Abstract
Background: Chronic HBV infection is a serious worldwide health problem that mainly causes liver cirrhosis and hepatocellular carcinoma (HCC). Few studies have explored how T cell exhaustion helps HBV avoid immune system attack and how to reverse that exhaustion. Recently, T cell immunoglobulin and immune receptor tyrosine-based inhibitory motif (ITIM) domain (TIGIT) have been identified as coinhibitory receptors, similar to PD-1. This study explores the expression of TIGIT and the T cell function changes in patients with chronic HBV infection. Results: In this study, we found that the expression of TIGIT on T cells increased significantly in patients with chronic HBV infection. High expression of TIGIT on T cells is associated with functional exhaustion. Importantly, this study demonstrates that blocking TIGIT can reverse T cell exhaustion and restore function in patients with chronic HBV infection. Conclusions: HBV induces T cell exhaustion by up-regulating the expression of TIGIT. Blocking the TIGIT/PVR signaling pathway can reverse T cell exhaustion, so this discovery provides an immunotherapy target to battle chronic HBV infection.
Keywords: T cell immunoglobulin and ITIM domain, TIGIT, programmed death receptor-1, PD-1, T cell exhaustion
Introduction
Hepatitis B virus (HBV) is the most common hepatotropic virus that causes liver inflammation and injury. In the stage of acute viral infection, the response of T cells to HBV infection is characterized by the activation of large numbers of epitope-specific CD4+ and CD8+T cells [1,2]. However, in the stage of chronic viral infection, the increase of antigen level and HBV viral load continue to stimulate T cells, leading to gradual loss of T cell function, which is defined as “T cell exhaustion” [3]. The “exhaustion” state of patients with chronic HBV infection is characterized by weak cytotoxic activity of effector cells, impaired production of cytokines, and continuous expression of multiple inhibitory receptors, such as cytotoxic T lymphocyte-associated antigen-4 (CTLA-4), lymphocyte activation gene-3 (LAG-3), T cell immunoglobulin-3 (TIM-3), and programmed cell death-1 (PD-1) [4-7].
When the immune system cannot eradicate an infection quickly, it will start a process of various actions to inhibit the infection and prevent immune-mediated tissue damage. This process is defined as exhaustion [8]. In patients with chronic HBV infection, the elevated expressions of PD-1, TIM-3, LAG-3, and CTLA-4 are associated with T cell exhaustion and persistent viral infection [8-12]. In addition, previous studies have demonstrated that blocking PD-1, Tim-3, LAG-3, and CTLA-4, whether alone or in combination, can improve the function of HBV-specific CD8+T cells in vitro [10-14]. It is worth noting that compared to other inhibitory receptors, the PD-1 pathway blockade leads to the strongest enhancement of T cell function in chronic HBV infection [11]. However, blocking the inhibitory receptors in patients with chronic HBV infection has only a limited effect on the recovery of HBV-specific CD8+T cell function [15], which indicates the necessity of exploring new inhibitory receptors.
TIGIT (T cell immunoglobulin and ITIM domain) was first identified as a new type of inhibitory receptor of CD28 family by bioinformatics algorithm in 2009 [16,17]. TIGIT is a coinhibitory receptor of the Ig superfamily, and is specifically expressed on T cells and natural killer (NK) cells [17-19]. In recent years, studies have reported that blocking both TIGIT and PD-1 pathways can reverse T cell exhaustion in HIV-infected patients [20,21]. However, there are few reports about the expression and function of TIGIT in patients with chronic HBV infection.
In this study, we analyzed the expression profile and function of TIGIT in peripheral T cells of patients with chronic HBV infection. We confirmed the effect of TIGIT blockade on the recovery of T cell function in patients with chronic HBV infection. Our study demonstrates that T cell exhaustion is the key to HBV immune escape, and blocking this checkpoint may reverse the functional impairment of T cells to HBV infection.
This study obtained approval from the Medical Ethics Committee of the Second Hospital of Nanjing in Jiangsu province of China (2017-LY-kt021), and informed consent was obtained from each enrolled subject. This study had no influence on the subsequent management of patients.
Materials and methods
Patient sample collection and isolation
Heparinized peripheral blood was collected from patients with chronic HBV infection and healthy donors. Patients with chronic HBV infection were defined as HBV surface antigen-positive at least six months before enrollment without evidence of cirrhosis or hepatocellular carcinoma (HCC) according to the criteria established by the Chinese Medical Association (Chinese Society of Hepatology and Chinese Society of Infectious Diseases) [22]. These donors were considered healthy, had had no infection or immunity in the past month, had no known immunodeficiency or any history of chemotherapy or radiotherapy, and had not received systemic steroids or any other immunosuppressive drugs in the past 6 months. All samples were obtained in accordance with the requirements of the ethics committee of the Second Hospital of Nanjing and processed in accordance with the principles of the International Conference on Harmonization Guidelines and the 1962 Declaration of Helsinki. All participants or their legal representatives voluntarily provided written informed consent before being included in the study. Venous blood was collected from each participant by venipuncture. Within 3 hours after collection, peripheral blood mononuclear cells (PBMCs)were extracted from blood samples with Lymphocyte separating solution [23]. Cell viability was detected by 0.4% trypan blue vital staining. All cells were cryopreserved in fetal bovine serum (FBS) containing 8% dimethyl sulfoxide (DMSO) and stored in liquid nitrogen until use.
T cell immunostaining and flow cytometric analysis
Cryopreserved PBMCs were thawed rapidly in warm 10% cRPMI (RPMI-1640) medium (GIBCO) supplemented with 10% FBS (GIBCO), 1% penicillin-streptomycin (GIBCO), 10 mM HEPES (GIBCO), 2 mM L-glutamine (GIBCO), and 10 μg/mL DNase I (Sigma-Aldrich, Dorset, United Kingdom), washed with PBS+2% FBS (GIBCO) (complete RPMI). The cells were stained with aqua fluorescent reactive dye Dead Cell Stain Kit (AARD; Invitrogen, Carlsbad, California), and then incubated with panels of conjugated anti-human monoclonal antibodies (mAbs) in the dark at 4°C for 30 min. The following directly conjugated mAbs used in this study were obtained from eBioScience (Inc., San Diego, CA): CD3 eFluor450 clone (OKT3), CD8a APC-eFluor® 780(OKT-8), TIGIT PE-Cyanine7 (MBSA43), Mouse IgG1 K Iso control PE-Cyanine7, Mouse IgG1 APC, and PE-conjugated anti-CD28 (Cat. No. 2071). CD45RA FITC (LeuTM-18, Cat. No. 347723) was obtained from IMMUNOTECH. PMA/Ionomycin mixture (250×) was obtained from MultiSciences. All cells were washed with PBS+2% FBS and then placed in 1% paraformaldehyde (PFA, Electron Microscopy Sciences, Hatfield, Pennsylvania), and data were obtained on a CANTO II flow cytometer (BD Biosciences) within 18 hours. For each sample, 100,000 to 500,000 lymphocyte events were gathered. The isotype control or fluorescence minus one (FMO) samples were prepared for gating. Flowjo software version 10.0 (Treestar, Ashland, Oregon, USA) was used to analyze the data.
