Abstract
Osteosarcoma is the most common primary malignant bone tumor that often occurs in children, adolescents, and young adults. Cannabidiol plays an essential role in cancer treatment. However, its effects on osteosarcoma have not yet been addressed. In the present study, we investigated the pharmacological effects of cannabidiol on osteosarcoma. We found that cannabidiol effectively suppressed the proliferation and colony formation of osteosarcoma cells. Further studies showed that cannabidiol significantly promoted cell apoptosis and changes in cell apoptosis-related gene proteins in vitro. In addition, cannabidiol administration inhibited tumor growth and promoted the apoptosis of osteosarcoma cells in a mouse xenograft model. The in vitro study also demonstrated that SP1 contributes to chromobox protein homolog 2 (CBX2) reduction in cannabidiol-treated MG63 and HOS cells, and that cannabidiol may recruit SP1 into the CBX2 promoter regions to downregulate CBX2 expression at the transcriptional level and promote osteosarcoma cell apoptosis. Further, the result showed that cannabidiol suppressed osteosarcoma cell migration. In summary, cannabidiol effectively promoted the apoptosis of osteosarcoma cells in vitro and in vivo and suppressed tumor growth in a mouse xenograft model by regulating the SP1-CBX2 axis. This finding provides novel therapeutic strategies for osteosarcoma in the clinic.
Keywords: Osteosarcoma, cannabidiol, apoptosis, SP1, CBX2
Introduction
Osteosarcoma is the most common bone tumor in children and adolescents. It originates in the mesenchyme and is characterized by malignant spindle stromal cells, which produce bone-like tissue [1]. It is the second leading cause of tumor-related deaths among teenagers [2,3]. The most well-known clinical symptoms of osteosarcoma are pain and local mass. However, it has a high rate of misdiagnosis and missed diagnosis in the early stages. As a result, the tumors grow quickly, leading to bone destruction and lung metastases [4,5]. Various factors, such as metabolic changes, immune evasion, activation of the DNA damage repair pathway, and the stemness of cancer cells, play vital roles in cancer cell resistance [6-8]. In particular, DNA damage can lead to malignant changes in cells. However, there are many tumor suppressor genes in the body, such as p53, which rarely promote tumor formation [9]. Apoptosis is a tightly regulated mechanism that contributes to many biological processes. It is an autonomous way of cell death under pathological or physiological conditions, regulated by internal genetic mechanisms. Apoptosis is related to the activation of caspase cascade reaction, among which caspase-3 is the key molecule in the caspase family that directly participates in the execution of cell apoptosis, and the cleavage and activation of caspase-3 can lead to cell disintegration [10]. Apoptosis plays an important role in the fate of tumor cells.
In order to seek potential therapeutic targets in osteosarcoma, we used bioinformatics methods to analyze differentially expressed genes (DEGs) between tumor and peri-tumor samples in osteosarcoma expression microarray datasets (GSE136088). Based on the screening results, we selected the Chromobox protein homolog 2 (CBX2) gene as a possible therapeutic target. CBX2 is a polycomb protein, which is located on human chromosome 17q25.3 [11]. The overexpression of CBX2 increases cell proliferation, migration, and invasion, suppress tumor suppressor genes, and maintains tumor stem cells in an undifferentiated type, resulting in high mortality [12]. Nevertheless, there are few studies supporting the effects of CBX2 on osteosarcoma [13].
Epigenetic modifications play an important role in transcription and gene expression in response to external stimuli. In cancer, transcriptomes are altered, promoting initiation and cancer progression [14]. Some studies have indicated that SP1 plays a pivotal role in transcriptional activation (DNA repair, cell growth, differentiation, and apoptosis) by repressing the tumor suppressor p53 protein [15]. In addition, Ding et al. suggested that the SP1-activated long non-coding RNA, CRNDE, contributes to osteosarcoma cell proliferation and invasion [16]. However, whether SP1 acts as a transcription factor that regulates the pathogenesis of osteosarcoma is still unknown.
Cannabidiol is a natural compound extracted from the cannabis plant, which has been used in cancer therapy [17]. For instance, Shrivastava et al. showed that cannabidiol activates endoplasmic reticulum stress and suppresses mTOR signaling in breast cancer cells, leading to apoptosis and autophagy [18]. Furthermore, Massi et al. demonstrated that cannabidiol induces apoptosis of human glioma cells by triggering the caspase cascade and producing reactive oxygen species [19]. Moreover, many studies have shown that cannabidiol has anti-proliferation, anti-metastasis, pro-apoptosis and pro-autophagy effects on multiple tumor models, in both in vitro cellular models and in vivo animal models [20,21]. Nonetheless, it is unknown if cannabidiol has a therapeutic effect on osteosarcoma.
Therefore, in this research, we explored the influence of cannabidiol treatment on osteosarcoma, in both in vitro and in vivo models, to elucidate the potential mechanisms. Our findings show for the first time that cannabidiol possesses antineoplastic properties due to its efficacy in promoting osteosarcoma cell apoptosis by targeting the SP1-CBX2 axis.
Materials and methods
Clinical tissue samples
The investigation of human tissue samples was approved by the Ethics and Scientific Committees of Harbin Medical University (KY2018-032). A total of 10 fresh samples and 9 corresponding normal samples (paracancerous tissue samples) were obtained from October 2018 to November 2020 from patients with osteosarcoma at the tissue bank of the 2nd Affiliated Hospital of Harbin Medical University. Tumor tissue samples and adjacent tissues were removed and immediately placed in liquid nitrogen for long-term storage until use. Prior to tissue collection, informed consent was obtained from cancer patients.
Tumor xenograft
Female BALB/c nude mice (aged 4-6 weeks, Vital River, Beijing, China) were used for the in vivo studies. Studies related to in vivo experiments were approved by the Animal Care and Use Committee of Harbin Medical University (SYDW2021-060). For xenografts, approximately 5×106 cultured MG63 cells in phosphate-buffered saline (PBS) were subcutaneously implanted in the right shoulder of nude mice. The nude mice were randomized into two groups (n=4), after the tumor volume reached up to 100 mm3. The animals were intraperitoneally injected with a control solution or cannabidiol (20 mg·kg-1·day-1) once a day for 15 days. Fifteen days later, the nude mice were sacrificed. Tumors removed from mice were measured and weighed. Then, immunohistochemical analysis and western blotting analysis were performed on tumor tissues. In addition, to verify that CBD plays a pro-apoptotic role via SP1-CBX2 signaling pathway in vivo, transfected or un-transfected MG-63 cells were subcutaneously planted in the right axilla of nude mice and divided into four groups (n=4) according to transfection and cannabidiol administration. The followed treatments and steps were the same as the aforementioned procedures.
Cell culture and transfection
Two human osteosarcoma cell lines (MG63 and HOS) were maintained in DMEM/F12 medium containing 10% FBS. All media were supplemented with 100 mg/mL penicillin and streptomycin. MG63 and HOS were obtained from the Shanghai Academy of Chinese Sciences. Small interfering ribonucleic acids (siRNAs) targeting CBX2 (CBX2-siRNA), SP1 (SP1-siRNA), and negative control (si-NC) were provided by Ribo Bio (Guangzhou, China). They were transiently transfected using Lipofectamine 2000TM transfection reagent (Invitrogen, Carlsbad, CA, USA) and reduced serum medium. The transfection medium was replaced with complete medium after 6 h, cannabidiol (10 µM) was then added, and the cells were harvested for RNA and protein extraction at 48 or 72 h after transfection. For constructing overexpression cell line, lentiviral packaging SP1 overexpressing plasmid (HANBIO, China) was transfected using the similar transfection protocol.
