Abstract
Introduction
Many stroke survivors require continuous outpatient rehabilitation therapy to maintain or improve their neurological functioning, independence, and quality of life. In Switzerland and many other countries, the shutdown to contain SARS-CoV-2 infections led to mobility restrictions and a decrease in therapy delivery. This study investigated the impact of the COVID-19 shutdown on stroke survivors’ access to therapy, physical activity, functioning and mood.
Methods
A prospective observational cohort study in stroke subjects. At 4 time-points (before, during, after the shutdown, and at 3-month follow-up), the amount of therapy, physical activities, motor function, anxiety, and depression were assessed.
Results
Thirty-six community-dwelling stroke subjects (median 70 years of age, 10 months post-stroke) were enrolled. Therapy reductions related to the shutdown were reported in 72% of subjects. This decrease was associated with significantly extended sedentary time and minimal deterioration in physical activity during the shutdown. Both parameters improved between reopening and 3-month follow-up. Depressive symptoms increased slightly during the observation period. Patients more frequently reported on self-directed training during shutdown.
Conclusion
The COVID-19 shutdown had measurable immediate, but no persistent, effects on post-stroke outcomes, except for depression. Importantly, a 2-month reduction in therapy may trigger improvements when therapy is fully re-initiated thereafter.
LAY ABSTRACT
In Switzerland and in many other countries, the shutdown to contain SARS-CoV-2 infections led to a reduced mobility and a reduction in therapy delivery. The impact of the shutdown on stroke survivors’ access to therapy, physical functioning, and mood was investigated in an observational study. Before, during, and after shutdown, the amount of therapy, motor functioning, and anxiety and depression were collected. Thirty-six community dwelling subjects were enrolled at a median of 10 months post-stroke. Therapy reductions related to the shutdown were reported in 72% of subjects. This was associated with an increased sedentary time and minimal deterioration in motor functioning, which improved after reopening. Depression increased slightly during the observation. Patients more frequently reported on self-directed training during shutdown. The COVID-19 shutdown had measurable immediate, but no persistent, effects on post-stroke outcomes, except depression. In fact, a 2-month reduction in therapy may trigger improvements when therapy is fully re-initiated thereafter.
Key words: stroke, rehabilitation, COVID-19, shutdown, outcome, assessment, physical activities
Stroke is the second largest cause of long-term disability worldwide, with approximately two-thirds of stroke survivors requiring inpatient rehabilitation to regain functioning and independence in daily life (1). In addition to improvements related to spontaneous recovery in the subacute phase, rehabilitation interventions were shown to be effective in the chronic phase (i.e. beyond 6 months) (2). At the beginning of the year 2020, the SARS-CoV-2 disease 2019 (COVID-19) pandemic spread worldwide. On 16 March 2020, the Swiss federal government issued containment measures, minimizing the mobility of the entire population (“shutdown”), including the closure of all non-essential business and industries, as well as research-related visits and a reduction in outpatient therapies. The aim of this shutdown was to curb the dispersion of the coronavirus and assure the provision of healthcare under pandemic circumstances. People at high risk, such as community-dwelling elderly people, were strongly recommended to stay at home under any circumstances unless they had to consult a physician. A partial opening of the shutdown was conducted after 27 April 2020, after which face-to-face visits were allowed, followed by a complete reopening by 6 June 2020 (3). The consequences of the interruption of post-stroke outpatient therapy in terms of patient outcome have not been well studied, as, even under normal circumstances, many physicians are reluctant to interrupt therapy out of fear of worsening deficits. This situation is amplified under the circumstances of a pandemic (4).
This pandemic-enforced observational study was used to investigate the consequences of an interruption of outpatient therapy in stroke survivors. It was hypothesized that discontinuation of rehabilitation services would lead to a decline in physical activity, functioning, and mood.
Aim
The primary objective of this prospective, longitudinal, observational cohort study was to investigate the impact of the COVID-19 shutdown on access to therapy, physical activity (given as sitting time and energy expenditure, measured by the metabolic equivalent of task (MET), which is an objective measure of the ratio of the rate at which a person expends energy), physical functioning and mood in stroke patients.