Isolation of PBMCs and stimulation of HLA-A2-restricted HBV peptides in vitro
PBMCs were separated from fresh heparinized blood by Ficoll-Hypaque density gradient centrifugation, and then resuspended in RPMI 1640 with 25 mM HEPES, 50 μg/mL of gentamicin, 2 mM L-glutamine, and 8% human serum (complete medium). For the expansion of CD8+T cells, PBMC were resuspended in complete medium supplemented with concentration 2×106/ml, sown at 200 μL/well into 96-well plates, and stimulated with HLA-A2-restricted HBV peptides (FLPSDFFPSV) in 10 μg/mL final concentration. Recombinant IL-2 (50 IU/mL), IL-7 (10 ng/mL), and IL-12 (10 ng/mL) were added on the 3rd and 6th days of culture, respectively. The immunological test was performed on the 10th day.
Intracellular staining and flow cytometry
PBMCs were cultured in RPMI-1640 medium (GIBCO) containing 10% FBS, stimulated with PMA/ionomycin (50 ng/mL and 1 μg/mL) or HBV peptides for 6 hours, and Golgiplug (BD Biosciences) for 5 hours. The cell surface was stained with CD3 eFluor450 clone (OKT3), CD8a APC-eFluor® 780(OKT-8), and TIGIT PE-Cyanine7 (MBSA43); intracellular staining was done with IL-2 PerCP-eFluor® 710(MQ1-17H12), IFN-γ-PE (4S.B3), and TNF-α-FITC (MAb11). The cell viability was measured with Fixable Viability Dye eFluor 450 (eBioScience). Finally, the cells were analyzed by 8-color BD flow cytometry.
Antibody blockade of T cell function in vitro
Cryopreserved PBMCs (6×105 cells) were blocked with anti-TIGIT mAb or IgG in wells of concave-bottomed 96-well plates (BD Falcon, Stockholm, Sweden) and cultured in a 5% CO2 incubator at 37°C for 40 min. Next, the peptides (10 μg/mL) were extracted from HBV epitope core-18 FLPSDFFPSV, env-183 FLLTRILTI, env-335 WLSLLVPFV, pol-455 GLSRYVARL peptides, and then put into the previous 96-well plates with concave bottom and incubated at 37°C for 96 hours in a 5% CO2 incubator. Then, similar lyophilized peptides were added into the 96-well plates with concave bottom at 37°C for 10 hours in a 5% CO2 incubator, and the Golgiplug was added into the concave-bottomed 96-well plates at 37°C for 6 hours in 5% CO2 incubator. Finally, the cells were washed and stained with LIVE/DEAD® Fixable Aqua Dead Cell Stain Kit and conjugated antibodies against CD3 and CD8 before intracellular staining of IFN-γ and IL-2 was carried out. As mentioned above, the cells were obtained by flow cytometry.
Statistical analysis
Prism Graphpad release 5.0 (Graphpad Software, San Diego, California) and SPSS 22.0 software (SPSS, Chicago, IL, USA) were used for statistical analysis. Under the assumption that the sample size and sample population obey the normal distribution, the independent-samples t test was selected to compare the two independent groups. In the event that the sample’s size and population do not obey the normal distribution, the Mann-Whitney U test was selected to compare the two independent groups. Repeated measures were analyzed byone-way ANOVA attended by Tukey’s multiple comparisons. Wilcoxon matched-pairs signed ranked test was used for pairing tests, and Spearman’s rho test was used for correlation analyses. The significance measures are denoted in our tables and figures as *P<0.05, **P<0.01, or ***P<0.001.
Results
Expression of TIGIT on T cells in patients with chronic HBV infection
To investigate whether TIGIT is involved in the pathogenesis of chronic HBV infection, we detected the expression of TIGIT on T cells of peripheral blood mononuclear cells (PBMCs) in patients with chronic HBV infection and healthy donors (HD). 50 patients with chronic HBV infection (42 males and 8 females, median age 42.34 years, range 25-76 years) were selected as the study group, and 20 healthy donors (13 males and 7 females, median age 39.94 years, range 21-50 years) formed the control group. The mean percentage of CD4+T cells was 65.08±11.29% in the chronic HBV infection group and 67.87±11.14% in the HD group (P=0.249). The mean percentage of CD8+T cells was 33.02%±11.18% in the chronic HBV infection group and 30.63%±10.66% in the HD group (P=0.302). Details of participants’ characteristics are shown in Table 1.
Table 1.
Participant characteristics
| Value | HBV-Uninfected Donors (HD; n=20) | Chronic hepatitis B (CHB; n=50) |
|---|---|---|
| Age (Years) | 29.94±2.45 | 42.34±1.43 |
| Gender (Male/Female) | 13/7 | 42/8 |
| ALT (IU/L) | 37.23±5.72 | 32.18±3.05 |
| AST (IU/L) | 31.44±3.31 | 33.84±4.08 |
| GGT (IU/L) | 66.16±19.04 | 39.79±5.35 |
| HBsAg | NA | 1045.16±251.95 |
| HBeAg | NA | 23.40±17.21 |
| HBcAb | NA | 9.44±2.93 |
| HBV viral load (copies/ml) Log10 | NA | 3.06±1.12 |
| CD4+T (%) | 67.87±11.14 | 64.26±11.26 |
| CD8+T (%) | 30.63±10.66 | 33.81±11.17 |
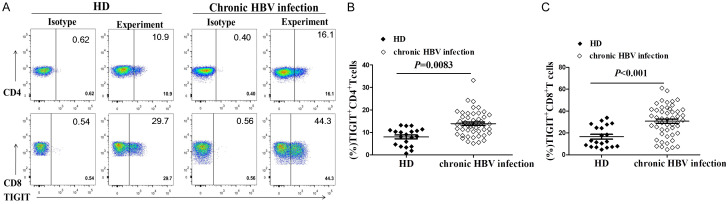
The percentage of TIGIT+CD4+T cells in the chronic HBV infection group was significantly higher than in the HD group (12.28±0.93% vs.7.98±0.86%, P=0.0083, Figure 1A, 1B). In addition, the percentage of TIGIT+CD8+T cells in the chronic HBV infection group was significantly higher than in the HD group (30.77±2.00% vs. 16.61±2.17%, P=0.0001, Figure 1A, 1C). These results suggest that the expression of TIGIT on CD4+T cells and CD8+T cells might be related to the pathogenesis of chronic HBV infection.
Figure 1.
