Summary
Background
Retrospective studies suggest that for patients with ulcerative colitis (UC) combination therapy with low-dose azathioprine and allopurinol (L-AZA/ALLO) may result in higher remission rates than monotherapy with azathioprine (AZA). We prospectively investigated the effects of these drugs for remission in patients with moderate-to-severe UC.
Methods
Open-label, unblinded, randomised, controlled, investigator-initiated, multicentre study conducted at eight hospital sites in Denmark. Adult patients with established UC, who were steroid dependent/refractory, thiopurine naïve, had a normal thiopurine methyltransferase, and achieved remission with steroids or infliximab were eligible for inclusion. Patients were randomly assigned by the investigators (1:1) to 52 weeks of treatment with once daily oral AZA (median dose 50 mg) combined with ALLO 100 mg versus AZA monotherapy (median dose 200 mg), using a computer-generated randomisation list with blocks of six. The trial was open without masking. All randomised patients who received at least one dose of study drug were included in primary and safety analyses (intention to treat population). The primary outcome was steroid and infliximab free remission after 52 weeks, defined as a Mayo Score of ≤1 and no rectal bleeding. The trial is completed and is registered in ClinicalTrials.gov (ClinicalTrials.gov NCT03101800).
Findings
Between January 9, 2017 and February 10, 2021, 47 patients were randomised to l-AZA/ALLO and 42 to AZA and received at least one dose of the study drug. After 52 weeks, 20 of 47 (43%) patients in the l-AZA/ALLO group and nine of 42 (21%) patients in the AZA group achieved remission (odds ratio 2·54 [95% CI 1·00 to 6.78, p < 0·048]). Fourteen patients (30%) in the l-AZA/ALLO group and 16 (38%) in the AZA group were withdrawn from the study due to adverse events.
Interpretation
This study suggests that after one year l-AZA/ALLO therapy may be associated with a beneficial effect on steroid- and infliximab-free clinical remission in patients with moderate-to-severe UC and should be considered as first line therapy.
Funding
Funding for AAUC was provided by The Capital Region of Denmark (Regionernes Medicinpulje (6062/16)).
Keywords: Ulcerative colitis, Azathioprine, Allopurinol, Reatment, Randomised trial
Research in context.
Evidence before this study
We previously undertook a pilot trial in which 46 patients were randomised to 24 weeks of treatment with low-dose azathioprine and allopurinol (L-AZA/ALLO) or azathioprine monotherapy (AZA). Remission rates in the two groups were 35% and 70%. A search of PubMed and the ICTRP trial search portal (conducted December 15, 2021) using the search terms “ulcerative colitis’’ AND “azathioprine’’ AND “allopurinol’’ yielded 23 records. No other relevant published trials were identified.
Added value of this study
This randomised, controlled trial found that in patients with moderate-to-severe UC and normal thiopurine methyltransferase (TPMT), who were naïve to thiopurines, l-AZA/ALLO combination therapy may be associated with a beneficial effect on steroid- and infliximab-free clinical remission (a Mayo Score of ≤1) after one year.
Implications of all the available evidence
This trial suggests that treatment with l-AZA/ALLO as compared to AZA may benefit treatment naïve patients with moderate-to-severe UC who have a normal TPMT.
Alt-text: Unlabelled box
Introduction
Azathioprine (AZA) and 6-mercaptopurine (6MP) are recommended for maintaining remission in moderate-to-severe UC among patients for whom aminosalicylates have failed or proved intolerable, or who require repeated courses of steroids.1,2 Furthermore, thiopurines are recommended as concomitant immunomodulation alongside infliximab treatment.3 Up to 60% of patients with inflammatory bowel disease (IBD) are treated with thiopurines during the course of their disease. Unfortunately, up to 45% of patients treated with conventional, weight-based monotherapy are classified as treatment failures due to an inadequate response or adverse events leading to the withdrawal of therapy.4‒6
Most patients whose AZA fails have a predominant methylator genotype (thiopurine shunter) associated with a normal thiopurine methyltransferase (TPMT, a key enzyme in the thiopurine metabolic pathway) genotype, leading to low erythrocyte 6-thioguanine nucleotide (6-TGN) values and high values of methylated metabolites (MeMP).7 Allopurinol (ALLO) was originally developed to optimize thiopurine treatment. In the last 20 years, the combination of low-dose AZA/ALLO (L-AZA/ALLO) has been assessed in patients with IBD who show an inadequate response to, or who experience adverse events during treatment with AZA. The combination of AZA and ALLO reverses the unfavourable metabolite profile, leading to higher levels of 6-TGN and lower MeMP, which reduces the risk of hepatotoxicity and other adverse events. In mostly retrospective studies evaluating thiopurine shunters, l-AZA/ALLO combination therapy appears to increase clinical remission rates and provide safe, long-term immunosuppression.8‒14
In a recent randomised prospective pilot study of 46 patients with either UC or Crohn's disease (CD), we found that l-AZA/ALLO therapy increased remission rates from 35 to 70% when compared to AZA monotherapy.6 The results suggest that for every three patients treated with l-AZA/ALLO instead of AZA, one additional patient will maintain remission. Despite this evidence for the improved efficacy of l-AZA/ALLO, thiopurine monotherapy continues to be recommended as the initial treatment for patients based on safety concerns and a lack of prospective studies in thiopurine-naïve patients. We therefore conducted a larger, randomised, open, controlled trial to evaluate the effects and safety of l-AZA/ALLO versus standard AZA in patients with moderate-to-severe UC.