Cell viability assay
MTT assay was applied to detect the effect of cannabidiol on MG63 and HOS cells. Briefly, cells were seeded in 96-well plates at a density of 8000 cells/well. Overnight, the solution was changed, and the complete medium with cannabidiol (2.5, 5, 10, 15, or 20 μM) or without cannabidiol was added to the 96-well plate for 48 h. Then, diluted MTT solution was added and incubated at 37°C for 4 h. After carefully discarding the supernatant, dimethyl sulfoxide was added to dissolve the crystals. Absorbance at 490 nm was measured using an enzyme microplate reader (Beckman Coulter, Brea, CA, USA).
Colony formation assay
The capacity of MG63 and HOS cells to proliferate following cannabidiol administration was determined using a colony formation assay. Briefly, cells were seeded in 6-well plates at a density of 1000 cells/well. In the cannabidiol-treated group, the medium was replaced with fresh medium containing cannabidiol (10, 15, or 20 μM) for approximately 12 days until clones were visible. After being rinsed twice with cold PBS, the cells were fixed with 4% paraformaldehyde. Crystal violet was used to stain the cells for 15 min. A total of 50 cells were counted in each of the colonies. Using a digital camera, the number of colonies generated was recorded and photographed.
Migration assay
The migratory capacity of MG63 and HOS cells was evaluated using wound healing assays following treatment with cannabidiol. On a 6-well plate, the cells were grown in DMEM/F12 medium containing 10% serum until cell confluence reached 80-90%. The medium was then removed, and fresh medium with or without cannabidiol (10 μM) was added and incubated for 6 h. Thereafter, the cell monolayer was scratched perpendicular to the 6-well plate using a 200 μL pipette tip. The cells were then rinsed three times with PBS and kept in serum-free DMEM/F12 conditions. At 0 and 24 h, wound closure was examined and photographed using a microscope.
Annexin V-FITC/propidium iodide (PI) double staining assay
The apoptosis detection kit (BD Biosciences, USA) was used to assess the apoptosis rate in cannabidiol-treated cells in accordance with the manufacturer’s instructions. In summary, cells were grown in 6-well plates at a density of 3.5×105 cells per well and treated with cannabidiol (10 µM) for 48 h. Cultured cells were washed twice with cold PBS, and then resuspended in 1× binding buffer. Annexin V-FITC and PI were then added to the cells, which were cultured for 15 min in the dark. Flow cytometry was used to measure the apoptotic cells (Beckman Coulter, USA).
Western blot
Western blot was performed on osteosarcoma cells and tumor tissue-derived proteins. Briefly, 10-15% sodium dodecyl sulfate polyacrylamide gel electrophoresis was used to separate equal amounts of proteins, followed by transfer of the proteins to a nitrocellulose membrane. Next, 5% skim milk in TBST was used to block non-specific binding, followed by incubation of the membrane with the indicated primary antibodies overnight at 4°C: rabbit anti-CBX2 antibody (Abcam, ab235305, Cambridge, MA, USA), rabbit anti-cleaved caspase-3 antibody (Abcam, ab184787, Cambridge, MA, USA), rabbit anti-Bax antibody (Abcam, ab182733, Cambridge, MA, USA), mouse anti-BCL2 antibody (Abcam, ab182858, Cambridge, MA, USA), and rabbit anti-SP1 antibody (Cell Signaling Technology, #9389S, USA). As an internal reference, glyceraldehyde-3-phosphate dehydrogenase antibody (Zhongshanjinqiao, TA-08 Beijing, China) was used. Membranes were rinsed three times with TBST before being incubated for 1 h at room temperature with an HRP-conjugated secondary antibody. Finally, detection was performed using the ECL western blotting detection system (BD), and a Tanon Chemiluminescence Imaging System was used to visualize the particular signals. Using the Image Lab (Bio-Rad) software, the images were opened and analyzed.
Chromatin immunoprecipitation (ChIP) assay
ChIP assay was carried out with the ChIP assay kit (Invitrogen, Carlsbad, CA, USA) following the manufacturers protocol. Chromatin solutions were sonicated and incubated with anti-SP1 antibody (Cell Signaling Technology, #18687, USA) or IgG antibody as a control, and rotated overnight at 4°C. Co-immunoprecipitated DNA was detected by qRT-PCR assay using CBX2 promoter specifc primers. RT-PCR products were separated on a 1.5% agarose gel and visualized by ethidium bromide staining. CBX2 primers (forward: 5’-CATTGTCCATTTCCCACCTC-3’; reverse: 5’-TTTCTCGGACTCCACTCACC-3’) flank the SP1 binding sites (-251 to -242 nt).
Real-time PCR analysis
Utilizing the TRIzol reagent (Invitrogen, CA, USA), total RNA was obtained from osteosarcoma cells and tumor tissues. The reverse transcription kit and SYBR Green PCR Master Mix (Dojima Hama, Osaka, Japan) were used to perform real-time PCR analysis of mRNA, as directed by the manufacturer. In addition, qRT-PCR was performed using the Applied Biosystems (Foster City, CA, USA) 7500 FAST Real-Time PCR System. The primer sequences were as follows: CBX2, forward: 5’-GCCATGTTCTTGCTACCCTG-3’; reverse: 5’-TGTGGAGGAAGAGGACGAAC-3’.
Immunohistochemistry assay
All tissues were formalin-fixed and paraffin-embedded. Five-micrometer-thick sections were obtained from the embedded tumor tissues. For immunohistochemical staining, the tissue sections underwent antigen retrieval. The slides were treated with 10% goat serum after inhibiting endogenous peroxidase activity, and then incubated with antibodies against CBX2 (1:400) and SP1 (1:400) at 4°C overnight. The secondary antibody was applied to sections after being washed with PBS three times, and diaminobenzidine (DAB) was used for staining. A microscope (Olympus DP73, Japan) was used to acquire all the images.
Statistical analyses
Two-tailed Student’s t-test and ANOVA were applied to calculate data differences. P<0.05 was defined as statistical significance. Data are expressed as the mean ± standard deviation (SD) or standard error of the mean (SEM). All analyses were performed using GraphPad Prism 7.0.
Results
Cannabidiol suppresses proliferation and migration of osteosarcoma cells
Cancer proliferation and metastasis are the primary causes of cancer-associated deaths. To study the effect of cannabidiol on the proliferation and metastasis of osteosarcoma cells, MTT, colony formation, and wound healing assays were performed. The results demonstrated that cannabidiol decreased MG63 and HOS cell viability in a dose-dependent manner, with a significant effect at 10 µM for 48 h (Figure 1A and 1B). Therefore, moving forward, we used this concentration and time. In addition, cannabidiol effectively inhibited the colony formation ability of MG63 and HOS cells at concentrations of 10, 15, and 20 µm (Figure 1C and 1D). Wound healing assays were performed to evaluate the migration ability of cells. As illustrated in Figure 1E and 1F, cannabidiol (10 µM) significantly reduced the number of MG63 and HOS cells at 24 h.
Figure 1.