METHODS
This observational study with one retrospective time-point was approved by the cantonal ethics committee Zurich (BASEC-Nr. 2020-00761) and was prospectively registered at ClinicalTrials. gov (Identifier: NCT04373109). Results are reported in alignment with the Strengthening the Reporting of Observational studies in Epidemiology (STROBE) checklist (see supplementary material). Stroke patients were enrolled in this study if they participated in an ongoing observational stroke rehabilitation research project (ClinicalTrials.gov Identifiers NCT03735901 and NCT03522519) at the University and University Hospital Zurich that was initiated before the SARS-CoV-2 pandemic. Inclusion criteria for the primary studies were: first-ever ischaemic or haemorrhagic stroke with motor impairment, independent living prior to the insult and no history of any other neurological disease. All subjects provided additional verbal and written consent for participation and further use of unencrypted health-related data according to the Declaration of Helsinki.
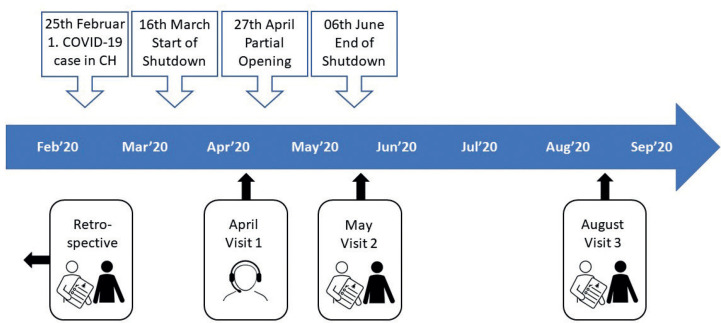
Stroke subjects were recruited by telephone from 20 April until 11 May 2020, and were prospectively monitored during and after the shutdown, as well as 3 months after the shutdown (Fig. 1). The face-to-face visits took place at the University hospital Zurich or the participants’ homes. As a retrospective baseline, data from the ongoing observational studies collected at a median of 160 days before study enrollment was used.
Fig. 1.
Timeline of pandemic-related national events in Switzerland (CH) and study visits.
Subject characteristics included in the analysis were the patient’s age (years), stroke type (ischaemic/haemorrhagic) (5), medical history, affected body side, hand dominance, disabilities, ambulation status, cognitive status, living arrangements, and length of hospital/rehabilitation stay.
The outcomes, measured at all 4 time-points of the study, included self-reported type and frequency of outpatient therapies, self-directed training, self-reported physical activity by use of the International Physical Activity Questionnaire (IPAQ) (6), psychological symptoms with the Hospital Anxiety and Depression Scale (HADS) (7), motor function with the Functional Ambulation Categories (FAC) (8), and upper limb usage with the Motor Activity Log – 14-Item Version (MAL) (9).
During face-to-face study visits, motor function was assessed by use of the Fugl-Meyer Motor Assessment (FMMA) (11), the Action Research Arm Test (ARAT) (12), and the Ten-Meter Walk Test (10MWT) (10).
Secondary outcomes consisted of the Patient-Reported Outcomes Measurement Information System – 29 Version (PROMIS–29) (11), the Fatigue Severity Scale (FSS) (12), Rivermead Mobility Index (RMI) (8), Activities-specific Balance Confidence Scale (ABC) (13), modified Rankin Scale (mRS) (14), National Institute of Health Stroke Scale (NIHSS) (15), self-reported leisure activities, and the reasoning for therapy reduction at visit 1.
Statistical analysis
To investigate the impact of the COVID-19 shutdown on therapy availability and intensity, physical functioning, and mental functions in subjects after stroke, linear mixed-effect model analysis was performed for each outcome variable to determine the effects of the fixed factors, time-points, self-directed training (yes/no) and therapy reduction (yes/no) by considering covariates of initial stroke severity (NIHSS), sex (male/female), days after stroke and age at stroke (in days). Linear mixed-effect model analysis enables the investigation of fixed and random effects simultaneously, is appropriate for longitudinal data, and was chosen as it considers the repeated or nested measures in our analysis and can deal with missing data. Each fixed and random effect is tested for significant influence on the outcome variable, given in estimated changes on the outcome variable and the corresponding p-values with a statistical significance of < 0.05. The random subject factor is based on varying intercepts of the individual lines, and thereby informs about between-subject variability, or, in other words, the individual-specific effects that are uncorrelated with the independent variable. The outcome data are presented in median and interquartile range (IQR) or frequencies. All statistical analyses were performed at an alphalevel of p < 0.05. The analysis was performed using R (version 4.0.3.; R Core Team (2020) (16), including the libraries readxl, dplyr, lme4, and lmerTest).