Hypothesis
In patients with moderate-to-severe UC and normal TPMT, treatment with l-AZA/ALLO has a beneficial effect on steroid- and infliximab-free remission after 52 weeks when compared with AZA monotherapy.
Methods
Patients
This randomised trial comparing 52 weeks of treatment with l-AZA/ALLO or just AZA was conducted in eight gastroenterology hospital centers (Supplementary Material 1) in Denmark between January 9, 2017 and February 10, 2021. The trial was approved by the local ethics committee (H-16,031,131, Hvidovre center), in accordance with the Declaration of Helsinki, and by the Danish Medical Agency. The study was reported to EudraCT (2016-002433-30), ClincalTrials.gov (NCT03101800), and the Danish Data Protection Agency. All participants provided their written informed consent. The study was conducted according to GCP-ICH guidelines and monitored by the three regional Good Clinical Practice units. Adult patients between 18 and 80 years old, who were naïve to thiopurine treatment and had (clinically and histologically) established UC, with a normal TPMT defined as a homozygous wildtype TPMT genotype or a phenotype with more than 14 U/ml erythrocytes, were eligible for inclusion. In addition, the patients were steroid dependent defined as a failure to taper the medicine after at least one course of high dose prednisolone/budesonide or the patients should be steroid refractory (defined as requiring infliximab (IFX) treatment because of a lack of response to steroids). Additional inclusion criteria were endoscopy showing active inflammation during the current disease flare-up and a negative stool test for pathogenic bacteria, including Clostridium difficile. Exclusion criteria were kidney disease with a GRF of 50 ml/min or less, persistently elevated alanine aminotransferase U/L (ALT), participation in other interventional clinical trials, pregnancy or breastfeeding, previous thiopurine treatment and treatment with biologics other than infliximab, or the absence of informed consent.
Study design
This was a randomised, investigator-initiated, parallel-arm, controlled, open trial among patients with UC. Patients who had obtained remission with steroids or infliximab were randomised 1:1 to low-dose AZA administered once daily at a dose of approximately 0·8 mg/kg combined with ALLO 100 mg, or to AZA administered once daily of approximately 2·5 mg/kg. This dosing was based on previous evidence and internationally recognized standards. Medication adherence was monitored by measuring thiopurine metabolites. Quantification of 6-TGN and MeMP was performed as described by Kirchherr et al.15 A 6-TGN value greater than 230 pmol/8 × 108 RBC was considered the therapeutic target.16 Participants were followed for 52 weeks and patient visits were planned at baseline (week 0) and after weeks 2, 4, 6, 8, 12, 16, 24, 38, and 52 weeks. At every visit (including at the time of a withdrawal (WD)) standard blood tests were carried out and a partial Mayo Score and any adverse events were recorded. Faecal calprotectin was measured at weeks 0, 12, 26, 52, or at WD and thiopurine metabolites were measured at weeks 6 and 52, or at WD. The metabolites results were blinded for the investigators. A sigmoidoscopy (or colonoscopy) was performed at week 52. Endoscopic inflammation was evaluated using the Mayo Endoscopic Subscore.17 The endoscopy was recorded and sent for central reading. Two experienced gastroenterologists scored the videos blinded (Supplementary Material 2). Four biopsies were taken from the rectum and, in cases of inflammation, from the most inflamed area. For evaluating the degree of histological inflammation we used a version of an existing scoring scheme by Noam Harpaz.18 We have used this scheme in two previous studies and found it suitable for our purposes.19,20 The tissue samples were reviewed by two experienced gastrointestinal pathologists unaware of any clinical information. All slides were stained with Hematoxylin and Eosin, and some with Weigert- Alcian-Sirius, and Periodic-acid-Schiff. Histological inflammatory activity was divided into four groups: (0) no neutrophil inflammation, (1) cryptitis or crypt abscesses in less than 10% of the illustrated crypts, (2) cryptitis or crypt abscesses in 10% to 50% of the illustrated crypts, and (3) cryptitis or crypt abscesses in more than 50% of the illustrated crypts and/or the presence of erosions or ulcers. The tissue samples showing the highest grade of inflammation determined the final score. Differences of interpretation were resolved by consensus.
Quality of life was assessed by the Short Health Scale (SHS)21 and the Short Inflammatory Bowel Disease Questionnaire (SIBDQ)22 at weeks 0 and 52 or WD.
Randomisation and masking
Patients were randomised using computer generated random numbers via a centralized randomisation service (https://www.sealedenvelope.com/). Patients were allocated 1:1 in blocks of 6. The allocation sequence was concealed in envelopes. To avoid tampering, the envelopes were serially numbered and opaque. The randomisation process was undertaken by investigators independently of the trial. As explained, the allocation was stratified but the number of participants allocated at some sites was small. The trial was open without blinding.
Withdrawal criteria
Withdrawal criteria were a partial Mayo Score of 3 or more from weeks 12–52, intolerable side effects from the medication, laboratory tests showing persistent effects on the liver (an ALT value greater than 100 U/L), increasing s-amylase (double that of normal values) or leukopenia (a total leukocyte count smaller than 3·0), irrespective of a patient's condition, abdominal surgery or severe infection.