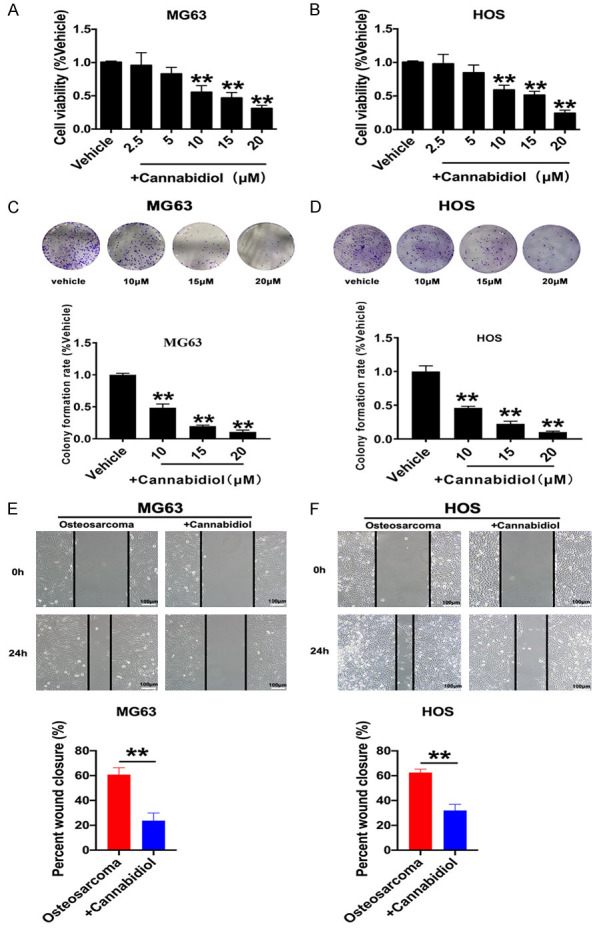
Cannabidiol suppresses osteosarcoma cell proliferation and migration. A, B. Different concentrations of cannabidiol (0, 2.5, 5, 10, 15, 20 µm) were added to MG63 and HOS cells and cultured for 48 h. Cell viability was measured by the MTT assay. **P<0.01 vs. Vehicle; n=6. C, D. Representative osteosarcoma cell colony formation images and statistical analysis of cell colony formation rate after adding different concentrations of cannabidiol. **P<0.01 vs. Vehicle; n=3. E, F. Wound closure of MG63 and HOS cells cultured with or without cannabidiol (10 µM) at 0 h and 24 h. Wound width = wound area/wound height in each group. Scale bar: 100 µm. *P<0.05, **P<0.01 vs. Vehicle; n=5. All results are presented as mean ± SEM.
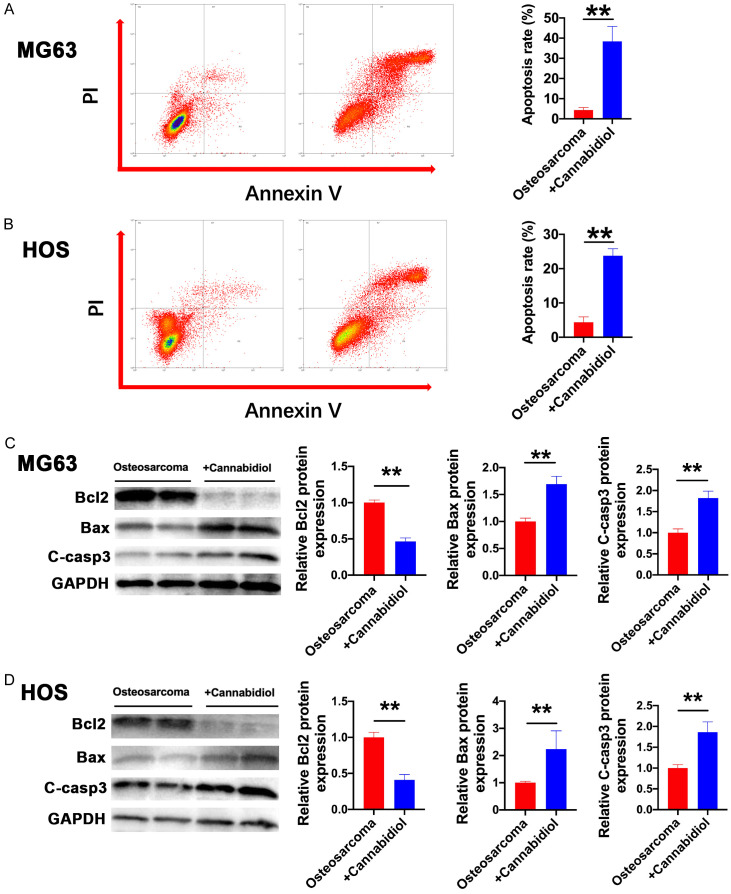
Cannabidiol enhances apoptotic cell death of osteosarcoma cells
To further differentiate the stages of apoptosis and the type of cell death, the effects of cannabidiol on cell death were determined in MG63 and HOS cells via Annexin V-FITC and PI double staining assays after 48 h of treatment. In comparison to that in the untreated group, the proportion of apoptotic MG63 and HOS cells increased substantially following cannabidiol treatment (Figure 2A and 2B). To confirm which proteins were involved in the apoptosis of MG63 and HOS cells induced by cannabidiol, we further screened proteins related to apoptosis. The protein levels of BCL2 were significantly reduced and those of BAX and cleaved caspase-3 were significantly upregulated by cannabidiol treatment for 48 h in MG63 and HOS cells (Figure 2C and 2D). These results revealed that cannabidiol induced osteosarcoma cell apoptosis by regulating BCL2/BAX/Casp3 signaling.
Figure 2.
Cannabidiol induces osteosarcoma cell apoptosis. A, B. Apoptosis of MG63 and HOS cells in response to cannabidiol treatment, as measured by flow cytometry. After cannabidiol treatment (10 µM) for 24 h, representative images (left) and the ratio (right) of apoptotic cells are shown. These histograms show the mean ± SD of three independent experiments. n=3 for each group. **P<0.01 vs. osteosarcoma. C, D. The protein levels of BCL-2, BAX, and cleaved caspase-3 in MG63 and HOS cells of the osteosarcoma and osteosarcoma + cannabidiol groups. n=3 for each group. **P<0.01 vs. osteosarcoma.
Cannabidiol significantly suppresses the tumor growth and promotes apoptosis of osteosarcoma cells in vivo
To further explore the effect of cannabidiol in vivo, we randomly divided the MG63 xenografted mice (n=4 per group) into the following two treatment groups: sham and cannabidiol. When compared to sham mice, intraperitoneal injection of 20 mg kg-1 cannabidiol once per day for 15 days resulted in a substantial reduction in average tumor size (Figure 3A and 3B). Similarly, there was a significant reduction in tumor weight in the cannabidiol treatment group (Figure 3C). These results showed that cannabidiol restrained the growth of osteosarcoma cells in vivo. Besides, we also discovered that the expression level of BCL2 decreased and those of BAX and cleaved caspase-3 increased in cannabidiol-treated tumor tissues, demonstrating clear pro-apoptosis effect (Figure 3D-F).
Figure 3.
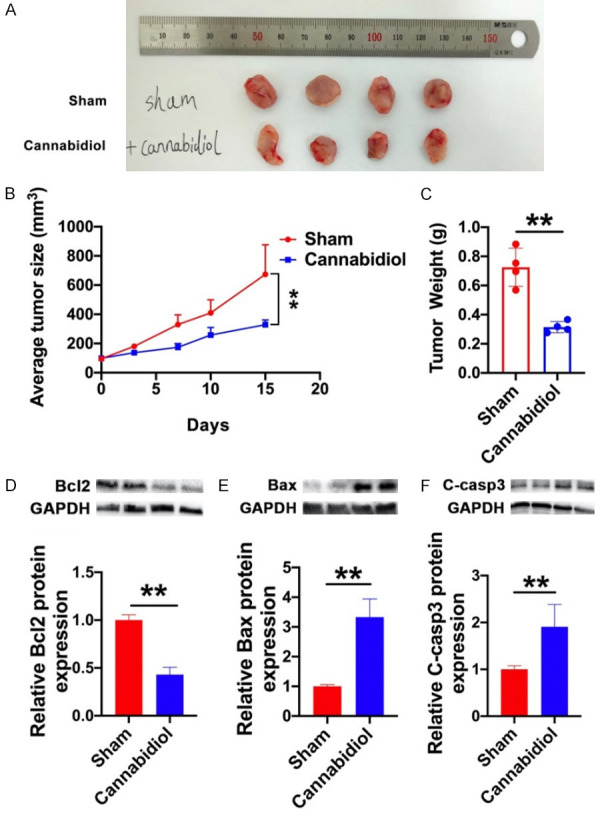
Cannabidiol influences tumor growth and apoptosis-related protein levels in established MG63 xenograft mice. After randomly grouping MG63 xenograft mice (n=4 per group), they were injected with either solvent (sham) or cannabidiol (20·mg-1·kg-1) for 15 days. A. The tumors of two groups were isolated and compared. B. Tumor growth curve of Sham group and Cannabidiol group. Tumor volume was measured every few days. C. Tumor weight was measured at the end of the experiment. D-F. The protein levels of Bcl2, BAX, and cleaved caspase-3 in the Sham and Cannabidiol groups. The data shown are mean ± SD. **P<0.01 vs. Sham.
