Abstract
This study investigated the effects of supplementation with a cyanidin- and delphinidin-rich extract (CDRE) on the postprandial dysmetabolism, inflammation, and redox and insulin signaling, triggered by the consumption of a high fat meal (HFM) in healthy individuals. Participants (n = 25) consumed a 1026-kcal HFM simultaneously with either the CDRE providing 320.4 mg of anthocyanins (90% cyanidin and delphinidin) or placebo. Diets were randomly assigned in a double blind, placebo-controlled crossover design. Blood was collected prior to (fasted, time 0), and for 5 h after meal consumption; plasma, serum, and peripheral blood mononuclear cells (PBMC) were isolated. AC metabolites were detected in serum as early as 30 min after CDRE consumption. The CDRE mitigated HFM-induced endotoxemia, reducing increases in plasma LPS and LPS-binding protein. The CDRE also reduced other events associated with HFM-triggered postprandial dysmetabolism including: i) plasma glucose and triglyceride increases; ii) TNFα and NOX4 upregulation in PBMC; and iii) JNK1/2 activation in PBMC. The CDRE did not significantly affect HFM-mediated increases in plasma insulin, GLP-1, GLP-2, GIP, and LDL- and HDL-cholesterol, and IKK phosphorylation in PBMC. In summary, dietary AC, i.e. cyanidin and delphinidin, exerted beneficial actions against unhealthy diets by modulating the associated postprandial dysmetabolism, endotoxemia, alterations of glycemia and lipidemia, and redox and insulin signaling.
Keywords: Flavonoids, Obesity, Inflammation, Endotoxemia, Oxidative stress, Antioxidants
Graphical abstract
Abbreviations
- AC
anthocyanidins
- AUC
area under the curve
- CDRE
cyanidin and delphinidin-rich extract
- GIP
gastric inhibitory polypeptide
- GLP-1
glucagon-like peptide-1
- GLP-2
glucagon-like peptide-2
- HFM
high fat meal
- IKK
IκB kinase
- IL-1β
interleukin-1 beta
- IL-8
interleukin-8
- IL-18
interleukin-18
- JNK1/2
c-Jun N-terminal kinases 1/2
- LBP
LPS-binding protein
- LPS
lipopolysaccharides
- PBMC
peripheral blood mononuclear cells
- PTP1B
protein tyrosine phosphate 1B
- NOX2
NADPH oxidase 2
- NOX4
NADPH oxidase 4
- T2D
type 2 diabetes
- TLR4
toll-like receptor 4
- TNFα
tumor necrosis factor alpha
1. Introduction
Unhealthy diets, e.g. Western style diets high in fats and carbohydrates, can trigger inflammatory conditions that are associated with the development of several pathologies including type 2 diabetes (T2D), nonalcoholic fatty liver disease (NAFLD), cardiovascular disease, cancer, and certain neuropathies [[1], [2], [3], [4], [5], [6], [7]]. Oral intake of bioactives to counteract inflammation is a strategy with the potential for high impact on human health, especially when these bioactives are present in diets and dietary components, e.g. fruits and vegetables, in amounts provided by realistic intakes.
Following the ingestion of any food, a physiological postprandial response occurs as a result of nutrient absorption and metabolism. However, excess consumption of lipids and/or carbohydrates leads to a series of short-term events described as postprandial dysmetabolism. These events include alterations in glucose and lipid metabolism, endotoxemia, inflammation and oxidative stress. Importantly, postprandial dysmetabolism has been associated with a higher risk, among other pathologies, for cardiovascular disease and mortality [[8], [9], [10]], nonalcoholic fatty liver disease [11], and T2D progression [12].
It is well documented that even a single meal high in fat and/or carbohydrates can lead to postprandial hyperglycemia, hypertriglyceridemia and/or endotoxemia [[13], [14], [15]]. High fat diets cause metabolic endotoxemia mainly through intestinal permeabilization [16] and/or co-transport of lipopolysaccharides (LPS) with chylomicrons [17]. Once in the circulation, endotoxins can reach different cells and organs where they promote, among other effects, the production of proinflammatory molecules, e.g. cytokines and chemokines, leading to systemic inflammation and oxidative stress, which directly affect metabolic pathways [7,18]. Additionally, postprandial dyslipidemia per se contributes to altered glucose metabolism, insulin resistance and inflammation [19]. Mechanisms involved in inflammation- and oxidative stress-associated insulin resistance include the activation of the redox sensitive kinases, i.e. c-Jun N-terminal kinases (JNK) and IκB kinase (IKK), and of transcription factor NF-κB downstream of IKK. The activation of both, JNK [20] and IKK [21], as well as the upregulation of the NF-κB-regulated protein tyrosine phosphatase 1B (PTP1B) [22], inhibit the insulin signaling pathway resulting in tissue insulin resistance.
As a counterbalance to the aforementioned proinflammatory consequences of high fat diets, the consumption of select fruits and vegetables could prevent and/or attenuate these unhealthy conditions. Evidence for the latter is complex when considering overall intakes, types of fruits and vegetables consumed, and other variables associated with population-based studies [[23], [24], [25]]. On the other hand, a large body of evidence from experimental animal models suggests consistent benefits of select phytochemicals against the development of obesity and associated pathologies mainly triggered by consumption of high fructose and/or high fat diets. Among those phytochemicals, anthocyanidins (AC) are being actively investigated for their potential to mitigate unhealthy conditions, particularly metabolic disorders. In this regard, mounting evidence supports a potential beneficial action of AC consumption on T2D [26] and cardiovascular disease [27]. Furthermore, consumption of AC-rich foods has been inversely correlated with overall mortality [28].
Chemically, AC are aglycones of anthocyanins, which are the flavonoids that provide color to grapes, dark berries (e.g. blueberries, black currants, and bilberries), purple/black corn, and black rice, among other fruits and vegetables. According to the number and position of functional groups around the flavonoid scaffold, AC are sub-classified into cyanidins, delphinidins, malvidins, petunidins, peonidins and pelargonidins [29]. These differences in substitutions have been shown to have a major impact on their mechanisms of action. In this regard, we recently reported that cyanidin and delphinidin were more effective than malvidin, petunidin and peonidin at mitigating inflammation, e.g. preventing tumor necrosis factor alpha (TNFα)-induced activation of transcription factor NF-κB in an intestinal cell (Caco-2) model [30].
Understanding the mechanisms by which AC modify cellular functions is crucial to define public health recommendations in terms of diets, i.e. foods and potential supplementation. Regulation of inflammation and oxidative stress are central mechanisms involved in the capacity of cyanidin- and delphinidin-rich extracts to mitigate the deleterious gastrointestinal and metabolic effects of chronic high fat dietary consumption in mice [[31], [32], [33]]. In terms of redox regulation, it is unfeasible that AC could act as direct antioxidants given their poor intestinal absorption that results in low tissue concentrations. However, AC and/or AC metabolites can act regulating redox homeostasis, e.g. modulating NADPH oxidase expression, oxidative stress and inflammation. Importantly, cyanidin and delphinidin have been shown to prevent high fat diet-induced endotoxemia in mice by regulating intestinal permeability [33] through redox-dependent and independent mechanisms [[34], [35], [36]].
This study investigated the beneficial effects of supplementation in healthy individuals with a cyanidin- and delphinidin-rich extract (CDRE), firstly, on parameters of inflammation, i.e. endotoxemia, and secondly, on parameters of lipid and glucose metabolism and redox signaling. These parameters were associated with changes in redox homeostasis and signaling. Volunteers consumed a 1026-kcal high-fat meal (HFM) simultaneously with either the CDRE, or a placebo in a randomly assigned, double blind, placebo-controlled crossover intervention. The CDRE mitigated HFM-induced acute endotoxemia and several parameters of postprandial dysmetabolism associated with the consumption of the HFM.
2. Materials and methods
2.1. Study design
The study was a randomly assigned, double blind, placebo-controlled crossover intervention comparing the effects of supplementation with CDRE or placebo. Each intervention (visit) lasted 5 h after consumption of the HFM and supplements, separated by a washout period of 7–30 d between visits. The study (registered at http://www.clinicaltrials.gov as NCT03309982) was conducted at the Ragle Facility at the University of California, Davis (UCD) in accordance with the Declaration of Helsinki guidelines. All procedures were approved by UCD IRB administration and UCD Social & Behavioral Committee. Written informed consent was obtained from the study volunteers. Clinical interventions were conducted between December 2017 and March 2018.
2.2. Study population
Twenty-five healthy volunteers 19–35 y, body mass index >21 and <29.9 kg/m2, were recruited from the Davis/Sacramento area to participate in the study. Exclusion criteria included: systolic blood pressure ≥160 mm Hg or diastolic blood pressure ≥95 mm Hg, fasting glucose blood concentration <50 mg/dL or >100 mg/dL, fasting serum triglycerides >150 mg/dL, severe or incompatible dietary restrictions, e.g. vegetarians, current use of herbal supplements, anti-inflammatory medications or medications that interfere with insulin metabolism, regular participation in endurance exercise activities, current tobacco smoker or user of tobacco products within the previous year, heavy alcohol daily intake or substance abuse or dependence, history of stroke, hepatic, kidney, thyroid disease or cancer, malabsorption or gastrointestinal tract disorders or surgery, or severe eating disorders, presence of depression, anxiety or other psychiatric conditions, diarrhea or oral antibiotic intake within the last 4 w, weight change (>5%) in the last 8 w, allergy or sensitivity to components in the CDRE and/or HFM. In addition, to be included in the final data analysis participants had to have a healthy metabolic status in all the determined parameters as confirmed by the study PI.