Expression of TIGIT in T cells elevated in chronic HBV infection. Cryopreserved PBMCs from 50 patients diagnosed with chronic HBV infection and 20 healthy donors were thawed and surface phenotypes were tested for TIGIT expression. (A) Representative flow cytometry flow plots showing TIGIT expression on CD4+ and CD8+T cells. Graphs show compiled data of TIGIT expression on (B) CD4+ and (C) CD8+T cells in the HBV-uninfected healthy donors group (HD, black diamond; n=20) and chronic HBV infection group (open diamond; n=50).
Analysis of TIGIT phenotype of T cell subsets in patients with chronic HBV infection
Previous studies have demonstrated that chronic HBV infection can lead to the expansion of immature effector CD4+ or CD8+T cells [24,25]. Our study found that the expression of TIGIT on T cells in patients with chronic HBV infected was elevated. In order to analyze the expression of TIGIT in different T cell subsets, T cells were further divided into the Naive group, Memory group, Intermediate group, and Effector group according to the expression of CD28 and CD45RA [24,25].
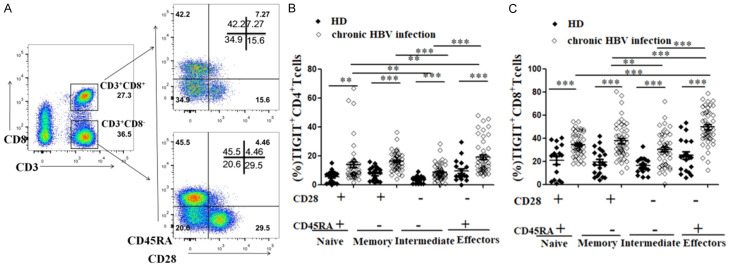
We analyzed the expression pattern of TIGIT in the above heterogeneous CD4+T cells and CD8+T cell subsets. Figure 2A shows a representative flow cytometry flow chart of co-expression of CD28 and CD45RA on CD4+ and CD8+T cells in chronic HBV infection. Compared to the HD group, TIGIT was highly expressed in four differentiation states of CD4+ and CD8+T cells in the chronic HBV infection group (Figure 2B, 2C). In addition, we compared the expression of TIGIT in four subsets of CD4+ and CD8+T cells in the chronic HBV infection group. It should be noted that the expression of TIGIT in effector CD4+ and CD8+T cell subsets of patients with chronic HBV infection was the highest, followed by expression in memory CD4+T cell subsets (Effector, Memory, Naïve, and Intermediate CD4+T cell subset results: 18.92%±1.60%, 16.18%±6.25%, 13.81%±1.88%, and 8.64%±0.80%, P<0.001) and CD8+T cell subsets (Effector, Memory, Naïve, and Intermediate CD8+T cell subset results: 49.61%±14.66%, 37.62%±15.29%, 34.45%±9.65%, and 30.42%±14.13%, P<0.001, Figure 2B, 2C).
Figure 2.
Expression of TIGIT is elevated at different stages of T cell differentiation in patients with chronic HBV infection. After thawing of frozen PBMCs, TIGIT expression on CD4+ and CD8+T cell subsets with different CD28 and CD45RA expression was detected. A. Representative flow cytometry flow plots show CD28 and CD45RA co-expression on CD4+ and CD8+T cells in chronic HBV infected patients. B. Graph shows compiled percentage of TIGIT+T cells in different CD4+T cell subsets in HD and chronic HBV infection groups. HBV-uninfected healthy donors (HD, black diamond; n=20), chronic HBV infection (open diamond; n=50). C. Graph shows compiled percentage of TIGIT+T cells in four CD8+T cell subsets in HD and chronic HBV infection groups. HBV-uninfected healthy donors (HD, black diamond; n=20), chronic HBV infection (open diamond; n=50). P values were calculated using independent-samples t test.
Furthermore, the expression of TIGIT on all of the Naive, Memory, Intermediate, and Effector subsets of T cells was significantly higher in the chronic HBV infection group than in the HD group. Additionally, the highest expression of TIGIT was found in the effector T cell subsets during chronic HBV infection, suggesting that the high expression of TIGIT may be related to the dysfunction of effector T cells in patients with chronic HBV infection.
Cytokine secretion by TIGIT+ and TIGIT-CD8+T cells after stimulation of HLA-A2-restricted HBV peptides
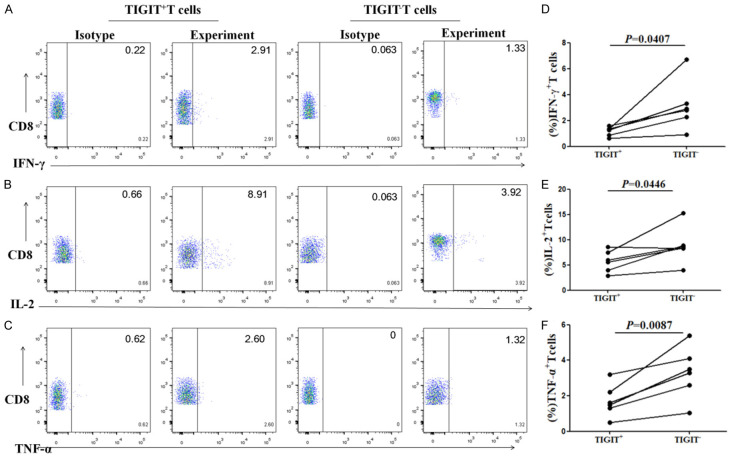
HBV-specific CTL can secrete antiviral cytokines such as IFN-γ, IL-2, and TNF-α, and it plays a role in virus clearance [8]. The dysfunction of cytokine secretion by exhausted HBV-specific CTLs in patients with chronic HBV infection could lead to the abnormal secretion of pro-inflammatory cytokines including decreases in IFN-γ, IL-2, and TNF-α [8]. The PBMCs from chronic HBV infection patients were stimulated by HLA-A2-restricted HBV peptides, and the cytokine secretion by TIGIT+ and TIGIT-CD8+T cells were compared to study the effect of TIGIT on the cytokine secretion of CD8+T cells. The percentage of IFN-γ secreting cells in the TIGIT+CD8+T cell group (1.16±0.14%) was significantly lower than in TIGIT-CD8+T cells stimulated by the antigenic peptides group (3.13±0.79%) after stimulation by HBV peptides. There was a significant difference between the two groups (P=0.0407, Figure 3A, 3D). The percentage of TNF-α secreting cells in the TIGIT+CD8+T cells group (1.72±0.37%) was significantly lower than that in TIGIT- the CD8+T cells stimulated by the antigenic peptides group (3.32±0.59%). There was a significant difference between the two groups (P=0.0087, Figure 3B, 3E). The percentage of IL-2 secreting cells in TIGIT+CD8+T cells stimulated by antigenic peptides group (5.73±0.86%) was significantly lower than that in TIGIT-CD8+T cells stimulated by antigenic peptides group (8.91±1.48%) after stimulation of HBV peptides. There was a significant difference between the two groups (P=0.0446, Figure 3C, 3F). These results suggest that the expression of TIGIT on CD8+T cells stimulated by antigenic peptides might be related to the inhibition of cytokines secreted by CD8+T cells stimulated by antigenic peptides.