CBX2 is upregulated in human osteosarcoma tissue
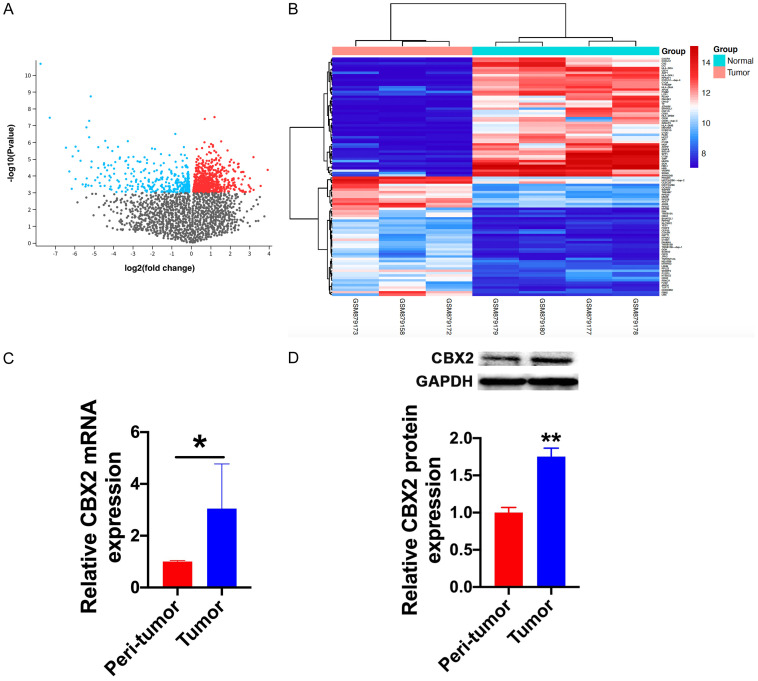
To further clarify the molecular mechanisms of cannabidiol-induced apoptosis, we assessed differentially expressed genes (DEGs) between tumor and peri-tumor samples in osteosarcoma expression microarray datasets (GSE136088). GEO2R is a data analysis system that enables the comparison of two or more sample groups in the Gene Expression Omnibus (GEO) series online to identify deregulated genes for the purpose of screening datasets. The DEGs are reflected in a volcano plot (Figure 4A) and a heat map (top 100 DEGs; Figure 4B). The expression of CBX2 in tumors was significantly elevated compared to that in peri-tumor tissues. Moreover, studies have shown that CBX2 is extensively involved in the regulation of cell apoptosis. Therefore, we hypothesized that cannabidiol regulates tumor cell apoptosis by regulating CBX2. To further confirm this assumption, RT-qPCR and western blotting were used to confirm CBX2 expression in clinical samples. CBX2 mRNA and protein levels were substantially elevated in osteosarcoma tissues as compared to those in neighboring normal tissues (Figure 4C and 4D).
Figure 4.
CBX2 is involved in the progression of osteosarcoma. A. DEGs are reflected in a volcano plot. B. Heat map of the top 100 DEGs. Red and blue indicate upregulation and downregulation, respectively. C. The mRNA levels of CBX2 in the peri-tumor and tumor groups. n=5 for each group. *P<0.05 vs. peri-tumor group. D. The protein levels of CBX2 in the peri-tumor and tumor groups. n=3 for each group. **P<0.01 vs. peri-tumor group.
Cannabidiol inhibits the protein expression of CBX2
To further verify whether CBX2 is a potential molecular target of cannabidiol in inhibiting osteosarcoma, we investigated the expression of CBX2 in the presence and absence of cannabidiol at both in vitro and in vivo levels. RT-qPCR and western blot results showed that the expression of CBX2 was lower in MG63 and HOS cells treated with cannabidiol than that in cells treated with the control solution, and the mRNA and protein levels of CBX2 were also downregulated in mouse osteosarcoma tissues treated with cannabidiol (Figure 5A-F). The results of immunohistochemistry were consistent with those of western blotting and RT-qPCR (Figure 5G).
Figure 5.
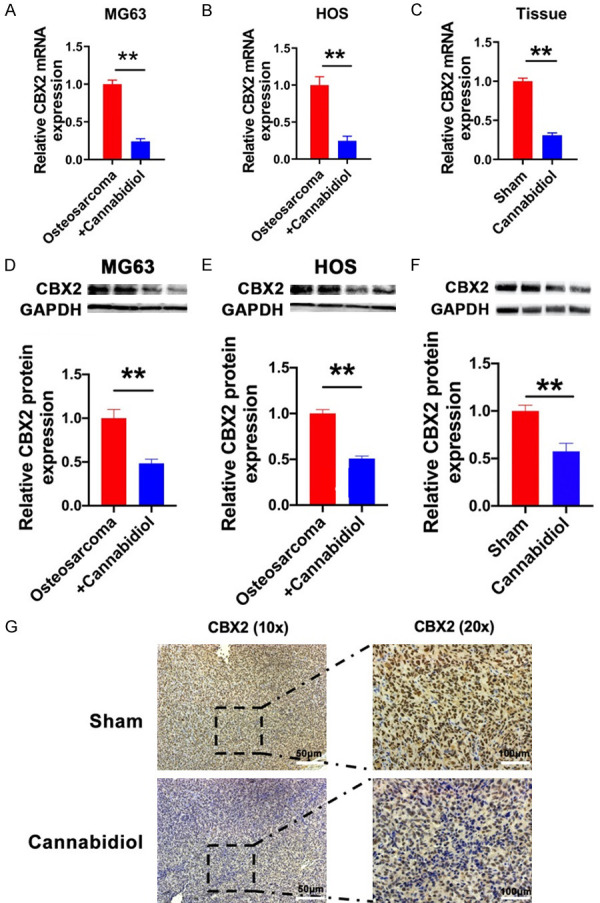
Cannabidiol regulates CBX2 in osteosarcoma. The mRNA levels of CBX2 in MG63 (A) and HOS (B) cells of the osteosarcoma and osteosarcoma + cannabidiol groups. n=5 for each group. **P<0.01 vs. osteosarcoma group. (C) The mRNA levels of CBX2 in the sham and cannabidiol groups. n=5 for each group. **P<0.01 vs. sham group. The protein expression of CBX2 in MG63 (D) and HOS (E) cells of the osteosarcoma and osteosarcoma + cannabidiol groups. n=3 for each group. **P<0.01 vs. osteosarcoma group. (F) The protein levels of CBX2 in each group. n=5 for each group. **P<0.01 vs. Sham. (G) Immunohistochemical results of CBX2 in a mouse osteosarcoma model. Scale bar: 100 µm.
CBX2 depletion induces apoptosis in osteosarcoma cell lines
To further investigate the role of CBX2 in the apoptosis of osteosarcoma cells, CBX2-siRNA was used, and the protein expression of CBX2 was reduced by approximately 42% after transfecting CBX2-siRNA for 72 h (Figure 6A). We found that CBX2 depletion in MG63 cells significantly changed the expression of apoptosis-related genes, among which the expression of BCL2 was suppressed (Figure 6B), and that of BAX (Figure 6C) and cleaved caspase-3 (Figure 6D) was increased at the protein level compared to that in the control. The experimental data showed that CBX2 was a key apoptotic inhibitory protein in osteosarcoma.
