Abstract
AIS is a heterogeneous 3D spinal deformity with Cobb angle ≥10°. It affects children in the age group of 10-16 years globally with 2-3% prevalence and significant female predominance. The exact etiology of AIS is not known however, it is supposed to be associated with factors such as anthropometric, metabolic, neuromuscular abnormalities and genetics. Objectives: To determine the prevalence of AIS and association of anthropometric factors with AIS in the studied population group. Methodology: Scoliosis screening of 9,500 individuals was carried out at different educational institutions of Jammu region in Jammu and Kashmir, India using a scoliosis-meter. The subjects were later examined radiologically. Results: In population of the region, AIS was most prevalent among all types of scoliosis with overall prevalence of 0.61%. The prevalence was observed to be lower in females (0.31%) than males (0.88%). Based on angle of trunk rotation (ATR), lumbar curves were more prevalent than thoracic curves. Average Cobb angle in males and females were 24.9° and 22.6°, respectively. BMI showed significant association with AIS in the age group of 12-16 years (P value =0.028). Furthermore, height was significantly associated with AIS in the overall screened population (P-value =0.029). Conclusions: The AIS patients in the Jammu region of India have unique clinical features. In contrast to the global prevalence data, the prevalence of AIS in females in the region was less in comparison to males. Based on epidemiological literature and our findings, we hypothesized that genetic factors might be a major contributor in the AIS pathogenesis along with other confounding factors such as height, BMI, ethnicity, etc.
Keywords: Adolescent idiopathic scoliosis, prevalence, Cobb angle, BMI, angle of trunk rotation, Jammu and Kashmir
Introduction
Adolescent idiopathic scoliosis (AIS) is a common spinal disorder that develops between 10 to 16 years of age and is characterized by a lateral curvature of spine with Cobb angle of more than 10°. There has been a wide variation in the prevalence of AIS in different populations of the world. The prevalence of AIS in general population lies between 2-4% [1]. Globally, AIS is more prevalent in females than in males with female/male ratio of 1.5 to 11 [2]. About 2-3% adolescent population have scoliosis with a Cobb angle of greater than 10°, 0.3-0.5% population with a Cobb angle of more than 20°, and less than 0.1% of the population have severe scoliosis, i.e., with a Cobb angle of more than 40° [3]. The prevalence of the AIS varies with the geography [2] and it is reported more in the northern latitudes than in the lower latitudes [4]. However, the incidence varied in different studies and populations of the world [5-14].
Despite being idiopathic there are several factors related to the possible etiology of AIS including hormones, biomechanics, anthropometric, metabolic, growth and neuromuscular abnormalities [15]. In addition to this, AIS is strongly considered to be associated with the genetic factors [16]. For centuries, the underlying genetic, cellular and molecular basis of AIS has remained uncertain. However, new approaches to genomics and system biology are helping to illuminate the genetic modifications underlying AIS risk [2]. The AIS heritability is indicated by its higher prevalence in the first degree relatives of individuals with scoliosis within a family than in general population and by the twin studies that show its higher concordance rate in the monozygotic twins than in dizygotic twins [3,17,18]. Certain genes that were identified with the pathogenesis of AIS on the basis of GWAS, whole-genome and exome sequencing as well as candidate gene approach include LBX1, GPR126, BNC2, CHL1, PAX1 and others, alike [19-25]. Candidate gene association studies have reported many genes which may play a functional role in the development of AIS. Some of the candidate genes that were evaluated for the susceptibility of AIS were supposed to be involved in different pathways like bone formation and bone metabolism, connective tissue structure, puberty and growth hormones, neurological and melatonin signaling pathways [26].
Early detection of AIS helps in preventing further progression of scoliosis in an individual either by non-surgical (bracing) or by surgical methods [27]. For early detection, school-based screening is an effective method and is recommended by the American Academy of Pediatrics, American Academy of Orthopaedic Surgeons and the Pediatric Orthopaedic Society of North America [28]. The Adam’s forward bend test and scoliometer measurement are used for scoliosis screening. The latter gives the angle of trunk rotation (ATR). The individual is considered to be scoliotic if the scoliosis meter reading is ≥7°, and the final confirmation for scoliosis is done by radiographic diagnosis [4].