Figure 3.
Secretion of IFN-γ, TNF-α, and IL-2 by TIGIT+CD8+T cells is lower than that of TIGIT-CD8+T cells after stimulation of HLA-A2-restricted HBV peptides. Representative flow cytometry flow plots show percentage of (A) IFN-γ, (B) IL-2, and (C) TNF-α expression on TIGIT+ and TIGIT-CD8+T cells after stimulation of HLA-A2-restricted HBV peptides. Graphs show the compiled percentage of (D) IFN-γ, (E) IL-2, and (F) TNF-α expression in TIGIT+ or TIGIT-CD8+T cells after stimulation of HLA-A2-restricted HBV peptides in chronic HBV infection group (n=6). P values were calculated using independent-samples t test.
TIGIT blockade on T cells reversed the cytokine secretion of HBV-specific CD8+T cells
Boni et al. [2] indicated that the blockade of the PD-1/PD-L1 pathway can restore exhausted T cell function in patients with chronic HBV infection. Through previous studies, the authors found that the expression of TIGIT on the surface of CD8+T cells may lead to dysfunction of CD8+T cells in vitro. It is unclear whether blocking TIGIT signaling pathways can affect the cytokine secretion of HBV-specific CTL in patients with chronic HBV infection. Therefore, we added anti-TIGIT mAb alone to PBMCs from 10 patients with chronic HBV infection to block TIGIT receptors in vitro. Then, the HBV peptide pool was added to the system and co-cultured with PBMCs for 96 hours. Finally, the expression of cytokines was detected by flow cytometry.
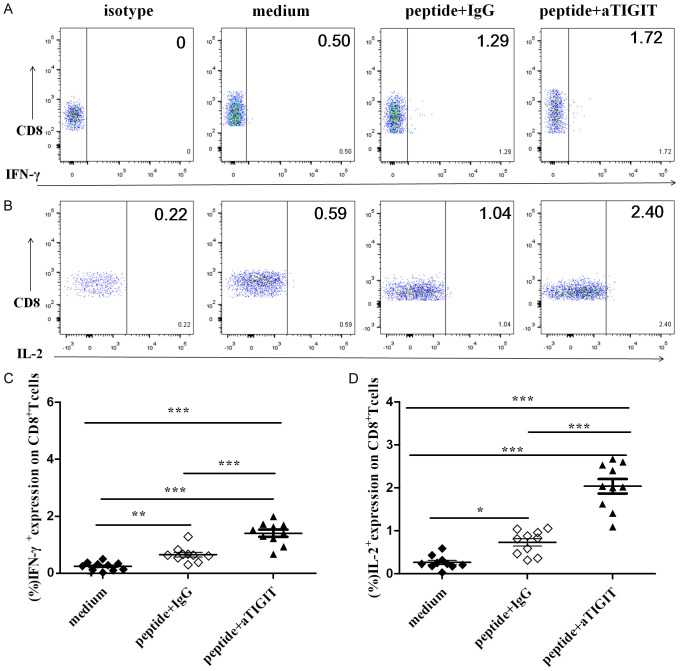
The percentage of IFN-γ+CD8+T cells after stimulation by the HBV peptide pool in blocking the TIGIT signaling pathway group (1.40±0.12%) was significantly higher than of the Ig group (0.65±0.08%). There was a significant difference between the two groups (P<0.001, Figure 4A, 4C). The percentage of CD8+T cells secreting IL-2 in the group blocking TIGIT signaling pathway (2.03±0.16%) was significantly higher than that in Ig group (0.73±0.08%). There was a significant difference between the two groups (P<0.001, Figure 4B, 4D). Therefore, blocking the TIGIT signaling pathway in patients with chronic HBV infection partially reverses the cytokine secretion function of HBV-specific CD8+T cells.
Figure 4.
Effect of blocking with anti-TIGIT mAbs on HBV-specific CTL responses in vitro. In the presence or absence of mAb blocking antibodies, the HBV peptide pool was added to stimulate PBMCs from patients with chronic HBV infection in vitro. Representative flow cytometry flow plots gated on CD8+T cells, showing (A) IFN-γ and (B) IL-2 responses for medium, HBV peptide pool stimulation with IgG, HBV peptide pool stimulation with anti-TIGIT mAb from a chronic HBV-infected individual. Compiled data showing variation in the percentage of (C) IFN-γ+ and (D) IL-2+ CD8+T cells in response to medium, HBV peptide pool stimulation with IgG, and HBV peptide pool stimulation with anti-TIGIT mAb (n=10). P values were calculated with repeated-measures one-way ANOVA test, followed by Tukey’s multiple comparisons test.
Relationship between increased TIGIT expression on T cells and clinical values in patients with chronic HBV infection
To investigate the correlation between the expression level of TIGIT on T cells and clinical indexes in patients with chronic HBV infection, the expression level of TIGIT on T cells, liver function, and HBV viral load were detected in 50 patients with chronic HBV infection. The characteristics of these 50 patients are listed in Table 1.
The results show that the percentage of TIGIT+CD4+T cells was positively correlated with the level of alanine-transaminase (ALT), gamma-glutamyl transpeptidase (GGT) and HBV viral load, but not with the level of aspartate-aminotransferase (AST) and HBsAg (Figure 5A-C and Supplementary Figure 1A, 1B). Similarly, the percentage of TIGIT+CD8+T cells was also positively correlated with the level of ALT and HBV viral load (Figure 5D, 5E), but not with the level of AST, GGT, and HBsAg (Supplementary Figure 1C-E). These results suggest that the expression of TIGIT on CD4+ and CD8+T cells in patients with chronic HBV infection positively correlates with liver inflammation and HBV viral load.
Figure 5.