Figure 6.
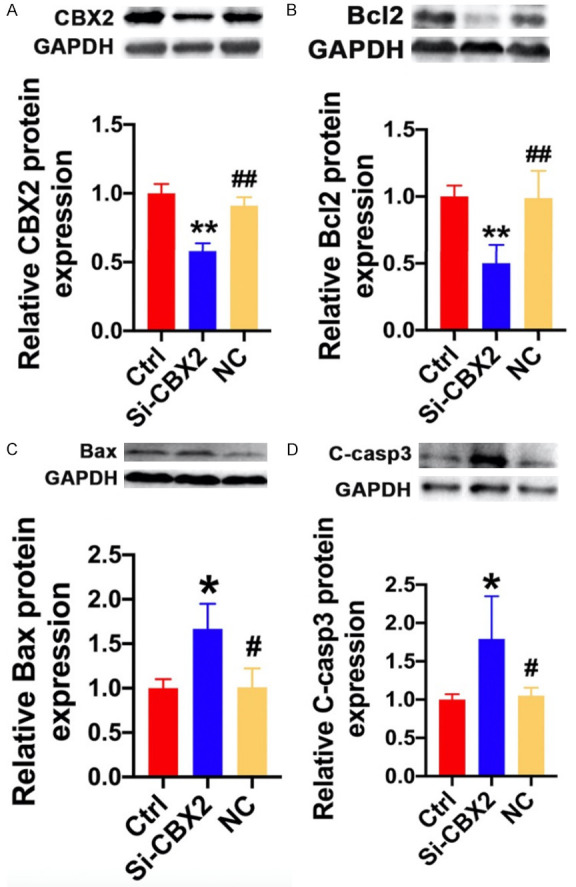
CBX2 inhibited osteosarcoma cell apoptosis. The protein levels of (A) CBX2, (B) BCL2, (C) BAX, and (D) cleaved caspase-3 in the Ctrl, CBX2-siRNA, and NC groups; n=3 for each group. mean ± SD, *P<0.05, **P<0.01 vs. Ctrl; #P<0.05, ##P<0.01 vs. CBX2-siRNA.
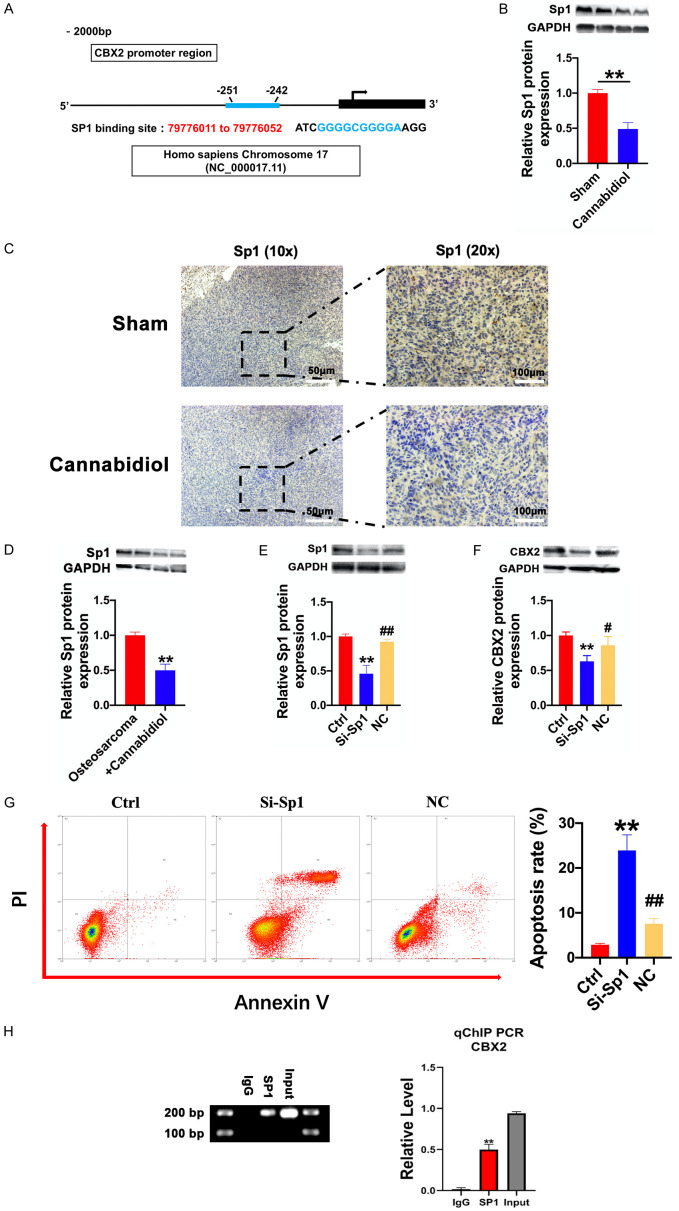
SP1 acts as an upstream regulator of CBX2 and directly binds to the CBX2 promoter
The concordant changes in CBX2 mRNA and protein levels in osteosarcoma tissues demonstrated the presence of CBX2 transcriptional regulation. The PROMO database analysis indicated that the proximal regions of CBX2 promoters (2000 bp upstream) harbor putative SP1 binding sequences, which are conserved among humans, rats, and mice (Figure 7A). Therefore, we examined the expression level of the transcription factor (SP1) protein in cannabidiol-treated tumor tissues and cell lines. As shown in Figure 7B and 7C, the protein levels of SP1 were downregulated in cannabidiol-treated tumor tissues. Similarly, the expression of SP1 in MG63 cells was decreased by cannabidiol treatment (Figure 7D). The expression of CBX2 was also suppressed at the protein level by silencing SP1 with siRNA (Figure 7E and 7F). Notably, Annexin V-FITC and PI staining assays showed that the percentage of apoptotic MG63 cells increased significantly in the SP1-siRNA transfected group compared to that in the un-transfected group (Figure 7G). In order to prove that CBX2 and SP1 have a direct effect, ChIP assay confirmed the recruitment of SP1 to the proximal promoter regions of CBX2 (Figure 7H). These results showed that SP1 regulated osteosarcoma cell apoptosis by acting as an upstream regulator of CBX2 and directly binds to the CBX2 promoter.
Figure 7.
SP1 positively regulates CBX2 protein expression by binding to the CBX2 promoter. A. Conservative SP1 DNA binding sites in the CBX2 DNA promoters. The sequences of these putative binding sites in the proximal region (2000 bp upstream) of CBX2 promoters are shown in the PROMO database. B. The protein expression of SP1 in tumor tissues of the sham and cannabidiol groups. n=6 for each group. **P<0.01 vs. Sham. C. Immunohistochemical results of SP1 in a mouse osteosarcoma model. Scale bar: 100 µm. D. The protein levels of SP1 in MG63 cells of the osteosarcoma and osteosarcoma + cannabidiol groups. n=4 for each group. **P<0.01 vs. osteosarcoma group. E. SP1-siRNA transfection efficiency in MG-63 cells; expression of SP1 protein in MG63 cells transfected with SP1-siRNA. **P<0.01 vs. Ctrl; ##P<0.01 vs. SP1-siRNA. Data are presented as mean ± SEM. F. The protein levels of CBX2 in MG63 cells of Ctrl, SP1-siRNA, and NC groups. n=6 for each group. **P<0.01 vs. CtrL; #P<0.05 vs. SP1-siRNA. Data are presented as mean ± SEM. G. The apoptosis of MG63 cells in Ctrl, SP1 siRNA, and NC groups was analyzed by flow cytometry. Representative images (left) and the ratio (right) of apoptotic cells in Ctrl, SP1-siRNA, and NC groups. The annexin V-positive/PI-negative and annexin V-positive/PI-positive fractions were plotted. The columns represent the mean ± SD of three independent experiments. n=3 for each group. **P<0.01 vs. Ctrl; ##P<0.01 vs. SP1-siRNA. H. The binding of SP1 to the proximal promoter regions of CBX2 DNA in MG-63 cells was determined using ChIP and quantitative chromatin immunoprecipitation (qChIP) assays. All values were initially expressed relative to relevant IgG DNA content. **P<0.01 vs. IgG.