Medication
Steroids were discontinued at week 12 and tapered according to a strict schedule (Supplementary Material 3). In the case of relapse, steroids could be increased/restarted with 20 mg and decreased by 5 mg per week. An inability to discontinue steroids by week 16 was deemed as treatment failure. Infliximab could be used as a therapy until week 26, at which time it was to be discontinued if the patients were in clinical remission. If not in remission the patients continued with infliximab and deemed as a treatment failure. The dosage of oral 5-ASA was required to be stable in the two weeks prior to the study and throughout its duration. The use of topical 5-ASA was permitted during the study, but not topical steroids.
Outcome measures
The outcomes were centrally assessed. The primary outcome was steroid- and infliximab-free remission at week 52, defined as a total Mayo Score of 1 or less and with no rectal bleeding.
Secondary outcomes were
-
•
A partial clinical response after 52 weeks (defined as a Mayo Score less than 3).
-
•
Endoscopic remission after 52 weeks (defined as a Mayo Subscore of 0).
-
•
Histological mucosal healing after 52 weeks (defined as a histological score of 0).
-
•
Faecal calprotectin levels after 52 weeks.
-
•
Quality of life after 52 weeks.
-
•
TGN and MEMP at week 6 and week 52.
-
•
Adverse events.
Adverse events were defined as any untoward or unfavourable medical occurrence, including any abnormal symptoms or disease, associated with the patient's participation in the research, whether or not they were considered related to study participation.
Sample size calculation
We conducted the sample size calculation with an alpha (two-sided) of 5% and power of 80%. The sample size calculation was based on our primary outcome (remission). Based on evidence from our pilot study,6 we set the response rate (to 60% in the intervention group (L-AZA/ALLO) and 33% in the control group (AZA). We assessed this difference as clinically meaningful. The required sample size was 84 patients (42 in each treatment group).
Statistical analyses
We used paper CRFs and data were double entered in our database by two independent investigators. We used the statistical package STATA version 17 for Windows. We conducted our analyses based on the intention-to-treat principle including all patients randomised who received at least one dose of the allocated study drug. There were no missing outcome data for the primary analyses or for the assessment of safety. For the endoscopic and histological assessments, we were only able to undertake per protocol analyses as only patients who completed the trial underwent endoscopy with biopsies at week 52. We summarized quantitative data using medians with 25th to 75th percentiles and binary data using proportions. The intervention and control groups were compared using Mann-Whitney or chi-square tests. A p value smaller than <0·05 vas considered statistically significant (2-sided alpha). Binary outcomes were analysed using multivariable and univariable logistic regression identify predictors (age, sex, BMI, smoking, disease duration, calprotectin at baseline and week 26, IFX) of our primary outcome (clinical remission at week 52). We have not allowed for multiplicity in the analyses of secondary outcomes.
Role of the funding source
This trial received a grant from the Capital Region of Denmark (Regionernes Medicinpulje (6062/16)).The funder of the study had no role in the study design, data collection, data analysis, data interpretation, or the writing of the report, and was not involved in the decision to submit the manuscript. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
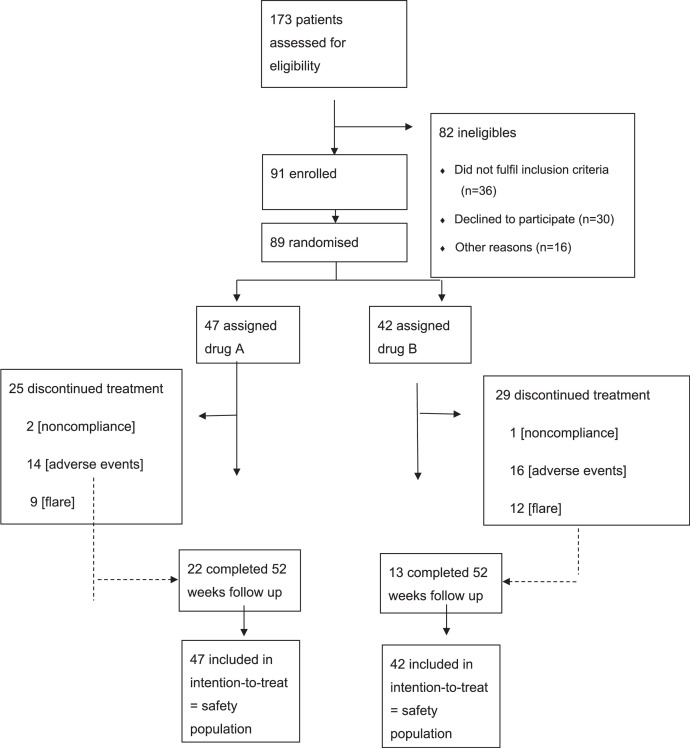
Between January 9, 2017 and February 10, 2021, 91 patients with UC were randomised to the l-AZA/ALLO or AZA groups. In total 89 patients received at least one dose of the study drug and were included in our intention to treat analyses (Figure 1). Forty-seven patients were allocated to l-AZA/ALLO and 42 to AZA. The baseline characteristics of the two groups were comparable (Table 1). The mean dose of azathioprine was 50 mg (25th to 75th percentile 50 to 50 mg) in the l-AZA/ALLO group and 200 mg (175 to 200 mg) in the AZA group. No protocol deviations were recorded in 35 (39%) of the 89 patients, 22 (four IFX) in the l-AZA/ALLO group and 13 (five IFX) in the AZA group. The study was not impacted by COVID-19.
Figure 1.
Trial profile
Table 1.