2.3. Sample size estimation
The number of participants was determined by a power calculation using data from two previously published studies determining the impact of high-fat meals on endotoxin levels in plasma [37,38]. Using this data, a sample size of 24 participants was calculated to detect a mean change of 0.12 EU/mL in the average endotoxin level with power equal to 0.80 and a Type I error of 0.05.
2.4. Recruitment and screening
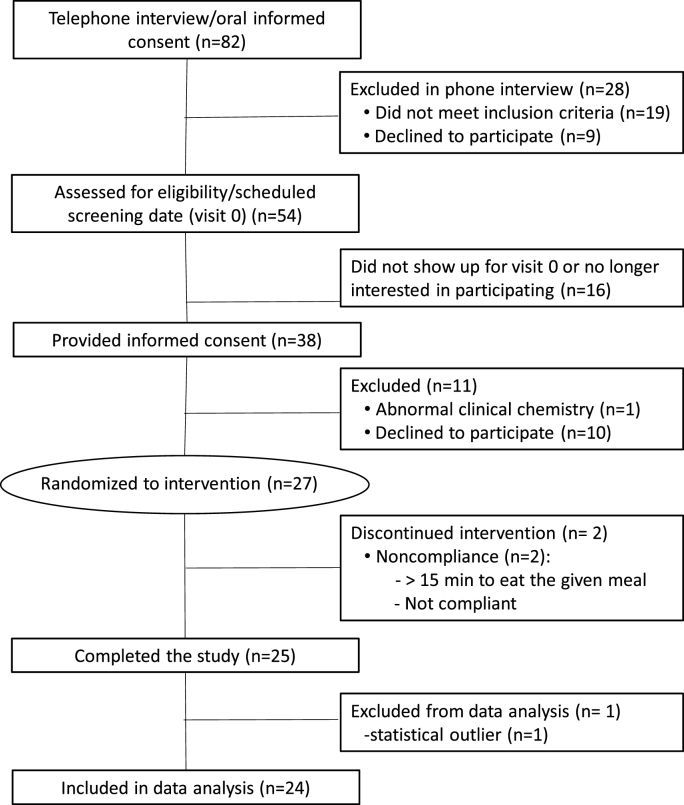
Recruitment and screening followed the Consolidated Standards of Reporting Trials strategy (CONSORT diagram Fig. 1). Briefly, female and male volunteers that showed interest in the study were provided with information about the design and the procedures. If willing to commit to the study, a screening phone interview was conducted to assess their potential eligibility. Those who showed interest and met the basics of the inclusion and exclusion criteria were called for an in-person visit (visit 0). Participants were asked to attend in a fasted state (≥12 h). The written informed consent was explained, and upon approval, anthropometric parameters were recorded and a finger-prick blood sample was obtained to determine glucose and triglycerides using a CardioCheck analyzer (PTS Diagnostic, IN, USA). Participants that met inclusion criteria were invited to be part of the clinical study and to schedule two in-person visits separated by a washout period of: i) at least 7 d to prevent significant carryover effects based on the fact that AC and their metabolites tend to disappear from circulation within 48 h of intake; ii) less than 30 d, to prevent major changes in lifestyle, especially those associated to periods of food overconsumption and seasonal influences.
Fig. 1.
CONSORT study flowchart.
2.5. Clinical interventions
The day of visit 0, participants were provided with dietary restriction and guideline instructions asking them to: i) not consume (poly)phenol-rich foods for 24 h before each of the study visits; ii) consume a similar low-fat dinner the evening before each meal (before starting the 12 h fasting); and iii) complete a 3-d food record before visits 1 and 2 (form provided) to assess compliance with the dietary requirements. The days of the study visits, upon arrival glucose and triglyceride levels were assessed in blood samples collected by finger prick using the CardioCheck analyzer to confirm that participants were in a fasted state and their adherence to the inclusion/exclusion criteria. Body weight and blood pressure were also determined. Participants were then asked to consume the placebo or CDRE following the randomization scheme. Powders were packaged in sealed black sachets, which were coded to blind the study personnel to the treatment. Study personnel dissolved the powder in 200 mL of water, which was provided to each participant along with the prepared HFM. The participants were asked to drink the CDRE supplement drink and then eat the HFM within 15 min. The HFM (320 g) consisted of English muffin bread, sausage, egg and cheese, obtained from the US market with carotenoid-free palm oil added to bring the total dietary fat to the desired level. The total energy content of the HFM was 1026 Kcal with 70.5 g of fat (29.8 g saturated fat), 270 mg cholesterol, 70.2 g carbohydrate, and 33 g protein with a total of 62% of the energy originated from fat, 25% from carbohydrates, and 13% from protein. Venous blood was taken at time 0 (baseline before meal intake) and 0.5, 1, 2, 3, and 5 h after consumption of the HFM, and collected in three separated tubes for plasma, serum and peripheral blood mononuclear cells (PBMC) isolation.
2.6. Composition of test product and placebo
The tested cyanidin and delphinidin-rich extract (CDRE) was a blend of anthocyanidin-rich powdered plant extracts. The CDRE powder (4 g) consisted of 1 g of AC-rich extracts (150 mg bilberry extract, 230 mg black currant extract, and 620 mg black rice extract) (Table 1) and 3 g of a mix of maltodextrins. The placebo powder (4 g) consisted of 3.85 g of the same maltodextrins included in the CDRE plus 125 mg of Red Dye No. 40, and 25 mg of Blue Dye No.1. CDRE and placebo were manufactured by Deseret Laboratories, Inc. (St. George, UT) exclusively for NSE Products, Inc. (Pharmanex), Provo, UT). The presence of (poly)-phenols in the CDRE was determined by HPLC (a service generously provided by Dr. Mary Ann Lila's lab, North Carolina State University).
Table 1.
CDRE anthocyanidin compositiona.
| Compound | Content (mg/g of CDRE) |
|---|---|
| Cyanidins | 166.4 ± 0.5 |
| Delphinidins | 121.7 ± 0.4 |
| Peonidins | 16.7 ± 0.1 |
| Petunidins | 5.1 ± .0.1 |
| Malvidin | 7.6 ± 0.1 |
| Other anthocyanins | 3.0 ± 0.1 |
| Total anthocyanins | 320.4 ± 0.7 |
Values are means ± SE.
Detailed concentration of anthocyanins in each individual extract is included in Supplemental Table S3, and (poly)phenol composition of the extracts in Supplemental Table S4.
2.7. Biochemical analyses
For plasma and serum isolation, blood samples were collected in EDTA/sodium citrate and in anti-coagulant free tubes, respectively. Immediately after collection, samples were centrifuged at room temperature for 15 min at 3000×g. Serum samples were acidified to pH 2.4 with formic acid to prevent anthocyanin degradation. The obtained plasma and serum were then stored at −80 °C until further analysis. The following parameters were measured in plasma by ELISA kits following the manufacturer's instructions: lipopolysaccharides (LPS) (Abbexa, TX, USA), LPS-binding protein (LBP), free fatty acid (FFA) (Abcam, CA, USA), gastric inhibitory polypeptide (GIP), glucagon-like peptide-1 (GLP-1), glucagon-like peptide-2 (GLP-2), insulin, leptin, adiponectin (Crystal Chem, IL, USA), and ghrelin (BioVendor, NC, USA). Triglycerides, total- and HDL-cholesterol (Wiener Lab, Santa Fe, Argentina), and glucose (Sigma-Aldrich, MO, USA) concentrations were measured by colorimetric assays following the manufacturer's instructions. LDL-cholesterol was estimated using the Friedewald equation.
2.8. PBMC isolation
Blood was collected in BD Vacutainer CPT Cell Preparation tubes with sodium citrate (BD, NJ, USA). Immediately after collection, blood samples were centrifuged at room temperature at 1800×g for 30 min. The PBMC left in the tube were collected and washed twice with phosphate-buffered saline (PBS) by centrifugation at room temperature for 15 min at 300×g. The pellet was resuspended in lysis buffer (Thermo Fisher Scientific, MA, USA) or TRIzol reagent (Invitrogen, CA, USA) for the subsequent extraction of protein or RNA, respectively.
2.9. Western blot analysis
Proteins from the PBMC were extracted with lysis buffer containing protease and phosphatase inhibitors (Thermo Fisher Scientific, MA, USA). From the total protein extracted, 30 μg were denatured with Laemmli buffer, separated by reducing 10% (w/v) polyacrylamide gel electrophoresis, and electroblotted onto polyvinylidene fluoride (PVDF) membranes. Membranes were blocked for 1 h in 5% (w/v) fat-free milk, and subsequently incubated in the presence of the corresponding primary antibody (1:1000 dilution) in BSA 5% (w/v) overnight at 4 °C. Primary antibodies for p-IKK (#2697), IKK (#2684), p-JNK 1/2 (#9255), JNK2 (#4672) and β-actin (#12620) were from Cell Signaling Technology (MA, USA). The antibody for PTP1B (sc-1718) was from Santa Cruz Biotechnology (TX, USA). After incubation for 90 min at room temperature in the presence of the corresponding secondary antibody (HRP conjugated) (1:10,000 dilution), the conjugates were visualized using an ECL system in a Phospho Imager 840 (Amersham Pharmacia Biotech. Inc., NJ, USA).