The annual scoliosis screening of all school aged children (preferred between 12-18 years) has been recommended by the Scoliosis Research Society (https://www.srs.org/) [4] and is a common practice in most developed countries. However, in India, so far, prevalence of scoliosis has been reported from only two small geographical regions (0.13% in Patiala city of Punjab and 0.2% in Assam) [29,30].
Lack of epidemiological studies on scoliosis explains its unawareness and ignorance in India. The present school screening study was performed in 125 educational institutions comprising 9,500 individuals to determine the prevalence of the AIS in Jammu region of India by using Adam’s forward bend test and radiological verification. Further, it was examined that whether or not the prevalence of scoliosis was influenced by gender in our population. We have also evaluated the possible association of AIS with anthropometric factors such as BMI, height and weight.
Materials and methods
Preparatory work
The study was approved by Institutional Ethics Review Board (IERB), Shri Mata Vaishno Devi University (IERB serial no: SMVDU/IERB/18/69) and communicated to Human Genetics Research Group (Figure S1). For carrying out awareness of scoliosis in target population, simplified brochures were designed which contained all the relevant information about scoliosis including its causes and consequences. Screening camps at different districts of Jammu region were held in collaboration with registered non-government organizations.
The parents of the children were informed a week before the screening by means of letters regarding the intentions of the study, its significance and the method of screening and diagnosis. Additionally, school authorities were also provided with the datasheet and consent form so as to get their consents and to have an idea about the parameters and the information collected during screening. Prior to the screening at schools, briefing up of the screening process and the details required during screening were properly demonstrated to the students.
Scoliosis screening program
The screening was carried out by the team of university researchers, trained in measuring the angle of trunk rotation using scoliosis-meter (Baseline®, USA), in 120 schools and 5 higher educational institutions located in various districts of the Jammu region of J&K. The students from the age group of 10 to 28 years were screened by adopting the random sampling method. The screening was carried out in a separate room using the Adam’s forward bend test, where an individual bends forward while his/her feet and hands joined together or feet kept shoulder width apart. Any asymmetry of the shoulder level or waist line was measured using scoliosis meter which ultimately gives the ATR.
Information about age, gender, ethnicity, family history, ancestral place and anthropometric measurements (including height, weight and BMI) of the children were collected. The status of neurological, muscular or skeletal diseases was also recorded. The angle of trunk rotation of 7° was taken as the cut off value for the scoliosis.
Radiological examination
The individuals with ATR ≥7° were referred to the hospitals for the confirmation of the condition by radiological examination. Allis test was done by clinicians to rule out any functional scoliosis that may arise because of leg length discrepancy followed by the X-ray of the complete pelvis to look for any malformation or injury in the pelvic region. Antero-posterior and lateral X-rays of the spine were taken for the confirmation of the scoliosis. Cobb angle of 10° or more was considered to be scoliotic. Vertebral anomalies were checked in the X-ray reports to rule out congenital scoliosis.
Statistical analyses
All statistical analysis was carried out using Microsoft Excel 2013, R programming on R studio version 1.2.5033 and SPSS v.23.0. Prevalence of AIS has been evaluated in the male, female and in the overall population using Microsoft Excel 2013. To evaluate the association of different confounding factors (anthropometric factors) with AIS multiple statistical tests were performed. The independent t-test was carried out to evaluate the association of anthropometric factors including height, weight and BMI with AIS in overall screened population and in the age group of 12 to 16 years. The test was aimed to determine whether the difference in the means of the confounding factors in cases and controls were statistically significant or not. To evaluate the strong relationship between anthropometric factors (BMI, height and weight) with ATR in cases, Pearson’s correlation coefficient was calculated using SPSS. The level of significance of ≤0.05 was considered as a criterion for the statistical significance for all statistical analysis. 3D scatter plot was generated using R studio to visualize the distribution of BMI in cases and controls in the age group of 12-16 years.
Results
In this study, 9,500 individuals participated in the screening program from various regions of the Jammu. There were 5,001 males and 4,499 females. The overall prevalence of scoliosis (including AIS, infantile, congenital and functional scoliosis) was 0.67%, whereas the prevalence of AIS was 0.61%. The prevalence of idiopathic scoliosis in males was 0.88%, whereas that of female was 0.31% (Table 1). The male/female ratio was 1.1:1 in the screened population. The population screened was ethnically diverse comprising several ethnic groups of J&K, including Rajputs, Brahmins, Scheduled Castes, Scheduled Tribes, Kashmiri Pandits and Kashmiri Muslims. We did not find association of scoliosis with any particular ethnic group.