RESULTS
Thirty-six stroke survivors were included 72–584 days (range) after their stroke, with 4 subjects in the early subacute phase (until 3 months), 6 subjects in the late subacute phase (until 6 months) and 26 at the chronic stage (beyond 6 months) (17). The time post-stroke had no effect on the rehabilitation outcome measures, but on self-reported sitting time (IPAQ) with estimates of –3 h of sitting time per day after stroke. Two included subjects that were allocated in a rehabilitation clinic, continued inpatient rehabilitation during the shutdown.
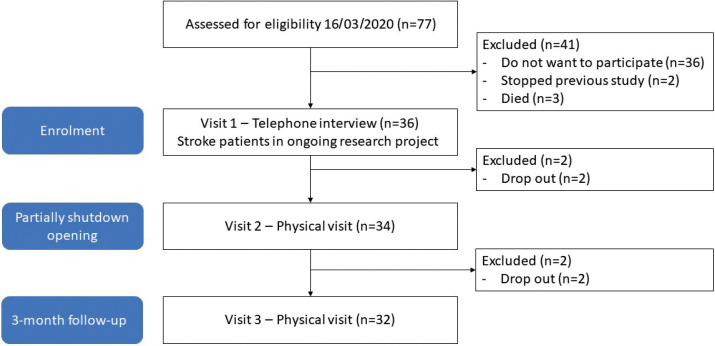
The participant’s baseline characteristics are shown in Table I and Table SI. Four participants declined participation in the follow-up measurements for personal reasons (Fig. 2). None of the included participants reported a COVID-19-infection, nor related symptoms.
Table I.
Demographic and baseline characteristics
| Characteristics | n = 36 |
|---|---|
| Age, years, median (Q1-Q3) | 70 (62-77) |
| Sex, men/woman, n | 19/17 |
| Handedness, left/ right, n | 3/33 |
| General health, Cumulative Illness Rating Scale, median (Q1-Q3) | 3 (2-6) |
| Stroke type, n (%) | |
| Ischaemic | 32 (88.9) |
| Haemorrhagic | 4 (11.1) |
| Time since stroke, median (Q1-Q3), days | 300 (171-428) |
| Stroke location, n (%), Bamford | |
| Lacunar stroke | 16 (44.4) |
| Total anterior circulation stroke | 8 (22.2) |
| Partial anterior circulation stroke | 10 (27.8) |
| Posterior circulation stroke | 2 (5.6) |
| Stroke severity hospital admission, NIHSS, median (Q1-Q3) | 2 (1-6) |
| Time in acute hospitalization, median (Q1-Q3), days | 9 (8-12) |
| Time in inpatient rehabilitation, median (Q1-Q3), days | 46 (29-77) |
| Time since last study visit, median (Q1-Q3), days | 160 (76-215) |
| Independent living (Home), n (%) | 29 (80.6) |
| Disability or dependence, modified Rankin Scale, Median (Q1-Q3) | 2 (1-3) |
| Ambulation, Functional Independence Categories, Median (Q1-Q3) | 5 (4-5) |
| Cognitive status, Montreal Cognitive Assessment, Median (Q1-Q3) | 27 (22.5-29) |
NIHSS: National Institute of Health Stroke Scale; N: number; Q: quartile.
Fig. 2.
Strengthening the Reporting of Observational studies in Epidemiology (STROBE) study flow-chart.
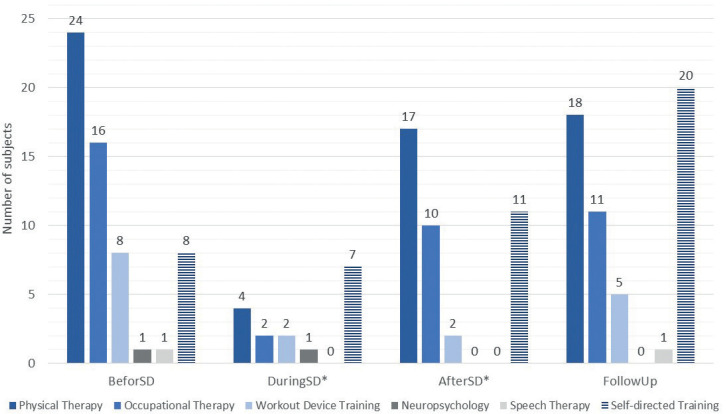
Twenty-six participants (72%) reported an overall reduction in therapy during the shutdown (visit 1). The decision to reduce therapy was made by the therapist in 11 cases, by the patient in 7 cases, and by other parties, such as healthcare institutions, in 8 cases, as reported by the participants. Significant shortening in physical (estimate: –51 min/week) and occupational therapy (estimate: –41 min/week) were followed by therapy re-initiation after the shutdown ended (estimate: physical therapy –42 min/week, occupational therapy: –37 min/week) and a 3-months follow-up (estimate: physical therapy –41 min/week, occupational therapy: –37 min/week) (Fig. 3). Patient-reported self-directed training increased in 13 subjects. Between baseline (before shutdown) and follow-up, the self-directed training increased non-significantly with estimates of –31 min/week.