2.10. RNA isolation and quantitative PCR (qPCR) analysis
Parameters of Inflammation, and NADPH oxidases were assessed by qPCR. RNA was extracted from PBMC using TRIzol reagent following the manufacturer's instructions. RNA was converted into cDNA using high-capacity cDNA Reverse Transcriptase (Applied Biosystems, NY, USA). RNA expression of IL-1β, IL-8, IL-18, TNFα, TLR-4, NOX2, and NOX4 was assessed by qPCR (iCycler, Bio-Rad, CA, USA) using primers described in Supplemental Table S1. β-actin was used as the housekeeping gene. The relative fold change in gene expression was calculated using the 2−ΔΔCt method [39].
2.11. Determination of AC and AC metabolites in serum
A broad-spectrum quantitative MS/MS assay was optimized to detect 150 analytes, which were quantified relative to 107 authentic commercial and synthetic standards (Supplemental Table S2). The metabolites were purified from 100 μL human serum by 96-well plate solid phase extraction (SPE; Strata™-X Polymeric Reversed Phase, microelution 2 mg/well). The solid phase extraction treated samples were chromatographically separated and quantified using Exion ultra-high performance liquid chromatography (UHPLC)-tandem mass spectrometry (SCIEX QTRAP 6500+ESI-MS/MS). Samples were injected into a Kinetex PFP UPLC column (1.7 μm, 100 Å, 100 mm × 2.1 mm; Phenomenex) with oven temperature maintained at 37 °C. Mobile phase A and B consisted of 0.1% (v/v) formic acid in water and 0.1% (v/v) formic acid in acetonitrile (respectively), with binary gradient ranging from 2% B to 90% B over 30 min and flow rate gradient from 0.55 ml/min to 0.75 ml/min. MS/MS scanning was accomplished using advanced scheduled multiple reaction monitoring (ADsMRM) with polarity switching, in Analyst (v.1.6.3, SCIEX) with quantitation using MultiQuant (v.3.0.2, SCIEX) software platforms.
2.12. Statistical analysis
Data are expressed as the means ± SE of 24 subjects. Analysis of variance (ANOVA) for repeated measures was used to evaluate the experimental effects of time (0, 0.5, 1, 2, 3, and 5 h), treatment (CDRE or placebo), and any interaction between these two factors, i. e. time-by-treatment. When significant main effects were identified, the Tukey Honest Significant Difference (HSD) post-hoc procedure was used to analyze significant differences among means. The individual time point data values for each blood parameter were used to compute an “Incremental Area Under Curve” (AUC) that represented the overall amount of change of each parameter. Post-hoc ANOVA and paired t-test were used for comparing AUC and other variables. p < 0.05 was considered statistically significant.
3. Results
3.1. Composition of the cyanidin- and delphinidin-rich extract (CDRE)
The total anthocyanin content in the CDRE was 320.4 mg/g (Table 1). Cyanidin and delphinidin glucosides constituted 90% (52% and 38%, respectively) of the total anthocyanins present in the CDRE (Supplementary Table S3). All other (poly)phenols present in the CDRE were below 3 mg/g. Ellagic acid (from black currant and bilberry extracts) and chlorogenic acid (from bilberry extract) were the primary phenolic acids; and myricetin-glucoside in black currant, the primary non-anthocyanin flavonoid present in the CDRE (Supplementary Table S4).
3.2. Participant flowchart and baseline characteristics
Out of the 82 subjects interviewed, 27 were enrolled, randomized to intervention and scheduled for visits 1 and 2 (Fig. 1). Two of these participants did not complete the study (one of them was unable to complete the HFM within the 15-min period during visit 1, and the other did not return for visit 2). A third participant completed the study, but was removed from the final analysis because their baseline insulin levels were at the lower limit of detection indicating possibly undiagnosed type 1 diabetes, an exclusion factor for participation in the study. The baseline characteristics of the participants were within normal clinical values, and did not change significantly from visit 1 to visit 2 (Table 2). No adverse events were reported as a result of the intervention.
Table 2.
Baseline characteristics of the participants in the study.
| Characteristic | |
|---|---|
| Subjects | 25 |
| Female | 14 |
| Male | 11 |
| Age (y) | 24.5 ± 0.9 |
| Body mass index (kg/m2) | 23.6 ± 0.4 |
| Blood pressure (mmHg) | |
| Systolic | 115 ± 2 |
| Diastolic | 73 ± 1 |
| Triglycerides (mg/dL) | 62.4 ± 6.3 |
| Total cholesterol (mg/dL) | 164.6 ± 6.4 |
| HDL-cholesterol (mg/dL) | 63.2 ± 2.5 |
| LDL-cholesterol (mg/dL) | 87.8 ± 6.0 |
| Glucose (mg/dL) | 80.3 ± 1.9 |
| Insulin (mU/dL) | 0.27 ± 0.04 |
| HOMA-b (%) | 8.6 ± 1.4 |
| HOMA-IR | 0.5 ± 0.1 |
Values are reported as n (categorical variables), and as means ± SE (continuous parameters).
3.3. Presence of AC metabolites in plasma after CDRE consumption
Metabolites associated to anthocyanin intake were observed to be higher in serum of participants who consumed the CDRE than in those on placebo (Supplemental Tables S5–S6). Fourteen metabolites displayed time, treatment, and time-by-treatment interactions (Table 3), including metabolites of hippuric acid, hydroxybenzoic acid, hydroxycinnamic acid, and a methylation and glucuronidation metabolite of delphinidin, i.e. malvidin-glucuronide.
Table 3.
Plasma metabolites determined in placebo- and CDRE-treated participants.
| Metabolite | AUC (nM.5 h) |
Cmax (nM) |
Tmax (h) |
||
|---|---|---|---|---|---|
| Placebo | CDRE | Change | Change | ||
| 5-hydroxybenzoic acid-3-sulfate | 3.5 | 31.0 | 27.5e | 15.0e | 0.5 |
| 4-methoxybenzoic acid-3-glucuronide | 10.3 | 97.5 | 87.2e | 37.8e | 0.5 |
| 4-methoxybenzoic acid-3-sulfate | 6.0 | 21.0 | 15.0e | 8.0e | 2.0 |
| 4-hydroxy-3-methoxybenzoic acid | 38.7 | 157.8 | 119.1e | 84.1e | 1.0 |
| 4-hydroxyhippuric acid | 90.6 | 124.0 | 33.5a | 40.3e | 1.0 |
| 4-hydroxy-3,5-dimethoxybenzoic acid | 5.3 | 9.8 | 4.5c | 3.4e | 0.5 |
| 3-hydroxy-4-methoxyphenylacetic acid | 19.7 | 27.8 | 8.1a | 6.3e | 0.5 |
| 3-hydroxy-4-methoxycinnamic acid | 8.8 | 16.3 | 7.5c | 4.1e | 0.5 |
| 3-methoxybenzoic acid-4-sulfate | 8.6 | 18.2 | 9.6b | −7.0 | 0.5 |
| Hydroxybenzoic acid-sulfatea | 1.3 | 1.5 | 0.2 | 8.6d | 2.0 |
| Methoxycinnamic acid-glucuronidea | 17.5 | 27.4 | 9.9 | 8.7a | 0.5 |
| Hydroxyhippuric acida | 35.7 | 34.5 | −1.2 | 5.4c | 0.5 |
| Dihydroxybenzoic acid-sulfatea | 2.5 | 14.0 | 11.6d | 4.9b | 2.0 |
| Malvidin glucuronide | 48.3 | 85.4 | 37.1 | 0.1 | 0.5 |
The shown compounds are those satisfying treatment (p ≤ 0.04), time (p ≤ 0.02), and time-by-treatment interaction (p ≤ 0.0001) effects that were subject to secondary ANOVA for AUC, Cmax and tmax. Significances for changes relative to the placebo are: ap ≤ 0.05, bp ≤ 0.01, cp ≤ 0.005, dp ≤ 0.001, and ep ≤ 0.0001.
Isomer of indeterminate structural orientation or hydroxyl, methoxyl, sulfate or glucuronide conjugating site.
Of those 14 metabolites: i) 10 showed significant treatment effects for changes in their AUC with differences in AUC between 4.5 and 119.1 nM × 5 h; and cumulative differences in serum metabolites added up to 325 nM × 5h; ii) 12 showed significant treatment effects for Cmax (Table 3); differences in Cmax were between 3.4 and 84.1 nM, and the total cumulative differences in serum metabolites was 240 nM; and iii) all of the tmax values were between 0.5 and 2 h, with the majority (60%) of these metabolites having a 0.5 h tmax (Table 3). Overall, serum metabolite concentrations generally peaked at 0.5 and 1 h after ingestion and remained elevated for 2–3 h before returning to baseline at 5 h; two acids (dihydroxybenzoic acid sulfate and hydroxybenzoic acid sulfate) remained elevated at 5 h (Supplemental Fig. S1).
One anthocyanidin, malvidin-glucuronide, displayed a time and time-by-treatment interaction effect (p < 0.01), and a treatment effect when time was collapsed (p < 0.02) (Supplemental Tables S5–S6). Further, malvidin-glucuronide did not display significant AUC or Cmax changes associated with CDRE treatment (Table 3).