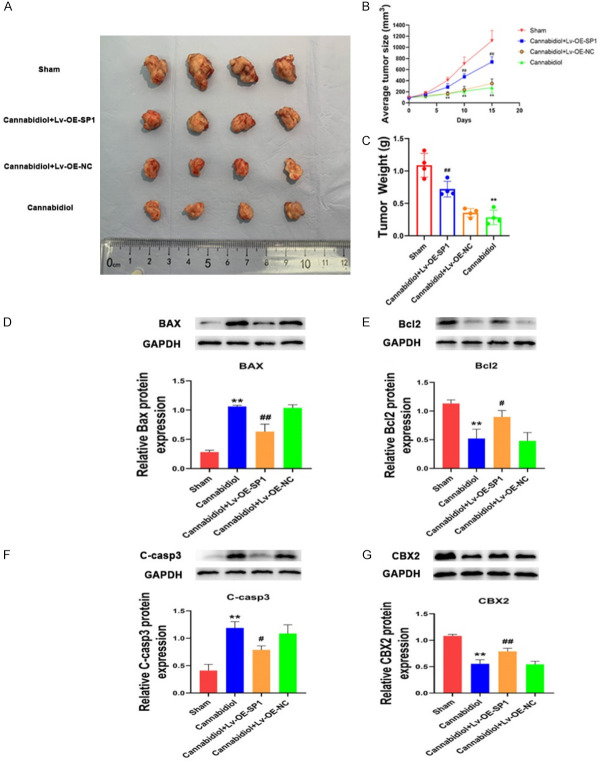
SP1 overexpression reverses the pro-apoptotic effect of cannabidiol in vivo
To further investigate the role of SP1-CBX2 pathway in apoptosis-promoting effect of cannabidiol at the animal levels. Female BALB/c nude mice were randomly divided into four groups: sham group, cannabidiol group, cannabidiol + SP1 overexpression lentivirus (Lv-OE-SP1) group and cannabidiol + negative control lentivirus (Lv-OE-NC) group. Cannabidiol treatment significantly decreased the growth of xenograft tumors in nude mice, whereas SP1 overexpression can partially reverse this phenotype (Figure 8A-C). The tumor tissues showed that the regulatory effect of cannabidiol on the protein levels of apoptosis-related Bax, Bcl-2 and cleaved caspase-3 were reversed by SP1 overexpression (Figure 8D-F). In addition, SP1 overexpression also reversed the downregulation of CBX2 by cannabidiol administration (Figure 8G). These findings suggested that the apoptosis-promoting effect of cannabidiol was mediated by SP1-CBX2 axis at the animal levels.
Figure 8.
SP1 overexpression reverses the pro-apoptotic effect of cannabidiol in vivo. A. The tumors of four groups were isolated and compared. B. Tumor growth curve of Sham group, Cannabidiol + Lv-OE-SP1 group, Cannabidiol + Lv-OE-NC group and Cannabidiol group (n=4 per group). Tumor volume was measured every few days. C. Tumor weight was measured at the end of the experiment. D-G. The protein levels of BAX, Bcl2, cleaved caspase-3 and CBX2 in the Sham group, Cannabidiol group, Cannabidiol + Lv-OE-SP1 group, and Cannabidiol + Lv-OE-NC group. The data shown are mean ± SD. **P<0.01 vs. Sham; #P<0.05, ##P<0.01 vs. Cannabidiol + Lv-OE-NC.
Discussion
In this research, we demonstrated that cannabidiol promoted apoptosis of osteosarcoma cells, and thus, showed for the first time that it effectively decreased tumor size and weight in MG63 xenograft mice by targeting the SP1-CBX2 axis (Figure 9). The main findings of the present study include the following: (1) Cannabidiol suppressed the proliferation and migration of MG63 and HOS cells in a concentration-dependent manner. (2) Cannabidiol suppressed tumor growth and promoted apoptosis via SP1-CBX2 axis in MG63 xenograft mice. (3) Cannabidiol promoted apoptosis of osteosarcoma cells by downregulating the protein expression of CBX2. (4) SP1 acted as an upstream transcriptional regulator of CBX2. This indicates the potential application of cannabidiol in the prevention and treatment of osteosarcoma.
Figure 9.
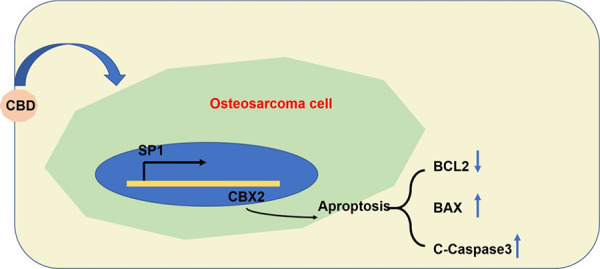
Schematic diagram of the mechanism of cannabidiol inhibiting osteosarcoma proliferation and promoting apoptosis of osteosarcoma cells by activating the SP1-CBX2 axis.
Osteosarcoma is a rare malignant bone tumor that can directly produce bone-like tissue. It is more common in adolescents and ranks first in the incidence of primary malignant bone tumors [22]. The etiology of osteosarcoma remains obscure in most patients, which may be associated with rapid bone proliferation, radiation exposure, and hereditary factors [23-25]. Currently, there are many limitations to the treatment of osteosarcoma. Although surgical treatment combined with postoperative chemotherapy and radiotherapy can cure some patients, local recurrence and post-reconstruction complications are common, presenting a significant challenge to treatment [26,27]. At present, there are several chemotherapeutic drugs for osteosarcoma, including doxorubicin [28], high-dose methotrexate [29], cisplatin [30], and ifosfamide. However, neoadjuvant chemotherapy has entered a plateau after decades of development. Even with combination chemotherapy, dose increase, or administration route changes, many patients still have poor chemotherapy response and severe side effects. Therefore, it is urgent to seek effective and low-toxicity drugs for the treatment of osteosarcoma.