Fig. 3.
Number of subjects performing therapy/ training per time-point (n=36). SD: shutdown.
The changes in physical activity, leisure activities, anxiety and depression, and upper and lower limb motor function and activities are shown in Table II, Fig. S1 and Table SII. The results of the linear mixed effect models per therapy are shown in Table SIII.
Table II.
Descriptive statistics of physical activity, anxiety and depression, and motor function per time-point
| Assessment | Retrospective data Median (Q1–Q3) | Visit 1 During shutdown Median (Q1–Q3) | p-value | Visit 2 Partially opening Median (Q1–Q3) | p-value | Visit 3 3-Month’s follow-up Median (Q1–Q3) | p-value |
|---|---|---|---|---|---|---|---|
| International Physical Activity Questionnaire | |||||||
| Sitting (Min/Weeks) | 2,460 (1,320–3,825) | 3,390 (2,625–4,200) | 0.001 | 3,360 (2,520–4,200) | 0.001 | 2,800 (1,680–3,570) | 0.557 |
| MET-min/Week | 551 (0–1,760) | 864 (399–2,523) | 0.390 | 903 (506–2,546) | 0.662 | 1,674 (978–3,080) | 0.015 |
| Hospital Anxiety and Depression Scale | |||||||
| Anxiety | 5.00 (3.00–7.50) | 3.00 (2.00–5.00) | 0.018 | 4.00 (1.00–6.00) | 0.113 | 3.00 (2.00–5.50) | 0.048 |
| Depression | 4.00 (2.00–6.00) | 4.00 (2.25–7.00) | 0.034 | 4.00 (2.00–7.00) | 0.047 | 4.00 (2.00–7.00) | 0.040 |
| Functional Ambulation Categories | 4.00 (2.00–5.00) | 5.00 (4.00–5.00) | 0.037 | 5.00 (3.50–5.00) | 0.002 | 5.00 (3.75–5.00) | 0.037 |
| modified Rankin Scale | 2.5 (1–4) | 2 (1–3) | < 0.001 | 2 (1–3) | 0.001 | 2 (1–3) | 0.001 |
| Motor Activity Log–14-Item Version | |||||||
| Amount of Use | 2.92 (1.19–4.55) | 3.81 (1.19–4.62) | 0.084 | 3.4 (1.57–4.56) | 0.317 | 3.46 (0.68–4.83) | 0.421 |
| Quality of Movements | 2.67 (1.04–3.79) | 3.25 (1.26–4.36) | 0.014 | 3.3 (1.25–4.00) | 0.106 | 3.17 (0.45–4.84) | 0.151 |
| Fugl-Meyer Motor Assessment | |||||||
| Lower Extremity Subscale | 28.00 (17.75–30.00) | NA | NA | 27.50 (21.25–29.00) | 0.150 | 27.00 (24.00–30.75) | 0.004 |
| Upper Extremity Subscale | 50.50 (27.00–59.25) | NA | NA | 50.00 (27.75–59.00) | 0.157 | 49.50 (31.00–57.00) | 0.019 |
| Action Research Arm Test | 42.00 20.00–55.00 | NA | NA | 44.50 (23.25–57.00) | 0.059 | 48.00 (22.00–57.00) | 0.070 |
| Ten-Meter Walk Test | 10.07 (7.93–11.57) | NA | NA | 10.40 (7.49–12.45) | 0.371 | 8.47 (7.61–10.92) | 0.005 |
MET: metabolic equivalent tasks; NA: not applicable; Q: quartile. The ten-meter walk test (10MWT) is reported in s. The p-value for each visit time-point reflects the statistical significance of differences in comparison with the retrospective data time-point. Bold font indicates p-values with a statistical significance of < 0.05.
