H, Hinkka-Yli-Salomäki S, McKeague IW, Surcel HM, Sourander A. Association of maternal insecticide levels with autism in offspring from a national birth cohort. Am J Psychiatry. 2018;175:1094–1101. doi: 10.1176/appi.ajp.2018.17101129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Philippat C, Barkoski J, Tancredi DJ, Elms B, Barr DB, Ozonoff S, Bennett DH, Hertz-Picciotto I. Prenatal exposure to organophosphate pesticides and risk of autism spectrum disorders and other non-typical development at 3 years in a high-risk cohort. Int J Hyg Environ Health. 2018;221:548–555. doi: 10.1016/j.ijheh.2018.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fountain C, Zhang Y, Kissin DM, Schieve LA, Jamieson DJ, Rice C, Bearman P. Association between assisted reproductive technology conception and autism in California, 1997-2007. Am J Public Health. 2015;105:963–971. doi: 10.2105/AJPH.2014.302383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Corsi DJ, Donelle J, Sucha E, Hawken S, Hsu H, El-Chaâr D, Bisnaire L, Fell D, Wen SW, Walker M. Maternal cannabis use in pregnancy and child neurodevelopmental outcomes. Nat Med. 2020;26:1536–1540. doi: 10.1038/s41591-020-1002-5. [DOI] [PubMed] [Google Scholar]
- 26.Wood AG, Nadebaum C, Anderson V, Reutens D, Barton S, O’Brien TJ, Vajda F. Prospective assessment of autism traits in children exposed to antiepileptic drugs during pregnancy. Epilepsia. 2015;56:1047–1055. doi: 10.1111/epi.13007. [DOI] [PubMed] [Google Scholar]
- 27.von Ehrenstein OS, Ling C, Cui X, Cockburn M, Park AS, Yu F, Wu J, Ritz B. Prenatal and infant exposure to ambient pesticides and autism spectrum disorder in children: population based case-control study. BMJ. 2019;364:l962. doi: 10.1136/bmj.l962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pu Y, Yang J, Chang L, Qu Y, Wang S, Zhang K, Xiong Z, Zhang J, Tan Y, Wang X, Fujita Y, Ishima T, Wang D, Hwang SH, Hammock BD. Maternal glyphosate exposure causes autism-like behaviors in offspring through increased expression of soluble epoxide hydrolase. Proc Natl Acad Sci U S A. 2020;117:11753–11759. doi: 10.1073/pnas.1922287117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Holmes AS, Blaxill MF, Haley BE. Reduced levels of mercury in first baby haircuts of autistic children. Int J Toxicol. 2003;22:277–285. doi: 10.1080/10915810305120. [DOI] [PubMed] [Google Scholar]
- 30.Majewska MD, Urbanowicz E, Rok-Bujko P, Namyslowska I, Mierzejewski P. Age-dependent lower or higher levels of hair mercury in autistic children than in healthy controls. Acta Neurobiol Exp (Wars) 2010;70:196–208. doi: 10.55782/ane-2010-1791. [DOI] [PubMed] [Google Scholar]
- 31.Lakshmi Priya MD, Geetha A. Level of trace elements (copper, zinc, magnesium and selenium) and toxic elements (lead and mercury) in the hair and nail of children with autism. Biol Trace Elem Res. 2011;142:148–158. doi: 10.1007/s12011-010-8766-2. [DOI] [PubMed] [Google Scholar]
- 32.Geier DA, Kern JK, King PG, Sykes LK, Geier MR. Hair toxic metal concentrations and autism spectrum disorder severity in young children. Int J Environ Res Public Health. 2012;9:4486–4497. doi: 10.3390/ijerph9124486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Crider A, Thakkar R, Ahmed AO, Pillai A. Dysregulation of estrogen receptor beta (ERβ), aromatase (CYP19A1), and ER co-activators in the middle frontal gyrus of autism spectrum disorder subjects. Mol Autism. 