Table 1.
Prevalence of scoliosis in the Jammu and Kashmir population
| Gender | No. of Individuals screened | No. of Scoliosis cases | No. of AIS cases | Prevalence of AIS (%) |
|---|---|---|---|---|
| Male | 5,001 | 47 | 44 | 0.88 |
| Female | 4,499 | 17 | 14 | 0.31 |
| Total | 9,500 | 64 | 58 | 0.61 |
AIS: adolescent idiopathic scoliosis.
Our study mainly focused on AIS and it was found to be more prevalent than other types of scoliosis in both males and females in this region (Table 2). We excluded all other types of scoliosis based on the clinical evaluation. Cases excluded from our study comprised two congenital scoliosis cases where vertebral fusion was the major reason. There were two kyphoscoliosis cases having congenital vertebral anomaly with profound kyphosis, two cases of functional scoliosis who had leg length discrepancy, and also a case with infantile idiopathic scoliosis who was diagnosed at the age of 2 years and had strong family history of scoliosis (Table 2). Notably, AIS is less prevalent in females than in males in contrast to the data reported elsewhere in the world [2].
Table 2.
Types of scoliosis in the Jammu and Kashmir population
| Gender | Types of scoliosis cases | ||||
|---|---|---|---|---|---|
|
| |||||
| No. of Infantile | No. of Congenital | No. of Kyphoscoliosis* | No. of Functional** | No. of Idiopathic | |
| Male | 1 | 0 | 1 | 1 | 44 |
| Female | 0 | 1 | 1 | 1 | 14 |
With fused vertebrae along with profound kyphosis;
due to leg length discrepancy.
The lumbar curve was more prominent than thoracic curve in both males and females, whereas only three male subjects were observed to have double curve (Table 3). Moreover, the average Cobb angle of all individuals with idiopathic scoliosis was 25.4°. The average Cobb angle was 24.9° in males with AIS, and 22.6° in females with AIS. All the individuals who were having ATR ≥7° had Cobb angle of >10°. These results suggest that based on the Cobb angle the curvature of spine was also greater in males than in females. To evaluate the association of anthropometric factors with AIS, an independent t-test was done for factors such as, height, weight and BMI between the affected individuals and the healthy controls in overall screened population. No significant association was observed for weight and BMI among AIS patients and healthy controls, with P-value of 0.453 and 0.610 respectively. However, height showed significant association with AIS having P-value of 0.029 where the mean height was higher in cases (163.0±10.81 cm) as compared to controls (159.79±20.32 cm) in the overall screened population. As AIS develops more frequently in the adolescent age (12-16 years), in order to evaluate the possible role of anthropometric factors in the development of AIS, we narrowed down our population size by selecting the population in adolescent age group (12-16 years) from the overall population screened. The association of anthropometric factors (height, weight and BMI) with AIS in the adolescent age group was carried out using independent t-test between the affected individuals and healthy controls. A significant association of BMI with AIS has been observed with P-value of 0.028. The AIS patients were found to have lower BMI (mean BMI of 17.67±2.79 kg/m2) as compared to the healthy controls (mean BMI of 19.01±4.06 kg/m2) in the adolescent age group. However, no significant associations were observed for height and weight (P-value of 0.291 and 0.302 respectively) (Table 4). A 3D scatter plot was generated to visualize the distribution of BMI among cases and controls in 12-16 years age group (Figure 1). It can be clearly seen from the graph that cases are concentrated more towards lower BMI as compare to that of controls. It was observed that majority of the cases (19) were underweight (BMI<18.5) followed by 5 cases in normal weight category with BMI in the range of 18.5 to 24.9 (Figure 1). In this study, we have also evaluated the correlation between BMI, height and weight with ATR in AIS cases. No signification association was observed for BMI with ATR (P-value =0.580, r=-0.071), height with ATR (P-value =0.415, r=-0.104) and weight with ATR (P-value =0.314, r=-0.129).
Table 3.
Curve types of AIS in the Jammu and Kashmir population
| Gender | Curve type of AIS* | ||
|---|---|---|---|
|
| |||
| No. of Thoracic | No. of Lumbar | No. of Double curve | |
| Male | 20 | 21 | 3 |
| Female | 5 | 9 | 0 |
AIS: adolescent idiopathic scoliosis.
On the basis of angle of trunk rotation.
Table 4.