Cannabidiol is the second most abundant type of cannabinoid, which has non-psychoactive effects and is advantageous for clinical applications compared to tetrahydrocannabinol [31,32]. Cannabidiol has neuroprotective, analgesic, anti-emetic, anti-spasmodic, and anti-inflammatory effects, and numerous studies have indicated that cannabidiol has antineoplastic potential both in vitro in various cell lines, and in vivo in several animal models [33]. Previous studies have demonstrated that cannabidiol has no significant cytotoxicity on normal tissues/cells, which proves the safety of cannabidiol as an anti-tumor drug [34]. Cannabidiol has high safety and has been used in clinical practice in some diseases. Both the US Food and Drug Administration (FDA) and the European Medicines Agency (EMA) have authorized cannabidiol (CBD) as an add-on treatment for two uncommon children epilepsies: Dravet Syndrome and Lennox-Gastaut Syndrome [35]. In clinical studies of malignant brain tumors, cannabidiol treatment concomitant to the standard therapeutic procedure has been shown to be effective in prolonging patients’ mean survival time [36]. However, the effects of cannabidiol on osteosarcoma have not yet been addressed. This study aimed at investigating the pharmacological actions of cannabidiol on osteosarcoma. We found that cannabidiol not only inhibited the cell viability of both MG63 cell lines and HOS cell lines in a concentration-dependent manner, but also induced apoptosis. To clarify the underlying molecular mechanisms of cannabidiol-induced apoptosis, we screened the genes involved in osteosarcoma through GEO databases. The results demonstrated that the mRNA and protein levels of CBX2 were substantially elevated in osteosarcoma tissues, MG63 xenograft mice, and osteosarcoma cell lines (MG63 cells and HOS cells), and that increasing CBX2 has anti-apoptotic potential via the expression of the transcription factor, SP1. Additionally, we found that SP1 was increased in osteosarcoma cells, which was suppressed by cannabidiol therapy. We validated SP1 binding to the proximal promoter regions of CBX2 based on the conservative binding sites of SP1 in the promoter region of CBX2 predicted by bioinformatics and ChIP assay. The results of in vivo experiment showed that cannabidiol could effectively inhibit tumor growth through the SP1-CBX2 axis, and rescue experiments also proved this result. These suggest that the SP1-CBX2 signaling pathway is involved in the pro-apoptotic effects of cannabidiol in vitro and in vivo. For the mechanism by which cannabidiol reduces the expression of SP1, we conjecture that there are two reasons. Cannabinoids (including cannabidiol and Δ-9-tetrahydrocannabinol) are hydrophobic compounds produced by the cannabis plant. The chemical structures of cannabidiol and Δ-9-tetrahydrocannabinol are very similar, differing only by the presence of an additional free hydroxyl group on one of the rings in cannabidiol (in Δ-9-tetrahydrocannabinol the equivalent oxygen forms part of a closed pyran ring) [37]. That hydroxyl group is the one which forms the hydrogen bond present in the cannabidiol-protein complex, and provides an additional intermolecular interaction for cannabidiol, which could account for the functional effects of the cannabidiol on SP1. In addition, the cannabinoid WIN 55,212-2 (WIN) induced repression of SP1 was due to protein phosphatase 2A (PP2A)-dependent downregulation of microRNA-27a (miR-27a) and induction of miR-27a-regulated zinc finger and BTB domain containing 10 (ZBTB10) [38]. The cannabinoid WIN and cannabidiol have certain similarities in structure and function, so we suggest that cannabidiol may downregulate the expression of SP1 in a similar way. In order to better understand this mechanism, further studies will be necessary. We will continue to explore this in future studies.
In conclusion, we revealed for the first time that cannabidiol treatment could not only induce apoptosis of osteosarcoma cells in vivo and in vitro, but also suppress tumor size and weight in a mouse xenograft model by regulating the SP1-CBX2 pathway. These findings provide evidence that cannabidiol has anti-tumor effects and highlight a new potential application of cannabidiol in osteosarcoma treatment.
Acknowledgements
This study was supported by the National Natural Science Foundation of China (No. 82072472). We wish to thank the Key Laboratory of Myocardial Ischemia, Harbin Medical University, Ministry of Education for providing experimental equipment and technical support.
Disclosure of conflict of interest
None.
References
- 1.Liao J, Han R, Wu Y, Qian Z. Review of a new bone tumor therapy strategy based on bifunctional biomaterials. Bone Res. 2021;9:18. doi: 10.1038/s41413-021-00139-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zheng D, Liu W, Xie W, Huang G, Jiang Q, Yang Y, Huang J, Xing Z, Yuan M, Wei M, Li Y, Yin J, Shen J, Shi Z. AHA1 upregulates IDH1 and metabolic activity to promote growth and metastasis and predicts prognosis in osteosarcoma. Signal Transduct Target Ther. 2021;6:25. doi: 10.1038/s41392-020-00387-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aljubran AH, Griffin A, Pintilie M, Blackstein M. Corrigendum to ‘Osteosarcoma in adolescents and adults: survival analysis with and without lung metastases’: annals of Oncology 2009; 20: 1136-1141. Ann Oncol. 2021;32:424. doi: 10.1016/j.annonc.2020.12.011. [DOI] [PubMed] [Google Scholar]
- 4.Zhong L, Liao D, Li J, Liu W, Wang J, Zeng C, Wang X, Cao Z, Zhang R, Li M, Jiang K, Zeng YX, Sui J, Kang T. Rab22a-NeoF1 fusion protein promotes osteosarcoma lung metastasis through its secretion into exosomes. Signal Transduct Target Ther. 2021;6:59. doi: 10.1038/s41392-020-00414-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Quadros M, Momin M, Verma G. Design strategies and evolving role of biomaterial assisted treatment of osteosarcoma. Mater Sci Eng C Mater Biol Appl. 2021;121:111875. doi: 10.1016/j.msec.2021.111875. [DOI] [PubMed] [Google Scholar]
- 6.Luengo A, Li Z, Gui DY, Sullivan LB, Zagorulya M, Do BT, Ferreira R, Naamati A, Ali A, Lewis CA, Thomas CJ, Spranger S, Matheson NJ, Vander Heiden MG. Increased demand for NAD relative to ATP drives aerobic glycolysis. Molecular cell. 2021;81:691–707. e6. doi: 10.1016/j.molcel.2020.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang M, Luo JL, Sun Q, Harber J, Dawson AG, Nakas A, Busacca S, Sharkey AJ, Waller D, Sheaff MT, Richards C, Wells-Jordan P, Gaba A, Poile C, Baitei EY, Bzura A, Dzialo J, Jama M, Le Quesne J, Bajaj A, Martinson L, Shaw JA, Pritchard C, Kamata T, Kuse N, Brannan L, De Philip Zhang P, Yang H, Griffiths G, Wilson G, Swanton C, Dudbridge F, Hollox EJ, Fennell DA. Clonal architecture in mesothelioma is prognostic and shapes the tumour microenvironment. Nat Commun. 2021;12:1751. doi: 10.1038/s41467-021-21798-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arai H, Elliott A, Xiu J, Wang J, Battaglin F, Kawanishi N, Soni S, Zhang W, Millstein J, Sohal D, Goldberg RM, Hall MJ, Scott AJ, Khushman Md, Hwang JJ, Lou E, Weinberg BA, Marshall JL, Lockhart AC, Stafford P, Zhang J, Moretto R, Cremolini C, Korn WM, Lenz HJ. The landscape of alterations in DNA damage response pathways in colorectal cancer. Clin Cancer Res. 2021;27:3234–3242. doi: 10.1158/1078-0432.CCR-20-3635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Helbling-Leclerc A, Garcin C, Rosselli F. Beyond DNA repair and chromosome instability-Fanconi anaemia as a cellular senescence-associated syndrome. Cell Death Differ. 2021;28:1159–1173. doi: 10.1038/s41418-021-00764-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Khalilzadeh B, Shadjou N, Kanberoglu GS, Afsharan H, de la Guardia M, Charoudeh HN, Ostadrahimi A, Rashidi MR. Advances in nanomaterial based optical biosensing and bioimaging of apoptosis via caspase-3 activity: a review. Mikrochim Acta. 2018;185:434. doi: 10.1007/s00604-018-2980-6. [DOI] [PubMed] [Google Scholar]