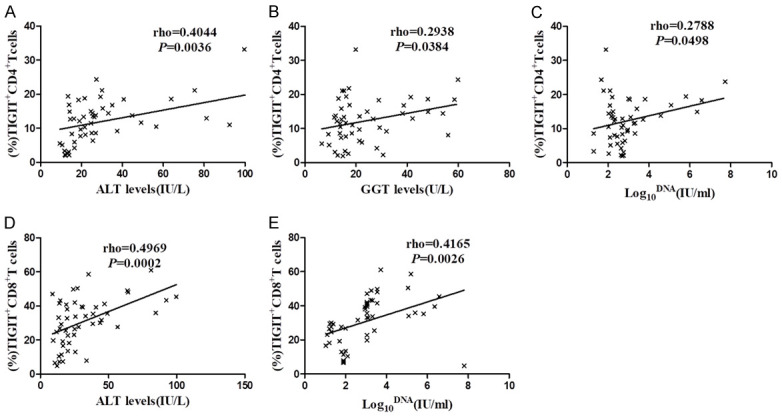
Relationship between the expression of elevated TIGIT on T cells and clinical parameters in patients with chronic HBV infection. Graphs show correlation between the percentage of TIGIT+CD4+T cells and (A) ALT (IU/L) levels, (B) GGT (U/L) levels, and (C) HBV viral load (log10 IU/ml) levels in chronic HBV infection group (n=50). Graphs show correlations between the percentage of TIGIT+CD8+T cells and (D) ALT (IU/L) levels, and (E) HBV viral load (log10 IU/ml) levels in chronic HBV infection group (n=50). Spearman’s rho tests were performed to detect correlations.
Discussion
The persistent expression of multiple inhibitory receptors in patients with chronic HBV infection is one of the characteristics of T cell exhaustion [1,3]. There have been many studies on the expression of PD-1 and other inhibitory receptors on T cells of patients with chronic HBV infection [26-28]. TIGIT is a member of the immunoglobulin superfamily, especially expressed in immune cells, as a coinhibitory receptor [17-19]. Previous studies have demonstrated that TIGIT is expressed on activated T cells, memory T cells, regulatory T (Treg) cell subsets, NK cells, and follicular helper T (Tfh) cells [16,29,30]. In addition to protecting against autoimmune diseases [31,32], TIGIT also plays an important role in cancer and chronic viral infections [21,33,34]. In a recent study, TIGIT blockade or deficiency was found to lead to chronic hepatitis, fibrosis, and even HCC in HBsAg-transgenic mice in a CD8+T cell-dependent manner. The expression of the TIGIT gene in intratumoral regions of HCC patients is also higher than in paracancerous regions [35].
In this study, we studied the phenotype and function of T cells expressing TIGIT in patients with chronic HBV infection. The important findings of this study are as follows. First, the expression of TIGIT on T cells is elevated. Second, the expression of TIGIT on the Effector T cell subset was higher than that on the Naïve, Memory, and Intermediate T cell subsets. Third, the high expression of TIGIT on T cells was related to the decrease of cytokines secreted by CD8+T cells after stimulation by HBV peptides. Moreover, blocking the TIGIT signaling pathway partially restores T cell function. Finally, in patients with chronic HBV infection, increased TIGIT expression on T cells is positively correlated with liver inflammation and HBV viral load.
In patients with adult T cell leukemia [36], rheumatoid arthritis [37], HIV [38], and primary and metastatic melanoma [39], the expansion of T cell immune checkpoints may affect the skewness of T cell subsets. Speculating that TIGIT could play a dominant role in the upregulation of effector T cell subsets in patients with chronic HBV infection, we analyzed the expression pattern of TIGIT in T cell subsets (naïve T cells, Memory T cells, Intermediate T cells, and Effector T cells). We found that TIGIT was highly expressed in four differentiation states of CD4+ and CD8+T cells in the chronic HBV infection group compared with the HD group, respectively. In addition, the expression of TIGIT on the effector of CD4+ and CD8+T cell subsets was the highest in patients with chronic HBV infection.
Strikingly, this study found that TIGIT expression was relatively stable in the naïve CD8+T cell population, but there was no significant difference in TIGIT expression between the CD8+T cells naive subsets and intermediate subsets, and their expression values were lower than those of the effector subsets and memory subsets. Similarly, Chew et al. showed that TIGIT expression was relatively stable in the naïve population (CD28+CD45RA+CD8+T cells) [21].
In addition, TIGIT expression significantly increased on the CD8+T cell effector subsets, with the highest expression of TIGIT on the effector CD8+T cell subset, followed by intermediate and transitional subset values during chronic HIV infection. These results are consistent with a role for TIGIT as a potential regulator of intermediate, transitional, and effector T cell responses. However, we found a significant difference in TIGIT expression between the CD4+T cells naive subsets and effector subsets, with the naïve subset’s value being lower than the effector subset. The reason might be that CD4+T cells include many cell types, including Tregs, Th1, Th2, Th17, and many others.
Previous studies have shown that the level of TIGIT expression can regulate CD4+T cell subsets. TIGIT is a newly identified coinhibitory receptor with up-regulated expression in effector Treg cells, and the marking Tregs specifically control Th1 and Th17 responses [40]. Additionally, gene expression analysis had shown that the frequency of TIGIT+CD4+T cells associated with a skewed CD4+T cell molecular profile indicates an exhausted Th1 profile [41]. TIGIT is critical for Th2 immunity. In vitro research has shown that the up-regulation of TIGIT expression in Th2 effector cells and its interaction with CD155 expressed in dendritic cells are important during the development of Th2 responses, blockade of TIGIT inhibited Th2 cells responses [42]. TIGIT is up-regulated in effector Treg cells, and this subset allows selectivity in inhibiting the response of Th1 and Th17 cells rather than Th2 cell response [30].
However, our study found a significant difference in TIGIT expression between CD4+T cell Naive subsets and Effector subsets, such that their values were lower than those of the Effector subset. Therefore, these results suggested that increasing TIGIT affects theskewing of T cell subsets, especially the Effector T cell subset. In other words, the high expression of TIGIT may be related to the dysfunction of Effector T cells in patients with chronic HBV infection. In addition to analyzing the general regulatory role of TIGIT as a coinhibitory receptor of CD8+T cells, the increased expression of TIGIT on CD4+T cells indicates a special regulatory role of TIGIT in shifting the cytokine balance, which has been reported in other studies and needs further study in patients with chronic HBV infection [6].
PD-1 is a receptor of the Ig superfamily. It has been demonstrated to play a major negative regulatory role in the T cell-mediated immune response [28]. Previous studies have found that PD-1 and other inhibitory receptors are co-expressed on T cells, leading to T cell exhaustion in chronic HBV infection [29,33,34]. In this study, the expression of TIGIT on CD4+ and CD8+T cells in the chronic HBV infection group was significantly higher than that of the HD group. Therefore, TIGIT may also be a marker of T cell exhaustion in patients with chronic HBV infection.