Independent t-test between cases and controls for height, weight and BMI in the age group of 12-16 years and overall population (10-28 years)
| Age group | N (No. of samples) | Average Height in cm (± SD) | Average Weight in kg (± SD) | Average BMI in Kg/m2 (±SD) | |
|---|---|---|---|---|---|
| Cases | 12-16 years | 24 | 159.91 (±11.94) | 45.04 (±7.4) | 17.67 (±2.79) |
| Controls | 5518 | 157.26 (±24.25) | 46.64 (±10.19) | 19.01 (±4.06) | |
| Independent t-test (P-Value) | 0.291 | 0.302 | 0.028 | ||
| Cases | 10-28 years | 58 | 163.0 (±10.81) | 51.86 (±12.43) | 19.56 (±4.43) |
| Controls | 9436 | 159.79 (±20.32) | 50.63 (±12.38) | 19.86 (±4.36) | |
| Independent t-test (P-Value) | 0.029 | 0.453 | 0.610 |
SD: standard deviation, BMI: body mass index.
Figure 1.
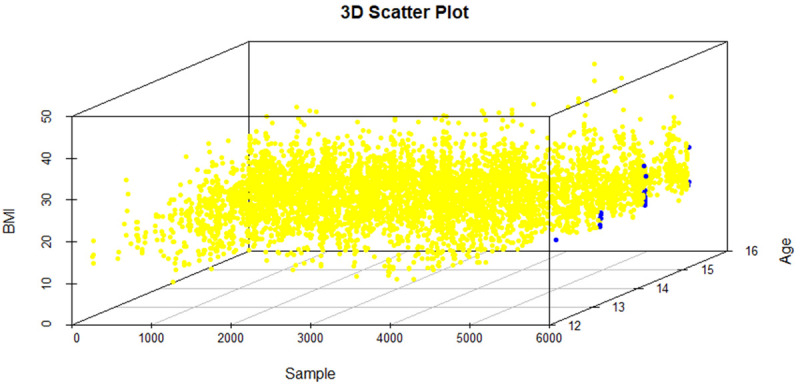
3D Scatter plot depicting the BMI of all the samples including both cases and controls in the age group of 12-16 years. Cases are represented with blue dots and controls are represented with yellow dots. It can be depicted from the figure that cases are more concentrated towards the lower BMI as compared to that of controls.
Discussion
The prevalence of scoliosis varies in different populations of the world. In India, till now the prevalence has been reported only from two small geographical regions, i.e., Patiala city of Punjab and in the state of Assam. The present study is the first report conducted for the screening of scoliosis and its prevalence in the population of J&K, India. Interestingly, we observed high prevalence of scoliosis in our target region as compared to its incidence which was 0.13% in the population of Patiala, Punjab [29] and 0.2% in Assam [30]. Similarly, studies have also reported less prevalence of scoliosis in the countries such as Japan (0.87%) [31], Saudi Arabia (0.78%) [32] and Singapore (0.27-2.49% in 9-13 years old female) [33].
In our study, we also observed that individuals in the pre-obesity category had minor ATR readings, which could be due to high soft tissue content that ultimately decreases the rotational deformity measured by Adam’s forward bend test [34].
In order to identify the predisposing factors of AIS, we examined the association of anthropometric factors (height, weight and BMI) with the AIS and observed the significant association of height with AIS in overall population and BMI with AIS in the children of adolescent age, i.e., 12-16 years when the AIS develops more frequently. However, the level of significance was not very high which suggested that height and BMI may act as a confounding factor for the manifestation of AIS but is not the only contributory factor for the AIS predisposition. Based on the findings reported in the literature [16], we hypothesize that genetic factors might be a major contributor in the pathogenesis of the AIS along with other confounding factors such as height, BMI, ethnicity, etc.
Conclusion
This study is the first report of AIS in the population of Jammu region, J&K, India. The prevalence of AIS was lower than the expected prevalence of the AIS globally with less prevalence of scoliosis in females (0.31%) than in males (0.88%). The confounding factor may not be the major contributor for the pathogenesis of AIS; instead genetics may play a major role in the AIS predisposition. The male prevalent AIS in our population could be attributed to the population structure and gene pool of the population group.
Future perspectives
As the prevalence of AIS observed in our study is contrasting to previous literature, it becomes pertinent to screen large sample size to confirm the result as well as replicate the study in another independent population group from India. Genetic studies are warranted to determine the genetic predisposition of AIS in our population group as well as to understand the cause of less prevalence of female AIS better.