3.4. Efficacy of CDRE consumption on postprandial endotoxemia
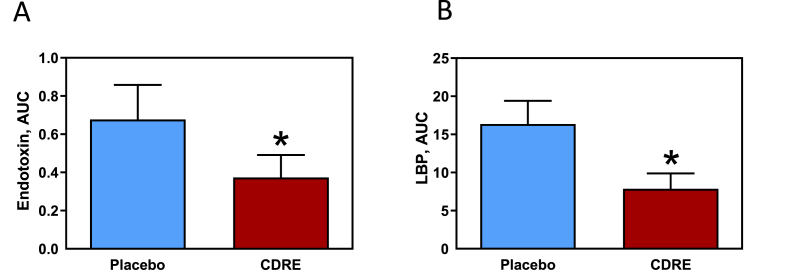
The primary end-point of the present study was to assess whether or not the CDRE could attenuate postprandial endotoxemia induced by consumption of a HFM. Postprandial endotoxemia was evaluated using two biomarkers, plasma LPS and LBP. For LPS, there was a significant effect for time (p < 0.01) where consumption of the HFM triggered increased plasma LPS levels in both groups. The treatment effect (p = 0.05) confirmed that plasma LPS concentrations were lower in the CDRE group compared to the placebo group. The time-by-treatment interaction was not significant (p = 0.14). The total AUC was calculated in order to evaluate the extent of exposure to LPS in each group over the 5-h postprandial time period. AUC values were 44% lower (0.38 ± 0.15 vs. 0.68 ± 0.12 EU/mL x 5 h) when participants received CDRE compared to when they received placebo with the HFM (p = 0.04) (Fig. 2A).
Fig. 2.
Effects of CDRE consumption on endotoxemia associated to a HFM in healthy participants. Incremental AUC for plasma endotoxin (A) and LBP (B) determined during the 5 h following the consumption of a HFM and placebo or CDRE. Results are shown as means ± SE. Units for incremental AUC/5 h are for endotoxin, endotoxin units (EU)/mL; and for LBP, μg/mL. *Significantly different from placebo (p < 0.05, one-way ANOVA).
Plasma LBP showed a significant treatment effect (p = 0.04), wherein average LBP concentrations were lower in the CDRE group compared to the placebo group. Time (p < 0.09) and time-by-treatment interaction (p = 0.08) were non-significant. Consistent with plasma LPS, and the observed treatment effect, a significant effect of CDRE was observed for the LBP AUC (p < 0.03). Thus, the CDRE treatment elicited a smaller LBP AUC response (52% reduction) over the 5-h postprandial time period compared to the placebo (7.9 ± 2.0 vs. 16.4 ± 3.0 μg/mL x 5 h) (Fig. 2B).
3.5. Efficacy of CDRE consumption on postprandial glucose metabolism
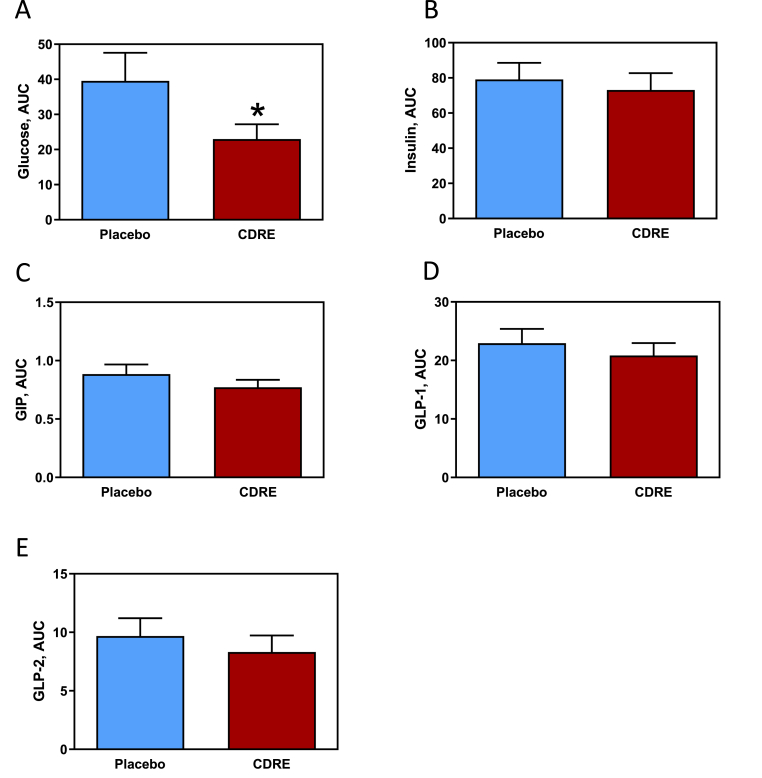
In addition to endotoxemia, ingestion of the HFM caused postprandial increases in biomarkers of glucose metabolism (Fig. 3). There was a statistically significant time effect (p < 0.01) where plasma glucose and insulin increased in both groups in response to the high-fat meal. For glucose, there were statistically significant treatment (p < 0.02) and time-by-treatment interaction (p < 0.02) effects. The CDRE effect for blood glucose AUC was significant (p < 0.02) with the average glucose concentrations for the CDRE group being 42% lower than for the placebo group across the 5-h post-HFM period (23.0 ± 4.4 vs. 39.6 ± 8.0 mg/dL, respectively) (Fig. 3A).
Fig. 3.
Effects of CDRE consumption on glucose dysmetabolism associated to a HFM in healthy participants. Incremental AUC for plasma glucose (A), insulin (B), GIP (C), GLP-1 (D), and GLP-2 (E) determined during the 5 h following the consumption of a HFM and placebo or CDRE. Results are shown as means ± SE. Units for incremental AUC/5 h are for glucose, mg/dL; insulin, mU/L; GIP, μM; GLP-1 and GLP-2, μg/mL. *Significantly different from placebo (p < 0.05, one-way ANOVA).
Postprandial concentrations of plasma insulin increased in both groups, showing a time-by-treatment interaction (p < 0.01). While insulin levels in both groups peaked at 30 min post-meal, Tukey HSD testing indicated that the CDRE treatment attenuated the spike, with the treatment group achieving statistically significantly lower insulin level compared to the placebo group at 30 min (p < 0.05). Unlike plasma glucose, there was no indication of a CDRE effect for the insulin AUC across the 5 h post-HFM (73.1 ± 9.6 and 79.1 ± 9.4 mU/L, for the CDRE and placebo groups respectively) (Fig. 3B).
The gut-secreted hormones GIP, GLP-1, and GLP-2 are involved in the regulation of insulin secretion and overall glucose homeostasis. A statistically significant time effect (p < 0.01) was observed for the three hormones. GIP exhibited non-significant treatment and time-by-treatment interaction effects (p = 0.20 and p = 0.08, respectively). For GLP-1 and GLP-2 the treatment and time-by-treatment interaction effects were non-significant (for treatment, p = 0.34 for both hormones; for time-by-treatment interaction effect, p < 0.09 for GLP-1 and p < 0.78 for GLP-2. For GIP, the AUC for the CDRE group was 13% lower than for the placebo group (0.77 ± 0.06 vs. 0.89 ± 0.08 μM; p = 0.05) (Fig. 3C). No differences in AUC were observed for GLP-1 or GLP-2 between groups (Fig. 3D and E).
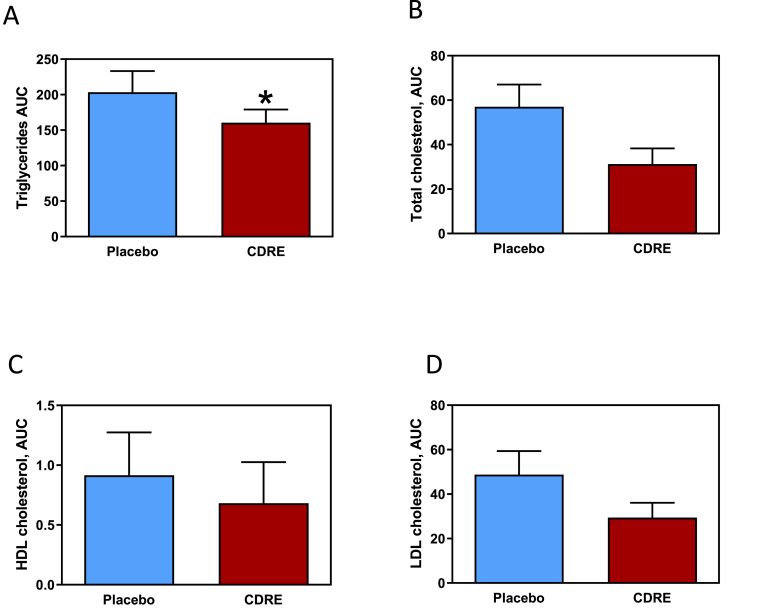
3.6. Efficacy of CDRE consumption on postprandial hyperlipidemia
Another hallmark of the ingestion of a high-fat meal is an increase in postprandial lipids (Fig. 4). Consumption of the HFM caused a statistically significant increase in blood triglycerides, total cholesterol, HDL-cholesterol and LDL-cholesterol (time effect p < 0.01). For triglycerides, no treatment (p = 0.50) or time-by-treatment interaction (p = 0.52) effects were observed. There was, however, a significant CDRE effect for AUC (p = 0.03) where the concentration of plasma triglycerides was lower for the CDRE than for the placebo (161.5 ± 18.5 vs. 203.5 ± 29.6 mg/dL for 5 h) (Fig. 4A). A statistically significant time-by-treatment interaction effect was observed for both plasma total cholesterol (p < 0.03) and for LDL-cholesterol (p < 0.03). For plasma HDL-cholesterol, the treatment (p = 0.38) and time-by-treatment interaction (p = 0.23) effects were not significant. The AUC for total cholesterol was lower than that for the placebo group, 31.2 ± 7.0 vs. 57.0 ± 10.0 mg/dL for 5 h, respectively (p < 0.06) (Fig. 4B). No differences in AUC were observed between groups for LDL- and HDL-cholesterol (Fig. 4C and D). There was a statistically significant time effect for plasma free fatty acids (p < 0.01), but no significant treatment (p = 0.12) or time-by-treatment interaction (p = 0.64) effects. Unlike other lipid markers, however, free fatty acids decreased between baseline and 30 min post-HFM in both groups (p < 0.05) and remained low, not returning to baseline until 5 h post-HFM. No effect of CDRE was detected on the values of free fatty acid at the different times; likewise AUC were similar for both groups for the hormones leptin, ghrelin, and adiponectin (Supplemental Table 7).