The synergistic effects of TIGIT expression on T cell function in AML patients [33], melanoma patients [43,44], and HIV patients [21,45] have been elucidated in the literature. Based on that, we speculated that the synergistic effect of TIGIT on T cells in patients with chronic HBV infection could inhibit the cytokines secreted by CD8+T cells. Therefore, we studied the effect of TIGIT expression on cytokine secretion of CD8+T cells in patients with chronic HBV infection in vitro, finding that the cytokine secretion of the TIGIT+CD8+T cell group was significantly reduced after stimulation of HBV peptides, suggesting that TIGIT could lead to the down-regulation of cytokine secretion of HBV-specific CD8+T cells. Moreover, blocking the TIGIT signaling pathway significantly increases the production of IL-2 in CD8+T cell groups stimulated by antigenic peptides-after stimulation by HBV peptides. It was deduced that anti-TIGIT mAb could partially restore the HBV-specific CD8+T cell response. This result is similar to previous reports [15,21,26,46-48], but not exactly the same, so it needs to be confirmed with enlarged samples. Other similar studies have found that TIGIT is associated with T cell exhaustion in AML patients [33] or melanoma patients [43]. In this study, our data clearly demonstrate the key role of TIGIT in regulating T cell exhaustion in chronic HBV infection.
Chew et al. [21] and Tanaka et al. [36] illustrated that the viral load was associated with high expression of TIGIT in HIV patients. Therefore, considering the persistence of the TIGIT pathway under precise conditions of virus inhibition, blocking TIGIT and other inhibitor receptor signaling pathways can be used to enhance the response of CD8+T cells [21,36]. Similar results were found in our study. The percentages of TIGIT+CD4+T cells and TIGIT+CD8+T cells were positively correlated with HBV viral load. Moreover, the percentage of TIGIT+T cells was positively correlated with ALT and GGT levels. Our results also indicate that the expression of TIGIT on CD4+ and CD8+T cells in patients with chronic HBV infection is positively correlated with liver inflammation and HBV viral load. However, the exact role of TIGIT in HBV clearance remains unclear.
Conclusions
This research elucidated the role of TIGIT in the pathogenesis of chronic HBV infection and dysfunction of T cells during chronic HBV infection. It is noted that blocking the TIGIT pathway can reverse T cell exhaustion and restore T cell function, which provides a basis for the treatment of chronic HBV infection.
Acknowledgements
This research was partially supported by grants from the Natural Science Foundation of Anhui Province (No. 2108085QH312 to Yan-Yan Wei), Nanjing Medical Science and Technology Development Foundation (No. YKK-20100 to Wei Ye), 333 Project of Jiangsu Province (Wei Ye), Graduate student scientific research innovation projects in Jiangsu province (KYCX21_1634 to Meng-Xuan Shan), and Project of Jiangsu Provincial Medical Youth Talent (Wei Ye).
Disclosure of conflict of interest
None.
Supporting Information
References
- 1.Ye B, Liu X, Li X, Kong H, Tian L, Chen Y. T-cell exhaustion in chronic hepatitis B infection: current knowledge and clinical significance. Cell Death Dis. 2015;6:e1694. doi: 10.1038/cddis.2015.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boni C, Fisicaro P, Valdatta C, Amadei B, Di Vincenzo P, Giuberti T, Laccabue D, Zerbini A, Cavalli A, Missale G, Bertoletti A, Ferrari C. Characterization of hepatitis B virus (HBV)-specific T-cell dysfunction in chronic HBV infection. J Virol. 2007;81:4215–4225. doi: 10.1128/JVI.02844-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wherry EJ. T cell exhaustion. Nat Immunol. 2011;12:492–499. doi: 10.1038/ni.2035. [DOI] [PubMed] [Google Scholar]
- 4.Brooks DG, Ha SJ, Elsaesser H, Sharpe AH, Freeman GJ, Oldstone MB. IL-10 and PD-L1 operate through distinct pathways to suppress T-cell activity during persistent viral infection. Proc Natl Acad Sci U S A. 2008;105:20428–20433. doi: 10.1073/pnas.0811139106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sakuishi K, Apetoh L, Sullivan JM, Blazar BR, Kuchroo VK, Anderson AC. Targeting Tim-3 and PD-1 pathways to reverse T cell exhaustion and restore anti-tumor immunity. J Exp Med. 2010;207:2187–2194. doi: 10.1084/jem.20100643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Anderson AC, Joller N, Kuchroo VK. Lag-3, Tim-3, and TIGIT: co-inhibitory receptors with specialized functions in immune regulation. Immunity. 2016;44:989–1004. doi: 10.1016/j.immuni.2016.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li FJ, Zhang Y, Jin GX, Yao L, Wu DQ. Expression of LAG-3 is coincident with the impaired effector function of HBV-specific CD8(+) T cell in HCC patients. Immunol Lett. 2013;150:116–122. doi: 10.1016/j.imlet.2012.12.004. [DOI] [PubMed] [Google Scholar]
- 8.Kim PS, Ahmed R. Features of responding T cells in cancer and chronic infection. Curr Opin Immunol. 2010;22:223–230. doi: 10.1016/j.coi.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Toh JWT, de Souza P, Lim SH, Singh P, Chua W, Ng W, Spring KJ. The potential value of immunotherapy in colorectal cancers: review of the evidence for programmed death-1 inhibitor therapy. Clin Colorectal Cancer. 2016;15:285–291. doi: 10.1016/j.clcc.2016.07.007. [DOI] [PubMed] [Google Scholar]
- 10.Cho H, Kang H, Lee HH, Kim CW. Programmed cell death 1 (PD-1) and cytotoxic t lymphocyte-associated antigen 4 (CTLA-4) in viral hepatitis. Int J Mol Sci. 2017;18:1517. doi: 10.3390/ijms18071517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nebbia G, Peppa D, Schurich A, Khanna P, Singh HD, Cheng Y, Rosenberg W, Dusheiko G, Gilson R, ChinAleong J, Kennedy P, Maini MK. Upregulation of the Tim-3/galectin-9 pathway of T cell exhaustion in chronic hepatitis B virus infection. PLoS One. 2012;7:e47648. doi: 10.1371/journal.pone.0047648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kennedy PTF, Sandalova E, Jo J, Gill U, Ushiro-Lumb I, Tan AT, Naik S, Foster GR, Bertoletti A. Preserved T-cell function in children and young adults with immune-tolerant chronic hepatitis B. Gastroenterology. 2012;143:637–645. doi: 10.1053/j.gastro.2012.06.009. [DOI] [PubMed] [Google Scholar]