Physical activity, as measured by MET-min/week of the IPAQ, increased non-significantly (+562 MET-min/week, p = 0.390) and immediately after the shut-down (+304 MET-min/week, p = 0.662), but increased 3-months later (+1,715 MET-min/week, p = 0.015). Female patients were less active than males (–1,891 MET-min/week; p = 0.033). Five days including 30 min of walking relate to around 500 MET-min/week, which represents moderate physical intensity (18). Sitting time increased during (+1190 min/week, p < 0.001), after the shutdown (+1195 min/week, p = 0.001) and 3-months later (+208 min/week, p = 0.557). Stroke severity (+75 min/week per point in NIHSS, p = 0.038), sex (female +1,125 min/week, p < 0.001) and days since stroke (–3 min/week per day after stroke, p = 0.016) significantly affected sitting time. The diminished physical activity in female participants could be explained by the more pronounced impairments, at baseline compared with the male participants with a mean NIHSS of 1.4 points in males and 4.1 points in females. Related to this, the significant effect of sex on the change in impairment is shown in Table SIV.
Depressive symptoms, as measured with the HADS depression subscale, increased slightly between baseline and shutdown (+1.26 points, p = 0.034), after shutdown (+1.28 points, p = 0.047) and follow-up (+1.37 points, p = 0.040). Also, the number of subjects who scored above the 8-point HADS depression cutoff score increased from 8-percent to 19-percent after the lockdown, as shown in the Table SV. All moodrelated outcomes (HADS anxiety and depression, PROMIS–29, and FSS) showed significant effects of the random subject factor (p ≤ 0.001).
Dependence in the daily activities, measured by mRS, improved significantly between baseline and 3-months follow-up (–0.66 points, p = 0.001) with significant effects of stroke severity (+0.19 points, p < 0.001), and the random subject factor (p < 0.001). The MAL quality of movements subscale improved between retrospective data and shutdown (+0.47 points; p = 0.014, significant effects of stroke severity, p < 0.001, and the random subject factor, p < 0.001). No deterioration in MAL was found during and after shutdown. The FMMA improved between end of shutdown and 3-months follow-up (+3.54 points; p = 0.019, lower extremity subscore +2.69 points; p = 0.004). Also walking speed in the 10MWT improved in the comparison of these time-points (–3.54 s, p = 0.005). All functional motor outcomes were significantly influenced by the factor of stroke severity, as measured by the initial NIHSS. In subjects who had reported reduction in therapy, a worsening of FMMA (–2.61) and ARAT (–5.68) and an improvement in 10MWT (–1.94 s) were not significant.
DISCUSSION
This study explored the influence of the COVID-19 shutdown on delivery of therapy in predominantly community-dwelling stroke survivors. The 2-month shutdown caused drastic reductions in therapy in 72% of subjects, mostly based on decisions made by the therapists (42%). Expectedly, subjects were less active during and after the shutdown. This was associated with a slight, but significant, worsening of depressive symptoms, which is in accordance with epidemiological studies in non-stroke populations (19). In contrast to our hypothesis, the self-reported quality of upper limb usage improved, and physical function did not deteriorate. Significant improvements in upper and lower limb motor function between the end of shutdown and 3 months thereafter are probably related to the re-initiation of rehabilitation therapies, as well as to increased self-directed training and increased physical activity.
On average, this studied cohort coped well with pandemic-related changes in daily living, but intersubject variability was large. Subjects with more severe stroke were more likely to fare poorly, as expected from the literature (20). For efficient usage of the healthcare resources, it might be especially important to identify those stroke subjects with capacities for independent training, in order to encourage them and those stroke subjects who require assistance either physically, or mentally.
To deliver rehabilitation for stroke patients in the community, personal protective equipment and infection control precautions are important (21) for assessing patients’ capabilities and providing hands-on therapy. New approaches, such as telerehabilitation (22) and technology-based self-training methods, should be investigated and included in therapeutic programmes, since they do not require patient mobility and do not carry the risk of spreading infections. Furthermore, technology-based self-training might increase the takeover of training in the patients’ real life.