Acknowledgements
We are grateful to all the individuals who have participated in the study. We are thankful to Shri Mata Vaishno Devi University for providing assistantship for the Ph.D. work. We would like to acknowledge Dr. Carol A. Wise for her continuous support for carrying out this study. We are also very thankful to Ms. Parul Priya and Ms. Ayushi, Ph.D research scholars at School of Languages and Literature, Shri Mata Vaishno Devi University, for assisting us in the language editing of the manuscript.
Disclosure of conflict of interest
None.
Supporting Information
References
- 1.Tambe A, Panikkar S, Millner P, Tsirikos A. Current concepts in the surgical management of adolescent idiopathic scoliosis. Bone Joint J. 2018;100:415–424. doi: 10.1302/0301-620X.100B4.BJJ-2017-0846.R2. [DOI] [PubMed] [Google Scholar]
- 2.Cheng JC, Castelein RM, Chu WC, Danielsson AJ, Dobbs MB, Grivas TB, Gurnett CA, Luk KD, Moreau A, Newton PO, Stokes IA, Weinstein SL, Burwell RG. Adolescent idiopathic scoliosis. Nat Rev Dis Primers. 2015;1:15030. doi: 10.1038/nrdp.2015.30. [DOI] [PubMed] [Google Scholar]
- 3.SOSORT guideline committee. Weiss HR, Negrini S, Rigo M, Kotwicki T, Hawes MC, Grivas TB, Maruyama T, Landauer F. Indications for conservative management of scoliosis (guidelines) Scoliosis. 2006;1:5. doi: 10.1186/1748-7161-1-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Adobor RD, Rimeslatten S, Steen H, Brox JI. School screening and point prevalence of adolescent idiopathic scoliosis in 4000 Norwegian children aged 12 years. Scoliosis. 2011;6:23. doi: 10.1186/1748-7161-6-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chan A, Moller J, Vimpani G, Paterson D, Southwood R, Sutherland A. The case for scoliosis screening in Australian adolescents. Med J Aust. 1986;145:379–383. doi: 10.5694/j.1326-5377.1986.tb112390.x. [DOI] [PubMed] [Google Scholar]
- 6.Penha PJ, Ramos NLJP, de Carvalho BKG, Andrade RM, Schmitt ACB, João SMA. Prevalence of adolescent idiopathic scoliosis in the State of São Paulo, Brazil. Spine (Phila Pa 1976) 2018;43:1710–1718. doi: 10.1097/BRS.0000000000002725. [DOI] [PubMed] [Google Scholar]
- 7.Hengwei F, Zifang H, Qifei W, Weiqing T, Nali D, Ping Y, Junlin Y. Prevalence of idiopathic scoliosis in Chinese schoolchildren: a large, population-based study. Spine (Phila Pa 1976) 2016;41:259–264. doi: 10.1097/BRS.0000000000001197. [DOI] [PubMed] [Google Scholar]
- 8.Zheng Y, Dang Y, Wu X, Yang Y, Reinhardt JD, He C, Wong M. Epidemiological study of adolescent idiopathic scoliosis in Eastern China. J Rehabil Med. 2017;49:512–519. doi: 10.2340/16501977-2240. [DOI] [PubMed] [Google Scholar]
- 9.Koukourakis I, Giaourakis G, Kouvidis G, Kivernitakis E, Blazos J, Koukourakis M. Screening school children for scoliosis on the island of Crete. J Spinal Disord. 1997;10:527–531. [PubMed] [Google Scholar]
- 10.Yawn BP. School-screening for scoliosis. A prospective epidemiological study in northwestern and central Greece. J Bone Joint Surg Am. 1998;80:1244. [PubMed] [Google Scholar]
- 11.Suh SW, Modi HN, Yang JH, Hong JY. Idiopathic scoliosis in Korean schoolchildren: a prospective screening study of over 1 million children. Eur Spine J. 2011;20:1087–1094. doi: 10.1007/s00586-011-1695-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lonstein JE, Bjorklund S, Wanninger MH, Nelson RP. Voluntary school screening for scoliosis in Minnesota. J Bone Joint Surg Am. 1982;64:481–488. [PubMed] [Google Scholar]