- 13.Wu W, Shi Y, Li S, Zhang Y, Liu Y, Wu Y, Chen Z. Blockade of Tim-3 signaling restores the virus-specific CD8(+) T-cell response in patients with chronic hepatitis B. Eur J Immunol. 2012;42:1180–1191. doi: 10.1002/eji.201141852. [DOI] [PubMed] [Google Scholar]
- 14.Bengsch B, Martin B, Thimme R. Restoration of HBV-specific CD8+T cell function by PD-1 blockade in inactive carrier patients is linked to T cell differentiation. J Hepatol. 2014;61:1212–1219. doi: 10.1016/j.jhep.2014.07.005. [DOI] [PubMed] [Google Scholar]
- 15.Amancha PK, Hong JJ, Rogers K, Ansari AA, Villinger F. In vivo blockade of the programmed cell death-1 pathway using soluble recombinant PD-1-Fc enhances CD4+ and CD8+T cell responses but has limited clinical benefit. J Immunol. 2013;191:6060–6070. doi: 10.4049/jimmunol.1302044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Levin SD, Taft DW, Brandt CS, Bucher C, Howard ED, Chadwick EM, Johnston J, Hammond A, Bontadelli K, Ardourel D, Hebb L, Wolf A, Bukowski TR, Rixon MW, Kuijper JL, Ostrander CD, West JW, Bilsborough J, Fox B, Gao Z, Xu W, Ramsdell F, Blazar BR, Lewis KE. Vstm3 is a member of the CD28 family and an important modulator of T-cell function. Eur J Immunol. 2011;41:902–915. doi: 10.1002/eji.201041136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stanietsky N, Simic H, Arapovic J, Toporik A, Levy O, Novik A, Levine Z, Beiman M, Dassa L, Achdout H, Stern-Ginossar N, Tsukerman P, Jonjic S, Mandelboim O. The interaction of TIGIT with PVR and PVRL2 inhibits human NK cell cytotoxicity. Proc Natl Acad Sci U S A. 2009;106:17858–17863. doi: 10.1073/pnas.0903474106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Boles KS, Vermi W, Facchetti F, Fuchs A, Wilson TJ, Diacovo TG, Cella M, Colonna M. A novel molecular interaction for the adhesion of follicular CD4 T cells to follicular DC. Eur J Immunol. 2009;39:695–703. doi: 10.1002/eji.200839116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yu X, Harden K, Gonzalez LC, Francesco M, Chiang E, Irving B, Tom I, Ivelja S, Refino CJ, Clark H, Eaton D, Grogan JL. The surface protein TIGIT suppresses T cell activation by promoting the generation of mature immunoregulatory dendritic cells. Nat Immunol. 2009;10:48–57. doi: 10.1038/ni.1674. [DOI] [PubMed] [Google Scholar]
- 20.Matter M, Odermatt B, Yagita H, Nuoffer JM, Ochsenbein AF. Elimination of chronic viral infection by blocking CD27 signaling. J Exp Med. 2006;203:2145–2155. doi: 10.1084/jem.20060651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chew GM, Fujita T, Webb GM, Burwitz BJ, Wu HL, Reed JS, Hammond KB, Clayton KL, Ishii N, Abdel-Mohsen M, Liegler T, Mitchell BI, Hecht FM, Ostrowski M, Shikuma CM, Hansen SG, Maurer M, Korman AJ, Deeks SG, Sacha JB, Ndhlovu LC. TIGIT marks exhausted T cells, correlates with disease progression, and serves as a target for immune restoration in HIV and SIV infection. PLoS Pathog. 2016;12:e1005349. doi: 10.1371/journal.ppat.1005349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hou J, Wang G, Wang F, Cheng J, Ren H, Zhuang H, Sun J, Li L, Li J, Meng Q, Zhao J, Duan Z, Jia J, Tang H, Sheng J, Peng J, Lu F, Xie Q, Wei L Chinese Society of Hepatology Chinese Medical Association and Chinese Society of Infectious Diseases Chinese Medical Association. Guideline of prevention and treatment for chronic hepatitis B (2015 update) J Clin Transl Hepatol. 2017;5:297–318. doi: 10.14218/JCTH.2016.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Henson SM, Franzese O, Macaulay R, Libri V, Azevedo RI, Kiani-Alikhan S, Plunkett FJ, Masters JE, Jackson S, Griffiths SJ, Pircher HP, Soares MV, Akbar AN. KLRG1 signaling induces defective Akt (ser473) phosphorylation and proliferative dysfunction of highly differentiated CD8+T cells. Blood. 2009;113:6619–6628. doi: 10.1182/blood-2009-01-199588. [DOI] [PubMed] [Google Scholar]
- 24.Sobao Y, Tomiyama H, Sugi K, Tokunaga M, Ueno T, Saito S, Fujiyama S, Morimoto M, Tanaka K, Takiguchi M. The role of hepatitis B virus-specific memory CD8 T cells in the control of viral replication. J Hepatol. 2002;36:105–115. doi: 10.1016/s0168-8278(01)00264-1. [DOI] [PubMed] [Google Scholar]
- 25.Appay V, Dunbar PR, Callan M, Klenerman P, Gillespie GM, Papagno L, Ogg GS, King A, Lechner F, Spina CA, Little S, Havlir DV, Richman DD, Gruener N, Pape G, Waters A, Easterbrook P, Salio M, Cerundolo V, McMichael AJ, Rowland-Jones SL. Memory CD8+T cells vary in differentiation phenotype in different persistent virus infections. Nat Med. 2002;8:379–385. doi: 10.1038/nm0402-379. [DOI] [PubMed] [Google Scholar]
- 26.Stecher C, Battin C, Leitner J, Zettl M, Grabmeier-Pfistershammer K, Holler C, Zlabinger GJ, Steinberger P. PD-1 blockade promotes emerging checkpoint inhibitors in enhancing T cell responses to allogeneic dendritic cells. Front Immunol. 2017;8:572. doi: 10.3389/fimmu.2017.00572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kaufmann DE, Walker BD. PD-1 and CTLA-4 inhibitory cosignaling pathways in HIV infection and the potential for therapeutic intervention. J Immunol. 2009;182:5891–5897. doi: 10.4049/jimmunol.0803771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zha Y, Blank C, Gajewski TF. Negative regulation of T-cell function by PD-1. Crit Rev Immunol. 2004;24:229–237. doi: 10.1615/critrevimmunol.v24.i4.10. [DOI] [PubMed] [Google Scholar]