Beyond the COVID-19 pandemic, the results of the current study provide important information about dose and interruption of maintenance therapy in stroke survivors. Before the shutdown, the median amount of outpatient physical and occupational therapy was 30–60 min per week, which is the typical intensity of maintenance outpatient therapy in Switzerland. While it might be sufficient to provide therapy on a weekly or bi-weekly basis in the chronic stage to monitor and prevent complications, such as pain or immobility, this therapy frequency probably cannot improve functional deficits (23). On the other hand, in our work, a 2-month interruption did not seem to make a difference (24). Even more importantly, interruptions may be useful, as the re-initiation of therapy led to functional improvements; a “vacation” effect (25). This effect was independent of the number of days post-stroke, in all outcomes (Tables SIII–SVII).
This prospective longitudinal study represents observations related to the event of the first COVID-19-related shutdown in 2020 in Switzerland. The results could be different if repeated in subsequent pandemic-related restrictions, thereby stressing the importance of monitoring effects on healthcare services, such as stroke rehabilitation, and the burden on specific subgroups of patients and stroke survivors.
Limitations
The small sample size and a potential recruitment bias must be considered when interpreting the findings. All included subjects were actively recruited from 2 previous cohorts, and their willingness to participate in the current project probably does not reflect the general population of stroke survivors. The subjects of the primary cohorts who declined to participate in the current study were more impaired (as shown in Table SI), which hampers the generalizability of these findings for this population. The fact that most of the outcome measures of physical functioning and mood were significantly influenced by the random individual subject factor underpins the need to monitor these aspects in subjects after stroke and to identify the individuals’ health-related barriers and facilitators.
Conclusion
The pandemic-related shutdown was associated with a marked reduction in outpatient physical and occupational therapy in 72% of community-dwelling stroke subjects. The participants became less physically active, were sitting more, and became slightly more depressed. Reinitiating therapy after the shutdown was associated with improvements in functional outcomes. The findings stress the need for personalization in therapy delivery and its form (e.g. self-training, telerehabilitation, hands-on therapy) and suggest that “vacation” from outpatient therapy can sometimes be helpful.
Supplementary Material
ACKNOWLEDGEMENTS
The authors thank all study participants for their participation in these delicate times.
Funding
This work was supported by the CRPP Stroke (University of Zurich) and the P&K Pühringer Foundation.
Footnotes
The authors have no conflicts of interest to declare.
REFERENCES
- 1.Winstein CJ, Stein J, Arena R, Bates B, Cherney LR, Cramer SC, et al. Guidelines for adult stroke rehabilitation and recovery: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2016; 47: e98-e169. [DOI] [PubMed] [Google Scholar]
- 2.Garcia-Rudolph A, Laxe S, Sauri J, Opisso E, Tormos JM, Bernabeu M. Evidence of chronic stroke rehabilitation interventions in activities and participation outcomes: systematic review of meta-analyses of randomized controlled trials. Eur J Phys Rehabil Med 2019; 55: 695–709. [DOI] [PubMed] [Google Scholar]
- 3.Eckert F, Mikosch H. Mobility and sales activity during the corona crisis: daily indicators for Switzerland. Swiss J Econ Stat 2020; 156: 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lebrasseur A, Fortin-Bédard N, Lettre J, Bussières E-L, Best K, Boucher N, et al. Impact of COVID-19 on people with physical disabilities: a rapid review. Disabil Health J 2020: 101014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bamford J, Sandercock P, Dennis M, Burn J, Warlow C. Classification and natural history of clinically identifiable subtypes of cerebral infarction. Lancet 1991; 337: 1521–1526. [DOI] [PubMed] [Google Scholar]
- 6.Craig CL, Marshall AL, Sjöström M, Bauman AE, Booth ML, Ainsworth BE, et al. International physical activity questionnaire: 12-country reliability and validity. Med Sci Sports Exercise 2003; 35: 1381–1395. [DOI] [PubMed] [Google Scholar]