Characteristics of Included Participants. Baseline characteristics of study participants reported as numbers or median values (25th to 75th percentiles) allocated to azathioprine and allopurinol (L-AZA/ALLO) versus azathioprine (AZA) alone in intention to treat study population.
| L-AZA/ALLO (N = 47) | AZA (N = 42) | |
|---|---|---|
| Sex (male/female) | 23/24 | 16/26 |
| Ethnicity (Caucasians) | 47 | 42 |
| Age (years) | 30 (25‒48) | 37 (29‒42) |
| Smoking status | ||
| Never smoked | 24 | 21 |
| Previous | 7 | 2 |
| Smoker (ongoing) | 17 | 18 |
| Body Mass Index (kg/m2) | 24 (22‒26) | 25 (22‒27) |
| Ulcerative Colitis Phenotype | ||
| E1 - proctitis | 1 | 3 |
| E2 - left-sided | 24 | 18 |
| E3 - extensive | 23 | 20 |
| Disease duration (years) | 1 (1‒8) | 2 (1‒6.5) |
| Partial Mayo Score (inclusion visit) | 5 (1‒6) | 5 (1‒7) |
| Previous systemic prednisolone (total number) | 2 (1‒3) | 2 (1‒3) |
| Concomitant oral 5-ASA | 40 | 32 |
| AZA dose | 50 (50‒50) | 200 (175‒200) |
| Infliximab treatment | 15 | 16 |
At week 52, 20 (43%) patients in the l-AZA/ALLO group and nine (21%) patients in the AZA group had achieved remission (odds ratio 2·54 [95% CI 1·00 to 6·78], p < 0·048, Table 2). Twenty-two (47%) patients in the l-AZA/ALLO group versus 13 (31%) patients in the AZA group achieved partial clinical remission defined as a Mayo Score < 3 (odds ratio 1·54 [95% CI 1·01 to 2·36], p < 0·034, Table 2). Use of IFX before week 26 did not predict remission (odds ratio 1·29 [95% CI 1·50 to 3·31], p = 0·602, Supplementary Material 5). No clear difference was observed between the two groups regarding endoscopic remission (odds ratio 0·64 [95% CI 0·13 to 3·13], p < 0·072) among the 29 patients in remission who underwent endoscopic assessment at week 52 (Table 2). Likewise, there was no clear difference regarding histological remission (odds ratio 2·00 [95% CI 0·45 to 8·98]) (p = 0·362, Table 2). The faecal calprotectin levels at week 52 was 121 mg/kg [30 to 1210] in the (L-AZA/ALLO) and 49 mg/kg [30 to 1800] in the AZA group (p = 0·757, Table 2). The l-AZA/ALLO group had higher levels of 6TGN and lower levels of MeMP compared with AZA at week 6 and week 52, p < 0·001 (Table 2). Most patients (94%) in the l-AZA/ALLO group who were in remission at week 52 achieved 6-TGN levels higher than 230 pmol/8 × 108 RBC compared to 57% in the AZA group. No differences were observed in haemoglobin, C reactive protein, albumin or mean corpuscular volume (MCV) or health related quality of life assessed using the SIBDQ and SHS between the two groups (Table 2, Supplementary Material 4).
Table 2.
Primary and secondary outcomes in patients allocated to l-azathioprine (L-AZA)/ allopurinol (ALLO) versus AZA at week 52 (n = number of patients) in intention to treat analysis. The table shows the median (25th to 75th percentile) 6-thioguanine (TGN) and methylmercaptopurine (MeMP) levels (pmol/8 × 108 RBC) at week 6 and week 52, calprotectin (mg/kg) at week 52 and the two health-related quality of life scores at week 52 short inflammatory bowel disease questionnaire (SIBDQ) and short health scale (SHS).
| Outcomes | AZA/ALLO n = 47 | AZA n = 42 | P value | Odds Ratio | 95% CI |
|---|---|---|---|---|---|
| Primary outcome | |||||
| Remission | 20 (43%) | 9 (21%) | 0·048 | 2.54 | 1.00–6.78 |
| Secondary outcomes | |||||
| Total Mayo score < 3 | 22 (47%) | 13 (31%) | 0·034 | 1.54 | 1.01–2.36 |
| Endoscopic Mayo 0 | 19 (41%) | 8 (19%) | 0·072 | 0.64 | 0.13–3.13 |
| Histological score 0 | 16 (34%) | 7 (17%) | 0·362 | 2.00 | 0.45–8.98 |
| 6TGN Week 6 | 454 (341‒568) | 330 (207‒412) | 0.001 | ||
| 6‒TGN Week 52 | 475 (356‒594) | 303 (199‒401) | 0.001 | ||
| MeMP Week 6 | 246 (156‒299) | 3134 (1836‒5987) | 0.001 | ||
| MeMP Week 52 | 113 (66‒267) | 3305 (1334‒6153) | 0·001 | ||
| Calprotectin | 121 (30‒1210) | 49 (30‒1800) | 0·757 | ||
| SIBDQ | 57 (36‒68) | 55 (47‒65) | 0·621 | ||
| SHS | 61 (14‒279) | 73 (27‒117) | 0·396 |
Nine patients in the l-AZA/ALLO group and 12 in the AZA group experienced a clinical relapse, which was defined as a partial Mayo Score of 3 or higher. Nine patients in each group were analysed after week 8, by which point the thiopurine treatment should have been effective. The two groups did not differ regarding time to relapse, partial Mayo Score, calprotectin values, 6-TGN and MeMP levels (data not shown). All patients had measurable 6-TGN and MeMP levels. One patient in the l-AZA/ALLO group and two in the AZA group underwent concomitant treatment with IFX.