Fig. 4.
Effects of CDRE consumption on hyperlipidemia associated to a HFM in healthy participants. Incremental AUC for plasma triglycerides (A), total cholesterol (B), HDL-cholesterol (C), and LDL-cholesterol (D) determined during the 5 h following the consumption of a HFM and placebo or CDRE. Results are shown as means ± SE. Units for incremental AUC/5 h are mg/dL.*Significantly different from placebo (p < 0.05, one-way ANOVA).
3.7. Efficacy of CDRE consumption on inflammation and oxidative stress biomarkers in PBMC
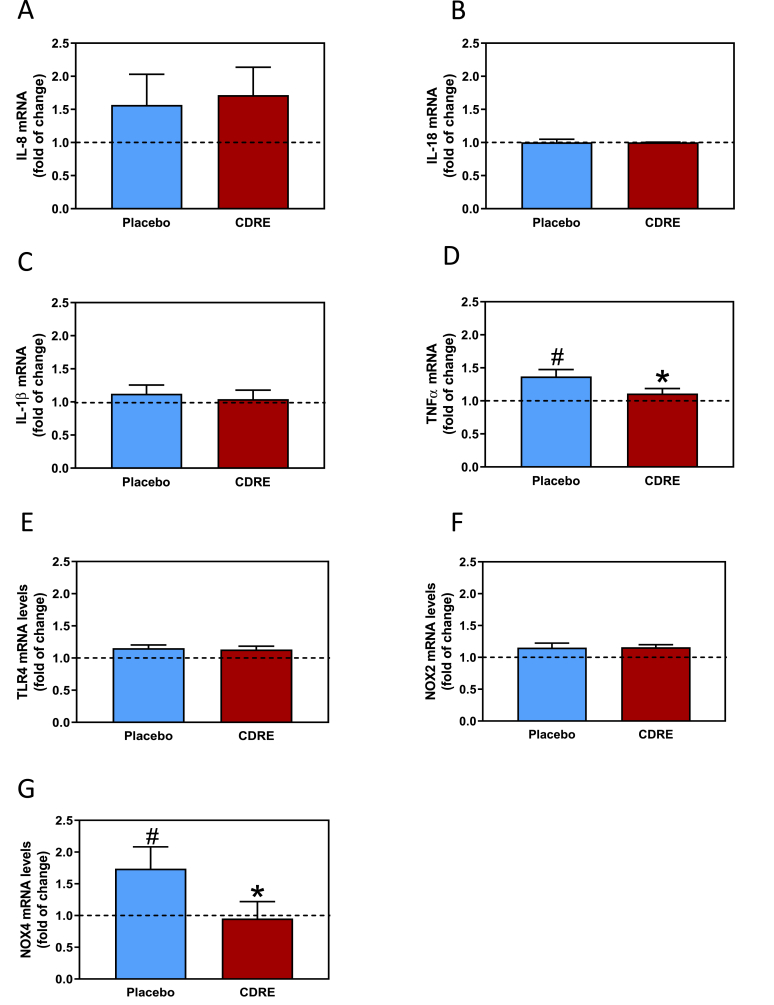
Other endpoints related to inflammation and oxidative stress were measured in 0 and 3-h postprandial PBMC. The consumption of the HFM and placebo led to increases in IL-8 (51%), TNFα (45%), and NOX4 (74%, p < 0.04) mRNA levels (Fig. 5A, D, and G). In CDRE-treated participants no significant HFM-mediated increases in TNFα and NOX4 were observed, while the increase in IL-8 was not affected. Neither the HFM nor the intake of placebo or CDRE affected mRNA levels of IL-18, IL-1β, TLR4, and NOX2 (Fig. 5B, C, E, and F).
Fig. 5.
Effects of CDRE consumption on inflammation and redox signaling associated to a HFM in PMBC from healthy participants. Changes in mRNA levels of IL-8 (A), IL-18 (B), IL-1β (C), TNFα (D), TLR4 (E), NOX2 (F), and NOX4 (G) determined in PMBC isolated 0 and 3 h after the consumption of a HFM and placebo or CDRE. Results are shown as means ± SE. Fold of change, indicates values at 3 h respect values at 0 h #Significantly different comparing 0 vs. 3 h; and *significant different from placebo (p < 0.05, one-way ANOVA).
3.8. Efficacy of CDRE consumption on insulin signaling biomarkers in PBMC
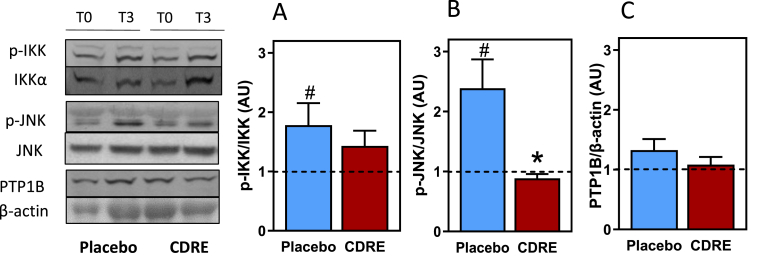
Consumption of the HFM caused 86 and 140% increases in IKK (Ser178/180) and JNK1/2 (Thr183/Tyr185) phosphorylation, respectively (p < 0.04 and p < 0.01) in PBMC from placebo-treated participants (Fig. 6). CDRE treatment reduced postprandial increases in IKK phosphorylation to 40% (Fig. 6A) and abolished that of JNK1/2 (Fig. 6B). Between placebo and CDRE groups differences were significant for JNK1/2 phosphorylation (p < 0.01). No changes induced by the HFM and placebo or CDRE were observed for PTP1B protein levels in PBMC (Fig. 6C).
Fig. 6.
Effects of CDRE consumption on changes in regulators of the insulin signaling pathway associated to a HFM in PMBC from healthy participants. Phosphorylation levels of IKK (A), and JNK1/2 (B), and protein levels of PTP1B (C) were measured by Western blot in PMBC isolated at time 0 and 3 h after the consumption of a HFM and placebo or CDRE. Representative images are shown in the left panel. Bands were quantified and values referred to total protein (IKK, JNK1/2) or β-actin levels (PTP1B). Results are shown as means ± SE. #Significantly different comparing 0 vs. 3 h; and *significant different from placebo (p < 0.05, one-way ANOVA).
4. Discussion
This study showed that the intake of a CDRE reduced inflammation and other metabolic postprandial changes associated with the consumption of a HFM in a young and metabolically healthy population. Thus, for the primary endpoint of the study, CDRE reduced HFM-induced increases in plasma LPS. The CDRE treatment also reduced the postprandial increases of other markers of inflammation, i.e. plasma LBP concentration, and TNFα levels in PBMC, as well as of cardiometabolic outcomes, i.e. plasma levels of glucose and triglycerides and cholesterol. In terms of parameters indicative of mechanisms of action, in PBMC the CDRE reduced HFM-mediated JNK1/2 increased phosphorylation, and NOX4 overexpression, which can both contribute to inflammation, oxidative stress, and insulin resistance.
The positive effects of AC consumption on markers of inflammation and cardiometabolic health have been extensively characterized in laboratory animals [[31], [32], [33],40,41] and in some nutrition intervention studies [26,27,42,43]. We previously observed that, among AC, delphinidin and cyanidin were particularly active in protecting the gastrointestinal tract from permeabilization and dysbiosis, endotoxemia, and the promotion of local and systemic inflammation and oxidative stress in mouse models of diet (high fat)-induced obesity [[30], [31], [32], [33],40]. The extract used in the present study was rich in delphinidin and cyanidin, which points to these AC as being responsible for altering serum AC metabolites. These plasma metabolites as well as unmodified AC present in the intestinal lumen can be likely involved in the observed amelioration of the negative effects of the acute fat load provided to the participants. Other (poly)phenols present in the CDRE at very minor amounts could have contributed modestly to the observed effects. Collectively, these (poly)phenols and their metabolites may also be important when considering long-term effects that could modulate the microbiome, absorptive mechanisms such as ATP-binding cassette and organic anion transporters, and phase II enzyme abundance or responsiveness [44]. In terms of the quantities of AC supplemented, the administered extract provides levels compatible with both, acute and long-term human consumption, and also agree with those used in other clinical studies [[45], [46], [47]]. Thus, the obtained results are of practical relevance when it comes to making recommendations for dietary intakes and/or the use of supplements. Further dose-response studies are warranted to establish the optimal amounts to be consumed.