2014;5:46. doi: 10.1186/2040-2392-5-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Krezel W, Dupont S, Krust A, Chambon P, Chapman PF. Increased anxiety and synaptic plasticity in estrogen receptor beta-deficient mice. Proc Natl Acad Sci U S A. 2001;98:12278–12282. doi: 10.1073/pnas.221451898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xu XJ, Zhang HF, Shou XJ, Li J, Jing WL, Zhou Y, Qian Y, Han SP, Zhang R, Han JS. Prenatal hyperandrogenic environment induced autistic-like behavior in rat offspring. Physiol Behav. 2015;138:13–20. doi: 10.1016/j.physbeh.2014.09.014. [DOI] [PubMed] [Google Scholar]
- 36.Xiang D, Lu J, Wei C, Cai X, Wang Y, Liang Y, Xu M, Wang Z, Liu M, Wang M, Liang X, Li L, Yao P. Berberine ameliorates prenatal dihydrotestosterone exposure-induced autism-like behavior by suppression of androgen receptor. Front Cell Neurosci. 2020;14:87. doi: 10.3389/fncel.2020.00087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zou Y, Lu Q, Zheng D, Chu Z, Liu Z, Chen H, Ruan Q, Ge X, Zhang Z, Wang X, Lou W, Huang Y, Wang Y, Huang X, Liu Z, Xie W, Zhou Y, Yao P. Prenatal levonorgestrel exposure induces autism-like behavior in offspring through ERβ suppression in the amygdala. Mol Autism. 2017;8:46. doi: 10.1186/s13229-017-0159-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Halladay AK, Bishop S, Constantino JN, Daniels AM, Koenig K, Palmer K, Messinger D, Pelphrey K, Sanders SJ, Singer AT, Taylor JL, Szatmari P. Sex and gender differences in autism spectrum disorder: summarizing evidence gaps and identifying emerging areas of priority. Mol Autism. 2015;6:36. doi: 10.1186/s13229-015-0019-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Frazier TW, Georgiades S, Bishop SL, Hardan AY. Behavioral and cognitive characteristics of females and males with autism in the Simons Simplex Collection. J Am Acad Child Adolesc Psychiatry. 2014;53:329–340. e1–3. doi: 10.1016/j.jaac.2013.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schaafsma SM, Gagnidze K, Reyes A, Norstedt N, Månsson K, Francis K, Pfaff DW. Sex-specific gene-environment interactions underlying ASD-like behaviors. Proc Natl Acad Sci U S A. 2017;114:1383–1388. doi: 10.1073/pnas.1619312114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hu VW, Sarachana T, Sherrard RM, Kocher KM. Investigation of sex differences in the expression of RORA and its transcriptional targets in the brain as a potential contributor to the sex bias in autism. Mol Autism. 2015;6:7. doi: 10.1186/2040-2392-6-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hoffman EJ, Turner KJ, Fernandez JM, Cifuentes D, Ghosh M, Ijaz S, Jain RA, Kubo F, Bill BR, Baier H, Granato M, Barresi MJF, Wilson SW, Rihel J, State MW, Giraldez AJ. Estrogens suppress a behavioral phenotype in zebrafish mutants of the autism risk gene, CNTNAP2. Neuron. 2016;89:725–733. doi: 10.1016/j.neuron.2015.12.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Turner TN, Wilfert AB, Bakken TE, Bernier RA, Pepper MR, Zhang Z, Torene RI, Retterer K, Eichler EE. Sex-based analysis of de novo variants in neurodevelopmental disorders. Am J Hum Genet. 2019;105:1274–1285. doi: 10.1016/j.ajhg.2019.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Robinson EB, Lichtenstein P, Anckarsäter H, Happé F, Ronald A. Examining and interpreting the female protective effect against autistic behavior. Proc Natl Acad Sci U S A. 2013;110:5258–5262. doi: 10.1073/pnas.1211070110. [DOI] [PMC free article] [PubMed] [Google Scholar]