Seventy-seven of patients reported at least one adverse event (AE) with a total of 164 AE, Table 3. There were no unexpected AEs. Twelve patients (four in the l-AZA/ALLO and eight in the AZA group) reported no AE. Fourteen (30%) patients in the l-AZA/ALLO group and 16 (38%) patients in the AZA group had to be withdrawn due to an AE, Table 3. Most of these AE were moderate and resolved themselves within a matter of days. There was no difference in the type of AE between the two groups. The most common AE were liver test abnormality (an ALT higher than 100 U/L), infection, and gastrointestinal intolerance. The AE reported in patients who were withdrawn from the study are shown in Table 3. There were seven serious AE leading to hospitalization, six of them are included in the total number of AE, of which three were in the l-AZA/ALLO group and three were in the AZA group. Of these seven patients, four had an infection (two with gastroenteritis, one with pneumonia, and one with sepsis of unknown focus), one had a flare-up, one had leucopenia, and one had an increased ALT. All of these patients recovered with no permanent harm done.
Table 3.
Adverse events. The table includes the number of patients allocated to l-azathioprine (L-AZA)/ allopurinol (ALLO) versus AZA with adverse events among patients who received at least one dose of the study drug. The numbers in the parentheses indicate the time to withdrawal of treatment (weeks) for patients who had to discontinue treatments.
| L-AZA/ALLO N = 47 | AZA N = 42 | |
|---|---|---|
| Total Adverse Events (n)* | 80 | 77 |
| Serious Adverse Events | 3 (6%) | 3 (7%) |
| Total number of withdrawals | 14 (30%) | 16 (38%) |
| Causes for withdrawals | ||
| Increased Alanine aminotransferase | 3 (3‒15 weeks) | 3 (3‒7 weeks) |
| Nausea | 6 (2‒10 weeks) | 6 (3‒27 weeks) |
| Infections | 2 (2‒10 weeks) | 3 (1‒5 weeks) |
| Leukopenia | 1 (8 weeks) | 1 (5 weeks) |
| Rash | 1 (10 weeks) | 0 |
| Fatigue | 1 (25 weeks) | 0 |
| Joint/muscle pain | 0 | 2 (6‒42 weeks) |
| Headache | 0 | 1 (7 weeks) |
Some patients had more than one adverse event.
Discussion
This is the first prospective study among thiopurine-naïve patients with moderate-to-severe UC to evaluate the effect of l-AZA/ALLO combination therapy versus AZA monotherapy on steroid- and infliximab-free clinical remission rates after one year. We found that l-AZA/ALLO treatment was associated with a possible beneficial effect on our primary endpoint, which was a total Mayo Score of 1 or less with no rectal bleeding. This was a strict endpoint indicating complete remission. Overall, 29 (33%) of the randomised patients achieved this endpoint, 20 (43%) of patients in the l-AZA/ALLO group versus nine (21%) patients in the AZA group. With a Mayo score of less than 3 (47%) in the L-AZA/ALLO group and 31% in the AZA group were in steroid/infliximab – free remission at study end. In a recent retrospective study including 4968 patients with UC in monotherapy with AZA or 6MP, 52·7% were in remission during the treatment time.23 This is clearly a higher proportion than our 31% but may be explained by the retrospective versus prospective study designs, in addition to the use of 6-MP in the retrospective study and that we saw a higher rate of AEs leading to discontinuation of treatment.
The effect of thiopurines on endoscopic and histological mucosal healing has not previously been reported. In patients with remission at week 52 we found endoscopic mucosa healing in 95% of the AZA/ALLO patients and in 89% in the AZA patients. Although this difference was not statistically significant (p = 0.07) it suggests that AZA/ALLO treatment is more favourable in obtaining endoscopic mucosal healing than AZA treatment. In the l-AZA/ALLO group 80% of the patients in clinical remission had histological mucosal healing versus 78% in the AZA group. We found no significant difference in health-related quality of life, as assessed by the SIBDQ or SHS, between the two groups of patients in remission. In a univariable regression analysis, clinical remission at week 52 was not predicted by age, sex, BMI, disease duration, smoking, calprotectin a baseline or infliximab treatment before week 26.
The sample size calculation before study initiation was based on our pilot study. We estimated 27% as the relevant target difference as well as what we believe was a clinically meaningful difference. In this study we found a target difference of 22%. The small difference in estimated versus observed outcome is probably explained by the difference in study duration, i.e. 24 weeks in the pilot study versus 52 weeks in the present study. In our pilot study which included patients with UC or CD, and where we optimized AZA treatment based on 6-TGN levels, we observed that most patients in the l-AZA/ALLO group achieved 6-TGN values greater than 230 pmol/8 × 108 RBC after the first dosing and did not require adjustments to their treatment.6 In the current study, the thiopurine metabolites were blinded, and no treatment optimization was allowed. Despite this, we found that 94% of the patients in the l-AZA/ALLO group achieved 6-TGN levels greater than 230 pmol/8 × 108 RBC, which was our therapeutic goal, compared to only 57% in the AZA group. In daily practice, not all clinicians have the capability to measure 6-TGN and MeMP metabolites, so it is a valuable observation that when using l-AZA/ALLO combination therapy there is no absolute need for therapeutic drug monitoring in patients in clinical remission.