- 7.Zigmond A, Snaith R. The Hospital Anxiety and Depression Scale. Acta Psychiatr Scand 1983; 67. [DOI] [PubMed] [Google Scholar]
- 8.Collen FM, Wade DT, Bradshaw CM. Mobility after stroke: reliability of measures of impairment and disability. Internat Disabil Stud 1990; 12: 6–9. [DOI] [PubMed] [Google Scholar]
- 9.Uswatte G, Taub E, Morris D, Vignolo M, McCulloch K. Reliability and validity of the upper-extremity Motor Activity Log–14 for measuring real-world arm use. Stroke 2005; 36: 2493–2496. [DOI] [PubMed] [Google Scholar]
- 10.Perera S, Mody SH, Woodman RC, Studenski SA. Meaningful change and responsiveness in common physical performance measures in older adults. J Am Geriatr Soc 2006; 54: 743–749. [DOI] [PubMed] [Google Scholar]
- 11.Fischer F, Gibbons C, Coste J, Valderas JM, Rose M, Leplege A. Measurement invariance and general population reference values of the PROMIS Profile 29 in the UK, France, and Germany. Qual Life Res 2018; 27: 999–1014. [DOI] [PubMed] [Google Scholar]
- 12.Krupp LB, LaRocca NG, Muir-Nash J, Steinberg AD. The fatigue severity scale. Application to patients with multiple sclerosis and systemic lupus erythematosus. Arch Neurol 1989; 46: 1121–1123. [DOI] [PubMed] [Google Scholar]
- 13.Powell LE, Myers AM. The Activities-specific Balance Confidence (ABC) Scale. J Gerontol A Biol Sci Med Sci 1995; 50A: M28–34. [DOI] [PubMed] [Google Scholar]
- 14.Bruno A, Akinwuntan AE, Lin C, Close B, Davis K, Baute V, et al. Simplified modified rankin scale questionnaire: reproducibility over the telephone and validation with quality of life. Stroke 2011; 42: 2276–2279. [DOI] [PubMed] [Google Scholar]
- 15.Lyden P, Brott T, Tilley B, Welch KM, Mascha EJ, Levine S, et al. Improved reliability of the NIH Stroke Scale using video training. NINDS TPA Stroke Study Group. Stroke 1994; 25: 2220–2226. [DOI] [PubMed] [Google Scholar]
- 16.R Core Team (2020). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL http://www.R-project.org/. [Google Scholar]
- 17.Bernhardt J, Hayward KS, Kwakkel G, Ward NS, Wolf SL, Borschmann K, et al. Agreed definitions and a shared vision for new standards in stroke recovery research: The Stroke Recovery and Rehabilitation Roundtable taskforce. Int J Stroke 2017; 12: 444–450. [DOI] [PubMed] [Google Scholar]
- 18.Craig CL, Marshall AL, Sjostrom M, Bauman AE, Booth ML, Ainsworth BE, et al. International physical activity questionnaire: 12-country reliability and validity. Med Sci Sports Exerc 2003; 35: 1381–1395. [DOI] [PubMed] [Google Scholar]
- 19.Pierce M, Hope H, Ford T, Hatch S, Hotopf M, John A, et al. Mental health before and during the COVID-19 pandemic: a longitudinal probability sample survey of the UK population. Lancet Psychiatry 2020; 7: 883–892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sommerlad A, Marston L, Huntley J, Livingston G, Lewis G, Steptoe A, et al. Social relationships and depression during the COVID-19 lockdown: longitudinal analysis of the COVID-19 Social Study. Psychol Med 2021: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ford GA, Hargroves D, Lowe D, Rooney G, Fisher R, Oatley H, et al. Restoration and recovery of stroke services during the COVID-19 pandemic 2020. Available from: https://www.oxfordahsn.org/wp-content/uploads/2020/07/Restoration-and-recovery-of-stroke-services-during-the-COVID-19-pandemic-July–2020–1.pdf.
- 22.Laver KE, Adey-Wakeling Z, Crotty M, Lannin NA, George S, Sherrington C. Telerehabilitation services for stroke. Cochrane Database Syst Rev 2020; 1: CD010255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lang CE, MacDonald JR, Gnip C. Counting repetitions: an observational study of outpatient therapy for people with hemiparesis post-stroke. J Neurol Phys Ther 2007; 31: 3–10. [DOI] [PubMed] [Google Scholar]
- 24.Ward NS, Brander F, Kelly K. Intensive upper limb neurorehabilitation in chronic stroke: outcomes from the Queen Square programme. J Neurol Neurosurg Psychiatry 2019; 90: 498–506. [DOI] [PubMed] [Google Scholar]
- 25.Hesse S, Welz A, Werner C, Quentin B, Wissel J. Comparison of an intermittent high-intensity vs continuous low-intensity physiotherapy service over 12 months in community-dwelling people with stroke: a randomized trial. Clin Rehabil 2011; 25: 146–156. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.