Postprandial dysmetabolism is associated with a higher risk for a number of pathologies [[8], [9], [10], [11], [12]], being characterized by undesired changes in glucose and lipid metabolism, inflammation and oxidative stress [48]. If these changes become chronic given the habitual consumption of high calorie (high fat and high carbohydrate) diets, e.g. Western style diets, they can lead to a perpetuating cycle of inflammation, loss of redox homeostasis, and development of chronic diseases [[31], [32], [33],49]. Overall, a sustained consumption of cyanidin and delphinidin through the diet or through supplementation could afford a suitable strategy to combat inflammation and associated metabolic changes. The AC-beneficial effects observed in a healthy cohort could be even more relevant in overweight/obese and metabolic-compromised populations, meriting further research in this area.
In the context of healthy individuals, the increased translocation of luminal endotoxins to the circulation would be mostly due to the co-transport of LPS with nascent chylomicrons [17,50], although an increased LPS paracellular transport could also contribute to the observed endotoxemia, and the consequent initiation of pro-inflammatory events. The increases in TNFα and NOX4 expression in PBMC provides additional support of both HFM-induced inflammation and altered cell redox regulation. The latter was also associated with the activation of NF-κB (IKK) and JNK1/2 pathways which are both sensitive to increases in cellular oxidants and changes in redox homeostasis. Consistently, the CDRE mitigated endotoxemia and reduced TNFα and NOX4 overexpression, and JNK phosphorylation, suggesting that AC can mitigate the inflammatory and pro-oxidant conditions triggered by an excess fat consumption. These effects on redox sensitive signaling support the antioxidant actions of AC, that may involve the direct quenching of oxidants in the intestinal lumen, but also to indirect antioxidant effects achievable at the very low concentrations of AC and AC metabolites present in cells and tissues [[34], [35], [36]].
Another important component of postprandial dysmetabolism is the associated dyslipidemia. Plasma triglycerides, and to a lesser extent total cholesterol, increased upon consumption of the HFM. Postprandial hypertriglyceridemia is associated with the development of cardiovascular disease [19,51], therefore its prevention by the CDRE is of high relevance. CDRE-mediated capacity to mitigate postprandial hypertriglyceridemia could be related to, among other factors, their capacity to inhibit pancreatic lipase [52], or any of the events involved in fatty acid absorption and chylomicron synthesis and secretion. In addition, although with a p < 0.06, the CDRE also reduced the HFM-related increase in plasma cholesterol, providing additional support for cardiovascular protection.
Although rich in fat, the provided meal also contained carbohydrates. Thus, a postprandial elevation in glucose and insulin plasma levels was observed upon consumption of the HFM. The CDRE attenuated the postprandial increase in plasma glucose; this reduction could be due, among other mechanisms, to the capacity of AC to inhibit luminal glycosidases [52] or to compete with the enterocyte glucose transporter [53]. In close relationship to systemic glucose homeostasis, CDRE intake cancelled the HFM-mediated activation (phosphorylation) of JNK1/2, and decreased IKK activation. Both kinases are directly involved in tissue insulin resistance, given that they phosphorylate the insulin receptor substrate 1 (IRS-1) in serine/threonine residues, thus inhibiting the insulin receptor pathway [20,21]. If extrapolated from PBMC to other tissues, the inhibition of JNK1/2 activation by the CDRE would translate to an improvement in tissue insulin sensitivity. In fact, in high fat diet-fed mice, chronic supplementation with a similar CDRE improved glucose tolerance and insulin sensitivity in the liver and adipose tissue, inhibiting tissue JNK1/2 and IKK activation [33].
In terms of the chemical species mediating the observed effects, it is possible that parent AC, present at high concentrations in the intestinal lumen, are directly involved in the regulation of epithelial barrier function, and other actions at the intestinal lining [54]. However, the involvement of AC microbial metabolites in the reported effects is also feasible. Thus, seventeen metabolites of AC identified in plasma, including, hydroxybenzoic acids, hydroxycinnamic acids, hydroxyphenylacetic acids, hippuric acids and malvidin-glucuronide, displayed plasma kinetic time curves (Table 3, Supplemental Fig. 1) consistent with providing an effect on most of the metabolic and inflammatory parameters evaluated in plasma. Previous studies showed that these and similar metabolites have multiple peak concentrations in the blood, including at various time-points [[55], [56], [57]], and their bioactivity is likely to be observed beyond the 5 h captured in the present study [[57], [58], [59]]. This is consistent with metabolites, such as hydroxybenzoic acid-sulfate, which in the present study remained elevated at the 5-h time point; suggesting that the effects of the treatment are likely to extend well beyond the postprandial state. Future studies should explore mechanisms associated with prolonged bioactivity.
Metabolites displaying significant treatment and time-by-treatment interactions, were primarily associated with phase II metabolism of microbial AC metabolites, such as hydroxyhippuric acids, and hydroxybenzoic acids. For example, malvidin-glucuronide is a methyl metabolite of delphinidin glycosides, which were the second most abundant anthocyanins in the treatment. It's unclear why metabolites of cyanidin were not identified, as it was found in highest abundance in the CDRE. However, peonidin metabolites were identified and peonidin is a methyl derivative of AC which could be derived from phase II methylation of cyanidin [[60], [61], [62]].
One strength of this study is the experimental design, including the randomization of participants/treatment, the use of a placebo to control changes in variables, the double blind, and the crossover that allowed for between- and within-participant comparisons. Additional strengths are the inclusion of both, men and women, and the determination of objective outcomes. Limitations of this study include: i) the uniformity of the participants in terms of age and health status; ii) the use of an extract that does not allow to accurately identify the chemical entities(s) responsible for the observed effects; iii) the acute characteristics of the study that does not allow for the extrapolation to long-term or chronic consumption of HFMs and/or CDRE.
In summary, the data presented support the protective actions of the assayed CDRE against endotoxemia, inflammation, oxidative stress, and alterations in lipid and glucose metabolism. The luminal presence of parent compounds, as well as the rapid increase in circulating AC metabolites, stress the involvement of AC in the observed effects. All the above strengthen the conclusion that the consumption of fruits and vegetables rich in specific AC can counterbalance the adverse consequences of the ingestion of unhealthy meals. Further studies of the long-term and dose-dependent effects of AC consumption, and their impact on metabolic health are warranted.
Author contributions
Idea and design of the study: PIO, AM, CGF; experimental work: EC, ED, JK, ZW, RG; data acquisition, review and analysis: EC, DEI, RG, CDK, CGF, PIO; manuscript writing: EC, CGF, PIO, AM; critical revision and input: SNH, SMW, AM, CDK, CGF, PIO. All authors revised the article, and approved the final version.
Declaration of competing interest
SNH, SMW, and RG are, and AM was employed by Pharmanex Research, NSE Products Inc., Provo, UT, USA. CGF and PIO have received research grants from NSE Products Inc. as well as from other food companies and government agencies with an interest in health and nutrition. CGF and PIO are members of the NSE Products Inc. Scientific Advisory Board.
Acknowledgements
The authors express a special thanks to all the participants in the study. Also thank Robert P. O'Donnell, Ph. D., for his assistance with the statistical analysis; and to Prof. Dr. Mary Ann Lila, North Carolina State University, for the characterization of the extracts used. Funding was provided by a research grant from Pharmanex Research, NSE Products Inc., Provo, UT, USA. PIO is correspondent researcher from CONICET, Argentina. Graphical abstract created with BioRender.com.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.redox.2022.102273.
Contributor Information
Cesar G. Fraga, Email: cgfraga@ucdavis.edu.