Twenty-one patients (24%) had to be prematurely withdrawn from the study due to a clinical relapse, which for the purposes of this study was defined as a partial Mayo Score of 3 or higher. This proportion was larger than what we observed in our pilot study, where only 8% of patients were withdrawn due to a relapse before week 24. As these current data are from a larger cohort and 52 weeks versus 24 weeks of study, this rate of relapse is the more reliable and is in accordance with data from a newly published retrospective study of 193 patients with UC, where a relapse rate of 17% after one year was observed among biologic-naïve patients treated with AZA.24
Eighteen patients, nine in each group (20%), had a Mayo Score of 3 or higher after week 8, at which point treatment with thiopurine was expected to be effective. Relapses were mostly observed after discontinuation of prednisolone therapy, and in two patients in the AZA group after stopping IFX at week 26. As the levels of 6-TGN in patients with a Mayo Score of 3 or higher were similar to those in patients with a Mayo Score of 1 or less, it suggests that at least 20% of patients with UC do not respond to treatment with thiopurines.
Calprotectin higher than 200 mg/kg at week 26, was a statistically significant predictor of relapse. This observation could be taken to indicate that increased calprotectin values among patients in clinical remission after a course of steroids/infliximab and concomitant thiopurine treatment, and with therapeutic 6-TGN levels, whether treated with AZA alone or an l-AZA/ALLO combination, should be considered for more intense treatment with optimization of thiopurine treatment (if possible) or initiation of biologics in order to prevent a relapse.
In this study of thiopurine-naïve patients with UC, 77 of all patients reported adverse events, leading to the withdrawal from the study of 38% of patients in the AZA group and 30% in the l-AZA/ALLO group. This rate was expected in the AZA monotherapy group and is similar to that reported in other prospective studies of thiopurine-naïve patients with IBD.5,6 However, this study also demonstrated that AE occur in a considerable number of patients undergoing treatment with l-AZA/ALLO. Most of the AE were observed in the first four months after initiating therapy. Only three patients were withdrawn after 26 weeks and metabolite measurements revealed that two of these patients had not taken their medication. AE very rarely occur beyond four months after initiating treatment, an observation reported elsewhere.25 These data support the findings from our pilot study that the safety of combination treatment with l-AZA/ALLO is at least as good as AZA monotherapy. Patients experiencing AE may benefit from changing their therapy to l-AZA/ALLO if previously treated with AZA alone. If previously treated with l-AZA/ALLO switching to 6MP is recommended, since shifting from AZA to 6MP has been shown to reduce AEs from AZA therapy by 50%.26 Alternatively, treatment with thioguanine (TG) is an option, although it does not seem to reduce AE or increase clinical remission rates any better than l-AZA/ALLO,27 but TG could be at treatment option for patients intolerant to ALLO.
There have been some concerns that higher 6-TGN values may increase the short-term risk for leucopenia and infections. We did not observe this in our study and nor was it found to be the case in the largest observational study published thus far.28 In fact, a recent nationwide inflammatory bowel disease cohort study showed that treatment with concomitant thiopurine-allopurinol is as safe as thiopurines alone.29
There are some limitations to the present study. First, it was not blinded. Second, and an important limitation, we did not optimize treatment based on 6-TGN and MeMP levels, as is standard in many centers. The reason for this is that if low values of 6-TGN are found in a patient undergoing AZA therapy, it is typically a result of thiopurine shunting. In that case, increasing the dose will often result in a modest increase in 6-TGN levels, but a more pronounced increase in MeMP levels. The correct way to overcome the problem is to shift to l-AZA/ALLO therapy, as has been reported several times in retrospective studies.8, 9, 10, 11, 12, 13, 14 A third limitation is that our study's randomisation was imbalanced. As the randomisation occurred in blocks of six, the imbalance happened due to some of the participating centers recruiting fewer patients than others. We should have instead managed the randomisation centrally. Fourth, as some centers included few patients there could have been a difference in the handling of the patients.
In conclusion, the results of this study show that among patients with moderate-to-severe UC l-AZA/ALLO therapy leads to higher remission rates after one year than AZA alone. Furthermore, the l-AZA/ALLO combination therapy is as safe as AZA monotherapy. Perhaps the time has come to recommend that patients with UC with a normal TPMT who are about to begin AZA treatment should instead start l-AZA/ALLO therapy. This recommendation is in line with newly published retrospective data from the UK in a large single center population of IBD patients.30 We do not recommend this approach if TPMT has not been measured because no data exist as to whether it could lead to unexpected AE in patients with a heterozygote TPMT genotype. When combination therapy is given, the dose of AZA should be 50 mg for patients weighing between 50 and 100 kg and 75 mg for patients over 100 kg, in combination with 100 mg allopurinol. Adopting this strategy doubles the likelihood of patients being in steroid- and infliximab-free clinical, endoscopic, and histological remission after one year. In cases of AE related to l-AZA/ALLO therapy, we recommend prescribing 6MP. This study also shows that at least 20% of patients do not respond to AZA treatment alone or to combination treatment with ALLO. Furthermore, increased faecal calprotectin (anything greater than 200 mg/kg) appears to be a surrogate marker for likely relapse, even before symptoms develop.
Contributors
MKK, KT, LLG and AMN designed the trial.
MKK, KT, SBT, JTB, JB, AE, IBG, KLH, AN, SW, LL, LLG and AMN helped plan the trial and were responsible for acquisition of data.
JKM developed the method for metabolites analysis.
PHI and HGRJ reviewed the tissue samples.
LLG performed the statistical analyses.
MKK, KT, LLG and AMN drafted the manuscript.
KLH, LLG and AMN assessed and verified the underlying data.