- 13.Jenyo MS, Asekun-Olarinmoye EO. Prevalence of scoliosis in secondary school children in Osogbo, Osun State, Nigeria. Afr J Med Med Sci. 2005;34:361–364. [PubMed] [Google Scholar]
- 14.Yılmaz H, Zateri C, Kusvuran Ozkan A, Kayalar G, Berk H. Prevalence of adolescent idiopathic scoliosis in Turkey: an epidemiological study. Spine J. 2020;20:947–955. doi: 10.1016/j.spinee.2020.01.008. [DOI] [PubMed] [Google Scholar]
- 15.Tarrant RC, Queally JM, Moore DP, Kiely PJ. Prevalence and impact of low body mass index on outcomes in patients with adolescent idiopathic scoliosis: a systematic review. Eur J Clin Nutr. 2018;72:1463–1484. doi: 10.1038/s41430-018-0095-0. [DOI] [PubMed] [Google Scholar]
- 16.Wise CA, Sepich D, Ushiki A, Khanshour AM, Kidane YH, Makki N, Gurnett CA, Gray RS, Rios JJ, Ahituv N, Solnica-Krezel L. The cartilage matrisome in adolescent idiopathic scoliosis. Bone Res. 2020;8:13. doi: 10.1038/s41413-020-0089-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yee A, Song YQ, Chan D, Cheung KM. Understanding the basis of genetic studies: adolescent idiopathic scoliosis as an example. Spine Deform. 2014;2:1–9. doi: 10.1016/j.jspd.2013.09.003. [DOI] [PubMed] [Google Scholar]
- 18.Simony A, Carreon LY, H Jmark K, Kyvik KO, Andersen MØ. Concordance rates of adolescent idiopathic scoliosis in a Danish Twin population. Spine (Phila Pa 1976) 2016;41:1503–1507. doi: 10.1097/BRS.0000000000001681. [DOI] [PubMed] [Google Scholar]
- 19.Liu S, Wu N, Zuo Y, Zhou Y, Liu J, Liu Z, Chen W, Liu G, Chen Y, Chen J, Lin M, Zhao Y, Ming Y, Yuan T, Li X, Xia Z, Yang X, Ma Y, Zhang J, Shen J, Li S, Wang Y, Zhao H, Yu K, Zhao Y, Weng X, Qiu G, Wu Z. Genetic polymorphism of LBX1 is associated with adolescent idiopathic scoliosis in Northern Chinese Han population. Spine (Phila Pa 1976) 2017;42:1125–1129. doi: 10.1097/BRS.0000000000002111. [DOI] [PubMed] [Google Scholar]
- 20.Kou I, Takahashi Y, Johnson TA, Takahashi A, Guo L, Dai J, Qiu X, Sharma S, Takimoto A, Ogura Y, Jiang H, Yan H, Kono K, Kawakami N, Uno K, Ito M, Minami S, Yanagida H, Taneichi H, Hosono N, Tsuji T, Suzuki T, Sudo H, Kotani T, Yonezawa I, Londono D, Gordon D, Herring JA, Watanabe K, Chiba K, Kamatani N, Jiang Q, Hiraki Y, Kubo M, Toyama Y, Tsunoda T, Wise CA, Qiu Y, Shukunami C, Matsumoto M, Ikegawa S. Genetic variants in GPR126 are associated with adolescent idiopathic scoliosis. Nat Genet. 2013;45:676–679. doi: 10.1038/ng.2639. [DOI] [PubMed] [Google Scholar]
- 21.Ogura Y, Takeda K, Kou I, Khanshour A, Grauers A, Zhou H, Liu G, Fan YH, Zhou T, Wu Z, Takahashi Y, Matsumoto M Japan Scoliosis Clinical Research Group (JSCRG); Texas Scottish Rite Hospital for Children Clinical Group (TSRHCCG) Einarsdottir E, Kere J, Huang D, Qiu G, Xu L, Qiu Y, Wise CA, Song YQ, Wu N, Su P, Gerdhem P, Watanabe K, Ikegawa S. An international meta-analysis confirms the association of BNC2 with adolescent idiopathic scoliosis. Sci Rep. 2018;8:4730. doi: 10.1038/s41598-018-22552-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sharma S, Gao X, Londono D, Devroy SE, Mauldin KN, Frankel JT, Brandon JM, Zhang D, Li QZ, Dobbs MB, Gurnett CA, Grant SF, Hakonarson H, Dormans JP, Herring JA, Gordon D, Wise CA. Genome-wide association studies of adolescent idiopathic scoliosis suggest candidate susceptibility genes. Hum Mol Genet. 