Patricia I. Oteiza, Email: poteiza@ucdavis.edu.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Gregor M.F., Hotamisligil G.S. Inflammatory mechanisms in obesity. Annu. Rev. Immunol. 2011;29:415–445. doi: 10.1146/annurev-immunol-031210-101322. [DOI] [PubMed] [Google Scholar]
- 2.Cani P.D., Amar J., Iglesias M.A., Poggi M., Knauf C., Bastelica D., Neyrinck A.M., Fava F., Tuohy K.M., Chabo C., Waget A., Delmee E., Cousin B., Sulpice T., Chamontin B., Ferrieres J., Tanti J.F., Gibson G.R., Casteilla L., Delzenne N.M., Alessi M.C., Burcelin R. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes. 2007;56:1761–1772. doi: 10.2337/db06-1491. [DOI] [PubMed] [Google Scholar]
- 3.Cani P.D., Jordan B.F. Gut microbiota-mediated inflammation in obesity: a link with gastrointestinal cancer. Nat. Rev. Gastroenterol. Hepatol. 2018;15:671–682. doi: 10.1038/s41575-018-0025-6. [DOI] [PubMed] [Google Scholar]
- 4.Guillemot-Legris O., Muccioli G.G. Obesity-induced neuroinflammation: beyond the hypothalamus. Trends Neurosci. 2017;40:237–253. doi: 10.1016/j.tins.2017.02.005. [DOI] [PubMed] [Google Scholar]
- 5.Hotamisligil G.S. Endoplasmic reticulum stress and the inflammatory basis of metabolic disease. Cell. 2010;140:900–917. doi: 10.1016/j.cell.2010.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lumeng C.N., Saltiel A.R. Inflammatory links between obesity and metabolic disease. J. Clin. Invest. 2011;121:2111–2117. doi: 10.1172/JCI57132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sethi J.K., Hotamisligil G.S. Metabolic messengers: tumour necrosis factor. Nat. Metabol. 2021;3:1302–1312. doi: 10.1038/s42255-021-00470-z. [DOI] [PubMed] [Google Scholar]
- 8.Bell D.S., O'Keefe J.H., Jellinger P. Postprandial dysmetabolism: the missing link between diabetes and cardiovascular events? Endocr. Pract. 2008;14:112–124. doi: 10.4158/EP.14.1.112. [DOI] [PubMed] [Google Scholar]
- 9.O'Keefe J.H., Bell D.S. Postprandial hyperglycemia/hyperlipidemia (postprandial dysmetabolism) is a cardiovascular risk factor. Am. J. Cardiol. 2007;100:899–904. doi: 10.1016/j.amjcard.2007.03.107. [DOI] [PubMed] [Google Scholar]
- 10.Lin H.J., Lee B.C., Ho Y.L., Lin Y.H., Chen C.Y., Hsu H.C., Lin M.S., Chien K.L., Chen M.F. Postprandial glucose improves the risk prediction of cardiovascular death beyond the metabolic syndrome in the nondiabetic population. Diabetes Care. 2009;32:1721–1726. doi: 10.2337/dc08-2337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Musso G., Bo S., Cassader M., De Michieli F., Gambino R. Impact of sterol regulatory element-binding factor-1c polymorphism on incidence of nonalcoholic fatty liver disease and on the severity of liver disease and of glucose and lipid dysmetabolism. Am. J. Clin. Nutr. 2013;98:895–906. doi: 10.3945/ajcn.113.063792. [DOI] [PubMed] [Google Scholar]
- 12.Sottero B., Gargiulo S., Russo I., Barale C., Poli G., Cavalot F. Postprandial dysmetabolism and oxidative stress in type 2 diabetes: pathogenetic mechanisms and therapeutic strategies. Med. Res. Rev. 2015;35:968–1031. doi: 10.1002/med.21349. [DOI] [PubMed] [Google Scholar]
- 13.Clemente-Postigo M., Queipo-Ortuño M.I., Murri M., Boto-Ordoñez M., Perez-Martinez P., Andres-Lacueva C., Cardona F., Tinahones F.J. Endotoxin increase after fat overload is related to postprandial hypertriglyceridemia in morbidly obese patients. J. Lipid Res. 2012;53:973–978. doi: 10.1194/jlr.P020909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ghanim H., Batra M., Abuaysheh S., Green K., Makdissi A., Kuhadiya N.D., Chaudhuri A., Dandona P. Antiinflammatory and ROS suppressive effects of the addition of fiber to a high-fat high-calorie meal. J. Clin. Endocrinol. Metab. 2017;102:858–869. doi: 10.1210/jc.2016-2669. [DOI] [PubMed] [Google Scholar]
- 15.Schmid A., Leszczak S., Ober I., Schäffler A., Karrasch T. Serum progranulin concentrations are not responsive during oral lipid tolerance test and oral glucose tolerance test. Horm. Metab. Res. 2015;47:571–576. doi: 10.1055/s-0034-1395679. [DOI] [PubMed] [Google Scholar]
- 16.Cani P.D., Bibiloni R., Knauf C., Waget A., Neyrinck A.M., Delzenne N.M., Burcelin R. Changes in gut microbiota control metabolic endotoxemia-induced inflammation in high-fat diet-induced obesity and diabetes in mice. Diabetes. 2008;57:1470–1481. doi: 10.2337/db07-1403. [DOI] [PubMed] [Google Scholar]
- 17.Ghoshal S., Witta J., Zhong J., de Villiers W., Eckhardt E. Chylomicrons promote intestinal absorption of lipopolysaccharides. J. Lipid Res. 2009;50:90–97. doi: 10.1194/jlr.M800156-JLR200. [DOI] [PubMed] [Google Scholar]
- 18.Boutagy N.E., McMillan R.P., Frisard M.I., Hulver M.W. Metabolic endotoxemia with obesity: is it real and is it relevant? Biochimie. 2016;124:11–20. doi: 10.1016/j.biochi.2015.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Higgins V., Adeli K. Postprandial dyslipidemia: pathophysiology and cardiovascular disease risk assessment. eJIFCC. 2017;28:168–184. [PMC free article] [PubMed] [Google Scholar]
- 20.Hirosumi J., Tuncman G., Chang L., Gorgun C.Z., Uysal K.T., Maeda K., Karin M., Hotamisligil G.S. A central role for JNK in obesity and insulin resistance. Nature. 2002;420:333–336. doi: 10.1038/nature01137. [DOI] [PubMed] [Google Scholar]
- 21.Yuan M., Konstantopoulos N., Lee J., Hansen L., Li Z.W., Karin M., Shoelson S.E. Reversal of obesity- and diet-induced insulin resistance with salicylates or targeted disruption of Ikkbeta. Science. 2001;293:1673–1677. doi: 10.1126/science.1061620. [DOI] [PubMed] [Google Scholar]
- 22.Haj F.G., Zabolotny J.M., Kim Y.B., Kahn B.B., Neel B.G. Liver-specific protein-tyrosine phosphatase 1B (PTP1B) re-expression alters glucose homeostasis of PTP1B-/-mice. J. Biol. Chem. 2005;280:15038–15046. doi: 10.1074/jbc.M413240200. [DOI] [PubMed] [Google Scholar]
- 23.Mytton O.T., Nnoaham K., Eyles H., Scarborough P., Ni Mhurchu C. Systematic review and meta-analysis of the effect of increased vegetable and fruit consumption on body weight and energy intake. BMC Publ. Health. 2014;14:886. doi: 10.1186/1471-2458-14-886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kaiser K.A., Brown A.W., Bohan Brown M.M., Shikany J.M., Mattes R.D., Allison D.B. Increased fruit and vegetable intake has no discernible effect on weight loss: a systematic review and meta-analysis. Am. J. Clin. Nutr. 2014;100:567–576. doi: 10.3945/ajcn.114.090548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schwingshackl L., Hoffmann G., Kalle-Uhlmann T., Arregui M., Buijsse B., Boeing H. Fruit and vegetable consumption and changes in anthropometric variables in adult populations: a systematic review and meta-analysis of prospective cohort Studies. PLoS One. 2015;10(10) doi: 10.1371/journal.pone.0140846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guo X., Yang B., Tan J., Jiang J., Li D. Associations of dietary intakes of anthocyanins and berry fruits with risk of type 2 diabetes mellitus: a systematic review and meta-analysis of prospective cohort studies. Eur. J. Clin. Nutr. 2016;70:1360–1367. doi: 10.1038/ejcn.2016.142. [DOI] [PubMed] [Google Scholar]
- 27.Cassidy A. Berry anthocyanin intake and cardiovascular health. Mol. Aspect. Med. 2018;61:76–82. doi: 10.1016/j.mam.2017.05.002. [DOI] [PubMed] [Google Scholar]
- 28.Ivey K.L., Jensen M.K., Hodgson J.M., Eliassen A.H., Cassidy A., Rimm E.B. Association of flavonoid-rich foods and flavonoids with risk of all-cause mortality. Br. J. Nutr. 2017;117:1470–1477. doi: 10.1017/S0007114517001325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Crozier A., Del Rio D., Clifford M.N. Bioavailability of dietary flavonoids and phenolic compounds. Mol. Aspect. Med. 2010;31:446–467. doi: 10.1016/j.mam.2010.09.007. [DOI] [PubMed] [Google Scholar]
- 30.Cremonini E., Mastaloudis A., Hester S.N., Verstraeten S.V., Anderson M., Wood S.M., Waterhouse A.L., Fraga C.G., Oteiza P.I. Anthocyanins inhibit tumor necrosis alpha-induced loss of Caco-2 cell barrier integrity. Food Funct. 2017;8:2915–2923. doi: 10.1039/c7fo00625j. [DOI] [PubMed] [Google Scholar]
- 31.Cremonini E., Iglesias D.E., Matsukuma K.E., Hester S.N., Wood S.M., Bartlett M., Fraga C.G., Oteiza P.I. Supplementation with cyanidin and delphinidin mitigates high fat diet-induced endotoxemia and associated liver inflammation in mice. Food Funct. 2022;13:781–794. doi: 10.1039/d1fo03108b. [DOI] [PubMed] [Google Scholar]
- 32.Cremonini E., Daveri E., Mastaloudis A., Adamo A.M., Mills D., Kalanetra K., Hester S.N., Wood S.M., Fraga C.G., Oteiza P.I. Anthocyanins protect the gastrointestinal tract from high fat diet-induced alterations in redox signaling, barrier integrity and dysbiosis. Redox Biol. 2019;26:101269. doi: 10.1016/j.redox.2019.101269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Daveri E., Cremonini E., Mastaloudis A., Hester S.N., Wood S.M., Waterhouse A.L., Anderson M., Fraga C.G., Oteiza P.I. Cyanidin and delphinidin modulate inflammation and altered redox signaling improving insulin resistance in high fat-fed mice. Redox Biol. 2018;18:16–24. doi: 10.1016/j.redox.2018.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fraga C.G., Oteiza P.I., Galleano M. Plant bioactives and redox signaling: (-)-epicatechin as a paradigm. Mol. Aspect. Med. 2018;61:31–40. doi: 10.1016/j.mam.2018.01.007. [DOI] [PubMed] [Google Scholar]