All authors critically revised the manuscript, had full access to all data in the study and take full responsibility for the decision to submit it for publication.
Declaration of interests
MKK, LLG, EA, IBG, LL, JTB, JKM, KLH, AN, PHI, and HGRJ: none. AMN, SBT: Has received a grant from The Regions Medicinepulje to conduct the study. SW: Has received personal fees from Takeda and Tillotts outside the submitted work. JB: Has been a consultant and/or received advisory board fees and/or research grants from Abbvie, MSD, Takeda, Janssen, Pfizer, Bristol Myers Squibb and Gilead. KT: Has received personal fees from Ferring Lægemidler A/S, Takeda, Tillotts and Pfizer outside the submitted work. AN: Has received grants from Pharmacosmos and non-financial support from Janssen and Takeda, outside the submitted work.
Acknowledgments
Funding
The Capital Regions of Denmark (Regions Medicinpulje (6062/16)).
Data sharing
The AAUC group will evaluate proposals submitted by qualified researchers to the corresponding author.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.eclinm.2022.101332.
Appendix. Supplementary materials
References
- 1.Rubin D., Ananthakrishnan A.N., Siegel C.A., Sauer B.G., Long M.D. ACG clinical guideline. Ulcerative colitis in adults. Am J Gastroenterol. 2019;114:384–413. doi: 10.14309/ajg.0000000000000152. [DOI] [PubMed] [Google Scholar]
- 2.Hanauer S.B., Sandborn W.J., Lichtenstein G.R. Evolving considerations for thiopurine therapy for inflammatory bowel diseases - a clinical practice update: commentary. Gastroenterology. 2019;156:36–42. doi: 10.1053/j.gastro.2018.08.043. [DOI] [PubMed] [Google Scholar]
- 3.Colombel J.F., Sandborn W.J., Reinisch W., et al. Infliximab, azathioprine, or combination therapy for Crohn's disease. N Engl J Med. 2010;362:1383–1395. doi: 10.1056/NEJMoa0904492. [DOI] [PubMed] [Google Scholar]
- 4.Smith M.A., Blaker P., Marinaki A.M., Anderson S.H., Irving P.M., Sanderson J.D. Optimising outcome on thiopurines in inflammatory bowel disease by co-prescription of allopurinol. J Crohns Colitis. 2012;6:905–912. doi: 10.1016/j.crohns.2012.02.007. [DOI] [PubMed] [Google Scholar]
- 5.Dassopoulos T., Dubinsky M.C., Bentsen J.L., et al. Randomised clinical trial: individualised vs. weight-based dosing of azathioprine in Crohn's disease. AP&T. 2014;39:163–175. doi: 10.1111/apt.12555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kiszka-Kanowitz M., Theede K., Mertz-Nielsen A. Randomized clinical trial: a pilot study comparing efficacy of low-dose azathioprine and allopurinol to azathioprine on clinical outcomes in inflammatory bowel disease. Scand J Gastroenterol. 2016;51:1470–1475. doi: 10.1080/00365521.2016.1216589. [DOI] [PubMed] [Google Scholar]
- 7.Elion G.B. The purine path to chemotherapy. Science. 1989;244:41–47. doi: 10.1126/science.2649979. [DOI] [PubMed] [Google Scholar]
- 8.Ansari A., Elliott T., Baburajan B., et al. Long term outcome of using allopurinol co-therapy as a strategy for overcoming thiopurine hepatotoxicity in treating inflammatory bowel disease. AP&T. 2008;28:734–741. doi: 10.1111/j.1365-2036.2008.03782.x. [DOI] [PubMed] [Google Scholar]
- 9.Sparrow M.P., Hande S.A., Friedman S., et al. Allopurinol safely and effectively optimizes tioguanine metabolites in inflammatory bowel disease patients not responding to azathioprine and mercaptopurine. AP&T. 2005;22:441–446. doi: 10.1111/j.1365-2036.2005.02583.x. [DOI] [PubMed] [Google Scholar]
- 10.Sparrow M.P., Hande S.A., Friedman S., Cao D., Hanauer S.B. Effect of allopurinol on clinical outcomes in inflammatory bowel disease nonresponders to azathioprine or 6-mercaptopurine. Clin Gastroenterol Hepatol. 2007;5:209–214. doi: 10.1016/j.cgh.2006.11.020. [DOI] [PubMed] [Google Scholar]
- 11.Leung Y., Sparrow M.P., Schwartz M., Hanauer S.B. Long term efficacy and safty of allopurinol and azathioprine or 6-mercaptopurine in patients with inflammatory bowel disease. J Crohns Colitis. 2009;3:162–167. doi: 10.1016/j.crohns.2009.02.003. [DOI] [PubMed] [Google Scholar]
- 12.Ansari A., Patel N., Sanderson J., O'Donohue J., Duley J.A., Florin T.H. Low-dose azathioprine or mercaptopurine in combination with allopurinol can bypass many adverse drug reactions in patients with inflammatory bowel disease. AP&T. 2010;31:640–647. doi: 10.1111/j.1365-2036.2009.04221.x. [DOI] [PubMed] [Google Scholar]
- 13.Friedman A.B., Brown S.J., Bampton P., et al. Randomised clinical trial: efficacy, safety, and dosage of adjunctive allopurinol in azthioprine/mercaptopurine nonresponders (AAA study) AP&T. 2018;47:1092–1102. doi: 10.1111/apt.14571. [DOI] [PubMed] [Google Scholar]