- 29.Joller N, Hafler JP, Brynedal B, Kassam N, Spoerl S, Levin SD, Sharpe AH, Kuchroo VK. Cutting edge: TIGIT has T cell-intrinsic inhibitory functions. J Immunol. 2011;186:1338–1342. doi: 10.4049/jimmunol.1003081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Joller N, Lozano E, Burkett PR, Patel B, Xiao S, Zhu C, Xia J, Tan TG, Sefik E, Yajnik V, Sharpe AH, Quintana FJ, Mathis D, Benoist C, Hafler DA, Kuchroo VK. Treg cells expressing the coinhibitory molecule TIGIT selectively inhibit proinflammatory Th1 and Th17 cell responses. Immunity. 2014;40:569–581. doi: 10.1016/j.immuni.2014.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bommarito D, Hall C, Taams LS, Corrigall VM. Inflammatory cytokines compromise programmed cell death-1 (PD-1)-mediated T cell suppression in inflammatory arthritis through up-regulation of soluble PD-1. Clin Exp Immunol. 2017;188:455–466. doi: 10.1111/cei.12949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McKinney EF, Lee JC, Jayne DR, Lyons PA, Smith KG. T-cell exhaustion, co-stimulation and clinical outcome in autoimmunity and infection. Nature. 2015;523:612–616. doi: 10.1038/nature14468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kong Y, Zhu L, Schell TD, Zhang J, Claxton DF, Ehmann WC, Rybka WB, George MR, Zeng H, Zheng H. T-Cell Immunoglobulin and ITIM Domain (TIGIT) associates with CD8+T-cell exhaustion and poor clinical outcome in AML patients. Clin Cancer Res. 2016;22:3057–3066. doi: 10.1158/1078-0432.CCR-15-2626. [DOI] [PubMed] [Google Scholar]
- 34.Johnston RJ, Comps-Agrar L, Hackney J, Yu X, Huseni M, Yang Y, Park S, Javinal V, Chiu H, Irving B, Eaton DL, Grogan JL. The immunoreceptor TIGIT regulates antitumor and antiviral CD8(+) T cell effector function. Cancer Cell. 2014;26:923–937. doi: 10.1016/j.ccell.2014.10.018. [DOI] [PubMed] [Google Scholar]
- 35.Zong L, Peng H, Sun C, Li F, Zheng M, Chen Y, Wei H, Sun R, Tian Z. Breakdown of adaptive immunotolerance induces hepatocellular carcinoma in HBsAg-tg mice. Nat Commun. 2019;10:221. doi: 10.1038/s41467-018-08096-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tanaka Y, Yamazaki R, Terasako-Saito K, Nakasone H, Akahoshi Y, Nakano H, Ugai T, Wada H, Yamasaki R, Ishihara Y, Kawamura K, Sakamoto K, Ashizawa M, Sato M, Kimura S, Kikuchi M, Kako S, Kanda J, Tanihara A, Nishida J, Kanda Y. Universal cytotoxic activity of a HTLV-1 Tax-specific T cell clone from an HLA-A*24:02(+) patient with adult T-cell leukemia against a variety of HTLV-I-infected T-cells. Immunol Lett. 2014;158:120–125. doi: 10.1016/j.imlet.2013.12.016. [DOI] [PubMed] [Google Scholar]
- 37.Cho BA, Sim JH, Park JA, Kim HW, Yoo WH, Lee SH, Lee DS, Kang JS, Hwang YI, Lee WJ, Kang I, Lee EB, Kim HR. Characterization of effector memory CD8+T cells in the synovial fluid of rheumatoid arthritis. J Clin Immunol. 2012;32:709–720. doi: 10.1007/s10875-012-9674-3. [DOI] [PubMed] [Google Scholar]
- 38.Bansal A, Sterrett S, Erdmann N, Westfall AO, Dionne-Odom J, Overton ET, Goepfert PA. Normal T-cell activation in elite controllers with preserved CD4+T-cell counts. AIDS. 2015;29:2245–2254. doi: 10.1097/QAD.0000000000000860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Anichini A, Molla A, Vegetti C, Bersani I, Zappasodi R, Arienti F, Ravagnani F, Maurichi A, Patuzzo R, Santinami M, Pircher H, Di Nicola M, Mortarini R. Tumor-reactive CD8+ early effector T cells identified at tumor site in primary and metastatic melanoma. Cancer Res. 2010;70:8378–8387. doi: 10.1158/0008-5472.CAN-10-2028. [DOI] [PubMed] [Google Scholar]
- 40.Lucca LE, Axisa PP, Singer ER, Nolan NM, Dominguez-Villar M, Hafler DA. TIGIT signaling restores suppressor function of Th1 Tregs. JCI Insight. 2019;4:e124427. doi: 10.1172/jci.insight.124427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rinaldi S, de Armas L, Dominguez-Rodriguez S, Pallikkuth S, Dinh V, Pan L, Grtner K, Pahwa R, Cotugno N, Rojo P, Nastouli E, Klein N, Foster C, De Rossi A, Giaquinto C, Rossi P, Palma P, Pahwa S EPIICAL consortium. T cell immune discriminants of HIV reservoir size in a pediatric cohort of perinatally infected individuals. PLoS Pathog. 2021;17:e1009533. doi: 10.1371/journal.ppat.1009533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kourepini E, Paschalidis N, Simoes DC, Aggelakopoulou M, Grogan JL, Panoutsakopoulou V. TIGIT enhances antigen-specific th2 recall responses and allergic disease. J Immunol. 2016;196:3570–3580. doi: 10.4049/jimmunol.1501591. [DOI] [PubMed] [Google Scholar]
- 43.Chauvin JM, Pagliano O, Fourcade J, Sun Z, Wang H, Sander C, Kirkwood JM, Chen TH, Maurer M, Korman AJ, Zarour HM. TIGIT and PD-1 impair tumor antigen-specific CD8(+) T cells in melanoma patients. J Clin Invest. 2015;125:2046–2058. doi: 10.1172/JCI80445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Goding SR, Wilson KA, Xie Y, Harris KM, Baxi A, Akpinarli A, Fulton A, Tamada K, Strome SE, Antony PA. Restoring immune function of tumor-specific CD4+T cells during recurrence of melanoma. J Immunol. 2013;190:4899–4909. doi: 10.4049/jimmunol.1300271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fromentin R, Bakeman W, Lawani MB, Khoury G, Hartogensis W, DaFonseca S, Killian M, Epling L, Hoh R, Sinclair E, Hecht FM, Bacchetti P, Deeks SG, Lewin SR, Sekaly RP, Chomont N. CD4+T cells expressing PD-1, TIGIT and LAG-3 contribute to HIV persistence during ART. PLoS Pathog. 2016;12:e1005761. doi: 10.1371/journal.ppat.1005761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rutishauser RL, Hartogensis W, Deguit CD, Krone M, Hoh R, Hecht FM, Pilcher CD, Bacchetti P, Deeks SG, Hunt PW, McCune JM. Early and delayed antiretroviral therapy results in comparable reductions in CD8(+) T cell exhaustion marker expression. AIDS Res Hum Retroviruses. 2017;33:658–667. doi: 10.1089/aid.2016.0324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zarour HM. Reversing T-cell dysfunction and exhaustion in cancer. Clin Cancer Res. 2016;22:1856–1864. doi: 10.1158/1078-0432.CCR-15-1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Raziorrouh B, Heeg M, Kurktschiev P, Schraut W, Zachoval R, Wendtner C, Wächtler M, Spannagl M, Denk G, Ulsenheimer A, Bengsch B, Pircher H, Diepolder HM, Gruner NH, Jung MC. Inhibitory phenotype of HBV-specific CD4+T-cells is characterized by high PD-1 expression but absent coregulation of multiple inhibitory molecules. PLoS One. 2014;9:e105703. doi: 10.1371/journal.pone.0105703. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.