2011;20:1456–1466. doi: 10.1093/hmg/ddq571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sharma S, Londono D, Eckalbar WL, Gao X, Zhang D, Mauldin K, Kou I, Takahashi A, Matsumoto M, Kamiya N, Murphy KK, Cornelia R TSRHC Scoliosis Clinical Group; Japan Scoliosis Clinical Research Group. Herring JA, Burns D, Ahituv N, Ikegawa S, Gordon D, Wise CA. A PAX1 enhancer locus is associated with susceptibility to idiopathic scoliosis in females. Nat Commun. 2015;6:6452. doi: 10.1038/ncomms7452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gao W, Peng Y, Liang G, Liang A, Ye W, Zhang L, Sharma S, Su P, Huang D. Association between common variants near LBX1 and adolescent idiopathic scoliosis replicated in the Chinese Han population. PLoS One. 2013;8:e53234. doi: 10.1371/journal.pone.0053234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kou I, Watanabe K, Takahashi Y, Momozawa Y, Khanshour A, Grauers A, Zhou H, Liu G, Fan YH, Takeda K, Ogura Y, Zhou T, Iwasaki Y, Kubo M, Wu Z, Matsumoto M Japan Scoliosis Clinical Research Group (JSCRG); Texas Scottish Rite Hospital for Children Clinical Group (TSRHCCG) Einarsdottir E, Kere J, Huang D, Qiu G, Qiu Y, Wise CA, Song YQ, Wu N, Su P, Gerdhem P, Ikegawa S. A multi-ethnic meta-analysis confirms the association of rs6570507 with adolescent idiopathic scoliosis. Sci Rep. 2018;8:11575. doi: 10.1038/s41598-018-29011-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gorman KF, Julien C, Moreau A. The genetic epidemiology of idiopathic scoliosis. Eur Spine J. 2012;21:1905–1919. doi: 10.1007/s00586-012-2389-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Komang-Agung IS, Dwi-Purnomo SB, Susilowati A. Prevalence rate of adolescent idiopathic scoliosis: results of school-based screening in Surabaya, Indonesia. Malays Orthop J. 2017;11:17–22. doi: 10.5704/MOJ.1711.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Burton MS. Diagnosis and treatment of adolescent idiopathic scoliosis. Pediatr Ann. 2013;42:224–228. doi: 10.3928/00904481-20131022-09. [DOI] [PubMed] [Google Scholar]
- 29.Mittal R, Aggerwal R, Sarwal A. School screening for scoliosis in India the evaluation of a scoliometer. Int Orthop. 1987;11:335–338. doi: 10.1007/BF00271310. [DOI] [PubMed] [Google Scholar]
- 30.Saikia K, Duggal A, Bhattacharya P, Borgohain M. Scoliosis: an epidemiological study of school children in lower Assam. Indian J Orthop. 2002;36:243–245. [Google Scholar]
- 31.Ueno M, Takaso M, Nakazawa T, Imura T, Saito W, Shintani R, Uchida K, Fukuda M, Takahashi K, Ohtori S, Kotani T, Minami S. A 5-year epidemiological study on the prevalence rate of idiopathic scoliosis in Tokyo: school screening of more than 250,000 children. J Orthop Sci. 2011;16:1–6. doi: 10.1007/s00776-010-0009-z. [DOI] [PubMed] [Google Scholar]
- 32.Abo-Bakr A, Al-Mazyiad A, Al-Hussein M, Al-Sudairy R, Krimli M, Patel PJ. Adolescent idiopathic scoliosis screening of schoolgirls. Ann Saudi Med. 1992;12:555–557. doi: 10.5144/0256-4947.1992.555. [DOI] [PubMed] [Google Scholar]
- 33.Yong F, Wong HK, Chow KY. Prevalence of adolescent idiopathic scoliosis among female school children in Singapore. Ann Acad Med Singap. 2009;38:1056–1063. [PubMed] [Google Scholar]
- 34.Margalit A, McKean G, Constantine A, Thompson CB, Lee RJ, Sponseller PD. Body mass hides the curve: thoracic scoliometer readings vary by body mass index value. J Pediatr Orthop B. 2017;37:e255–e260. doi: 10.1097/BPO.0000000000000899. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