- 14.Hoentjen F., Seinen M.L., Hanauer S.B., et al. Safety and effectiveness of long-term allopurinol-thiopurine maintenance treatment in inflammatory bowel disease. Inflamm Bowel Dis. 2013;19:363–369. doi: 10.1002/ibd.23021. [DOI] [PubMed] [Google Scholar]
- 15.Kirchherr H., Shipkova M., von Ahsen N. Improved method for therapeutic drug monitoring of 6-thioguanine nucleotides and 6-methylmercaptopurine in whole-blood by LC/MSMS using isotope-labeled internal standards. Ther Drug Monit. 2013;35:313–321. doi: 10.1097/FTD.0b013e318283ed5d. [DOI] [PubMed] [Google Scholar]
- 16.Casteele N.V., Herfarth H., Katz J., Falck-Ytter Y., Singh S. American gastroenterological association institute technical review on the role of therapeutic drug monitoring in the management of inflammatory bowel diseases. Gastroenterology. 2017;153:835–857. doi: 10.1053/j.gastro.2017.07.031. [DOI] [PubMed] [Google Scholar]
- 17.D'Haens G., Sandborn W.J., Feagan B.G., et al. A review of activity indices and efficacy end points for clinical trials of medical theraphy in adults with ulcerative colitis. Gastroenterology. 2007;132:763–786. doi: 10.1053/j.gastro.2006.12.038. [DOI] [PubMed] [Google Scholar]
- 18.Fiel M., Qin L., Suriawinata A., et al. Histological grading of disease activity in chronic IBD: inter- and intra-observer variation among pathologists with different levels of experience. Mod Pathol. 2003;16:118A. [Google Scholar]
- 19.Theede K., Holck S., Ibsen P., Kallemose T., Nordgaard-Lassen I., Nielsen A.M. Level of faecal calprotectin correlates with endoscopic and histologic inflammation and identifies patients with mucosal healing of ulcerative colitis. Clin Gastroenterol Hepatol. 2015;13:1929–1936. doi: 10.1016/j.cgh.2015.05.038. [DOI] [PubMed] [Google Scholar]
- 20.Theede K., Holck S., Ibsen P., Kallemose T., Nordgaard-Lassen I., Nielsen A.M. Faecal calprotectin predicts relapse and histological mucosal healing in ulcerative colitis. Inflam Bowel Dis. 2016;16:1042–1048. doi: 10.1097/MIB.0000000000000736. [DOI] [PubMed] [Google Scholar]
- 21.Hjortswang H., Järnerot G., Curman B., et al. The short health scale: a valid measure of subjective health in ulcerative colitis. Scand J Gastroenterol. 2006;41:1196–1203. doi: 10.1080/00365520600610618. [DOI] [PubMed] [Google Scholar]
- 22.Irvine E.J., Zhou Q., Thompson A.K. The short inflammatory bowel disease questionnaire is reliable and responsive to clinically important change in ulcerative colitis. Am J Gastroenterol. 1996;91:1571–1578. doi: 10.1111/j.1572-0241.2001.04682.x. [DOI] [PubMed] [Google Scholar]
- 23.Stournaras E., Qian W., Pappas A., Hong Y.Y., et al. Thiopurine monotherapy is effective in ulcerative colitis but significantly less so in Crohn's disease: long-term outcomes for 11 928 patients in the UK inflammatory bowel disease. Gut. 2021;70:677–686. doi: 10.1136/gutjnl-2019-320185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Satohiro M., Hirosato M. Real-world long-term remission maintenance for 10 years with thiopurines in ulcerative colitis. Crohns Colitis. 2021;3:1–9. doi: 10.1093/crocol/otab003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kreijne J.E., de Vries A.C., de Veer R.C., et al. Limited added value of laboratory monitoring in thiopurine maintenance monotherapy in inflammatory bowel disease patients. AP&T. 2020;51:1353–1364. doi: 10.1111/apt.15734. [DOI] [PubMed] [Google Scholar]
- 26.Hindorf U., Johansson M., Eriksson A., Kvifors E., Almer S.H. Mercaptopurine treatment should be considered in azathioprine intolerant patients with inflammatory bowel disease. AP&T. 2009;15:654–6127. doi: 10.1111/j.1365-2036.2008.03925.x. [DOI] [PubMed] [Google Scholar]
- 27.Biemans V.B.C., Savelkoul E., Gabriëls M., et al. A comparative analysis of tioguanine versus low-dose thiopurines combined with allopurinol in inflammatory bowel disease patients. AP&T. 2020;51:1076–86.28. doi: 10.1111/apt.15730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pavlidis P., Stamoulos P., Abdulrehman A., et al. Long-term safety and efficacy of low-dose azathioprine and allopurinol co-therapy in inflammatory bowel disease: a large observational study. Inflamm Bowel Dis. 2016;22:1639–1646. doi: 10.1097/MIB.0000000000000827. [DOI] [PubMed] [Google Scholar]
- 29.Thomsen S.B., Allin K.H., Burisch J., et al. Outcome of concomitant treatment with thiopurines and allopurinol in patients with inflammatory bowel disease: a nationwide Danish cohort study. United Eur Gastroenterol J. 2020;8:68–76. doi: 10.1177/2050640619868387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.van Liege E., Bayoumy A.B., Mulder C.J.J., et al. Azathioprine with Allopurinol is a promising first-line therapy for inflammatory bowel diseases. Dig Dis Sci. 2021 doi: 10.1007/s10620-021-07273-y. Nov 2Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.