- 11.Mello JB, Ramos Cirilo PD, Michelin OC, Custódio Domingues MA, Cunha Rudge MV, Rogatto SR, Maestá I. Genomic profile in gestational and non-gestational choriocarcinomas. Placenta. 2017;50:8–15. doi: 10.1016/j.placenta.2016.12.009. [DOI] [PubMed] [Google Scholar]
- 12.Jangal M, Lebeau B, Witcher M. Beyond EZH2: is the polycomb protein CBX2 an emerging target for anti-cancer therapy? Expert Opin Ther Targets. 2019;23:565–578. doi: 10.1080/14728222.2019.1627329. [DOI] [PubMed] [Google Scholar]
- 13.Han Q, Li C, Cao Y, Bao J, Li K, Song R, Chen X, Li J, Wu X. CBX2 is a functional target of miRNA let-7a and acts as a tumor promoter in osteosarcoma. Cancer Med. 2019;8:3981–3991. doi: 10.1002/cam4.2320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fernando N, Sciumè G, O’Shea JJ, Shih HY. Multi-dimensional gene regulation in innate and adaptive lymphocytes: a view from regulomes. Front Immunol. 2021;12:655590. doi: 10.3389/fimmu.2021.655590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Koutsodontis G, Tentes I, Papakosta P, Moustakas A, Kardassis D. Sp1 plays a critical role in the transcriptional activation of the human cyclin-dependent kinase inhibitor p21 (WAF1/Cip1) gene by the p53 tumor suppressor protein. J Bio Chem. 2001;276:29116–29125. doi: 10.1074/jbc.M104130200. [DOI] [PubMed] [Google Scholar]
- 16.Ding Q, Mo F, Cai X, Zhang W, Wang J, Yang S, Liu X. LncRNA CRNDE is activated by SP1 and promotes osteosarcoma proliferation, invasion, and epithelial-mesenchymal transition via Wnt/β-catenin signaling pathway. J Cell Biochem. 2020;121:3358–3371. doi: 10.1002/jcb.29607. [DOI] [PubMed] [Google Scholar]
- 17.Seltzer ES, Watters AK, MacKenzie D, Granat LM, Zhang D. Cannabidiol (CBD) as a promising anti-cancer drug. Cancers. 2020;12:3203. doi: 10.3390/cancers12113203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shrivastava A, Kuzontkoski PM, Groopman JE, Prasad A. Cannabidiol induces programmed cell death in breast cancer cells by coordinating the cross-talk between apoptosis and autophagy. Mol Cancer Ther. 2011;10:1161–1172. doi: 10.1158/1535-7163.MCT-10-1100. [DOI] [PubMed] [Google Scholar]
- 19.Massi P, Vaccani A, Bianchessi S, Costa B, Macchi P, Parolaro D. The non-psychoactive cannabidiol triggers caspase activation and oxidative stress in human glioma cells. Cell Mol Life Sci. 2006;63:2057–2066. doi: 10.1007/s00018-006-6156-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sharma A, Kumar A, Li C, Panwar Hazari P, Mahajan SD, Aalinkeel R, Sharma RK, Swihart MT. A cannabidiol-loaded Mg-gallate metal-organic framework-based potential therapeutic for glioblastomas. J Mater Chem B. 2021;9:2505–2514. doi: 10.1039/d0tb02780d. [DOI] [PubMed] [Google Scholar]
- 21.Kis B, Ifrim FC, Buda V, Avram S, Pavel IZ, Antal D, Paunescu V, Dehelean CA, Ardelean F, Diaconeasa Z, Soica C, Danciu C. Cannabidiol-from plant to human body: a promising bioactive molecule with multi-target effects in Cancer. Int J Mol Sci. 2019;20:5905. doi: 10.3390/ijms20235905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ma B, Zhu J, Zhao A, Zhang J, Wang Y, Zhang H, Zhang L, Zhang Q. Raddeanin A, a natural triterpenoid saponin compound, exerts anticancer effect on human osteosarcoma via the ROS/JNK and NF-κB signal pathway. Toxicol Appl Pharmacol. 2018;353:87–101. doi: 10.1016/j.taap.2018.05.025. [DOI] [PubMed] [Google Scholar]
- 23.Yang C, Tian Y, Zhao F, Chen Z, Su P, Li Y, Qian A. Bone microenvironment and osteosarcoma metastasis. Int J Mol Sci. 2020;21:6985. doi: 10.3390/ijms21196985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hmada YA, Bernieh A, Morris RW, Lewin J, Allen T. Chondroblastoma-like Osteosarcoma. Arch Pathol Lab Med. 2020;144:15–17. doi: 10.5858/arpa.2019-0191-RA. [DOI] [PubMed] [Google Scholar]
- 25.Corre I, Verrecchia F, Crenn V, Redini F, Trichet V. The osteosarcoma microenvironment: a complex but targetable ecosystem. Cells. 2020;9:976. doi: 10.3390/cells9040976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reed DR, Hayashi M, Wagner L, Binitie O, Steppan DA, Brohl AS, Shinohara ET, Bridge JA, Loeb DM, Borinstein SC, Isakoff MS. Treatment pathway of bone sarcoma in children, adolescents, and young adults. Cancer. 2017;123:2206–2218. doi: 10.1002/cncr.30589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Crompton JG, Ogura K, Bernthal NM, Kawai A, Eilber FC. Local control of soft tissue and bone sarcomas. J. Clin. Oncol. 2018;36:111–117. doi: 10.1200/JCO.2017.75.2717. [DOI] [PubMed] [Google Scholar]
- 28.Buondonno I, Gazzano E, Tavanti E, Chegaev K, Kopecka J, Fanelli M, Rolando B, Fruttero R, Gasco A, Hattinger C, Serra M, Riganti C. Endoplasmic reticulum-targeting doxorubicin: a new tool effective against doxorubicin-resistant osteosarcoma. Cell Mol Life Sci. 2019;76:609–625. doi: 10.1007/s00018-018-2967-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang B, Zhang Y, Li R, Li J, Lu X, Zhang Y. The efficacy and safety comparison of first-line chemotherapeutic agents (high-dose methotrexate, doxorubicin, cisplatin, and ifosfamide) for osteosarcoma: a network meta-analysis. J Orthop Surg Res. 2020;15:51. doi: 10.1186/s13018-020-1576-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu Q, Wang K. The induction of ferroptosis by impairing STAT3/Nrf2/GPx4 signaling enhances the sensitivity of osteosarcoma cells to cisplatin. Cell Biol Int. 2019;43:1245–1256. doi: 10.1002/cbin.11121. [DOI] [PubMed] [Google Scholar]
- 31.Lukhele ST, Motadi LR. Cannabidiol rather than cannabis sativa extracts inhibit cell growth and induce apoptosis in cervical cancer cells. BMC Complement Altern Med. 2016;16:335. doi: 10.1186/s12906-016-1280-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Huang CC, Tung TH, Huang CC, Lin SY, Chao SC, Chiu SP, Lee SP, Lo CM. Electrochemical assessment of anticancer compounds on the human tongue squamous carcinoma cells. Sensors (Basel) 2020;20:2632. doi: 10.3390/s20092632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McAllister SD, Soroceanu L, Desprez PY. The antitumor activity of plant-derived non-psychoactive cannabinoids. J Neuroimmune Pharmacol. 2015;10:255–267. doi: 10.1007/s11481-015-9608-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bergamaschi MM, Queiroz RH, Zuardi AW, Crippa JA. Safety and side effects of cannabidiol, a cannabis sativa constituent. Curr Drug Saf. 2011;6:237–249. doi: 10.2174/157488611798280924. [DOI] [PubMed] [Google Scholar]
- 35.Wise J. European drug agency approves cannabis-based medicine for severe forms of epilepsy. BM. 2019;366:l5708. doi: 10.1136/bmj.l5708. [DOI] [PubMed] [Google Scholar]
- 36.Likar R, Koestenberger M, Stultschnig M, Nahler G. Concomitant treatment of malignant brain tumours with CBD-a case series and review of the literature. Anticancer Res. 2019;39:5797–5801. doi: 10.21873/anticanres.13783. [DOI] [PubMed] [Google Scholar]
- 37.Sait LG, Sula A, Ghovanloo MR, Hollingworth D, Ruben PC, Wallace BA. Cannabidiol interactions with voltage-gated sodium channels. Elife. 2020;9:e58593. doi: 10.7554/eLife.58593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sreevalsan S, Safe S. The cannabinoid WIN 55,212-2 decreases specificity protein transcription factors and the oncogenic cap protein eIF4E in colon cancer cells. Mol Cancer Ther. 2013;12:2483–2493. doi: 10.1158/1535-7163.MCT-13-0486. [DOI] [PMC free article] [PubMed] [Google Scholar]