- 35.Fraga C.G., Galleano M., Verstraeten S.V., Oteiza P.I. Basic biochemical mechanisms behind the health benefits of polyphenols. Mol. Aspect. Med. 2010;31:435–445. doi: 10.1016/j.mam.2010.09.006. [DOI] [PubMed] [Google Scholar]
- 36.Galleano M., Verstraeten S.V., Oteiza P.I., Fraga C.G. Antioxidant actions of flavonoids: thermodynamic and kinetic analysis. Arch. Biochem. Biophys. 2010;501(1):23–30. doi: 10.1016/j.abb.2010.04.005. [DOI] [PubMed] [Google Scholar]
- 37.Executive summary of the third report of the National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, tnd treatment of high blood cholesterol in adults (adult treatment panel III) JAMA. 2001;285:2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 38.Ghanim H., Sia C.L., Upadhyay M., Korzeniewski K., Viswanathan P., Abuaysheh S., Mohanty P., Dandona P. Orange juice neutralizes the proinflammatory effect of a high-fat, high-carbohydrate meal and prevents endotoxin increase and Toll-like receptor expression. Am. J. Clin. Nutr. 2010;91:940–949. doi: 10.3945/ajcn.2009.28584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rao X., Huang X., Zhou Z., Lin X. An improvement of the 2∧(-delta delta CT) method for quantitative real-time polymerase chain reaction data analysis. Biostat. Bioinforma. Biomath. 2013;3:71–85. [PMC free article] [PubMed] [Google Scholar]
- 40.Cremonini E., Daveri E., Mastaloudis A., Oteiza P.I. (-)-Epicatechin and anthocyanins modulate GLP-1 metabolism: evidence from C57BL/6J mice and GLUTag cells. J. Nutr. 2021;151:1497–1506. doi: 10.1093/jn/nxab029:. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lee S., Keirsey K.I., Kirkland R., Grunewald Z.I., Fischer J.G., de La Serre C.B. Blueberry supplementation influences the gut microbiota, inflammation, and insulin resistance in high-fat-diet-fed rats. J. Nutr. 2018;148:209–219. doi: 10.1093/jn/nxx027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Riazi K., Raman M., Taylor L., Swain M.G., Shaheen A.A. Dietary patterns and components in nonalcoholic fatty liver disease (NAFLD): what key messages can health care providers offer? Nutrients. 2019;11:2878. doi: 10.3390/nu11122878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yang L., Ling W., Du Z., Chen Y., Li D., Deng S., Liu Z., Yang L. Effects of anthocyanins on cardiometabolic health: a systematic review and meta-analysis of randomized controlled trials. Adv. Nutr. 2017;8:684–693. doi: 10.3945/an.116.014852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Williamson G., Kay C.D., Crozier A. The bioavailability, transport, and bioactivity of dietary flavonoids: a review from a historical perspective. Compr. Rev. Food Sci. Food Saf. 2018;17:1054–1112. doi: 10.1111/1541-4337.12351. [DOI] [PubMed] [Google Scholar]
- 45.Aboonabi A., Aboonabi A. Anthocyanins reduce inflammation and improve glucose and lipid metabolism associated with inhibiting nuclear factor-kappaB activation and increasing PPAR-γ gene expression in metabolic syndrome subjects. Free Radic. Biol. Med. 2020;150:30–39. doi: 10.1016/j.freeradbiomed.2020.02.004. [DOI] [PubMed] [Google Scholar]
- 46.Guo Y., Zhang P., Liu Y., Zha L., Ling W., Guo H. A dose-response evaluation of purified anthocyanins on inflammatory and oxidative biomarkers and metabolic risk factors in healthy young adults: a randomized controlled trial. Nutrition. 2020;74:110745. doi: 10.1016/j.nut.2020.110745. [DOI] [PubMed] [Google Scholar]
- 47.Yang L., Ling W., Yang Y., Chen Y., Tian Z., Du Z., Chen J., Xie Y., Liu Z., Yang L. Role of of purified anthocyanins in improving cardiometabolic risk factors in Chinese men and women with prediabetes or early untreated diabetes. A randomized controlled trial. Nutrients. 2017;9(10):1104. doi: 10.3390/nu9101104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schwander F., Kopf-Bolanz K.A., Buri C., Portmann R., Egger L., Chollet M., McTernan P.G., Piya M.K., Gijs M.A., Vionnet N., Pralong F., Laederach K., Vergères G. A dose-response strategy reveals differences between normal-weight and obese men in their metabolic and inflammatory responses to a high-fat meal. J. Nutr. 2014;144:1517–1523. doi: 10.3945/jn.114.193565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Oteiza P.I., Fraga C.G., Galleano M. Linking biomarkers of oxidative stress and disease with flavonoid consumption: from experimental models to humans. Redox Biol. 2021;42:101914. doi: 10.1016/j.redox.2021.101914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vors C., Pineau G., Drai J., Meugnier E., Pesenti S., Laville M., Laugerette F., Malpuech-Brugère C., Vidal H., Michalski M.C. Postprandial endotoxemia linked with chylomicrons and lipopolysaccharides handling in obese versus lean men: a lipid dose-effect trial. J. Clin. Endocrinol. Metab. 2015;100:3427–3435. doi: 10.1210/jc.2015-2518. [DOI] [PubMed] [Google Scholar]
- 51.Grundy S.M., Stone N.J., Bailey A.L., Beam C., Birtcher K.K., Blumenthal R.S., Braun L.T., de Ferranti S., Faiella-Tommasino J., Forman D.E., Goldberg R., Heidenreich P.A., Hlatky M.A., Jones D.W., Lloyd-Jones D., Lopez-Pajares N., Ndumele C.E., Orringer C.E., Peralta C.A., Saseen J.J., Smith S.C., Jr., Sperling L., Virani S.S., Yeboah J. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: executive summary: a report of the American College of Cardiology/American Heart association Task force on clinical practice guidelines. J. Am. Coll. Cardiol. 2019;73(24):3168–3209. doi: 10.1016/j.jacc.2018.11.002. [DOI] [PubMed] [Google Scholar]
- 52.You Q., Chen F., Wang X., Luo P.G., Jiang Y. Inhibitory effects of muscadine anthocyanins on alpha-glucosidase and pancreatic lipase activities. J. Agric. Food Chem. 2011;59:9506–9511. doi: 10.1021/jf201452v. [DOI] [PubMed] [Google Scholar]
- 53.Kwon O., Eck P., Chen S., Corpe C.P., Lee J.H., Kruhlak M., Levine M. Inhibition of the intestinal glucose transporter GLUT2 by flavonoids. Faseb. J. 2007;21:366–377. doi: 10.1096/fj.06-6620com. [DOI] [PubMed] [Google Scholar]
- 54.Oteiza P.I., Fraga C.G., Mills D.A., Taft D.H. Flavonoids and the gastrointestinal tract: local and systemic effects. Mol. Aspect. Med. 2018;61:41–49. doi: 10.1016/j.mam.2018.01.001. [DOI] [PubMed] [Google Scholar]
- 55.Czank C., Cassidy A., Zhang Q., Morrison D.J., Preston T., Kroon P.A., Botting N.P., Kay C.D. Human metabolism and elimination of the anthocyanin, cyanidin-3-glucoside: a (13)C-tracer study. Am. J. Clin. Nutr. 2013;97:995–1003. doi: 10.3945/ajcn.112.049247. [DOI] [PubMed] [Google Scholar]
- 56.de Ferrars R.M., Czank C., Zhang Q., Botting N.P., Kroon P.A., Cassidy A., Kay C.D. The pharmacokinetics of anthocyanins and their metabolites in humans. Br. J. Pharmacol. 2014;171:3268–3282. doi: 10.1111/bph.12676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rodriguez-Mateos A., Del Pino-García R., George T.W., Vidal-Diez A., Heiss C., Spencer J.P. Impact of processing on the bioavailability and vascular effects of blueberry (poly)phenols. Mol. Nutr. Food Res. 2014;58:1952–1961. doi: 10.1002/mnfr.201400231. [DOI] [PubMed] [Google Scholar]
- 58.Curtis P.J., van der Velpen V., Berends L., Jennings A., Feelisch M., Umpleby A.M., Evans M., Fernandez B.O., Meiss M.S., Minnion M., Potter J., Minihane A.M., Kay C.D., Rimm E.B., Cassidy A. Blueberries improve biomarkers of cardiometabolic function in participants with metabolic syndrome-results from a 6-month, double-blind, randomized controlled trial. Am. J. Clin. Nutr. 2019;109:1535–1545. doi: 10.1093/ajcn/nqy380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nieman D.C., Gillitt N.D., Chen G.Y., Zhang Q., Sha W., Kay C.D., Chandra P., Kay K.L., Lila M.A. Blueberry and/or banana consumption mitigate arachidonic, cytochrome P450 oxylipin generation during recovery from 75-km cycling: a randomized trial. Front. Nutr. 2020;7:121. doi: 10.3389/fnut.2020.00121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wu X., Cao G., Prior R.L. Absorption and metabolism of anthocyanins in elderly women after consumption of elderberry or blueberry. J. Nutr. 2002;132:1865–1871. doi: 10.1093/jn/132.7.1865. [DOI] [PubMed] [Google Scholar]
- 61.Kay C.D., Mazza G.J., Holub B.J. Anthocyanins exist in the circulation primarily as metabolites in adult men. J. Nutr. 2005;135:2582–2588. doi: 10.1093/jn/135.11.2582. [DOI] [PubMed] [Google Scholar]
- 62.Fernandes I., Azevedo J., Faria A., Calhau C., de Freitas V., Mateus N. Enzymatic hemisynthesis of metabolites and conjugates of anthocyanins. J. Agric. Food Chem. 2009;57:735–745. doi: 10.1021/jf802844p. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.